Enhancing the Dielectric Properties of Recycled Polyolefin Streams Through Blending
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
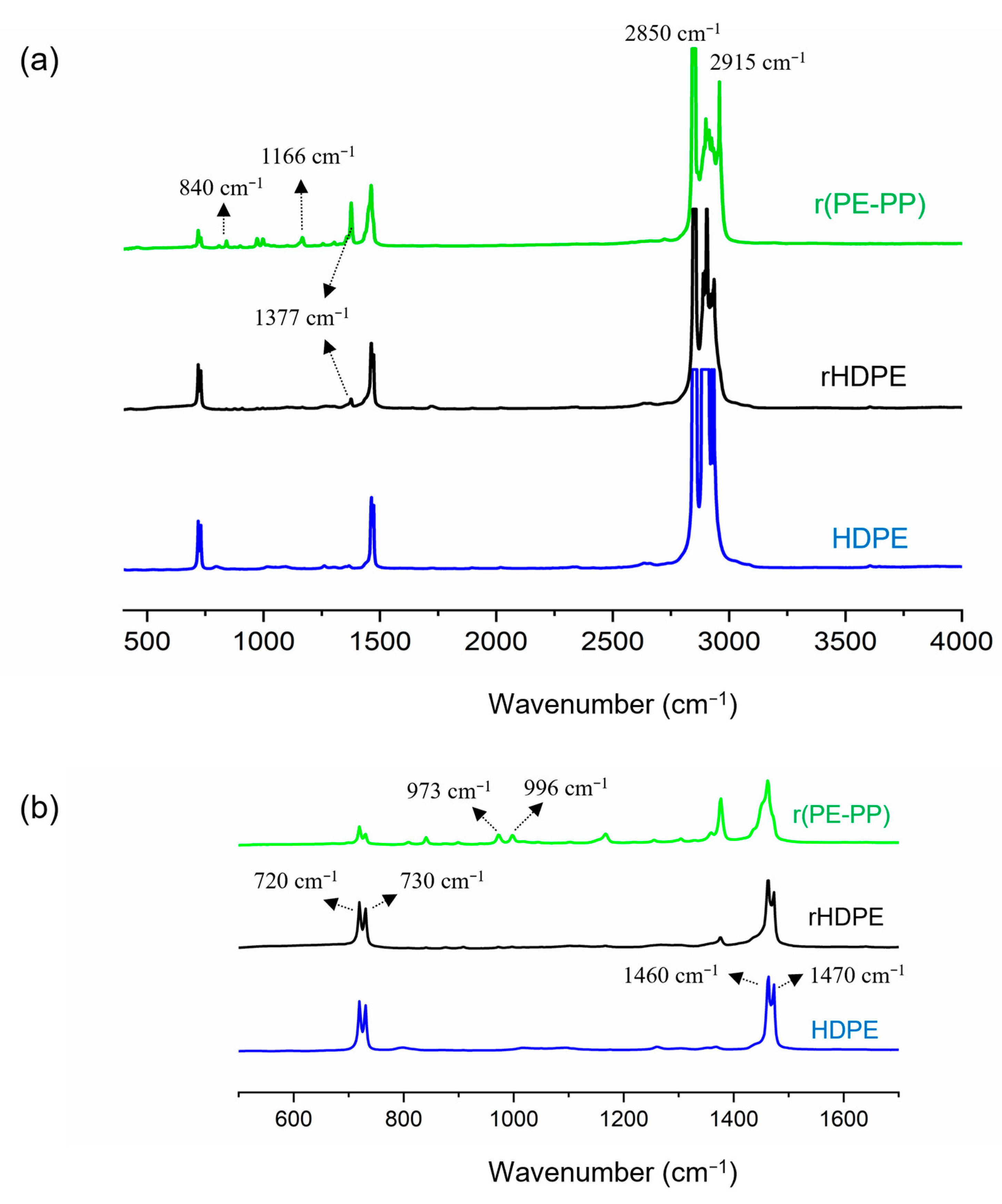
2.3.1. Chemical Characterization
2.3.2. Thermal Characterization
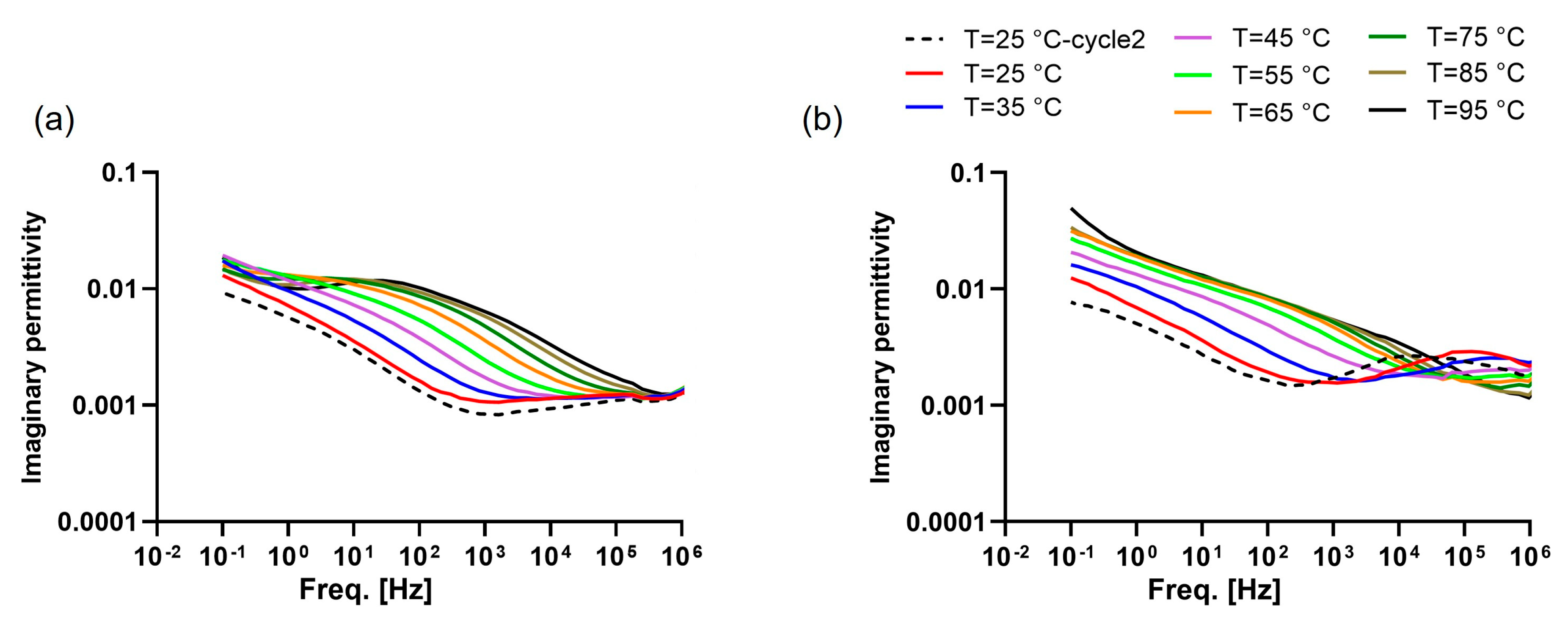
2.3.3. Dielectric Response
- (1)
- The dielectric response was assessed at various temperatures ranging from 25 °C to 95 °C across a frequency spectrum from 10−1 to 106 Hz with steps of 10 °C between each isothermal scan, with the measuring chamber continuously purged with dry nitrogen. After reaching the highest temperature, the sample cooled down to 25 °C, and the dielectric spectrum was measured again in order to examine the effect of the heating process on the dielectric response.
- (2)
- The samples were kept at a constant temperature of 70 °C, and the dielectric response was measured every 10 min to analyze the effect of high temperature on dielectric response.
