Soil Moisture Influence on the FTIR Spectrum of Salt-Affected Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling Sites
2.2. Laboratory Experimental Design
2.2.1. Analysis of Soil Characteristics in the Laboratory
2.2.2. Soil Salinity Experiment
- Salinity level 1 (slevel 1): 100 g of air-dried and sieved soil, to which 0.35 g salt was added (NaCl or Na2SO4 or Na2CO3 or a mixture of salts). Water was added until saturated soil moisture content;
- Salinity level 1 (slevel 2): 100 g air-dried and sieved soil, to which 1.75 g salt was added (NaCl or Na2SO4 or Na2CO3 or a mixture of salts). Water was added until saturated soil moisture content;
- Salinity level 1 (slevel 3): 100 gr air-dried and sieved soil, to which 3.5 g salt was added (NaCl or Na2SO4 or Na2CO3 or a mixture of salts). Water was added until saturated soil moisture content;
- Salinity level 1 (slevel 4): 100 g of air-dried and sieved soil, to which 7 g salt was added (NaCl or Na2SO4 or Na2CO3 or a mixture of salts). Water was added until saturated soil moisture content.
2.2.3. Soil Spectral Measurements and Analysis
- Sampling day 1: The soil samples were saturated with water. The soil moisture and FTIR spectrum were measured;
- Sampling days 2–4: The soil samples were left to air-dry. The soil moisture and FTIR spectrum were measured. The duration from 1st to 2nd day, 2nd to 3rd and 3rd to 4th was one day and night;
- Sampling days 5–6: The soil samples were dried at 105 °C until constant weight. The soil moisture, FTIR spectrum and electrical conductivity were measured. The duration from the 4th to 5th day was 2 days and nights.
3. Results
3.1. Salinity and Moisture Characteristics
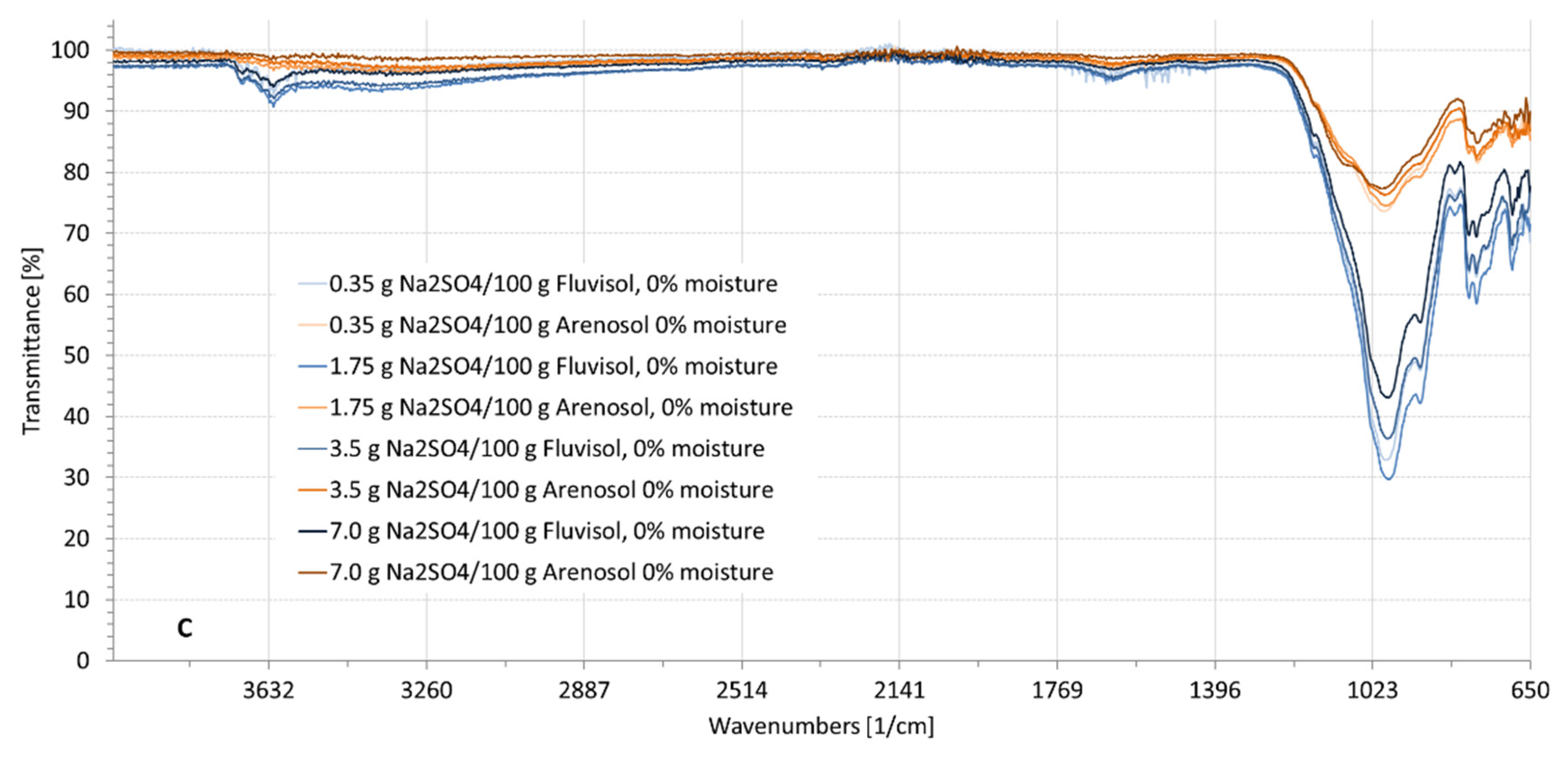
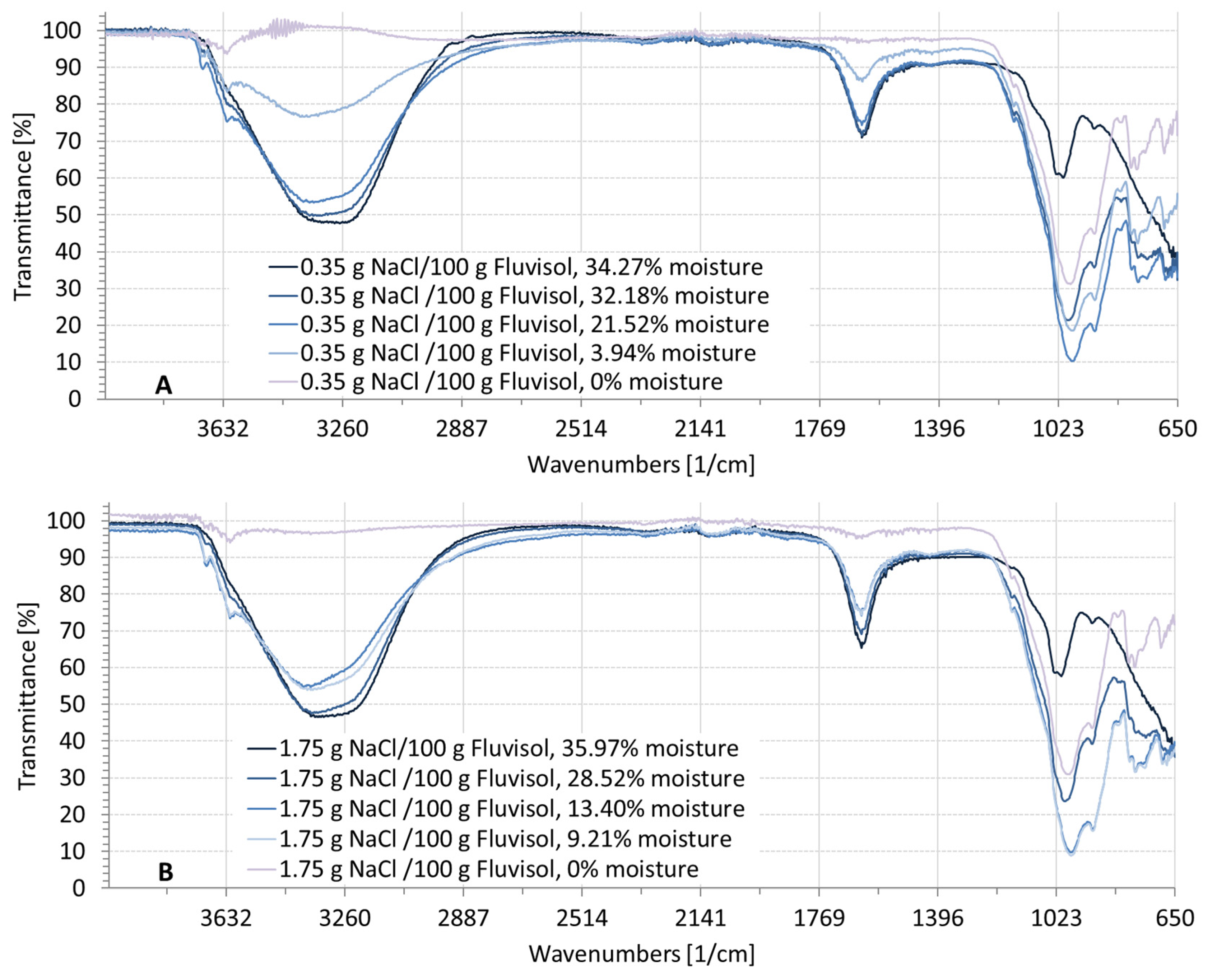
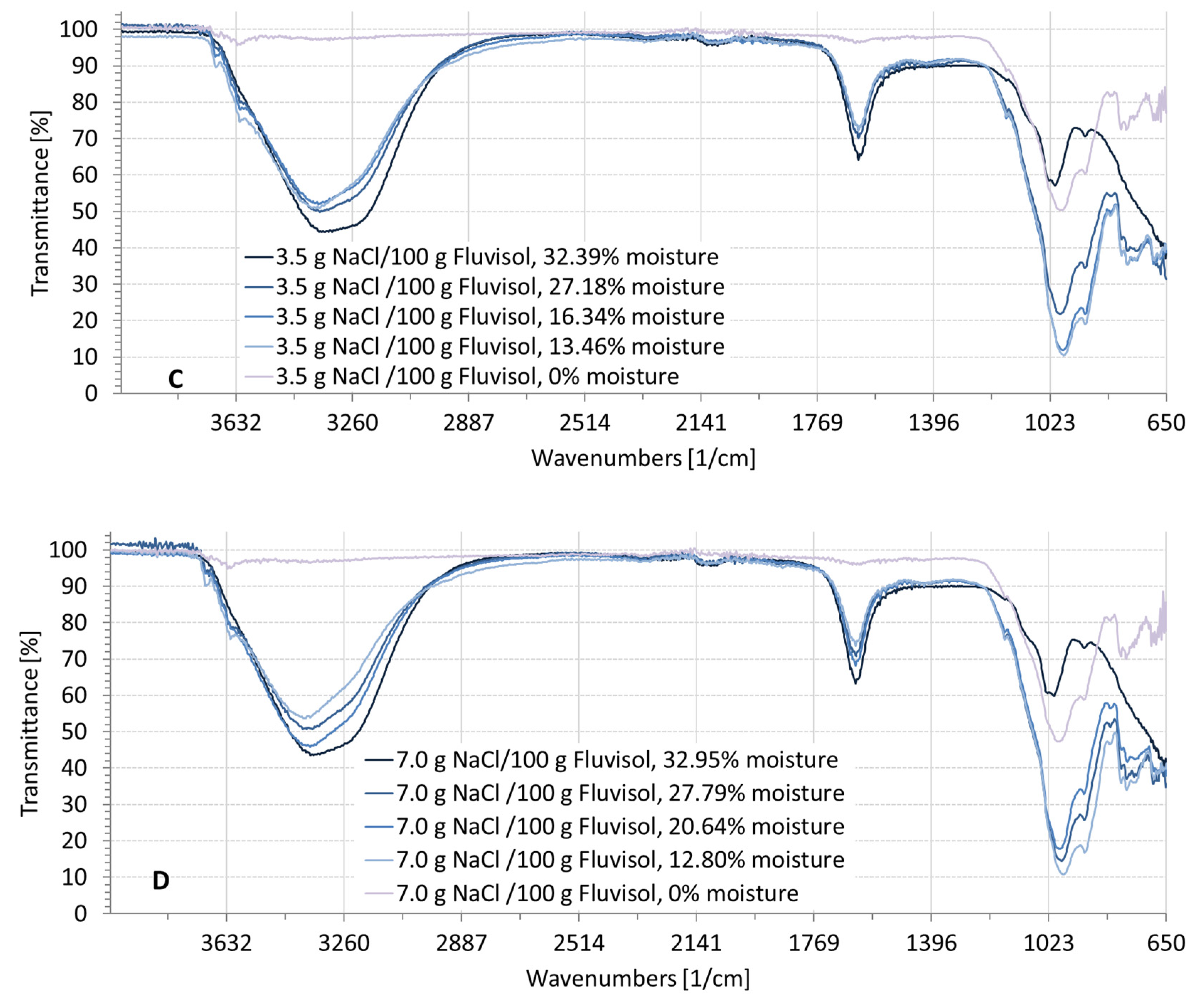
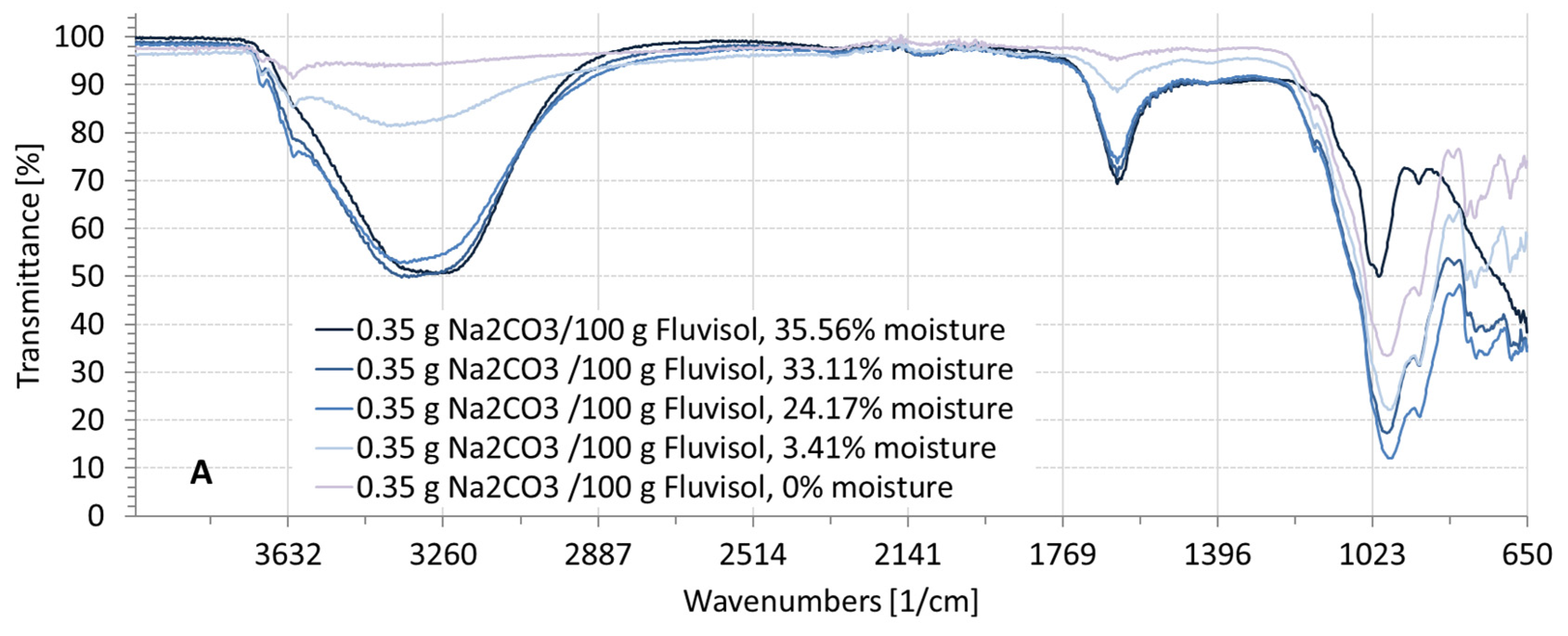
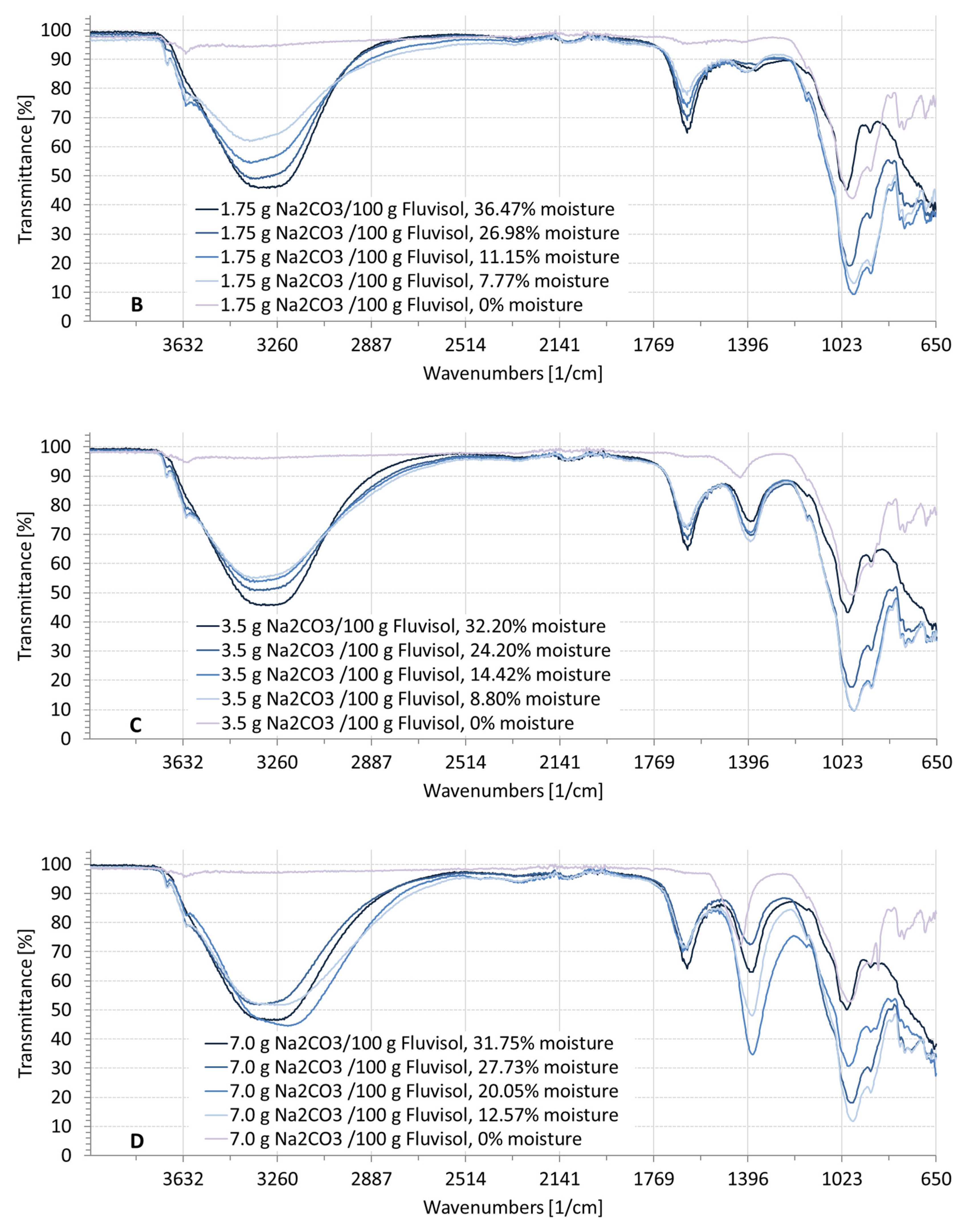
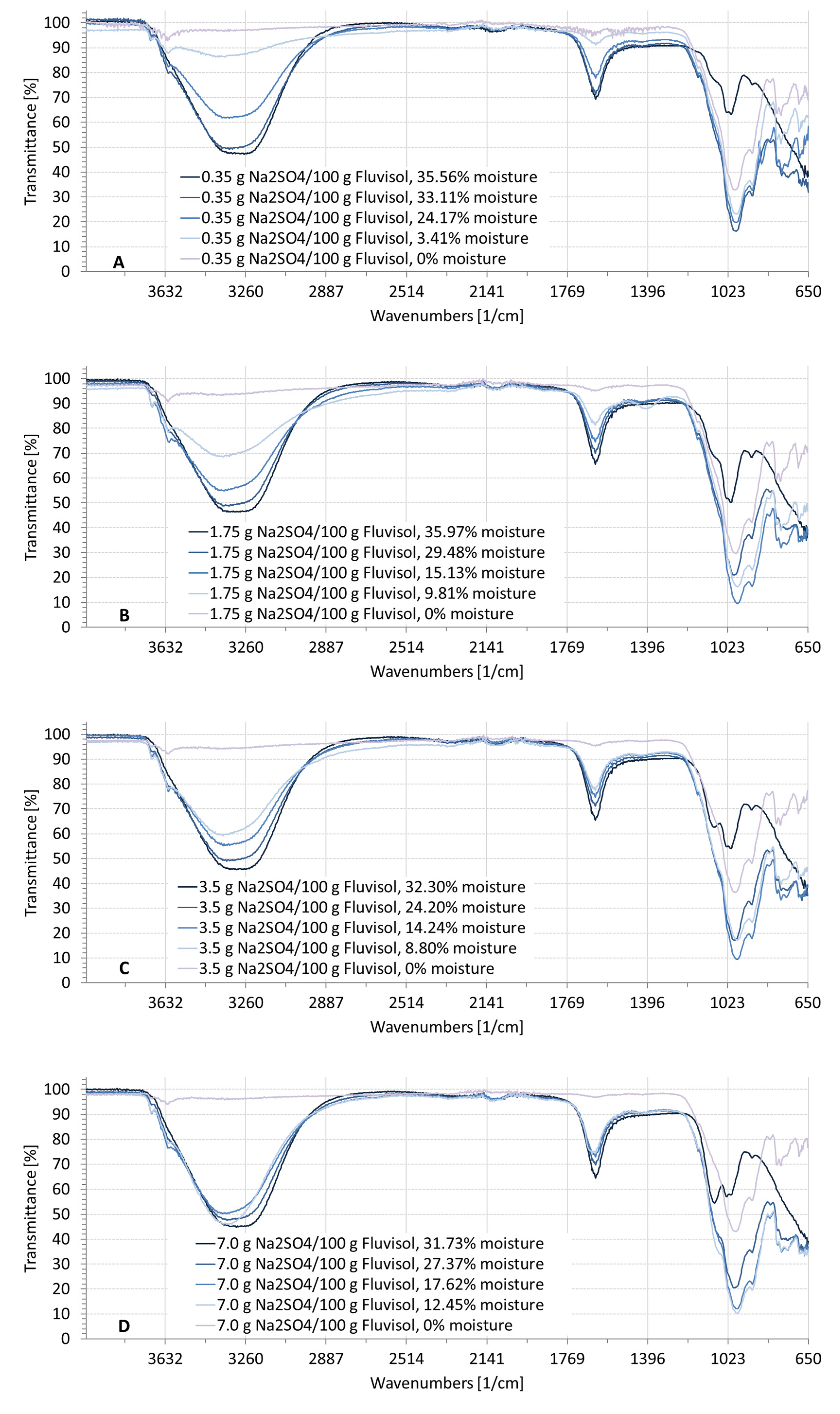
3.2. Spectral Characteristics of Soil Samples Contaminated with NaCl, Na2SO4, Na2CO3
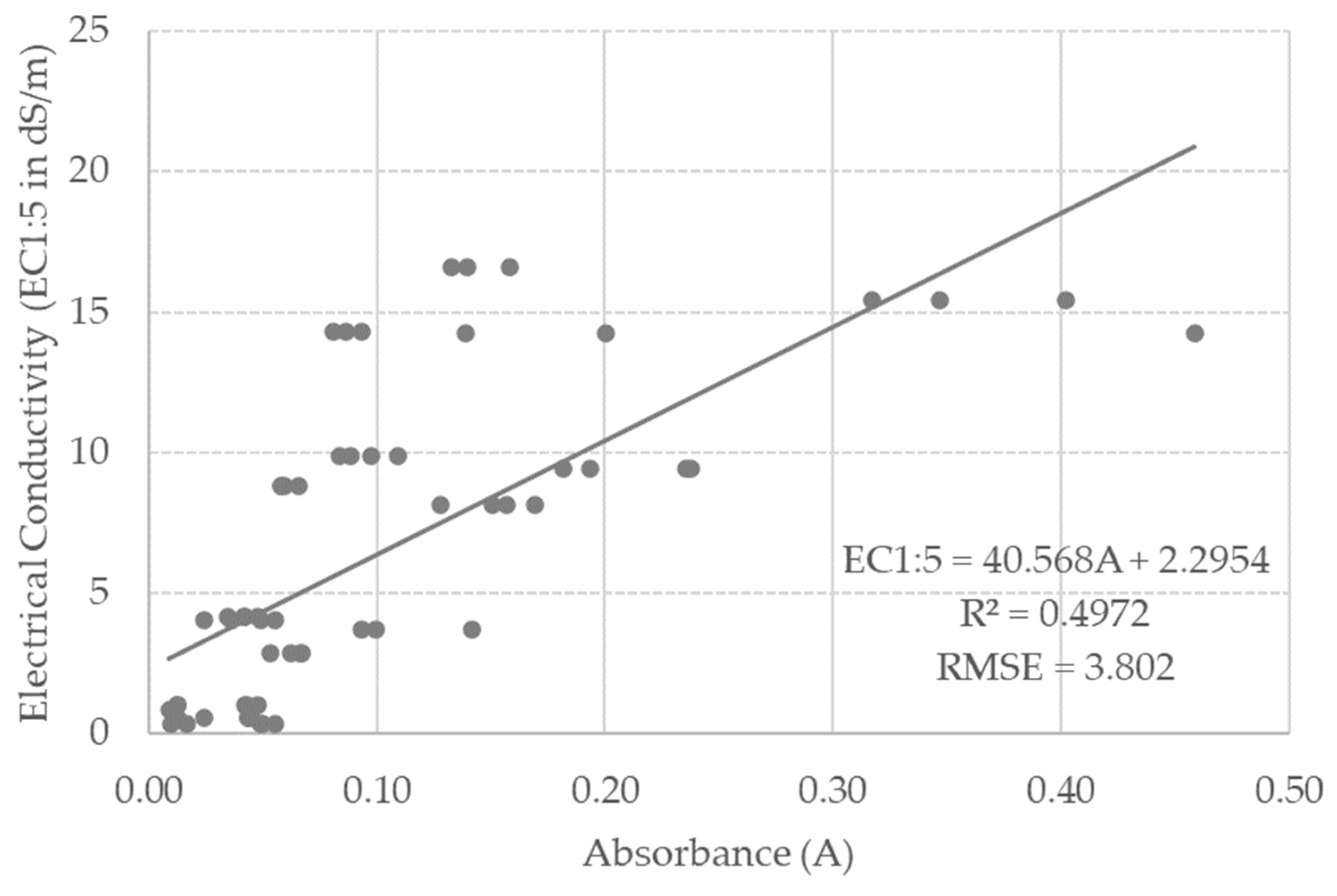
