Predicting Soil Respiration from Plant Productivity (NDVI) in a Sub-Arctic Tundra Ecosystem
Abstract
:1. Introduction
2. Materials and Methods
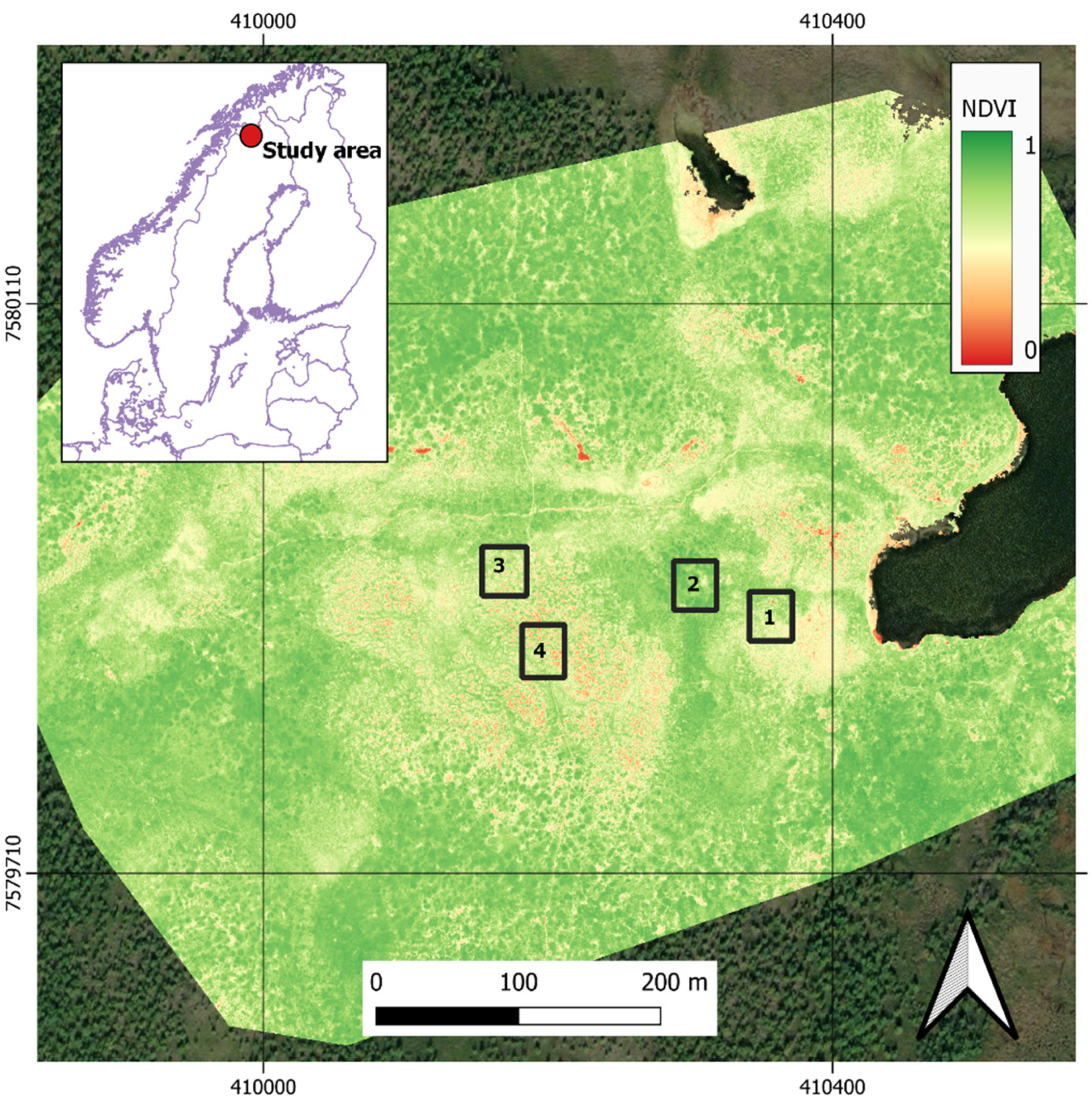
2.1. Site Description and Vegetation Survey
2.2. NDVI and Land Surface Greenness
2.3. Soil Respiration
2.4. Soil Cores: Moisture, Organic Matter, and Elemental Analysis
2.5. Statistical Analysis
3. Results
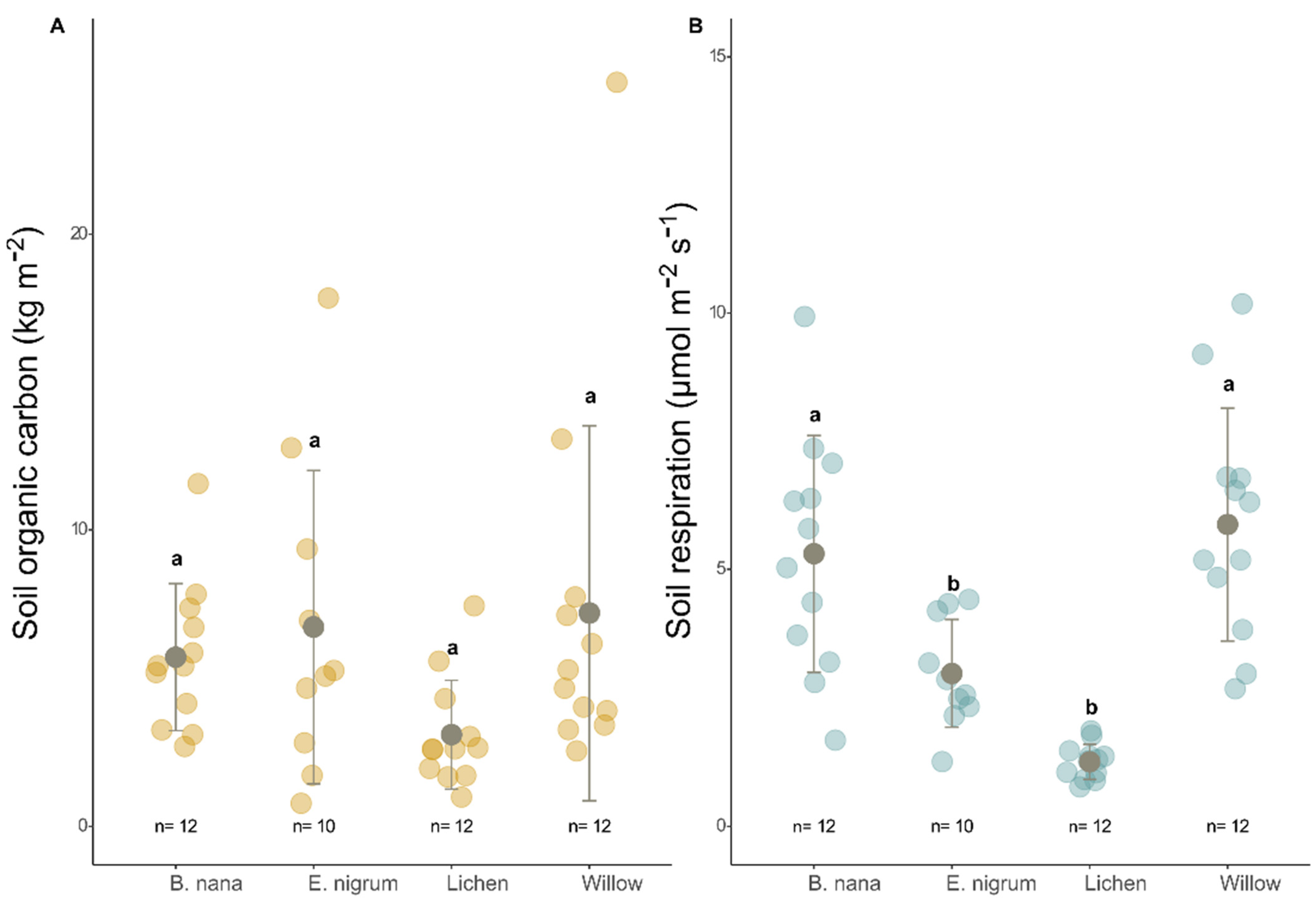
3.1. Organic Matter and Carbon
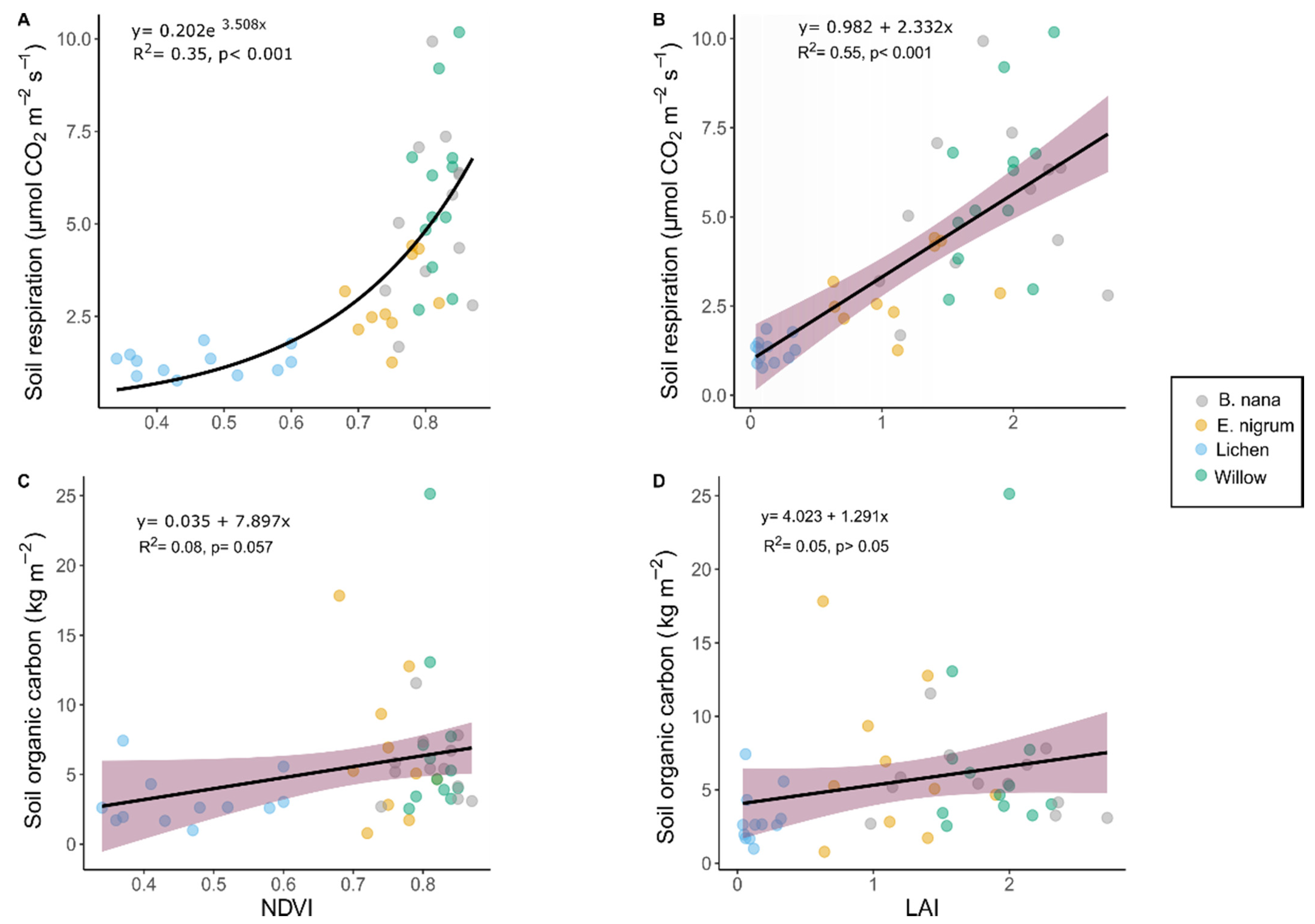
3.2. Soil Respiration, Organic Carbon, and Vegetation Indices
4. Discussion
4.1. Predicting Soil Respiration in a Highly Heterogeneous Environment
4.2. Soil Organic Carbon and Vegetation Indices Constraints
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landrum, L.; Holland, M.M. Extremes become routine in an emerging new Arctic. Nat. Clim. Chang. 2020, 10, 1108–1115. [Google Scholar] [CrossRef]
- Årthun, M.; Eldevik, T.; Smedsrud, L.H. The role of Atlantic heat transport in future Arctic winter sea ice loss. J. Clim. 2019, 32, 3327–3341. [Google Scholar] [CrossRef]
