Testing Proximal Optical Sensors on Quinoa Growth and Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant Sampling and Weather Measurements
2.3. Proximal Optical Sensing
2.4. Data Analysis
3. Results
3.1. Soil and Weather Conditions
3.2. Proximal Optical Sensor Readings
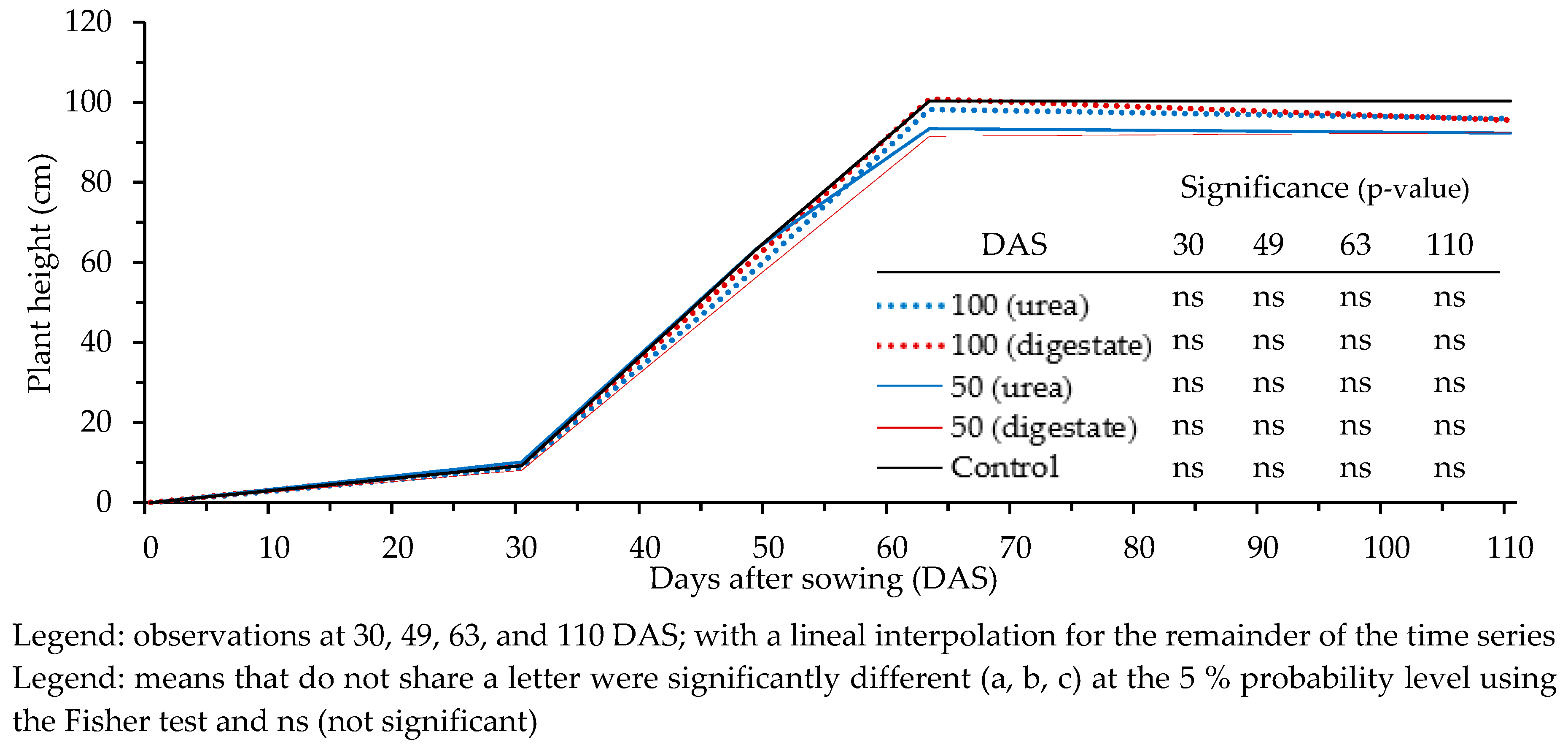
3.3. Effect of N Fertilization on Crop Growth and Development
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ogungbenle, H.N. Nutritional evaluation and functional properties of quinoa (Chenopodium quinoa) flour. Int. J. Food Sci. Nutr. 2003, 54, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. The Future of Food and Agriculture—Trends and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Pe’er, G.; Zinngrebe, Y.; Moreira, F.; Sirami, C.; Schindler, S.; Müller, R.; Hansjürgens, B. A greener path for the EU Common Agricultural Policy. Science 2019, 365, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Kanter, D.R.; Zhang, X.; Mauzerall, D.L. Reducing nitrogen pollution while decreasing farmers’ costs and increasing fertilizer industry profits. J. Environ. Qual. 2015, 44, 325–335. [Google Scholar] [CrossRef]
- Rütting, T.; Aronsson, H.; Delin, S. Efficient use of nitrogen in agriculture. Nutr. Cycl. Agroecosyst. 2018, 110, 1–5. [Google Scholar] [CrossRef] [Green Version]
- McBratney, A.; Whelan, B.; Ancev, T.; Bouma, J. Future directions of precision agriculture. Precis. Agric. 2005, 6, 7–23. [Google Scholar] [CrossRef]
- Ali, A.M.; Ibrahim, S.M. Wheat seed yield and nitrogen uptake prediction using at Leaf and GreenSeeker portable optical sensors at jointing growth stage. Inf. Process. Agric. 2019, in press. [Google Scholar]
- Chetan, H.T.; Potdar, M.P. Yield prediction models in maize using SPAD and NDVI. Res. Environ. Life Sci. 2016, 9, 1002–1004. [Google Scholar]
- Franzen, D.; Kitchen, N.; Holland, K.; Schepers, J.; Raun, W. Algorithms for in-season nutrient management in cereals. Agron. J. 2016, 108, 1775–1781. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, J.L.; Zarco-Tejada, P.J.; López-Herrera, P.J.; Pérez-Martín, E.; Alonso-Ayuso, M.; Quemada, M. Airborne and ground level sensors for monitoring nitrogen status in a maize crop. Biosyst. Eng. 2017, 160, 124–133. [Google Scholar] [CrossRef]
- Sharma, L.K.; Bali, S.K. A review of methods to improve nitrogen use efficiency in agriculture. Sustainability 2018, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- Povh, F.P.; dos Anjos, W.D.P.G. Optical sensors applied in agricultural crops. Opt. Sens.-New Dev. Pract. Appl. 2014, 141–163. [Google Scholar] [CrossRef] [Green Version]
