Spatial Patterns of Structural Complexity in Differently Managed and Unmanaged Beech-Dominated Forests in Central Europe
Abstract
1. Introduction
- (i)
- Do differently managed beech-dominated forests differ in the complexity of their lower, middle, and upper forest stratum?
- (ii)
- Are differences in the structural complexity of different forest strata characterized by differences in the density and distribution of plant material?
2. Materials and Methods
2.1. Study Sites
2.2. Terrestrial Laser Scanning and Sampling Design
2.3. Data Processing
2.4. Statistics
3. Results
3.1. Db of the Lower, Middle, and Upper Forest Stratum of Differently Managed and Unmanaged Beech-Dominated Stands
3.2. Spatial Patterns and Density of Vegetation in Differently Managed and Unmanaged Beech-Dominated Stands Within the Lower, Middle, and Upper Forest Strata
3.2.1. Lower Forest Stratum (≤33% of Relative Stand Height)
3.2.2. Middle Forest Stratum (34–66% of Rel. Stand Height)
3.2.3. Upper Forest Stratum (67–100% Rel. Stand Height)
4. Discussion
4.1. Spatial Patterns of Structural Complexity of the Lower, Middle, and Upper Stratum of Different Forest Types
4.2. Methodical Considerations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brang, P.; Spathelf, P.; Larsen, J.B.; Bauhus, J.; Boncina, A.; Chauvin, C.; Drössler, L.; García-Güemes, C.; Heiri, C.; Kerr, G.; et al. Suitability of close-to-nature silviculture for adapting temperate European forests to climate change. Forestry 2014, 87, 492–503. [Google Scholar] [CrossRef]
- Messier, C.C.; Puettmann, K.J.; Coates, K.D. Managing Forests as Complex Adaptive Systems: Building Resilience to the Challenge of Global Change; Routledge: Abigdon, UK, 2013; pp. 187–214. [Google Scholar]
- Puettmann, K.J.; Coates, K.D.; Messier, C.C. A Critique of Silviculture: Managing for Complexity; Island Press: Washington, DC, USA, 2009; pp. 107–108. [Google Scholar]
- Schütz, J.-P. Silvicultural tools to develop irregular and diverse forest structures. Forestry 2002, 75, 329–337. [Google Scholar] [CrossRef]
- Gadow, K.; Zhang, C.Y.; Wehenkel, C.; Pommerening, A.; Corral-Rivas, J.; Korol, M.; Myklush, S.; Hui, G.Y.; Kiviste, A.; Zhao, X.H. Forest structure and diversity. In Continuous Cover Forestry; Pukkala, T., von Gadow, K., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 23, pp. 29–83. [Google Scholar]
- Kint, V.; Robert, D.W.; Noël, L. Evaluation of sampling methods for the estimation of structural indices in forest stands. Ecol. Model. 2004, 180, 461–476. [Google Scholar] [CrossRef]