- Overland, J.; Dunlea, E.; Box, J.E.; Corell, R.; Forsius, M.; Kattsov, V.; Olsen, M.S.; Pawlak, J.; Reiersen, L.O.; Wang, M. The urgency of Arctic change. Polar Sci. 2019, 21, 6–13. [Google Scholar] [CrossRef]
- Haine, T.W.N.; Martin, T. The Arctic-Subarctic sea ice system is entering a seasonal regime: Implications for future Arctic amplification. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Cohen, J.; Screen, J.A.; Furtado, J.C.; Barlow, M.; Whittleston, D.; Coumou, D.; Francis, J.; Dethloff, K.; Entekhabi, D.; Overland, J.; et al. Recent Arctic amplification and extreme mid-latitude weather. Nat. Geosci. 2014, 7, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Serreze, M.C.; Barry, R.G. Processes and impacts of Arctic amplification: A research synthesis. Glob. Planet. Chang. 2011, 77, 85–96. [Google Scholar] [CrossRef]
- Box, J.E.; Colgan, W.T.; Christensen, T.R.; Schmidt, N.M.; Lund, M.; Parmentier, F.J.W.; Brown, R.; Bhatt, U.S.; Euskirchen, E.S.; Romanovsky, V.E.; et al. Key indicators of Arctic climate change: 1971–2017. Environ. Res. Lett. 2019, 14, 045010. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; McGuire, A.D.; Schädel, C.; Grosse, G.; Harden, J.W.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M.; et al. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef]
