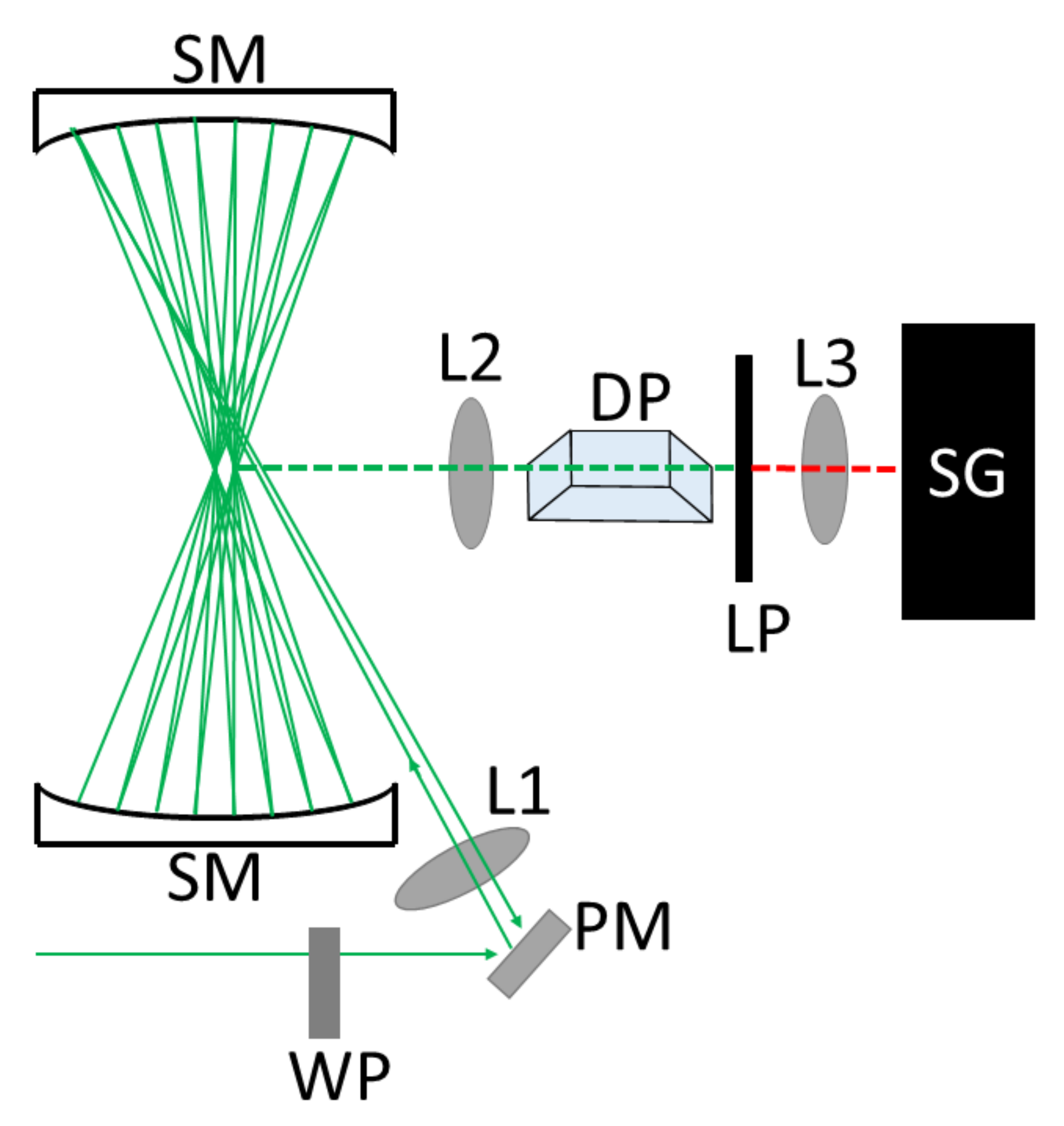
1. Introduction
Small changes in temperature can have non-linear effects on a number of processes. One such case is the nucleation of non-sea-salt sulfate particles from sulfuric acid in the atmosphere, where a decrease in temperature of a couple of degrees Celsius can result in an order of magnitude increase in new particle formation [
1]. As a result, fluctuations in temperature can result in enhanced nucleation rates of particles. For example, Platis et. al. [
2] observed a new particle formation event in an inversion layer with large fluctuations in temperature. However, often measurements of the inversion layer are limited by coarse time resolution, and the direct relation between temperature fluctuations and new particle formation is unknown. Also, fluctuations in water vapor saturation ratio are dependent on temperature fluctuations [
3]. The conditions most favorable for new particle formation involving water are those where saturation ratio and temperature are anti-correlated [
2,
4]. Temperature fluctuations can also affect the supersaturation in clouds, which in turn determines the activation and eventual growth of cloud droplets. In fact, thermodynamic fluctuations due to turbulent mixing in clouds may broaden the size distribution of droplets, which has implications for precipitation and the cloud optical properties [
5,
6]. In order to understand the effect that temperature fluctuations can have on the evolution of aerosol and cloud droplets, continuous monitoring of temperature would be ideal. This can be difficult above the ground level using direct means of temperature measurements, which are often performed by aircraft and radiosondes. Temperature fluctuations can be difficult for aircraft to measure, especially in clouds where condensation can limit the time response and accuracy of temperature measurements [
7]. While research aircraft are capable of high temporal and spatial resolution measurements in the horizontal direction (along the flight path), the range-resolved resolution of these measurements is poor [
8]. Remote sensing techniques cannot just provide vertical profiles, but can also monitor the evolution of those vertical profiles over time.