3.3. Model Calibration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shahbaz, M.; Ashraf, M. Improving Salinity Tolerance in Cereals. Crit. Rev. Plant Sci. 2013, 32, 237–249. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Blumwald, E. Developing Salt-Tolerant Crop Plants: Challenges and Opportunities. Trends Plant Sci. 2005, 10, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A.; Kempen, B.; de Sousa, L. Global Mapping of Soil Salinity Change. Remote Sens. Environ. 2019, 231, 111260. [Google Scholar] [CrossRef]
- Gupta, A.; Shaw, B.P. Biochemical and Molecular Characterisations of Salt Tolerance Components in Rice Varieties Tolerant and Sensitive to NaCl: The Relevance of Na+ Exclusion in Salt Tolerance in the Species. Funct. Plant Biol. 2021, 48, 72. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene Expression Profiling of Plants under Salt Stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil Salinity: A Threat to Global Food Security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Gobin, A.; Hien, L.T.T.; Hai, L.T.; Linh, P.H.; Thang, N.N.; Vinh, P.Q. Adaptation to Land Degradation in Southeast Vietnam. Land 2020, 9, 302. [Google Scholar] [CrossRef]
- Hai, L.T.; Gobin, A.; Hens, L. Risk Assessment of Desertification for Binh Thuan Province, Vietnam. Hum. Ecol. Risk Assess. Int. J. 2013, 19, 1544–1556. [Google Scholar] [CrossRef]
- Herbert, E.R.; Boon, P.; Burgin, A.J.; Neubauer, S.C.; Franklin, R.B.; Ardón, M.; Hopfensperger, K.N.; Lamers, L.P.; Gell, P. A Global Perspective on Wetland Salinization: Ecological Consequences of a Growing Threat to Freshwater Wetlands. Ecosphere 2015, 6, 1–43. [Google Scholar] [CrossRef]
- Metternicht, G.I.; Zinck, J.A. Remote Sensing of Soil Salinity: Potentials and Constraints. Remote Sens. Environ. 2003, 85, 1–20. [Google Scholar] [CrossRef]
- Hien, L.T.T.; Gobin, A.; Huong, P.T.T. Spatial Indicators for Desertification in Southeast Vietnam. Nat. Hazards Earth Syst. Sci. 2019, 19, 2325–2337. [Google Scholar] [CrossRef] [Green Version]
- Gobin, A.; Nguyen, H.T.; Pham, V.Q.; Pham, H.T.T. Heavy Rainfall Patterns in Vietnam and Their Relation with ENSO Cycles: Heavy Rainfall Patterns in Vietnam. Int. J. Climatol. 2016, 36, 1686–1699. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014; ISBN 978-92-5-108369-7. [Google Scholar]
