What Agriculture Can Learn from Native Ecosystems in Building Soil Organic Matter: A Review
Abstract
:1. Introduction
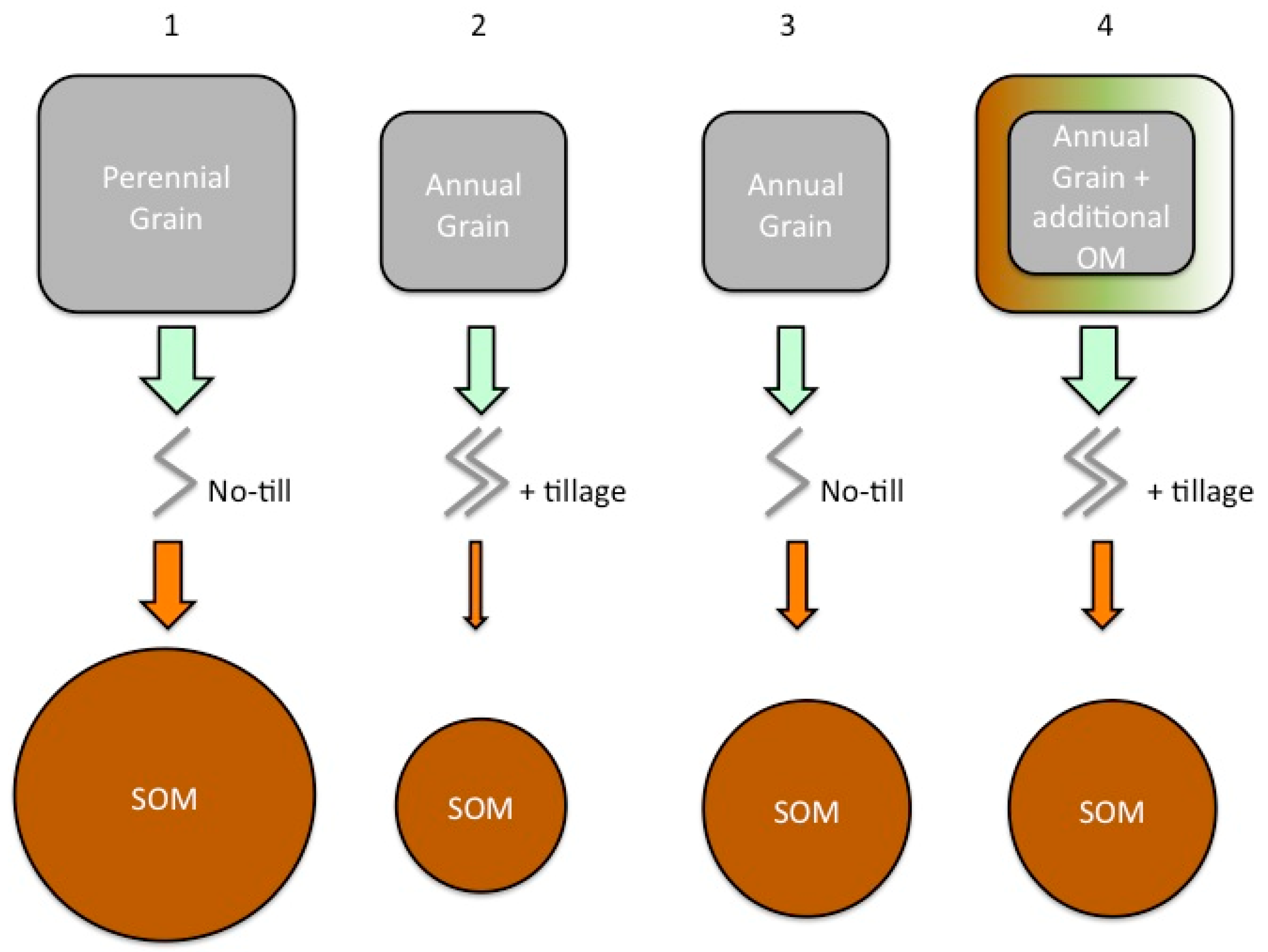
- The quantity of C inputs to soil are reduced in annual cropping systems compared to perennial native vegetation.