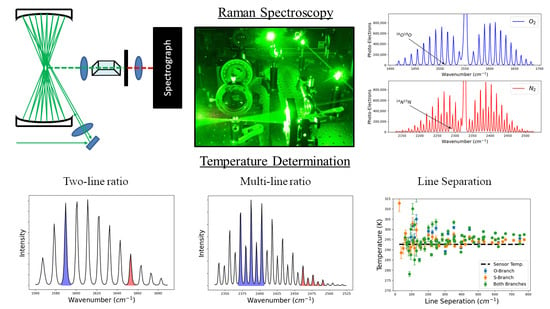
Raman and Rayleigh scattering techniques are used in a variety of applications where temperature must be determined remotely [
9]. Some of these techniques have been developed to measure the temperature during combustion processes, while others have been developed to measure atmospheric temperature. For example, Rayleigh scattering is often used to measure changes in molecular number density in flames which are in turn related to temperature using the ideal gas law under the assumption that the medium being measured has constant pressure and known molecular composition [
10,
11]. Additionally, changes in laser intensity, and elastic scattering from large particles, must be accounted for when using Rayleigh techniques; in fact, cloud droplets and dust, can degrade the precision of the technique [
9]. In an alternative approach, the intensity of pure rotational Raman (PRR) transitions can be used to determine the temperature of flames and the atmosphere without needing to make assumptions about the pressure or the composition of the gas [
12]. PRR light detection and ranging (LiDAR) is the most widely accepted remote air temperature measurement technique used by the atmospheric LiDAR community [
13,
14,
15]. A significant advantage of Raman-based techniques is the ability to take a ratio of two portions of the scattered spectrum, eliminating the need to monitor the laser intensity. However, the close proximity of the rotational Raman lines to elastic scattering presents a significant challenge especially in turbid environments, such as clouds. Modern narrow band-pass filters have allowed the technique to be employed with minimal systematic errors in aerosol layers and optically thin clouds [
16]. Several methods have been developed to extract temperature information from the vibrational spectra of N
and O
, which are spectrally separated from elastic scattering [
17]. The spectral separation allows for the use of optical filters that attenuate elastically scattered light better to reduce the systematic errors imposed by large particle scattering. The Stoke’s vibrational line has also been used for satellite-based temperature determination in the stratosphere using a similar principle to the Rayleigh methods described above, but Rayleigh measurements are necessary to estimate density [
18]. Temperature can also be determined by taking a ratio of the Stoke’s and anti-Stoke’s pure vibrational scattering; however this technique is not tenable at atmospherically relevant temperatures due to weak anti-Stoke’s signal [
9]. Several hybrid methods have also been developed to estimate atmospheric temperature. Su et al. compared the Stoke’s Q-Branch Raman transition to a PRR line with a high rotational quantum number to determine atmospheric temperature up to a height of 22 km. The technique determined the temperature within a cloud to within 1.5 K of a radiosonde measurement [
19].
