Cold Wave-Induced Reductions in NDII and ChlRE for North-Western Pacific Mangroves Varies with Latitude and Climate History
Abstract
:1. Introduction
2. Materials and Methods
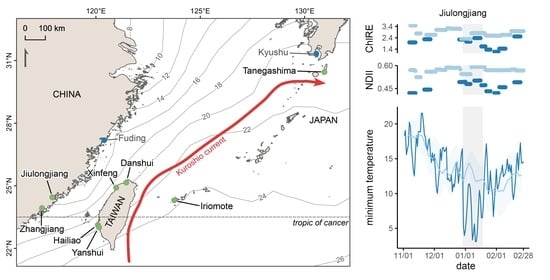
2.1. Sites Selection and Description
2.2. Data
2.2.1. Mangrove Stand Identification and Satellite Imagery
2.2.2. Climate Characteristics
2.3. Processing
2.3.1. Image Correction
2.3.2. Vegetation Indices
2.4. Analysis
2.4.1. Identification of Cold Wave Events
2.4.2. Baseline of Vegetation Indices and Change Detection
2.4.3. Change Detection and Difference with Baseline
2.4.4. Differences between Sites and Relationships with Site Characteristics
3. Results
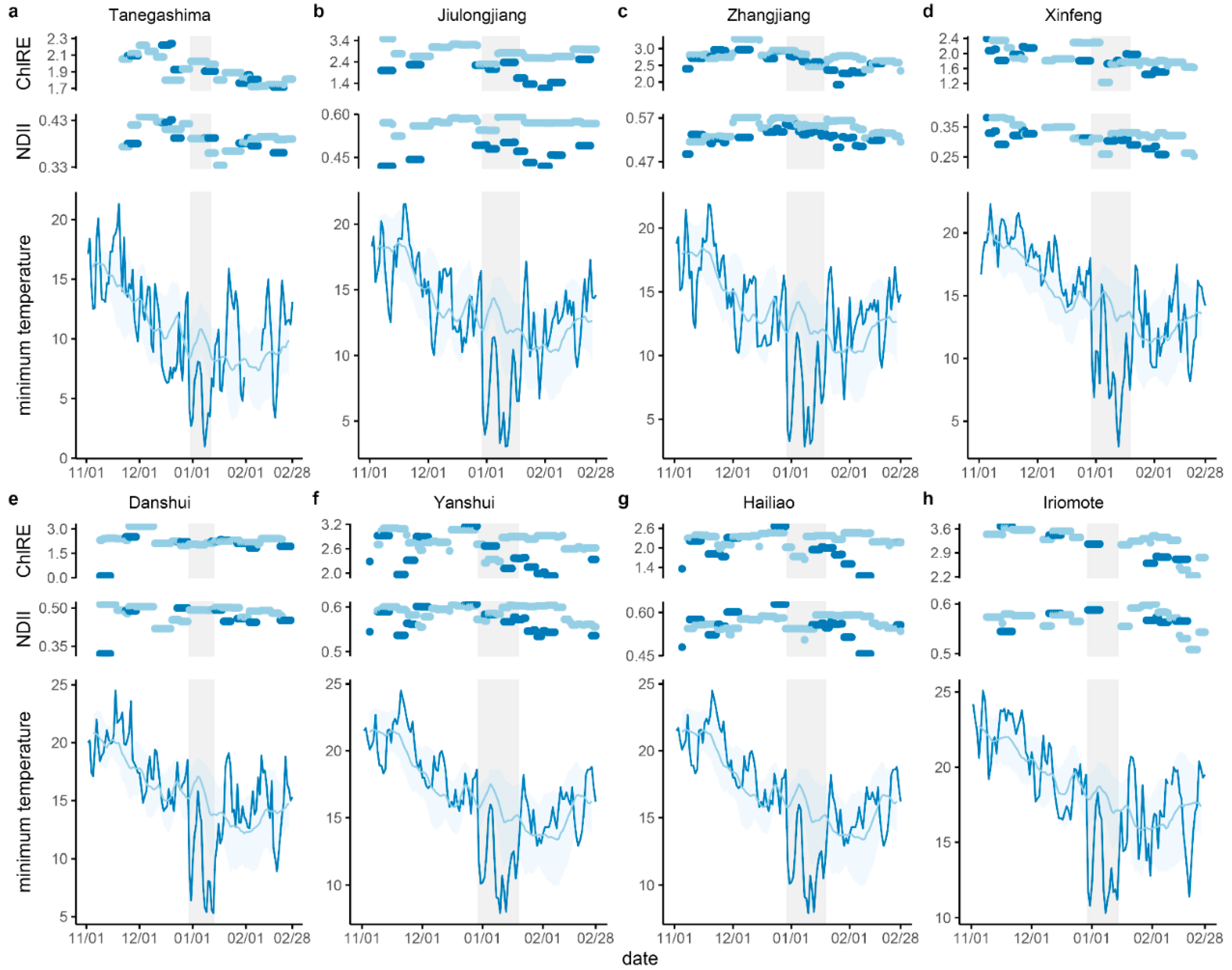
3.1. Temperature, Rainfall, and Windspeed during the Cold Wave Event
3.2. Vegetation Indices Change Following Cold
3.3. Relationships between Vegetation Indices
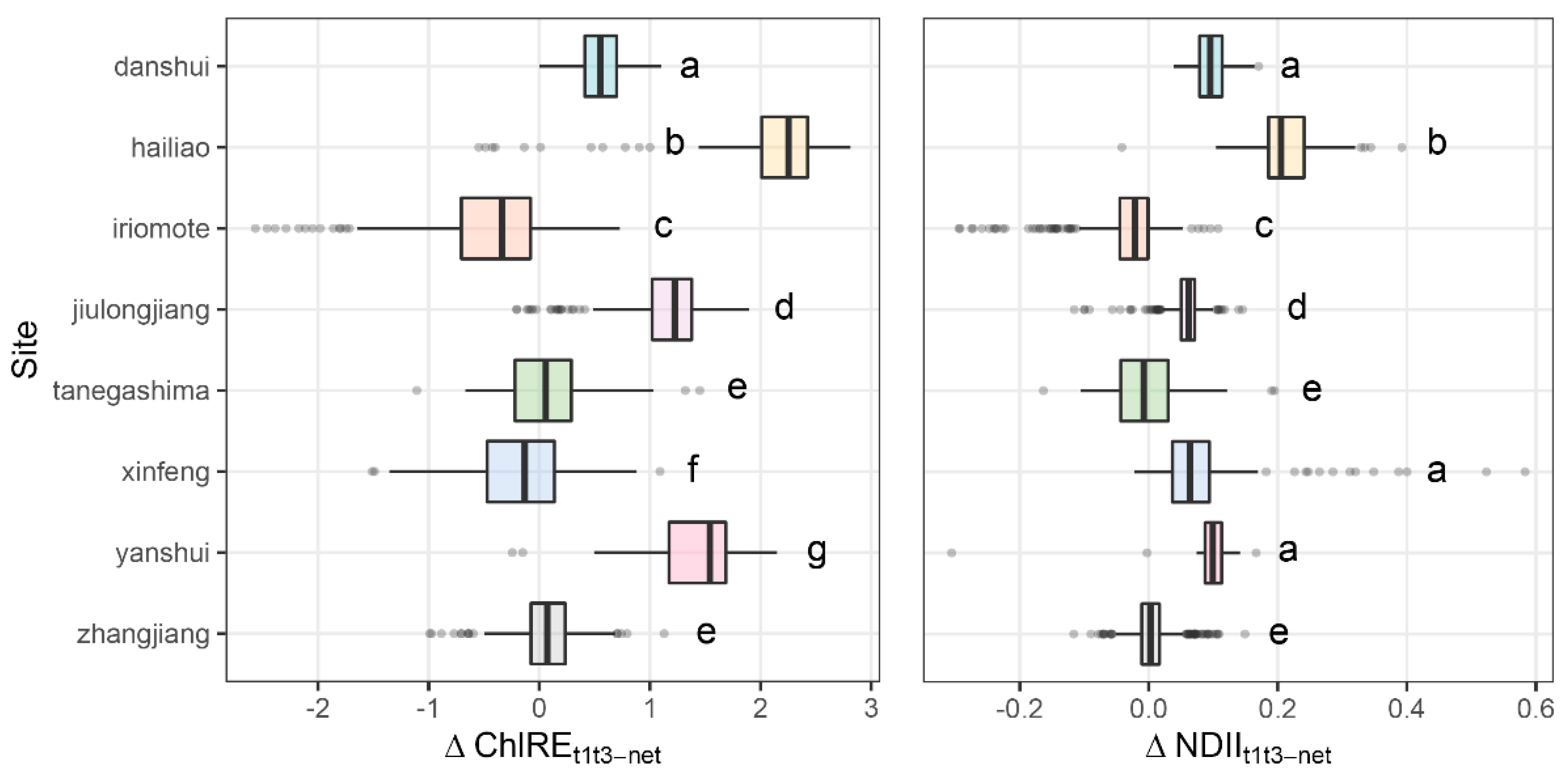
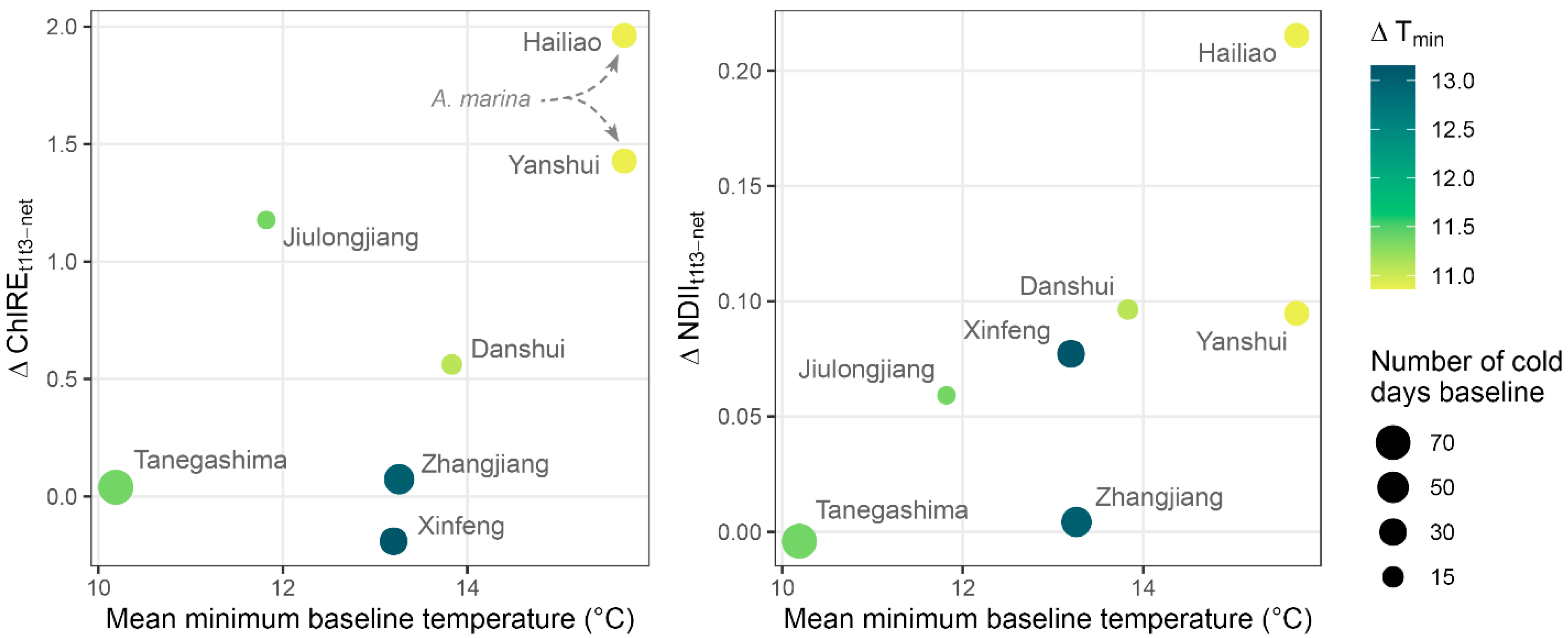
3.4. Canopy Change and Sites Climate
4. Discussion
4.1. Effects of Cold Wave on Mangrove Canopies
4.2. Sites Characteristics Dictate Vegetation Response to Cold
4.3. Complementarity of Vegetation Indices to Monitor Vegetation Disturbance
4.4. Implications for the Future of North Asian Mangroves
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spalding, M.; Blasco, F.; Field, C. World Mangrove Atlas; The International Society for Mangrove Ecosystems: Okinawa, Japan, 1997. [Google Scholar]
- Duke, N.C.; Ball, M.C.; Ellison, J.C. Factors influencing biodiversity and distributional gradients in mangroves. Glob. Ecol. Biogeogr. Lett. 1998, 7, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Krauss, K.W.; Lovelock, C.E.; McKee, K.L.; López-Hoffman, L.; Ewe, S.M.L.; Sousa, W.P. Environmental drivers in mangrove establishment and early development: A review. Aquat. Bot. 2008, 89, 105–127. [Google Scholar] [CrossRef]
- Wu, Y.; Ricklefs, R.E.; Huang, Z.; Zan, Q.; Yu, S. Winter temperature structures mangrove species distributions and assemblage composition in China. Glob. Ecol. Biogeogr. 2018, 27, 1492–1506. [Google Scholar] [CrossRef]
- Osland, M.J.; Feher, L.C.; Griffith, K.T.; Cavanaugh, K.C.; Enwright, N.M.; Day, R.H.; Stagg, C.L.; Krauss, K.W.; Howard, R.J.; Grace, J.B.; et al. Climatic controls on the global distribution, abundance, and species richness of mangrove forests. Ecol. Monogr. 2017, 87, 341–359. [Google Scholar] [CrossRef] [Green Version]
- Ellis, W.L.; Bowles, J.W.; Erickson, A.A.; Stafford, N.; Bell, S.S.; Thomas, M. Alteration of the chemical composition of mangrove (Laguncularia racemosa) leaf litter fall by freeze damage. Estuar. Coast. Shelf Sci. 2006, 68, 363–371. [Google Scholar] [CrossRef]
- Stuart, S.A.; Choat, B.; Martin, K.C.; Holbrook, N.M.; Ball, M.C. The role of freezing in setting the latitudinal limits of mangrove forests. New Phytol. 2007, 173, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; You, S.; Wang, Y.; Huang, L.; Wang, M. Influence of frost on nutrient resorption during leaf senescence in a mangrove at its latitudinal limit of distribution. Plant Soil 2011, 342, 105–115. [Google Scholar] [CrossRef]
