Depth-Specific Soil Electrical Conductivity and NDVI Elucidate Salinity Effects on Crop Development in Reclaimed Marsh Soils
Abstract
1. Introduction
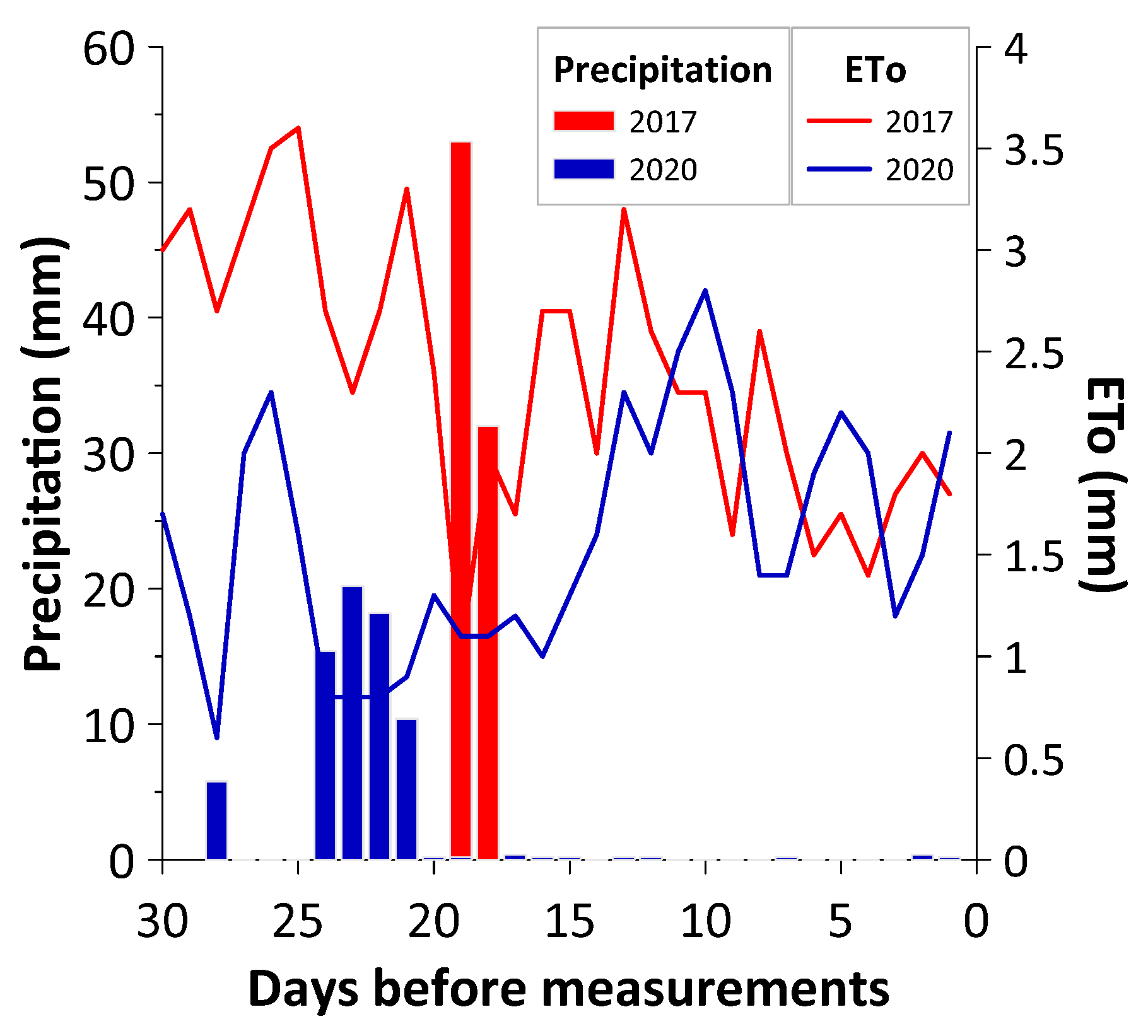
2. Materials and Methods
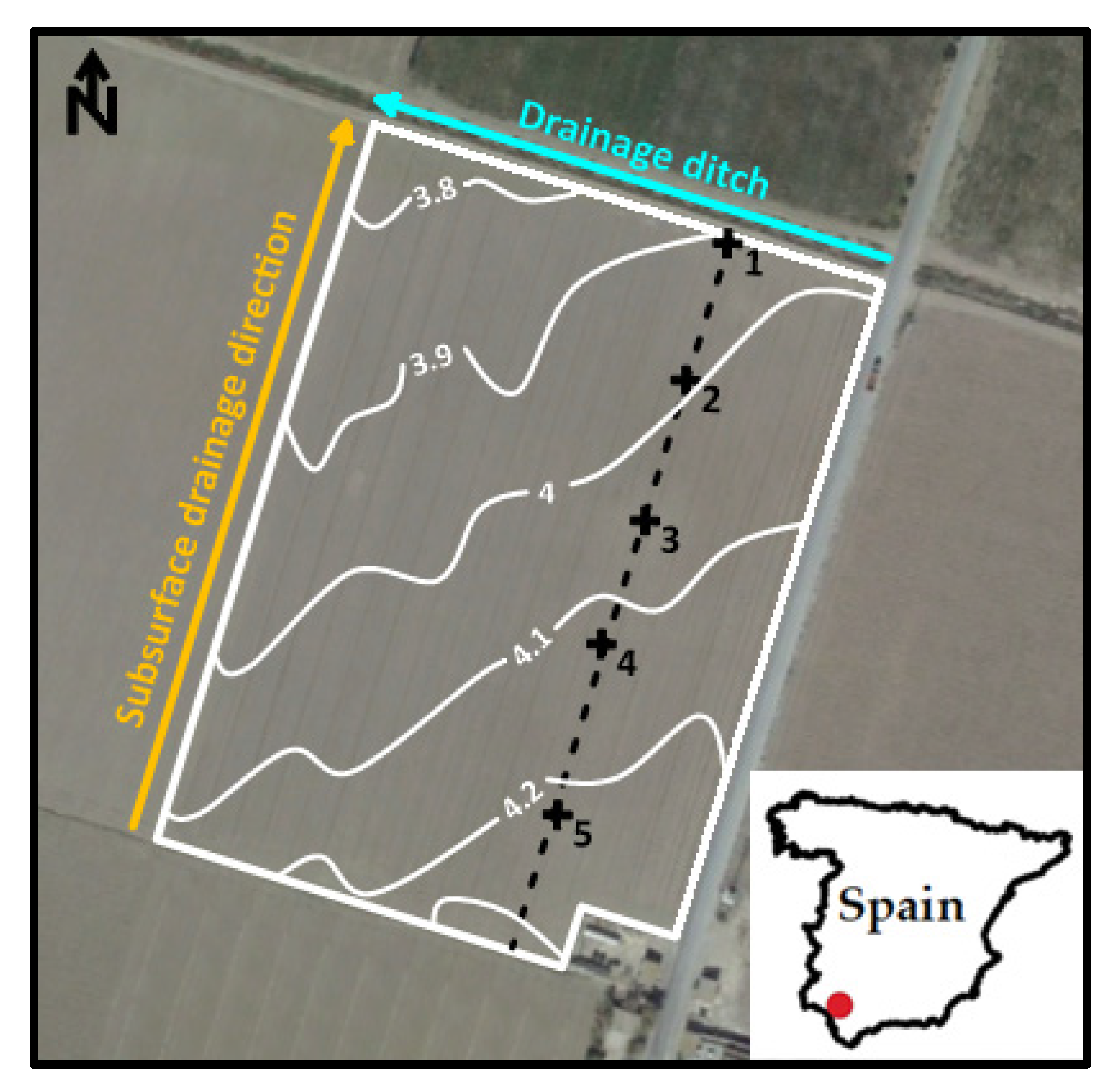
2.1. Field Description
2.2. Electromagnetic Induction Sensing of ECa and Inversion
2.3. Soil Sampling and Laboratory Analysis
2.4. Estimation of Soil Salinity Status
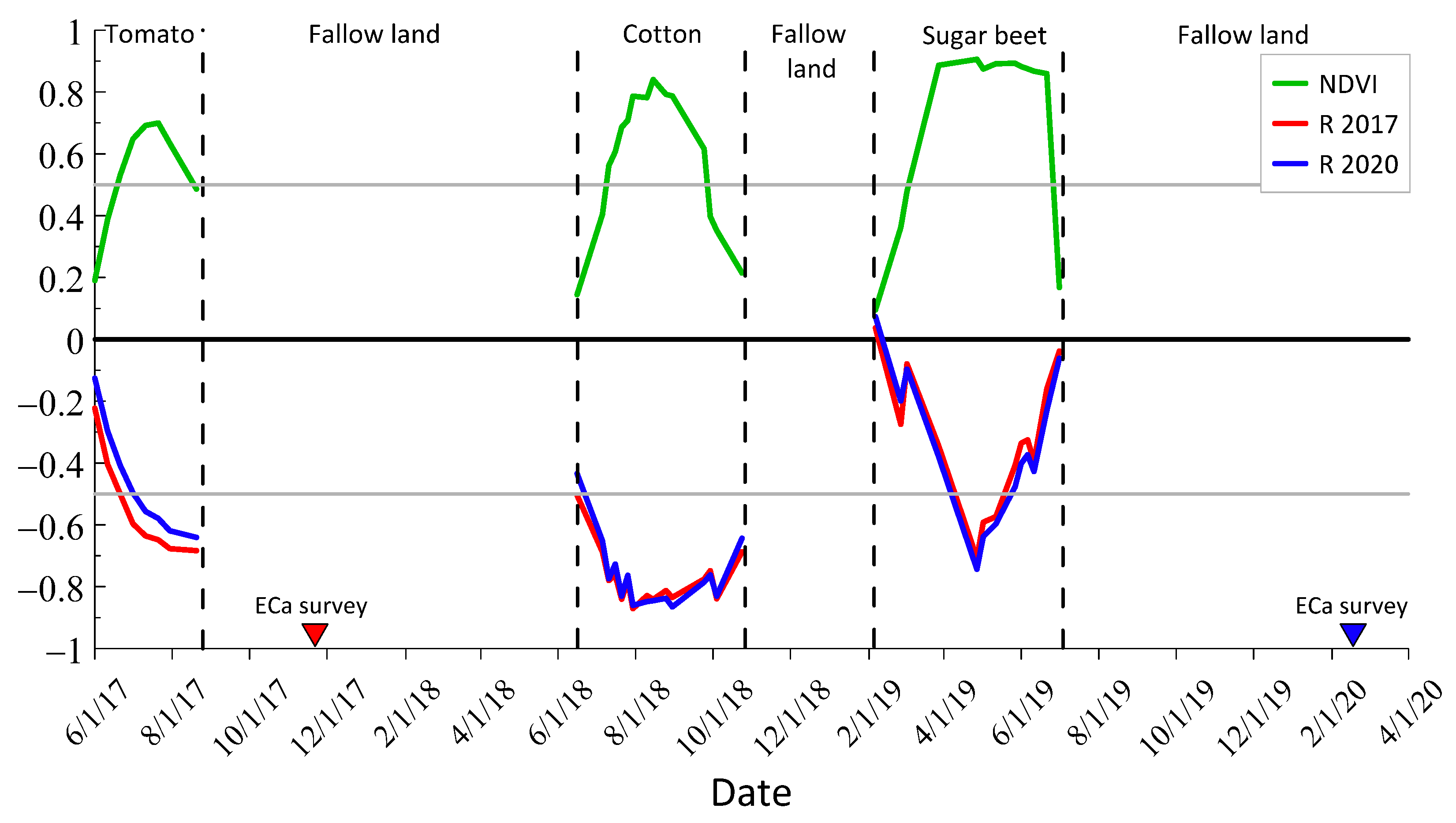
2.5. NDVI Imagery Processing and Analysis
3. Results and Discussion
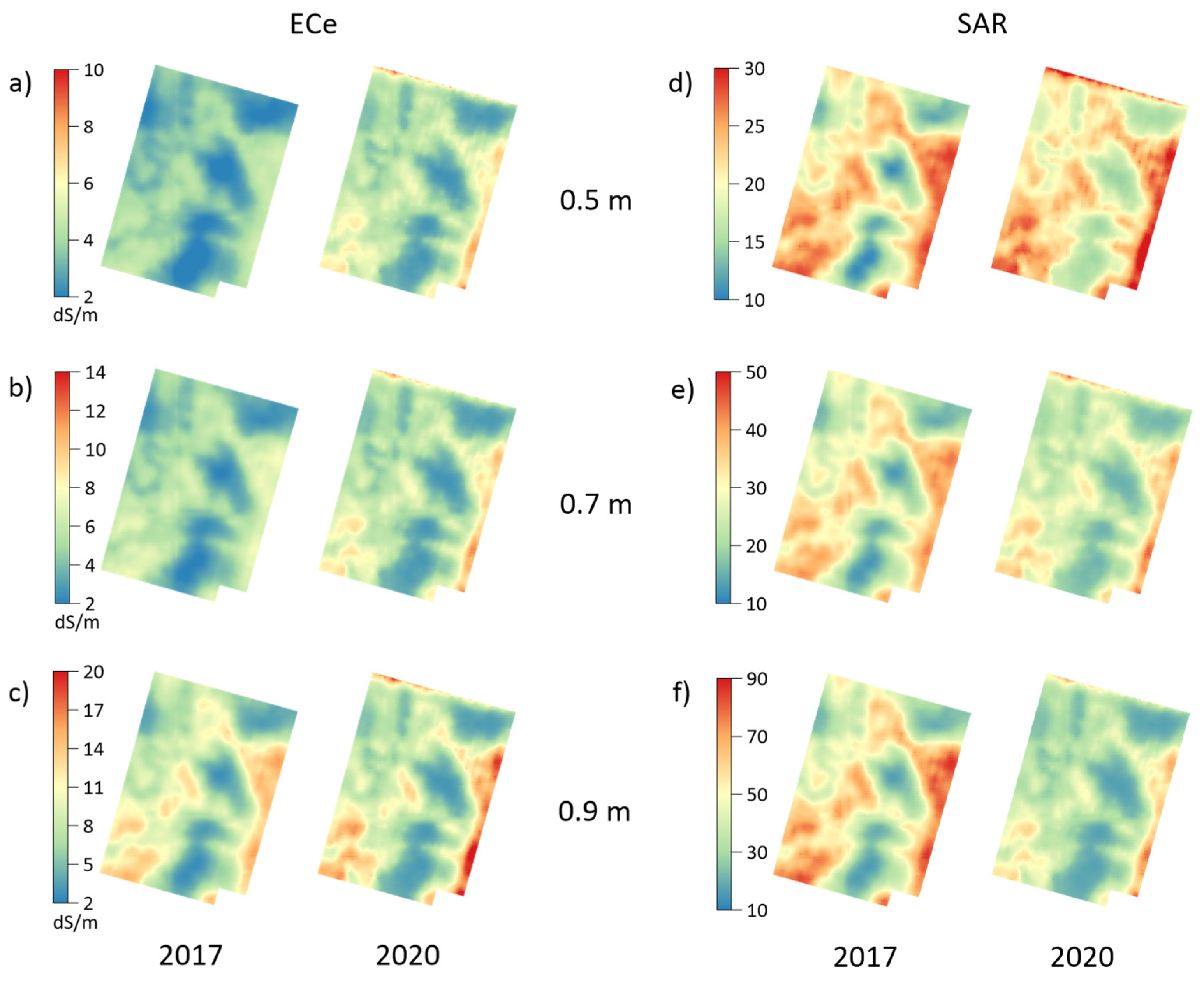
3.1. Soil Properties in 2017 and 2020
3.2. Depth-Specific Increments of Soil Properties between 2017 and 2020
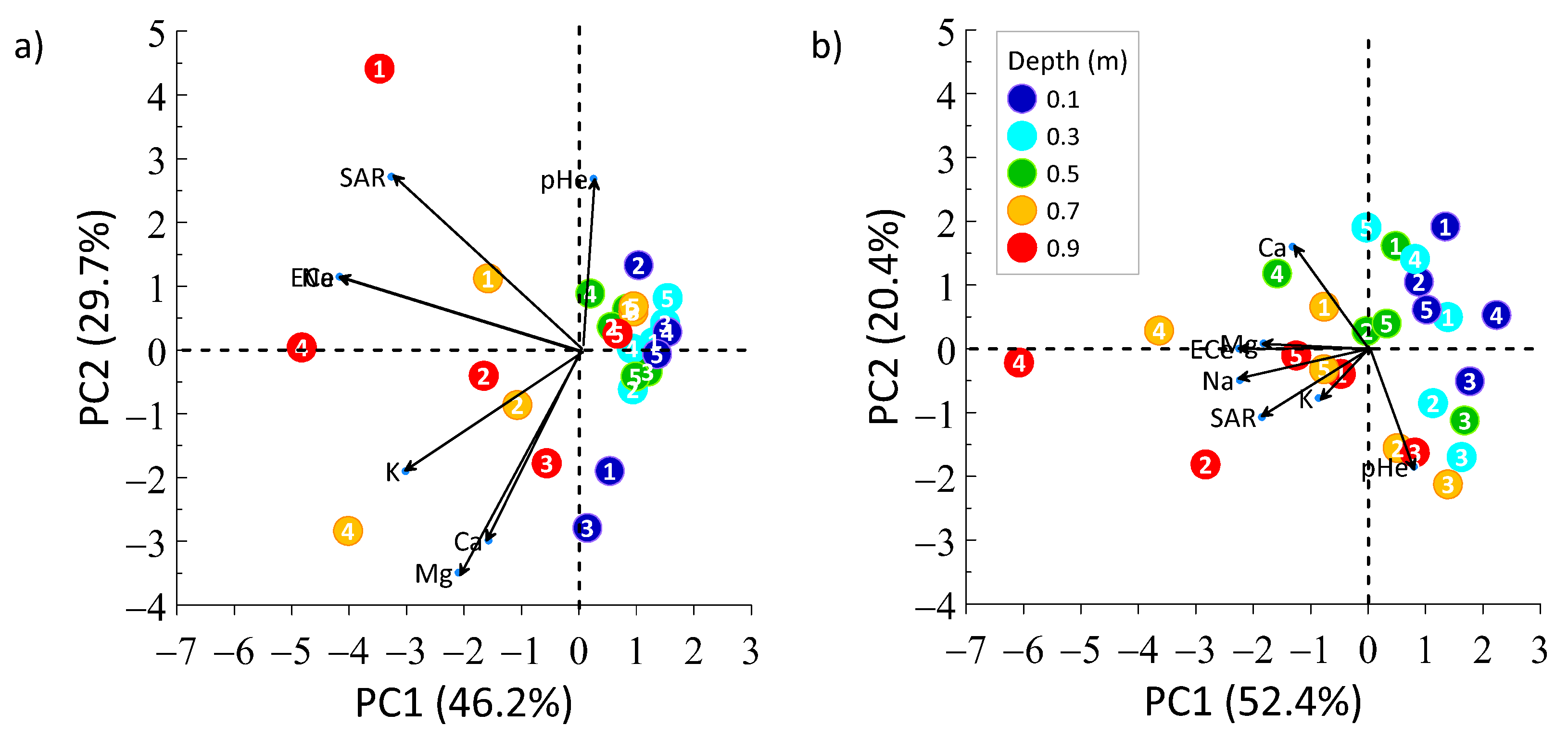
3.3. PCA of Soil Properties in 2017 and 2020
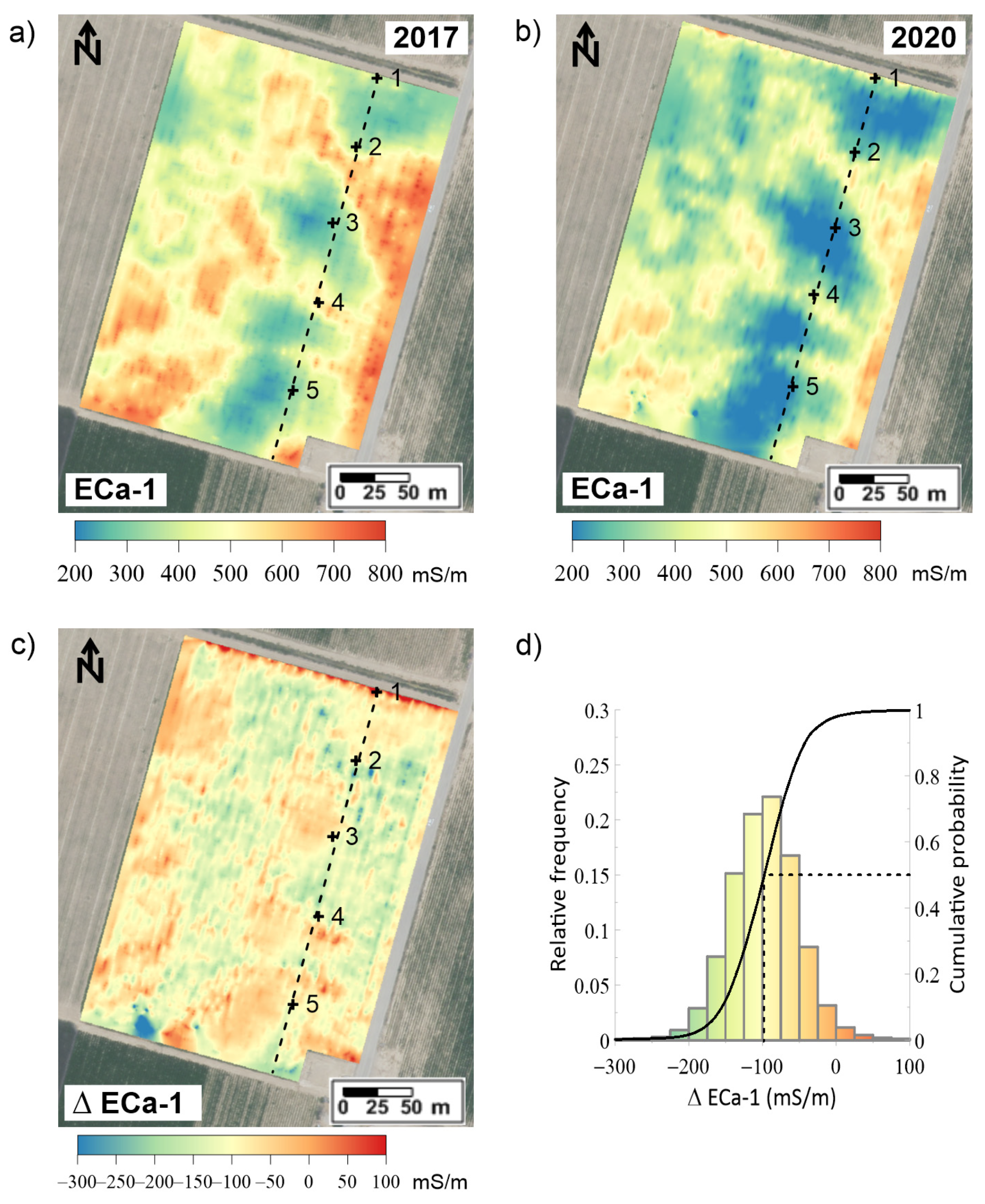
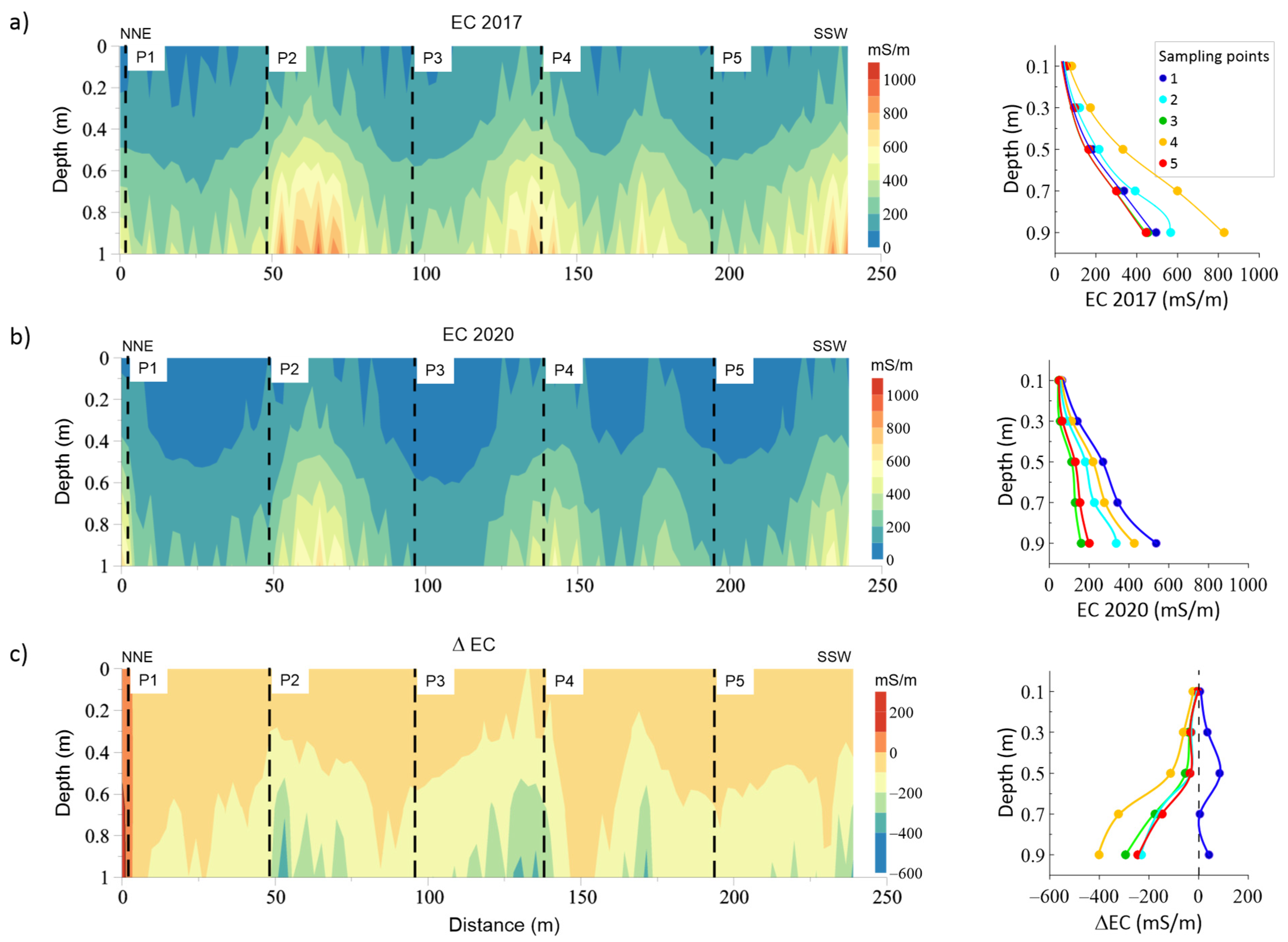
3.4. ECa in 2017 and 2020
3.5. 2D inversion of EMI Datasets: EC Variations along a Transect and Calibration
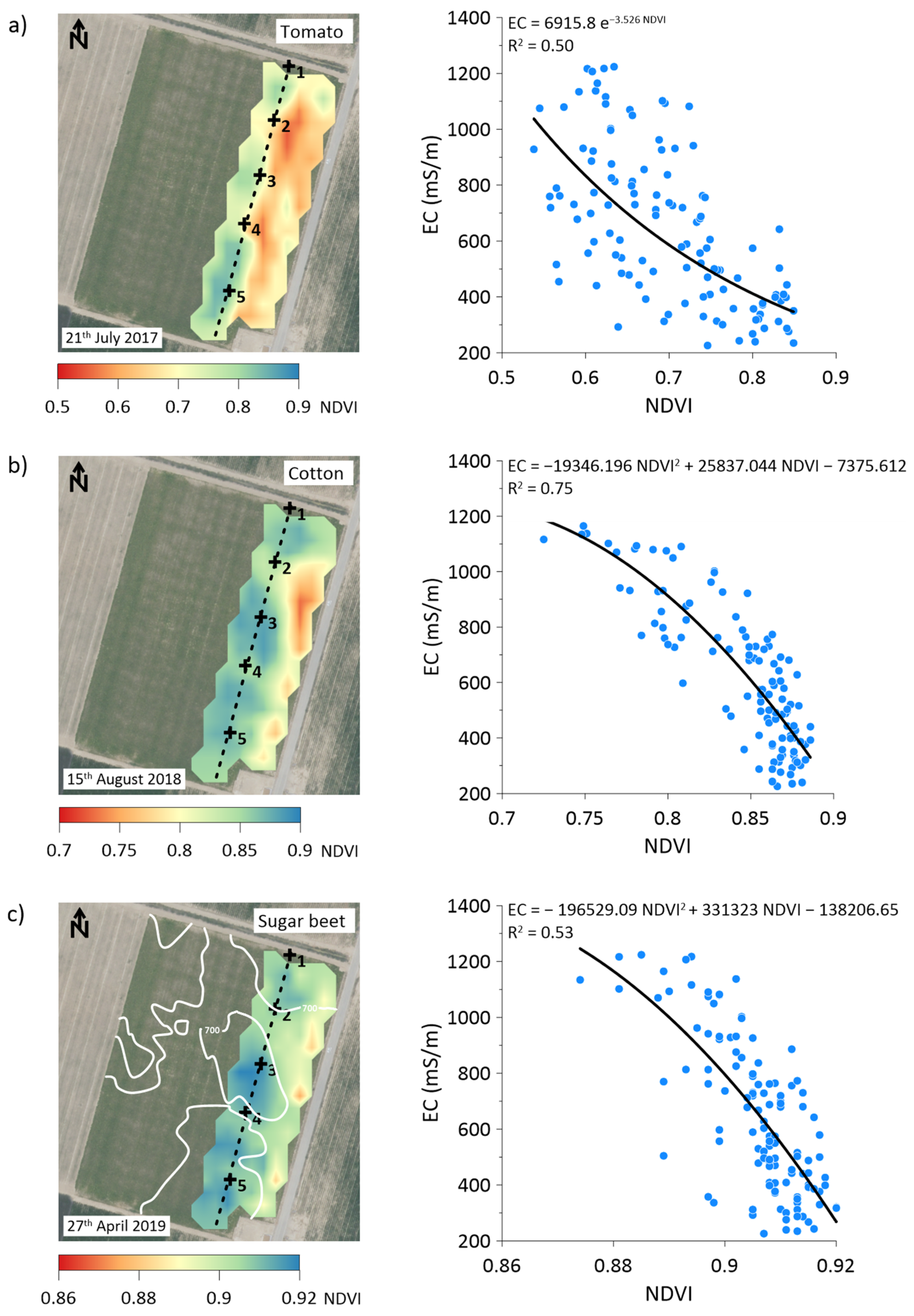
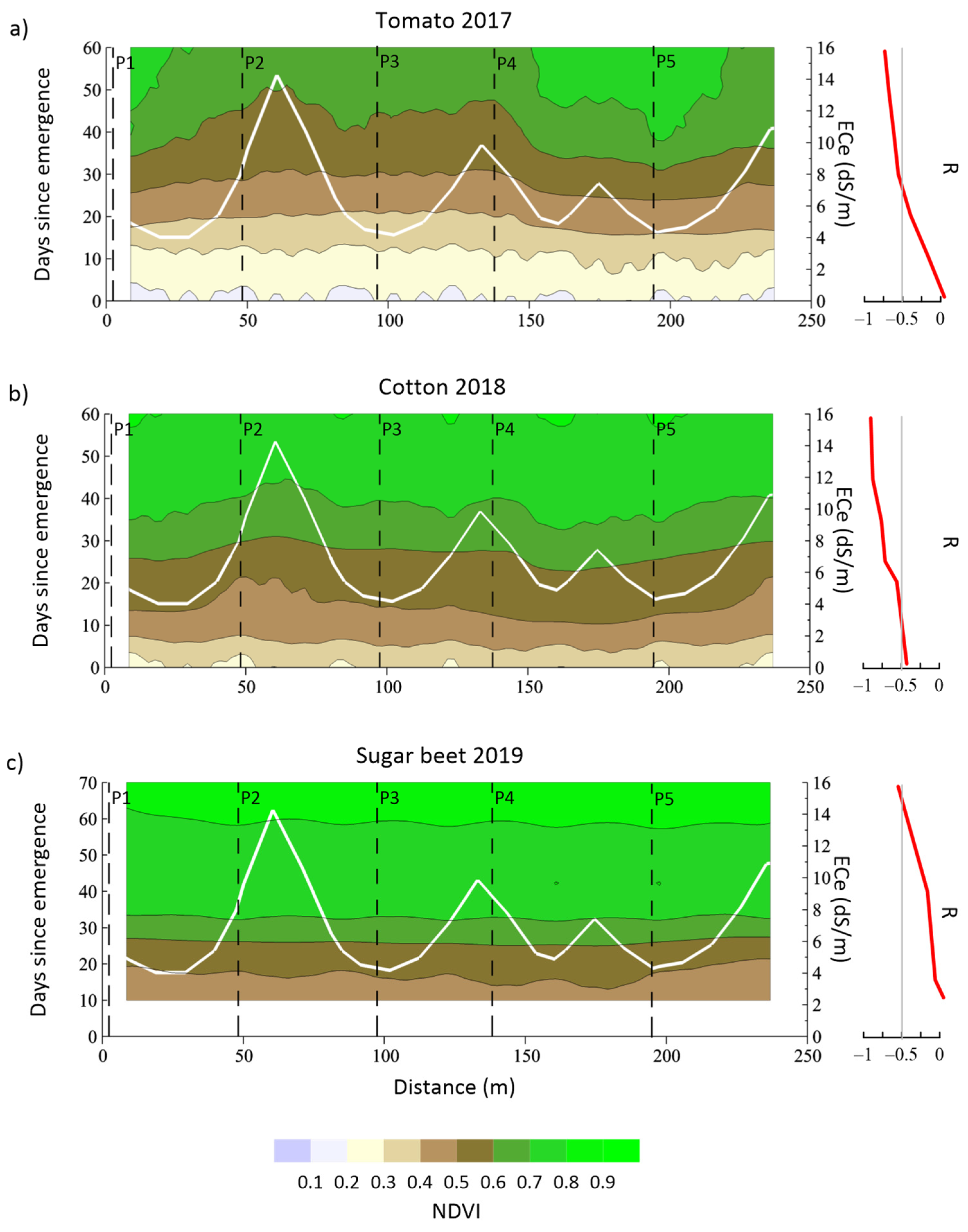