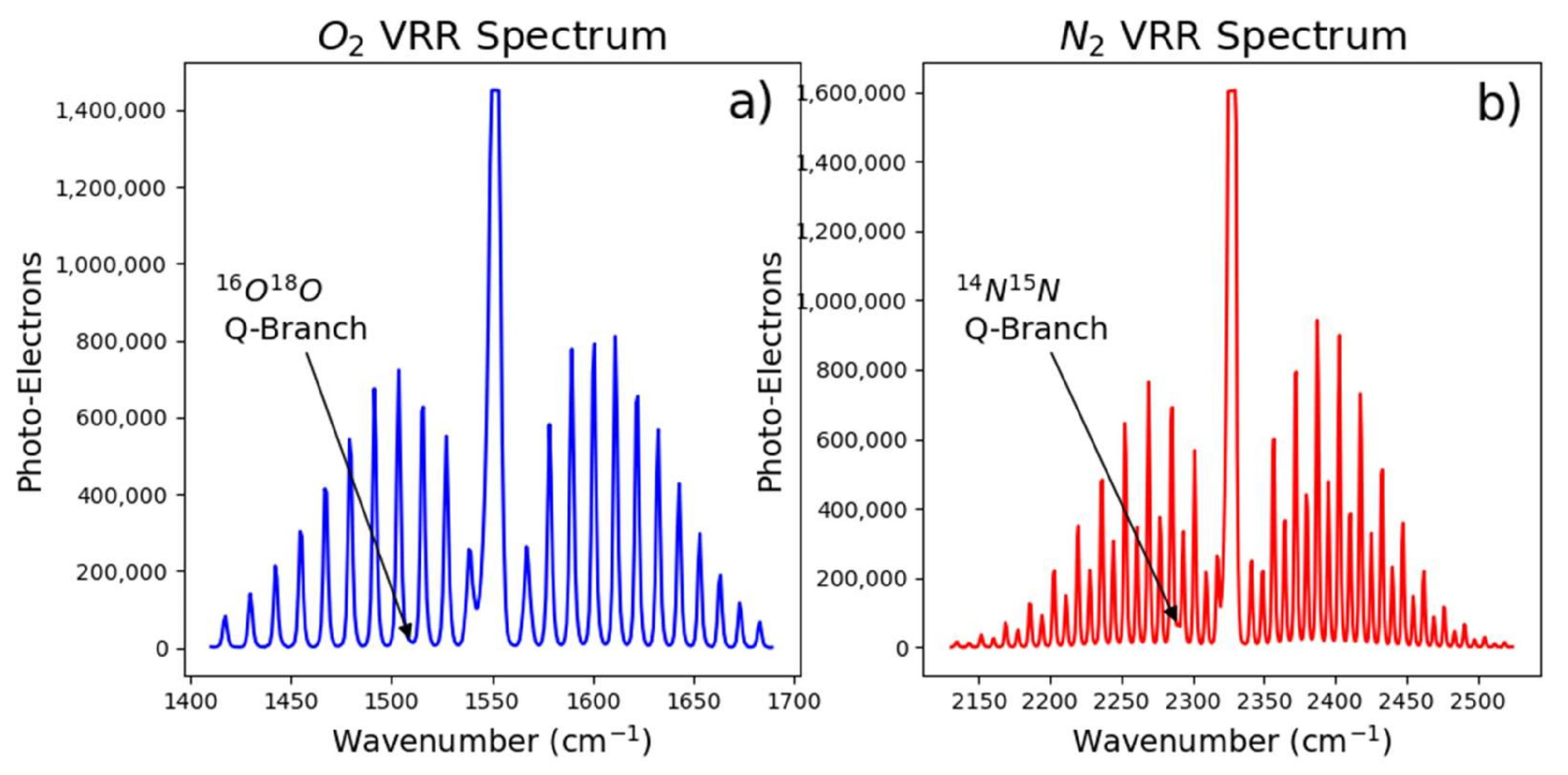
Temperature can be determined from the vibro-rotational S- and O-branches of the vibro-rotational Raman (VRR) spectrum of O
and N
in a similar fashion to the PRR spectrum. For both cases, Maxwell-Boltzmann statistics dictates that molecules populating lower energy rotational states will begin to populate higher energy rotational states as temperature increases [
20]. The VRR spectrum is spectrally located further from the excitation wavelength than the PRR spectrum. This is especially advantageous in environments including particulate matter, such as aerosol or droplets, as the filters needed to isolate the VRR spectrum will be less prone to allowing elastic scattering signal to leak to the photodetector. Along with being spectrally distant from the elastic scattering band, the vibro-rotational spectra of O
and N
are also spectrally separated from each other, unlike in PRR spectra. The PRR spectrum includes lines from every Raman active gaseous constituent in the atmosphere. In fact, for an excitation source at 532 nm the vibro-rotational Raman (VRR) spectra of O
and N
can be found well separated from each other between 575 and 585 nm and 600 and 615 nm, respectively [
21]. Because the Raman lines from one atmospheric constituent will not overlap with the Raman lines from another atmospheric constituent, direct calculations of temperature from the VRR spectra is more simple than from the PRR spectra. However, the VRR lines are about two orders of magnitude weaker than the PRR lines, representing a significant disadvantage. Therefore, we are not suggesting methods that employ VRR to derive temperature should replace the PRR method in every case; however, the VRR method can be significantly advantageous in situations where elastic scattering could drastically deteriorate the accuracy and sensitivity of the PRR method.
