Multi-Temporal UAV Data and Object-Based Image Analysis (OBIA) for Estimation of Substrate Changes in a Post-Bleaching Scenario on a Maldivian Reef
Abstract
1. Introduction
2. Materials and Methods
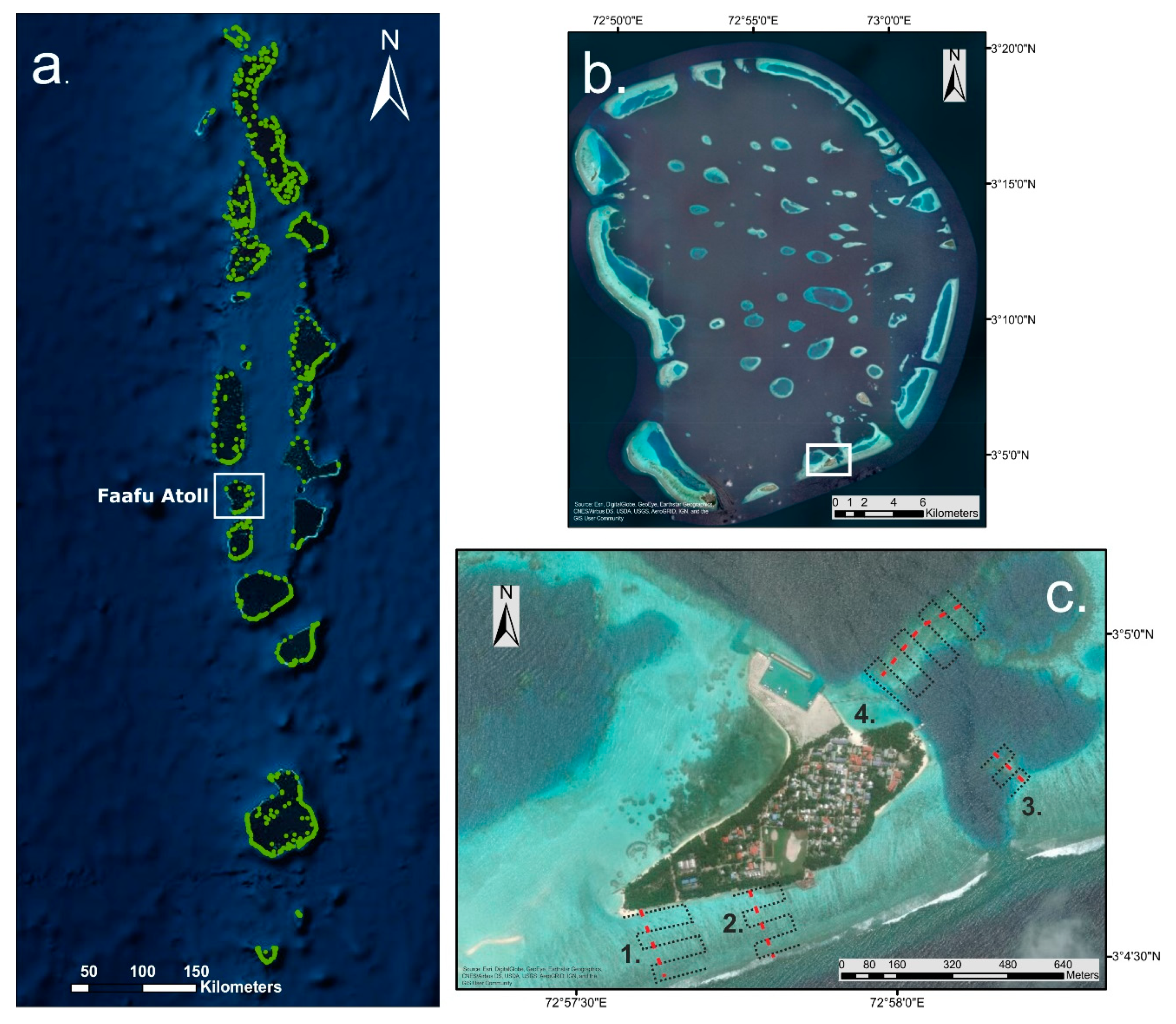
2.1. Study Area
2.2. UAV Data Collection and Field Survey
2.3. Flight Planning
2.4. SfM Processing and Georeferencing
2.5. OBIA and Classification of High-Resolution Orthomosaics
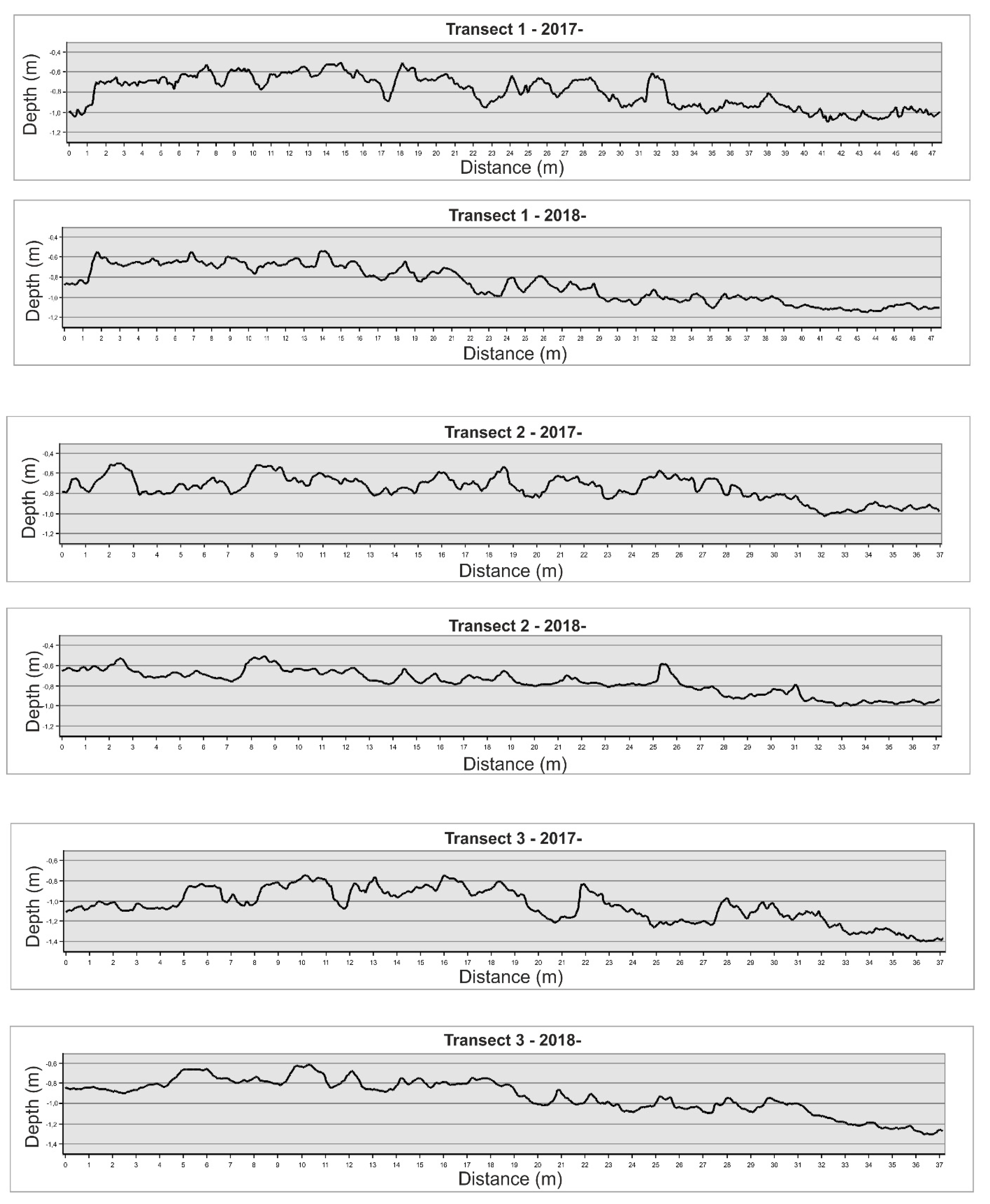
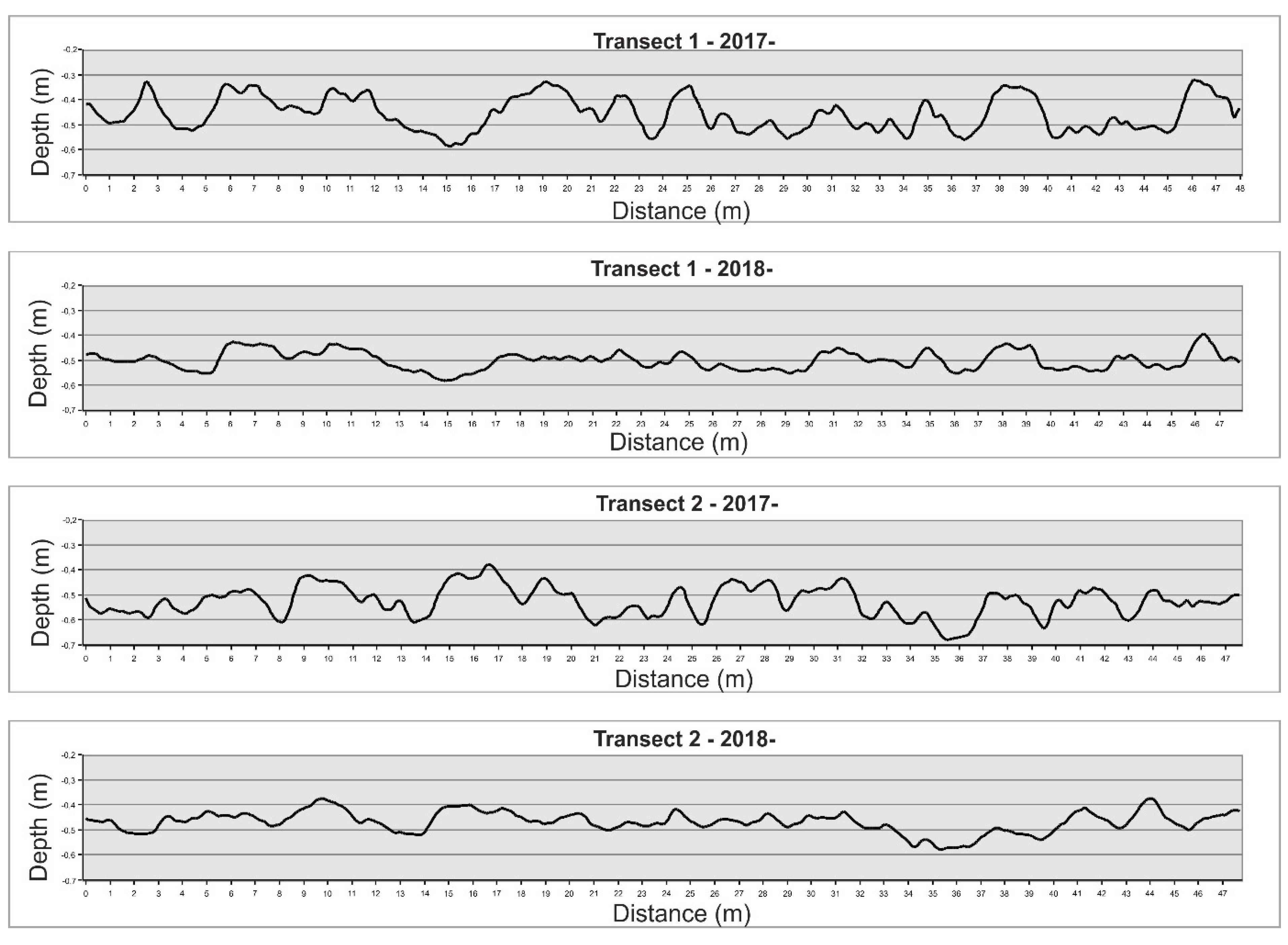
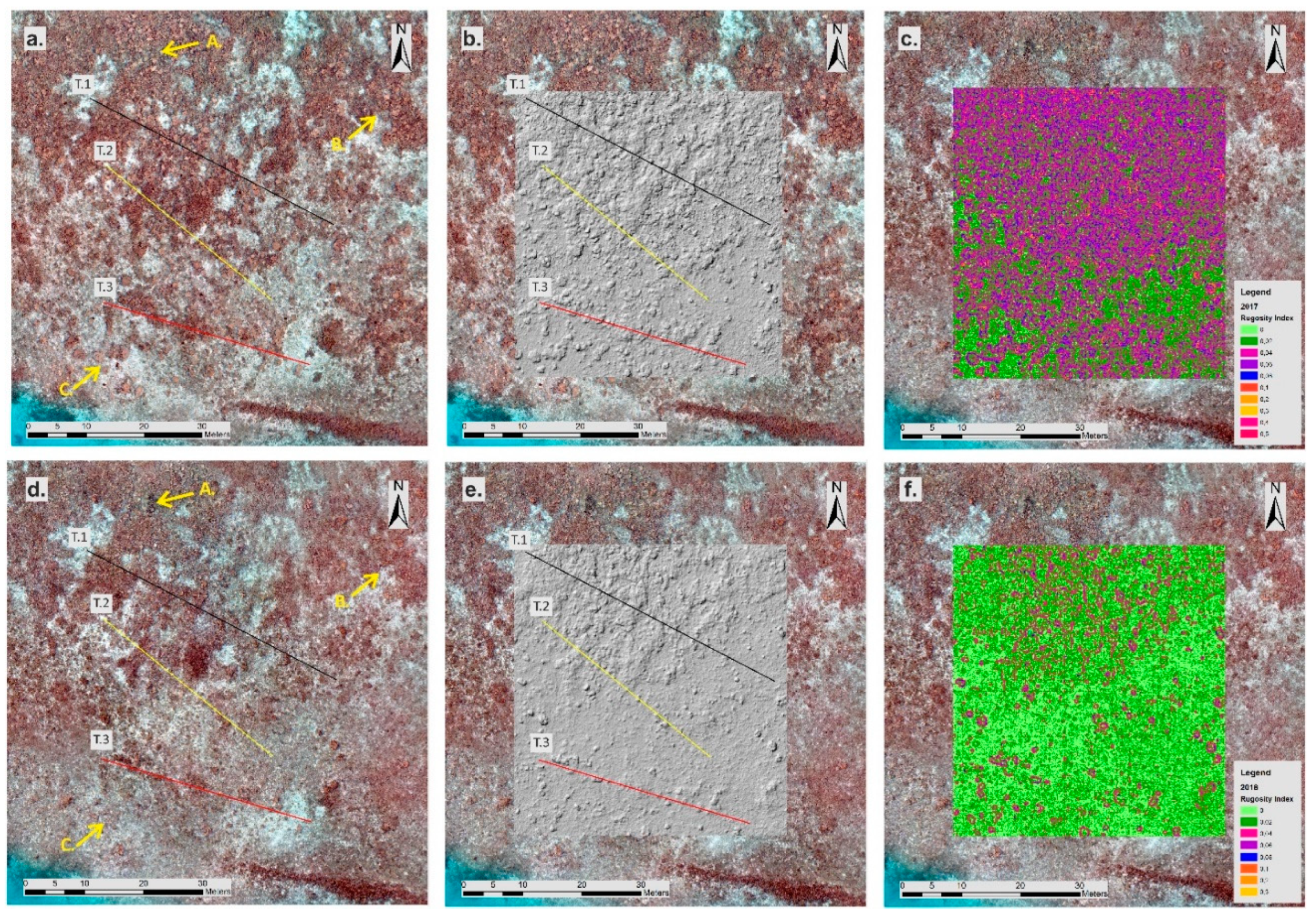
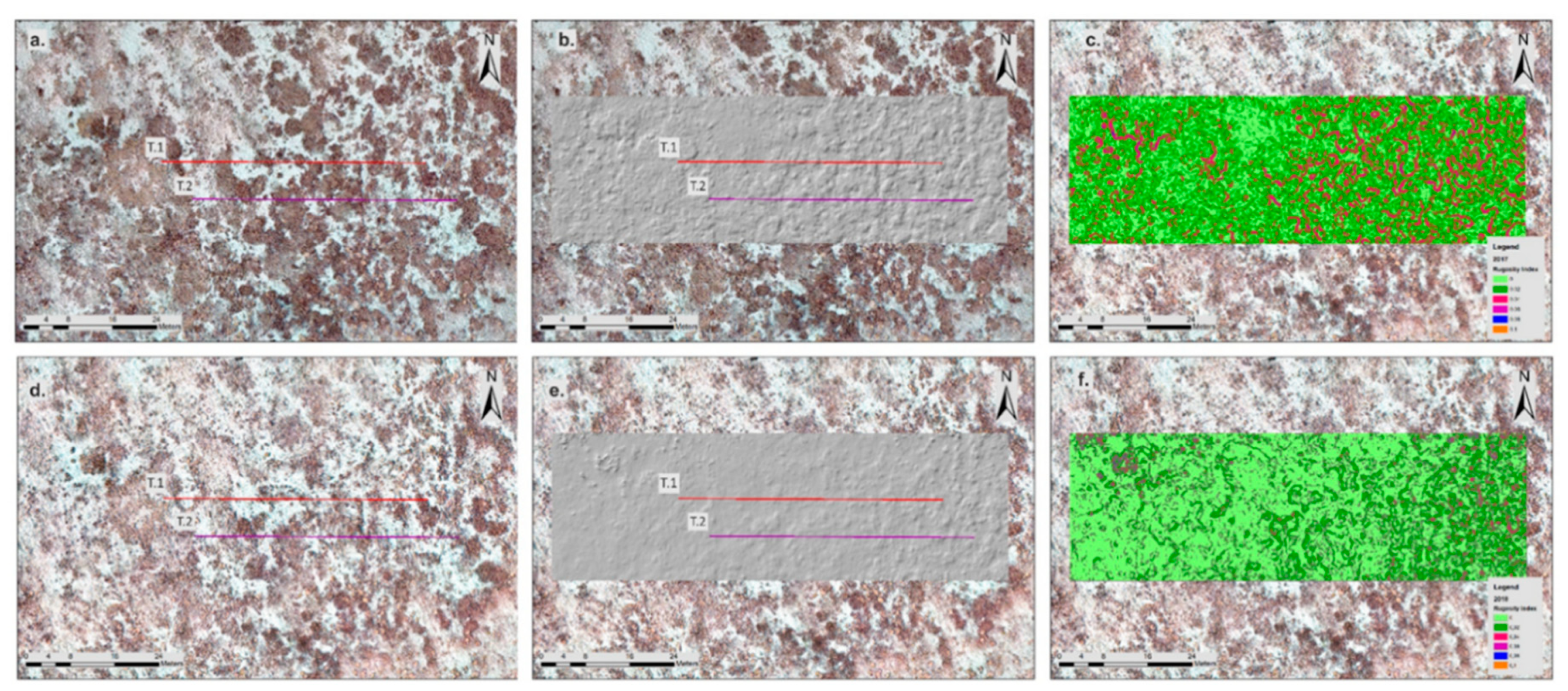
2.6. DTM Analysis and Structure Complexity Evaluation
2.7. Map Accuracy Assessment
3. Results
3.1. Map Accuracy
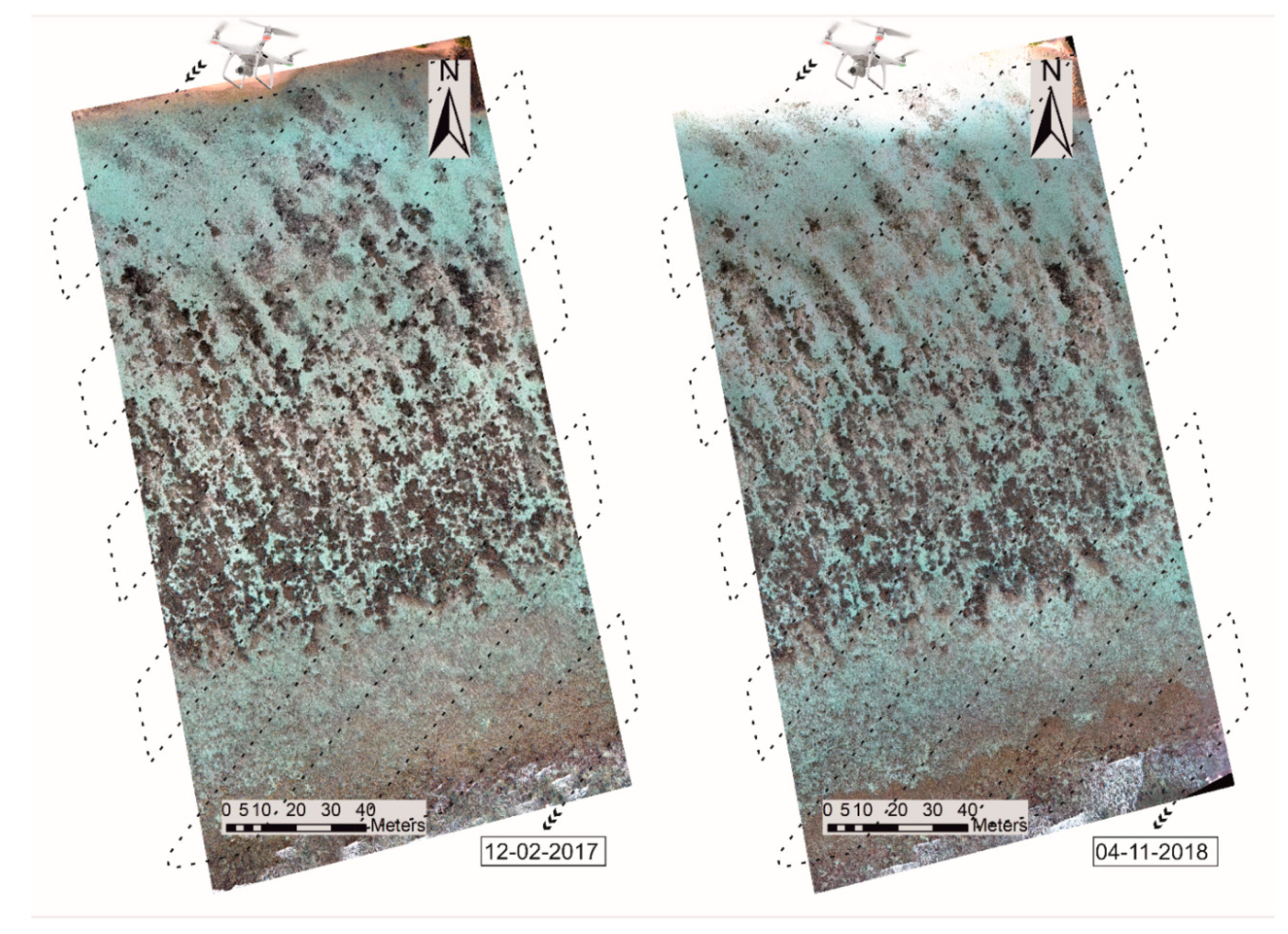
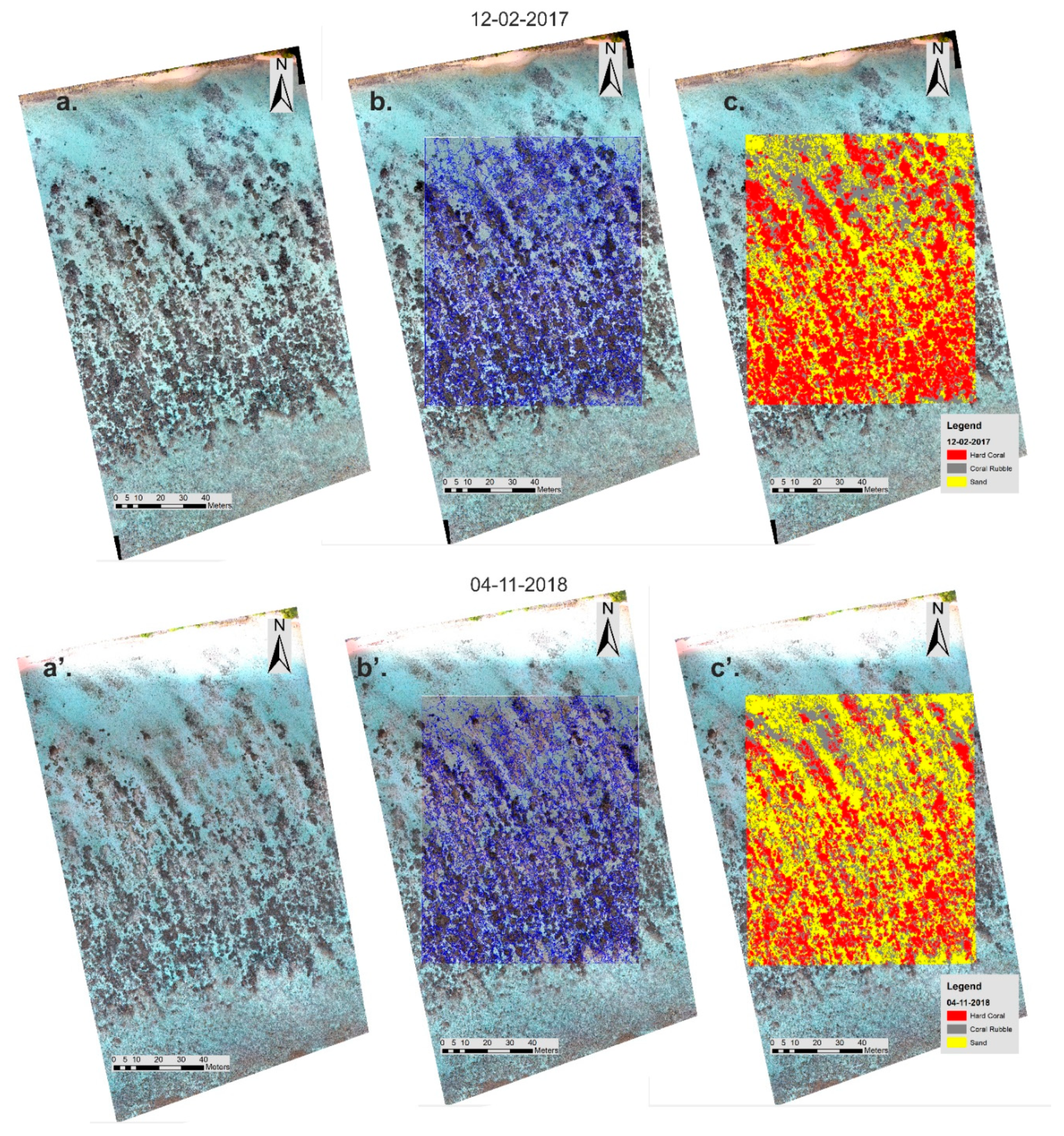
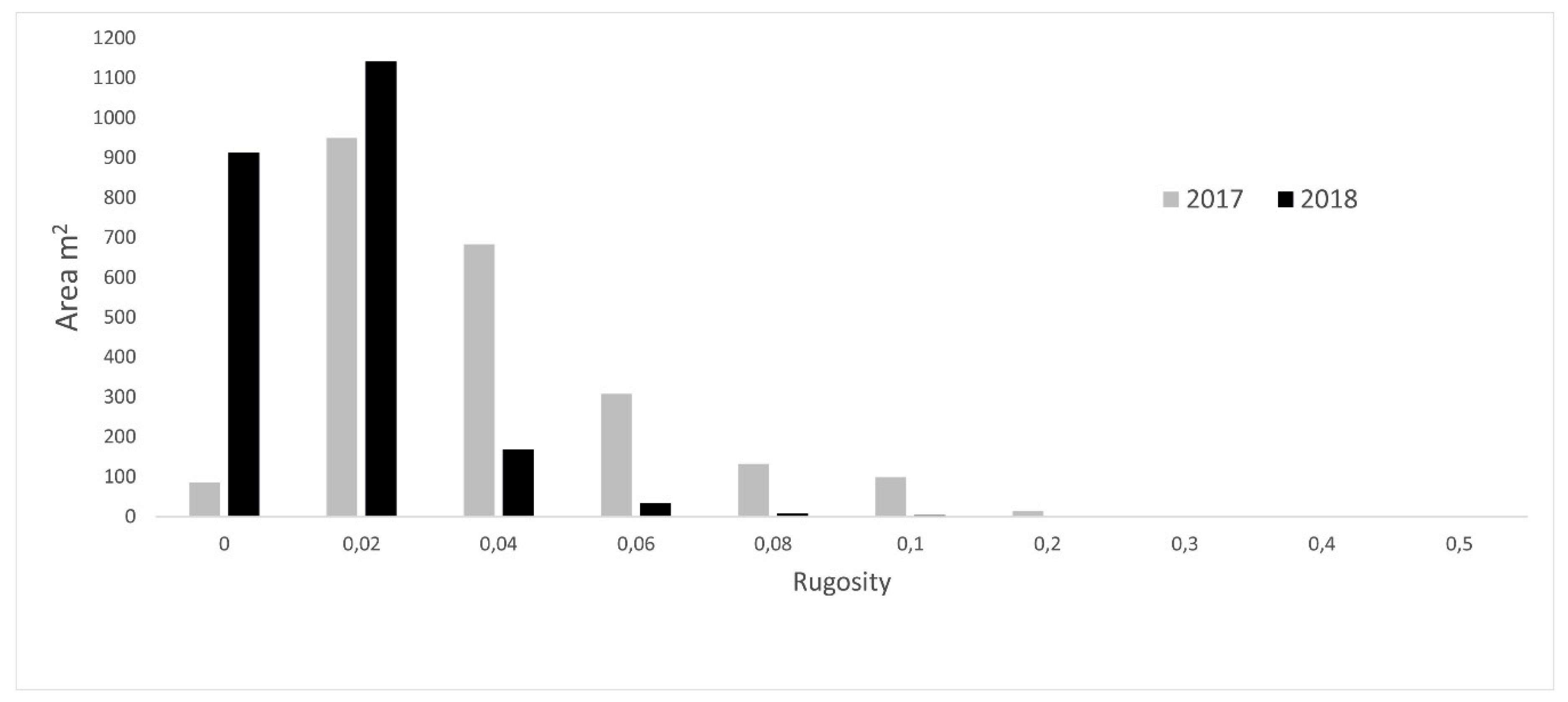
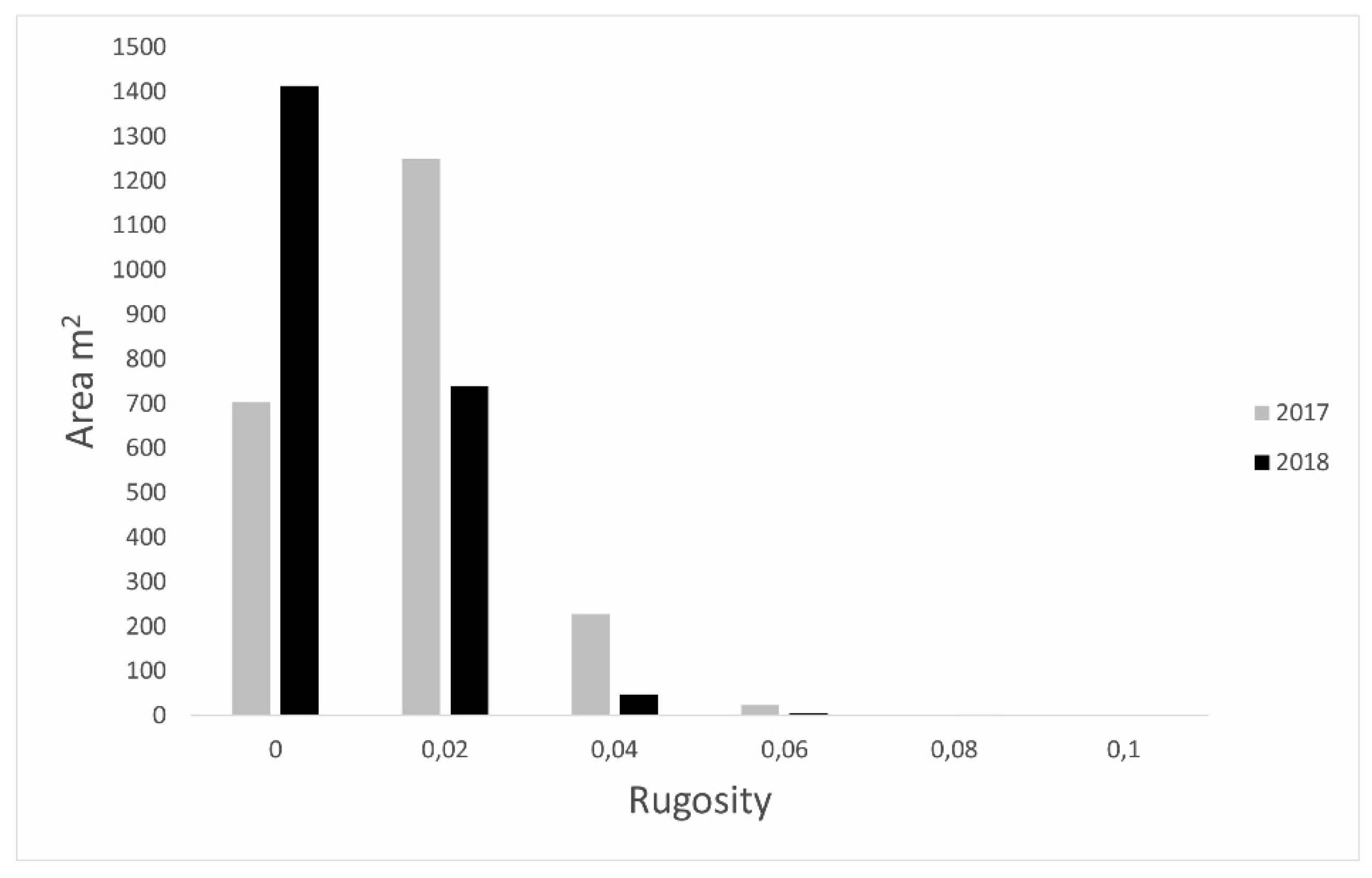
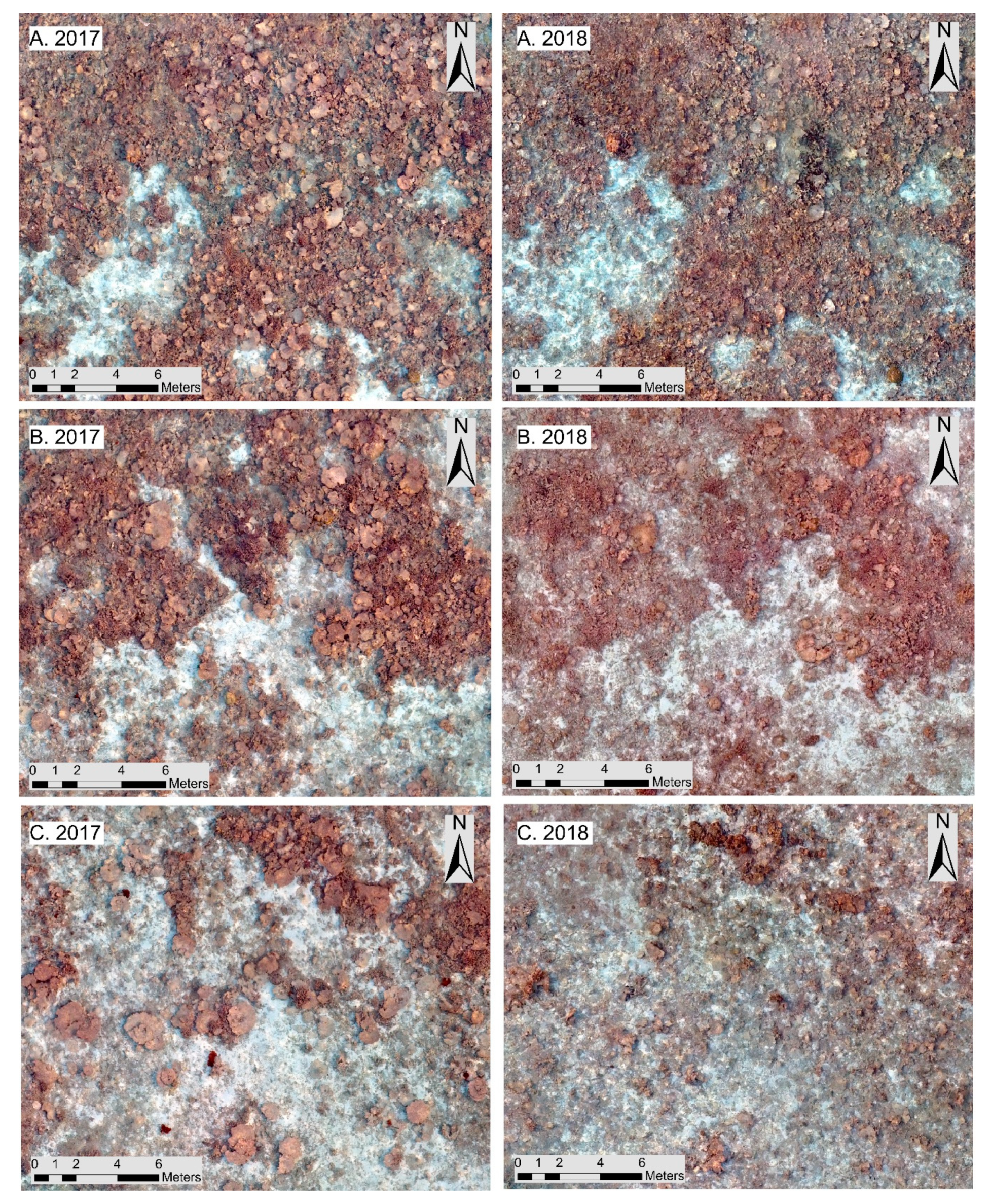
3.2. High-Resolution Orthomosaics, Substrate Type Maps and Structural Complexity Variation
4. Discussion
4.1. UAV Surveys and Image Processing
4.2. Classification and Map Comparison
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Workstation Configuration | |||
|---|---|---|---|
| Workstation | CPU | RAM Memory | GPU |
| Dell XPS 15” | Intel® Core™ i7-7700HQ CPU 2.80 GHz | 32 Gb DDR3 Ram | NVIDIA(R) GeForce(R) GTX 1050 Ti with 4 GB GDDR5 |
| DJI Phantom 4 Images Acquisition | SfM Processing in Agisoft Photoscan Pro | OBIA Workflow in eCognition 9.5 | Maps Comparison and DTM Processing in AgcGis 10.6 | Overall Processing Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aligment1 | DTM1 | Orthomosaic1 | Multiresolution Segmentation1 | Selecting Training Areas2 | Define Statistics2 | Nearest Neighbor Classification1 | Bentic Assemblages Maps Comparison2 | Structural Complesity Evaluation2 | |||||
| Processing Time for Area 1 | 21 min | 24 min | 122 min | 1.32 min | 5.20 min | 11.06 min | ≈ 16 min | ≈ 30 min | 23 min | ≈ 20 min | ≈ 30 min | ≈ 303 min | |
| Processing Time for Area 2 | 19 min | 19 min | 98 min | 1 min | 3.54 min | 8.34 min | ≈ 15 min | ≈ 30 min | 20 min | ≈ 20 min | ≈ 30 min | ≈ 263 min | |
| Processing Time for Area 3 | 11 min | 8 min | 26 min | 0.22 min | 2.05 min | 3.23 min | ≈ 10 min | ≈ 30 min | 15 min | ≈ 20 min | ≈ 30 min | ≈ 155 min | |
| Processing Time for Area 4 | 23 min | 25 min | 142 min | 2 min | 9 min | 28.13 min | ≈ 20 min | ≈ 30 min | 30 min | ≈ 20 min | ≈ 30 min | ≈ 360 min | |