3.6. Correlation between Depth-Specific EC and NDVI
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Assouline, S.; Russo, D.; Silber, A.; Or, D. Balancing water scarcity and quality for sustainable irrigated agriculture. Water Resour. Res. 2015, 51, 3419–3436. [Google Scholar] [CrossRef]
- Hopmans, J.W.; Qureshi, A.S.; Kisekka, I.; Munns, R.; Grattan, S.R.; Rengasamy, P.; Taleisnik, E. Critical knowledge gaps and research priorities in global soil salinity. Adv. Agron. 2021, 169, 1–191. [Google Scholar]
- Corwin, D.L.; Yemoto, K. Salinity: Electrical Conductivity and Total Dissolved Solids. In Methods of Soil Analysis; SSSA Book Ser. 5; SSSA: Madison, WI, USA, 2017; Volume 2. [Google Scholar]
- Visconti, F.; de Paz, J.M. Field Comparison of Electrical Resistance, Electromagnetic Induction, and Frequency Domain Reflectometry for Soil Salinity Appraisal. Soil Syst. 2020, 4, 61. [Google Scholar]
- Doolittle, J.A.; Brevik, E.C. The use of electromagnetic induction techniques in soils studies. Geoderma 2014, 223–225, 33–45. [Google Scholar] [CrossRef]