- Markley, J.L.; McMillan, C.; Thompson, G.A., Jr. Latitudinal differentiation in response to chilling temperatures among populations of three mangroves, Avicennia germinans, Laguncularia racemosa, and Rhizophora mangle, from the western tropical Atlantic and Pacific Panama. Can. J. Bot. 1982, 60, 2704–2715. [Google Scholar] [CrossRef]
- Peng, Y.-L.; Wang, Y.-S.; Fei, J.; Sun, C.-C.; Cheng, H. Ecophysiological differences between three mangrove seedlings (Kandelia obovata, Aegiceras corniculatum, and Avicennia marina) exposed to chilling stress. Ecotoxicology 2015, 24, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, W.; Li, Q.Q.; Zhang, Y.; Yang, S.; Osland, M.J.; Huang, J.; Peng, C. Mangrove species’ responses to winter air temperature extremes in China. Ecosphere 2017, 8, e01865. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, C.; Chen, J.; Qiu, J.; Huang, Z.; Wang, Q.; Ye, Y. Cold acclimation improves photosynthesis by regulating the ascorbate–glutathione cycle in chloroplasts of Kandelia obovata. J. For. Res. 2019, 30, 755–765. [Google Scholar] [CrossRef]
- Gu, X.; Yang, C.; Zhao, H.; Hu, N.; Krauss, K.W.; Deng, C.; Chen, L. Sap flow evidence of chilling injury and recovery in mangroves following a spring cold spell. Trees 2021, 35, 907–917. [Google Scholar] [CrossRef]
- Kao, W.-Y.; Shih, C.-N.; Tsai, T.-T. Sensitivity to chilling temperatures and distribution differ in the mangrove species Kandelia candel and Avicennia marina. Tree Physiol. 2004, 24, 859–864. [Google Scholar] [CrossRef]
- Devaney, J.L.; Pullen, J.; Feller, I.C.; Parker, J.D. Low humidity and hypersalinity reduce cold tolerance in mangroves. Estuar. Coast. Shelf Sci. 2021, 248, 107015. [Google Scholar] [CrossRef]
- Stevens, P.W.; Fox, S.L.; Montague, C.L. The interplay between mangroves and saltmarshes at the transition between temperate and subtropical climate in Florida. Wetlands Ecol. Manag. 2006, 14, 435–444. [Google Scholar] [CrossRef]
- Osland, M.J.; Enwright, N.; Day, R.H.; Doyle, T.W. Winter climate change and coastal wetland foundation species: Salt marshes vs. mangrove forests in the southeastern United States. Glob. Chang. Biol. 2013, 19, 1482–1494. [Google Scholar] [CrossRef]
- Saintilan, N.; Wilson, N.C.; Rogers, K.; Rajkaran, A.; Krauss, K.W. Mangrove expansion and salt marsh decline at mangrove poleward limits. Glob. Chang. Biol. 2014, 20, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Cavanaugh, K.C.; Kellner, J.R.; Forde, A.J.; Gruner, D.S.; Parker, J.D.; Rodriguez, W.; Feller, I.C. Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. Proc. Natl. Acad. Sci. USA 2014, 111, 723–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavanaugh, K.C.; Parker, J.D.; Cook-Patton, S.C.; Feller, I.C.; Williams, A.P.; Kellner, J.R. Integrating physiological threshold experiments with climate modeling to project mangrove species’ range expansion. Glob. Chang. Biol. 2015, 21, 1928–1938. [Google Scholar] [CrossRef] [Green Version]
- Osland, M.J.; Day, R.H.; Hall, C.T.; Brumfield, M.D.; Dugas, J.L.; Jones, W.R. Mangrove expansion and contraction at a poleward range limit: Climate extremes and land-ocean temperature gradients. Ecology 2017, 98, 125–137. [Google Scholar] [CrossRef]