References
- Perry, C.T.; Alvarez-Filip, L. Changing geo-ecological functions of coral reefs in the Anthropocene. Funct. Ecol. 2019, 33, 976–988. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Chase, T.J.; Dietzel, A.; Hill, T.; Hoey, A.S.; Hoogenboom, M.O.; Jacobson, M.; et al. Global warming impairs stock–recruitment dynamics of corals. Nature 2019, 568, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Fine, M.; Hoegh-Guldberg, O.; Meroz-Fine, E.; Dove, S. Ecological changes over 90 years at Low Isles on the Great Barrier Reef. Nat. Commun. 2019, 10, 4409. [Google Scholar] [CrossRef] [PubMed]
- Ateweberhan, M.; McClanahan, T.R. Relationship between historical sea-surface temperature variability and climate change-induced coral mortality in the western Indian Ocean. Mar. Pollut. Bull. 2010. [Google Scholar] [CrossRef]
- Cowburn, B.; Moritz, C.; Grimsditch, G.; Solandt, J. Evidence of coral bleaching avoidance, resistance and recovery in the Maldives during the 2016 mass-bleaching event. Mar. Ecol. Prog. Ser. 2019. [Google Scholar] [CrossRef]
- Harrison, H.B.; Álvarez-Noriega, M.; Baird, A.H.; Heron, S.F.; MacDonald, C.; Hughes, T.P. Back-to-back coral bleaching events on isolated atolls in the Coral Sea. Coral Reefs 2018. [Google Scholar] [CrossRef]
- Weiler, B.A.; Van Leeuwen, T.E.; Stump, K.L. The extent of coral bleaching, disease and mortality for data-deficient reefs in Eleuthera, The Bahamas after the 2014–2017 global bleaching event. Coral Reefs 2019. [Google Scholar] [CrossRef]
- Pisapia, C.; Burn, D.; Pratchett, M.S. Changes in the population and community structure of corals during recent disturbances (February 2016–October 2017) on Maldivian coral reefs. Sci. Rep. 2019, 9, 8402. [Google Scholar] [CrossRef]
- Magel, J.M.T.; Burns, J.H.R.; Gates, R.D.; Baum, J.K. Effects of bleaching-associated mass coral mortality on reef structural complexity across a gradient of local disturbance. Sci. Rep. 2019, 9, 2512. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Paddack, M.J.; Collen, B.; Robertson, D.R.; Côté, I.M. Simplification of Caribbean reef-fish assemblages over decades of coral reef degradation. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Nash, K.L. The importance of structural complexity in coral reef ecosystems. Coral Reefs 2013, 32, 315–326. [Google Scholar] [CrossRef]
