Assessing Salt Marsh Vulnerability Using High-Resolution Hyperspectral Imagery
Abstract
1. Introduction
2. Methods
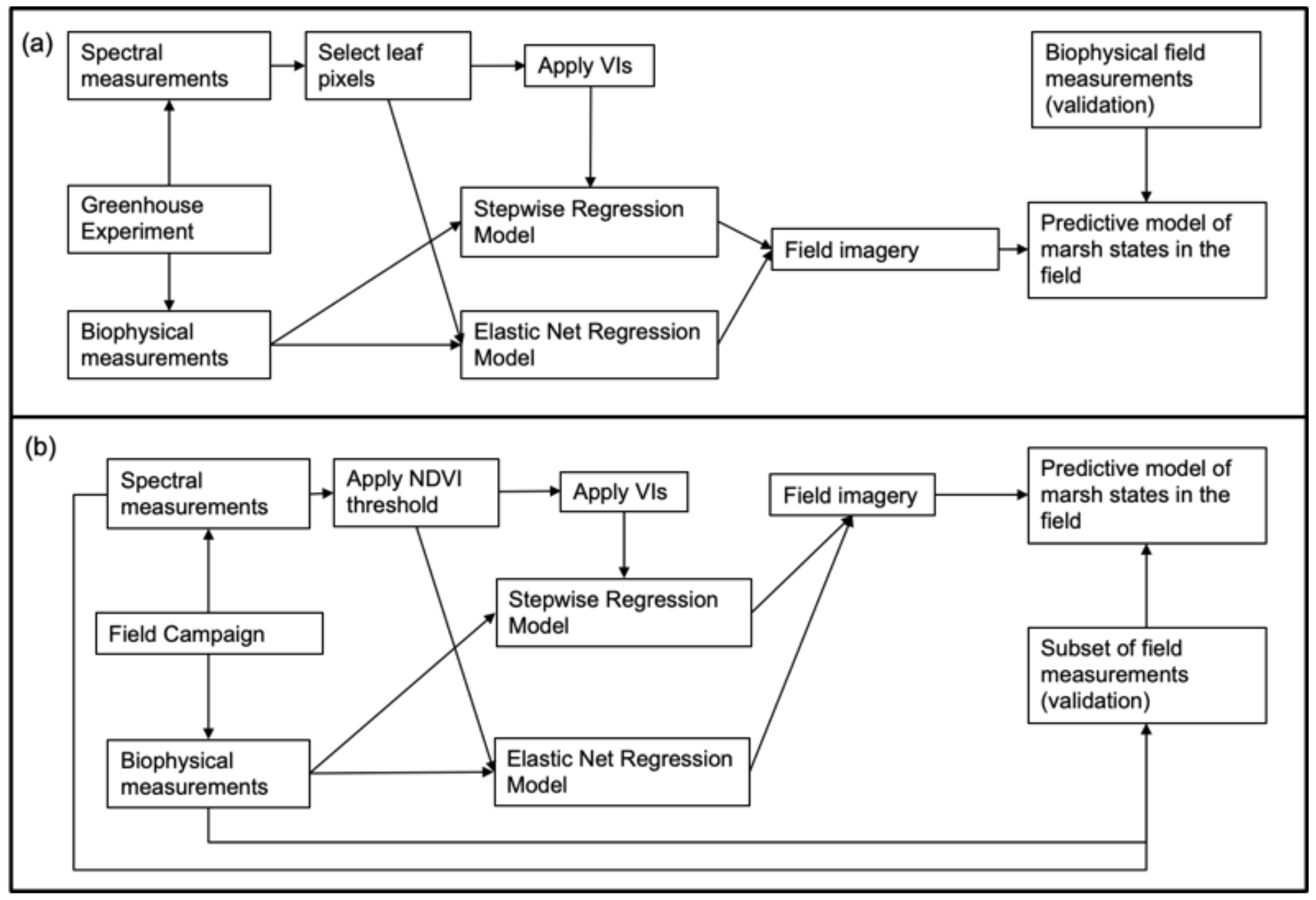
2.1. Greenhouse Experiment
2.1.1. Experimental Design
2.1.2. Statistics
2.1.3. Greenhouse Imagery Collection
2.2. Field Campaign
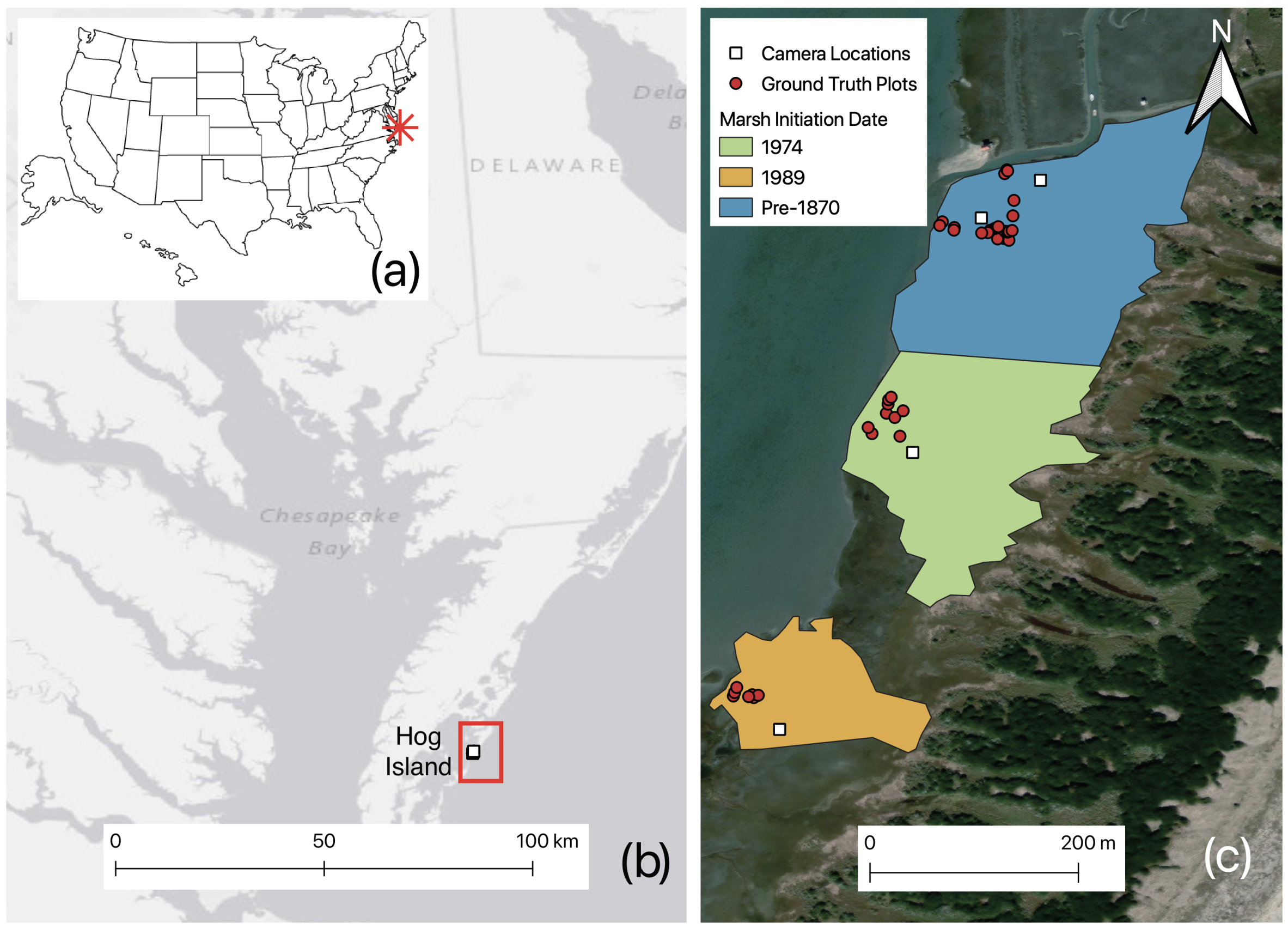
2.2.1. Site Description
2.2.2. Field Imagery Collection
2.3. Imagery Analysis
3. Results
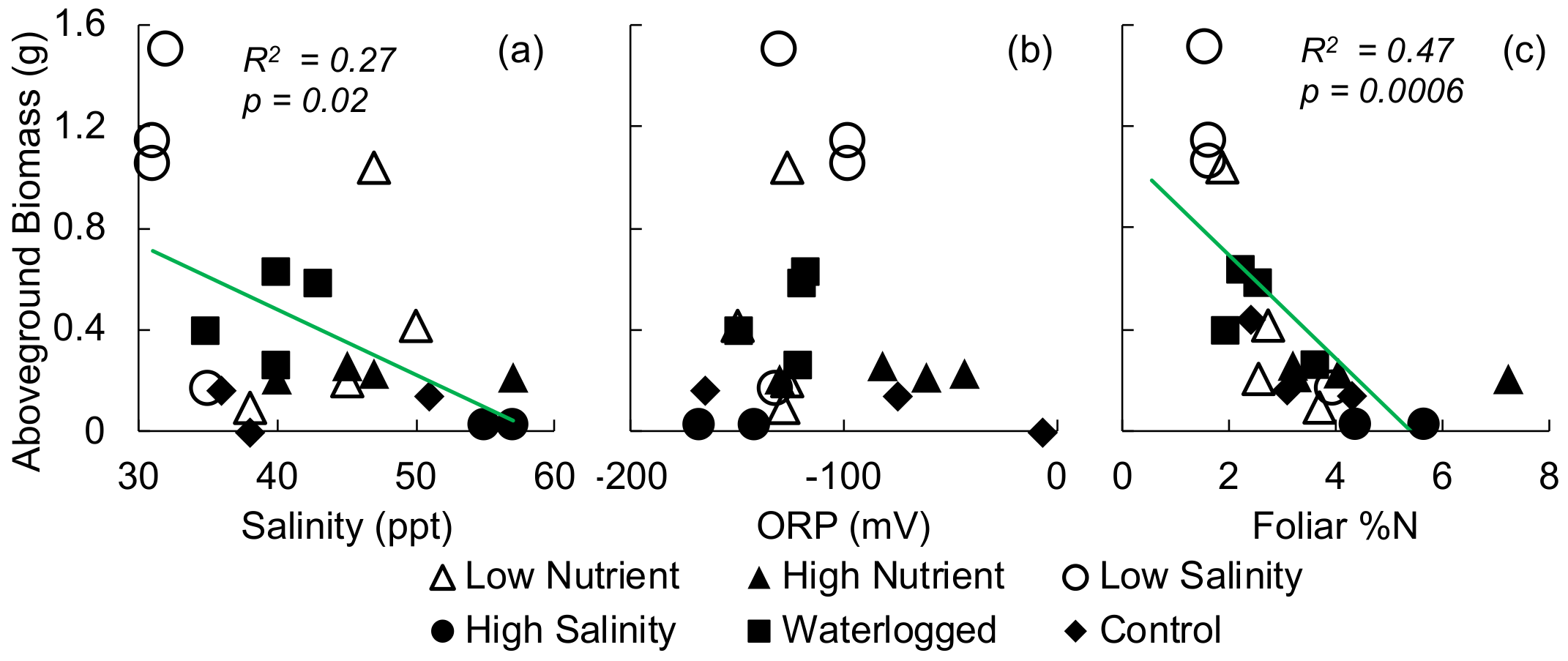
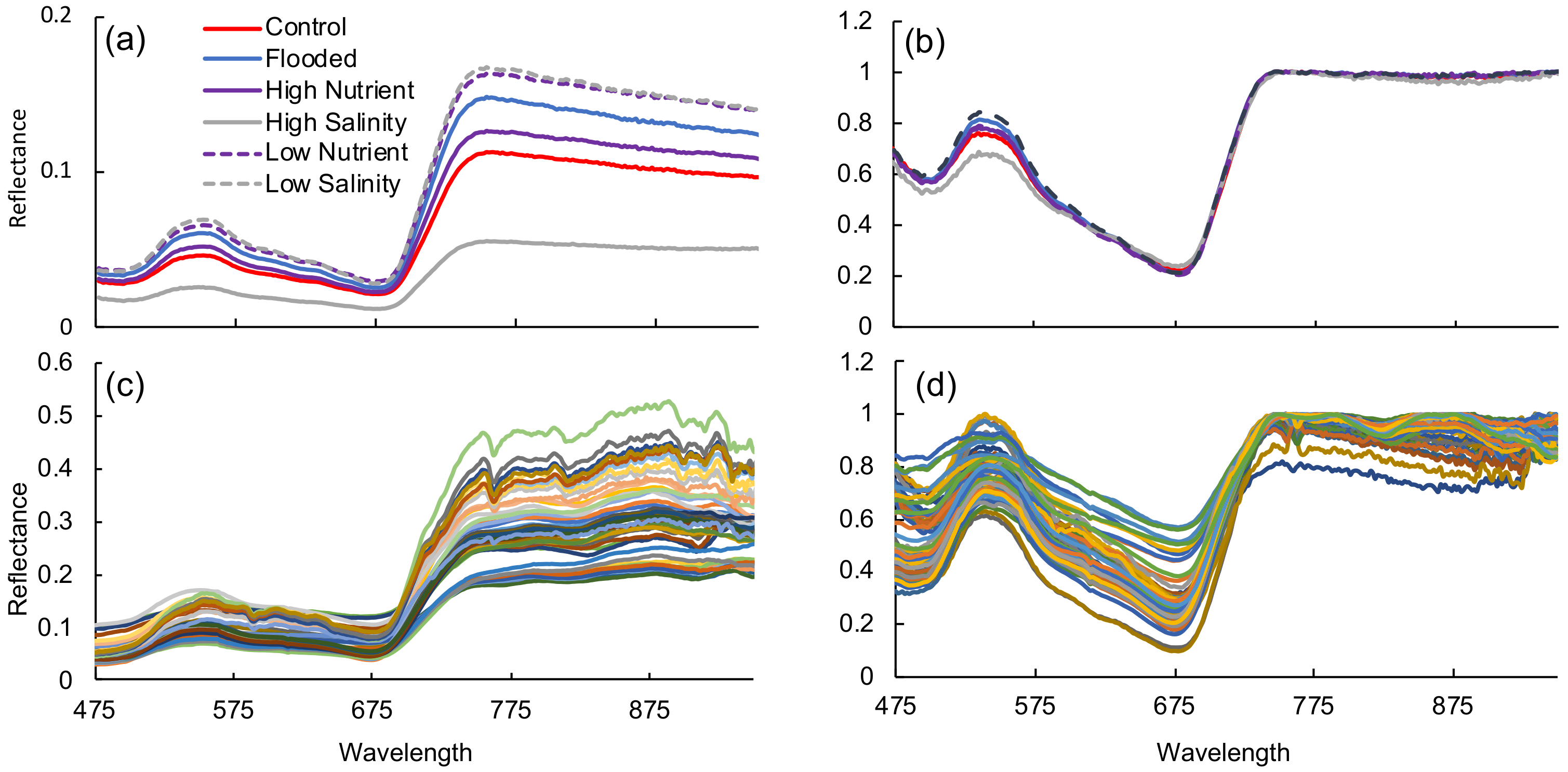
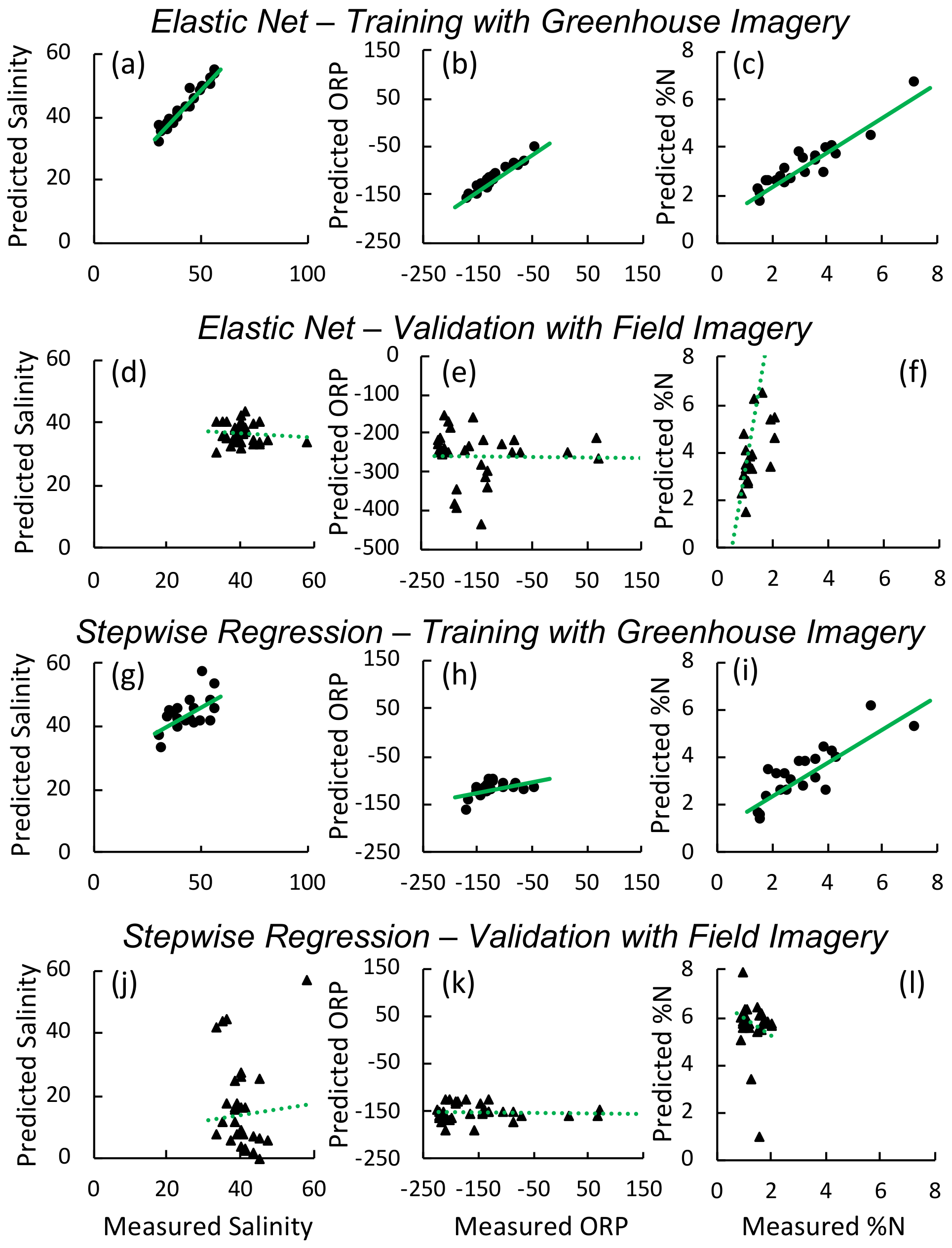
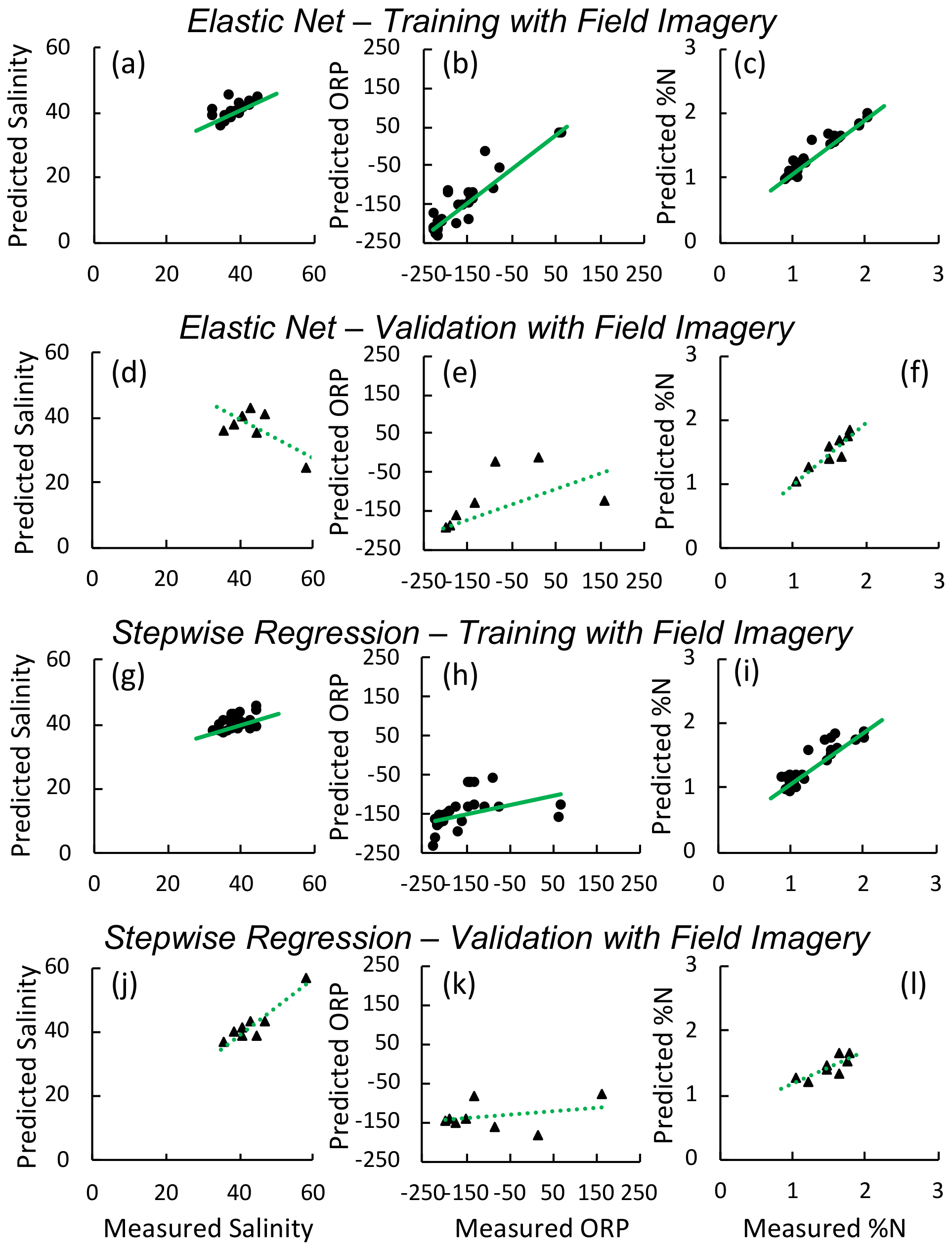
3.1. Greenhouse Experiment
3.2. Field
4. Discussion
4.1. Spectral Response to Stress
4.2. Nitrogen
4.3. Salinity
4.4. Waterlogging
4.5. Limitations of Models
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| NDVI | Normalized Difference Vegetation Index |
| NIR | Near Infrared |
| BRDF | Bidirectional Reflectance Distribution Function |
References
- Morris, J.T.; Sundareshwar, P.V.; Nietch, C.T.; Kjerfve, B.; Cahoon, D.R. Responses of Coastal Wetlands to Rising Sea Level. Ecology 2002, 83, 2869–2877. [Google Scholar] [CrossRef]
- McKee, K.L.; Mendelssohn, I.A.; Materne, M.D. Acute salt marsh dieback in the Mississippi River deltaic plain: A drought-induced phenomenon? Glob. Ecol. Biogeogr. 2004, 13, 65–73. [Google Scholar] [CrossRef]
- Gedan, K.B.; Silliman, B.R.; Bertness, M.D. Centuries of Human-Driven Change in Salt Marsh Ecosystems. Annu. Rev. Mar. Sci. 2009, 1, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.H.; Wilson, A.M.; Morris, J.T. Hydrologic variability in a salt marsh: Assessing the links between drought and acute marsh dieback. Estuarine Coast. Shelf Sci. 2012, 111, 95–106. [Google Scholar] [CrossRef]
- Alber, M.; Swenson, E.M.; Adamowicz, S.C.; Mendelssohn, I.A. Salt Marsh Dieback: An overview of recent events in the US. Estuarine Coast. Shelf Sci. 2008, 80, 1–11. [Google Scholar] [CrossRef]
- Bertness, M.D.; Silliman, B.R. Consumer Control of Salt Marshes Driven by Human Disturbance. Conserv. Biol. 2008, 22, 618–623. [Google Scholar] [CrossRef]
- Boesch, D.; Turner, R. Dependence of Fishery Species on Salt Marshes: The Role of Food and Refuge. Estuaries 1984, 7, 460–468. [Google Scholar] [CrossRef]
- Barbier, E.B.; Koch, E.W.; Silliman, B.R.; Hacker, S.D.; Wolanski, E.; Primavera, J.; Granek, E.F.; Polasky, S.; Aswani, S.; Cramer, L.A.; et al. Coastal ecosystem-based management with nonlinear ecological functions and values. Science 2008, 319, 321–323. [Google Scholar] [CrossRef]
- Koch, M.S.; Mendelssohn, I.A.; McKee, K.L. Mechanism for the hydrogen sulfide-induced growth limitation in wetland macrophytes. Limnol. Oceanogr. 1990, 35, 399–408. [Google Scholar] [CrossRef]
- Morgan, P.A.; Burdick, D.M.; Short, F.T. The Functions and Values of Fringing Salt Marshes in Northern New England, USA. Estuaries Coasts 2009, 32, 483–495. [Google Scholar] [CrossRef]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 2003, 17, 1111. [Google Scholar] [CrossRef]
