The Effects of Climate and Bioclimate on COVID-19 Cases in Poland
Abstract
:1. Introduction
2. Materials and Methods
3. The COVID-19 Pandemic in Poland
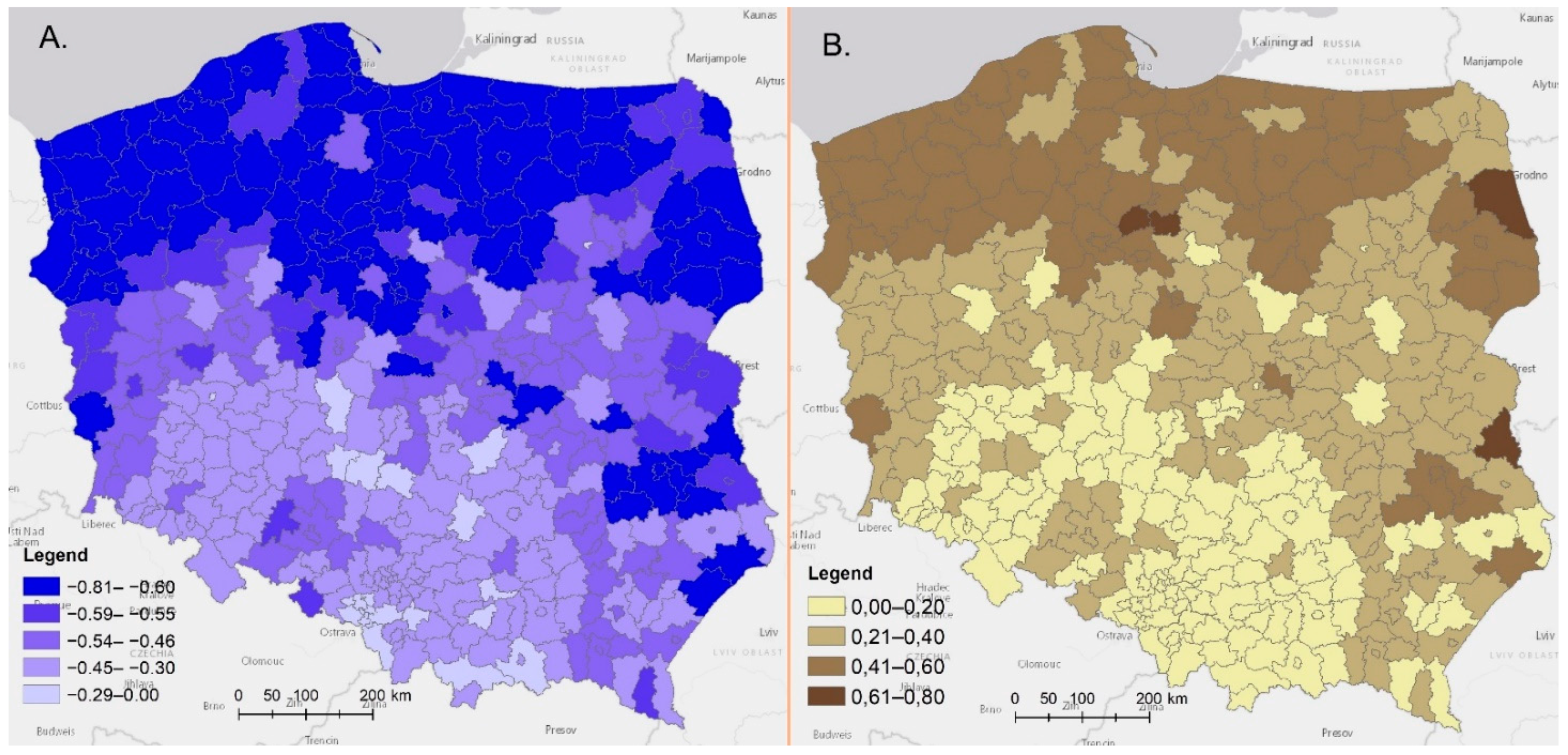
4. Climatic and Bioclimatic Conditions in Poland
4.1. Temperatures
4.2. UV Radiation at the Surface
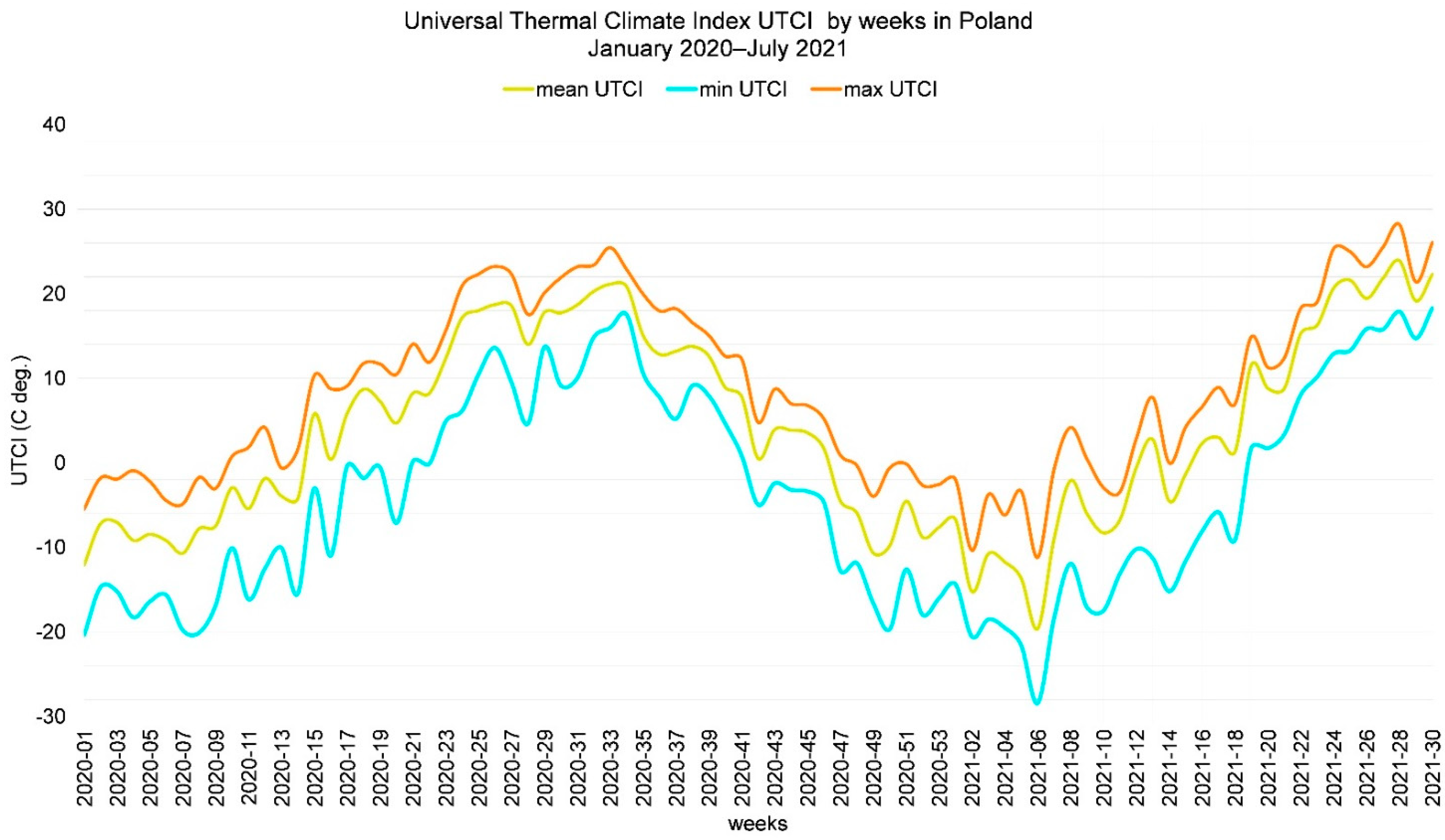
4.3. Universal Thermal Climate Index
5. General Approach
6. Results
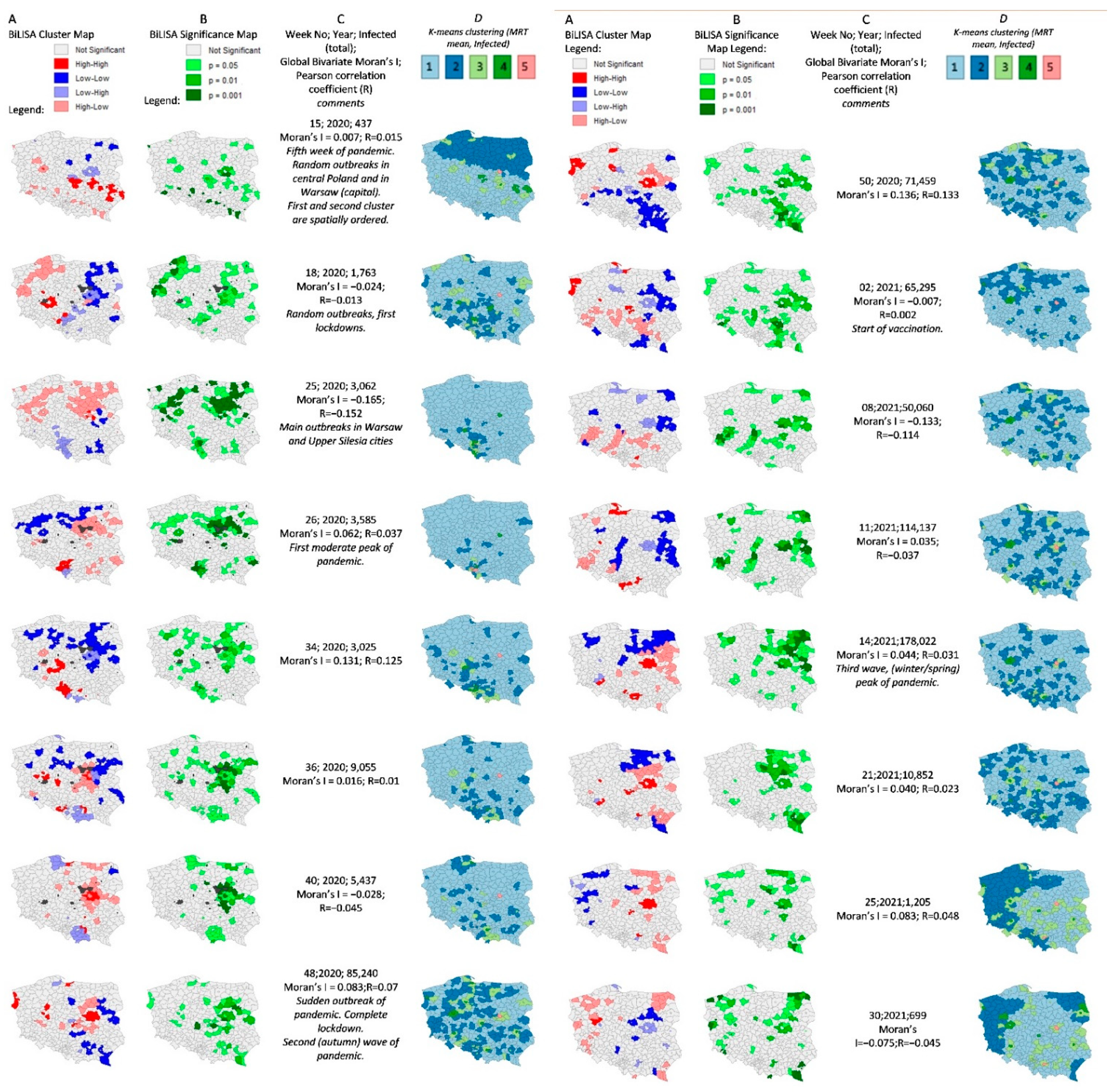
Results of Detailed Spatial Analyses
7. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamerius, J.; Nelson, M.I.; Zhou, S.Z.; Viboud, C.; Miller, M.A.; Alonso, W.J. Global Influenza Seasonality: Reconciling Patterns across Temperate and Tropical Regions. Environ. Health Perspect. 2011, 119, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fdez-Arroyabe, P. Influenza epidemics and Spanish climatic domains. Health 2012, 4, 941–945. [Google Scholar] [CrossRef] [Green Version]
- Price, R.H.M.; Graham, C.; Ramalingam, S. Association between viral seasonality and meteorological factors. Sci. Rep. 2019, 9, 929. [Google Scholar] [CrossRef] [PubMed]
- du Prel, J.; Puppe, W.; Gröndahl, B.; Knuf, M.; Weigl, J.A.I.; Schaaff, F.; Schmitt, H. Are Meteorological Parameters Associated with Acute Respiratory Tract Infections? Clin. Infect. Dis. 2009, 49, 861–868. [Google Scholar] [CrossRef] [Green Version]
- Lindner-Cendrowska, K. Wpływ warunków meteorologicznych na zachorowalność na grypę w wybranych polskich miastach = Impact of meteorological conditions on influenza morbidity in the selected Polish cities. Prz. Geogr. 2021, 93, 103–122. [Google Scholar] [CrossRef]