- Brang, P. Virgin forests as a knowledge source for central European silviculture: Reality or myth? For. Snow Landsc. Res. 2005, 79, 19–32. [Google Scholar]
- Gustafsson, L.; Baker, S.C.; Bauhus, J.; Beese, W.J.; Brodie, A.; Kouki, J.; Lindenmayer, D.B.; Lõhmus, A.; Pastur, G.M.; Messier, C.; et al. Retention Forestry to Maintain Multifunctional Forests: A World Perspective. BioScience 2012, 62, 633–645. [Google Scholar] [CrossRef]
- Pommerening, A. Approaches to quantifying forest structures. Forestry 2002, 75, 305–324. [Google Scholar] [CrossRef]
- Glatthorn, J.; Feldmann, E.; Pichler, V.; Hauck, M.; Leuschner, C. Biomass Stock and Productivity of Primeval and Production Beech Forests: Greater Canopy Structural Diversity Promotes Productivity. Ecosystems 2017, 110, 106. [Google Scholar] [CrossRef]
- Gough, C.M.; Atkins, J.W.; Fahey, R.T.; Hardiman, B.S. High rates of primary production in structurally complex forests. Ecology 2019, 100, e02864. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Uhl, E.; Dauber, E. Long-term stand dynamics of managed spruce–fir–beech mountain forests in Central Europe: Structure, productivity and regeneration success. Forestry 2015, 88, 407–428. [Google Scholar] [CrossRef]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Franklin, J.F.; van Pelt, R. Spatial Aspects of Structural Complexity in Old-Growth Forests. J. For. 2004, 102, 22–28. [Google Scholar] [CrossRef]
- Ehbrecht, M.; Schall, P.; Ammer, C.; Fischer, M.; Seidel, D. Effects of structural heterogeneity on the diurnal temperature range in temperate forest ecosystems. For. Ecol. Manag. 2019, 432, 860–867. [Google Scholar] [CrossRef]
- Parker, G.G.; Harmon, M.E.; Lefsky, M.A.; Chen, J.; van Pelt, R.; Weis, S.B.; Thomas, S.C.; Winner, W.E.; Shaw, D.C.; Frankling, J.F. Three-dimensional Structure of an Old-growth Pseudotsuga-Tsuga Canopy and Its Implications for Radiation Balance, Microclimate, and Gas Exchange. Ecosystems 2004, 7, 440–453. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötzschner, F.; Verkerk, P.J.; Bauhus, J.; Buchwald, E.; Chaskovsky, O.; et al. Where are Europe’s last primary forests? Divers. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef]
- Bauhus, J.; Puettmann, K.; Messier, C. Silviculture for old-growth attributes. For. Ecol. Manag. 2009, 258, 525–537. [Google Scholar] [CrossRef]
- Commarmot, B. Structures of virgin and managed beech forests in Uholka (Ukraine) and Sihlwald (Switzerland): A comparative study. For. Snow Landsc. Res. 2005, 79, 45–56. [Google Scholar]
- Kucbel, S.; Jaloviar, P.; Saniga, M.; Vencurik, J.; Klimaš, V. Canopy gaps in an old-growth fir-beech forest remnant of Western Carpathians. Eur. J. For. Res. 2010, 129, 249–259. [Google Scholar] [CrossRef]