- (3)
- The samples were subjected to heating and cooling cycles between 25 °C and 70 °C to mitigate the limits of evaluating the dielectric response at higher temperatures observed in the previous step. In each cycle, the sample was held at 70 °C for 10 min, then cooled back to 25 °C before measurements were performed at this temperature.
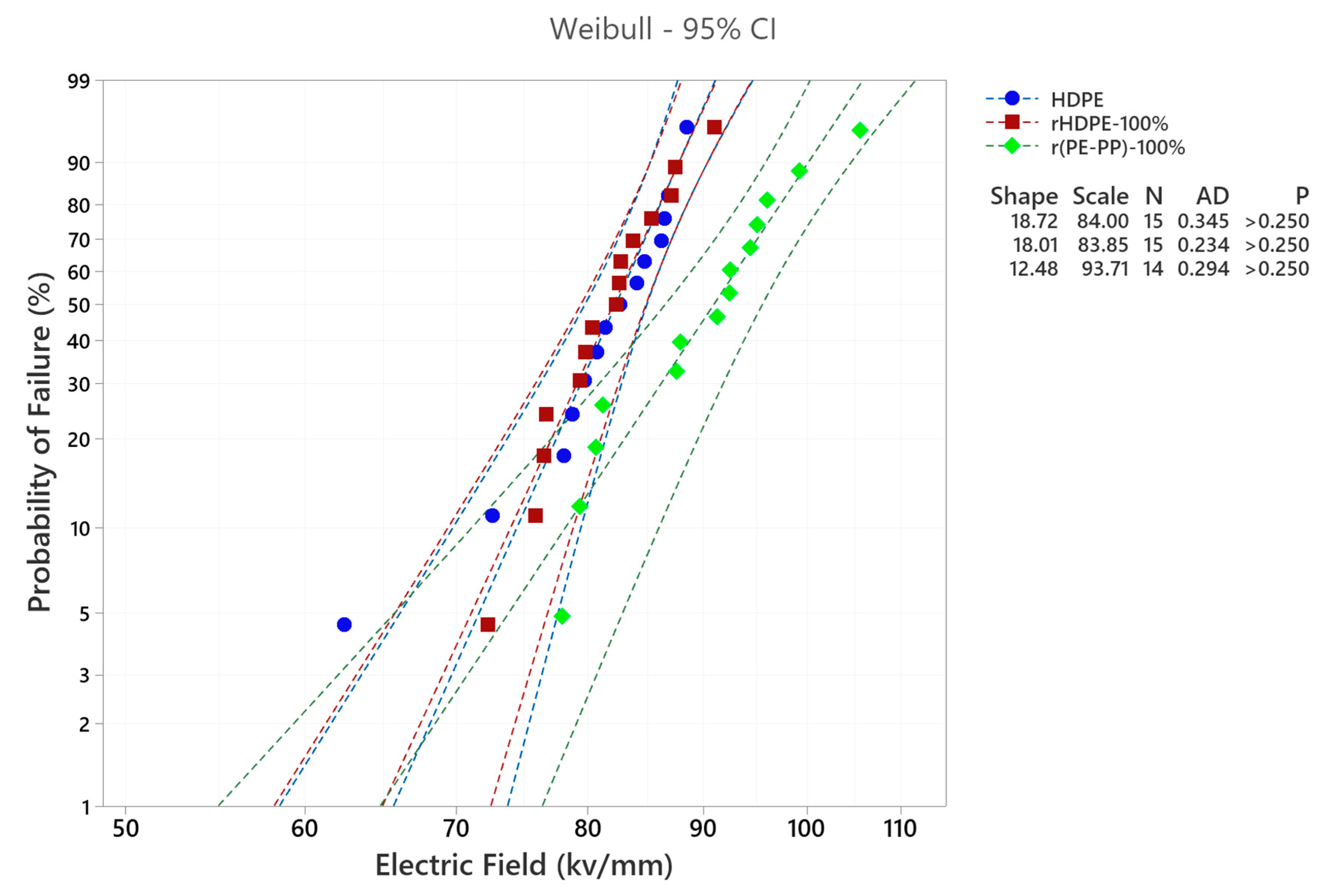
2.3.4. Dielectric Breakdown Strength
3. Results and Discussion
3.1. Chemical Characterization of Recycled Samples
3.2. Thermal Analysis (DSC, TGA, Pyrolysis) of Recycled HDPE
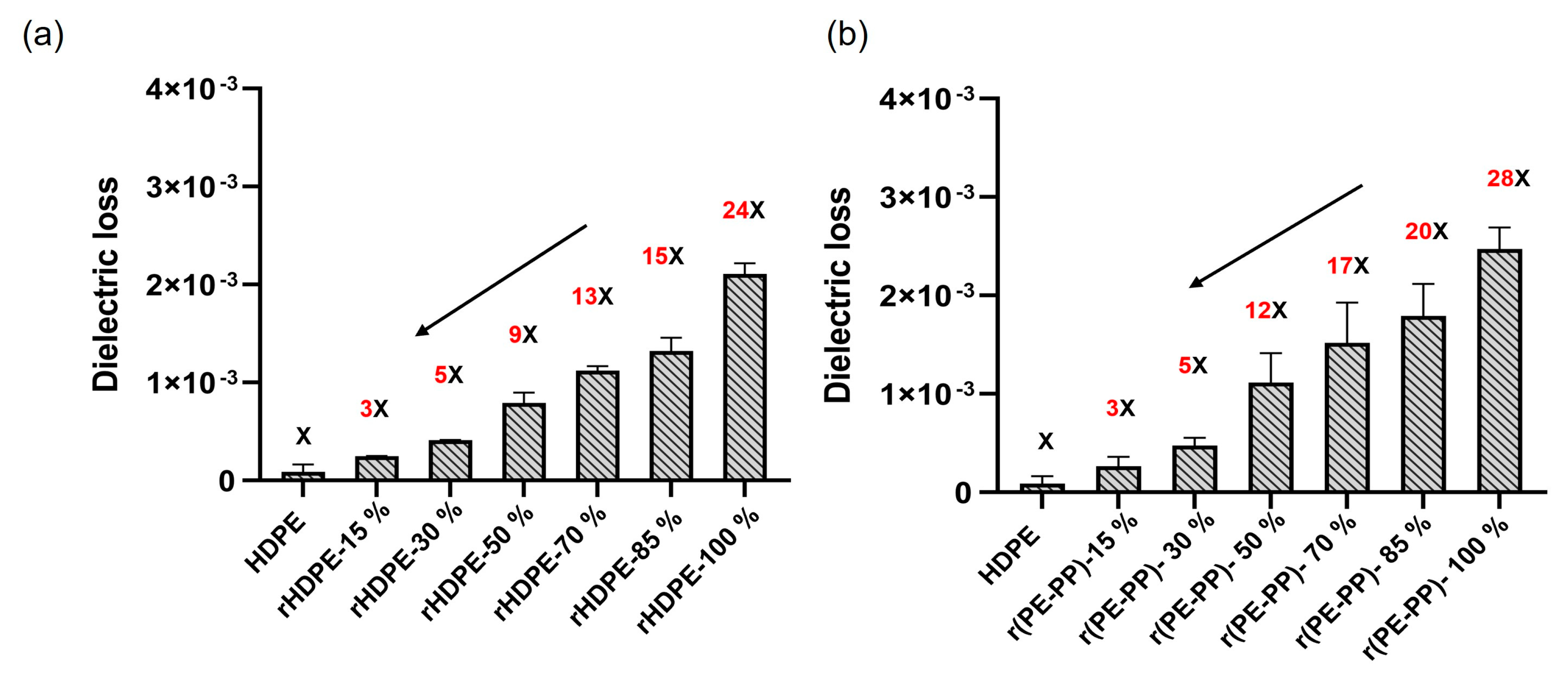
3.3. Dielectric Properties of Recycled Materials
3.4. Blending
3.4.1. Thermal Analysis
3.4.2. Dielectric Behavior of Blended Material
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Jiang, P.; Tanaka, T. Material progress toward recyclable insulation of power cables. Part 1: Polyethylene-based thermoplastic materials: Dedicated to the 80th birthday of professor Toshikatsu Tanaka. IEEE Electr. Insul. Mag. 2019, 35, 7–19. [Google Scholar] [CrossRef]