- The KIAS-Study Group; Jaakkola, K.; Saukkoriipi, A.; Jokelainen, J.; Juvonen, R.; Kauppila, J.; Vainio, O.; Ziegler, T.; Rönkkö, E.; Jaakkola, J.J.; et al. Decline in temperature and humidity increases the occurrence of influenza in cold climate. Environ. Health 2014, 13, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindner-Cendrowska, K.; Bröde, P. Impact of biometeorological conditions and air pollution on influenza-like illnesses incidence in Warsaw. Int. J. Biometeorol. 2021, 65, 929–944. [Google Scholar] [CrossRef]
- Burra, P.; Soto-Díaz, K.; Chalen, I.; Gonzalez-Ricon, R.J.; Istanto, D.; Caetano-Anollés, G. Temperature and Latitude Correlate with SARS-CoV-2 Epidemiological Variables but not with Genomic Change Worldwide. Evol. Bioinform. 2021, 17, 117693432198969. [Google Scholar] [CrossRef]
- Ma, Y.; Pei, S.; Shaman, J.; Dubrow, R.; Chen, K. Role of meteorological factors in the transmission of SARS-CoV-2 in the United States. Nat. Commun. 2021, 12, 3602. [Google Scholar] [CrossRef]
- Shi, P.; Dong, Y.; Yan, H.; Zhao, C.; Li, X.; Liu, W.; He, M.; Tang, S.; Xi, S. Impact of temperature on the dynamics of the COVID-19 outbreak in China. Sci. Total Environ. 2020, 728, 138890. [Google Scholar] [CrossRef] [PubMed]
- Prata, D.N.; Rodrigues, W.; Bermejo, P.H. Temperature significantly changes COVID-19 transmission in (sub)tropical cities of Brazil. Sci. Total Environ. 2020, 729, 138862. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xie, J.; Huang, F.; Cao, L. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci. Total Environ. 2020, 727, 138704. [Google Scholar] [CrossRef]
- Pani, S.K.; Lin, N.-H.; Ravindra Babu, S. Association of COVID-19 pandemic with meteorological parameters over Singapore. Sci. Total Environ. 2020, 740, 140112. [Google Scholar] [CrossRef] [PubMed]