- The physical disturbance of tillage increases SOM decomposition rates. It does this in part by destroying aggregates and physical structure, exposing previously physically protected SOM to decomposition.
- Tillage and lack of plant cover on agricultural fields enable soil erosion to occur which can remove large quantities of SOM.
1.1. Background
1.2. SOM and Ecological Intensification
2. Approaches to Rebuilding SOM in Croplands
2.1. Reducing SOM Losses from Microbial Respiration
2.2. Increasing SOM by Increasing Crop Inputs
2.3. Increasing SOM by Increasing Off-Farm Inputs of Organic Matter
3. Perennials Address the Root of the Problem
Potential Soil Carbon Accumulation with Perennial Grains
4. The Role of Diversity in Improving SOM
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scharlemann, J.P.W.; Tanner, E.V.J.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Trumbore, S. Radiocarbon and soil carbon dynamics. Annu. Rev. Earth Planet. Sci. 2009, 37, 47–66. [Google Scholar] [CrossRef]
- Vermeulen, S.J.; Campbell, B.M.; Ingram, J.S.I. Climate change and food systems. Annu. Rev. Environ. Resour. 2012, 37, 195–222. [Google Scholar] [CrossRef]
- Lal, R. Global potential of soil carbon sequestration to mitigate the greenhouse effect. Crit. Rev. Plant Sci. 2003, 22, 151–184. [Google Scholar] [CrossRef]
- Cole, C.V.; Duxbury, J.; Freney, J.; Heinemeyer, O.; Minami, K.; Mosier, A.; Paustian, K.; Rosenberg, N.; Sampson, N.; Sauerbeck, D.; et al. Global estimates of potential mitigation of greenhouse gas emissions by agriculture. Nutr. Cycl. Agroecosyst. 1997, 49, 221–228. [Google Scholar] [CrossRef]
- Le Quéré, C.; Moriarty, R.; Andrew, R.M.; Peters, G.P.; Ciais, P.; Friedlingstein, P.; Jones, S.D.; Sitch, S.; Tans, P.; Arneth, A.; et al. Global carbon budget 2014. Earth Syst. Sci. Data 2015, 7, 47–85. [Google Scholar] [CrossRef]
- IPCC. Agriculture, Forestry and Other Land Use. In Climate Change 2014 Synthesis Report; IPCC: Geneva, Switzerland, 2014; Chapter 11. [Google Scholar]
- McLauchlan, K. The nature and longevity of agricultural impacts on soil carbon and nutrients: A review. Ecosystems 2006, 9, 1364–1382. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson: New York, NY, USA, 2016. [Google Scholar]
- Post, W.M.; Izaurralde, R.C.; Jastrow, J.D.; McCarl, B.A.; Amonette, J.E.; Bailey, V.L.; Jardine, P.M.; West, T.O.; Zhou, J. Enhancement of carbon sequestration in US soils. BioScience 2004, 54, 895–908. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hillel, D. Out of the Earth; University of California Press: Berkeley, CA, USA, 1991. [Google Scholar]
- Montgomery, D.R. Dirt, the Erosion of Civilization; University of California Press: Berkeley, CA, USA, 2007. [Google Scholar]
- Grandy, A.S.; Robertson, G.P. Land-use intensity effects on soil organic carbon accumulation rates and mechanisms. Ecosystems 2007, 10, 58–73. [Google Scholar] [CrossRef]
- Haas, H.J.; Evans, C.E. Nitrogen and Carbon Changes in Great Plains Soils as Influenced by Cropping and Soil Treatments; USDA Technical Bulletin No. 1164; U.S. Government Printing Office: Washington, DC, USA, 1957.