- Chmura, G.L. What do we need to assess the sustainability of the tidal salt marsh carbon sink? Ocean. Coast. Manag. 2013, 83, 25–31. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Hopkinson, C.; Cai, W.J.; Hu, X. Carbon Sequestration in Wetland Dominated Coastal Systems—A Global Sink of Rapidly Diminishing Magnitude. Curr. Opin. Environ. Sustain. 2012, 4, 186–194. [Google Scholar] [CrossRef]
- Turner, R.E.; Howes, B.L.; Teal, J.M.; Milan, C.S.; Swenson, E.M.; Tonerb, D.D.G. Salt marshes and eutrophication: An unsustainable outcome. Limnol. Oceanogr. 2009, 54, 1634–1642. [Google Scholar] [CrossRef]
- Turner, R.E.; Swenson, E.M.; Milan, C.S.; Lee, J.M.; Oswald, T.A. Below-ground biomass in healthy and impaired salt marshes. Ecol. Res. 2004, 19, 29–35. [Google Scholar] [CrossRef]
- Deegan, L.A.; Johnson, D.S.; Warren, R.S.; Peterson, B.J.; Fleeger, J.W.; Fagherazzi, S.; Wollheim, W.M. Coastal eutrophication as a driver of salt marsh loss. Nature 2012, 490, 388–392. [Google Scholar] [CrossRef]
- Hester, M.W.; Mendelssohn, I.A.; McKee, K.L. Intraspecific Variation in Salt Tolerance and Morphology in Panicum hemitomon and Spartina alterniflora (Poaceae). Int. J. Plant Sci. 1998, 159, 127–138. [Google Scholar] [CrossRef]
- Linthurst, R.A.; Seneca, E.D. Aeration, Nitrogen and Salinity as Determinants of Spartina alterniflora Loisel. Growth Response. Estuaries 1981, 4, 53. [Google Scholar] [CrossRef]
- Brown, C.; Pezeshki, S.; DeLaune, R. The effects of salinity and soil drying on nutrient uptake and growth of Spartina alterniflora in a simulated tidal system. Environ. Exp. Bot. 2006, 58, 140–148. [Google Scholar] [CrossRef]
- Artigas, F.J.; Yang, J. Spectral discrimination of marsh vegetation types in the New Jersey Meadowlands, USA. Wetlands 2006, 26, 271–277. [Google Scholar] [CrossRef]
- Bachmann, C.; Donato, T.; Lamela, G.; Rhea, W.; Bettenhausen, M.; Fusina, R.; Du Bois, K.; Porter, J.; Truitt, B. Automatic classification of land cover on Smith Island, VA, using HyMAP imagery. IEEE Trans. Geosci. Remote. Sens. 2002, 40, 2313–2330. [Google Scholar] [CrossRef]
- Bachmann, C.; Bettenhausen, M.; Fusina, R.; Donato, T.; Russ, A.; Burke, J.; Lamela, G.; Rhea, W.; Truitt, B.; Porter, J. A credit assignment approach to fusing classifiers of multiseason hyperspectral imagery. IEEE Trans. Geosci. Remote. Sens. 2003, 41, 2488–2499. [Google Scholar] [CrossRef]
- Cheng, W.C.; Chang, J.C.; Chang, C.P.; Su, Y.; Tu, T.M. A Fixed-Threshold Approach to Generate High-Resolution Vegetation Maps for IKONOS Imagery. Sensors 2008, 8, 4308–4317. [Google Scholar] [CrossRef] [PubMed]
- Klemas, V.V. Remote Sensing of Wetlands: Case Studies Comparing Practical Techniques. J. Coast. Res. 2011, 27. [Google Scholar] [CrossRef]
- Hladik, C.; Schalles, J.; Alber, M. Salt marsh elevation and habitat mapping using hyperspectral and LIDAR data. Remote. Sens. Environ. 2013, 139, 318–330. [Google Scholar] [CrossRef]