- Isaia, G.; Diémoz, H.; Maluta, F.; Fountoulakis, I.; Ceccon, D.; di Sarra, A.; Facta, S.; Fedele, F.; Lorenzetto, G.; Siani, A.M. Does solar ultraviolet radiation play a role in COVID-19 infection and deaths? An environmental ecological study in Italy. Sci. Total Environ. 2021, 757, 143757. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Dey, S.; Ghosh, A.R. Are population size and diverse climatic conditions the driving factors for next COVID-19 pandemic epicenter in India? Results Phys. 2021, 26, 104454. [Google Scholar] [CrossRef]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; McIntyre, R.S.; Choo, F.N.; Tran, B.; Ho, R.; Sharma, V.K.; et al. A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav. Immun. 2020, 87, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Bannister-Tyrrell, M.; Meyer, A.; Faverjon, C.; Cameron, A. Preliminary evidence that higher temperatures are associated with lower incidence of COVID-19, for cases reported globally up to 29th February 2020. medRxiv 2020. [Google Scholar] [CrossRef]
- Rice, B.L.; Annapragada, A.; Baker, R.E.; Bruijning, M.; Dotse-Gborgbortsi, W.; Mensah, K.; Miller, I.F.; Motaze, N.V.; Raherinandrasana, A.; Rajeev, M.; et al. Variation in SARS-CoV-2 outbreaks across sub-Saharan Africa. Nat. Med. 2021, 27, 447–453. [Google Scholar] [CrossRef]
- Suvvari, T.K.; Kutikuppala, L.V.S.; Jonna, S.; Kashif, M.S. Impact of environmental factors on COVID-19 pandemic: A narrative review. MGM J. Med Sci. 2021, 8, 151. [Google Scholar] [CrossRef]