- Davidson, E.A.; Ackerman, I.L. Changes in soil carbon inventories following cultivation of previously untilled soils. Biogeochem. 1993, 20, 161–193. [Google Scholar] [CrossRef]
- Mazoyer, M.; Roudart, L. The History of World Agriculture: From the Neolithic Age to the Present Crisis; Earthscan: London, UK, 2006. [Google Scholar]
- Wilken, G.C. Good Farmers: Traditional Agricultural Resource Management in Mexico and Central America; University of California Press: Berkeley, CA, USA, 1987. [Google Scholar]
- Howard, A. An Agricultural Testament; Oxford University Press: London, UK, 1943. [Google Scholar]
- Rodale, J.I. Pay Dirt: Farming & Gardening with Compost; The Devin-Adair Company: New York, NY, USA, 1947. [Google Scholar]
- Jackson, W. New Roots for Agriculture; University of Nebraska Press: Lincoln, NE, USA, 1980. [Google Scholar]
- Gliessman, S.R. Agroecology. The Ecology of Sustainable Food System; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Royal Society. Reaping the Benefits; RS Policy Document 11/09; The Royal Society: London, UK, 2009. [Google Scholar]
- Sommer, R.; Bossio, D. Dynamics and climate change mitigation potential of soil organic carbon sequestration. J. Environ. Manag. 2014, 144, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Crowther, T.W.; Todd-Brown, K.E.O.; Rowe, C.W.; Wieder, W.R.; Carey, J.C.; Machmuller, M.B.; Snoek, B.L.; Fang, S.; Zhou, G.; Allison, S.D.; et al. Quantifying global soil carbon losses in response to warming. Nature 2016, 540, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Tittonell, P.; Giller, K.E. When yield gaps are poverty traps: The paradigm of ecological intensification in African smallholder agriculture. Field Crops Res. 2013, 143, 76–90. [Google Scholar] [CrossRef]
- Cassman, K.G. Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. PNAS 1999, 96, 5952–5959. [Google Scholar] [CrossRef] [PubMed]
- Tittonell, P. Ecological intensification: Local innovation to address global challenges. Sustain. Agric. Rev. 2016, 19, 1–34. [Google Scholar]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, M.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Gomiero, T. Soil degradation, land scarcity and food security: Reviewing a complex challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef]
- Dore, T.; Makowski, D.; Malezieux, E.; Munier-Jolain, N.; Tchamitchian, M.; Tittonell, P. Facing up to the paradigm of ecological intensification in agronomy: Revisiting methods, concepts and knowledge. Eur. J. Agron. 2011, 34, 197–210. [Google Scholar] [CrossRef]
- Tittonell, P. Ecological intensification of agriculture—Sustainable by nature. Curr. Opin. Environ. Sustain. 2014, 8, 53–61. [Google Scholar] [CrossRef]
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological intensification: Harnessing ecosystem services for food security. TREE 2013, 28, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Rusch, A.; Bommarco, R.; Ekbom, B. Conservation biological control in agricultural landscapes. In Insect-Plant Interactions in a Crop Protection Perspective; Advances in Botanical Research Series; Sauvion, N., Calatayud, P.-A., Thiery, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 81. [Google Scholar]
- Brady, M.V.; Hedlund, K.; Cong, R.G.; Hemerik, L.; Hotes, S.; Machado, S.; Mattsson, L.; Schulz, E.; Thomsen, I.K. Valuing supporting soil ecosystem services in agriculture: A natural capital approach. Agron. J. 2015, 107, 1809–1821. [Google Scholar] [CrossRef]
- Crews, T.E.; Blesh, J.; Culman, S.W.; Hayes, R.C.; Jensen, E.S.; Mack, M.C.; Peoples, M.B.; Schipanski, M.E. Going where no grains have gone before: From early to mid-succession. Agric. Ecosyst. Environ. 2016, 223, 223–238. [Google Scholar] [CrossRef]
