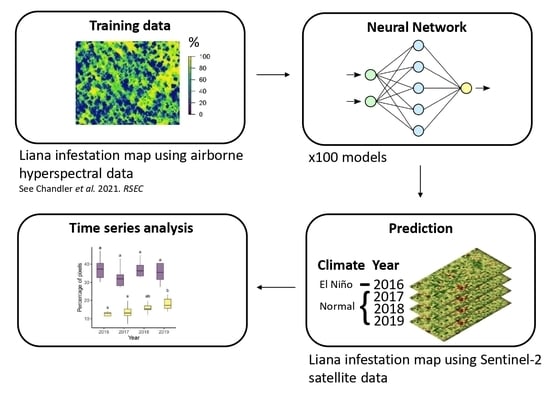
Detection of Spatial and Temporal Patterns of Liana Infestation Using Satellite-Derived Imagery
Abstract
:1. Introduction
2. Materials and Methods
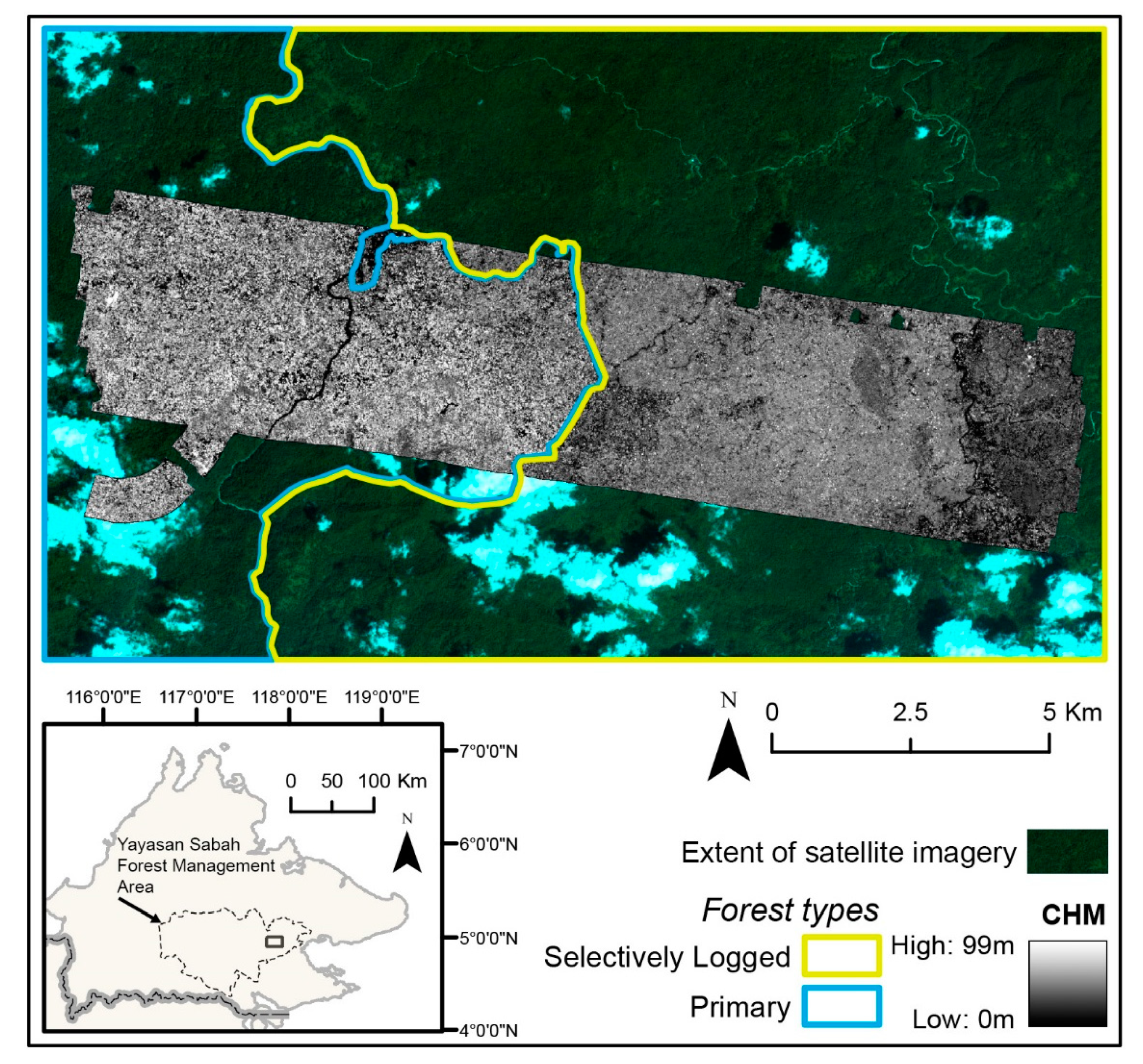
2.1. Study Area
2.2. Airborne-Derived Liana Infestation Assessment
2.2.1. Airborne Hyperspectral and LiDAR Data
2.2.2. Field Data Collection
2.2.3. Modelling Liana Infestation
2.3. Satellite-Derived Liana Infestation Assessment
2.3.1. Satellite-Based Data
2.3.2. Spectral Reflectance for Lianas versus Trees
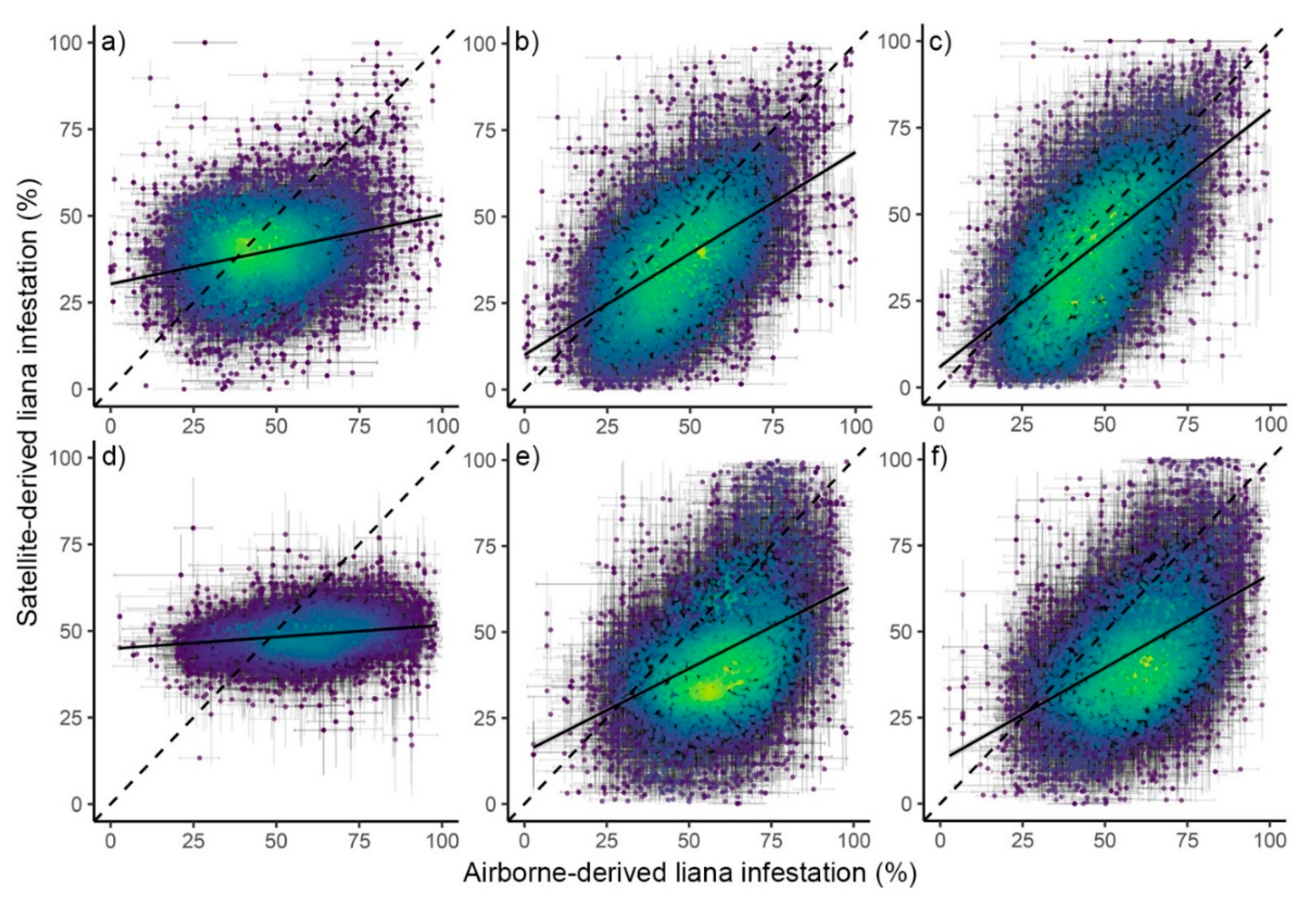
2.3.3. Modelling Liana Infestation
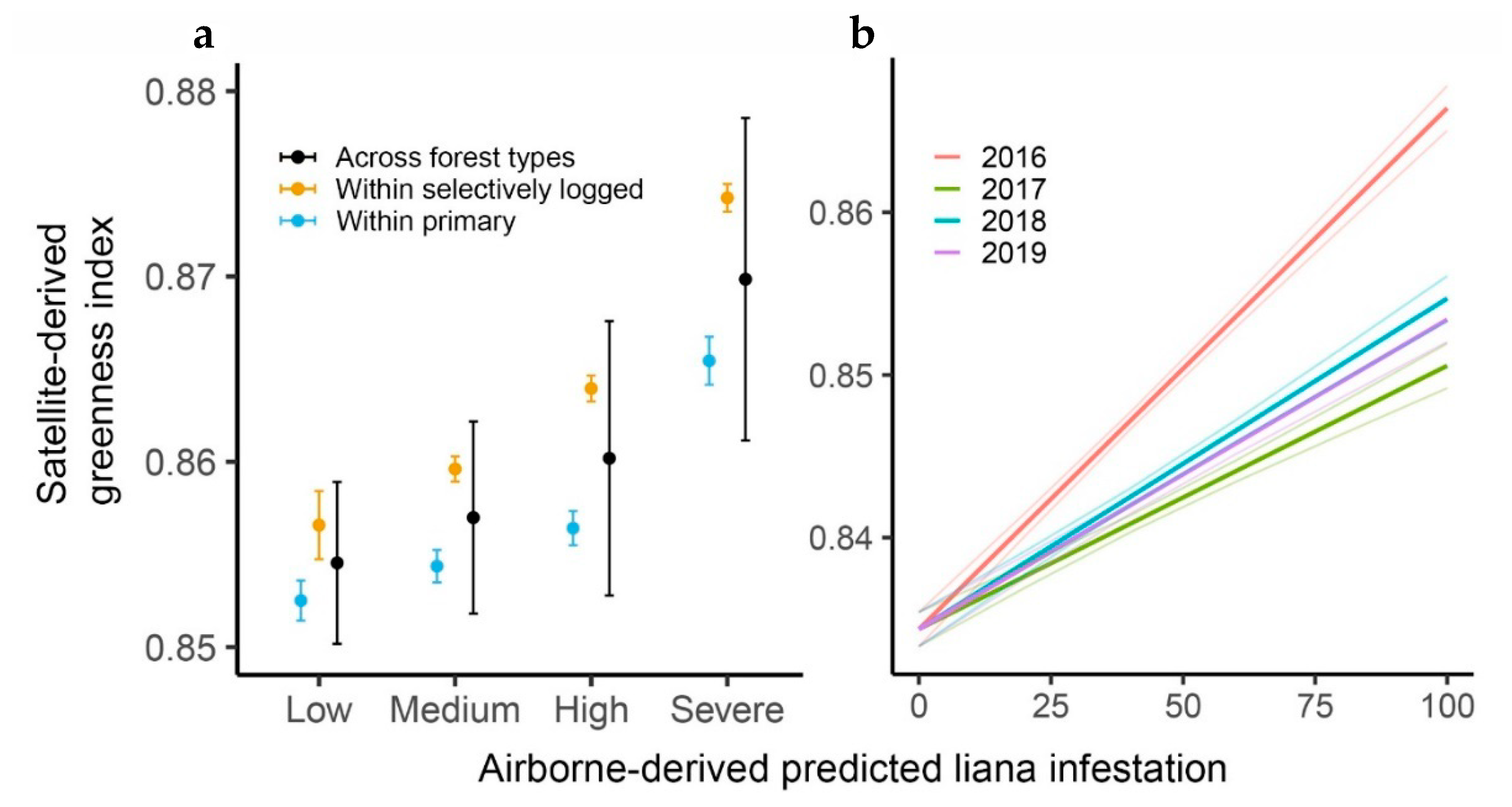
2.3.4. Temporal Change in Liana Infestation
2.3.5. Accuracy Assessment
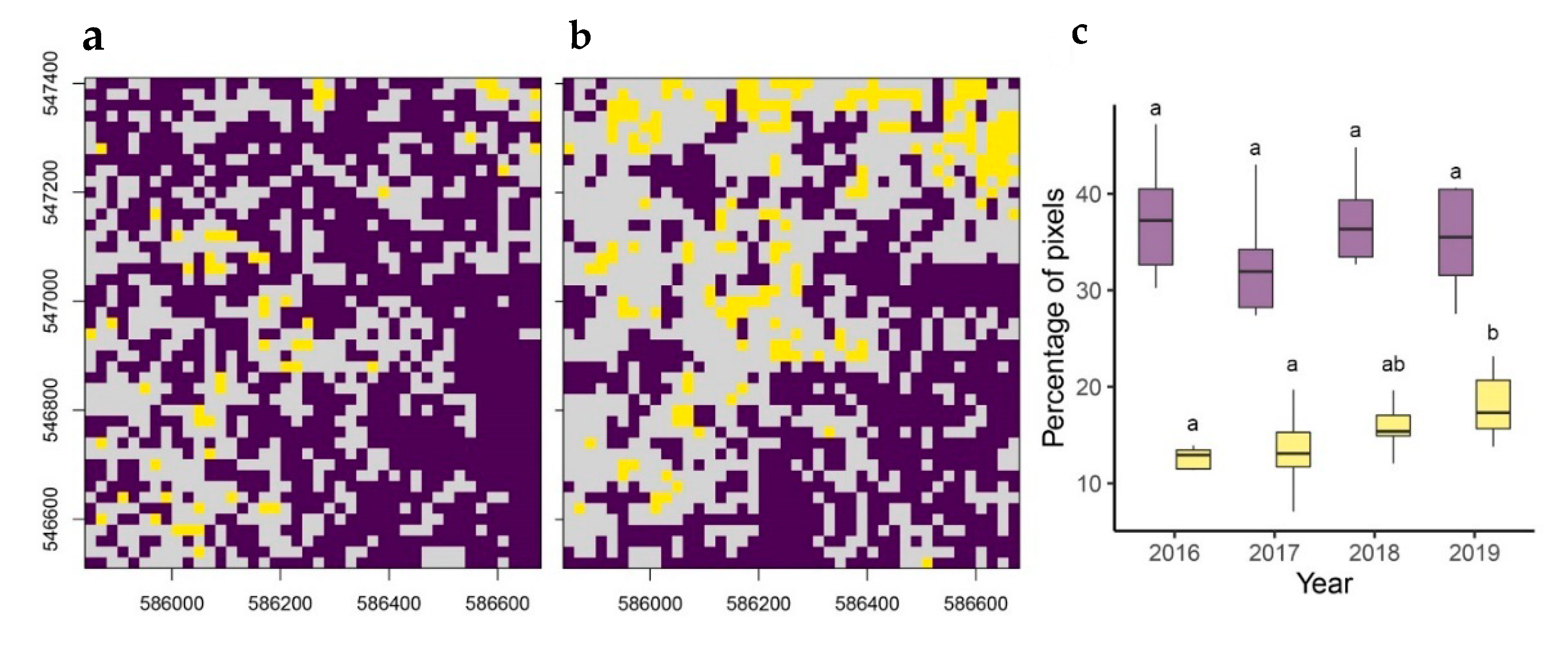
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Salicrup, D.R. Effect of liana cutting on tree regeneration in a liana forest in Amazonian Bolivia. Ecology 2001, 82, 389–396. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Mangan, S.A.; Dalling, J.W.; Baldeck, C.A.; Hubbell, S.P.; Ledo, A.; Muller-Landau, H.; Tobin, M.F.; Aguilar, S.; Brassfield, D. Liana abundance, diversity, and distribution on Barro Colorado Island, Panama. PLoS ONE 2012, 7, e52114. [Google Scholar] [CrossRef] [Green Version]
- Schnitzer, S.A.; Bongers, F. The ecology of lianas and their role in forests. Trends Ecol. Evol. 2002, 17, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Tobin, M.F.; Wright, A.J.; Mangan, S.A.; Schnitzer, S.A. Lianas have a greater competitive effect than trees of similar biomass on tropical canopy trees. Ecosphere 2012, 3, 1–11. [Google Scholar] [CrossRef]
- Van der Heijden, G.; Phillips, O. Liana infestation impacts tree growth in a lowland tropical moist forest. Biogeosciences 2009, 6, 2217–2226. [Google Scholar] [CrossRef] [Green Version]
- Grauel, W.T.; Putz, F.E. Effects of lianas on growth and regeneration of Prioria copaifera in Darien, Panama. Ecol. Manag. 2004, 190, 99–108. [Google Scholar] [CrossRef]