- Steiger, E.; Mussgnug, T.; Kroll, L.E. Causal graph analysis of COVID-19 observational data in German districts reveals effects of determining factors on reported case numbers. PLoS ONE 2021, 16, e0237277. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, J.; Flet-Berliac, Y.; Seligmann, H. Temperature Decreases Spread Parameters of the New COVID-19 Case Dynamics. Biology 2020, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Xiao, S.; Shi, R.; Ward, M.P.; Chen, Y.; Tu, W.; Su, Q.; Wang, W.; Wang, X.; Zhang, Z. COVID-19 transmission in Mainland China is associated with temperature and humidity: A time-series analysis. Sci. Total Environ. 2020, 728, 138778. [Google Scholar] [CrossRef]
- Sobral, M.F.F.; Duarte, G.B.; da Penha Sobral, A.I.G.; Marinho, M.L.M.; de Souza Melo, A. Association between climate variables and global transmission oF SARS-CoV-2. Sci. Total Environ. 2020, 729, 138997. [Google Scholar] [CrossRef] [PubMed]
- Tosepu, R.; Gunawan, J.; Effendy, D.S.; Lestari, H.; Bahar, H.; Asfian, P. Correlation between weather and COVID-19 pandemic in Jakarta, Indonesia. Sci. Total Environ. 2020, 725, 138436. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jing, W.; Liu, J.; Ma, Q.; Yuan, J.; Wang, Y.; Du, M.; Liu, M. Effects of temperature and humidity on the daily new cases and new deaths of COVID-19 in 166 countries. Sci. Total Environ. 2020, 729, 139051. [Google Scholar] [CrossRef]
- Auler, A.; Cássaro, F.; Da Silva, V.; Pires, L. Evidence that high temperatures and intermediate relative humidity might favor the spread of COVID-19 in tropical climate: A case study for the most affected Brazilian cities. Sci. Total Environ. 2020, 729, 139090. [Google Scholar] [CrossRef]