- Valipour, M.; Bateni, S.M.; Jun, C. Global Surface Temperature: A New Insight. Climate 2021, 9, 81. [Google Scholar] [CrossRef]
- Hugelius, G.; Strauss, J.; Zubrzycki, S.; Harden, J.W.; Schuur, E.A.G.; Ping, C.L.; Schirrmeister, L.; Grosse, G.; Michaelson, G.J.; Koven, C.D.; et al. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences 2014, 11, 6573–6593. [Google Scholar] [CrossRef] [Green Version]
- Köchy, M.; Hiederer, R.; Freibauer, A. Global distribution of soil organic carbon—Part 1: Masses and frequency distributions of SOC stocks for the tropics, permafrost regions, wetlands, and the world. Soil 2015, 1, 351–365. [Google Scholar] [CrossRef] [Green Version]
- Mishra, U.; Hugelius, G.; Shelef, E.; Yang, Y.; Strauss, J.; Lupachev, A.; Harden, J.W.; Jastrow, J.D.; Ping, C.-L.; Riley, W.J.; et al. Spatial heterogeneity and environmental predictors of permafrost region soil organic carbon stocks. Sci. Adv. 2021, 7, eaaz5236. [Google Scholar] [CrossRef]
- IPCC, Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, 2013. Available online: https://www.ipcc.ch/report/ar5/wg1/ (accessed on 19 April 2021).