- Ingwell, L.L.; Joseph Wright, S.; Becklund, K.K.; Hubbell, S.P.; Schnitzer, S.A. The impact of lianas on 10 years of tree growth and mortality on Barro Colorado Island, Panama. J. Ecol. 2010, 98, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Phillips, O.L.; Vásquez Martínez, R.; Monteagudo Mendoza, A.; Baker, T.R.; Núñez Vargas, P. Large lianas as hyperdynamic elements of the tropical forest canopy. Ecology 2005, 86, 1250–1258. [Google Scholar] [CrossRef] [Green Version]
- Van der Heijden, G.M.; Powers, J.S.; Schnitzer, S.A. Lianas reduce carbon accumulation and storage in tropical forests. Proc. Natl. Acad. Sci. USA 2015, 112, 13267–13271. [Google Scholar] [CrossRef] [Green Version]
- Phillips, O.L.; Martínez, R.V.; Arroyo, L.; Baker, T.R.; Killeen, T.; Lewis, S.L.; Malhi, Y.; Mendoza, A.M.; Neill, D.; Vargas, P.N. Increasing dominance of large lianas in Amazonian forests. Nature 2002, 418, 770–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnitzer, S.A.; Bongers, F. Increasing liana abundance and biomass in tropical forests: Emerging patterns and putative mechanisms. Ecol. Lett. 2011, 14, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Laurance, W.F.; Pérez-Salicrup, D.; Delamônica, P.; Fearnside, P.M.; D’Angelo, S.; Jerozolinski, A.; Pohl, L.; Lovejoy, T.E. Rain forest fragmentation and the structure of Amazonian liana communities. Ecology 2001, 82, 105–116. [Google Scholar] [CrossRef]
- Bongers, F.; Ewango, C.E.; van der Sande, M.T.; Poorter, L. Liana species decline in Congo basin contrasts with global patterns. Ecology 2020, 101, e03004. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.J.; Calderón, O.; Hernández, A.; Paton, S. Are lianas increasing in importance in tropical forests? A 17-year record from Panama. Ecology 2004, 85, 484–489. [Google Scholar] [CrossRef]
- Laurance, W.F.; Andrade, A.S.; Magrach, A.; Camargo, J.L.; Valsko, J.J.; Campbell, M.; Fearnside, P.M.; Edwards, W.; Lovejoy, T.E.; Laurance, S.G. Long-term changes in liana abundance and forest dynamics in undisturbed Amazonian forests. Ecology 2014, 95, 1604–1611. [Google Scholar] [CrossRef] [Green Version]