- Byrd, K.B.; O’Connell, J.L.; Di Tommaso, S.; Kelly, M. Evaluation of sensor types and environmental controls on mapping biomass of coastal marsh emergent vegetation. Remote. Sens. Environ. 2014, 149, 166–180. [Google Scholar] [CrossRef]
- Byrd, K.B.; Ballanti, L.; Thomas, N.; Nguyen, D.; Holmquist, J.R.; Simard, M.; Windham-Myers, L. A remote sensing-based model of tidal marsh aboveground carbon stocks for the conterminous United States. Isprs J. Photogramm. Remote. Sens. 2018, 139, 255–271. [Google Scholar] [CrossRef]
- DiGiacomo, A.E.; Bird, C.N.; Pan, V.G.; Dobroski, K.; Atkins-Davis, C.; Johnston, D.W.; Ridge, J.T. Modeling Salt Marsh Vegetation Height Using Unoccupied Aircraft Systems and Structure from Motion. Remote Sens. 2020, 12, 2333. [Google Scholar] [CrossRef]
- Ramsey, E., III; Rangoonwala, A. Leaf Optical Property Changes Associated with the Occurrence of Spartina alterniflora Dieback in Coastal Louisiana Related to Remote Sensing Mapping. Photogramm. Eng. Remote. Sens. 2005, 71, 299–311. [Google Scholar] [CrossRef]
- Ramsey, E., III; Rangoonwala, A. Canopy reflectance related to marsh dieback onset and progression in coastal Louisiana. Photogramm. Eng. Remote. Sens. 2006, 22, 641–652. [Google Scholar] [CrossRef]
- Ramsey, E.; Rangoonwala, A. Characterizing the marsh dieback spectral response at the plant and canopy level with hyperspectral and temporal remote sensing data. In Proceedings of the 2008 IEEE/OES US/EU-Baltic International Symposium, Tallinn, Estonia, 27–29 May 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 1–8. [Google Scholar]
- Marsh, A.; Blum, L.K.; Christian, R.R.; Ramsey, E.; Rangoonwala, A. Response and resilience of Spartina alterniflora to sudden dieback. J. Coast. Conserv. 2016, 20, 335–350. [Google Scholar] [CrossRef]
- Miller, G.; Morris, J.; Wang, C. Mapping salt marsh dieback and condition in South Carolina’s North Inlet-Winyah Bay National Estuarine Research Reserve using remote sensing. Aims Environ. Sci. 2017, 4, 677–689. [Google Scholar] [CrossRef]
- Badura, G.P.; Bachmann, C.M.; Tyler, A.C.; Goldsmith, S.; Eon, R.S.; Lapszynski, C.S. A Novel Approach for Deriving LAI of Salt Marsh Vegetation Using Structure From Motion and Multiangular Spectra. IEEE J. Sel. Top. Appl. Earth Obs. Remote. Sens. 2019, 12, 599–613. [Google Scholar] [CrossRef]
- Eon, R.S.; Goldsmith, S.; Bachmann, C.M.; Tyler, A.C.; Lapszynski, C.S.; Badura, G.P.; Osgood, D.T.; Brett, R. Retreival of salt marsh above-ground biomass from high-spatial resolution, multi-view hyperspectral imagery using PROSAIL. Remote Sens. 2019, 11, 1385. [Google Scholar] [CrossRef]
- LaCapra, V.C.; Melack, J.M.; Gastil, M.; Valeriano, D. Remote sensing of foliar chemistry of inundated rice with imaging spectrometry. Remote Sens. Environ. 1996, 55, 50–58. [Google Scholar] [CrossRef]
- Tian, Y.; Yao, X.; Yang, J.; Cao, W.; Zhu, Y. Extracting Red Edge Position Parameters from Ground- and Space-Based Hyperspectral Data for Estimation of Canopy Leaf Nitrogen Concentration in Rice. Plant Prod. Sci. 2011, 14, 270–281. [Google Scholar] [CrossRef]