- Pedrera-Parrilla, A.; Van De Vijver, E.; Van Meirvenne, M.; Espejo-Pérez, A.J.; Giráldez, J.V.; Vanderlinden, K. Apparent electrical conductivity measurements in an olive orchard under wet and dry soil conditions: Significance for clay and soil water content mapping. Precis. Agric. 2016, 17, 531–545. [Google Scholar] [CrossRef]
- Triantafilis, J.; Laslett, G.M.; McBratney, A.B. Calibrating an electromagnetic induction instrument to measure salinity in soil under irrigated cotton. Soil Sci. Soc. Am. J. 2000, 64, 1009–1017. [Google Scholar] [CrossRef]
- Nogués, J.; Robinson, D.A.; Herrero, J. Incorporating electromagnetic induction methods into regional soil salinity survey of irrigation districts. Soil Sci. Soc. Am. J. 2006, 70, 2075–2085. [Google Scholar]
- Corwin, D.L.; Scudiero, E. Field-Scale Apparent Soil Electrical Conductivity. In Methods Soil Analysis; SSSA Book Ser. 5; SSSA: Madison, WI, USA, 2016; Volume 1. [Google Scholar]
- Binley, A.; Hubbard, S.S.; Huisman, J.A.; Revil, A.; Robinson, D.A.; Singha, K.; Slater, L.D. The emergence of hydrogeophysics for improved understanding of subsurface processes over multiple scales. Water Resour. Res. 2015, 51, 3837–3866. [Google Scholar] [CrossRef]