- Mao, L.; Foong, S.Y. Tracing ancestral biogeography of Sonneratia based on fossil pollen and their probable modern analogues. Palaeoworld 2013, 22, 133–143. [Google Scholar] [CrossRef]
- Li, M.S.; Lee, S.Y. Mangroves of China: A brief review. For. Ecol. Manag. 1997, 96, 241–259. [Google Scholar] [CrossRef]
- Cook-Patton, S.C.; Lehmann, M.; Parker, J.D. Convergence of three mangrove species towards freeze-tolerant phenotypes at an expanding range edge. Funct. Ecol. 2015, 29, 1332–1340. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Preziosi, R.F.; Rowntree, J.K.; Feller, I.C. Is the central-marginal hypothesis a general rule? Evidence from three distributions of an expanding mangrove species, Avicennia germinans (L.) L. Mol. Ecol. 2020, 29, 704–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor-Guzman, J.; Dash, J.; Atkinson, P.M. Remote sensing of mangrove forest phenology and its environmental drivers. Remote Sens. Environ. 2018, 205, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Jia, M.; Wang, Z.; Zhang, Y.; Mao, D.; Wang, C. Monitoring loss and recovery of mangrove forests during 42 years: The achievements of mangrove conservation in China. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 535–545. [Google Scholar] [CrossRef]
- Younes, N.; Northfield, T.D.; Joyce, K.E.; Maier, S.W.; Duke, N.C.; Lymburner, L. A novel approach to modelling mangrove phenology from satellite images: A case study from Northern Australia. Remote Sens. 2020, 12, 4008. [Google Scholar] [CrossRef]
- Lagomasino, D.; Fatoyinbo, T.; Lee, S.; Feliciano, E.; Trettin, C.; Simard, M. A comparison of mangrove canopy height using multiple independent measurements from land, air, and space. Remote Sens. 2016, 8, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asbridge, E.; Lucas, R.; Rogers, K.; Accad, A. The extent of mangrove change and potential for recovery following severe Tropical Cyclone Yasi, Hinchinbrook Island, Queensland, Australia. Ecol. Evol. 2018, 8, 10416–10434. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Durgan, S.D.; Lagomasino, D. Modeling risk of mangroves to tropical cyclones: A case study of Hurricane Irma. Estuar. Coast. Shelf Sci. 2019, 224, 108–116. [Google Scholar] [CrossRef]
- Friess, D.A.; Rogers, K.; Lovelock, C.E.; Krauss, K.W.; Hamilton, S.E.; Lee, S.Y.; Lucas, R.; Primavera, J.; Rajkaran, A.; Shi, S. The state of the world’s mangrove forests: Past, present, and future. Annu. Rev. Environ. Resour. 2019, 44, 89–115. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.D.; Yokoya, N.; Bui, D.T.; Yoshino, K.; Friess, D.A. Remote sensing approaches for monitoring mangrove species, structure, and biomass: Opportunities and challenges. Remote Sens. 2019, 11, 230. [Google Scholar] [CrossRef] [Green Version]
- Kuenzer, C.; Bluemel, A.; Gebhardt, S.; Quoc, T.V.; Dech, S. Remote sensing of mangrove ecosystems: A review. Remote Sens. 2011, 3, 878. [Google Scholar] [CrossRef] [Green Version]
- Giri, C. Observation and monitoring of mangrove forests using remote sensing: Opportunities and challenges. Remote Sens. 2016, 8, 783. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Jia, M.; Yin, D.; Tian, J. A review of remote sensing for mangrove forests: 1956–2018. Remote Sens. Environ. 2019, 231, 111223. [Google Scholar] [CrossRef]