- He, L.; Song, X.; Feng, W.; Guo, B.B.; Zhang, Y.S.; Wang, Y.H.; Wang, C.Y.; Guo, T.C. Improved remote sensing of leaf nitrogen concentration in winter wheat using multi-angular hyperspectral data. Remote Sens. Environ. 2016, 174, 122–133. [Google Scholar] [CrossRef]
- O’Connell, J.L.; Mishra, D.R.; Cotten, D.L.; Wang, L.; Alber, M. The Tidal Marsh Inundation Index (TMII): An inundation filter to flag flooded pixels and improve MODIS tidal marsh vegetation time-series analysis. Remote Sens. Environ. 2017, 201, 34–46. [Google Scholar] [CrossRef]
- Zhang, M.; Ustin, S.L.; Rejmankova, E.; Sanderson, E.W. Monitoring Pacific Coast Salt Marshes Using Remote Sensing. Ecol. Appl. 1997, 7, 1039–1053. [Google Scholar] [CrossRef]
- Anderson, J.E.; Perry, J.E. Characterization of wetland plant stress using leaf spectral reflectance: Implications for wetland remote sensing. Wetlands 1996, 16, 477–487. [Google Scholar] [CrossRef]
- Smith, K.L.; Steven, M.D.; Colls, J.J. Spectral responses of pot-grown plants to displacement of soil oxygen. Int. J. Remote. Sens. 2004, 25, 4395–4410. [Google Scholar] [CrossRef]
- Naidoo, G.; Mckee, K.L.; Mendelssohn, I.A. Anatomical and metabolic responses to waterlogging and salinity in Spartina alterniflora and S. patens (Poaceae). Am. J. Bot. 1992, 79, 765–770. [Google Scholar] [CrossRef]
- MacTavish, R.M.; Cohen, R.A. A Simple, Inexpensive, and Field-Relevant Microcosm Tidal Simulator for Use in Marsh Macrophyte Studies. Appl. Plant Sci. 2014, 2, 1400058. [Google Scholar] [CrossRef] [PubMed]
- MacTavish, R.M.; Cohen, R.A. Water column ammonium concentration and salinity influence nitrogen uptake and growth of Spartina alterniflora. J. Exp. Mar. Biol. Ecol. 2017, 488, 52–59. [Google Scholar] [CrossRef]
- Berg, P.; McGlathery, K.J. A high-resolution pore water sampler for sandy sediments. Limnol. Oceanogr. 2001, 46, 203–210. [Google Scholar] [CrossRef]
- Solórzano, L. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol. Oceanogr. 1969, 14, 799–801. [Google Scholar] [CrossRef]
- Lichtenhaler, H.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 99–114. [Google Scholar] [CrossRef]
- Bachmann, C.M.; Eon, R.S.; Lapszynski, C.S.; Badura, G.P.; Vodacek, A.; Hoffman, M.J.; McKeown, D.; Kremens, R.L.; Richardson, M.; Bauch, T.; et al. A Low-Rate Video Approach to Hyperspectral Imaging of Dynamic Scenes. J. Imaging 2019, 5, 6. [Google Scholar] [CrossRef]
- Day, F.P.; Crawford, E.R.; Dilustro, J.J. Aboveground Plant Biomass Change along a Coastal Barrier Island Dune Chronosequence over a Six-Year Period. J. Torrey Bot. Soc. 2001, 128, 197. [Google Scholar] [CrossRef]
- Hayden, B.; Dueser, J.; Shugart, H. Long Term Research at the Virginia Coast Reserve: Modeling a highly dynamic environment. Bioscience 1991, 41, 310–318. [Google Scholar] [CrossRef]
- Walsh, J.P. Low Marsh Succession along an Over-Wash Salt Marsh Chronosequence. Ph.D. Thesis, University of Virginia, Charlottesville, VA, USA, 1998. [Google Scholar]