- Wilson, S.K.; Graham, N.A.J.; Pratchett, M.S.; Jones, G.P.; Polunin, N.V.C. Multiple disturbances and the global degradation of coral reefs: Are reef fishes at risk or resilient? Glob. Chang. Biol. 2006. [Google Scholar] [CrossRef]
- Sheppard, C.R.C.; Spalding, M.; Bradshaw, C.; Wilson, S. Erosion vs. recovery of coral reefs after 1998 El Niño: Chagos reefs, Indian Ocean. AMBIO 2002. [Google Scholar] [CrossRef]
- Harris, D.L.; Rovere, A.; Casella, E.; Power, H.; Canavesio, R.; Collin, A.; Pomeroy, A.; Webster, J.M.; Parravicini, V. Coral reef structural complexity provides important coastal protection from waves under rising sea levels. Sci. Adv. 2018, 4, 1–7. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Wilson, S.K.; Jennings, S.; Polunin, N.V.C.; Bijoux, J.P.; Robinson, J. Dynamic fragility of oceanic coral reef ecosystems. Proc. Natl. Acad. Sci. USA 2006. [Google Scholar] [CrossRef]
- Andréfouët, S.; Riegl, B. Remote sensing: A key tool for interdisciplinary assessment of coral reef processes. Coral Reefs 2004, 23, 1–4. [Google Scholar] [CrossRef]
- Witze, A. Reefs mapped from above. Nature 2016, 534, 13–14. [Google Scholar] [CrossRef]
- Green, E.P.; Mumby, P.J.; Edwards, A.J.; Clark, C.D. A review of remote sensing for the assessment and management of tropical coastal resources. Coast. Manag. 1996. [Google Scholar] [CrossRef]
- Mumby, P.J.; Skirving, W.; Strong, A.E.; Hardy, J.T.; LeDrew, E.F.; Hochberg, E.J.; Stumpf, R.P.; David, L.T. Remote sensing of coral reefs and their physical environment. Mar. Pollut. Bull. 2004, 48, 219–228. [Google Scholar] [CrossRef]
- Hedley, J.D.; Roelfsema, C.; Brando, V.; Giardino, C.; Kutser, T.; Phinn, S.; Mumby, P.J.; Barrilero, O.; Laporte, J.; Koetz, B. Coral reef applications of Sentinel-2: Coverage, characteristics, bathymetry and benthic mapping with comparison to Landsat 8. Remote Sens. Environ. 2018, 216, 598–614. [Google Scholar] [CrossRef]
- Traganos, D.; Aggarwal, B.; Poursanidis, D.; Topouzelis, K.; Chrysoulakis, N.; Reinartz, P. Towards global-scale seagrass mapping and monitoring using Sentinel-2 on Google Earth Engine: The case study of the Aegean and Ionian Seas. Remote Sens. 2018, 10, 1227. [Google Scholar] [CrossRef]
- Li, J.; Schill, S.R.; Knapp, D.E.; Asner, G.P. Object-based mapping of coral reef habitats using planet dove satellites. Remote Sens. 2019, 11, 1445. [Google Scholar] [CrossRef]