- Sjöberg, Y.; Siewert, M.B.; Rudy, A.C.A.; Paquette, M.; Bouchard, F.; Malenfant-Lepage, J.; Fritz, M. Hot trends and impact in permafrost science. Permafr. Periglac. Process. 2020, 31, 461–471. [Google Scholar] [CrossRef]
- Williams, M.; Street, L.E.; Van Wijk, M.T.; Shaver, G.R. Identifying differences in carbon exchange among arctic ecosystem types. Ecosystems 2006, 9, 288–304. [Google Scholar] [CrossRef]
- Davidson, S.J.; Santos, M.J.; Sloan, V.L.; Watts, J.D.; Phoenix, G.K.; Oechel, W.C.; Zona, D. Mapping arctic tundra vegetation communities using field spectroscopy and multispectral satellite data in North Alaska, USA. Remote Sens. 2016, 8, 978. [Google Scholar] [CrossRef] [Green Version]
- Pettorelli, N.; Laurance, W.F.; O’brien, T.G.; Wegmann, M.; Nagendra, H.; Turner, W. Satellite remote sensing for applied ecologists: Opportunities and challenges. J. Appl. Ecol. 2014, 51, 839–848. [Google Scholar] [CrossRef]
- Epstein, H.E.; Raynolds, M.K.; Walker, D.A.; Bhatt, U.S.; Tucker, C.J.; Pinzon, J.E. Dynamics of aboveground phytomass of the circumpolar Arctic tundra during the past three decades. Environ. Res. Lett. 2012, 7, 015506. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Kerby, J.T.; Phoenix, G.K.; Bjerke, J.W.; Epstein, H.E.; Assmann, J.J.; John, C.; Andreu-Hayles, L.; Angers-Blondin, S.; Beck, P.S.A.; et al. Complexity revealed in the greening of the Arctic. Nat. Clim. Chang. 2020, 10, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Myneni, R.B.; Keeling, C.D.; Tucker, C.J.; Asrar, G.; Nemani, R.R. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 1997, 386, 698–702. [Google Scholar] [CrossRef]
- Tucker, C.J.; Slayback, D.A.; Pinzon, J.E.; Los, S.O.; Myneni, R.B.; Taylor, M.G. Higher northern latitude normalized difference vegetation index and growing season trends from 1982 to 1999. Int. J. Biometeorol. 2001, 45, 184–190. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Chen, Y.; Shi, T.; Luo, M.; Ju, Q.L.; Zhang, H.; Wang, S. Prediction of soil organic carbon based on Landsat 8 monthly NDVI data for the Jianghan Plain in Hubei Province, China. Remote Sens. 2019, 11, 1683. [Google Scholar] [CrossRef] [Green Version]
- Siewert, M.B.; Olofsson, J. Scale-dependency of Arctic ecosystem properties revealed by UAV. Environ. Res. Lett. 2020, 15, 129601. [Google Scholar] [CrossRef]
- Juutinen, S.; Virtanen, T.; Kondratyev, V.; Laurila, T.; Linkosalmi, M.; Mikola, J.; Nyman, J.; Räsänen, A.; Tuovinen, J.P.; Aurela, M. Spatial variation and seasonal dynamics of leaf-Area index in the arctic tundra-implications for linking ground observations and satellite images. Environ. Res. Lett. 2017, 12, 095002. [Google Scholar] [CrossRef]
- Virtanen, T.; Ek, M. The fragmented nature of tundra landscape. Int. J. Appl. Earth Obs. Geoinf. 2014, 27, 4–12. [Google Scholar] [CrossRef]
- Siewert, M.B. High-resolution digital mapping of soil organic carbon in permafrost terrain using machine learning: A case study in a sub-Arctic peatland environment. Biogeosciences 2018, 15, 1663–1682. [Google Scholar] [CrossRef] [Green Version]
- Anisimov, O.A.; Vaughan, D.G. Polar regions (Arctic and Antarctic). Clim. Chang. 2007, 15, 653–685. [Google Scholar]
- ACIA, Arctic Climate Impact Assessment. ACIA Overview Report, 2005. Available online: http://www.amap.no/documents/doc/arctic-arctic-climate-impact-assessment/796 (accessed on 27 June 2020).