- Schlesinger, W.H. Carbon balance in terrestrial detritus. Annu. Rev. Ecol. Syst. 1977, 8, 51–81. [Google Scholar] [CrossRef]
- Johnston, A.E.; Poulton, P.R.; Coleman, K. Soil organic matter: Its importance in sustainable agriculture and carbon dioxide fluxes. Adv. Agron. 2009, 101, 1–57. [Google Scholar]
- Scott, D.A.; Baer, S.G.; Blair, J.M. Recovery and relative influence of root, microbial and structural properties of soil on physically sequestered carbon stocks in restored grassland. Soil Sci. Soc. Am. J. 2017, 81, 50–60. [Google Scholar] [CrossRef]
- Stockman, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; Remy de Courcelles, V.D.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 16, 80–99. [Google Scholar] [CrossRef]
- Kallenback, C.M.; Frey, S.D.; Grandy, S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Kleber, M. What is recalcitrant soil organic matter? Environ. Chem. 2010, 7, 1–13. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kogel-Knabner, I.; Lehmann, J.; Manning, D.; et al. Soil organic matter persistence as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Dungait, J.; Hopkins, D.; Gregory, A.; Whitmore, A. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Vitousek, P.M. Principles of Terrestrial Ecology, 2nd ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Hobbie, S.E.; Ogdahl, M.; Chorover, J.; Chadwick, O.A.; Oleksyn, J.; Zytkowiak, R.; Reich, P.B. Tree species effects on soil organic matter dynamics: The role of soil cation composition. Ecosystems 2007, 10, 999–1018. [Google Scholar] [CrossRef]
- Field, C.B. Sharing the garden. Science 2001, 294, 2490–2491. [Google Scholar] [CrossRef] [PubMed]
- Saugier, B.; Roy, J.; Mooney, H.A. Estimations of global terrestrial productivity: Converging toward a single number? In Terrestrial Global Productivity; Roy, J., Saugier, B., Mooney, H.A., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 543–557. [Google Scholar]
- Johnson, J.M.F.; Allmaras, R.R.; Reicosky, D.C. Estimating source carbon from crop residues, roots and rhizodeposits using the national grain-yield database. Agron. J. 2006, 98, 622–636. [Google Scholar] [CrossRef]
- Baker, J.M.; Ochsner, T.E.; Venterea, R.T.; Griffis, T.J. Tillage and soil carbon sequestration—What do we really know? Agric. Ecosyst. Environ. 2007, 118, 1–5. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K.; Doran, J.W. Aggregation and soil organic matter accumulation in cultivated and native grassland soils. Soil Sci. Soc. Am. J. 1998, 62, 1367–1377. [Google Scholar] [CrossRef]
- Grandy, A.S.; Neff, J.C. Molecular C dynamics downstream: The biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci. Total Environ. 2008, 404, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Janzen, H.H. The soil carbon dilemma: Shall we hoard it or use it? Soil Biol. Biochem. 2006, 38, 419–424. [Google Scholar] [CrossRef]
- Lal, R.; Griffin, M.; Apt, J.; Lave, L.; Morgan, M.G. Managing soil carbon. Science 2004, 304, 393. [Google Scholar] [CrossRef] [PubMed]
- Ogle, S.M.; Breidt, F.J.; Paustian, K. Agricultural management impacts on soil organic carbon storage under moist and dry climatic conditions of temperate and tropical regions. Biogeochemistry 2005, 72, 87–121. [Google Scholar] [CrossRef]
- VandenBygaart, A.J. The myth that no-till can mitigate global climate change. Agric. Ecosyst. Environ. 2016, 216, 98–99. [Google Scholar] [CrossRef]