- De Beurs, K.M.; McThompson, N.S.; Owsley, B.C.; Henebry, G.M. Hurricane damage detection on four major Caribbean islands. Remote Sens. Environ. 2019, 229, 1–13. [Google Scholar] [CrossRef]
- Otero, V.; Van De Kerchove, R.; Satyanarayana, B.; Mohd-Lokman, H.; Lucas, R.; Dahdouh-Guebas, F. An analysis of the early regeneration of mangrove forests using Landsat time series in the Matang Mangrove Forest Reserve, Peninsular Malaysia. Remote Sens. 2019, 11, 774. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Thapa, B.; Ross, M.; Gann, D. Remote sensing of seasonal changes and disturbances in mangrove forest: A case study from South Florida. Ecosphere 2016, 7, e01366. [Google Scholar] [CrossRef] [Green Version]
- Thapa, B. Spatio-Temporal Analysis of Chilling Events in Mangrove Forests of South Florida. Master’s Thesis, Florida International University, Miami, FL, USA, 2014. [Google Scholar]
- Liu, K.; Liu, L.; Liu, H.; Li, X.; Wang, S. Exploring the effects of biophysical parameters on the spatial pattern of rare cold damage to mangrove forests. Remote Sens. Environ. 2014, 150, 20–33. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.Q.; Zhang, Y.H.; Huang, L.; Zhao, C.L.; Yang, S.C.; Yang, Z.W.; Chen, Y.C.; Xu, H.L.; Zhong, C.R.; et al. Damage to mangroves from extreme cold in early 2008 in southern China. Chin. J. Plant Ecol. 2010, 34, 186–194. [Google Scholar] [CrossRef]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Nakasuga, T. Analysis of the mangrove stand. Sci. Bull. Fac. Agric. Univ. Ryukyus Okinawa 1979, 26, 413–519. [Google Scholar]
- Alongi, D.M.; Pfitzner, J.; Trott, L.A.; Tirendi, F.; Dixon, P.; Klumpp, D.W. Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang Estuary, China. Estuar. Coast. Shelf Sci. 2005, 63, 605–618. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Wu, Q.; Fang, B.; Lin, P. The growth of Kandelia candel seedlings in mangrove habitats of the Zhangjiang estuary in Fujian, China. Acta Ecol. Sin. 2006, 26, 1648–1655. [Google Scholar] [CrossRef]
- Hsueh, M.-L.; Lee, H.-H. Diversity and distribution of the mangrove forests in Taiwan. Wetlands Ecol. Manag. 2000, 8, 233–242. [Google Scholar] [CrossRef]
- Hurrell, J.W.; Hack, J.J.; Shea, D.; Caron, J.M.; Rosinski, J. A new sea surface temperature and sea ice boundary dataset for the community atmosphere model. J. Clim. 2008, 21, 5145–5153. [Google Scholar] [CrossRef]
- Shea, D.; Hurrell, J.; Phillips, A. Merged Hadley-OI Sea Surface Temperature and Sea Ice Concentration Data Set, 2nd ed.; UCAR/NCAR—DASH Repository: Boulder, CO, USA, 2021. [Google Scholar] [CrossRef]
- Sheue, C.-R.; Liu, H.-Y.; Yong, J.W.H. Kandelia obovata (Rhizophoraceae), a new mangrove species from Eastern Asia. Taxon 2003, 52, 287–294. [Google Scholar] [CrossRef]
- Wang, L.; Shi, C.; Tian, J.; Song, X.; Jia, M.; Li, X.; Liu, X.; Zhong, R.; Yin, D.; Yang, S.; et al. Researches on mangrove forest monitoring methods based on multi-source remote sensing. Biodivers. Sci. 2018, 26, 838–849. [Google Scholar] [CrossRef]
- Ho, C.-Y. Estimation of Carbon Sequestration for a Mangrove Wetland in Southern Taiwan. Master’s Thesis, Chia-Nan University of Pharmacy and Science, Tainan, Taiwan, 2012. [Google Scholar]