- Colaço, A.F.; Bramley, R.G. Do crop sensors promote improved nitrogen management in seed crops? Field Crop. Res. 2018, 218, 126–140. [Google Scholar] [CrossRef]
- Ravier, C.; Quemada, M.; Jeuffroy, M.H. Use of a chlorophyll meter to assess nitrogen nutrition index during the growth cycle in winter wheat. Field Crop. Res. 2017, 214, 73–82. [Google Scholar] [CrossRef]
- Ortuzar-Iragorri, M.A.; Aizpurua, A.; Castellón, A.; Alonso, A.; Estavillo, J.M.; Besga, G. Use of an N-tester chlorophyll meter to tune a late third nitrogen application to wheat under humid Mediterranean conditions. J. Plant Nutr. 2018, 41, 627–635. [Google Scholar] [CrossRef]
- Bijay-Singh, V.S.; Yadvinder-Singh, A.K.; Sharma, S.; Thind, H.S.; Choudhary, O.P.; Vashistha, M. Site-specific Fertilizer Nitrogen Management in Irrigated Wheat using Chlorophyll Meter (SPAD meter) in the North-western India. J. Indian Soc. Soil Sci. 2018, 66, 53–65. [Google Scholar] [CrossRef]
- Yue, X.; Hu, Y.; Zhang, H.; Schmidhalter, U. Evaluation of Both SPAD Reading and SPAD Index on Estimating the Plant Nitrogen Status of Winter Wheat. Int. J. Plant Prod. 2020, 14, 67–75. [Google Scholar] [CrossRef]
- Ling, Q.; Huang, W.; Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 2011, 107, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Padilla, F.M.; Gallardo, M.; Peña-Fleitas, M.T.; De Souza, R.; Thompson, R.B. Proximal optical sensors for nitrogen management of vegetable crops: A review. Sensors 2018, 18, 2083. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Thind, H.S. A framework for refining nitrogen management in dry direct-seeded rice using GreenSeeker™ optical sensor. Comput. Electron. Agric. 2015, 110, 114–120. [Google Scholar] [CrossRef]
- Govaerts, B.; Verhulst, N. The Normalized Difference Vegetation Index (NDVI) GreenseekerTM Handheld Sensor: Toward the Integrated Evaluation of Crop Management Part A: Concepts and Case Studies; International Maize and Wheat Improvement Center (CIMMYT): Texcoco, Mexico, 2010. [Google Scholar]
- Stone, M.L.; Solie, J.B.; Whitney, R.W.; Raun, W.R.; Lees, H.L. Sensors for Detection of Nitrogen in Winter Wheat (No. 961757); SAE Technical Paper; Oklahoma State University: Stillwater, OK, USA, 1996. [Google Scholar]
- Solie, J.B.; Raun, W.R.; Whitney, R.W.; Stone, M.L.; Ringer, J.D. Optical sensor-based field element size and sensing strategy for nitrogen application. Trans. ASAE 1996, 39, 1983–1992. [Google Scholar] [CrossRef]