- Jüni, P.; Rothenbühler, M.; Bobos, P.; Thorpe, K.E.; da Costa, B.R.; Fisman, D.N.; Slutsky, A.S.; Gesink, D. Impact of climate and public health interventions on the COVID-19 pandemic: A prospective cohort study. CMAJ 2020, 192, E566–E573. [Google Scholar] [CrossRef] [PubMed]
- Briz-Redón, Á.; Serrano-Aroca, Á. A spatio-temporal analysis for exploring the effect of temperature on COVID-19 early evolution in Spain. Sci. Total Environ. 2020, 728, 138811. [Google Scholar] [CrossRef]
- Shahzad, F.; Shahzad, U.; Fareed, Z.; Iqbal, N.; Hashmi, S.H.; Ahmad, F. Asymmetric nexus between temperature and COVID-19 in the top ten affected provinces of China: A current application of quantile-on-quantile approach. Sci. Total Environ. 2020, 736, 139115. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, M.; Jahangiri, M.; Najafgholipour, M. The sensitivity and specificity analyses of ambient temperature and population size on the transmission rate of the novel coronavirus (COVID-19) in different provinces of Iran. Sci. Total Environ. 2020, 728, 138872. [Google Scholar] [CrossRef] [PubMed]
- CovsirPhy Development Team CovsirPhy, Python Package for COVID-19 Analysis with SIR-Derived ODE Models. Available online: https://github.com/lisphilar/covid19-sir (accessed on 4 February 2021).
- Hirokazu, T. CovsirPhy Version [2.15.0] COVID-19 Data with SIR Model (Software). 2021. Available online: https://www.kaggle.com/lisphilar/covid-19-data-with-sir-model (accessed on 5 February 2021).
- Guidotti, E.; Ardia, D. COVID-19 Data Hub. J. Open Source Softw. 2020, 5, 2376. [Google Scholar] [CrossRef]
- Raport Zakażeń Koronawirusem (SARS-CoV-2)—Koronawirus: Informacje i Zalecenia—Portal Gov.pl. Available online: https://www.gov.pl/web/koronawirus/wykaz-zarazen-koronawirusem-sars-cov-2 (accessed on 7 February 2021).