- Wang, M.; Cao, W.; Guan, Q.; Wu, G.; Wang, F. Assessing changes of mangrove forest in a coastal region of southeast China using multi-temporal satellite images. Estuar. Coast. Shelf Sci. 2018, 207, 283–292. [Google Scholar] [CrossRef]
- Kao, W.Y.; Chang, K.W. Stable carbon isotope ratio and nutrient contents of the Kandelia candel mangrove populations of different growth forms. Bot. Bull. Acad. Sin. 1998, 39, 39–45. [Google Scholar]
- Lin, C.-W.; Kao, Y.-C.; Chou, M.-C.; Wu, H.-H.; Ho, C.-W.; Lin, H.-J. Methane emissions from subtropical and tropical mangrove ecosystems in Taiwan. Forests 2020, 11, 470. [Google Scholar] [CrossRef] [Green Version]
- Simpson, R.H.; Riehl, H. The Hurricane and Its Impact; Louisiana State University Press: Baton Rouge, LA, USA, 1981; p. 398. [Google Scholar]
- Bunting, P.; Rosenqvist, A.; Lucas, R.M.; Rebelo, L.-M.; Hilarides, L.; Thomas, N.; Hardy, A.; Itoh, T.; Shimada, M.; Finlayson, C.M. The Global Mangrove Watch—A new 2010 Global baseline of mangrove extent. Remote Sens. 2018, 10, 1669. [Google Scholar] [CrossRef] [Green Version]
- Ranghetti, L.; Boschetti, M.; Nutini, F.; Busetto, L. “sen2r”: An R toolbox for automatically downloading and preprocessing Sentinel-2 satellite data. Comput. Geosci. 2020, 139, 104473. [Google Scholar] [CrossRef]
- Ji, L.; Senay, G.B.; Verdin, J.P. Evaluation of the Global Land Data Assimilation System (GLDAS) air temperature data products. J. Hydrometeorol. 2015, 16, 2463–2480. [Google Scholar] [CrossRef]
- Hardisky, M.A.; Klemas, V.; Smart, R.M. The influence of soil salinity, growth form, and leaf moisture on the spectral reflectance of Spartina alterniflora canopies. Photogramm. Eng. Remote Sens. 1983, 49, 77–83. [Google Scholar]
- Cheng, Y.-B.; Zarco-Tejada, P.J.; Riaño, D.; Rueda, C.A.; Ustin, S.L. Estimating vegetation water content with hyperspectral data for different canopy scenarios: Relationships between AVIRIS and MODIS indexes. Remote Sens. Environ. 2006, 105, 354–366. [Google Scholar] [CrossRef]
- Gao, B.-C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Wang, W.; Qu, J.J.; Hao, X.; Liu, Y.; Stanturf, J.A. Post-hurricane forest damage assessment using satellite remote sensing. Agr. For. Meteorol. 2010, 150, 122–132. [Google Scholar] [CrossRef]
- Gang, C.; Pan, S.; Tian, H.; Wang, Z.; Xu, R.; Bian, Z.; Pan, N.; Yao, Y.; Shi, H. Satellite observations of forest resilience to hurricanes along the northern Gulf of Mexico. For. Ecol. Manag. 2020, 472, 118243. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef] [Green Version]
- Dou, Z.; Cui, L.; Li, J.; Zhu, Y.; Gao, C.; Pan, X.; Lei, Y.; Zhang, M.; Zhao, X.; Li, W. Hyperspectral estimation of the chlorophyll content in short-term and long-term restorations of mangrove in Quanzhou Bay estuary, China. Sustainability 2018, 10, 1127. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Qin, Q.; Ren, H.; Zhang, T.; Chen, S. Red-edge band vegetation indices for leaf area index estimation from Sentinel-2/MSI imagery. IEEE Trans. Geosci. Remote Sens. 2020, 58, 826–840. [Google Scholar] [CrossRef]
- Hijmans, R.J. Package ‘Raster’. R Package Version 2.9-23. 2019. Available online: https://cran.r-project.org/web/packages/raster/index.html (accessed on 20 July 2019).