- Inman, D.; Khosla, R.; Reich, R.M.; Westfall, D.G. Active remote sensing and seed yield in irrigated maize. Precis. Agric. 2007, 8, 241–252. [Google Scholar] [CrossRef]
- Marti, J.; Bort, J.; Slafer, G.A.; Araus, J.L. Can wheat yield be assessed by early measurements of Normalized Difference Vegetation Index? Ann. Appl. Biol. 2007, 150, 253–257. [Google Scholar] [CrossRef]
- Li, F.; Mistele, B.; Hu, Y.; Chen, X.; Schmidhalter, U. Reflectance estimation of canopy nitrogen content in winter wheat using optimised hyperspectral spectral indices and partial least squares regression. Eur. J. Agron. 2014, 52, 198–209. [Google Scholar] [CrossRef]
- Thind, H.S.; Kumar, A.; Gupta, R.K.; Kaul, A.; Vashistha, M. Fixed-time adjustable dose site-specific fertilizer nitrogen management in transplanted irrigated rice (Oryza sativa L.) in South Asia. Field Crop. Res. 2012, 126, 63–69. [Google Scholar]
- Aranguren, M.; Castellón, A.; Aizpurua, A. Crop Sensor-Based In-Season Nitrogen Management of Wheat with Manure Application. Remote Sens. 2019, 11, 1094. [Google Scholar] [CrossRef] [Green Version]
- Quemada, M.; Pancorbo, J.L.; Alonso-Ayuso, M.; Gabriel, J.L.; López-Herrera, J.; Pérez-Martín, E. Vegetation indices from remote sensing imagery as proxies for yield and seed N in wheat. In Precision Agriculture’19; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; pp. 57–70. [Google Scholar]
- Wang, K.; Huggins, D.R.; Tao, H. Rapid mapping of winter wheat yield, protein, and nitrogen uptake using remote and proximal sensing. Int. J. Appl. Earth Obs. Geoinf. 2019, 82, 101921. [Google Scholar] [CrossRef]
- Teal, R.K.; Tubana, B.; Girma, K.; Freeman, K.W.; Arnall, D.B.; Walsh, O.; Raun, W.R. In-season prediction of corn seed yield potential using normalized difference vegetation index. Agron. J. 2006, 98, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Freeman, K.W.; Girma, K.; Arnall, D.B.; Mullen, R.W.; Martin, K.L.; Teal, R.K.; Raun, W.R. By-plant prediction of corn forage biomass and nitrogen uptake at various growth stages using remote sensing and plant height. Agron. J. 2007, 99, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Molin, J.P.; Frasson, F.R.; Amaral, L.R.; Povh, F.P.; Salvi, J.V. Capability of an optical sensor in verifying the sugarcane response to nitrogen rates. Rev. Bras. Eng. Agrícola Ambient. 2010, 14, 1345–1349. [Google Scholar] [CrossRef]