- Hersbach, H.; Bell, B.; Berrisford, P.; Hirahara, S.; Horányi, A.; Muñoz-Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Schepers, D. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc. 2020, 146, 1999–2049. [Google Scholar] [CrossRef]
- Di Napoli, C.; Barnard, C.; Prudhomme, C.; Cloke, H.L.; Pappenberger, F. ERA5-HEAT: A global gridded historical dataset of human thermal comfort indices from climate reanalysis. Geosci. Data J. 2021, 8, 2–10. [Google Scholar] [CrossRef]
- Kántor, N.; Unger, J. The most problematic variable in the course of human-biometeorological comfort assessment—The mean radiant temperature. Cent. Eur. J. Geosci. 2011, 3, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Thorsson, S.; Rocklöv, J.; Konarska, J.; Lindberg, F.; Holmer, B.; Dousset, B.; Rayner, D. Mean radiant temperature—A predictor of heat related mortality. Urban Clim. 2014, 10, 332–345. [Google Scholar] [CrossRef]
- Błażejczyk, K.; Jendritzky, G.; Bröde, P.; Fiala, D.; Havenith, G.; Epstein, Y.; Psikuta, A.; Kampmann, B. An introduction to the universal thermal climate index (UTCI). Geogr. Pol. 2013, 86, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Dana Tomlin, C. Map Algebra. In International Encyclopedia of Geography: People, the Earth, Environment and Technology; Richardson, D., Castree, N., Goodchild, M.F., Kobayashi, A., Liu, W., Marston, R.A., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2017; pp. 1–17. ISBN 978-0-470-65963-2. [Google Scholar]
- Ozesmi, S.L.; Bauer, M.E. Satellite remote sensing of wetlands. Wetl. Ecol. Manag. 2002, 10, 381–402. [Google Scholar] [CrossRef]
- Sherrouse, B.C.; Clement, J.M.; Semmens, D. A GIS application for assessing, mapping, and quantifying the social values of ecosystem services. Appl. Geogr. 2011, 31, 748–760. [Google Scholar] [CrossRef]
- De Smith, M.J.; Goodchild, M.F.; Longley, P.A. Geospatial Analysis: A Comprehensive Guide to Principles, Techniques and Software Tools, 6th ed.; Drumlin Security: London, UK, 2018; ISBN 978-1-912556-05-2. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IMGW-PIB. Climate of Poland 2020; Institute of Meteorology and Water Management—National Research Institute: Warsaw, Poland, 2021; p. 24. Available online: https://www.imgw.pl/sites/default/files/2021-04/imgw-pib-klimat-polski-2020-opracowanie-final-eng-rozkladowki-min.pdf (accessed on 1 September 2021).
- Werner, P.A. Tracing and Modeling of the COVID-19 Pandemic Infections in Poland Using Spatial Interactions Models. In Computational Science and Its Applications—ICCSA 2021; Gervasi, O., Murgante, B., Misra, S., Garau, C., Blečić, I., Taniar, D., Apduhan, B.O., Rocha, A.M.A.C., Tarantino, E., Torre, C.M., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2021; Volume 12954, pp. 641–657. ISBN 978-3-030-86978-6. [Google Scholar]
- Sajadi, M.M.; Habibzadeh, P.; Vintzileos, A.; Shokouhi, S.; Miralles-Wilhelm, F.; Amoroso, A. Temperature, Humidity and Latitude Analysis to Predict Potential Spread and Seasonality for COVID-19; Social Science Research Network: Rochester, NY, USA, 2020; Available online: https://www.clinicadelviaggiatore.com/wp-content/uploads/2020/04/Temperature-humidity-and-latitude-analysis-to-predict-potential-spread-and-seasonality-for-COVID-19.pdf (accessed on 1 September 2021).