- Di Vittorio, A.V.; Negrón-Juárez, R.I.; Higuchi, N.; Chambers, J.Q. Tropical forest carbon balance: Effects of field-and satellite-based mortality regimes on the dynamics and the spatial structure of Central Amazon forest biomass. Environ. Res. Lett. 2014, 9, 034010. [Google Scholar] [CrossRef] [Green Version]
- Castro-Esau, K.; Sánchez-Azofeifa, G.; Caelli, T. Discrimination of lianas and trees with leaf-level hyperspectral data. Remote Sens. Environ. 2004, 90, 353–372. [Google Scholar] [CrossRef]
- Sánchez-Azofeifa, G.; Castro-Esau, K. Canopy observations on the hyperspectral properties of a community of tropical dry forest lianas and their host trees. Int. J. Remote Sens. 2006, 27, 2101–2109. [Google Scholar] [CrossRef]
- Hesketh, M.; Sánchez-Azofeifa, G.A. The effect of seasonal spectral variation on species classification in the Panamanian tropical forest. Remote Sens. Environ. 2012, 118, 73–82. [Google Scholar] [CrossRef]
- Guzman, Q.J.; Rivard, B.; Sánchez-Azofeifa, G.A. Discrimination of liana and tree leaves from a Neotropical Dry Forest using visible-near infrared and longwave infrared reflectance spectra. Remote Sens. Environ. 2018, 219, 135–144. [Google Scholar] [CrossRef]
- Kalacska, M.; Bohlman, S.; Sanchez-Azofeifa, G.A.; Castro-Esau, K.; Caelli, T. Hyperspectral discrimination of tropical dry forest lianas and trees: Comparative data reduction approaches at the leaf and canopy levels. Remote Sens. Environ. 2007, 109, 406–415. [Google Scholar] [CrossRef]
- Guzmán, Q.; Antonio, J.; Sánchez-Azofeifa, G.A.; Rivard, B. Differences in leaf temperature between lianas and trees in the neotropical canopy. Forests 2018, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Azofeifa, A.; Rankine, C.; do Espirito Santo, M.M.; Fatland, R.; Garcia, M. Wireless sensing networks for environmental monitoring: Two case studies from tropical forests. In Proceedings of the 2011 IEEE Seventh International Conference on eScience, Washington, DC, USA, 5–8 December 2011; pp. 70–76. [Google Scholar]