- Food and Agriculture Organization. Mapping of Salt-Affected Soils. Technical Specifications and Country Guidelines; FAO: Rome, Italy, 2020. [Google Scholar]
- Abrol, I.P.; Yadav, J.S.P.; Massoud, F.I. Salt-Affected Soils and Their Management; FAO: Rome, Italy, 1988. [Google Scholar]
- Paul, B.K.; Rashid, H. Climatic Hazards in Coastal Bangladesh. Sci. Dir. 2017, 153–182. [Google Scholar]
- Munns, R. Genes and Salt Tolerance: Bringing Them Together. N. Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Hunt, G.R. Visible and Near-Infrared Spectra of Minerals and Rocks: IV. Sulphides and Sulphates. Mod. Geol. 1971, 3, 1–14. [Google Scholar]
- Hunt, G.R.; Salisbury, J.W. Visible and near Infrared Spectra of Minerals and Rocks. II. Carbonates. Mod. Geol. 1971, 2, 23–30. [Google Scholar]
- Csillag, F.; Pásztor, L.; Biehl, L.L. Spectral Band Selection for the Characterization of Salinity Status of Soils. Remote sens. Environ. 1993, 43, 231–242. [Google Scholar] [CrossRef]
- Farifteh, J.; van der Meer, F.; van der Meijde, M.; Atzberger, C. Spectral Characteristics of Salt-Affected Soils: A Laboratory Experiment. Geoderma 2008, 145, 196–206. [Google Scholar] [CrossRef]
- Beck, R.H. Spectral Characteristics of Soils Related to the Interaction of Soil Moisture, Organic Carbon, and Clay Content; Paper 100; LARS Technical Reports: West Lafayette, IN, USA, 1975. [Google Scholar]
- Condit, H.R. The Spectral Reflectance of American Soils. Photogramm. Eng. 1970, 36, 955–966. [Google Scholar]
- Everitt, J.H.; Escobar, D.E.; Gerbermann, A.H.; Alaniz, M.A. Detecting Saline Soils with Video Imagery. Photogramm. Eng. Remote Sens. 1988, 54, 1283–1287. [Google Scholar]
- Melendez-Pastor, I.; Navarro-Pedreño, J.; Gómez, I.; Koch, M. Identifying Optimal Spectral Bands to Assess Soil Properties with VNIR Radiometry in Semi-Arid Soils. Geoderma 2008, 147, 126–132. [Google Scholar] [CrossRef]