- Hayes, M.A.; Shor, A.C.; Jesse, A.; Miller, C.; Kennedy, J.P.; Feller, I. The role of glycine betaine in range expansions; protecting mangroves against extreme freeze events. J. Ecol. 2019, 108, 61–69. [Google Scholar] [CrossRef]
- Zheng, C.; Ye, Y.; Liu, W.; Tang, J.; Zhang, C.; Qiu, J.; Chen, J. Recovery of photosynthesis, sucrose metabolism, and proteolytic enzymes in Kandelia obovata from rare cold events in the northernmost mangrove, China. Ecol. Process. 2016, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Suwa, R.; Hagihara, A. Seasonal changes in canopy photosynthesis and foliage respiration in a Rhizophora stylosa stand at the northern limit of its natural distribution. Wetl. Ecol. Manag. 2008, 16, 313. [Google Scholar] [CrossRef]
- Suwa, R.; Khan, M.N.I.; Hagihara, A. Canopy photosynthesis, canopy respiration and surplus production in a subtropical mangrove Kandelia candel forest, Okinawa Island, Japan. Mar. Ecol. Prog. Ser. 2006, 320, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Herberich, E.; Sikorski, J.; Hothorn, T. A robust procedure for comparing multiple means under heteroscedasticity in unbalanced designs. PLoS ONE 2010, 5, e9788. [Google Scholar] [CrossRef] [Green Version]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Zeileis, A.; Köll, S.; Graham, N. Various versatile variances: An object-oriented implementation of clustered covariances in R. J. Stat. Softw. 2020, 95, 36. [Google Scholar] [CrossRef]
- Mfilinge, P.L.; Meziane, T.; Bachok, Z.; Tsuchiya, M. Litter dynamics and particulate organic matter outwelling from a subtropical mangrove in Okinawa Island, South Japan. Estuar. Coast. Shelf Sci. 2005, 63, 301–313. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, Y.P.; Chen, G.C. Litter production and litter elemental composition in two rehabilitated Kandelia obovata mangrove forests in Jiulongjiang Estuary, China. Mar. Environ. Res. 2013, 83, 63–72. [Google Scholar] [CrossRef]
- Radabaugh, K.R.; Moyer, R.P.; Chappel, A.R.; Dontis, E.E.; Russo, C.E.; Joyse, K.M.; Bownik, M.W.; Goeckner, A.H.; Khan, N.S. Mangrove damage, delayed mortality, and early recovery following Hurricane Irma at two landfall sites in Southwest Florida, USA. Estuar. Coasts 2020, 43, 1104–1118. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Lin, P. Effect of soil salinity on cold tolerance of mangrove Kandelia candel. Chin. J. Oceanol. Limnol. 2005, 23, 98–103. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, W.; Qiu, J.; Huang, L.; Huang, X.; Chen, S. Comparison of physiological characteristics of Kandelia obovata at different ages in winter in the northernmost mangrove transplanted area of China. Acta Ecol. Sin. 2013, 33, 132–138. [Google Scholar] [CrossRef]
- Mabry, C.M.; Hamburg, S.P.; Lin, T.-C.; Horng, F.-W.; King, H.-B.; Hsia, Y.-J. Typhoon disturbance and stand-level damage patterns at a subtropical forest in Taiwan. Biotropica 1998, 30, 238–250. [Google Scholar] [CrossRef]
- Lin, T.-C.; Hamburg, S.P.; Lin, K.-C.; Wang, L.-J.; Chang, C.-T.; Hsia, Y.-J.; Vadeboncoeur, M.A.; Mabry McMullen, C.M.; Liu, C.-P. Typhoon disturbance and forest dynamics: Lessons from a Northwest Pacific subtropical forest. Ecosystems 2011, 14, 127–143. [Google Scholar] [CrossRef]
- Osland, M.J.; Hartmann, A.M.; Day, R.H.; Ross, M.S.; Hall, C.T.; Feher, L.C.; Vervaeke, W.C. Microclimate influences mangrove freeze damage: Implications for range expansion in response to changing macroclimate. Estuar. Coasts 2019, 42, 1084–1096. [Google Scholar] [CrossRef]
- Song, W.; Feng, J.; Krauss, K.W.; Zhao, Y.; Wang, Z.; Lin, G. Non-freezing cold event stresses can cause significant damage to mangrove seedlings: Assessing the role of warming and nitrogen enrichment in a mesocosm study. Environ. Res. Commun. 2020, 2, 031003. [Google Scholar] [CrossRef]