- Waite, C.E.; van der Heijden, G.M.; Field, R.; Boyd, D.S. A view from above: Unmanned aerial vehicles (UAV s) provide a new tool for assessing liana infestation in tropical forest canopies. J. Appl. Ecol. 2019, 56, 902–912. [Google Scholar] [CrossRef]
- Li, W.; Campos-Vargas, C.; Marzahn, P.; Sanchez-Azofeifa, A. On the estimation of tree mortality and liana infestation using a deep self-encoding network. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 1–13. [Google Scholar] [CrossRef]
- Yuan, X.; Laakso, K.; Marzahn, P.; Sanchez-Azofeifa, G.A. Canopy Temperature Differences between Liana-Infested and Non-Liana Infested Areas in a Neotropical Dry Forest. Forests 2019, 10, 890. [Google Scholar] [CrossRef] [Green Version]
- Foster, J.R.; Townsend, P.A.; Zganjar, C.E. Spatial and temporal patterns of gap dominance by low-canopy lianas detected using EO-1 Hyperion and Landsat Thematic Mapper. Remote Sens. Environ. 2008, 112, 2104–2117. [Google Scholar] [CrossRef]
- Marvin, D.C.; Asner, G.P.; Schnitzer, S.A. Liana canopy cover mapped throughout a tropical forest with high-fidelity imaging spectroscopy. Remote Sens. Environ. 2016, 176, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Chandler, C.J.; van der Heijden, G.M.F.; Boyd, D.S.; Cutler, M.E.J.; Costa, H.; Nilus, R.; Foody, G.M. Remote sensing liana infestation in an aseasonal tropical forest: Addressing mismatch in spatial units of analyses. Remote Sens. Ecol. Conserv. 2021. [Google Scholar] [CrossRef]
- Asner, G.P. Biophysical and biochemical sources of variability in canopy reflectance. Remote Sens. Environ. 1998, 64, 234–253. [Google Scholar] [CrossRef]
- Tangki, H.; Chappell, N.A. Biomass variation across selectively logged forest within a 225-km2 region of Borneo and its prediction by Landsat TM. Ecol. Manag. 2008, 256, 1960–1970. [Google Scholar] [CrossRef]
- Huete, A.R.; Kim, Y.; Ratana, P.; Didan, K.; Shimabukuro, Y.E.; Miura, T. Assessment of phenologic variability in Amazon tropical rainforests using hyperspectral Hyperion and MODIS satellite data. In Hyperspectral Remote Sensing of Tropical and Sub-Tropical Forests; CRC Press: Boca Raton, FL, USA, 2008; pp. 233–259. [Google Scholar]