- Mahajan, G.R.; Das, B.; Gaikwad, B.; Murgaonkar, D.; Desai, A.; Morajkar, S.; Patel, K.P.; Kulkarni, R.M. Monitoring Properties of the Salt-Affected Soils by Multivariate Analysis of the Visible and near-Infrared Hyperspectral Data. CATENA 2021, 198, 105041. [Google Scholar] [CrossRef]
- Hunt, G.R. Visible and Near-Infrared Spectra of Minerals and Rocks: V. Halides, Phosphates, Arsenates, Venadates and Borates. Mod. Geol. 1972, 3, 121–132. [Google Scholar]
- Crowley, J.K. Visible and Near-Infrared (0.4–2.5 Μm) Reflectance Spectra of Playa Evaporite Minerals. J. Geophys. Res. Solid Earth 1991, 96, 16231–16240. [Google Scholar] [CrossRef]
- Mougenot, B.; Pouget, M.; Epema, G.F. Remote Sensing of Salt Affected Soils. Remote Sens. Rev. 1993, 7, 241–259. [Google Scholar] [CrossRef]
- Howari, F.M.; Goodell, P.C.; Miyamoto, S. Spectral Properties of Salt Crusts Formed on Saline Soils. J. Environ. Qual. 2002, 31, 1453–1461. [Google Scholar] [CrossRef]
- Farifteh, J. Imaging Spectroscopy of Salt-Affected Soils: Model-Based Integrated Method. Doctoral Dissertation 143, Utrecht University, Utrecht, The Netherlands, ITC (Faculty of Geo-Information Science and Earth Observation, University of Twente), Enschede, The Netherlands, May 2007. [Google Scholar]
- Gobin, A.; Campling, P.; Deckers, J.; Feyen, J. Quantifying Soil Morphology in Tropical Environments Methods and Application in Soil Classification. Soil Sci. Soc. Am. J. 2000, 64, 1423–1433. [Google Scholar] [CrossRef] [Green Version]
- Twomey, S.A.; Bohren, C.F.; Mergenthaler, J.L. Reflectance and Albedo Differences between Wet and Dry Surfaces. Appl. Opt. 1986, 25, 431. [Google Scholar] [CrossRef]
- Lobell, D.B.; Asner, G.P. Moisture Effects on Soil Reflectance. Soil Sci. Soc. Am. J. 2002, 66, 722–727. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Chabrillat, S.; Demattê, J.A.M.; Taylor, G.R.; Hill, J.; Whiting, M.L.; Sommer, S. Using Imaging Spectroscopy to Study Soil Properties. Remote Sens. Environ. 2009, 113, S38–S55. [Google Scholar] [CrossRef]
- Liu, W.; Baret, F.; Gu, X.; Zhang, B.; Tong, Q.; Zheng, L. Evaluation of Methods for Soil Surface Moisture Estimation from Reflectance Data. Int. J. Remote Sens. 2003, 24, 2069–2083. [Google Scholar] [CrossRef]