- Trotsiuk, V.; Hobi, M.L.; Commarmot, B. Age structure and disturbance dynamics of the relic virgin beech forest Uholka (Ukrainian Carpathians). For. Ecol. Manag. 2012, 265, 181–190. [Google Scholar] [CrossRef]
- Zenner, E.K.; Hibbs, D.E. A new method for modeling the heterogeneity of forest structure. For. Ecol. Manag. 2000, 129, 75–87. [Google Scholar] [CrossRef]
- Witté, I.; Kneeshaw, D.; Messier, C. Do partial cuts create forest complexity? A new approach to measuring the complexity of forest patterns using photographs and the mean information gain. For. Chron. 2013, 89, 340–349. [Google Scholar] [CrossRef]
- Du Preez, C. A new arc–chord ratio (ACR) rugosity index for quantifying threedimensional landscape structural complexity. Lands. Ecol. 2015, 30, 181–192. [Google Scholar] [CrossRef]
- Atkins, J.W.; Bohrer, G.; Fahey, R.T.; Hardiman, B.S.; Morin, T.H.; Stovall, A.E.L.; Zimmerman, N.; Gough, C.M. Quantifying vegetation and canopy structural complexity from terrestrial LiDAR data using the forestr r package. Methods Ecol. Evol. 2018, 9, 2057–2066. [Google Scholar] [CrossRef]
- Ehbrecht, M.; Schall, P.; Juchheim, J.; Ammer, C.; Seidel, D. Effective number of layers: A new measure for quantifying three-dimensional stand structure based on sampling with terrestrial LiDAR. For. Ecol. Manag. 2016, 380, 212–223. [Google Scholar] [CrossRef]
- Seidel, D.; Ehbrecht, M.; Puettmann, K. Assessing different components of three-dimensional forest structure with single-scan terrestrial laser scanning: A case study. For. Ecol. Manag. 2016, 381, 196–208. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. Stochastic models for the Earth’s relief, the shape and the fractal dimension of the coastlines, and the number-area rule for islands. Proc. Natl. Acad. Sci. USA 1975, 72, 3825–3828. [Google Scholar] [CrossRef]
- Boudon, F.; Godin, C.; Pradal, C.; Puech, O.; Sinoquet, H. Estimating the fractal dimension of plants using the two-surface method: An analysis based on 3D-digitized tree foliage. Fractals 2011, 14, 149–163. [Google Scholar] [CrossRef]
- Dutilleul, P.; Han, L.; Valladares, F.; Messier, C. Crown traits of coniferous trees and their relation to shade tolerance can differ with leaf type: A biophysical demonstration using computed tomography scanning data. Front. Plant Sci. 2015, 6, 172. [Google Scholar] [CrossRef]