- Lamb, D. Natural regeneration and secondary forests. In Regreening the Bare Hills; Springer: Dordrecht, The Netherlands, 2011; pp. 157–209. [Google Scholar]
- Baccini, A.; Goetz, S.; Walker, W.; Laporte, N.; Sun, M.; Sulla-Menashe, D.; Hackler, J.; Beck, P.; Dubayah, R.; Friedl, M. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Clim. Chang. 2012, 2, 182. [Google Scholar] [CrossRef]
- Pinter, P.J., Jr.; Jackson, R.D.; Elaine Ezra, C.; Gausman, H.W. Sun-angle and canopy-architecture effects on the spectral reflectance of six wheat cultivars. Int. J. Remote Sens. 1985, 6, 1813–1825. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E. Contrasting leaf chemical traits in tropical lianas and trees: Implications for future forest composition. Ecol. Lett. 2012, 15, 1001–1007. [Google Scholar] [CrossRef]
- Sánchez-Azofeifa, G.A.; Castro, K.; Wright, S.J.; Gamon, J.; Kalacska, M.; Rivard, B.; Schnitzer, S.A.; Feng, J.L. Differences in leaf traits, leaf internal structure, and spectral reflectance between two communities of lianas and trees: Implications for remote sensing in tropical environments. Remote Sens. Environ. 2009, 113, 2076–2088. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, G.; Payne, J.; Sinun, W.; Mosigil, G.; Walsh, R.P. Changes in forest land use and management in Sabah, Malaysian Borneo, 1990–2010, with a focus on the Danum Valley region. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 3168–3176. [Google Scholar] [CrossRef] [PubMed]
- Kettle, C.J.; Maycock, C.R.; Burslem, D. New directions in dipterocarp biology and conservation: A synthesis. Biotropica 2012, 44, 658–660. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Philipson, C.D.; Reynolds, G.; Dzulkifli, D.; Snaddon, J.L.; Ong, R.; Hector, A. Positive effects of liana cutting on seedlings are reduced during El Niño-induced drought. J. Appl. Ecol. 2019, 56, 891–901. [Google Scholar] [CrossRef]