- Triantafilis, J.; Monteiro Santos, F.A. Electromagnetic conductivity imaging (EMCI) of soil using a DUALEM-421 and inversion modelling software (EM4Soil). Geoderma 2013, 211–212, 28–38. [Google Scholar] [CrossRef]
- McLachlan, P.; Blanchy, G.; Binley, A. EMagPy: Open-source standalone software for processing, forward modeling and inversion of electromagnetic induction data. Comput. Geosci. 2021, 146, 104561. [Google Scholar]
- Jadoon, K.Z.; Moghadas, D.; Jadoon, A.; Missimer, T.M.; Al-Mashharawi, S.K.; McCabe, F.M. Estimation of soil salinity in a drip irrigation system by using joint inversion of multicoil electromagnetic induction measurements. Water Resour. Res. 2015, 51, 3490–3504. [Google Scholar] [CrossRef]
- Zare, E.; Huang, J.; Monteiro Santos, F.M.; Triantafilis, J. Mapping salinity in three dimensions using a DUALEM-421 and electromagnetic inversion software. Soil Sci. Soc. Am. J. 2015, 79, 1729–1740. [Google Scholar] [CrossRef]
- Koganti, T.; Narjary, B.; Zare, E.; Pathan, A.L.; Huang, J.; Triantafilis, J. Quantitative mapping of soil salinity using the DUALEM-21S instrument and EM inversion software. Land Degrad. Dev. 2018, 29, 1768–1781. [Google Scholar] [CrossRef]
- Farzamian, M.; Paz, M.C.; Paz, A.M.; Castanheira, N.L.; Gonçalves, M.C.; Monteiro Santos, F.A.; Triantafilis, J. Mapping soil salinity using electromagnetic conductivity imaging—A comparison of regional and location-specific calibrations. Land Degrad. Dev. 2019, 30, 3317. [Google Scholar]
- Paz, A.M.; Castanheira, N.; Farzamian, M.; Paz, M.C.; Gonçalves, M.C.; Monteiro Santos, F.A.; Triantafilis, J. Prediction of soil salinity and sodicity using electromagnetic conductivity imaging. Geoderma 2020, 361, 114086. [Google Scholar] [CrossRef]
- Huang, S.; Tang, L.; Hupy, J.P.; Wang, Y.; Shao, G. A commentary review on the use of normalized difference vegetation index (NDVI) in the era of popular remote sensing. J. For. Res. 2021, 32, 1–6. [Google Scholar]
- Serrano, J.M.; Shaidian, S.; Marques da Silva, J.; Carvalho, M. Monitoring of soil organic carbon over 10 years in a Mediterranean silvo-pastoral system: Potential evaluation for differential management. Precis. Agric. 2016, 17, 274–295. [Google Scholar] [CrossRef]
- Pedrera-Parrilla, A.; Martínez, G.; Espejo-Pérez, A.J.; Gómez, J.A.; Giráldez, J.V.; Vanderlinden, K. Mapping impaired olive tree development using electromagnetic induction surveys. Plant Soil 2014, 384, 381–400. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Moreno, F.; Martín, J.; Mudarra, J.L. A soil sequence in the natural and reclaimed marshes of the Guadalquivir river, Seville (Spain). Catena 1981, 8, 201–211. [Google Scholar] [CrossRef][Green Version]
- Domínguez, R.; Campillo, C.D.; Peña, F.; Delgado, A. Effect of soil properties and reclamation practices on phosphorus dynamics in reclaimed calcareous marsh soils from the Guadalquivir Valley, SW Spain. Arid Land Res. Manag. 2001, 15, 203–221. [Google Scholar] [CrossRef]
- Google Earth. Available online: https://earth.google.com/web (accessed on 12 January 2022).