- Amaral, L.R.; Portz, G.; Rosa, H.J.A.; Molin, J.P. Use of active crop canopy reflectance sensor for nitrogen sugarcane fertilization. In Proceedings of the 11th International Conference on Precision Agriculture, Monticello, IL, USA, 15–18 July 2012; p. 15. [Google Scholar]
- Lofton, J.; Tubana, B.S.; Kanke, Y.; Teboh, J.; Viator, H.; Dalen, M. Estimating sugarcane yield potential using an in-season determination of normalized difference vegetative index. Sensors 2012, 12, 7529–7547. [Google Scholar] [CrossRef] [Green Version]
- Amaral, L.R.; Molin, J.P.; Portz, G.; Finazzi, F.B.; Cortinove, L. Comparison of crop canopy reflectance sensors used to identify sugarcane biomass and nitrogen status. Precis. Agric. 2015, 16, 15–28. [Google Scholar] [CrossRef]
- Schmidt, J.; Beegle, D.; Zhu, Q.; Sripada, R. Improving in-season nitrogen recommendations for maize using an active sensor. Field Crop. Res. 2011, 120, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Xue, L.; Li, G.; Qin, X.; Yang, L.; Zhang, H. Topdressing nitrogen recommendation for early rice with an active sensor in south China. Precis. Agric. 2014, 15, 95–110. [Google Scholar] [CrossRef]
- Bavec, F.; Bavec, M. Chlorophyll meter readings of winter wheat cultivars and seed yield prediction. Commun. Soil Sci. Plant Anal. 2001, 32, 2709–2719. [Google Scholar] [CrossRef]
- Vidal, I.; Longeri, L.; Hétier, J.M. Nitrogen uptake and chlorophyll meter measurements in spring wheat. Nutr. Cycl. Agroecosyst. 1999, 55, 1–6. [Google Scholar] [CrossRef]
- Shepherd, M.J.; Lindsey, L.E.; Lindsey, A.J. Soybean Canopy Cover Measured with Canopeo Compared with Light Interception. Agric. Environ. Lett. 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.S.; Choi, S.C.; Silva, R.R.; Kang, J.W.; Eom, J.H.; Kim, C. Case study: Estimation of sorghum biomass using digital image analysis with Canopeo. Biomass Bioenergy 2017, 105, 207–210. [Google Scholar] [CrossRef]
- Sosa-Zuniga, V.; Brito, V.; Fuentes, F.; Steinfort, U. Phenological growth stages of quinoa (Chenopodium quinoa) based on the BBCH scale. Ann. Appl. Biol. 2017, 171, 117–124. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Bach, A.P. The influence of temperature on seed germination rate in quinoa (Chenopodium quinoa Willd. Seed Sci. Technol. 1998, 26, 515–523. [Google Scholar]
- Jamieson, P.D.; Porter, J.R.; Wilson, D.R. A test of the computer simulation model ARCWHEAT1 on wheat crops grown in New Zealand. Field Crop. Res. 1991, 27, 337–350. [Google Scholar] [CrossRef]