- Wedding, L.M.; Friedlander, A.M.; McGranaghan, M.; Yost, R.S.; Monaco, M.E. Using bathymetric lidar to define nearshore benthic habitat complexity: Implications for management of reef fish assemblages in Hawaii. Remote Sens. Environ. 2008. [Google Scholar] [CrossRef]
- Casella, E.; Collin, A.; Harris, D.; Ferse, S.; Bejarano, S.; Parravicini, V.; Hench, J.L.; Rovere, A. Mapping coral reefs using consumer-grade drones and structure from motion photogrammetry techniques. Coral Reefs 2016. [Google Scholar] [CrossRef]
- Murfitt, S.L.; Allan, B.M.; Bellgrove, A.; Rattray, A.; Young, M.A.; Ierodiaconou, D. Applications of unmanned aerial vehicles in intertidal reef monitoring. Sci. Rep. 2017, 7, 10259. [Google Scholar] [CrossRef] [PubMed]
- Ventura, D.; Bonifazi, A.; Gravina, M.F.; Belluscio, A.; Ardizzone, G. Mapping and classification of ecologically sensitive marine habitats using unmanned aerial vehicle (UAV) imagery and Object-Based Image Analysis (OBIA). Remote Sens. 2018, 10, 1331. [Google Scholar] [CrossRef]
- Colomina, I.; Molina, P. Unmanned aerial systems for photogrammetry and remote sensing: A review. ISPRS J. Photogramm. Remote Sens. 2014, 92, 79–97. [Google Scholar] [CrossRef]
- Hamylton, S.M. Mapping coral reef environments: A review of historical methods, recent advances and future opportunities. Prog. Phys. Geogr. 2017, 41, 803–833. [Google Scholar] [CrossRef]
- Nowak, M.M.; Dziób, K.; Bogawski, P. Unmanned Aerial Vehicles (UAVs) in environmental biology: A review. Eur. J. Ecol. 2019, 4, 56–74. [Google Scholar] [CrossRef]
- Calders, K.; Phinn, S.; Ferrari, R.; Leon, J.; Armston, J.; Asner, G.P.; Disney, M. 3D Imaging Insights into Forests and Coral Reefs. Trends Ecol. Evol. 2020, 35, 6–9. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Wilson, S.K.; Pratchett, M.S.; Polunin, N.V.C.; Spalding, M.D. Coral mortality versus structural collapse as drivers of corallivorous butterflyfish decline. Biodivers. Conserv. 2009. [Google Scholar] [CrossRef]
- Risk, M.J. Fish Diversity on a Coral Reef in the Virgin Islands. Atoll Res. Bull. 1972. [Google Scholar] [CrossRef]
- Friedman, A.; Pizarro, O.; Williams, S.B.; Johnson-Roberson, M. Multi-Scale Measures of Rugosity, Slope and Aspect from Benthic Stereo Image Reconstructions. PLoS ONE 2012, 7, e50440. [Google Scholar] [CrossRef]