- Cecon, V.S.; Da Silva, P.F.; Vorst, K.L.; Curtzwiler, G.W. The effect of post-consumer recycled polyethylene (PCRPE) on the properties of polyethylene blends of different densities. Polym. Degrad. Stab. 2021, 190, 109627. [Google Scholar] [CrossRef]
- Zhang, J.; Hirschberg, V.; Rodrigue, D. Blending Recycled High-Density Polyethylene HDPE (rHDPE) with Virgin (vHDPE) as an Effective Approach to Improve the Mechanical Properties. Recycling 2023, 8, 2. [Google Scholar] [CrossRef]
- Jones, H.; McClements, J.; Ray, D.; Hindle, C.S.; Kalloudis, M.; Koutsos, V. Thermomechanical Properties of Virgin and Recycled Polypropylene—High-Density Polyethylene Blends. Polymers 2023, 15, 4200. [Google Scholar] [CrossRef]
- Yin, S.; Tuladhar, R.; Shi, F.; Shanks, R.A.; Combe, M.; Collister, T. Mechanical reprocessing of polyolefin waste: A review. Polym. Eng. Sci. 2015, 55, 2899–2909. [Google Scholar] [CrossRef]
- Sultana, S.M.N.; Helal, E.; Gutiérrez, G.; David, E.; Moghimian, N.; Demarquette, N.R. Effect of Few-Layer Graphene on the Properties of Mixed Polyolefin Waste Stream. Crystals 2023, 13, 358. [Google Scholar] [CrossRef]
- Sultana, S.M.N.; Helal, E.; Gutiérrez, G.; David, E.; Moghimian, N.; Demarquette, N.R. The Influence of a Commercial Few-Layer Graphene on the Photodegradation Resistance of a Waste Polyolefins Stream and Prime Polyolefin Blends. Recycling 2024, 9, 29. [Google Scholar] [CrossRef]
- Shirzaei Sani, I.; Demarquette, N.R.; David, E. Investigation and characterization of dielectric, thermal, and chemical properties of recycled high-density polyethylene blended with virgin polyethylene. Polym. Eng. Sci. 2023, 63, 3254–3267. [Google Scholar] [CrossRef]
- ASTM D-3418; Standard Test Method for Transition Annual Book of ASTM. ASTM: Philadelphia, PA, USA, 2015.
- ASTM D-149; Standard Test Method for Dielectric Breakdown Voltage and Dielectric Strength of Solid Electrical Insulating Materials at Commercial Power Frequencies. ASTM: Philadelphia, PA, USA, 2020.
- IEEE-930; IEEE Guide for the Statistical Analysis of Electrical Insulation Breakdown Data. Electrical Insulation Society: New York, NY, USA, 2005.