- Peng, J.; Biswas, A.; Jiang, Q.; Zhao, R.; Hu, J.; Hu, B.; Shi, Z. Estimating Soil Salinity from Remote Sensing and Terrain Data in Southern Xinjiang Province, China. Geoderma 2019, 337, 1309–1319. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Patkin, K.; Banin, A.; Karnieli, A. Mapping of Several Soil Properties Using DAIS-7915 Hyperspectral Scanner Data-a Case Study over Clayey Soils in Israel. Int. J. Remote Sens. 2002, 23, 1043–1062. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Heller, D.; Chudnovsky, A. A Novel Method of Classifying Soil Profiles in the Field Using Optical Means. Soil Sci. Soc. Am. J. 2008, 72, 1113–1123. [Google Scholar] [CrossRef]
- Muller, E.; Decamps, H. Modeling Soil Moisture–Reflectance. Remote Sens. Environ. 2001, 76, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Zhang, D.; Fang, Y.; Wu, J.; Huang, J. Comparison of Partial Least Square Regression, Support Vector Machine, and Deep-Learning Techniques for Estimating Soil Salinity from Hyperspectral Data. J. Appl. Rem. Sens. 2018, 12, 1. [Google Scholar] [CrossRef]
- Peng, X.; Xu, C.; Zeng, W.; Wu, J.; Huang, J. Elimination of the Soil Moisture Effect on the Spectra for Reflectance Prediction of Soil Salinity Using External Parameter Orthogonalization Method. J. Appl. Remote Sens. 2016, 10, 015014. [Google Scholar] [CrossRef]
- Blommaert, J.; Lesschaeve, S.; Tavares, J.L.; Nuyts, D.; Delaure, B.; Gobin, A.; Dries, J.C.; De Vos, L. Satirim: Towards a Thermal IR Small Satellites Constellation. In Proceedings of the 2021 IEEE International Geoscience and Remote Sensing Symposium IGARSS, Brussels, Belgium, 11 July 2021; IEEE: Brussels, Belgium, 2021; pp. 1765–1768. [Google Scholar]
- Hoa, P.; Giang, N.; Binh, N.; Hai, L.; Pham, T.-D.; Hasanlou, M.; Tien Bui, D. Soil Salinity Mapping Using SAR Sentinel-1 Data and Advanced Machine Learning Algorithms: A Case Study at Ben Tre Province of the Mekong River Delta (Vietnam). Remote Sens. 2019, 11, 128. [Google Scholar] [CrossRef] [Green Version]
- TCVN 4046-85; Soil—Method of Sampling. State Science and Technology Committee: Hanoi, Vietnam, 1985.