- Powlson, D.S.; Stirling, C.M.; Jat, M.L.; Gerard, B.G.; Palm, C.A.; Sanchez, P.A.; Cassman, K.G. Limited potential of no-till agriculture for climate change mitigation. Nat. Clim. Chang. 2014, 4, 678–683. [Google Scholar] [CrossRef]
- Dolan, M.S.; Clapp, C.E.; Allmaras, R.R.; Baker, J.M.; Molina, J.A.E. Soil organic carbon and nitrogen in a Minnesota soil as related to tillage, residue and nitrogen management. Soil Tillage Res. 2006, 89, 221–231. [Google Scholar] [CrossRef]
- VandenBygaart, A.J.; Gregorich, E.G.; Angers, D.A. Influence of agricultural management on soil organic carbon: A compendium and assessment of Canadian studies. Can. J. Soil Sci. 2003, 83, 363–380. [Google Scholar] [CrossRef]
- Gál, A.; Vyn, T.J.; Michéli, E.; Kladivko, E.J.; McFee, W.W. Soil carbon and nitrogen accumulation with long-term no-till versus moldboard plowing overestimated with tilled-zone sampling depths. Soil Tillage Res. 2007, 96, 42–51. [Google Scholar] [CrossRef]
- Syswerda, S.P.; Corbin, A.T.; Mokma, D.L.; Kravchenko, A.N.; Robertson, G.P. Agricultural management and soil carbon storage in surface vs. deep layers. Soil Sci. Soc. Am. J. 2011, 75, 92–101. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Robertson, G.P. Whole-profile soil carbon stocks: The danger of assuming too much from analyses of too little. Soil Sci. Soc. Am. J. 2011, 75, 235–240. [Google Scholar] [CrossRef]
- Six, J.; Ogle, S.M.; Breidt, F.J.; Conant, R.T.; Mosier, A.R.; Paustian, K. The potential to mitigate global warming with no-tillage management is only realized when practised in the long term. Glob. Chang. Biol. 2004, 10, 155–160. [Google Scholar] [CrossRef]
- Powlson, D.S.; Glendining, M.J.; Coleman, K.; Whitmore, A.P. Implications for soil properties of removing cereal straw: Results from long-term studies. Agron. J. 2011, 103, 279–287. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Crop residue removal for bioenergy reduces soil carbon pools: How can we offset carbon loss? BioEnergy Res. 2013, 6, 358–371. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops—A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- West, T.O.; Post, W.M. Soil organic carbon sequestration rates by tillage and crop rotation: A global data analysis. Soil Sci. Soc. Am. J. 2002, 66, 1930–1946. [Google Scholar] [CrossRef]
- Beniston, J.W.; DuPont, S.T.; Glover, J.D.; Lal, R.; Dungait, J.A.J. Soil organic carbon dynamics 75 years after land-use change in perennial grassland and annual wheat agricultural systems. Biogeochemistry 2014, 120, 27–49. [Google Scholar] [CrossRef]
- Kell, D.B. Breeding crop plants with deep roots: their role in sustainable carbon, nutrient and water sequestration. Ann. Bot. 2011, 108, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B. Large-scale sequestration of atmospheric carbon via plant roots in natural and agricultural ecosystems: Why and how. Philos. Trans. R. Soc. B 2012, 367, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- King, F.H. Farmers of Forty Centuries; Dover Publications: New York, NY, USA, 2004. [Google Scholar]
- Schlesinger, W. Carbon sequestration in soils. Science 1999, 284, 2095. [Google Scholar] [CrossRef]
- DeLonge, M.S.; Ryals, R.; Silver, W.L. A lifecycle model to evaluate carbon sequestration potential and greenhouse gas dynamics of managed grasslands. Ecosystems 2013, 16, 962–979. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Cropland soil carbon dynamics. In Recarbonization of the Biosphere; Lal, R., Lorenz, K., Huttl, R.F., Schneider, B.U., von Braun, J., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar]
- FAOSTAT. Food and Agriculture Data. Available online: www.fao.org/faostat/en/#home (accessed on 27 December 2016).