- Jonckheere, I.; Nackaerts, K.; Muys, B.; van Aardt, J.; Coppin, P. A fractal dimension-based modelling approach for studying the effect of leaf distribution on LAI retrieval in forest canopies. Ecol. Model. 2006, 197, 179–195. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, X.; Chen, J.; Huang, H.; Yang, X. Estimating fractal dimensions of tree crowns in 3-D space based on structural relationships. For. Chron. 2014, 90, 177–183. [Google Scholar] [CrossRef]
- Dorji, Y.; Annighöfer, P.; Ammer, C.; Seidel, D. Response of Beech (Fagus sylvatica L.) Trees to Competition—New Insights from Using Fractal Analysis. Remote Sens. 2019, 11, 2656. [Google Scholar] [CrossRef]
- Seidel, D. A holistic approach to determine tree structural complexity based on laser scanning data and fractal analysis. Ecol. Evol. 2018, 102, 3. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.; Ehbrecht, M.; Annighöfer, P.; Ammer, C. From tree to stand-level structural complexity—Which properties make a forest stand complex? Agric. For. Meteorol. 2019, 278, 107699. [Google Scholar] [CrossRef]
- Zemp, D.C.; Ehbrecht, M.; Seidel, D.; Ammer, C.; Craven, D.; Erkelenz, J.; Irawan, B.; Sundawati, L.; Hölscher, D.; Kreft, H. Mixed-species tree plantings enhance structural complexity in oil palm plantations. Agric. Ecosyst. Environ. 2019, 283, 106564. [Google Scholar] [CrossRef]
- Ehbrecht, M.; Schall, P.; Ammer, C.; Seidel, D. Quantifying stand structural complexity and its relationship with forest management, tree species diversity and microclimate. Agric. For. Meteorol. 2017, 242, 1–9. [Google Scholar] [CrossRef]
- Willim, K.; Stiers, M.; Annighöfer, P.; Ammer, C.; Ehbrecht, M.; Kabal, M.; Stillhard, J.; Seidel, D. Assessing understory complexity in beech-dominated Forests (Fagus sylvatica L.)-from managed to primary forests. Sensors 2019, 19, 1684. [Google Scholar] [CrossRef]
- Stiers, M.; Willim, K.; Seidel, D.; Ehbrecht, M.; Kabal, M.; Ammer, C.; Annighöfer, P. A quantitative comparison of the structural complexity of managed, lately unmanaged and primary European beech (Fagus sylvatica L.) forests. For. Ecol. Manag. 2018, 430, 357–365. [Google Scholar] [CrossRef]
- Camarretta, N.; Harrison, P.A.; Bailey, T.; Potts, B.; Lucieer, A.; Davidson, N.; Hunt, M. Monitoring forest structure to guide adaptive management of forest restoration: A review of remote sensing approaches. New For. 2019, 18, 305. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Entscheidungshilfen zur Behandlung und Entwicklung von Buchenbeständen. Available online: https://www.nw-fva.de/fileadmin/user_upload/Verwaltung/Publikationen/Merkblaetter/Bu_Nds_Entscheidungshilfen_zur_Behandlung_und_Entwicklung_von_Buchenbestaenden.pdf (accessed on 30 July 2018).