- Burns, J.; Delparte, D.; Gates, R.D.; Takabayashi, M. Integrating structure-from-motion photogrammetry with geospatial software as a novel technique for quantifying 3D ecological characteristics of coral reefs. PeerJ 2015. [Google Scholar] [CrossRef] [PubMed]
- Leon, J.X.X.; Roelfsema, C.M.; Saunders, M.I.; Phinn, S.R. Measuring coral reef terrain roughness using ‘Structure-from-Motion’ close-range photogrammetry. Geomorphology 2015, 242, 21–28. [Google Scholar] [CrossRef]
- Ferrari, R.; DElparte, D.; Kapono, L.; Belt, M.; Gates, R.D.; Takabayashi, M. Quantifying multiscale habitat structural complexity: A cost-effective framework for underwater 3D modelling. Remote Sens. 2016, 8, 113. [Google Scholar] [CrossRef]
- Storlazzi, C.D.; Dartnell, P.; Hatcher, G.A.; Gibbs, A.E. End of the chain? Rugosity and fine-scale bathymetry from existing underwater digital imagery using structure-from-motion (SfM) technology. Coral Reefs 2016. [Google Scholar] [CrossRef]
- Burns, J.H.R.; Delparte, D.; Kapono, L.; Belt, M.; Gates, R.D.; Takabayashi, M. Assessing the impact of acute disturbances on the structure and composition of a coral community using innovative 3D reconstruction techniques. Methods Oceanogr. 2016, 16, 49–59. [Google Scholar] [CrossRef]
- Couch, C.S.; Burns, J.H.R.; Liu, G.; Steward, K.; Gutlay, T.N.; Kenyon, J.; Eakin, C.M.; Kosaki, R.K. Mass coral bleaching due to unprecedented marine heatwave in Papahānaumokuākea Marine National Monument (Northwestern Hawaiian Islands). PLoS ONE 2017. [Google Scholar] [CrossRef]
- Ferrari, R.; Figueira, W.F.; Pratchett, M.S.; Boube, T.; Adam, A.; Kobelkowsky-Vidro, T.; Doo, S.S.; Atwood, T.B.; Byrne, M. 3D photogrammetry quantifies growth and external erosion of individual coral colonies and skeletons. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- Naseer, A.; Hatcher, B.G. Inventory of the Maldives? Coral reefs using morphometrics generated from Landsat ETM+ imagery. Coral Reefs 2004, 23, 161–168. [Google Scholar] [CrossRef]
- Fallati, L.; Savini, A.; Sterlacchini, S.; Galli, P. Land use and land cover (LULC) of the Republic of the Maldives: First national map and LULC change analysis using remote-sensing data. Environ. Monit. Assess. 2017, 189, 417. [Google Scholar] [CrossRef] [PubMed]
- Hicks, F. Ecosystem Services Assessment of North Ari Atoll Ecosystem Services Assessment of North Ari Atoll Tundi Agardy; IUCN: Gland, Switzerland, 2017. [Google Scholar]