- Baker, R.E.; Yang, W.; Vecchi, G.A.; Metcalf, C.J.E.; Grenfell, B.T. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. Science 2020, 369, 315–319. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community Transmission and Viral Load Kinetics of the SARS-CoV-2 Delta (B.1.617.2) Variant in Vaccinated and Unvaccinated Individuals in the UK: A Prospective, Longitudinal, Cohort Study. Lancet Infect. Dis. 2021, S1473309921006484. [Google Scholar] [CrossRef]
- Ledford, H. Six Months of COVID Vaccines: What 1.7 Billion Doses Have Taught Scientists. Nature 2021, 594, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Acharya, C.B.; Schrom, J.; Mitchell, A.M.; Coil, D.A.; Marquez, C.; Rojas, S.; Wang, C.Y.; Liu, J.; Pilarowski, G.; Solis, L.; et al. No Significant Difference in Viral Load Between Vaccinated and Unvaccinated, Asymptomatic and Symptomatic Groups When Infected with SARS-CoV-2 Delta Variant. MedRxiv 2021. [Google Scholar] [CrossRef]
- Brown, C.M.; Vostok, J.; Johnson, H.; Burns, M.; Gharpure, R.; Sami, S.; Sabo, R.T.; Hall, N.; Foreman, A.; Schubert, P.L.; et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Tuel, A.; Eltahir, E.A.B. On the Environmental Determinants of COVID-19 Seasonality. Geohealth 2021, 5, e2021GH000413. [Google Scholar] [CrossRef]
- Fontal, A.; Bouma, M.J.; San-José, A.; López, L.; Pascual, M.; Rodó, X. Climatic signatures in the different COVID-19 pandemic waves across both hemispheres. Nat. Comput. Sci. 2021, 1, 655–665. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.; Li, C.; Zhao, Y.; Wang, D.; Huang, Z.; Yang, K. The role of seasonality in the spread of COVID-19 pandemic. Environ. Res. 2021, 195, 110874. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.J.; Gomez, A.C.R.; Bansal, S.; Ryan, S.J. Misconceptions about weather and seasonality must not misguide COVID-19 response. Nat. Commun. 2020, 11, 4312. [Google Scholar] [CrossRef]







| JAN | FEB | MAR | APR | MAY | JUN | JUL | AUG | SEP | OCT | NOV | DEC | YEAR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2.2 | 4.0 | 4.5 | 8.7 | 11.0 | 17.7 | 18.2 | 19.8 | 15.1 | 10.5 | 5.6 | 1.9 | 9.9 |
| Anomalies, 1981–2010 | 3.7 | 4.6 | 1.7 | 0.6 | −2.3 | 1.7 | −0.1 | 2.0 | 1.8 | 1.9 | 2.2 | 2.2 | 1.6 |
| Correlations | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infected | Failed | Mean rt | Min rt | Max rt | Mean nsat | Min nsat | Max nsat | Mean dpt | Min dpt | Max dpt | Mean UTCI | Min UTCI | Max UTCI | Mean UV | Min UV | Max UV | ||
| Infected | Pearson’s Correlation | 1 | 0.841 ** | −0.694 ** | −0.703 ** | −0.676 ** | −0.655 ** | −0.662 ** | −0.614 ** | −0.491 ** | −0.527 ** | −0.521 ** | −0.651 ** | −0.636 ** | −0.660 ** | −0.666 ** | −0.651 ** | −0.657 ** |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| N (weeks) | 65 | 65 | 65 | 65 | 65 | 64 | 64 | 64 | 64 | 64 | 64 | 65 | 65 | 65 | 64 | 64 | 64 | |
| Failed | Pearson Correlation | 0.841 ** | 1 | −0.667 ** | −0.688 ** | −0.630 ** | −0.644 ** | −0.643 ** | −0.585 ** | −0.498 ** | −0.520 ** | −0.510 ** | −0.649 ** | −0.625 ** | −0.636 ** | −0.585 ** | −0.575 ** | −0.586 ** |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| N (weeks) | 65 | 65 | 65 | 65 | 65 | 64 | 64 | 64 | 64 | 64 | 64 | 65 | 65 | 65 | 64 | 64 | 64 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, P.A.; Skrynyk, O.; Porczek, M.; Szczepankowska-Bednarek, U.; Olszewski, R.; Kęsik-Brodacka, M. The Effects of Climate and Bioclimate on COVID-19 Cases in Poland. Remote Sens. 2021, 13, 4946. https://doi.org/10.3390/rs13234946
Werner PA, Skrynyk O, Porczek M, Szczepankowska-Bednarek U, Olszewski R, Kęsik-Brodacka M. The Effects of Climate and Bioclimate on COVID-19 Cases in Poland. Remote Sensing. 2021; 13(23):4946. https://doi.org/10.3390/rs13234946
Chicago/Turabian StyleWerner, Piotr A., Oleh Skrynyk, Mariusz Porczek, Urszula Szczepankowska-Bednarek, Robert Olszewski, and Małgorzta Kęsik-Brodacka. 2021. "The Effects of Climate and Bioclimate on COVID-19 Cases in Poland" Remote Sensing 13, no. 23: 4946. https://doi.org/10.3390/rs13234946
APA StyleWerner, P. A., Skrynyk, O., Porczek, M., Szczepankowska-Bednarek, U., Olszewski, R., & Kęsik-Brodacka, M. (2021). The Effects of Climate and Bioclimate on COVID-19 Cases in Poland. Remote Sensing, 13(23), 4946. https://doi.org/10.3390/rs13234946