- Stiers, M.; Willim, K.; Seidel, D.; Ammer, C.; Kabal, M.; Stillhard, J.; Annighöfer, P. Analyzing Spatial Distribution Patterns of European Beech (Fagus sylvatica L.) Regeneration in Dependence of Canopy Openings. Forests 2019, 10, 637. [Google Scholar] [CrossRef]
- Fischer, M.; Bossdorf, O.; Gockel, S.; Hänsel, F.; Hemp, A.; Hessenmöller, D.; Korte, G.; Nieschulze, J.; Pfeiffer, S.; Prati, D.; et al. Implementing large-scale and long-term functional biodiversity research: The Biodiversity Exploratories. Basic Appl. Ecol. 2010, 11, 473–485. [Google Scholar] [CrossRef]
- Bartsch, N.; von Lüpke, B.; Röhrig, E. Waldbau auf ökologischer Grundlage; Eugen Ulmer KG: Stuttgart, Germany, 2006; p. 549. [Google Scholar]
- Schall, P.; Gossner, M.M.; Heinrichs, S.; Fischer, M.; Boch, S.; Prati, D.; Jung, K.; Baumgartner, V.; Blaser, S.; Böhm, S.; et al. The impact of even-aged and uneven-aged forest management on regional biodiversity of multiple taxa in European beech forests. J. Appl. Ecol. 2018, 109, 267–278. [Google Scholar] [CrossRef]
- Korpel’, S. Die Urwälder der Westkarpaten; Gustav Fischer Verlag: Stuttgart, Germany, 1995; pp. 132–148. [Google Scholar]
- Juchheim, J.; Ammer, C.; Schall, P.; Seidel, D. Canopy space filling rather than conventional measures of structural diversity explains productivity of beech stands. For. Ecol. Manag. 2017, 395, 19–26. [Google Scholar] [CrossRef]
- Seidel, D.; Leuschner, C.; Scherber, C.; Beyer, F.; Wommelsdorf, T.; Cashman, M.J.; Fehrmann, L. The relationship between tree species richness, canopy space exploration and productivity in a temperate broad-leaf mixed forest. For. Ecol. Manag. 2013, 310, 366–374. [Google Scholar] [CrossRef]
- Béland, M.; Widlowski, J.L.; Fournier, R.A. A model for deriving voxel-level tree leaf area density estimates from ground-based LiDAR. Environ. Model. Softw. 2014, 51, 184–189. [Google Scholar] [CrossRef]
- Sarkar, N.; Chaudhuri, B.B. An efficient differential box-counting approach to compute fractal dimension of image. IEEE Trans. Syst. Man Cybern. 1994, 24, 115–120. [Google Scholar] [CrossRef]
- Stinglwagner, G.; Haseder, I.; Erlbeck, R. Das Kosmos Wald & Forst-Lexikon; Kosmos: Stuttgart, Germany, 2009; p. 76. [Google Scholar]
- Leibundgut, H. Empfehlungen für die Baumklassenbildung und Methodik bei Versuchen über die Wirkung von Waldpflegemassnahmen; IUFRO-Congr: Oxford, UK, 1956; pp. 92–94. [Google Scholar]
- Clark, P.J.; Evans, F.C. Distance to Nearest Neighbor as a Measure of Spatial Relationships in Populations. Ecology 1954, 35, 445–453. [Google Scholar] [CrossRef]