- Glover, J.D.; Reganold, J.P.; Bell, L.W.; Borevitz, J.; Brummer, E.C.; Buckler, E.S.; Cox, C.M.; Cox, T.S.; Crews, T.E.; Culman, S.W.; et al. Increased food and ecosystem security via pernnial grains. Science 2010, 328, 1638–1639. [Google Scholar] [CrossRef] [PubMed]
- Batello, C.; Wade, L.; Cox, S.; Pogna, N.; Bozzini, A.; Choptiany, J. Perennial Crops for Food Security; FAO: Rome, Italy, 2013. [Google Scholar]
- Kantar, M.B.; Tyl, C.E.; Dorn, K.M.; Zhang, X.; Jungers, J.M.; Kaser, J.M.; Schendel, R.R.; Eckberg, J.O.; Runck, B.C.; Bunzel, M.; et al. Perennial grain and oilseed crops. Annu. Rev. Plant Biol. 2016, 67, 703–729. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.P.; Hamilton, S.K.; del Grosso, S.J.; Parton, W.J. The biogeochemistry of bioenergy landscapes: Carbon, nitrogen, and water considerations. Ecol. Appl. 2011, 21, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Van Tassel, D.L.; DeHaan, L.R.; Cox, T.S. Missing domesticated plant forms: Can artificial selection fill the gap? Evol. Appl. 2010, 3, 434–452. [Google Scholar] [CrossRef] [PubMed]
- DeHaan, L.R.; van Tassel, D.L. Useful insights from evolutionary biology for developing perennial grain crops. Am. J. Bot. 2014, 101, 1801–1819. [Google Scholar] [CrossRef] [PubMed]
- DeHaan, L.R.; Van Tassel, D.L.; Anderson, J.A.; Asselin, S.R.; Barnes, R.; Baute, G.J.; Cattani, D.J.; Culman, S.W.; Dorn, K.M.; Hulke, B.S.; et al. Pipeline for grain domestication. Crop Sci. 2016, 56, 917–930. [Google Scholar] [CrossRef]
- Gomiero, T. Are biofuels an effective and viable energy strategy for industrialized societies? A reasoned overview of potentials and limits. Sustainability 2015, 7, 8491–8521. [Google Scholar] [CrossRef]
- Lin, B.B. Resilience in agriculture through crop diversification: Adaptive management for environmental change. Bioscience 2011, 61, 183–193. [Google Scholar] [CrossRef]
- Berry, W. The Unsettling of America, Culture and Agriculture; Sierra Club Books: Washington, DC, USA, 1977. [Google Scholar]
- Kämpf, I.; Hölzel, N.; Störrle, M.; Broll, G.; Kiehl, K. Potential of temperate agricultural soils for carbon sequestration: A meta-analysis of land-use effects. Sci. Total Environ. 2016, 566–567, 428–435. [Google Scholar]
- Kurganova, I.; de Gerenyu, V.L.; Six, J.; Kuzyakov, Y. Carbon cost of collective farming collapse in Russia. GCB 2014, 20, 928–947. [Google Scholar] [CrossRef] [PubMed]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Chang. Biol. 2000, 6, 317–328. [Google Scholar] [CrossRef]
- Conant, R.T.; Paustian, K.; Elliott, E.T. Grassland management and conversion into grassland: Effects on soil carbon. Ecol. Appl. 2001, 11, 343–355. [Google Scholar] [CrossRef]
- Kucharik, C.J. Impact of prairie age and soil order on carbon and nitrogen sequestration. Soil Sci. Soc. Am. J. 2007, 71, 430–441. [Google Scholar] [CrossRef]
- VandenBygaart, A.J.; McConkey, B.G.; Angers, D.A.; Smith, W.; de Gooijer, H.; Bentham, M.; Martin, T. Soil carbon change factors for the Canadian agriculture national greenhouse gas inventory. Can. J. Soil Sci. 2008, 88, 671–680. [Google Scholar] [CrossRef]
- Matamala, R.; Jastrow, J.D.; Miller, R.M.; Garten, C.T. Temporal changes in C and N stocks of restored prairie: Implications for C sequestration strategies. Ecol. Appl. 2009, 18, 1470–1488. [Google Scholar] [CrossRef]