- Pisapia, C.; Burn, D.; Yoosuf, R.; Najeeb, A.; Anderson, K.D.; Pratchett, M.S. Coral recovery in the central Maldives archipelago since the last major mass-bleaching, in 1998. Sci. Rep. 2016, 6, 34720. [Google Scholar] [CrossRef] [PubMed]
- Saponari, L.; Montalbetti, E.; Galli, P.; Strona, G.; Seveso, D.; Dehnert, I.; Montano, S. Monitoring and assessing a 2-year outbreak of the corallivorous seastar Acanthaster planci in Ari Atoll, Republic of Maldives. Environ. Monit. Assess. 2018. [Google Scholar] [CrossRef] [PubMed]
- Montalbetti, E.; Saponari, L.; Montano, S.; Maggioni, D.; Dehnert, I.; Galli, P.; Seveso, D. New insights into the ecology and corallivory of Culcita sp. (Echinodermata: Asteroidea) in the Republic of Maldives. Hydrobiologia 2019, 827, 353–365. [Google Scholar] [CrossRef]
- Bruckner, A.W.; Coward, G.; Bimson, K.; Rattanawongwan, T. Predation by feeding aggregations of Drupella spp. inhibits the recovery of reefs damaged by a mass bleaching event. Coral Reefs 2017. [Google Scholar] [CrossRef]
- Levy, J.; Hunter, C.; Lukacazyk, T.; Franklin, E.C. Assessing the spatial distribution of coral bleaching using small unmanned aerial systems. Coral Reefs 2018. [Google Scholar] [CrossRef]
- Doukari, M.; Batsaris, M.; Papakonstantinou, A.; Topouzelis, K. A Protocol for Aerial Survey in Coastal Areas Using UAS. Remote Sens. 2019, 11, 1913. [Google Scholar] [CrossRef]
- Montano, S.; Strona, G.; Seveso, D.; Galli, P. First report of coral diseases in the Republic of Maldives. Dis. Aquat. Organ. 2012. [Google Scholar] [CrossRef]
- Seveso, D.; Montano, S.; Strona, G.; Orlandi, I.; Galli, P.; Vai, M. The susceptibility of corals to thermal stress by analysing Hsp60 expression. Mar. Environ. Res. 2014. [Google Scholar] [CrossRef]
- Montano, S.; Fattorini, S.; Parravicini, V.; Berumen, M.L.; Galli, P.; Maggioni, D.; Arrigoni, R.; Seveso, D.; Strona, G. Corals hosting symbiotic hydrozoans are less susceptible to predation and disease. Proc. R. Soc. B Biol. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Saliu, F.; Montano, S.; Garavaglia, M.G.; Lasagni, M.; Seveso, D.; Galli, P. Microplastic and charred microplastic in the Faafu Atoll, Maldives. Mar. Pollut. Bull. 2018, 136, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.dji.com/it/ground-station-pro (accessed on 18 January 2017).