- Tyler, A.C.; Zieman, J.C. Patterns of development in the creekbank region of a barrier island Spartina alterniflora marsh. Mar. Ecol. Prog. Ser. 1999, 180, 161–177. [Google Scholar] [CrossRef]
- Goldsmith, S. Decadal Changes in Salt Marsh Succession and Assessing Salt Marsh Vulnerability Using High-Resolution Hyperspectral Imagery; Rochester Institute of Technology, ProQuest Dissertations: Rochester, NY, USA, 2019. [Google Scholar]
- Savitzky, A.; Golay, M.J. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer Series in Statistics; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Main, R.; Cho, M.A.; Mathieu, R.; O’Kennedy, M.M.; Ramoelo, A.; Koch, S. An investigation into robust spectral indices for leaf chlorophyll estimation. ISPRS J. Photogramm. Remote. Sens. 2011, 66, 751–761. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote. Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Penuelas, J.; Pinol, J.; Ogaya, R.; Filella, I. Estimation of plant water concentration by the reflectance Water Index WI (R900/R970). Int. J. Remote. Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Daughtry, C. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Ju, C.H.; Tian, Y.C.; Yao, X.; Cao, W.X.; Zhu, Y.; Hannaway. Estimating Leaf Chlorophyll Content Using Red Edge Parameters. Pedosphere 2010, 20, 633–644. [Google Scholar] [CrossRef]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Thenot, F.; Méthy, M.; Winkel, T. The Photochemical Reflectance Index (PRI) as a water-stress index. Int. J. Remote. Sens. 2002, 23, 5135–5139. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of Leaf-Area Index from Quality of Light on the Forest Floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Motohka, T.; Nasahara, K.N.; Oguma, H.; Tsuchida, S. Applicability of Green-Red Vegetation Index for Remote Sensing of Vegetation Phenology. Remote Sens. 2010, 2, 2369–2387. [Google Scholar] [CrossRef]
- Haboudane, D. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide Dynamic Range Vegetation Index for Remote Quantification of Biophysical Characteristics of Vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef]
- Jensen, J.R. Remote Sensing of the Environment: An Earth Resource Perspective, 2nd ed.; Pearson: Upper Saddle River, NJ, USA, 2006. [Google Scholar]
- Hilker, T.; Coops, N.C.; Nesic, Z.; Wulder, M.A.; Black, A.T. Instrumentation and approach for unattended year round tower based measurements of spectral reflectance. Comput. Electron. Agric. 2007, 56, 72–84. [Google Scholar] [CrossRef]
- Knipling, E. Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens. Environ. 1970, 3, 155–159. [Google Scholar] [CrossRef]
- Siciliano, D.; Wasson, K.; Potts, D.C.; Olsen, R. Evaluating hyperspectral imaging of wetland vegetation as a tool for detecting estuarine nutrient enrichment. Remote Sens. Environ. 2008, 112, 4020–4033. [Google Scholar] [CrossRef]