- Cabrera-Bosquet, L.; Molero, G.; Stellacci, A.; Bort, J.; Nogues, S.; Araus, J. NDVI as a potential tool for predicting biomass, plant nitrogen content and growth in wheat genotypes subjected to different water and nitrogen conditions. Cereal Res. Commun. 2011, 39, 147–159. [Google Scholar] [CrossRef]
- Jacobsen, S.E. The worldwide potential for quinoa (Chenopodium quinoa Willd.). Food Rev. Int. 2003, 19, 167–177. [Google Scholar] [CrossRef]
- Parks, S.E.; Irving, D.E.; Milham, P.J. A critical evaluation of on-farm rapid tests for measuring nitrate in leafy vegetables. Sci. Hortic. 2012, 134, 1–6. [Google Scholar] [CrossRef]
- Zhu, J.; Tremblay, N.; Liang, Y. A corn nitrogen status indicator less affected by soil water content. Agron. J. 2011, 103, 890–898. [Google Scholar] [CrossRef]
- Holland, K.H.; Schepers, J.S. Use of a virtual-reference concept to interpret active crop canopy sensor data. Precis. Agric. 2013, 14, 71–85. [Google Scholar] [CrossRef]
- Shaver, T.M.; Khosla, R.; Westfall, D.G. Evaluation of two ground-based active crop canopy sensors in maize: Growth stage, row spacing, and sensor movement speed. Soil Sci. Soc. Am. J. 2010, 74, 2101–2108. [Google Scholar] [CrossRef]
- Ali, A.M.; Thind, H.S.; Sharma, S. Prediction of dry direct-seeded rice yields using chlorophyll meter, leaf color chart and GreenSeeker optical sensor in northwestern India. Field Crop. Res. 2014, 161, 11–15. [Google Scholar] [CrossRef]
- Sibley, A.M.; Grassini, P.; Thomas, N.E.; Cassman, K.G. Testing remote sensing approaches for assessing yield variability among maize fields. Agron. J. 2014, 106, 24–32. [Google Scholar] [CrossRef]
- Condorelli, G.E.; Maccaferri, M.; Newcomb, M.; Andrade-Sanchez, P.; White, J.W.; French, A.N.; Tuberosa, R. Comparative aerial and ground based high throughput phenotyping for the genetic dissection of NDVI as a proxy for drought adaptive traits in durum wheat. Front. Plant Sci. 2018, 9, 893. [Google Scholar] [CrossRef]
- Li, F.; Zhang, H.; Jia, L.; Bareth, G.; Miao, Y.; Chen, X. Estimating winter wheat biomass and nitrogen status using an active crop sensor. Intell. Autom. Soft Comput. 2010, 16, 1221–1230. [Google Scholar]
- Fabbri, C.; Napoli, M.; Mancini, M.; Brandani, G.; Vivoli, R.; Orlandini, S. Adopting precision agriculture to improve the cultivation of old wheat varieties in Tuscany (Italy). In Precision Agriculture’19; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; pp. 309–315. [Google Scholar]