- Arrouays, D.; Balesdent, J.; Germon, J.C.; Jayet, P.A.; Soussana, J.F.; Stengel, P. (Eds.) Increasing carbon stocks in French Agricultural Soils. In Mitigation of the Greenhouse Effect; Synthesis of an Assessment Report by the French Institute of Agricultural Research; INRA: Paris, France, 2002. [Google Scholar]
- IPCC. Land Use, Land-Use Change and Forestry (LULUCF); Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Agostini, F.; Gregory, A.S.; Richter, G.M. Carbon sequestration by perennial energy crops: Is the jury still out? Bioenergy Res. 2015, 8, 1057–1080. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Dunn, J.B.; Kwon, H.; Mueller, S.; Wander, M.M. Soil carbon sequestration and land use change associated with biofuel production: Empirical evidence. GCB Bioenergy 2016, 8, 66–80. [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Lee, J.; Hopmans, J.W.; Rolston, D.E.; Baer, S.G.; Six, J. Determining soil carbon stock changes: Simple bulk density corrections fail. Agric. Ecosyst. Environ. 2009, 134, 251–256. [Google Scholar] [CrossRef]
- Soussana, J.-F.; Loiseau, P.; Vuichard, N.; Ceschia, E.; Balesdent, J.; Chevallier, T.; Arrouays, D. Carbon cycling and sequestration opportunities in temperate grasslands. Soil Use Manag. 2004, 20, 219–230. [Google Scholar] [CrossRef]
- Kirby, C.A.; Arichardson, A.E.; Wade, L.J.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Carbon-nutrient stoichiometry to increase soil carbon sequesration. Soil Biol. Chem. 2013, 20, 77–86. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Qi, L.; Mei, X.; Liao, J.; Ding, X.; Deng, W.; Fan, L.; He, X.; Vivanco, J.M.; et al. Plant-plant-microbe mechanisms involved in soil-born disease suppression on a maize and pepper intercropping system. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Gurr, G.M.; Lu, Z.; Zheng, X.; Xu, H.; Zhu, P.; Chen, G.; Yao, X.; Cheng, J.; Zhu, Z.; Catindig, J.L.; et al. Multi-country evidence that crop diversification promotes ecological intensification of agriculture. Nat. Plants 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H.; McBride, J.R.; Shurin, J.B.; Bever, J.D.; Crews, T.E.; Tilman, G.D. Crop diversification can contribute to disease risk control in sustainable biofuels production. Front. Ecol. Environ. 2015, 13, 561–567. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Matulich, K.L.; Hooper, D.U.; Byrnes, J.E.; Duffy, E.; Gamfeldt, L.; Balvanera, P.; O’Connor, M.I.; Gonzalez, A. The functional role of producer diversity in ecosystems. Am. J. Bot. 2011, 98, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Hill, J.; Lehman, C. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science 2006, 314, 1598–1600. [Google Scholar] [CrossRef] [PubMed]
- Ampleman, M.D.; Crawford, K.M.; Fike, D.A. Differential soil organic carbon storage at forb and grass-dominated plant communities, 33 years after tallgrass prairie restoration. Plant Soil 2014, 374, 899–913. [Google Scholar] [CrossRef]
- Richardson, A.E.; Kirby, C.A.; Banerjee, S.; Kirkegaard, J.A. The inorganic nutrient cost of building soil carbon. Carbon Manag. 2014, 3, 265–268. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347. [Google Scholar] [CrossRef] [PubMed]
- Grandy, A.S.; Robertson, G.P. Initial cultivation of a temperate-region soil immediately accelerates aggregate turnover and CO2 and N2O fluxes. Glob. Chang. Biol. 2006, 12, 1507–1520. [Google Scholar] [CrossRef]