- ISO 11464:2006; Soil Quality—Pretreatment of Samples for Physico-Chemical Analysis. ISO: Geneva, Switzerland, 2006.
- TCVN 4080:2011; Soil Quality—Determination of Moisture and Absolute Dryness Coefficient. Ministry of Science and Technology: Hanoi, Vietnam, 2011.
- TCVN 8567:2010; Soil Quality—Method for Determination of Particle Size Dístribution. Ministry of Science and Technology: Hanoi, Vietnam, 2010.
- ISO 11265:1994; Soil Quality—Determination of the Specific Electrical Conductivity. ISO: Geneva, Switzerland, 1994.
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Gross, J.; Ligges, U. Tests for Normality. R Package Version 1.0-4. 2015. Available online: https://rdrr.io/cran/nortest/ (accessed on 6 April 2022).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.0. 2021. Available online: https://cran.r-project.org/web/packages/rstatix/index.html (accessed on 6 April 2022).
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots. R Package Version 0.4.0. 2020. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 6 April 2022).
- Gobin, A.M.; Campling, P.; Deckers, J.A.; Poesen, J.; Feyen, J. Soil Erosion Assessment at the Udi-Nsukka Cuesta (Southeastern Nigeria). Land Degrad. Dev. 1999, 10, 141–160. [Google Scholar] [CrossRef]
- Stoner, E.R.; Baumgardner, M.F. Characteristic Variations in Reflectance of Surface Soils. Soil Sci. Soc. Am. J. 1981, 45, 1161–1165. [Google Scholar] [CrossRef] [Green Version]
- Corwin, D.L.; Lesch, S.M. Apparent Soil Electrical Conductivity Measurements in Agriculture. Comput. Electron. Agric. 2005, 46, 11–43. [Google Scholar] [CrossRef]