- Montandon, L.M.; Small, E.E. The impact of soil reflectance on the quantification of the green vegetation fraction from NDVI. Remote Sens. Environ. 2008, 112, 1835–1845. [Google Scholar] [CrossRef]
- Hogrefe, K.R.; Patil, V.P.; Ruthrauff, D.R.; Meixell, B.W.; Budde, M.E.; Hupp, J.W.; Ward, D.H. Normalized Difference Vegetation Index as an estimator for abundance and quality of avian herbivore forage in Arctic Alaska. Remote Sens. 2017, 9, 1234. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Ge, X.; Shen, P.; Li, W.; Liu, X.; Cao, Q.; Tian, Y. Predicting Rice Seed Yield Based on Dynamic Changes in Vegetation Indexes during Early to Mid-Growth Stages. Remote Sens. 2019, 11, 387. [Google Scholar] [CrossRef] [Green Version]
- Schepers, J.S.; Blackmer, T.M.; Wilhelm, W.W.; Resende, M. Transmittance and reflectance measurements of cornleaves from plants with different nitrogen and water supply. J. Plant Physiol. 1996, 148, 523–529. [Google Scholar] [CrossRef] [Green Version]
- El-Shikha, D.M.; Waller, P.; Hunsaker, D.; Clarke, T.; Barnes, E. Ground-based remote sensing for assessing water and nitrogen status of broccoli. Agric. Water Manag. 2007, 92, 183–193. [Google Scholar] [CrossRef]
- Noulas, C. Chapter 6.1.6. In State of the Art Report on Quinoa around the World in 2013 Greece; Bazile, D., Bertero, H.D., Nieto, C., Eds.; FAO, CIRAD: Santiago du Chili, Chile, 2015; pp. 492–510. [Google Scholar]
- Alvar-Beltrán, J.; Dao, A.; Marta, A.D.; Saturnin, C.; Casini, P.; Sanou, J.; Orlandini, S. Effect of Drought, Nitrogen Fertilization, Temperature, and Photoperiodicity on Quinoa Plant Growth and Development in the Sahel. Agronomy 2019, 9, 607. [Google Scholar] [CrossRef] [Green Version]
- Serrano, L.; Filella, I.; Penuelas, J. Remote sensing of biomass and yield of winter wheat under different nitrogen supplies. Crop Sci. 2000, 40, 723–731. [Google Scholar] [CrossRef] [Green Version]
- Alvar-Beltrán, J.; Saturnin, C.; Dao, A.; Dalla Marta, A.; Sanou, J.; Orlandini, S. Effect of drought and nitrogen fertilisation on quinoa (Chenopodium quinoa Willd.) under field conditions in Burkina Faso. Ital. J. Agrometeorol. 2019, 33–43. [Google Scholar] [CrossRef]
- Pulvento, C.; Riccardi, M.; Lavini, A.; d’Andria, R.; Iafelice, G.; Marconi, E. Field trial evaluation of two chenopodium quinoa genotypes grown under rain-fed conditions in a typical Mediterranean environment in South Italy. J. Agron. Crop Sci. 2010, 196, 407–411. [Google Scholar] [CrossRef]

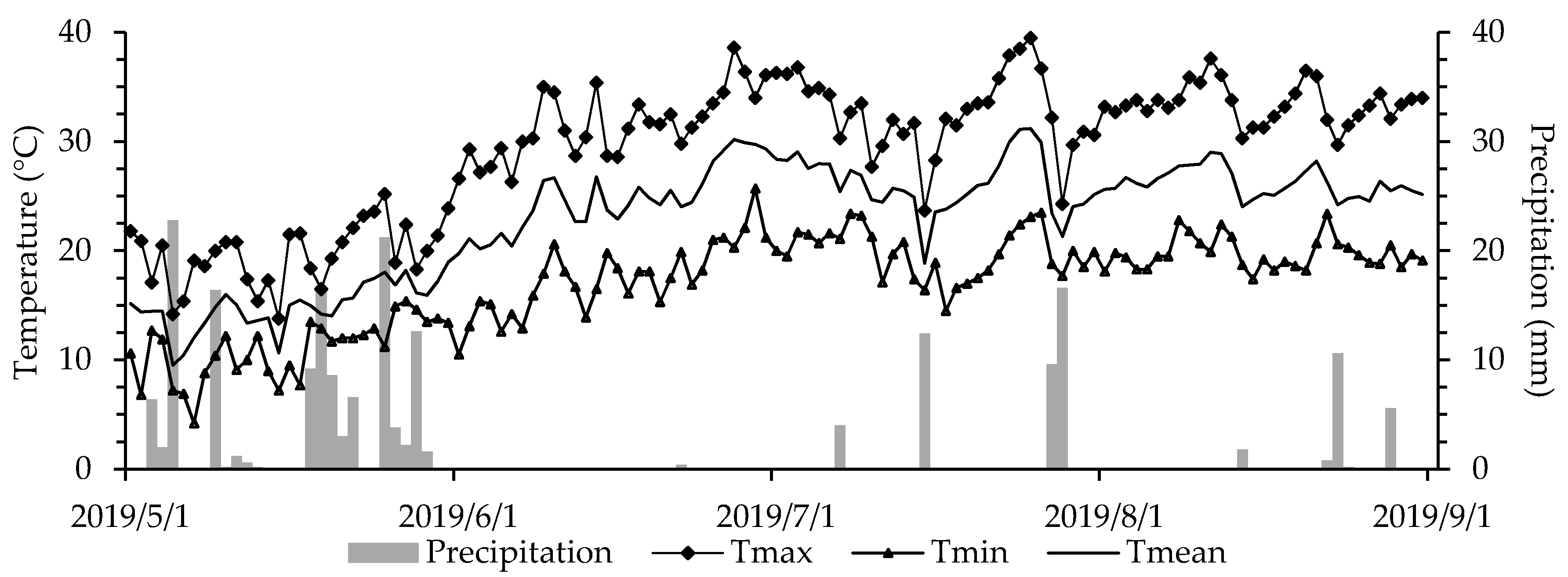
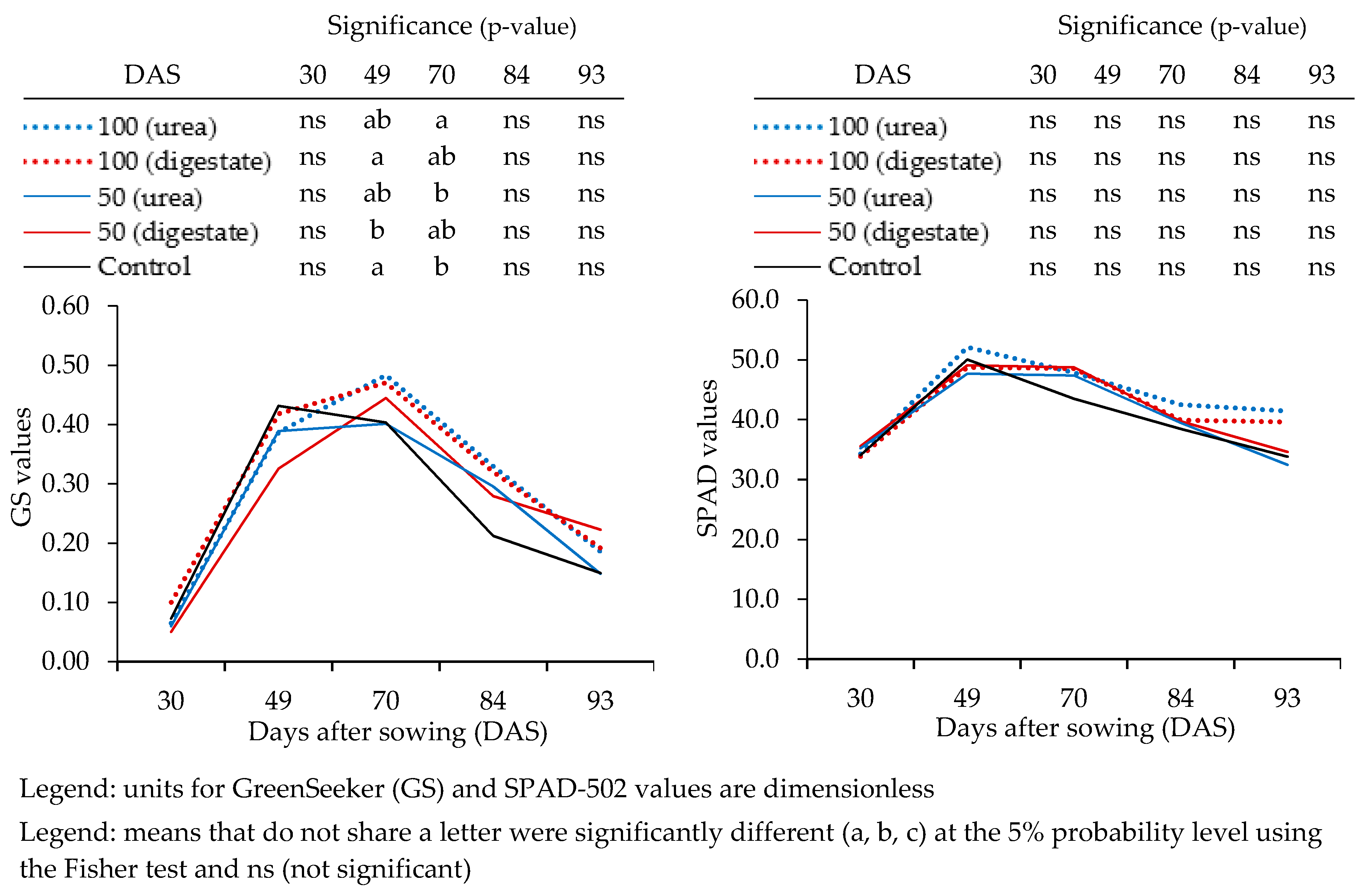