- Whitmore, T. Tropical Rain Forests of the Far East, 2nd ed.; Clarendon: Oxford, UK, 1984. [Google Scholar]
- Chapman, S.; Syktus, J.I.; Trancoso, R.; Salazar, A.; Thatcher, M.J.; Watson, J.E.; Meijaard, E.; Sheil, D.; Dargusch, P.; McAlpine, C.A. Compounding impact of deforestation on Borneo’s climate during El Niño events. Environ. Res. Lett. 2020, 15, 084006. [Google Scholar] [CrossRef] [Green Version]
- Walsh, R.; Newbery, D. The ecoclimatology of Danum, Sabah, in the context of the world’s rainforest regions, with particular reference to dry periods and their impact. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1999, 354, 1869–1883. [Google Scholar] [CrossRef] [Green Version]
- Nunes, M.H.; Both, S.; Bongalov, B.; Brelsford, C.; Khoury, S.; Burslem, D.F.; Philipson, C.; Majalap, N.; Riutta, T.; Coomes, D.A. Changes in leaf functional traits of rainforest canopy trees associated with an El Niño event in Borneo. Environ. Res. Lett. 2019, 14, 085005. [Google Scholar] [CrossRef]
- Foody, G.M. Fully fuzzy supervised classification of land cover from remotely sensed imagery with an artificial neural network. Neural Comput. Appl. 1997, 5, 238–247. [Google Scholar] [CrossRef]
- Foody, G.M. Mapping land cover from remotely sensed data with a softened feedforward neural network classification. J. Intell. Robot. Syst. 2000, 29, 433–449. [Google Scholar] [CrossRef]
- Thirumalai, K.; DiNezio, P.N.; Okumura, Y.; Deser, C. Extreme temperatures in Southeast Asia caused by El Niño and worsened by global warming. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Woebbecke, D.M.; Meyer, G.E.; Von Bargen, K.; Mortensen, D. Color indices for weed identification under various soil, residue, and lighting conditions. Trans. Asae 1995, 38, 259–269. [Google Scholar] [CrossRef]
- Cliff, N. Ordinal Methods for Behavioral Data Analysis; Psychology Press: New York, NY, USA, 2014. [Google Scholar]
- Piñeiro, G.; Perelman, S.; Guerschman, J.P.; Paruelo, J.M. How to evaluate models: Observed vs. predicted or predicted vs. observed? Ecol. Model. 2008, 216, 316–322. [Google Scholar] [CrossRef]