- Schipanski, M.E.; MacDonald, G.K.; Rosenzweig, S.; Chappell, M.J.; Bennett, E.M.; Kerr, R.B.; Blesh, J.; Crews, T.; Drinkwater, L.; Lundgren, J.G.; et al. Realizing resilient food systems. Bioscience 2016, 66, 600–610. [Google Scholar] [CrossRef]
- Head, J.W. International Law and Agroecological Husbandry: Building Legal Foundations for a New Agriculture; Earthscan: New York, NY, USA, 2017. [Google Scholar]


| Ecosystem Type | kg SOM m−2 |
|---|---|
| Tropical forest | 17.9 |
| Temperate forest | 20.3 |
| Boreal forest | 25.7 |
| Woodland and shrubland | 11.9 |
| Tropical savanna | 6.4 |
| Temperate grassland | 33.1 |
| Tundra and alpine | 37.2 |
| Desert scrub | 9.7 |
| Study Type | Geographic Areas | Mean C Accumulation t ha−1 Year−1 | Depths 1 Sampled (cm) | No. Studies or Sites Included | Reference |
|---|---|---|---|---|---|
| Annual crops to perennial pasture or restored native grassland | |||||
| Meta-analysis | Central Europe, N. America, Russia | 0.72 | 0–30 | 273 | [89] |
| Meta-analysis | Russia | 0.96 | 20 | 45 | [90] |
| Meta-analysis | Tropical to temperate | 0.33 | 5–300 | 39 | [91] |
| Meta-analysis | Americas, UK, Australia | 1.01 | NR 2 | 23 | [92] |
| Review | N. Midwest USA | 0.44–0.5 | 25 | 39 | [93] |
| Review | W. Canada | 0.59 | NR | 17 | [94] |
| Chronosequences | Illinois, USA | 0.43 | 100 | 16 | [95] |
| Review | France | 0.50 | NR | - | [96] |
| Review | NR | 0.3–1.0 | NR | - | [97] |
| Annual crops to perennial bioenergy crops | |||||
| Meta-analysis | NR | 1.14–1.88 | 0–150 | 23 | [98] |
| Meta-analysis | N. & S. America, Europe | ||||
| Miscanthus | S. Africa, Asia | 1.09 | 100 | 13 | [99] |
| Switchgrass | 1.28 | 100 | 40 | [99] | |
| Assumptions | Area | Potential C Accumulation | SOC Accumulation Year−1 Until Equilibrium Reached | ||
|---|---|---|---|---|---|
| 30 Years | 60 Years | 90 Years | |||
| Conservative | Global (Pg) | 13 | 0.42 | 0.21 | 0.14 |
| Baseline 1 | Average t ha−1 | 11.9 | 0.40 | 0.20 | 0.13 |
| Full profile | Global (Pg) | 25 | 0.84 | 0.42 | 0.28 |
| C accumulation 2 | Average t ha−1 | 23.9 | 0.80 | 0.40 | 0.27 |
| 50% SOC lost | Global (Pg) | 21 | 0.70 | 0.35 | 0.23 |
| with conversion 3 | Average t ha−1 | 19.9 | 0.66 | 0.33 | 0.22 |
| 90% of potential | Global (Pg) | 16 | 0.54 | 0.27 | 0.18 |
| Accumulation 4 | Average t ha−1 | 15.3 | 0.51 | 0.26 | 0.17 |
| Maximum | Global (Pg) | 54 | 1.80 | 0.90 | 0.60 |
| Accumulation 5 | Average t ha−1 | 51.2 | 1.70 | 0.85 | 0.57 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crews, T.E.; Rumsey, B.E. What Agriculture Can Learn from Native Ecosystems in Building Soil Organic Matter: A Review. Sustainability 2017, 9, 578. https://doi.org/10.3390/su9040578
Crews TE, Rumsey BE. What Agriculture Can Learn from Native Ecosystems in Building Soil Organic Matter: A Review. Sustainability. 2017; 9(4):578. https://doi.org/10.3390/su9040578
Chicago/Turabian StyleCrews, Timothy E., and Brian E. Rumsey. 2017. "What Agriculture Can Learn from Native Ecosystems in Building Soil Organic Matter: A Review" Sustainability 9, no. 4: 578. https://doi.org/10.3390/su9040578
APA StyleCrews, T. E., & Rumsey, B. E. (2017). What Agriculture Can Learn from Native Ecosystems in Building Soil Organic Matter: A Review. Sustainability, 9(4), 578. https://doi.org/10.3390/su9040578