- Red de Información Agroclimática de Andalucía (RIA). Available online: https://ifapa.junta-andalucia.es/agriculturaypesca/ifapa/riaweb/web/inicio_estaciones (accessed on 10 September 2021).
- González Jiménez, A.; Pachepsky, Y.; Gómez Flores, J.L.; Ramos Rodríguez, M.; Vanderlinden, K. Correcting on-the-go field measurement–coordinate mismatch by minimizing nearest neighbor difference. Sensors 2022, 22, 1496. [Google Scholar] [CrossRef]
- Rhoades, J.D. Soluble salts. In Methods of Soil Analysis, 2nd ed.; Page, A.L., Ed.; Agronomy Monograph No 9; American Society of Agronomy: Madison, WI, USA, 1982; Part 2, pp. 167–179. [Google Scholar]
- Sposito, G. The Chemistry of Soils, 2nd ed.; Oxford University Press: New York, NY, USA, 2008; pp. 296–315. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Phil. Trans. R. Soc. A 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Legates, D.R.; McCabe, G.J., Jr. Evaluating the use of “goodness-of-fit” measures in hydrologic and hydroclimatic model validation. Water Resour. Res. 1999, 35, 233–242. [Google Scholar] [CrossRef]
- Ritter, A.; Muñoz-Carpena, R. Performance evaluation of hydrological models: Statistical significance for reducing subjectivity in goodness-of-fit assessments. J. Hydrol. 2013, 480, 33–45. [Google Scholar] [CrossRef]
- Google Earth Engine. Available online: https://code.earthengine.google.com/ (accessed on 15 October 2021).
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S. Salinity effects on nitrogen metabolism in plants–focusing on the activities of nitrogen metabolizing enzymes: A review. J. Plant. Nutr. 2018, 41, 1065–1081. [Google Scholar] [CrossRef]



| Mean | Depth (m) | Sampling Points | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.3 | 0.5 | 0.7 | 0.9 | 1 | 2 | 3 | 4 | 5 | |||
| 2017 | ECe | 3.62 | 1.62a | 1.61a | 2.50a | 4.92ab | 7.46b | 4.82a | 3.74a | 1.83a | 5.73a | 1.99a |
| SAR | 24.0 | 8.3a | 10.5a | 14.7ab | 28.0ab | 58.6b | 42.7a | 19.7a | 11.1a | 31.8a | 14.9a | |
| Na+ | 740 | 236a | 287a | 462a | 1045ab | 1662b | 975a | 706a | 373a | 1250a | 388a | |
| Ca2+ | 38.4 | 37.9a | 23.1a | 44.3a | 48.1a | 38.8a | 37.7a | 38.6a | 38.2a | 45.6a | 32.0a | |
| Mg2+ | 30.9 | 35.1a | 22.4a | 23.4a | 38.7a | 34.8a | 28.9a | 32.3a | 35.7a | 39.4a | 18.1a | |
| K+ | 28.4 | 24.6a | 19.7a | 20.8a | 42.7a | 34.1a | 24.0a | 32.6a | 28.6a | 39.7a | 16.8a | |
| pHe | 8.6 | 8.4a | 8.5a | 8.9a | 8.7a | 8.4a | 9.0a | 8.7a | 8.3a | 8.4a | 8.5a | |
| 2020 | ECe | 3.40 | 1.37a | 2.76ab | 2.89ab | 4.37ab | 5.60b | 2.88ab | 3.15ab | 1.62a | 5.92b | 3.43ab |
| SAR | 17.6 | 8.8a | 15.5ab | 14.7ab | 22.7bc | 26.4c | 15.8a | 20.3a | 14.4a | 20.3a | 17.3a | |
| Na+ | 735 | 343a | 448a | 644a | 919ab | 1323b | 642a | 848a | 444a | 1051a | 692a | |
| Ca2+ | 57.2 | 60.0a | 46.2a | 69.4a | 47.5a | 62.8a | 58.0ab | 51.1ab | 31.7a | 80.4b | 64.8ab | |
| Mg2+ | 45.5 | 35.7ab | 25.1a | 46.5ab | 49.9ab | 70.5b | 47.2a | 55.6a | 30.7a | 56.4a | 37.9a | |
| K+ | 33.2 | 28.5a | 27.7a | 34.2a | 37.1a | 38.7a | 14.5a | 25.0ab | 44.0b | 41.3b | 41.5b | |
| pHe | 7.8 | 7.8a | 7.7a | 7.8a | 7.9a | 8.0a | 7.5a | 8.2b | 8.6bc | 7.4a | 7.4a | |
| t-test | ECe | 0.203 | 0.007 | 0.379 | 0.629 | 0.290 | 0.298 | 0.256 | 0.525 | 0.729 | 0.043 | |
| SAR | 0.854 | 0.228 | 0.992 | 0.455 | 0.256 | 0.347 | 0.865 | 0.118 | 0.189 | 0.347 | ||
| Na+ | 0.025 | 0.010 | 0.032 | 0.533 | 0.450 | 0.439 | 0.076 | 0.088 | 0.309 | 0.005 | ||
| Ca2+ | 0.270 | 0.357 | 0.355 | 0.973 | 0.184 | 0.359 | 0.580 | 0.492 | 0.155 | 0.163 | ||
| Mg2+ | 0.957 | 0.778 | 0.121 | 0.489 | 0.098 | 0.367 | 0.157 | 0.636 | 0.364 | 0.038 | ||
| K+ | 0.709 | 0.291 | 0.130 | 0.678 | 0.490 | 0.194 | 0.413 | 0.148 | 0.742 | 0.004 | ||
| pHe | 0.237 | 0.056 | 0.028 | 0.029 | 0.389 | 2.0E-04 | 0.079 | 0.287 | 0.005 | 0.006 | ||
| ECa-1 | ECa-3 | |||
|---|---|---|---|---|
| 2017 | 2020 | 2017 | 2020 | |
| m* | 474.0 | 376.0 | 566.8 | 484.2 |
| min | 204.9 | 96.1 | 337.1 | 266.3 |
| max | 831.0 | 753.1 | 790.0 | 780.7 |
| med | 477.5 | 376.3 | 574.4 | 491.1 |
| s | 118.7 | 109.3 | 98.3 | 100.0 |