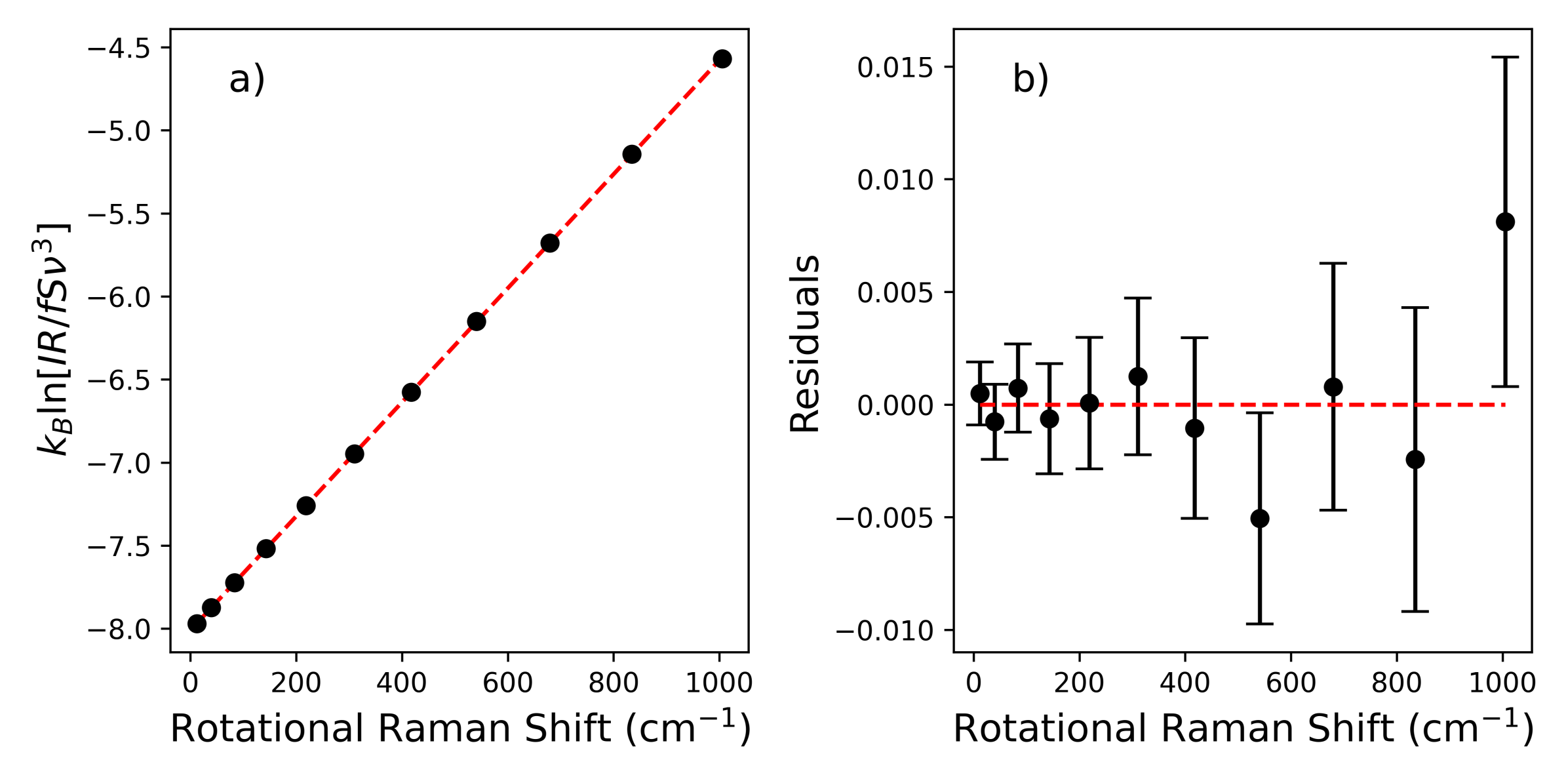
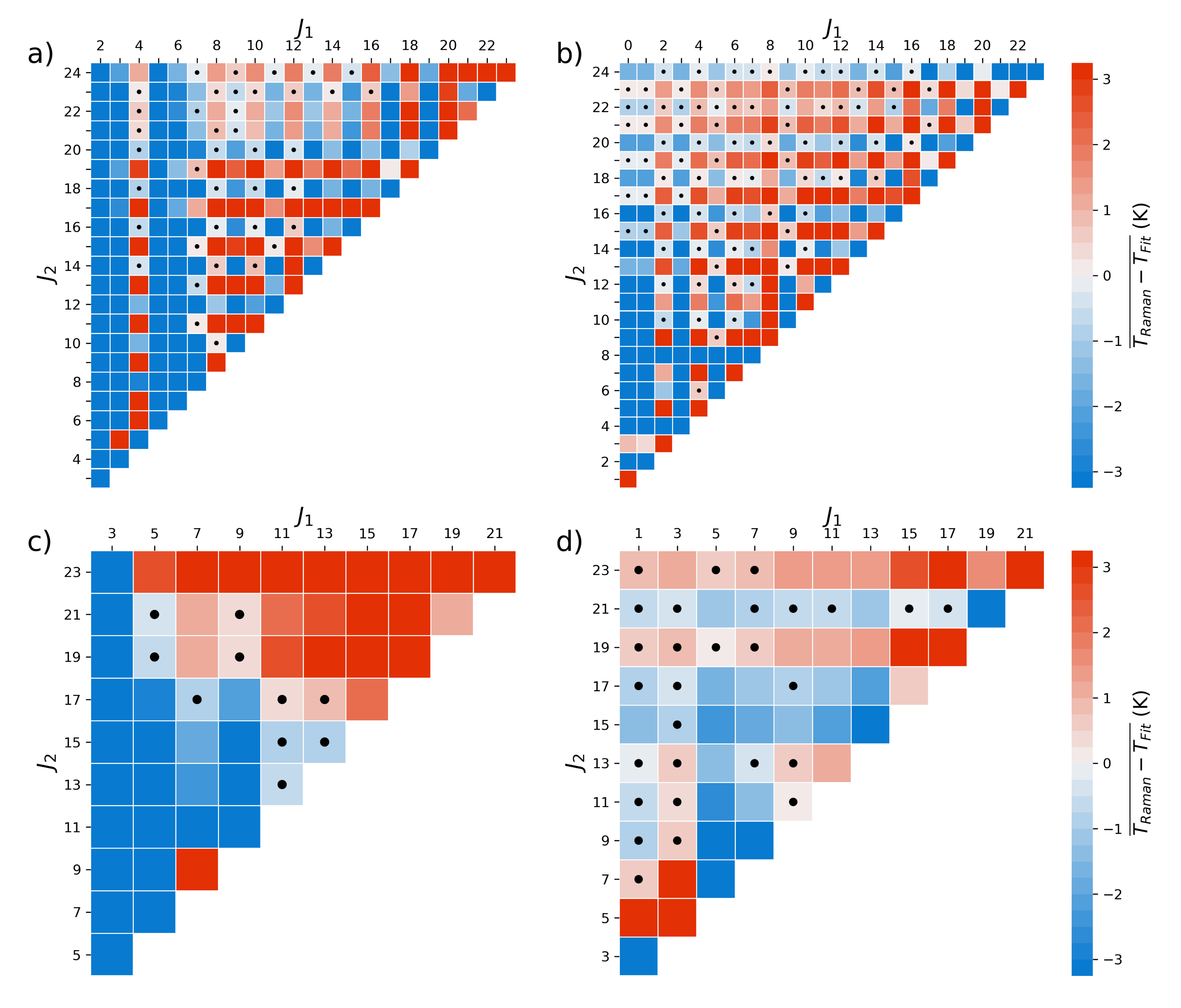
An important consideration for applications reliant on the intensity of VRR lines is the fact that the Raman cross-section is dependent on the rotational quantum number, J, due to vibrational-rotational coupling. The coupling is a direct consequence of diatomic molecules behaving such as non-rigid rotors. Ustav and Varghese determined the temperature of gases in flames by simultaneously fitting the intensity profiles of the S-, O-, and Q- branches [
22], though they note that temperature determination is mostly influenced by the shape of the Q-Branch. A Lidar system was also developed that determines temperature by taking a ratio of individual VRR N
lines as well as fitting the intensity of individual N
lines [
23]. While they showed that their system was within 2.2 K of a radiosonde up to 7 km, Liu and Yi treat the Nitrogen molecule as a rigid rotor that we show lead to significant bias. This bias may have been offset by the low spectral-resolution of their system which could result in overlap errors from adjacent lines.
In this work, we demonstrate how temperature can be derived from the fully resolved O- and S- branches in the Raman fundamental band of N
and O
. Our methods use the integrated intensity of individual lines within the VRR spectra to determine the temperature. The intensity of each line depends on the Raman cross-section of the molecule, the Maxwell-Boltzmann distribution, the VRR line strength, the nuclear spin statistics, and other factors as can be seen from Equation (
1) below. Each of these components can be expressed mathematically from first principles, which allows for temperature to be inferred from VRR spectra without the need for ad hoc temperature calibration. We start our discussion by considering two theoretical approaches to the problem. First, molecules are treated as rigid rotors, an approach typically used in PRR applications. While non-rigidity can affect the intensities of PRR lines, it is usually believed to be small enough to be ignored. However, the vibrational-rotational coupling is stronger in the VRR spectra and must be accounted for [
24,
25]. We show that this approach leads to significant biases in temperatures derived from the S- and the O-branch. Then, we examine the case of molecular non-rigidity where the Raman cross-section is dependent on the rotational quantum number. The correction is shown to improve the accuracy of temperatures determined from the VRR spectra, as well as the agreement between temperatures derived from the S-branch and those derived from the O-branch. From there we set out to derive and implement four separate methods that infer temperature from the S- and O- branches of N
and O
. An inter-comparison between the different methods allow us to determine the relative accuracy and precision of each method.