- Siewert, M.B.; Hanisch, J.; Weiss, N.; Kuhry, P.; Maximov, T.C.; Hugelius, G. Comparing carbon storage of Siberian tundra and taiga permafrost ecosystems at very high spatial resolution. J. Geophys. Res. Biogeosci. 2015, 120, 1973–1994. [Google Scholar] [CrossRef] [Green Version]
- Wookey, P.A.; Aerts, R.; Bardgett, R.D.; Baptist, F.; Bråthen, K.; Cornelissen, J.H.C.; Gough, L.; Hartley, I.P.; Hopkins, D.W.; Lavorel, S.; et al. Ecosystem feedbacks and cascade processes: Understanding their role in the responses of Arctic and alpine ecosystems to environmental change. Glob. Chang. Biol. 2009, 15, 1153–1172. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhuang, Q.; Schaphoff, S.; Sitch, S.; Sokolov, A.; Kicklighter, D.; Melillo, J. Uncertainty analysis of vegetation distribution in the northern high latitudes during the 21st century with a dynamic vegetation model. Ecol. Evol. 2012, 2, 593–614. [Google Scholar] [CrossRef] [Green Version]
- Hartley, I.P.; Garnett, M.H.; Sommerkorn, M.; Hopkins, D.W.; Fletcher, B.J.; Sloan, V.L.; Phoenix, G.K.; Wookey, P.A. A potential loss of carbon associated with greater plant growth in the European Arctic. Nat. Clim. Chang. 2012, 2, 875–879. [Google Scholar] [CrossRef]
- Frost, G.V.; Epstein, H.E. Tall shrub and tree expansion in Siberian tundra ecotones since the 1960s. Glob. Chang. Biol. 2014, 20, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Heijmans, M.M.P.D.; Mommer, L.; Van Ruijven, J.; Maximov, T.C.; Berendse, F. Belowground plant biomass allocation in tundra ecosystems and its relationship with temperature. Environ. Res. Lett. 2016, 11, 055003. [Google Scholar] [CrossRef]
- Wang, P.; Limpens, J.; Mommer, L.; van Ruijven, J.; Nauta, A.L.; Berendse, F.; Schaepman-Strub, G.; Blok, D.; Maximov, T.C.; Heijmans, M.M.P.D. Above- and below-ground responses of four tundra plant functional types to deep soil heating and surface soil fertilization. J. Ecol. 2017, 105, 947–957. [Google Scholar] [CrossRef] [Green Version]
- Iversen, C.M.; Sloan, V.L.; Sullivan, P.F.; Euskirchen, E.S.; Mcguire, A.D.; Norby, R.J.; Walker, A.P.; Warren, J.M.; Wullschleger, S.D. The unseen iceberg: Plant roots in arctic tundra. New Phytol. 2015, 205, 34–58. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.; Serra-Diaz, J.M.; Syphard, A.D.; Regan, H.M. Global change and terrestrial plant community dynamics. Proc. Natl. Acad. Sci. USA 2016, 113, 3725–3734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, T.C.; Subke, J.A.; Wookey, P.A. Rapid carbon turnover beneath shrub and tree vegetation is associated with low soil carbon stocks at a subarctic treeline. Glob. Chang. Biol. 2015, 21, 2070–2081. [Google Scholar] [CrossRef] [Green Version]
- Grogan, P.; Chapin, F.S. Arctic soil respiration: Effects of climate and vegetation depend on season. Ecosystems 1999, 2, 451–459. [Google Scholar] [CrossRef]
- Box, E.O. Plant functional types and climate at the global scale. J. Veg. Sci. 1996, 7, 309–320. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Epstein, H.E.; Box, E.O.; Euskirchen, E.S.; Goswami, S.; Iversen, C.M.; Kattge, J.; Norby, R.J.; Van Bodegom, P.M.; Xu, X. Plant functional types in Earth system models: Past experiences and future directions for application of dynamic vegetation models in high-latitude ecosystems. Ann. Bot. 2014, 114, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Station, A.S. Support for Polar Research—Polar Research Secretariat, (n.d.). Available online: https://polar.se/stod-till-polarforskning/ (accessed on 7 March 2021).
- Parker, T.C.; Clemmensen, K.E.; Friggens, N.L.; Hartley, I.P.; Johnson, D.; Lindahl, B.D.; Olofsson, J.; Siewert, M.B.; Street, L.E.; Subke, J.; et al. Rhizosphere allocation by canopy-forming species dominates soil CO2 efflux in a subarctic landscape. New Phytol. 2020, 227, 1818–1830. [Google Scholar] [CrossRef] [Green Version]
- Callaghan, T.V.; Jonasson, C.; Thierfelder, T.; Yang, Z.; Hedenås, H.; Johansson, M.; Molau, U.; van Bogaert, R.; Michelsen, A.; Olofsson, J.; et al. Ecosystem change and stability over multiple decades in the Swedish subarctic: Complex processes and multiple drivers. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, D.A.; Raynolds, M.K.; Daniëls, F.J.A.; Einarsson, E.; Elvebakk, A.; Gould, W.A.; Katenin, A.E.; Kholod, S.S.; Markon, C.J.; Melnikov, E.S.; et al. The other members of the CAVM Team, The Circumpolar Arctic vegetation map. J. Veg. Sci. 2005, 16, 267–282. [Google Scholar] [CrossRef]