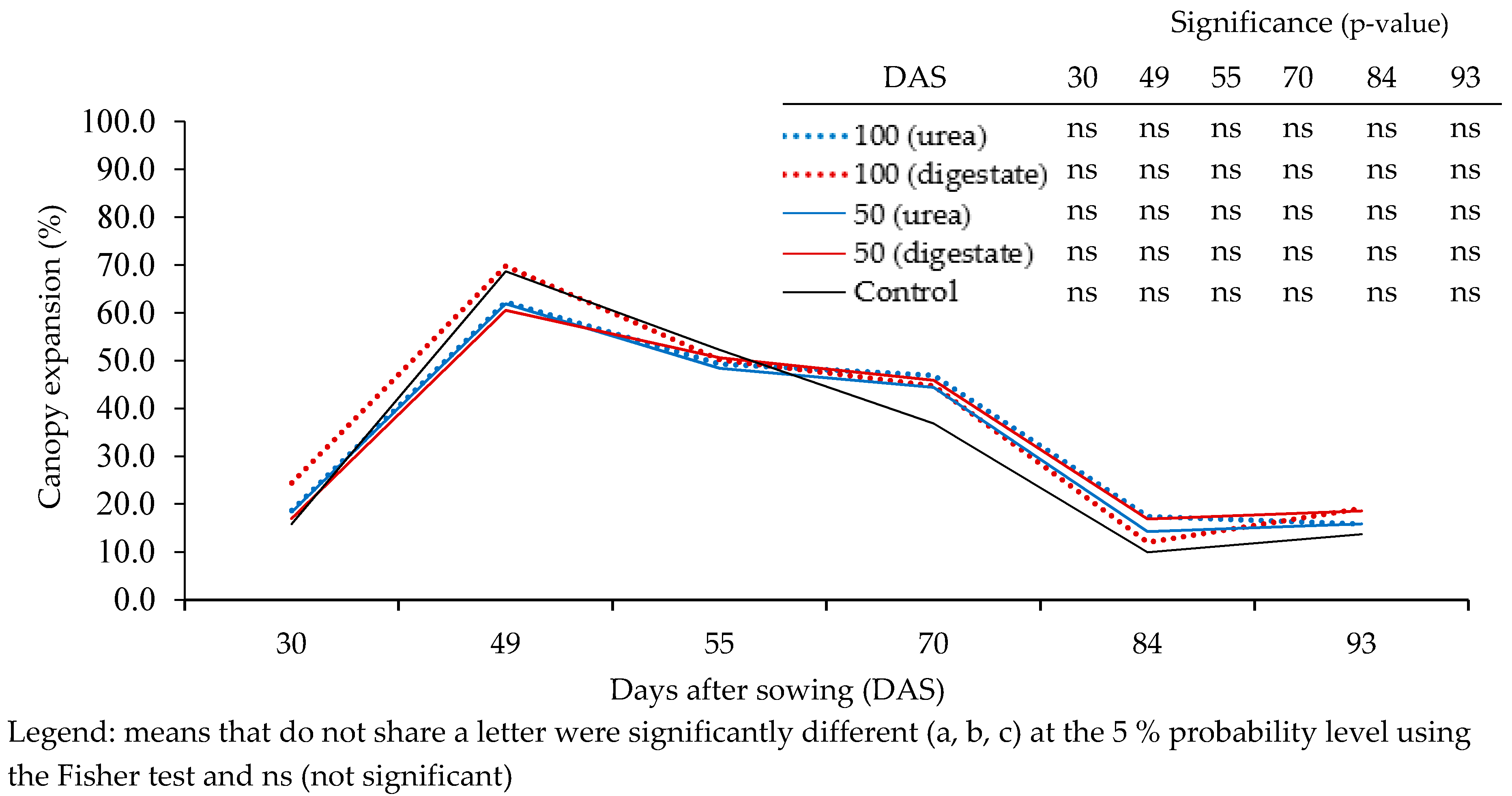

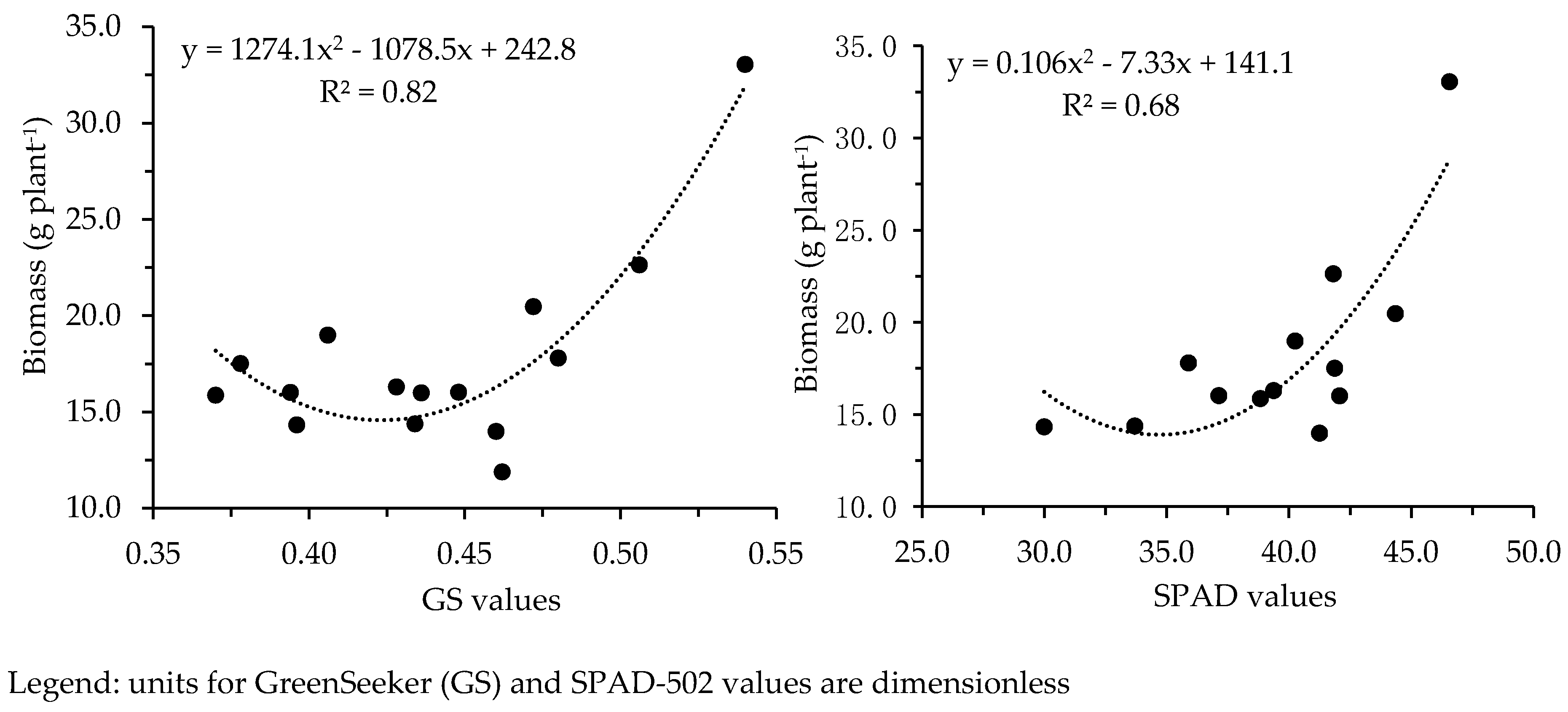
| Measurement Date (DAS) | CGDD | Agronomic Practices | Parameter(s), Devices and Number (#) of Samples Per Plot |
|---|---|---|---|
Before sowing 0 7 16 28 30 33 49 55 63 70 77 84 93 110 After harvesting | - - 76 189 377 414 482 833 991 1184 1330 1499 1658 1870 2263 | - Sowing date - - 1st fertilization Weeding - - 2nd fertilization Weeding - - - - - - Harvest - | Soil samples (#3) - BBCH-10 (#38) BBCH-11 (#38) BBCH-14 (#38) PH; NDVI and Canopy cover (#5); Chlorophyll (#25) BBCH-51 (#38) BBCH-60 (#38); PH; NDVI; Canopy cover (#5); Chlorophyll (#25) Canopy cover (#5) PH (#5) NDVI and Canopy cover (#5); Chlorophyll (#25) BBCH-81 (#38) NDVI and Canopy cover (#5); Chlorophyll (#25) NDVI and Canopy cover (#5); Chlorophyll (#25) BBCH-93 (#38); PH, BP, LP, WP, SD (#5) SYP (#38); TSW (#38) |
| Parameter | Unit | Characteristics |
|---|---|---|
| Sand Silt Clay Texture C N C/N | % % % % % | 56 29 15 Sandy-Loam 1.94 0.15 12.9 |
| SPAD vs. GreenSeeker | Canopeo vs. GreenSeeker | SPAD vs. Canopeo | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Reg. Line | R2 | Sig. | Reg. Line | R2 | Sig. | Reg. Line | R2 | Sig. |
| 100 (urea) | y = 35.8x + 33.3 | 0.77 | * | y = 90.5x + 6.0 | 0.50 | ns | y = 0.28x + 34.7 | 0.75 | * |
| 100 (digestate) | y = 41.2x + 29.5 | 0.90 | ** | y = 96.0x + 5.2 | 0.40 | ns | y = 0.21x + 34.8 | 0.62 | ns |
| 50 (urea) | y = 42.2x + 29.5 | 0.84 | ** | y = 105.1x + 3.8 | 0.56 | ns | y = 0.29x + 31.5 | 0.78 | * |
| 50 (digestate) | y = 39.3x + 31.1 | 0.66 | * | y = 92.8x + 7.2 | 0.44 | ns | y = 0.32x + 31.4 | 0.86 | ** |
| Control | y = 41.2x + 29.5 | 0.89 | ** | y = 134.5x − 4.8 | 0.70 | * | y = 0.25x + 32.6 | 0.87 | ** |
| Fertilizer Type | N Rate (kg N ha−1) | Yield (g Plant−1) | Biomass (g Plant−1) | 1000-Seed Weight (g) | Harvest Index (%) | Branches Plant (Number) | Panicle Length (cm) | Panicle Width (mm) | Stem Diameter (mm) |
|---|---|---|---|---|---|---|---|---|---|
| Digestate | 100 | 3.96 ± 0.69 | 19.16 ± 4.28 | 1.27 ± 0.15 | 20.9 ± 2.42 | 10.0 ± 2.42ab | 21.7 ± 2.44 | 43.5 ± 9.5 | 7.33 ± 1.21 |
| 50 | 3.75 ± 0.77 | 15.94 ± 1.91 | 1.37 ± 0.20 | 23.6 ± 3.46 | 9.8 ± 1.73ab | 19.7 ± 2.53 | 61.3. ± 17.5 | 7.60 ± 1.83 | |
| Urea | 100 | 4.47 ± 2.11 | 20.32 ± 11.22 | 1.39 ± 0.03 | 22.7 ± 2.22 | 11.7 ± 1.62a | 21.7 ± 1.50 | 56.0 ± 15.2 | 7.80 ± 1.83 |
| 50 | 4.26 ± 0.53 | 17.05 ± 1.69 | 1.32 ± 0.09 | 25.0 ± 3.07 | 10.3 ± 2.04ab | 22.7 ± 1.30 | 49.9 ± 2.0 | 8.47 ± 1.10 | |