- Visconti, F.; Miguel de Paz, J. Prediction of the Soil Saturated Paste Extract Salinity from Extractable Ions, Cation Exchange Capacity, and Anion Exclusion. Soil Res. 2012, 50, 536. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A.K. The Need for the Spectral Characterization of Dominant Salts and Recommended Methods of Soil Sampling and Analysis for the Proper Spectral Evaluation of Salt Affected Soils Using Hyper -Spectral Remote Sensing. Remote Sens. Lett. 2022, 13, 588–598. [Google Scholar] [CrossRef]
- Allbed, A.; Kumar, L.; Sinha, P. Mapping and Modelling Spatial Variation in Soil Salinity in the Al Hassa Oasis Based on Remote Sensing Indicators and Regression Techniques. Remote Sens. 2014, 6, 1137–1157. [Google Scholar] [CrossRef] [Green Version]
- Alqasemi, A.S.; Ibrahim, M.; Fadhil Al-Quraishi, A.M.; Saibi, H.; Al-Fugara, A.; Kaplan, G. Detection and Modeling of Soil Salinity Variations in Arid Lands Using Remote Sensing Data. Open Geosci. 2021, 13, 443–453. [Google Scholar] [CrossRef]
- Shahrayini, E.; Noroozi, A.A. Modeling and Mapping of Soil Salinity and Alkalinity Using Remote Sensing Data and Topographic Factors: A Case Study in Iran. Environ. Model. Assess. 2022. [Google Scholar] [CrossRef]

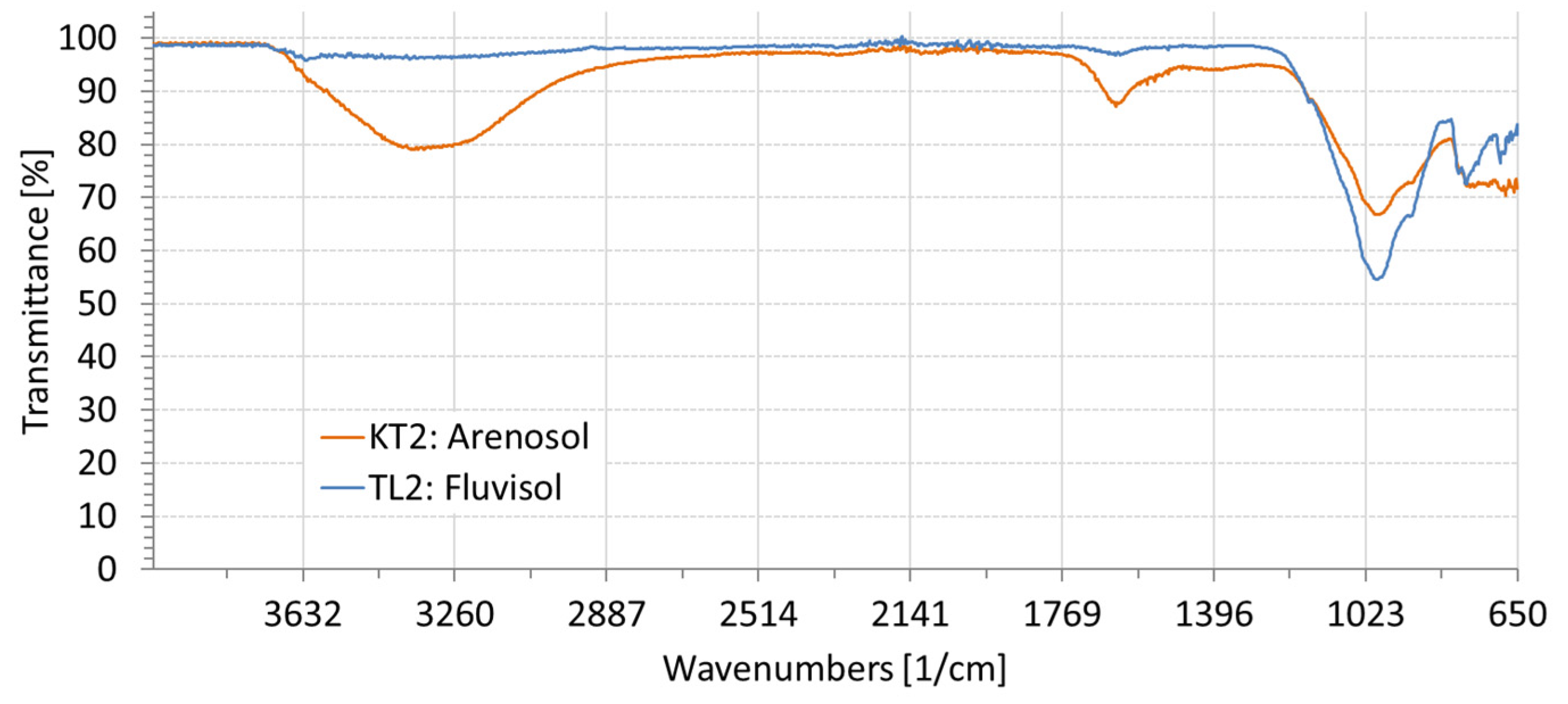
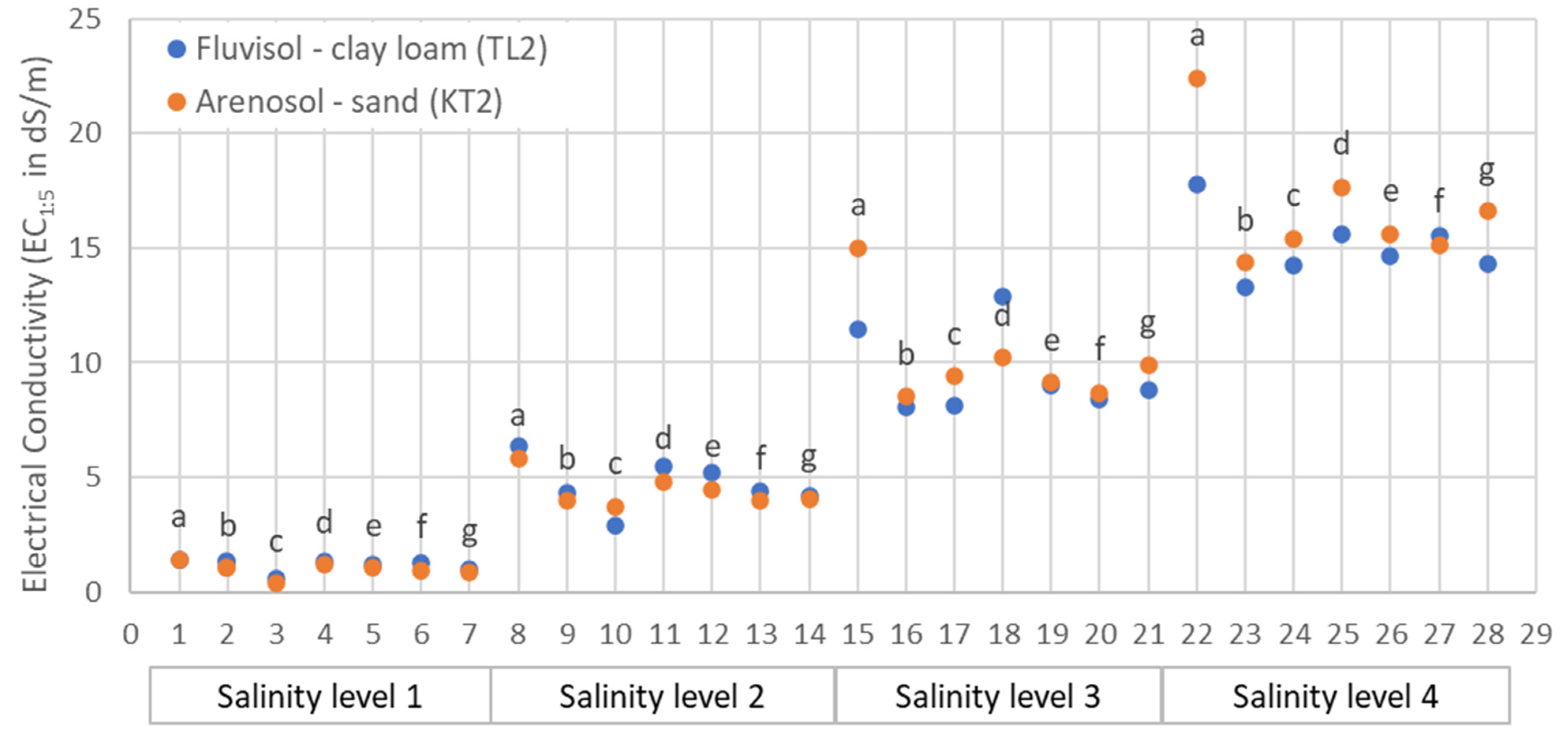
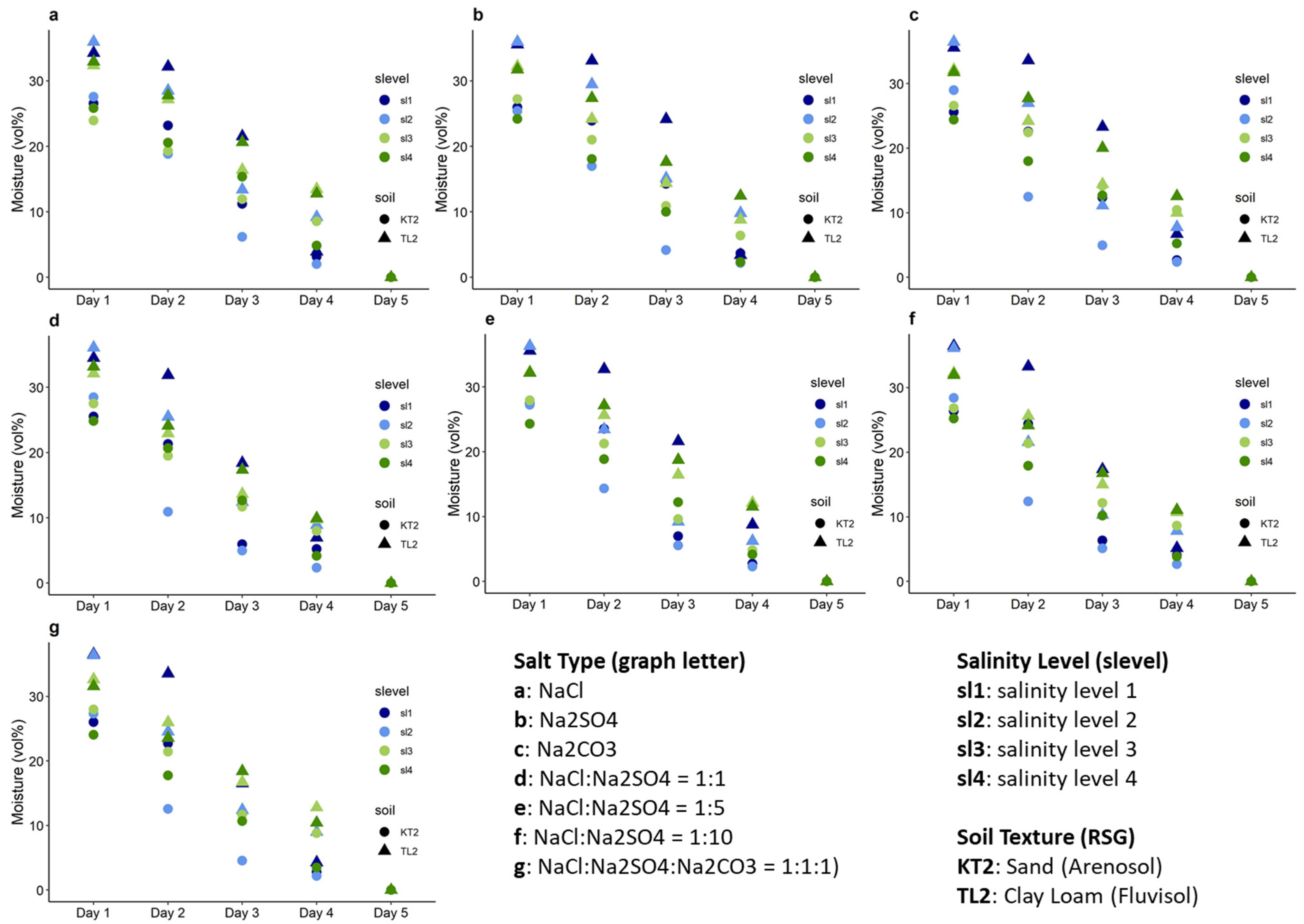
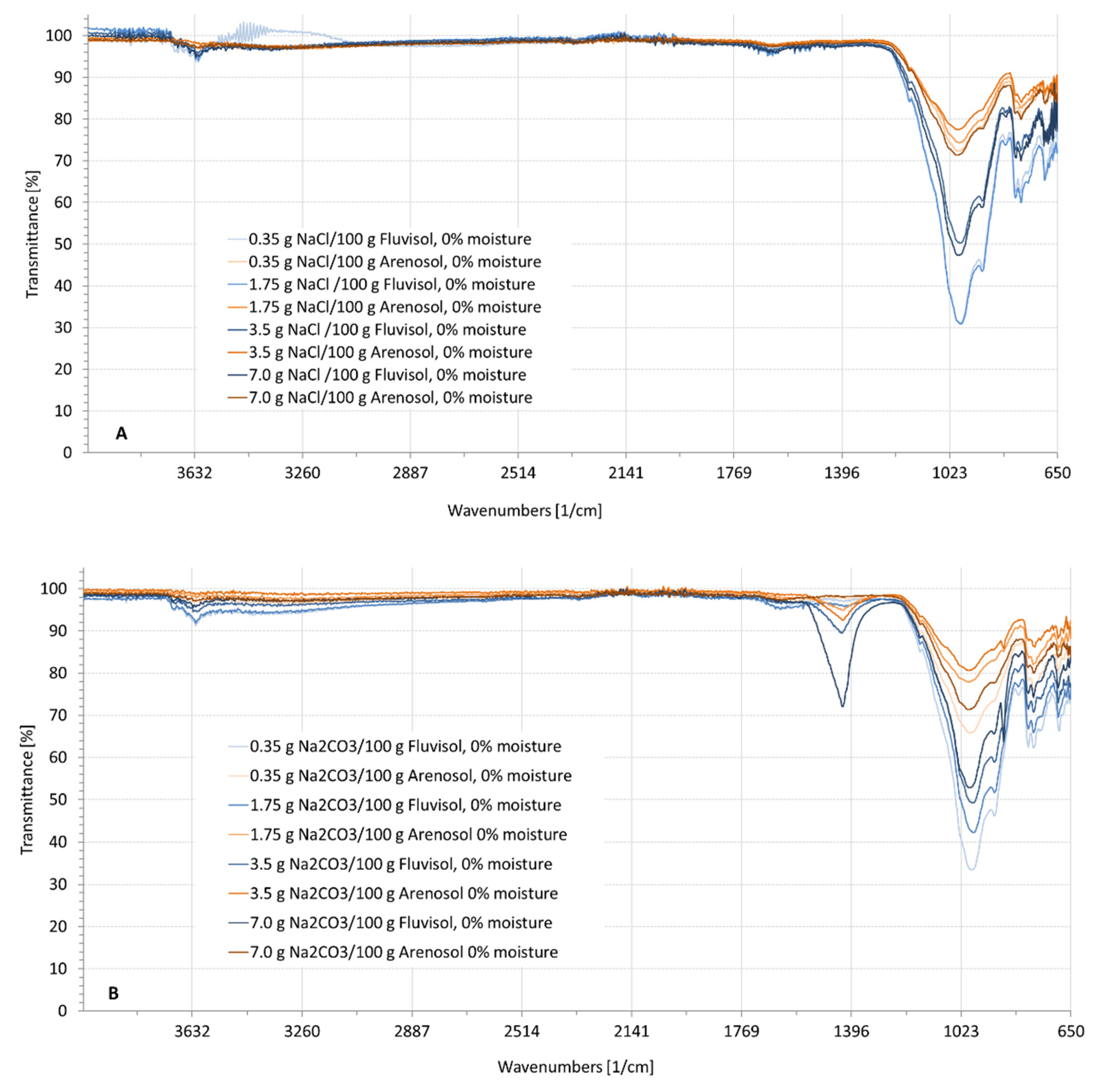




| ID | Location | Clay (%) | Silt (%) | Sand (%) | EC1:5 (dS/m) | Moisture (%) | Textural Class | RSG |
|---|---|---|---|---|---|---|---|---|
| gt1 | Giao Thuy | 10.40 | 10.26 | 79.34 | 0.30 | 20.1 | Sandy Loam | Fluvisol |
| gt2 | Giao Thuy | 9.52 | 1.24 | 89.24 | 0.24 | 21.8 | Loamy Sand | Fluvisol |
| gt3 | Giao Thuy | 8.76 | 2.52 | 88.72 | 0.28 | 21.9 | Loamy Sand | Fluvisol |
| vb1 | Vinh Bao | 25.44 | 40.32 | 34.24 | 0.54 | 31.4 | Loam | Fluvisol |
| tl1 | Tien Lang | 28.82 | 28.10 | 43.08 | 0.32 | 33.9 | Clay Loam | Arenosol |