- Available online: www.agisoft.com (accessed on 22 March 2017).
- Benassi, F.; Dall’Asta, E.; Diotri, F.; Forlani, G.; Morra di Cella, U.; Roncella, R.; Santise, M. Testing accuracy and repeatability of UAV blocks oriented with gnss-supported aerial triangulation. Remote Sens. 2017, 9, 172. [Google Scholar] [CrossRef]
- Cook, K.L. An evaluation of the effectiveness of low-cost UAVs and structure from motion for geomorphic change detection. Geomorphology 2017, 278, 195–208. [Google Scholar] [CrossRef]
- Bonali, F.L.; Tibaldi, A.; Marchese, F.; Fallati, L.; Russo, E.; Corselli, C.; Savini, A. UAV-based surveying in volcano-tectonics: An example from the Iceland rift. J. Struct. Geol. 2019, 121, 46–64. [Google Scholar] [CrossRef]
- Verhoeven, G. Taking computer vision aloft—Archaeological three-dimensional reconstructions from aerial photographs with photoscan. Archaeol. Prospect. 2011, 18, 67–73. [Google Scholar] [CrossRef]
- Brunier, G.; Fleury, J.; Anthony, E.J.; Gardel, A.; Dussouillez, P. Close-range airborne Structure-from-Motion Photogrammetry for high-resolution beach morphometric surveys: Examples from an embayed rotating beach. Geomorphology 2016, 261, 76–88. [Google Scholar] [CrossRef]
- Available online: https://emlid.com/reachrs/ (accessed on 8 January 2017).
- Hill, A.C.; Limp, F.; Casana, J.; Laugier, E.J.; Williamson, M. A New Era in Spatial Data Recording: Low-Cost GNSS. Adv. Archaeol. Pract. 2019, 7, 169–177. [Google Scholar] [CrossRef]
- Available online: https://webapp.geod.nrcan.gc.ca/geod/tools-outils/ppp.php?locale=en (accessed on 22 November 2017).
- Available online: http://www.ecognition.com/suite/ecognition-developer (accessed on 3 April 2019).
- Roelfsema, C.; Kovacs, E.; Roos, P.; Terzano, D.; Lyons, M.; Phinn, S. Use of a semi-automated object based analysis to map benthic composition, Heron Reef, Southern Great Barrier Reef. Remote Sens. Lett. 2018, 9, 324–333. [Google Scholar] [CrossRef]
- Liu, D.; Xia, F. Assessing object-based classification: Advantages and limitations. Remote Sens. Lett. 2010, 1, 187–194. [Google Scholar] [CrossRef]
- Naylor, A.K. Island morphology, reef resources, and development paths in the Maldives. Prog. Phys. Geogr. 2015, 39, 728–749. [Google Scholar] [CrossRef]
- Sappington, J.M.; Longshore, K.M.; Thompson, D.B. Quantifying Landscape Ruggedness for Animal Habitat Analysis: A Case Study Using Bighorn Sheep in the Mojave Desert. J. Wildl. Manag. 2007, 71, 1419–1426. [Google Scholar] [CrossRef]
- Price, D.M.; Robert, K.; Callaway, A.; Lo Iacono, C.; Hall, R.A.; Huvenne, A.A.I. Using 3D photogrammetry from ROV video to quantify cold-water coral reef structural complexity and investigate its influence on biodiversity and community assemblage. Coral Reefs 2019. [Google Scholar] [CrossRef]
- Anelli, M.; Juilla, T.; Fallati, L.; Galli, P.; Rossini, M.; Colombo, R. Towards new applications of underwater photogrammetry for investigating coral reef morphology and habitat complexity in the Myeik Archipelago, Myanmar. Geocarto Int. 2017, 6049, 459–472. [Google Scholar] [CrossRef]
- Kattenborn, T.; Lopatin, J.; Förster, M.; Braun, A.C.; Fassnacht, F.E. UAV data as alternative to field sampling to map woody invasive species based on combined Sentinel-1 and Sentinel-2 data. Remote Sens. Environ. 2019, 227, 61–73. [Google Scholar] [CrossRef]
- Congalton, R.G.; Green, K. Assessing the Accuracy of Remotely Sensed Data. Assess. Accuracy Remote. Sensed Data 2008. [Google Scholar] [CrossRef]
- Fallati, L.; Polidori, A.; Salvatore, C.; Saponari, L.; Savini, A.; Galli, P. Anthropogenic Marine Debris assessment with Unmanned Aerial Vehicle imagery and deep learning: A case study along the beaches of the Republic of Maldives. Sci. Total Environ. 2019, 133581. [Google Scholar] [CrossRef]
- Martin, C.; Parkes, S.; Zhang, Q.; Zhang, X.; McCabe, M.F.; Duarte, C.M. Use of unmanned aerial vehicles for efficient beach litter monitoring. Mar. Pollut. Bull. 2018, 131, 662–673. [Google Scholar] [CrossRef]
- Ventura, D.; Bruno, M.; Jona Lasinio, G.; Belluscio, A.; Ardizzone, G. A low-cost drone based application for identifying and mapping of coastal fish nursery grounds. Estuar. Coast. Shelf Sci. 2016, 171, 85–98. [Google Scholar] [CrossRef]
- Papakonstantinou, A.; Topouzelis, K.; Pavlogeorgatos, G. Coastline Zones Identification and 3D Coastal Mapping Using UAV Spatial Data. ISPRS Int. J. Geo-Inf. 2016, 5, 75. [Google Scholar] [CrossRef]
- Kiszka, J.J.; Mourier, J.; Gastrich, K.; Heithaus, M.R. Using unmanned aerial vehicles (UAVs) to investigate shark and ray densities in a shallow coral lagoon. Mar. Ecol. Prog. Ser. 2016, 560, 237–242. [Google Scholar] [CrossRef]
- Colefax, A.P.; Butcher, P.A.; Kelaher, B.P. The potential for unmanned aerial vehicles (UAVs) to conduct marine fauna surveys in place of manned aircraft. ICES J. Mar. Sci. 2018, 75, 1–8. [Google Scholar] [CrossRef]
- Raoult, V.; Tosetto, L.; Williamson, J. Drone-Based High-Resolution Tracking of Aquatic Vertebrates. Drones 2018, 2, 37. [Google Scholar] [CrossRef]
- Lowe, M.K.; Adnan, F.A.F.; Hamylton, S.M.; Carvalho, R.C.; Woodroffe, C.D. Assessing Reef-Island Shoreline Change Using UAV-Derived Orthomosaics and Digital Surface Models. Drones 2019, 3, 44. [Google Scholar] [CrossRef]
- Laporte-Fauret, Q.; Marieu, V.; Castelle, B.; Michalet, R.; Bujan, S.; Rosebery, D. Low-Cost UAV for high-resolution and large-scale coastal dune change monitoring using photogrammetry. J. Mar. Sci. Eng. 2019, 7, 63. [Google Scholar] [CrossRef]
- Tibaldi, A.; Bonali, F.L.; Russo, E.; Fallati, L. Surface deformation and strike-slip faulting controlled by dyking and host rock lithology: A compendium from the Krafla Rift, Iceland. J. Volcanol. Geotherm. Res. 2020, 395, 106835. [Google Scholar] [CrossRef]
- Baron, J.; Hill, D.J.; Elmiligi, H. Combining image processing and machine learning to identify invasive plants in high-resolution images. Int. J. Remote Sens. 2018, 39, 5099–5118. [Google Scholar] [CrossRef]
- Parsons, M.; Bratanov, D.; Gaston, K.J.; Gonzalez, F. UAVs, hyperspectral remote sensing, and machine learning revolutionising reef monitoring. Sensors 2018, 18, 2026. [Google Scholar] [CrossRef]
- Collin, A.; Dubois, S.; James, D.; Houet, T. Improving Intertidal Reef Mapping Using UAV Surface, Red Edge, and Near-Infrared Data. Drones 2019, 3, 67. [Google Scholar] [CrossRef]