- Magney, T.S.; Bowling, D.R.; Logan, B.A.; Grossmann, K.; Stutz, J.; Blanken, P.D.; Burns, S.P.; Cheng, R.; Garcia, M.A.; Kӧhler, P.; et al. Mechanistic evidence for tracking the seasonality of photosynthesis with solar-induced fluorescence. Proc. Natl. Acad. Sci. USA 2019, 116, 11640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, G.H.; Colombo, R.; Middleton, E.M.; Rascher, U.; van der Tol, C.; Nedbal, L.; Goulas, Y.; Pérez-Priego, O.; Damm, A.; Meroni, M.; et al. Remote sensing of solar-induced chlorophyll fluorescence (SIF) in vegetation: 50 years of progress. Remote Sens. Environ. 2019, 231, 111177. [Google Scholar] [CrossRef]
- Zhu, X.; Hou, Y.; Zhang, Y.; Lu, X.; Liu, Z.; Weng, Q. Potential of sun-induced chlorophyll fluorescence for indicating mangrove canopy photosynthesis. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG006159. [Google Scholar] [CrossRef]
- Burrows, M.T.; Schoeman, D.S.; Buckley, L.B.; Moore, P.; Poloczanska, E.S.; Brander, K.M.; Brown, C.; Bruno, J.F.; Duarte, C.M.; Halpern, B.S.; et al. The Pace of Shifting Climate in Marine and Terrestrial Ecosystems. Science 2011, 334, 652–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickey, S.M.; Phinn, S.R.; Callow, N.J.; Van Niel, K.P.; Hansen, J.E.; Duarte, C.M. Is climate change shifting the poleward limit of mangroves? Estuar. Coasts 2017, 40, 1215–1226. [Google Scholar] [CrossRef]
- Wakushima, S.; Kuraishi, S.; Sakurai, N. Soil salinity and pH in Japanese mangrove forests and growth of cultivated mangrove plants in different soil conditions. J. Plant Res. 1994, 107, 39–46. [Google Scholar] [CrossRef]
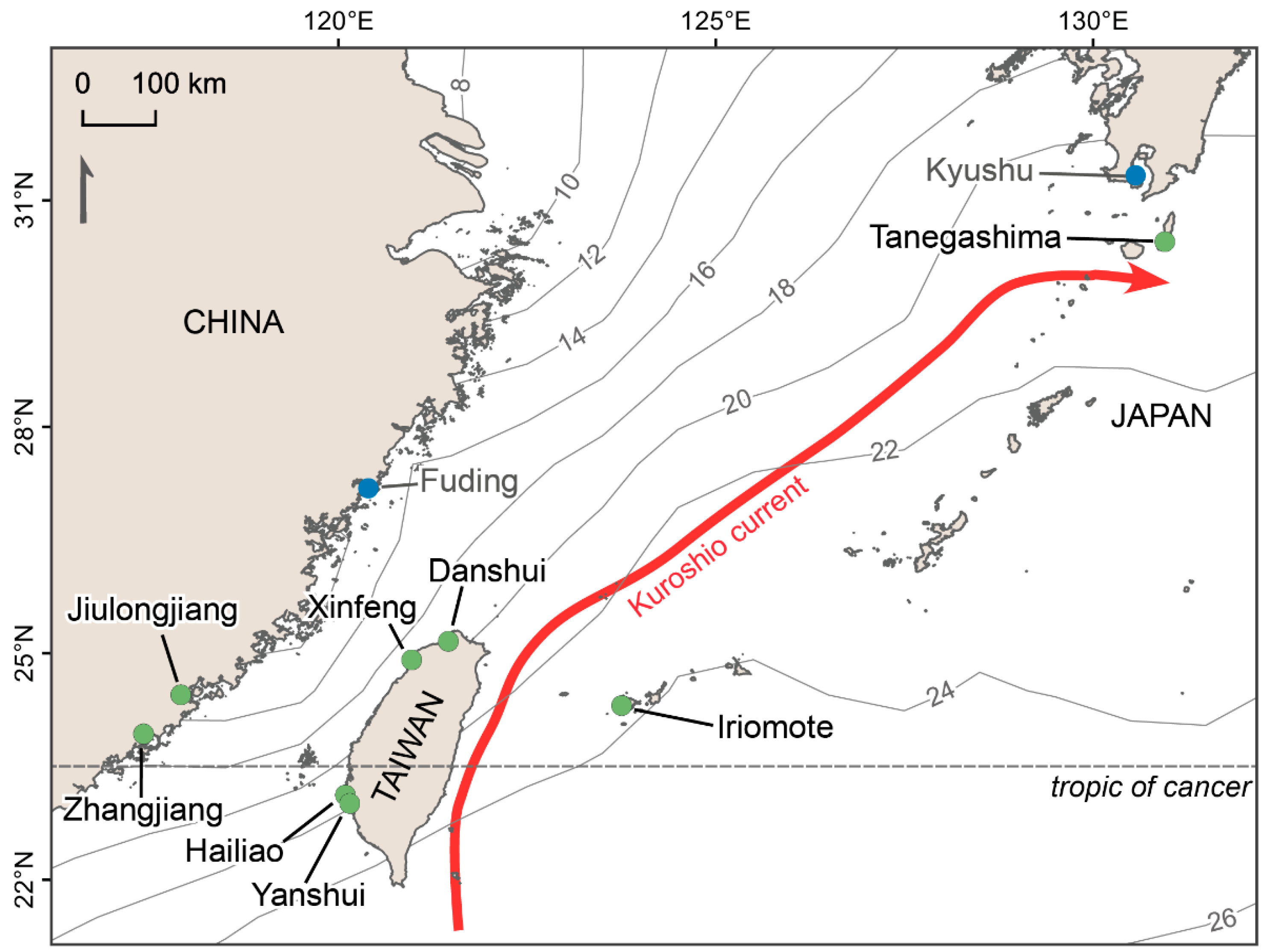
| Site | Coordinates Latitude, Longitude | Dominant Species | Weather Station | ||
|---|---|---|---|---|---|
| Name | Elevation (m) | Distance (km) | |||
| Danshui | 25.15266, 121.45770 | K. obovata | Danshui | 19 | 1 |
| Xinfeng | 24.90940, 120.96920 | K. obovata, A. marina | Shiangshan | 15 | 19 |
| Hailiao | 23.11786, 120.09012 | A. marina | Yongkang | 8.1 | 17 |
| Yanshui | 23.00215, 120.15111 | A. marina | Yongkang | 8.1 | 9 |
| Japan | |||||
| Tanegashima | 30.45068, 130.95517 | K. obovata | Kaminaka | 150 | 7 |
| Iriomote | 24.29597, 123.74971 | K. obovata, R. mucronata, Bruguiera gymnorhiza | Iriomotejima | 10 | 15 |
| China | |||||
| Jiulongjiang | 24.44507, 117.91111 | K. obovata | – | – | – |
| Zhangjiang | 23.92832, 117.41522 | K. obovata, A. marina | – | – | – |
| Site | 2021 Temperature (°C) | 2015–2020 Temperature (°C) | |||||
|---|---|---|---|---|---|---|---|
| Mean | Min | Date | Mean (±SD) | Mean Min (±SD) | Min | ||
| Tanegashima | 3.1 | 1.0 | 8 January | 12.37 (±2.12) | 10.19 (±2.18) | −0.3 | 11.37 |
| Iriomote | 11.6 | 10.3 | 8 January | 20.34 (±1.97) | 18.26 (±1.93) | 8.1 | 10.04 |
| Danshui | 12.1 | 5.3 | 13 January | 16.38 (±3.02) | 13.83 (±3.15) | 3.8 | 11.08 |
| Xinfeng | 11.2 | 3.0 | 13 January | 16.15 (±2.47) | 13.20 (±3.01) | 2.9 | 13.15 |
| Yanshui | 13.6 | 7.9 | 10 January | 18.76 (±2.79) | 15.70 (±2.88) | 5.7 | 10.86 |
| Hailiao | 13.6 | 7.9 | 10 January | 18.76 (±2.79) | 15.70 (±2.88) | 5.7 | 10.86 |
| Jiulongjiang | 7.8 | 3.1 | 11 January | 14.46 (±2.77) | 11.82 (±3.34) | 1.02 | 11.36 |
| Zhangjiang | 7.0 | 2.9 | 8 January | 15.92 (±2.58) | 13.26 (±2.67) | 0.4 | 13.02 |
| Site | ΔVIt1t2 | ΔVIt1t3 | ||||
|---|---|---|---|---|---|---|
| 2015–2020 Baseline | 2021 | 95%CI | 2015–2020 Baseline | 2021 | 95%CI | |
| ChlRE | ||||||
| Tanegashima | −0.19 (0.043) | 0.02 (0.014) | 0.12;0.30 | 0.08 (0.03) | 0.12 (0.02) | −0.03;0.11 |
| Iriomote | 0.22 (0.015) | 0.82 (0.015) | 0.56;0.64 | 1.13 (0.02) | 0.70 (0.02) | −0.49;−0.37 |
| Danshui | −0.28 (0.021) | −0.03 (0.018) | 0.19;0.30 | −0.19 (0.03) | 0.38 (0.03) | 0.48;0.64 |
| Xinfeng | 0.52 (0.035) | −0.17 (0.02) | −0.77;−0.62 | 0.49 (0.03) | 0.30 (0.01) | −0.26;−0.12 |
| Yanshui | −0.19 (0.028) | 0.79 (0.03) | 0.89;1.06 | −0.21 (0.03) | 1.22 (0.04) | 1.33;1.52 |
| Hailiao | −0.36 (0.040) | 0.67 (0.04) | 0.91;1.13 | −0.44 (0.04) | 1.53 (0.06) | 1.81;2.11 |
| Jiulongjiang | −0.54 (0.01) | −0.13 (0.01) | 0.38;0.43 | −0.37 (0.01) | 0.80 (0.01) | 1.15;1.20 |