- Van Wijk, M.T.; Williams, M. Optical Instruments for Measuring Leaf Area Index in Low Vegetation: Application in Arctic Ecosystems. Ecol. Soc. Am. 2005, 15, 1462–1470. [Google Scholar] [CrossRef] [Green Version]
- Kutsch, W.L.; Bahn, M.; Heinemeyer, A. Soil Carbon Dynamics: An Integrated Methodology; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar] [CrossRef]
- Ball, D.F. Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soils. J. Soil Sci. 1964, 15, 84–92. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Ge, L.; Lafleur, P.M.; Humphreys, E.R. Respiration from soil and ground cover vegetation under tundra shrubs. Arct. Antarct. Alp. Res. 2017, 49, 537–550. [Google Scholar] [CrossRef]
- Lara, M.J.; Mcguire, A.D.; Euskirchen, E.S.; Sloan, V.; Wullschleger, S.D. Local-Scale Arctic tundra heterogneity affects regional-scale carbon dynamics. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Grosse, G.; Harden, J.; Turetsky, M.; McGuire, A.D.; Camill, P.; Tarnocai, C.; Frolking, S.; Schuur, E.A.G.; Jorgenson, T.; Marchenko, S.; et al. Vulnerability of high-latitude soil organic carbon in North America to disturbance. J. Geophys. Res. Biogeosci. 2011, 116, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Herndon, E.M.; Mann, B.F.; Chowdhury, T.R.; Yang, Z.; Wullschleger, S.D.; Graham, D.; Liang, L.; Gu, B. Pathways of anaerobic organic matter decomposition in tundra soils from Barrow, Alaska. J. Geophys. Res. G Biogeosci. 2015, 120, 2345–2359. [Google Scholar] [CrossRef] [Green Version]
- Stow, D.A.; Hope, A.; McGuire, D.; Verbyla, D.; Gamon, J.; Huemmrich, F.; Houston, S.; Racine, C.; Sturm, M.; Tape, K.; et al. Remote sensing of vegetation and land-cover change in Arctic Tundra Ecosystems. Remote Sens. Environ. 2004, 89, 281–308. [Google Scholar] [CrossRef] [Green Version]
- Callaghan, T.; Björn, L.O.; Callaghan, T.; Chernov, Y.; Chapin, T.; Christensen, T.R.; Ims, R.; Jolly, D.; Jonasson, S.; Matveyeva, N.; et al. Arctic tundra and polar desert ecosystems. In Arctic Climate Impact Assessment (ACIA): Scientific Report; Symon, C., Arris, L., Heal, B., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 243–352. [Google Scholar]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Karhu, K.; Auffret, M.D.; Dungait, J.A.J.; Hopkins, D.W.; Prosser, J.I.; Singh, B.K.; Subke, J.A.; Wookey, P.A.; Agren, G.I.; Sebastià, M.T.; et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 2014, 513, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Hartley, I.P.; Hopkins, D.W.; Garnett, M.H.; Sommerkorn, M.; Wookey, P.A. Soil microbial respiration in arctic soil does not acclimate to temperature. Ecol. Lett. 2008, 11, 1092–1100. [Google Scholar] [CrossRef] [Green Version]
- Nobrega, S.; Grogan, P. Landscape and Ecosystem-Level Controls on Net Carbon Dioxide Exchange along a Natural Moisture Gradient in Canadian Low Arctic Tundra. Ecosystems 2008, 11, 377–396. [Google Scholar] [CrossRef]
- Oberbauer, S.F.; Tweedie, C.E.; Welker, J.M.; Fahnestock, J.T.; Henry, G.H.R.; Webber, P.J.; Hollister, R.D.; Walker, M.D.; Kuchy, A.; Elmore, E.; et al. Tundra CO2 fluxes in response to experimental warming across latitudinal and moisture gradients. Ecol. Monogr. 2007, 77, 221–238. [Google Scholar] [CrossRef] [Green Version]
- Davidson, E.A.; Belk, E.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Chang. Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Raich, J.W.; Potter, C.S. Global patterns of carbon dioxide emissions from soils. Glob. Biogeochem. Cycles 1995, 9, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Almagro, M.; López, J.; Querejeta, J.I.; Martínez-Mena, M. Temperature dependence of soil CO2 efflux is strongly modulated by seasonal patterns of moisture availability in a Mediterranean ecosystem. Soil Biol. Biochem. 2009, 41, 594–605. [Google Scholar] [CrossRef]
- Chang, Z.; Feng, Q.; Si, J.; Su, Y.; Xi, H.; Li, J. Analysis of the spatial and temporal changes in soil CO2 flux in alpine meadow of Qilian Mountain. Environ. Geol. 2009, 58, 483–490. [Google Scholar] [CrossRef]
- Elmendorf, S.C.; Henry, G.H.R.; Hollister, R.D.; Björk, R.G.; Bjorkman, A.D.; Callaghan, T.V.; Collier, L.S.; Cooper, E.J.; Cornelissen, J.H.C.; Day, T.A.; et al. Global assessment of experimental climate warming on tundra vegetation: Heterogeneity over space and time. Ecol. Lett. 2012, 15, 164–175. [Google Scholar] [CrossRef]