- Donnelly, K. Simulations to determine the variance and edge-effect of total nearest neighbour distance. In Simulation Studies in Archaeology; Hodder, I., Ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1978; pp. 91–95. [Google Scholar]
- Pommerening, A.; Stoyan, D. Edge-correction needs in estimating indices of spatial forest structure. Can. J. For. Res. 2006, 36, 1723–1739. [Google Scholar] [CrossRef]
- Nagel, T.A.; Svoboda, M.; Kobal, M. Disturbance, life history traits, and dynamics in an old-growth forest landscape of southeastern Europe. Ecol. Appl. 2014, 24, 663–679. [Google Scholar] [CrossRef]
- Schelhaas, M.J.; Nabuurs, G.J.; Schuck, A. Natural disturbances in the European forests in the 19th and 20th centuries. Glob. Chang. Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Emborg, J.; Christensen, M.; Heilmann-Clausen, J. The structural dynamics of Suserup Skov, a near-natural temperate deciduous forest in Denmark. For. Ecol. Manag. 2000, 126, 173–189. [Google Scholar] [CrossRef]
- Feldmann, E.; Drößler, L.; Hauck, M.; Kucbel, S.; Pichler, V.; Leuschner, C. Canopy gap dynamics and tree understory release in a virgin beech forest, Slovakian Carpathians. For. Ecol. Manag. 2018, 415–416, 38–46. [Google Scholar] [CrossRef]
- Schröter, M.; Härdtle, W.; von Oheimb, G. Crown plasticity and neighborhood interactions of European beech (Fagus sylvatica L.) in an old-growth forest. Eur. J. For. Res. 2012, 131, 787–798. [Google Scholar] [CrossRef]
- Drößler, L. Struktur und Dynamik von zwei Buchenurwäldern in der Slowakei. Ph.D. Thesis, Georg-August-University, Göttingen, Germany, 2006. [Google Scholar]
- Hobi, M.L.; Ginzler, C.; Commarmot, B.; Bugmann, H. Gap pattern of the largest primeval beech forest of Europe revealed by remote sensing. Ecosphere 2015, 6, art76. [Google Scholar] [CrossRef]
- Hobi, M.L.; Commarmot, B.; Bugmann, H. Pattern and process in the largest primeval beech forest of Europe (Ukrainian Carpathians). J. Veg. Sci. 2015, 26, 323–336. [Google Scholar] [CrossRef]
- Kenderes, K.; Mihók, B.; Standovár, T. Thirty years of gap dynamics in a central european beech forest reserve. Forestry 2008, 81, 111–123. [Google Scholar] [CrossRef]
- Meyer, P.; Vath, T.; Burghard, V.L. Die Struktur albanischer Rotbuchen-Urwälder – Ableitungen für eine naturnahe Buchenwirtschaft. Forstwissenschaftliches Centralblatt 2003, 122, 47–58. [Google Scholar] [CrossRef]
- Nagel, T.A.; Svoboda, M.; Rugani, T.; Diaci, J. Gap regeneration and replacement patterns in an old-growth Fagus–Abies forest of Bosnia–Herzegovina. Plant Ecol. 2010, 208, 307–318. [Google Scholar] [CrossRef]