- Chen, Y.J.; Cao, K.F.; Schnitzer, S.A.; Fan, Z.X.; Zhang, J.L.; Bongers, F. Water-use advantage for lianas over trees in tropical seasonal forests. New Phytol. 2015, 205, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Maréchaux, I.; Bartlett, M.K.; Iribar, A.; Sack, L.; Chave, J. Stronger seasonal adjustment in leaf turgor loss point in lianas than trees in an Amazonian forest. Biol. Lett. 2017, 13, 20160819. [Google Scholar] [CrossRef] [PubMed]
- Van der Sande, M.T.; Poorter, L.; Schnitzer, S.A.; Engelbrecht, B.M.; Markesteijn, L. The hydraulic efficiency–safety trade-off differs between lianas and trees. Ecology 2019, 100, e02666. [Google Scholar] [PubMed] [Green Version]
- Pérez-Salicrup, D.R.; Sork, V.L.; Putz, F.E. Lianas and Trees in a Liana Forest of Amazonian Bolivia. Biotropica 2001, 33, 34–47. [Google Scholar] [CrossRef]
- Saleska, S.R.; Didan, K.; Huete, A.R.; Da Rocha, H.R. Amazon forests green-up during 2005 drought. Science 2007, 318, 612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, L.O.; Malhi, Y.; Aragão, L.E.; Ladle, R.; Arai, E.; Barbier, N.; Phillips, O. Remote sensing detection of droughts in Amazonian forest canopies. New Phytol. 2010, 187, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S.A.; van der Heijden, G.M. Lianas have a seasonal growth advantage over co-occurring trees. Ecology 2019, 100, e02655. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.J.; Sun, I.; Pickering, M.; Fletcher, C.D.; Chen, Y.-Y. Long-term changes in liana loads and tree dynamics in a Malaysian forest. Ecology 2015, 96, 2748–2757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAlpine, C.A.; Johnson, A.; Salazar, A.; Syktus, J.; Wilson, K.; Meijaard, E.; Seabrook, L.; Dargusch, P.; Nordin, H.; Sheil, D. Forest loss and Borneo’s climate. Environ. Res. Lett. 2018, 13, 044009. [Google Scholar] [CrossRef] [Green Version]
- Gaveau, D.L.; Locatelli, B.; Salim, M.A.; Yaen, H.; Pacheco, P.; Sheil, D. Rise and fall of forest loss and industrial plantations in Borneo (2000–2017). Conserv. Lett. 2019, 12, e12622. [Google Scholar] [CrossRef]