- Perkins, S.R.; Owens, M.K. Growth and Biomass Allocation of Shrub and Grass Seedlings in Response to Predicted Changes in Precipitation Seasonality. Plant Ecol. 2003, 168, 107–120. Available online: https://link.springer.com/content/pdf/10.1023%2FA%3A1024447305422.pdf (accessed on 10 February 2021). [CrossRef]
- Langford, Z.; Kumar, J.; Hoffman, F.M.; Norby, R.J.; Wullschleger, S.D.; Sloan, V.L.; Iversen, C.M. Mapping Arctic Plant Functional Type Distributions in the Barrow Environmental Observatory Using. Remote Sens. 2016, 8, 733. [Google Scholar] [CrossRef] [Green Version]
- Druel, A.; Peylin, P.; Krinner, G.; Ciais, P.; Viovy, N.; Peregon, A.; Bastrikov, V.; Kosykh, N.; Mironycheva-Tokareva, N. Towards a more detailed representation of high-latitude vegetation in the global land surface model ORCHIDEE (ORC-HL-VEGv1.0). Geosci. Model Dev. 2017, 10, 4693–4722. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, M.V.; Strimbeck, R.; Nystuen, K.O.; Kapas, R.E.; Enquist, B.J.; Graae, B.J. Draining the Pool? Carbon Storage and Fluxes in Three Alpine Plant Communities. Ecosystems 2018, 21, 316–330. [Google Scholar] [CrossRef]
- Oleson, K.; Lawrence, D.M.; Bonan, G.B.; Drewniak, B.; Huang, M.; Koven, C.D.; Levis, S.; Li, F.; Rilet, W.J.; Subin, Z.M.; et al. Technical Description of Version 4.5 of the Community Land Model (CLM) (No. NCAR/TN-503+STR); UCAR: Boulder, CO, USA, 2013. [Google Scholar] [CrossRef]
- Chapin, F.S.; Bret-Harte, M.S.; Hobbie, S.E.; Zhong, H. Plant functional types as predictors of transient responses of arctic vegetation to global change. J. Veg. Sci. 1996, 7, 347–358. [Google Scholar] [CrossRef]
- Street, L.E.; Subke, J.A.; Baxter, R.; Dinsmore, K.J.; Knoblauch, C.; Wookey, P.A. Ecosystem carbon dynamics differ between tundra shrub types in the western Canadian Arctic. Environ. Res. Lett. 2018, 13, 084014. [Google Scholar] [CrossRef]
- Högberg, P.; Nordgren, A.; Buchmann, N.; Taylor, A.F.S.; Ekblad, A.; Högberg, M.N.; Nyberg, G.; Ottosson-Löfvenius, M.; Read, D.J. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 2001, 411, 789–792. [Google Scholar] [CrossRef]
- Scott-Denton, L.E.; Rosenstiel, T.N.; Monson, R.K. Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Glob. Chang. Biol. 2006, 12, 205–216. [Google Scholar] [CrossRef]
- Lees, K.J.; Quaife, T.; Artz, R.R.E.; Khomik, M.; Clark, J.M. Potential for using remote sensing to estimate carbon fluxes across northern peatlands—A review. Sci. Total Environ. 2018, 615, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; He, J.S.; Niu, Z. Estimating the spatial pattern of soil respiration in Tibetan alpine grasslands using Landsat TM images and MODIS data. Ecol. Indic. 2013, 26, 117–125. [Google Scholar] [CrossRef]
- Huang, N.; Gu, L.; Niu, Z. Estimating soil respiration using spatial data products: A case study in a deciduous broadleaf forest in the Midwest USA. J. Geophys. Res. 2014, 119, 6393–6408. [Google Scholar] [CrossRef]
- Bradley-Cook, J.I.; Virginia, R.A. Landscape variation in soil carbon stocks and respiration in an Arctic tundra ecosystem, west Greenland. Arct. Antarct. Alp. Res. 2018, 50, S100024. [Google Scholar] [CrossRef] [Green Version]
- Raynolds, M.; Walker, D.; Verbyla, D.; Munger, C. Patterns of change within a tundra landscape: 22-year landsat NDVI trends in an area of the northern foothills of the brooks range, Alaska. Arct. Antarct. Alp. Res. 2013, 45, 249–260. [Google Scholar] [CrossRef] [Green Version]
- De Deyn, G.B.; Cornelissen, J.H.C.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef]
- Sloan, V.L.; Fletcher, B.J.; Press, M.C.; Williams, M.; Phoenix, G.K. Leaf and fine root carbon stocks and turnover are coupled across Arctic ecosystems. Glob. Chang. Biol. 2013, 19, 3668–3676. [Google Scholar] [CrossRef]
- Mishra, U.; Jastrow, J.D.; Matamala, R.; Hugelius, G.; Koven, C.D.; Harden, J.W.; Ping, C.L.; Michaelson, G.J.; Fan, Z.; Miller, R.M.; et al. Empirical estimates to reduce modeling uncertainties of soil organic carbon in permafrost regions: A review of recent progress and remaining challenges. Environ. Res. Lett. 2013, 8, 035020. [Google Scholar] [CrossRef]
- Angelopoulou, T.; Tziolas, N.; Balafoutis, A.; Zalidis, G.; Bochtis, D. Remote sensing techniques for soil organic carbon estimation: A review. Remote Sens. 2019, 11, 676. [Google Scholar] [CrossRef] [Green Version]