- Berger, A.L.; Puettmann, K. Overstory Composition and Stand Structure Influence Herbaceous Plant Diversity in the Mixed Aspen Forest of Northern Minnesota. Am. Nat. 2000, 143, 111–125. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Sullivan, D.S.; Lindgren, P.M. Stand structure and small mammals in young lodgepole pine forest: 10-year results after thinning. Ecol. Appl. 2001, 11, 1151–1173. [Google Scholar] [CrossRef]
- Von Oheimb, G.; Westphal, C.; Tempel, H.; Härdtle, W. Structural pattern of a near-natural beech forest (Fagus sylvatica) (Serrahn, North-east Germany). For. Ecol. Manag. 2005, 212, 253–263. [Google Scholar] [CrossRef]
- Rugani, T.; Diaci, J.; Hladnik, D. Gap Dynamics and Structure of Two Old-Growth Beech Forest Remnants in Slovenia. PLoS ONE 2012, 8, e52641. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.G.; Brown, M.J. Forest canopy stratification-Is it useful? Am. Nat. 2000, 155, 473–484. [Google Scholar] [CrossRef] [PubMed]
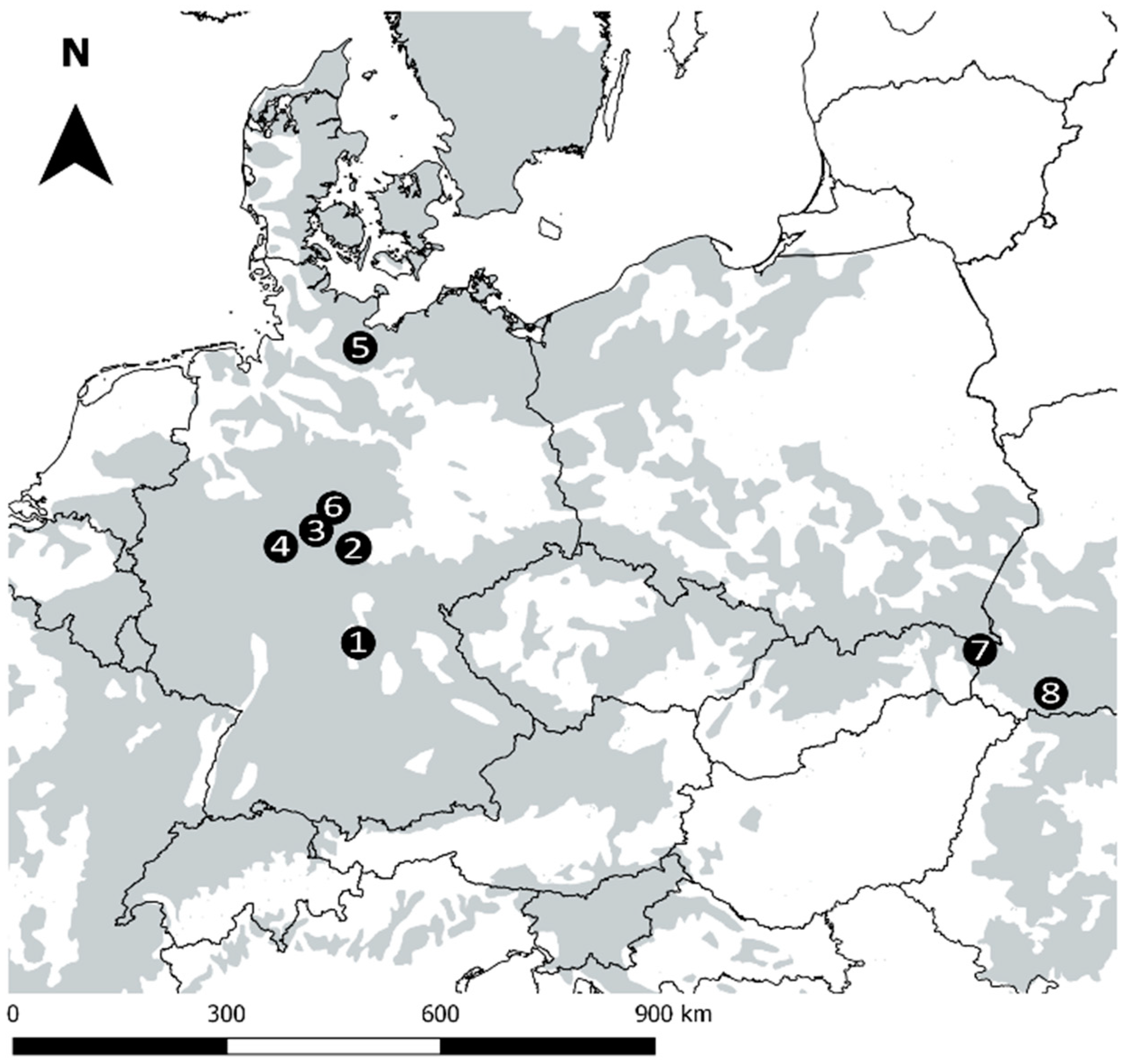

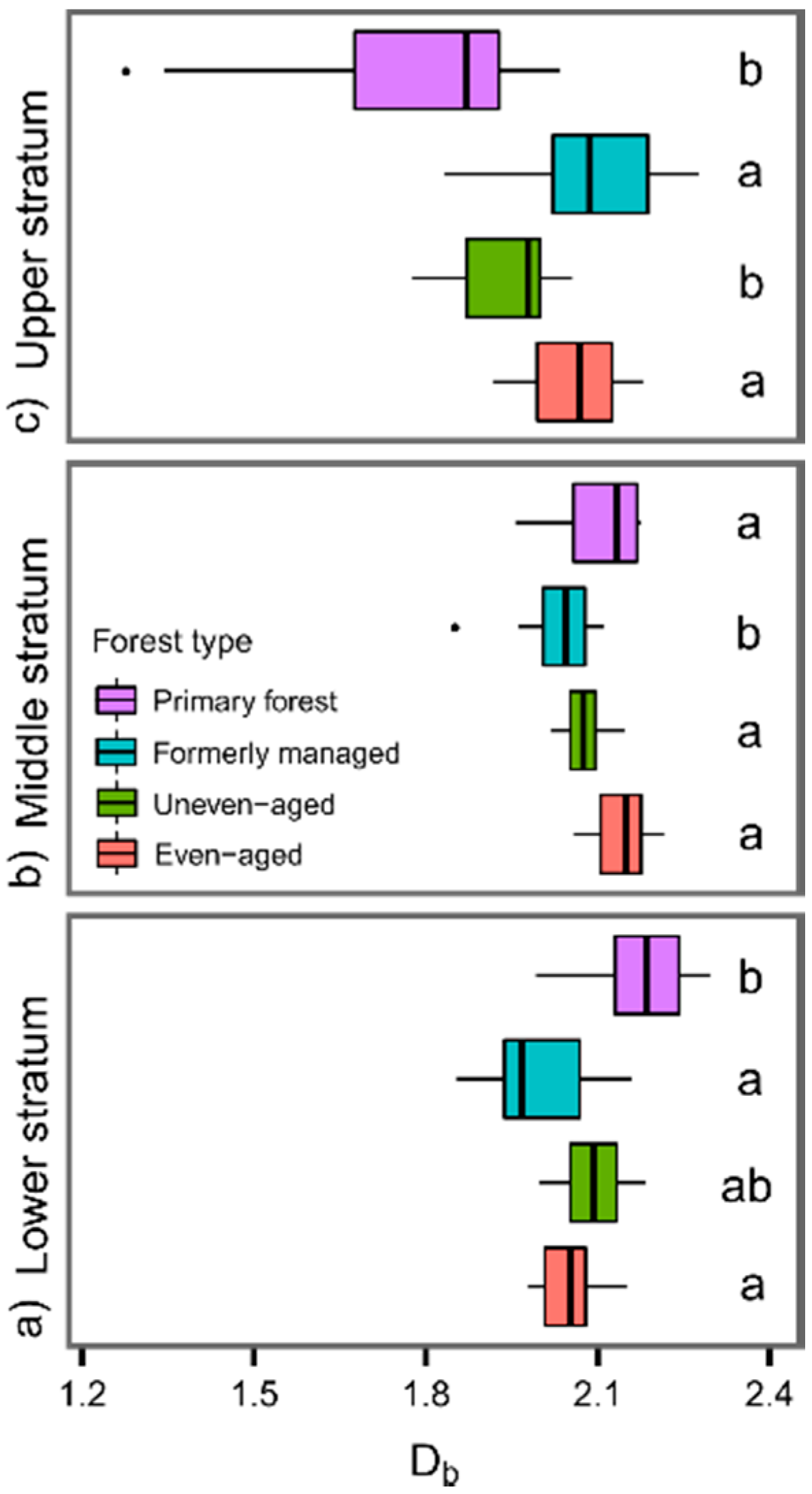
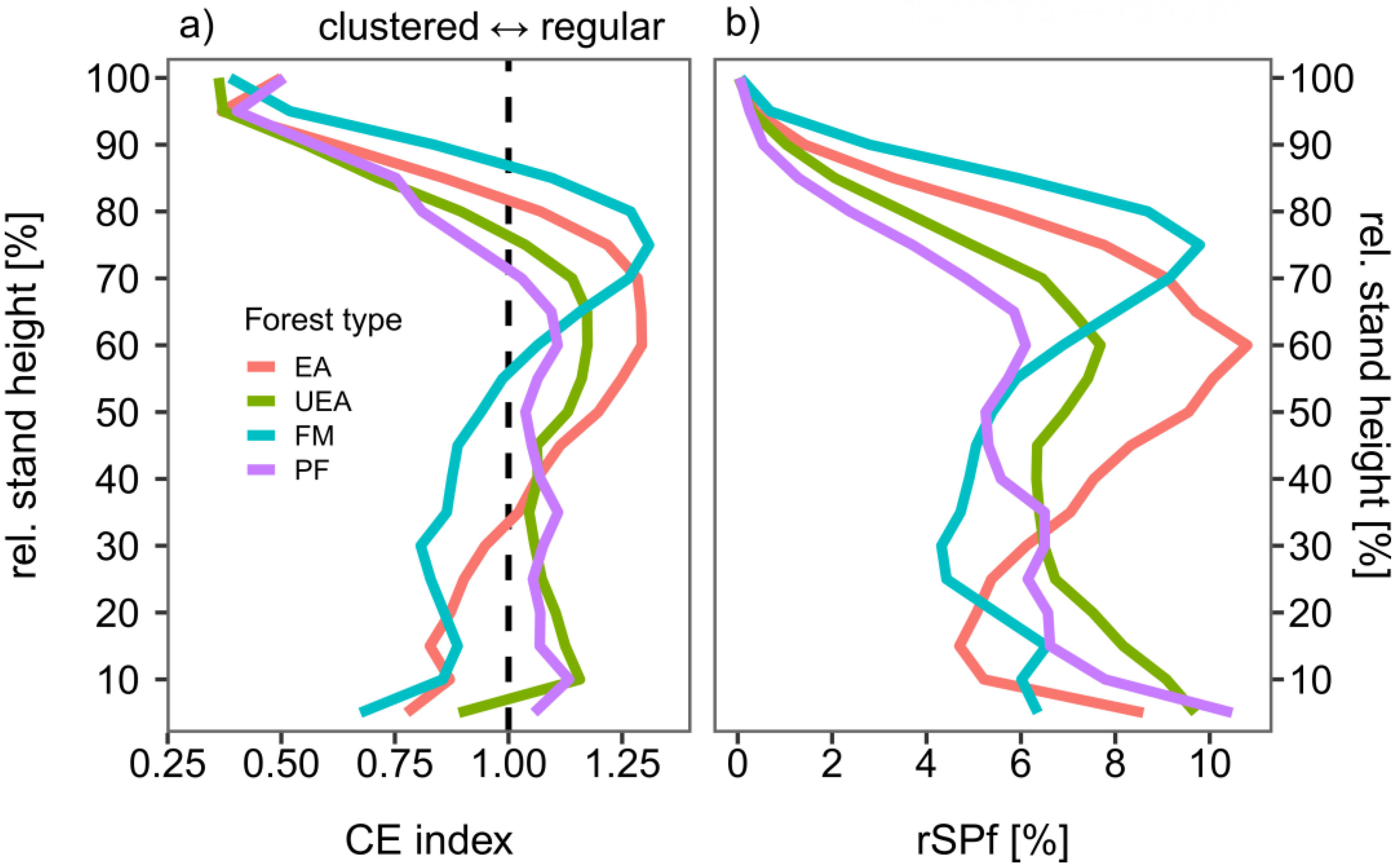
| Location | Study Sites | MAT (°C) | MAP (mm) | Elevation (m a.s.l.) | Forest Type | Age (Years) | No. of Plots |
|---|---|---|---|---|---|---|---|
| Germany | Ebrach | 7–8 | 850 | 320–480 | UEA | 111 | 4 |
| Hainich | 6.5–8 | 500–800 | 285–550 | UEA FM | 160–186 150–182 | 4 * 4 + 3 * | |
| Hann. Münden | 6.5–7.5 | 750–1050 | 270–410 | EA | 81 | 4 | |
| Kellerwald | 6–8 | 600–800 | 540–635 | FM | 184 | 4 | |
| Lübeck | 8–8.5 | 625–725 | 40–90 | EA | 131 | 4 | |
| Reinhausen | 8 | 740 | 190–310 | EA | 98 | 4 | |
| Slovakia | Rožok | 6–7 | 780 | 580–745 | PF | ~220 | 4 |
| Ukraine | Uholka | 7 | 1407 | 700–840 | PF | ~350 | 4 |
| Lower Stratum | Middle Stratum | Upper Stratum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Site | Db | rSPf (%) | CE | Height Limits (m) | Db | rSPf (%) | CE | Height Limits (m) | Db | rSPf (%) | CE | Height Limits (m) | |
| Ebrach (UEA) | 2.14 ± 0.05 | 7.04 ± 1.80 | 1.06 ± 0.15 | Min: 1 Max: 12.1 ± 0.6 | 2.10 ± 0.04 | 5.55 ± 0.91 | 1.07 ± 0.07 | Min: 12.1 ± 0.6 Max: 24.3 ± 1.89 | 1.99 ± 0.01 | 2.41 ± 2.24 | 0.76 ± 0.31 | Min: 24.3 ± 1.89 Max: 35.7 ± 2.15 | |
| Hainich | (UEA) | 2.05 ± 0.04 | 8.85 ± 1.37 | 1.07 ± 0.05 | Min: 1 Max: 11.15 ± 0.93 | 2.05 ± 0.02 | 8.21 ± 1.16 | 1.16 ± 0.08 | Min: 11.15 ± 0.93 Max: 23.56 ± 0.61 | 1.89 ± 0.10 | 2.83 ± 1.50 | 0.72 ± 0.14 | Min: 23.56 ± 0.61 Max: 33.9 ± 2.75 |
| (FM)) | 1.96 ± 0.09 | 4.61 ± 2.22 | 0.78 ± 0.16 | Min: 1 Max: 11.73 ± 0.3 | 2.01 ± 0.08 | 5.45 ± 1.42 | 0.92 ± 0.13 | Min: 11.73 ± 0.3 Max: 22.45 ± 1.88 | 2.13 ± 0.13 | 6.28 ± 2.04 | 1.03 ± 0.14 | Min: 22.45 ± 1.88 Max: 35.8 ± 0.92 | |