- Marimon, B.S.; Oliveira-Santos, C.; Marimon-Junior, B.H.; Elias, F.; de Oliveira, E.A.; Morandi, P.S.; Prestes, N.C.d.S.; Mariano, L.H.; Pereira, O.R.; Feldpausch, T.R. Drought generates large, long-term changes in tree and liana regeneration in a monodominant Amazon forest. Plant Ecol. 2020, 1–15. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Wu, T.; Zhang, H. An analysis of shadow effects on spectral vegetation indexes using a ground-based imaging spectrometer. IEEE Geosci. Remote Sens. Lett. 2015, 12, 2188–2192. [Google Scholar] [CrossRef]
| Contrast (Years) | Estimate | SE | df | t | p |
|---|---|---|---|---|---|
| 2016–2017 | 0.01583 | 0.00158 | 34852 | 10.038 | <0.0001 |
| 2016–2018 | 0.01170 | 0.00158 | 34852 | 7.415 | <0.0001 |
| 2016–2019 | 0.01300 | 0.00158 | 34852 | 8.240 | <0.0001 |
| 2017–2018 | −0.00414 | 0.00158 | 34852 | −2.623 | 0.0524 |
| 2017–2019 | −0.00284 | 0.00158 | 34852 | −1.798 | 0.4330 |
| 2018–2019 | 0.00130 | 0.00158 | 34852 | 0.825 | 1.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandler, C.J.; van der Heijden, G.M.F.; Boyd, D.S.; Foody, G.M. Detection of Spatial and Temporal Patterns of Liana Infestation Using Satellite-Derived Imagery. Remote Sens. 2021, 13, 2774. https://doi.org/10.3390/rs13142774
Chandler CJ, van der Heijden GMF, Boyd DS, Foody GM. Detection of Spatial and Temporal Patterns of Liana Infestation Using Satellite-Derived Imagery. Remote Sensing. 2021; 13(14):2774. https://doi.org/10.3390/rs13142774
Chicago/Turabian StyleChandler, Chris J., Geertje M. F. van der Heijden, Doreen S. Boyd, and Giles M. Foody. 2021. "Detection of Spatial and Temporal Patterns of Liana Infestation Using Satellite-Derived Imagery" Remote Sensing 13, no. 14: 2774. https://doi.org/10.3390/rs13142774
APA StyleChandler, C. J., van der Heijden, G. M. F., Boyd, D. S., & Foody, G. M. (2021). Detection of Spatial and Temporal Patterns of Liana Infestation Using Satellite-Derived Imagery. Remote Sensing, 13(14), 2774. https://doi.org/10.3390/rs13142774