5. Conclusions
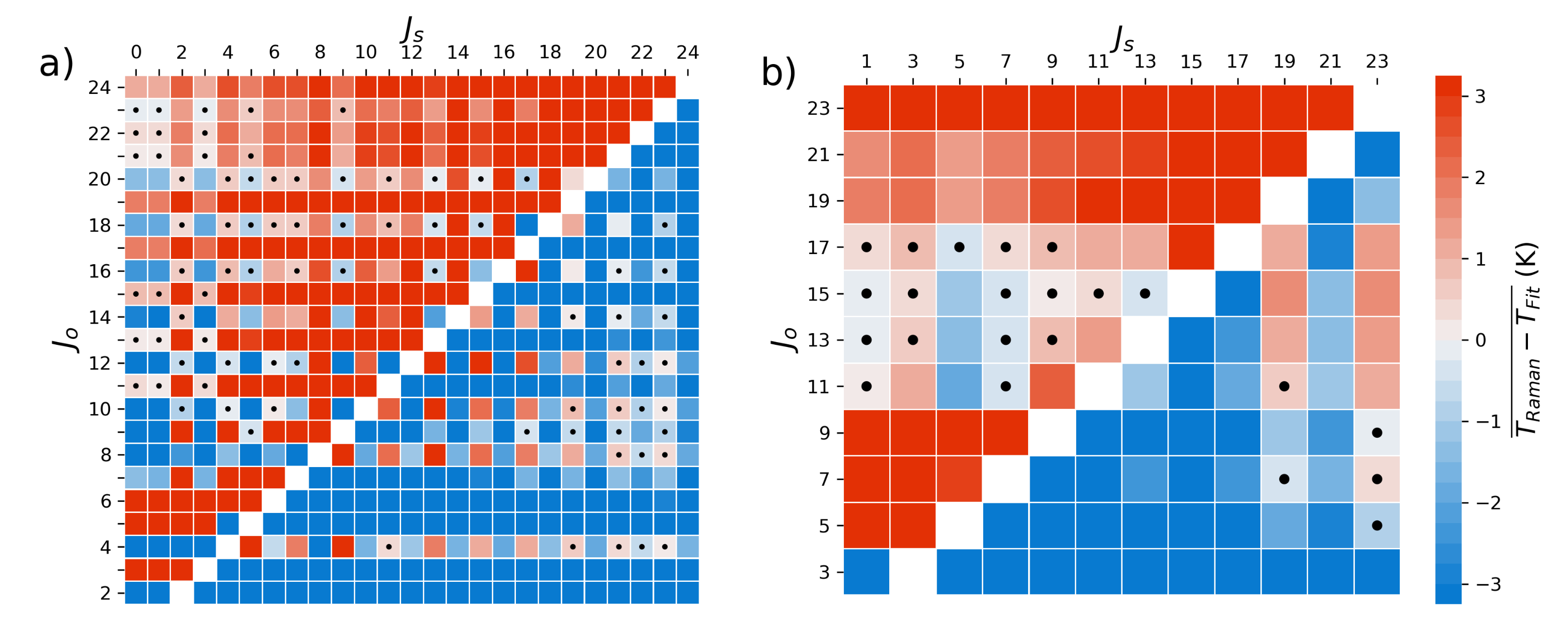
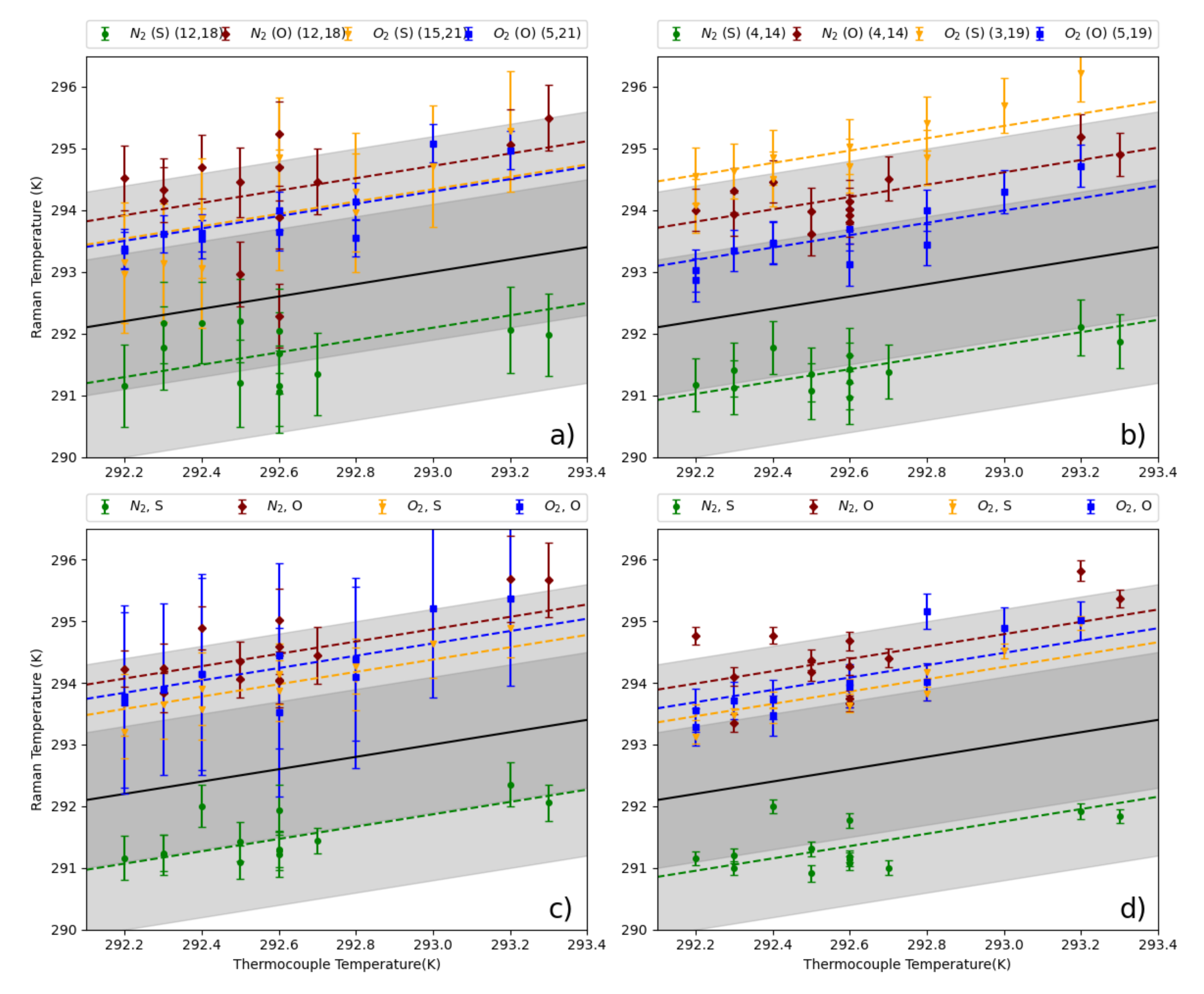
Our experimental setup and measurement procedure allowed for high resolution measurement of VRR spectra of N and O. Using the fundamental properties of these molecules, we were able to determine atmospheric temperature within the scattering region of our multi-pass cell. For both gases, treating the molecules as non-rigid rotors not only improved the self-consistency of temperature derived from Raman measurements, but also improved the the agreement between Raman derived temperatures and the thermocouple. However, the VRR spectra of O, specifically the S-Branch, would be best for determining atmospheric temperature with high precision without the need for ad hoc temperature calibration. The S-Branch provided the best results in this work, largely due to higher line intensities and a greater number of viable lines due to less influence from overlapping vibrational lines. We found that greater resolution is likely needed when determining temperature from individual N lines. A more accurate estimate of might further improve the temperature inferred from N VRR spectra. Regardless, the N S-branch would likely provide the most precise temperature measurement for applications where a calibration can be performed. This is because this branch would have the strongest signal out of the four branches explored.