| tl2 | Tien Lang | 37.08 | 38.82 | 24.10 | 0.51 | 34.5 | Clay Loam | Fluvisol |
| th1 | Tien Hai | 8.02 | 1.52 | 90.46 | 0.65 | 20.4 | Sand | Arenosol |
| th2 | Tien Hai | 9.38 | 2.46 | 88.16 | 0.44 | 28.2 | Loamy Sand | Fluvisol |
| kt1 | Kien Thuy | 19.8 | 19.82 | 60.38 | 0.60 | 24.2 | Sandy Loam | Arenosol |
| kt2 | Kien Thuy | 9.14 | 0.24 | 90.62 | 0.12 | 21.1 | Sand | Arenosol |
| tt1 | Thai Thuy | 18.58 | 18.02 | 63.40 | 0.29 | 25.1 | Sandy Loam | Fluvisol |
| ID | EC1:5 (dS/m) | ECe (dS/m) | Temperature (°C) | Salinity Class |
|---|---|---|---|---|
| TL2 | 0.51 | 7.5 | 23.6 | Moderately saline |
| KT2 | 0.12 | 2.2 | 23.6 | Slightly saline |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hien, L.T.T.; Gobin, A.; Lim, D.T.; Quan, D.T.; Hue, N.T.; Thang, N.N.; Binh, N.T.; Dung, V.T.K.; Linh, P.H. Soil Moisture Influence on the FTIR Spectrum of Salt-Affected Soils. Remote Sens. 2022, 14, 2380. https://doi.org/10.3390/rs14102380
Hien LTT, Gobin A, Lim DT, Quan DT, Hue NT, Thang NN, Binh NT, Dung VTK, Linh PH. Soil Moisture Influence on the FTIR Spectrum of Salt-Affected Soils. Remote Sensing. 2022; 14(10):2380. https://doi.org/10.3390/rs14102380
Chicago/Turabian StyleHien, Le Thi Thu, Anne Gobin, Duong Thi Lim, Dang Tran Quan, Nguyen Thi Hue, Nguyen Ngoc Thang, Nguyen Thanh Binh, Vu Thi Kim Dung, and Pham Ha Linh. 2022. "Soil Moisture Influence on the FTIR Spectrum of Salt-Affected Soils" Remote Sensing 14, no. 10: 2380. https://doi.org/10.3390/rs14102380
APA StyleHien, L. T. T., Gobin, A., Lim, D. T., Quan, D. T., Hue, N. T., Thang, N. N., Binh, N. T., Dung, V. T. K., & Linh, P. H. (2022). Soil Moisture Influence on the FTIR Spectrum of Salt-Affected Soils. Remote Sensing, 14(10), 2380. https://doi.org/10.3390/rs14102380