- Hedley, J.; Roelfsema, C.; Koetz, B.; Phinn, S. Capability of the Sentinel 2 mission for tropical coral reef mapping and coral bleaching detection. Remote Sens. Environ. 2012, 120, 145–155. [Google Scholar] [CrossRef]
- Roelfsema, C.; Phinn, S.; Jupiter, S.; Comley, J.; Albert, S. Mapping coral reefs at reef to reef-system scales, 10s-1000s km2, using object-based image analysis. Int. J. Remote Sens. 2013. [Google Scholar] [CrossRef]
- Roelfsema, C.; Kovacs, E.; Ortiz, J.C.; Wolff, N.H.; Callaghan, D.; Wettle, M.; Ronan, M.; Hamylton, S.M.; Mumby, P.J.; Phinn, S. Coral reef habitat mapping: A combination of object-based image analysis and ecological modelling. Remote Sens. Environ. 2018, 208, 27–41. [Google Scholar] [CrossRef]
- Kalacska, M.; Lucanus, O.; Sousa, L.; Vieira, T.; Arroyo-Mora, J. Freshwater Fish Habitat Complexity Mapping Using Above and Underwater Structure-From-Motion Photogrammetry. Remote Sens. 2018, 10, 1912. [Google Scholar] [CrossRef]
- Blaschke, T. Object based image analysis for remote sensing. ISPRS J. Photogramm. Remote Sens. 2010, 65, 2–16. [Google Scholar] [CrossRef]
- Marine Research Center. Status of Coral Bleaching in the Maldives; Marine Research Center: Malé, Maldives, 2016; pp. 47 pages. [Google Scholar]
- Saponari, L.; Dehnert, I.; Galli, P.; Montano, S. Assessing population collapse of Drupella spp. (Mollusca: Gastropoda) in the shallow reef after a catastrophic bleaching event in the Republic of Maldives. Under Rev. 2020. [Google Scholar]
- Perry, C.T.; Morgan, K.M. Bleaching drives collapse in reef carbonate budgets and reef growth potential on southern Maldives reefs. Sci. Rep. 2017, 7, 40581. [Google Scholar] [CrossRef]
- Roth, F.; Saalmann, F.; Thomson, T.; Coker, D.J.; Villalobos, R.; Jones, B.H.; Wild, C.; Carvalho, S. Coral reef degradation affects the potential for reef recovery after disturbance. Mar. Environ. Res. 2018, 142, 48–58. [Google Scholar] [CrossRef]
- Newman, S.P.; Meesters, E.H.; Dryden, C.S.; Williams, S.M.; Sanchez, C.; Mumby, P.J.; Polunin, N.V.C. Reef flattening effects on total richness and species responses in the Caribbean. J. Anim. Ecol. 2015, 84, 1678–1689. [Google Scholar] [CrossRef]
- James, R.M.; Robson, S. Mitigating systematic error in topographic models derived from UAV and ground-based image networks. Earth Surf. Process. Landf. 2014, 39, 1413–1420. [Google Scholar] [CrossRef]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Barid, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef]
- Gilmour, J.P.; Smith, L.D.; Heyward, A.J.; Baird, A.H.; Pratchett, M.S. Recovery of an isolated coral reef system following severe disturbance. Science 2013. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.A.J.; Nash, K.L.; Kool, J.T. Coral reef recovery dynamics in a changing world. Coral Reefs 2011. [Google Scholar] [CrossRef]
- Aslam, M.; Kench, P.S. Reef island dynamics and mechanisms of change in Huvadhoo Atoll, Republic of Maldives, Indian Ocean. Anthropocene 2017, 18, 57–68. [Google Scholar] [CrossRef]



| 2017 | 2018 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AREA 1 | AREA 2 | AREA 3 | AREA 4 | AREA 1 | AREA 2 | AREA 3 | AREA 4 | ||
| UAV Images Acquisition | Flying Date | 11-Feb | 12-Feb | 05-Feb | 18-Feb | 04-Nov | 14-Oct | 05-Nov | |
| Flying Altitude | 35 m | 35 m | |||||||
| Frontal Overlap | 85% | 85% | |||||||
| Lateral Overlap | 75% | 75% | |||||||
| Speed | 2.2 m/s | 2.2 m/s | |||||||
| Shutter Interval | 2.0 s | 2.0 s | |||||||
| Image Resolution | 1.5 cm/px | 1.5 cm/px | |||||||
| Covered Area | 4.98 Ha | 4.46 Ha | 1.20 Ha | 5.37 Ha | 5.52 Ha | 4.38 Ha | 1.49 Ha | 5.39 Ha | |
| Images Numbers | 495 | 431 | 123 | 662 | 550 | 425 | 186 | 664 | |
| SfM Processing | Alignment and Dense Cloud processing accuracy | High | High | ||||||
| Dense Cloud Points | 87,138,680 | 56,885,927 | 12,412,455 | 96,498,907 | 89,836,998 | 54,737,644 | 15,038,040 | 96,149,457 | |
| DTM Resolution cm/pix | 2.94 | 3.16 | 2.97 | 2.86 | 3.08 | 3.17 | 3.1 | 2.9 | |
| Ortomosaic Resolution cm/pix | 1.49 | 1.58 | 1.43 | 1.36 | 1.54 | 1.58 | 1.55 | 1.45 | |
| Accuracy Table | |||||||
|---|---|---|---|---|---|---|---|
| Control Reference | |||||||
| Benthic maps | Hard Coral | Coral Rubble | Sand | Total | User Accuracy (%) | Producer Accuracy (%) | |
| Hard Coral | 349 | 39 | 21 | 409 | 85 | 86 | |
| Coral Rubble | 52 | 169 | 35 | 256 | 66 | 78 | |
| Sand | 2 | 7 | 58 | 67 | 86 | 51 | |
| Total | 403 | 215 | 114 | 732 | |||
| Overall Accuracy | Kappa Index | ||||||
| 79% | 0.56 | ||||||
| Linear Distance (m) | True Distance (m) | Linear Rugosity | Average Slope | |
|---|---|---|---|---|
| T.1 2017 | 47,395 | 49,422 | 1042 | 23,878 |
| T.1 2018 | 47,395 | 48,189 | 1016 | 13,941 |
| T.2 2017 | 36,682 | 38,014 | 1036 | 21,763 |
| T.2 2018 | 36,682 | 37,219 | 1014 | 12,939 |
| T.3 2017 | 37,083 | 38,406 | 1035 | 21,407 |
| T.3 2018 | 37,083 | 37,684 | 1015 | 13,756 |
| Linear Distance (m) | True Distance (m) | Linear Rugosity | Average Slope | |
|---|---|---|---|---|
| T.1 2017 | 47,705 | 48,057 | 1007 | 9647 |
| T.1 2018 | 47,705 | 47,809 | 1002 | 4908 |
| T.2 2017 | 47,705 | 48,004 | 1006 | 8815 |
| T.2 2018 | 47,705 | 47,801 | 1002 | 5078 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallati, L.; Saponari, L.; Savini, A.; Marchese, F.; Corselli, C.; Galli, P. Multi-Temporal UAV Data and Object-Based Image Analysis (OBIA) for Estimation of Substrate Changes in a Post-Bleaching Scenario on a Maldivian Reef. Remote Sens. 2020, 12, 2093. https://doi.org/10.3390/rs12132093
Fallati L, Saponari L, Savini A, Marchese F, Corselli C, Galli P. Multi-Temporal UAV Data and Object-Based Image Analysis (OBIA) for Estimation of Substrate Changes in a Post-Bleaching Scenario on a Maldivian Reef. Remote Sensing. 2020; 12(13):2093. https://doi.org/10.3390/rs12132093
Chicago/Turabian StyleFallati, Luca, Luca Saponari, Alessandra Savini, Fabio Marchese, Cesare Corselli, and Paolo Galli. 2020. "Multi-Temporal UAV Data and Object-Based Image Analysis (OBIA) for Estimation of Substrate Changes in a Post-Bleaching Scenario on a Maldivian Reef" Remote Sensing 12, no. 13: 2093. https://doi.org/10.3390/rs12132093
APA StyleFallati, L., Saponari, L., Savini, A., Marchese, F., Corselli, C., & Galli, P. (2020). Multi-Temporal UAV Data and Object-Based Image Analysis (OBIA) for Estimation of Substrate Changes in a Post-Bleaching Scenario on a Maldivian Reef. Remote Sensing, 12(13), 2093. https://doi.org/10.3390/rs12132093