- O’Connell, J.L.; Byrd, K.B.; Kelly, M. Remotely-Sensed Indicators of N-Related Biomass Allocation in Schoenoplectus acutus. PLoS ONE 2014, 9, e90870. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.; Morris, J. The influence of salinity on the kinetics of NH4+ uptake in Spartina alterniflora. Oecologia 1991, 85, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Read, J.J.; Tarpley, L.; McKinion, J.M.; Reddy, K.R. Narrow-Waveband Reflectance Ratios for Remote Estimation of Nitrogen Status in Cotton. J. Environ. Qual. 2002, 31, 1442. [Google Scholar] [CrossRef]
- Cabrera-Bosquet, L.; Molero, G.; Stellacci, A.; Bort, J.; Nogués, S.; Araus, J. NDVI as a potential tool for predicting biomass, plant nitrogen content and growth in wheat genotypes subjected to different water and nitrogen conditions. Cereal Res. Commun. 2011, 39, 147–159. [Google Scholar] [CrossRef]
- Giurgevich, J.; Dunn, E. Seasonal patterns of CO2 and water vapor exchange of the tall and short height forms of Spartina alterniflora Loisel in a Georgia salt marsh. Oecologia 1979, 43, 139–156. [Google Scholar] [CrossRef]
- Levering, C.; Thomson, W. The ultrastructure of the salt gland of Spartina foliosa. Planta 1971, 97, 183–196. [Google Scholar] [CrossRef]
- Longstreth, D.J.; Strain, B.R. Effects of salinity and illumination on photosynthesis and water balance of Spartina alterniflora Loisel. Oecologia 1977, 31, 191–199. [Google Scholar] [CrossRef]
- Longstreth, D.J.; Nobel, P.S. Salinity Effects on Leaf Anatomy: Consequences for Photosynthesis. Plant Physiol. 1979, 63, 700–703. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zeng, S.L.; Gao, Y.; Ouyang, Z.T.; Li, B.; Fang, C.M.; Zhao, B. Using hyperspectral vegetation indices as a proxy to monitor soil salinity. Ecol. Indic. 2011, 11, 1552–1562. [Google Scholar] [CrossRef]
- Esteban, R.; Fernández-marín, B.; Hernandez, A.; Jiménez, E.T.; León, A.; García-mauriño, S.; Silva, C.D.; Dolmus, J.R.; Dolmus, C.M.; Molina, M.J.; et al. Salt crystal deposition as a reversible mechanism to enhance photoprotection in black mangrove. Trees 2013, 27, 229–237. [Google Scholar] [CrossRef]
- Mendelssohn, I.A.; McKee, K.L.; Patrick, W.H. Oxygen Deficiency in Spartina alterniflora Roots: Metabolic Adaptation to Anoxia. Sci. New Ser. 1981, 214, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, S. Photosynthesis and root growth in Spartina alterniflora in relation to root zone aeration. Photosynthetica 1997, 34, 107–114. [Google Scholar] [CrossRef]
- Burdick, D.M. Root Aerenchyma Development in Spartina Patens in Response to Flooding. Am. J. Bot. 1989, 76, 777. [Google Scholar] [CrossRef]
- Maricle, B.R.; Lee, R.W. Aerenchyma development and oxygen transport in the estuarine cordgrasses Spartina alterniflora and S. anglica. Aquat. Bot. 2002, 74, 109–120. [Google Scholar] [CrossRef]
- Burdick, D.M.; Mendelssohn, I.A. Waterlogging responses in dune, swale and marsh populations of Spartina patens under field conditions. Oecologia 1987, 74, 321–329. [Google Scholar] [CrossRef]