- Lamichhane, S.; Kumar, L.; Wilson, B. Digital soil mapping algorithms and covariates for soil organic carbon mapping and their implications: A review. Geoderma 2019, 352, 395–413. [Google Scholar] [CrossRef]
- Siewert, M.B.; Lantuit, H.; Richter, A.; Hugelius, G. Permafrost causes unique fine-scale spatial variability across tundra soils. Glob. Biogeochem. Cycles 2021, 35, e2020GB006659. [Google Scholar] [CrossRef]
- Aldana-Jague, E.; Heckrath, G.; Macdonald, A.; van Wesemael, B.; Van Oost, K. UAS-based soil carbon mapping using VIS-NIR (480–1000 nm) multi-spectral imaging: Potential and limitations. Geoderma 2016, 275, 55–66. [Google Scholar] [CrossRef]
- Beamish, A.; Raynolds, M.K.; Epstein, H.; Frost, G.V.; Macander, M.J.; Bergstedt, H.; Bartsch, A.; Kruse, S.; Miles, V.; Tanis, C.M.; et al. Recent trends and remaining challenges for optical remote sensing of Arctic tundra vegetation: A review and outlook. Remote Sens. Environ. 2020, 246, 111872. [Google Scholar] [CrossRef]
| B. nana (N= 12) | E. nigrum (N= 10) | Lichen (N= 12) | Willow (N= 12) | Overall (N= 46) | ||
|---|---|---|---|---|---|---|
| Fine Roots | ||||||
| Mean (SD) | 146 (87.8) | 146 (87.8) | 146 (87.8) | 146 (87.8) | 93.2 (77.4) | |
| Median [Min, Max] | 125 [42.8, 216] | 125 [42.8, 216] | 125 [42.8, 216] | 125 [42.8, 216] | 78.9 [14.0, 316] | |
| LAI | ||||||
| Mean (SD) | 1.82 (0.563) | 1.13 (0.413) | 0.146 (0.111) | 1.87 (0.276) | 1.25 (0.807) | |
| Median [Min, Max] | 1.88 [0.980, 2.72] | 1.11 [0.630, 1.90] | 0.105 [0.040, 0.340] | 1.95 [1.51, 2.31] | 1.41 [0.040, 2.72] | |
| NDVI | ||||||
| Mean (SD) | 0.813 (0.0427) | 0.751 (0.0431) | 0.461 (0.0961) | 0.818 (0.0221) | 0.709 (0.161) | |
| Median [Min, Max] | 0.820 [0.740, 0.870] | 0.750 [0.680, 0.820] | 0.450 [0.340, 0.600] | 0.815 [0.780, 0.850] | 0.780 [0.340, 0.870] | |
| SOM | ||||||
| Mean (SD) | 13.0 (6.49) | 14.4 (11.4) | 6.70 (3.99) | 15.7 (13.9) | 12.4 (9.98) | |
| Median [Min, Max] | 12.0 [5.89, 25.4] | 11.3 [1.64, 39.1] | 5.68 [1.86, 16.2] | 10.9 [5.51, 55.1] | 9.73 [1.64, 55.1] | |
| SOC | ||||||
| Mean (SD) | 5.71 (2.47) | 6.72 (5.29) | 3.09 (1.83) | 7.19 (6.33) | 5.63 (4.51) | |
| Median [Min, Max] | 5.42 [2.69, 11.6] | 5.17 [0.780, 17.8] | 2.62 [0.990, 7.43] | 4.97 [2.54, 25.1] | 4.66 [0.780, 25.1] | |
| Soil Moisture | ||||||
| Mean (SD) | 63.0 (7.32) | 57.3 (13.0) | 44.0 (14.3) | 62.9 (12.0) | 56.8 (14.0) | |
| Median [Min, Max] | 64.4 [49.4, 72.1] | 61.5 [28.3, 67.8] | 43.7 [22.8, 63.2] | 65.3 [31.7, 75.0] | 60.8 [22.8, 75.0] | |
| Soil Respiration | ||||||
| Mean (SD) | 5.30 (2.31) | 2.98 (1.05) | 1.26 (0.340) | 5.87 (2.27) | 3.89 (2.54) | |
| Median [Min, Max] | 5.41 [1.68, 9.93] | 2.71 [1.26, 4.41] | 1.29 [0.770, 1.86] | 5.75 [2.68, 10.2] | 3.19 [0.770, 10.2] | |
| Soil Temperature | ||||||
| Mean (SD) | 10.9 (1.22) | 12.9 (1.31) | 16.6 (1.38) | 10.7 (0.883) | 12.8 (2.69) | |
| Median [Min, Max] | 11.0 [8.43, 12.8] | 13.3 [11.1, 14.9] | 16.2 [14.6, 18.7] | 10.9 [8.93, 11.8] | 11.8 [8.43, 18.7] | |
| Parameter Values | Standard Error | CI (Lower) | CI (Upper) | |||
|---|---|---|---|---|---|---|
| NDVI | 2.20 ** | 0.81 | 0.58 | 4.32 | ||
| log (Rs) | Soil temperature | 0.0644 *** | 0.0191 | 0.0247 | 0.1010 | |
| Constant | −1.38 | 0.73 | −3.04 | 0.055 | ||
| ICC (adjusted) | 0.495 | |||||
| Soil moisture | 0.0264 *** | 0.0055 | 0.0154 | 0.0372 | ||
| log (SOC) | Fine roots | 0.00205 * | 0.00101 | 0.00010 | 0.00407 | |
| Constant | −0.209 | 0.340 | −0.863 | 0.454 | ||
| ICC (adjusted) | 0.414 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, O.; Parker, T.C.; Siewert, M.B.; Subke, J.-A. Predicting Soil Respiration from Plant Productivity (NDVI) in a Sub-Arctic Tundra Ecosystem. Remote Sens. 2021, 13, 2571. https://doi.org/10.3390/rs13132571
Azevedo O, Parker TC, Siewert MB, Subke J-A. Predicting Soil Respiration from Plant Productivity (NDVI) in a Sub-Arctic Tundra Ecosystem. Remote Sensing. 2021; 13(13):2571. https://doi.org/10.3390/rs13132571
Chicago/Turabian StyleAzevedo, Olivia, Thomas C. Parker, Matthias B. Siewert, and Jens-Arne Subke. 2021. "Predicting Soil Respiration from Plant Productivity (NDVI) in a Sub-Arctic Tundra Ecosystem" Remote Sensing 13, no. 13: 2571. https://doi.org/10.3390/rs13132571
APA StyleAzevedo, O., Parker, T. C., Siewert, M. B., & Subke, J.-A. (2021). Predicting Soil Respiration from Plant Productivity (NDVI) in a Sub-Arctic Tundra Ecosystem. Remote Sensing, 13(13), 2571. https://doi.org/10.3390/rs13132571