| Control | 0 | 4.22 ± 0.63 | 15.94 ± 1.91 | 1.28 ± 0.00 | 22.4 ± 3.27 | 8.3 ± 0.50b | 20.9 ± 1.47 | 43.1 ± 6.91 | 6.93 ± 0.31 |
| Digestate | 3.86 ± 0.66 | 17.55 ± 3.45 | 1.32 ± 0.17 | 22.3 ± 3.40 | 9.9 ± 1.89ab | 20.7 ± 2.50 | 52.4 ± 15.9 | 7.47 ± 1.40 | |
| Urea | 4.37 ± 1.38 | 18.69 ± 7.40 | 1.36 ± 0.07 | 23.9 ± 2.72 | 11.0 ± 1.82a | 22.2 ± 1.37 | 52.9 ± 10.3 | 8.13 ± 1.40 | |
| Control | 4.22 ± 0.63 | 15.94 ± 1.59 | 1.28 ± 0.00 | 22.4 ± 3.27 | 8.3 ± 0.50b | 20.9 ± 1.47 | 43.1 ± 6.91 | 6.93 ± 0.31 | |
| 100 | 4.22 ± 1.43 | 19.74 ± 7.62 | 1.33 ± 0.12 | 21.8 ± 2.30 | 10.8 ± 2.06 | 21.7 ± 1.81 | 49.8 ± 13.2 | 7.57 ± 1.41 | |
| 50 | 4.00 ± 0.65 | 16.49 ± 1.72 | 1.35 ± 0.14 | 24.3 ± 3.40 | 10.0 ± 1.71 | 21.2 ± 2.46 | 55.6 ± 12.8 | 8.03 ± 1.43 | |
| 0 | 4.22 ± 0.63 | 15.94 ± 1.59 | 1.28 ± 0.00 | 22.4 ± 3.27 | 8.3 ± 0.50 | 20.9 ± 1.47 | 43.1 ± 6.91 | 6.93 ± 0.31 |
| POSs | Advantages | Disadvantages |
|---|---|---|
| SPAD-502 | Precise on measuring N content evolution. Accurate on predicting in-season biomass at harvest. | Not accurate on predicting in-season yields at harvest under heat-stress conditions. Small sample area. |
| GreenSeeker | Precise on measuring N content evolution Accurate on predicting in-season biomass at harvest. Significant canopy areas can be measured | Not accurate on predicting in-season yields at harvest under heat-stress conditions. |
| Canopeo App. | Precise on monitoring canopy cover expansion. | Frequent weeding required to obtain accurate results. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvar-Beltrán, J.; Fabbri, C.; Verdi, L.; Truschi, S.; Dalla Marta, A.; Orlandini, S. Testing Proximal Optical Sensors on Quinoa Growth and Development. Remote Sens. 2020, 12, 1958. https://doi.org/10.3390/rs12121958
Alvar-Beltrán J, Fabbri C, Verdi L, Truschi S, Dalla Marta A, Orlandini S. Testing Proximal Optical Sensors on Quinoa Growth and Development. Remote Sensing. 2020; 12(12):1958. https://doi.org/10.3390/rs12121958
Chicago/Turabian StyleAlvar-Beltrán, Jorge, Carolina Fabbri, Leonardo Verdi, Stefania Truschi, Anna Dalla Marta, and Simone Orlandini. 2020. "Testing Proximal Optical Sensors on Quinoa Growth and Development" Remote Sensing 12, no. 12: 1958. https://doi.org/10.3390/rs12121958
APA StyleAlvar-Beltrán, J., Fabbri, C., Verdi, L., Truschi, S., Dalla Marta, A., & Orlandini, S. (2020). Testing Proximal Optical Sensors on Quinoa Growth and Development. Remote Sensing, 12(12), 1958. https://doi.org/10.3390/rs12121958