- Morris, J.T.; Dacey, J.W.H. Effects of O2 on Ammonium uptake and Root Respiration by Spartina alterniflora. Am. J. Bot. 1984, 71, 979–985. [Google Scholar] [CrossRef]
- Naumann, J.C.; Anderson, J.E.; Young, D.R. Linking physiological responses, chlorophyll fluorescence and hyperspectral imagery to detect salinity stress using the physiological reflectance index in the coastal shrub, Myrica cerifera. Remote Sens. Environ. 2008, 112, 3865–3875. [Google Scholar] [CrossRef]
- Mendelssohn, I.A. The influence of nitrogen level, form, and application method on the growth response ofSpartina alterniflora in North Carolina. Estuaries 1979, 2, 106–112. [Google Scholar] [CrossRef]
- Linthurst, R.A. An evaluation of aeration, nitrogen, pH and salinity as factors affecting Spartina alterniflora growth: A summary. In Estuarine Perspectives; Elsevier: Amsterdam, The Netherlands, 1980; pp. 235–247. [Google Scholar]

| Vegetation Index | Definition | Use | Source |
|---|---|---|---|
| Red edge position linear interpolation (REP) | Chlorophyll concentration | [60] | |
| Normalized Difference Vegetation Index (NDVI) | Green biomass, chlorophyll concentration | [61] | |
| Water Index (WI) | Leaf water content | [62] | |
| Optimized Soil Adjusted Vegetation Index (OSAVI) | Green biomass | [63] | |
| Optimized Soil Adjusted Vegetation Index 2 (OSAVI2) | Green biomass | [64] | |
| Modified Chlorophyll Absorption Reflectance Index (MCARI) | Chlorophyll concentration, leaf area index | [65] | |
| Red Edge Symmetry (RES) | Chlorophyll concentration | [66] | |
| Photochemical Reflectance Index (PRI) | Light-use efficiency, plant stress | [67,68] | |
| Ratio Vegetation Index (RVI) | Green biomass | [69] | |
| Green-Red Vegetation Index (GRVI) | Green biomass | [70] | |
| Modified Soil Adjusted Vegetation Index 2 (MSAVI2) | Green biomass | [71] | |
| Wide Dynamic Range Normalized Difference Vegetation Index (WDR NDVI) | Green biomass | [72] |
| F | p-Value | Control | High Nutrient | Low Nutrient | High Salinity | Low Salinity | Flooded | |
|---|---|---|---|---|---|---|---|---|
| AG Biomass | 3.2 | 0.04 | 0.25 (0.1) | 0.23 (0.01) | 0.44 (0.01) | 0.03 (0.21) | 0.97 (0.28) | 0.46 (0.09) |
| BG Biomass | 0.64 | 0.67 | 3.31 (0.75) | 3.71 (0.45) | 3.3 (0.44) | 2.81 (0.04) | 4.15 (0.62) | 3.57 (0.37) |
| Foliar | 2.75 | 0.06 | 3.26 (0.55) | 4.45 (0.95) | 2.71 (0.38) | 5.03 (0.64) | 2.19 (0.59) | 2.62 (0.36) |
| Aerial N | 2.63 | 0.07 | 0.72 (0.17) | 1.0 (0.18) | 0.99 (0.36) | 0.14 (0.01) | 1.65 (0.36) | 1.16 (0.18) |
| Chl | 0.95 | 0.95 | 38 (11) | 31 (7) | 33 (4) | 51 (19) | 40 (6) | 29 (3) |
| N:Chl | 3.07 | 0.04 | 0.09 (0.02) | 0.15 (0.02) | 0.09 (0.02) | 0.11 (0.03) | 0.06 (0.02) | 0.09 (0.02) |
| Salinity | 6.23 | 0.003 | 47 (6) | 47 (4) | 45 (3) | 56 (1) | 32 (1) | 40 (2) |
| ORP | 2.57 | 0.08 | −120 (45) | −79 (19) | −132 (6) | −154 (13) | −114 (10) | −126 (8) |
| 0.7 | 0.63 | 9.6 (5.3) | 13.6 (2.8) | 8.7 (2.2) | 17.1 (10.8) | 9.9 (2.3) | 8.6 (1.7) |
| Variable | BIC | AICc | RMSE Training | Training | RMSE Validation | Validation | Factors | |