| CV | 0.25 | 0.29 | 0.17 | 0.21 |
| kurtosis | −0.826 | −0.779 | −0.885 | −0.866 |
| skewness | 0.019 | 0.128 | −0.185 | −0.121 |
| Depth (m) | |||||||
|---|---|---|---|---|---|---|---|
| 0–0.9 | 0.1 | 0.3 | 0.5 | 0.7 | 0.9 | ||
| 2017 | ECe | 0.91 | 0.05 | 0.91 | 0.96 | 0.99 | 0.98 |
| SAR | 0.88 | 0.33 | 0.72 | 0.96 | 0.93 | 0.97 | |
| Na+ | 0.91 | 0.22 | 0.94 | 0.94 | 0.98 | 0.99 | |
| Ca2+ | 0.46 | −0.51 | −0.09 | −0.74 | 0.98 | 0.24 | |
| Mg2+ | 0.46 | −0.58 | 0.71 | 0.33 | 0.99 | −0.06 | |
| K+ | 0.63 | −0.55 | 0.91 | 0.93 | 0.66 | 0.82 | |
| pHe | −0.19 | 0.35 | −0.50 | 0.67 | −0.25 | −0.17 | |
| 2020 | ECe | 0.89 | 0.12 | 0.27 | 0.92 | 0.73 | 0.91 |
| SAR | 0.88 | 0.04 | −0.22 | 0.55 | 0.81 | 0.94 | |
| Na+ | 0.95 | −0.18 | 0.06 | 0.86 | 0.82 | 0.99 | |
| Ca2+ | 0.36 | −0.14 | 0.19 | 0.97 | 0.76 | 0.85 | |
| Mg2+ | 0.73 | −0.22 | −0.05 | 0.75 | 0.84 | 0.99 | |
| K+ | 0.35 | −0.74 | −0.91 | −0.55 | 0.00 | 0.35 | |
| pHe | −0.18 | −0.45 | −0.45 | −0.53 | −0.52 | −0.51 | |
| EC | ECe | SAR | ||||
|---|---|---|---|---|---|---|
| RMSE | MAE | NSE | RMSE | MAE | NSE | |
| 2017 | 1.44 | 1.08 | 0.74 | 14.67 | 9.16 | 0.57 |
| Topsoil | 0.61 | 0.47 | −2.05 | 4.33 | 3.03 | −1.30 |
| Subsoil | 1.4 | 1.23 | 0.71 | 11.6 | 8.26 | 0.51 |
| 2020 | 1.35 | 1.09 | 0.74 | 6.85 | 5.75 | 0.70 |
| Topsoil | 0.86 | 0.7 | 0.06 | 7.16 | 5.75 | −0.12 |
| Subsoil | 1.59 | 1.35 | 0.69 | 4.78 | 3.89 | 0.67 |
| R | 2017 | |||||
|---|---|---|---|---|---|---|
| Days since Emergence/Transplanting | EC 0.1 | EC 0.3 | EC 0.5 | EC 0.7 | EC 0.9 | |
| Tomato 2017 | 10 | −0.14 | −0.16 | −0.15 | −0.16 | −0.17 |
| 20 | −0.38 | −0.39 | −0.38 | −0.38 | −0.40 | |
| 30 | −0.54 | −0.55 | −0.55 | −0.54 | −0.56 | |
| 40 | −0.60 | −0.61 | −0.60 | −0.60 | −0.61 | |
| 50 | −0.66 | −0.68 | −0.67 | −0.67 | −0.68 | |
| 60 | −0.71 | −0.73 | −0.72 | −0.72 | −0.73 | |
| Cotton 2018 | 5 | −0.39 | −0.40 | −0.41 | −0.42 | −0.43 |
| 20 | −0.47 | −0.49 | −0.49 | −0.50 | −0.51 | |
| 25 | −0.67 | −0.69 | −0.69 | −0.70 | −0.71 | |
| 30 | −0.59 | −0.62 | −0.62 | −0.62 | −0.65 | |
| 35 | −0.70 | −0.73 | −0.73 | −0.74 | −0.76 | |
| 40 | −0.79 | −0.79 | −0.79 | −0.79 | −0.80 | |
| 45 | −0.79 | −0.84 | −0.84 | −0.85 | −0.87 | |
| 55 | −0.53 | −0.59 | −0.59 | −0.60 | −0.63 | |
| 60 | −0.85 | −0.88 | −0.88 | −0.88 | −0.90 | |
| Sugar beet 2019 | 5 | 0.11 | 0.12 | 0.11 | 0.11 | 0.11 |
| 30 | 0.07 | 0.06 | 0.06 | 0.05 | 0.03 | |
| 60 | −0.35 | −0.42 | −0.41 | −0.43 | −0.46 | |
| 70 | −0.57 | −0.55 | −0.55 | −0.55 | −0.53 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Flores, J.L.; Ramos Rodríguez, M.; González Jiménez, A.; Farzamian, M.; Herencia Galán, J.F.; Salvatierra Bellido, B.; Cermeño Sacristan, P.; Vanderlinden, K. Depth-Specific Soil Electrical Conductivity and NDVI Elucidate Salinity Effects on Crop Development in Reclaimed Marsh Soils. Remote Sens. 2022, 14, 3389. https://doi.org/10.3390/rs14143389
Gómez Flores JL, Ramos Rodríguez M, González Jiménez A, Farzamian M, Herencia Galán JF, Salvatierra Bellido B, Cermeño Sacristan P, Vanderlinden K. Depth-Specific Soil Electrical Conductivity and NDVI Elucidate Salinity Effects on Crop Development in Reclaimed Marsh Soils. Remote Sensing. 2022; 14(14):3389. https://doi.org/10.3390/rs14143389
Chicago/Turabian StyleGómez Flores, José Luis, Mario Ramos Rodríguez, Alfonso González Jiménez, Mohammad Farzamian, Juan Francisco Herencia Galán, Benito Salvatierra Bellido, Pedro Cermeño Sacristan, and Karl Vanderlinden. 2022. "Depth-Specific Soil Electrical Conductivity and NDVI Elucidate Salinity Effects on Crop Development in Reclaimed Marsh Soils" Remote Sensing 14, no. 14: 3389. https://doi.org/10.3390/rs14143389
APA StyleGómez Flores, J. L., Ramos Rodríguez, M., González Jiménez, A., Farzamian, M., Herencia Galán, J. F., Salvatierra Bellido, B., Cermeño Sacristan, P., & Vanderlinden, K. (2022). Depth-Specific Soil Electrical Conductivity and NDVI Elucidate Salinity Effects on Crop Development in Reclaimed Marsh Soils. Remote Sensing, 14(14), 3389. https://doi.org/10.3390/rs14143389