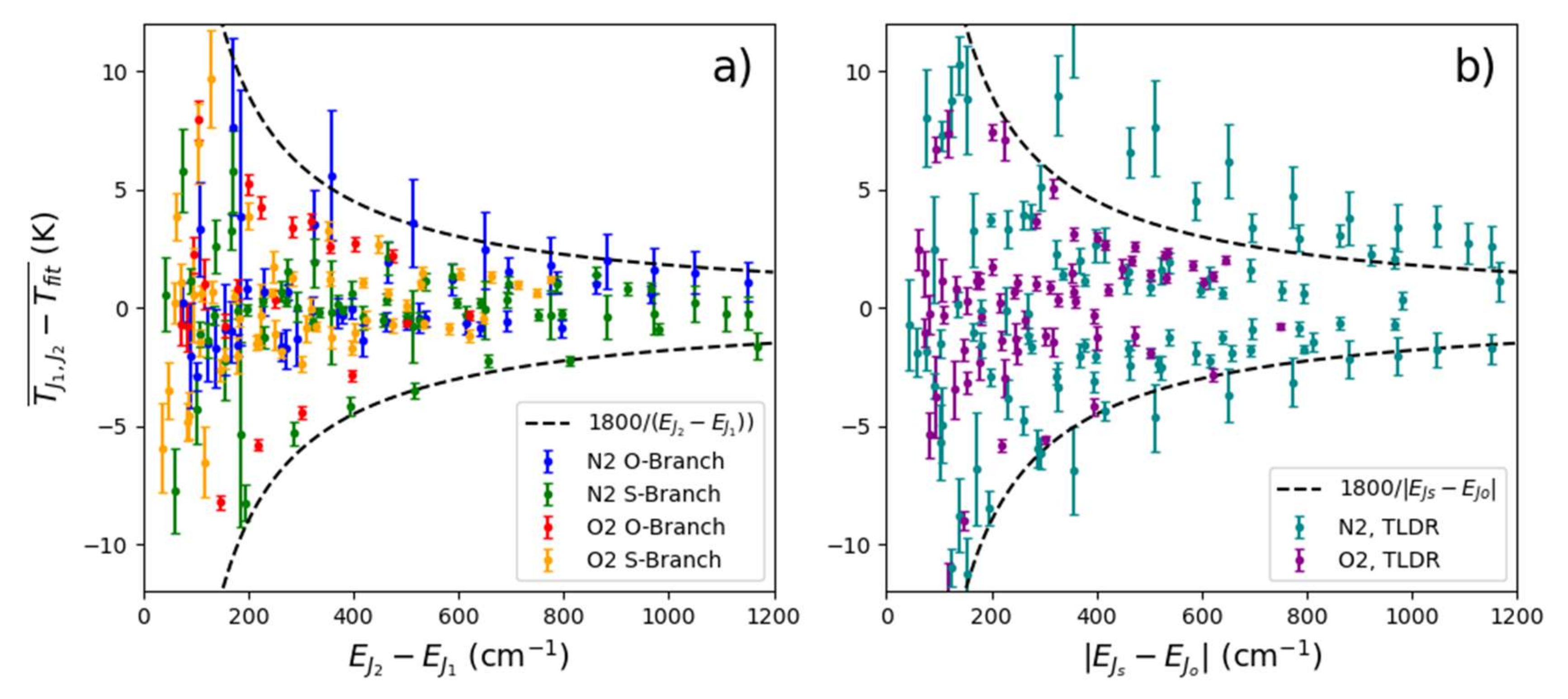
We derived four methods of ascertaining atmospheric temperature from the VRR spectra of N and O. These methods could be a starting point in the development of an instrument for contactless measurements of atmospheric temperature using VRR spectra of N or O, though further work is necessary to scale these methods to a field deployable instrument. Our analysis to find the optimal line combinations was performed for a limited range of temperature values. The best line combinations will need to be revisited for different temperature regimes and we provided a theoretical approach to estimate the optimal line pairs. However, our work shows that regardless of temperature, well separated line combinations are ideal. Of the methods implemented in this work the multi-line ratio is the best in terms of attainable precision and practical applicability. A future implementation would still require well-characterized filters that isolate specific line combinations. A calibrated version of the multi-line method can also be envisioned, where two sections of the spectra are isolated and a calibration constant allows for temperature to be determined, just like in PRR methods. We anticipate that the VRR method would be applicable to the same range of temperatures that purely rotational Raman can be, since the formulation is similar. Methods employing interference filters would have orders of magnitude greater transmission than our spectrograph setup, which would allow for higher time resolution. It would be interesting to apply such a setup to investigate the effects of temperature fluctuations on atmospheric processes. Line-fitting and two-line ratios can also be effective techniques, though they may only be viable options when using a polychromator or other light dispersion techniques. These methods do not have the strict filter requirements of the multi-line method.
It is worth reiterating, again, that VRR-based temperature measurement will not replace PRR-based temperature measurements. However, we believe there are situations where the elastic scattering signal will be too strong for PRR-based measurement to be reliable. Determining temperature from VRR spectra may be useful in the development of short range LiDAR systems that investigate turbid environments, such as near clouds. Additionally, VRR spectra could be used for temperature measurements in combustion diagnostics, especially in cases where incomplete combustion leads to the production of particles that could increase the elastic scattering coefficient in the measurement media and degrade the precision of other techniques.