| Zhangjiang | 0.49 (0.01) | 0.37 (0.01) | −0.14;−0.10 | 0.32 (0.01) | 0.39 (0.01) | 0.05;0.09 |
| NDII | ||||||
| Tanegashima | 0.05 (0.006) | −0.0001 (0.002) | −0.06;−0.04 | 0.02 (0.004) | 0.02 (0.004) | −0.01;0.01 |
| Iriomote | −0.02 (0.002) | 0.01 (0.002) | 0.03;0.04 | 0.05 (0.002) | 0.02 (0.002) | −0.04;−0.03 |
| Danshui | −0.05 (0.005) | 0.01 (0.002) | 0.05;0.07 | −0.04 (0.004) | 0.06 (0.004) | 0.09;0.11 |
| Xinfeng | −0.03 (0.003) | 0.02 (0.003) | 0.04;0.06 | −0.02 (0.004) | 0.06 (0.002) | 0.07;0.09 |
| Yanshui | −0.03 (0.002) | 0.03 (0.007) | 0.05;0.08 | −0.03 (0.002) | 0.06 (0.006) | 0.08;0.11 |
| Hailiao | −0.05 (0.002) | 0.08 (0.008) | 0.11;0.15 | −0.04 (0.003) | 0.17 (0.004) | 0.20;0.23 |
| Jiulongjiang | −0.05 (0.001) | −0.01 (0.001) | 0.03;0.04 | −0.03 (0.001) | 0.03 (0.001) | 0.06;0.06 |
| Zhangjiang | 0.02 (0.001) | 0.02 (0.001) | −0.001;0.004 | 0.03 (0.001) | 0.04 (0.001) | 0.002;0.01 |
| Site | ChlRE | NDII | ||||
|---|---|---|---|---|---|---|
| 95%CI | 95%CI | |||||
| Tanegashima | 0.21 (0.04) | 0.04 (0.03) | −0.28;−0.06 | −0.05 (0.006) | −0.004 (0.005) | 0.03;0.06 |
| Iriomote | 0.60 (0.02) | −0.43 (0.03) | −1.09;−0.97 | 0.03 (0.002) | −0.03 (0.003) | −0.07;−0.06 |
| Danshui | 0.25 (0.03) | 0.56 (0.04) | 0.22;0.41 | 0.06 (0.006) | 0.10 (0.005) | 0.02;0.05 |
| Xinfeng | −0.70 (0.04) | −0.19 (0.03) | 0.41;0.60 | 0.05 (0.004) | 0.08 (0.005) | 0.01;0.04 |
| Yanshui | 0.97 (0.04) | 1.43 (0.05) | 0.32;0.59 | 0.06 (0.008) | 0.09 (0.006) | 0.01;0.05 |
| Hailiao | 1.02 (0.05) | 1.96 (0.10) | 0.72;1.16 | 0.13 (0.008) | 0.22 (0.007) | 0.07;0.11 |
| Jiulongjiang | 0.41 (0.01) | 1.18 (0.02) | 0.74;0.81 | 0.04 (0.001) | 0.06 (0.001) | 0.02;0.03 |
| Zhangjiang | −0.12 (0.01) | 0.07 (0.01) | 0.16;0.22 | 0.002 (0.001) | 0.004 (0.001) | −0.001;0.01 |
| Site | ||
|---|---|---|
| Tanegashima | 0.73 (<0.01) | 0.29 (<0.01) |
| Iriomote | 0.18 (<0.01) | 0.29 (<0.01) |
| Danshui | 0.78 (<0.01) | −0.10 (0.52) |
| Xinfeng | 0.90 (<0.01) | 0.61 (<0.01) |
| Yanshui | 0.71 (<0.01) | 0.03 (0.81) |
| Hailiao | 0.34 (0.01) | −0.16 (0.19) |
| Jiulongjiang | 0.87 (<0.01) | 0.32 (<0.01) |
| Zhangjiang | 0.82 (<0.01) | 0.30 (<0.01) |
| Predictor | Estimate | SE | t-Value | p |
|---|---|---|---|---|
| Intercept | 5.98 | 0.16 | 38.23 | <0.01 |
| Mean minimum temperature baseline | 0.10 | 0.01 | 11.56 | <0.01 |
| Number of cold days baseline | −0.01 | 0.01 | −19.22 | <0.01 |
| −0.52 | 0.01 | −39.15 | <0.01 | |
| Statistics | n = 1736; Residual standard error = 0.4226; df = 1732; Mult. R2= 0.6744; Adj. R2 = 0.6738; F = 1196; p < 0.01; AIC = −2986.30 | |||
| Intercept | 0.13 | 0.02 | 7.01 | <0.01 |
| Mean minimum temperature baseline | 0.02 | 0.001 | 18.3 | <0.01 |
| Number of cold days baseline | −0.001 | 0.0001 | −11.1 | <0.01 |
| −0.02 | 0.002 | −15.35 | <0.01 | |
| Statistics | n = 1736; Residual standard error = 0.0519; df = 1732; Mult. R2= 0.3761; Adj. R2 = 0.3750; F = 348; p < 0.01; AIC = −10,269.23 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peereman, J.; Hogan, J.A.; Lin, T.-C. Cold Wave-Induced Reductions in NDII and ChlRE for North-Western Pacific Mangroves Varies with Latitude and Climate History. Remote Sens. 2021, 13, 2732. https://doi.org/10.3390/rs13142732
Peereman J, Hogan JA, Lin T-C. Cold Wave-Induced Reductions in NDII and ChlRE for North-Western Pacific Mangroves Varies with Latitude and Climate History. Remote Sensing. 2021; 13(14):2732. https://doi.org/10.3390/rs13142732
Chicago/Turabian StylePeereman, Jonathan, J. Aaron Hogan, and Teng-Chiu Lin. 2021. "Cold Wave-Induced Reductions in NDII and ChlRE for North-Western Pacific Mangroves Varies with Latitude and Climate History" Remote Sensing 13, no. 14: 2732. https://doi.org/10.3390/rs13142732
APA StylePeereman, J., Hogan, J. A., & Lin, T.-C. (2021). Cold Wave-Induced Reductions in NDII and ChlRE for North-Western Pacific Mangroves Varies with Latitude and Climate History. Remote Sensing, 13(14), 2732. https://doi.org/10.3390/rs13142732