| Hann. Münden (EA) | 2.05 ± 0.05 | 6.76 ± 4.23 | 0.87 ± 0.15 | Min: 1 Max: 11.4 ± 0.51 | 2.16 ± 0.05 | 10.51 ± 2.75 | 1.22 ± 0.15 | Min: 11.4 ± 0.51 Max: 22.9 ± 1.09 | 2.07 ± 0.05 | 4.42 ± 4.01 | 0.8 ± 0.41 | Min: 22.9 ± 1.09 Max: 33.55 ± 1.59 | |
| Kellerwald (FM) | 2.06 ± 0.10 | 7.12 ± 4.95 | 0.89 ± 0.26 | Min: 1 Max: 10.15 ± 0.41 | 2.07 ± 0.02 | 6.51 ± 1.60 | 1.05 ± 0.11 | Min: 10.15 ± 0.41 Max: 20.5 ± 0.82 | 2.01 ± 0.12 | 3.58 ± 3.3 | 0.86 ± 0.34 | Min: 20.5 ± 0.82 Max: 29.9 ± 1.25 | |
| Lübeck (EA) | 2.09 ± 0.05 | 6 ± 2.39 | 0.92 ± 0.21 | Min: 1 Max: 11.85 ± 0.71 | 2.10 ± 0.05 | 6.80 ± 2.54 | 1.08 ± 0.19 | Min: 11.85 ± 0.71 Max: 23.83 ± 1.31 | 2.10 ± 0.10 | 4.62 ± 4.16 | 0.96 ± 0.40 | Min: 23.83 ± 1.31 Max: 35.15 ± 2.16 | |
| Reinhausen (EA) | 2.02 ± 0.03 | 4.77 ± 2 | 0.80 ± 0.07 | Min: 1 Max: 11.6 ± 0.49 | 2.17 ± 0.02 | 9.69 ± 2.61 | 1.22 ± 0.17 | Min: 11.6 ± 0.49 Max: 23.25 ± 0.89 | 1.99 ± 0.07 | 2.79 ± 3.08 | 0.8 ± 0.37 | Min: 23.25 ± 0.89 Max: 34.15 ± 1.48 | |
| Rožok (PF) | 2.10 ± 0.08 | 5.14 ± 3.38 | 0.87 ± 0.19 | Min: 1 Max: 14.5 ± 0.48 | 2.15 ± 0.03 | 6.71 ± 2.04 | 1.11 ± 0.17 | Min: 14.5 ± 0.48 Max: 29.1 ± 0.90 | 1.92 ± 0.08 | 2.57 ± 2.88 | 0.7 ± 0.39 | Min: 29.1 ± 0.90 Max: 42.85 ± 1.43 | |
| Uholka (PF) | 2.25 ± 0.04 | 9.55 ± 2.61 | 1.28 ± 0.15 | Min: 1 Max: 15.3 ± 0.5 | 2.05 ± 0.10 | 4.80 ± 2.66 | 1.07 ± 0.24 | Min: 15.3 ± 0.5 Max: 30.75 ± 0.91 | 1.59 ± 0.34 | 1.15 ± 1.57 | 0.68 ± 0.30 | Min: 30.75 ± 0.91 Max: 45.3 ± 1.35 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willim, K.; Stiers, M.; Annighöfer, P.; Ehbrecht, M.; Ammer, C.; Seidel, D. Spatial Patterns of Structural Complexity in Differently Managed and Unmanaged Beech-Dominated Forests in Central Europe. Remote Sens. 2020, 12, 1907. https://doi.org/10.3390/rs12121907
Willim K, Stiers M, Annighöfer P, Ehbrecht M, Ammer C, Seidel D. Spatial Patterns of Structural Complexity in Differently Managed and Unmanaged Beech-Dominated Forests in Central Europe. Remote Sensing. 2020; 12(12):1907. https://doi.org/10.3390/rs12121907
Chicago/Turabian StyleWillim, Katharina, Melissa Stiers, Peter Annighöfer, Martin Ehbrecht, Christian Ammer, and Dominik Seidel. 2020. "Spatial Patterns of Structural Complexity in Differently Managed and Unmanaged Beech-Dominated Forests in Central Europe" Remote Sensing 12, no. 12: 1907. https://doi.org/10.3390/rs12121907
APA StyleWillim, K., Stiers, M., Annighöfer, P., Ehbrecht, M., Ammer, C., & Seidel, D. (2020). Spatial Patterns of Structural Complexity in Differently Managed and Unmanaged Beech-Dominated Forests in Central Europe. Remote Sensing, 12(12), 1907. https://doi.org/10.3390/rs12121907