|---|---|---|---|---|---|---|---|---|
| Elastic Net | Foliar | 54.5 | 55.8 | 0.5 | 0.85 | 3.7 | 0.39 | ”651’, 672’ **, 775”, 880”, 948”” |
| Salinity | 151 | 149.8 | 6.1 | 0.46 | 3.3 | < 0.1 | ”478’, 622”, 842”” | |
| Redox | 169.5 | 171.8 | 9.9 | 0.9 | 71.8 | < 0.1 | ”573”, 755”, 830’, 909”, 918”” | |
| Stepwise | Foliar | 66.1 | 66.4 | 0.9 | 0.7 | 1.3 | < 0.1 | ”OSAVI2 **, PRI *, RVI **, GRVI ** |
| Salinity | 152.2 | 153.4 | 7.2 | 0.4 | 11.6 | 0.15 | ”REP **, WI *, OSAVI2 ** | |
| Redox | 197.9 | 199.4 | 29.8 | 0.23 | 16.2 | < 0.1 | WI * |
| Initiation Date | Biomass | Height | Density | ORP | Salinity | |
|---|---|---|---|---|---|---|
| 1989 | 135.4 ± 21.8 [76.4–213.3] | 33.6 ± 2.4 [27.2–41.2] | 40.3 ± 7 [15–57] | n.d. | −19 ± 32 [−104–69] | 42 ± 1 [40–45] |
| 1974 | 104.1 ± 29.4 [17.5–243.2] | 28.2 ± 2.9 [20.1–42.5] | 157 ± 20 [88–248] | 1.61 ± 0.03 [1.5–1.76] | −99 ± 38 [−177–162] | 42 ± 1 [36–47] |
| 1845 | 130.4 ± 27.1 [7.8–569.9] | 36 ± 2.8 [14.9–68.7] | 103 ± 29 [5–484] | 1.32 ± 0.07 [0.91–2.05] | −192 ± 6 [−222–131] | 39 ± 1 [33–58] |
| Variable | BIC | AICc | RMSE Training | Training | RMSE Validation | Validation | Factors | |
|---|---|---|---|---|---|---|---|---|
| Elastic Net | Foliar | −27.5 | −18.5 | 0.1 | 0.97 | 0.1 | 0.74 | 415”, 449” **, 624”, 803”, 825” *, 869” **, 908’, 907’, 931’**” |
| Salinity | 77.9 | 99 | 0.5 | 0.96 | 5.3 | 0.4 | ”434” **, 464” **, 475” **, 598” **, 608”, 628”, 699”, 803” **, 843” **, 849”, 916”” | |
| Redox | 232.3 | 253.4 | 13 | 0.97 | 120 | 0.22 | ”463” *, 473” **, 795” **, 809” **, 830” **, 851”, 857”, 868”, 884’ *, 955”, 961” **” | |
| Stepwise | Foliar | −5.3 | −8.9 | 0.2 | 0.76 | 0.1 | 0.99 | ”WI *, OSAVI *, GRVI *, MSAVI2 *” |
| Salinity | 159.4 | 155.5 | 3.3 | 0.6 | 1.9 | 0.61 | ”NDVI *, WI,” WDR-NDVI * | |
| Redox | 337.6 | 334.6 | 87.2 | 0.2 | 39.5 | < 0.1 | PRI * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldsmith, S.B.; Eon, R.S.; Lapszynski, C.S.; Badura, G.P.; Osgood, D.T.; Bachmann, C.M.; Tyler, A.C. Assessing Salt Marsh Vulnerability Using High-Resolution Hyperspectral Imagery. Remote Sens. 2020, 12, 2938. https://doi.org/10.3390/rs12182938
Goldsmith SB, Eon RS, Lapszynski CS, Badura GP, Osgood DT, Bachmann CM, Tyler AC. Assessing Salt Marsh Vulnerability Using High-Resolution Hyperspectral Imagery. Remote Sensing. 2020; 12(18):2938. https://doi.org/10.3390/rs12182938
Chicago/Turabian StyleGoldsmith, Sarah B., Rehman S. Eon, Christopher S. Lapszynski, Gregory P. Badura, David T. Osgood, Charles M. Bachmann, and Anna Christina Tyler. 2020. "Assessing Salt Marsh Vulnerability Using High-Resolution Hyperspectral Imagery" Remote Sensing 12, no. 18: 2938. https://doi.org/10.3390/rs12182938
APA StyleGoldsmith, S. B., Eon, R. S., Lapszynski, C. S., Badura, G. P., Osgood, D. T., Bachmann, C. M., & Tyler, A. C. (2020). Assessing Salt Marsh Vulnerability Using High-Resolution Hyperspectral Imagery. Remote Sensing, 12(18), 2938. https://doi.org/10.3390/rs12182938