- Dissado, L.A.; Fothergill, J.C. Electrical Degradation and Breakdown in Polymers. Statistical Features of Breakdown; IET: London, UK, 1992; pp. 319–355. [Google Scholar]
- Gala, A.; Guerrero, M.; Serra, J.M. Characterization of post-consumer plastic film waste from mixed MSW in Spain: A key point for the successful implementation of sustainable plastic waste management strategies. Waste Manag. 2020, 111, 22–33. [Google Scholar] [CrossRef]
- Smith, B. The Infrared Spectra of Polymers II: Polyethylene. Spectroscopy 2021, 36, 24–29. [Google Scholar] [CrossRef]
- Andreassen, E. Infrared and Raman Spectroscopy of Polypropylene. In Polypropylene: An A–Z Reference; Karger-Kocsis, J., Ed.; Springer: Dordrecht, The Netherlands, 1999; pp. 320–328. [Google Scholar]
- Camacho, W.; Karlsson, S. NIR, DSC, and FTIR as quantitative methods for compositional analysis of blends of polymers obtained from recycled mixed plastic waste. Polym. Eng. Sci. 2001, 41, 1626–1635. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, L.; Sutton, D.; Wang, X.; Lin, T. Needleless Melt-Electrospinning of Polypropylene Nanofibres. J. Nanomater. 2012, 2012, 382639. [Google Scholar] [CrossRef]
- Heydariaraghi, M.; Ghorbanian, S.; Hallajisani, A.; Salehpour, A. Fuel properties of the oils produced from the pyrolysis of commonly-used polymers: Effect of fractionating column. J. Anal. Appl. Pyrolysis 2016, 121, 307–317. [Google Scholar] [CrossRef]
- Gall, M.; Freudenthaler, P.J.; Fischer, J.; Lang, R.W. Characterization of Composition and Structure–Property Relationships of Commercial Post-Consumer Polyethylene and Polypropylene Recyclates. Polymers 2021, 13, 1574. [Google Scholar] [CrossRef]
- Smith, B. The infrared spectra of polymers III: Hydrocarbon polymers. Spectroscopy 2021, 6, 22–25. [Google Scholar] [CrossRef]
- Turku, I.; Kärki, T.; Rinne, K.; Puurtinen, A. Characterization of plastic blends made from mixed plastics waste of different sources. Waste Manag. Res. 2017, 35, 200–206. [Google Scholar] [CrossRef]
- Thoden van Velzen, E.U.; Chu, S.; Alvarado Chacon, F.; Brouwer, M.T.; Molenveld, K. The impact of impurities on the mechanical properties of recycled polyethylene. Packag. Technol. Sci. 2021, 34, 219–228. [Google Scholar] [CrossRef]
- Dakin, T.W. Conduction and polarization mechanisms and trends in dielectric. IEEE Electr. Insul. Mag. 2006, 22, 11–28. [Google Scholar] [CrossRef]
- Ciuprina, F.; Plesa, I.; Notingher, P.V.; Zaharescu, T.; Rain, P.; Panaitescu, D. Dielectric properties of LDPE-SiO2 nanocomposites. In Proceedings of the 2010 10th IEEE International Conference on Solid Dielectrics, Potsdam, Germany, 4–9 July 2010; pp. 1–4. [Google Scholar]
- Zois, H.; Apekis, L.; Omastova, M. Electrical properties and percolation phenomena in carbon black filled polymer composites. In Proceedings of the 10th International Symposium on Electrets (ISE 10) (Cat. No.99 CH36256), Athens, Greece, 22–24 September 1999; pp. 529–532. [Google Scholar]
- Tomer, V.; Polizos, G.; Randall, C.A.; Manias, E. Polyethylene nanocomposite dielectrics: Implications of nanofiller orientation on high field properties and energy storage. J. Appl. Phys. 2011, 109, 074113. [Google Scholar] [CrossRef]
- Tripathi, N.; Thakur, A.K.; Shukla, A.; Marx, D.T. Dielectric, transport and thermal properties of clay based polymer- nanocomposites. Polym. Eng. Sci. 2018, 58, 220–227. [Google Scholar] [CrossRef]
- Böhning, M.; Goering, H.; Fritz, A.; Brzezinka, K.-W.; Turky, G.; Schönhals, A.; Schartel, B. Dielectric Study of Molecular Mobility in Poly(propylene-graft-maleic anhydride)/Clay Nanocomposites. Macromolecules 2005, 38, 2764–2774. [Google Scholar] [CrossRef]
- Cheng, Y.; Bai, L.; Yu, G.; Zhang, X. Effect of particles size on dielectric properties of nano-ZnO/LDPE composites. Materials 2018, 12, 5. [Google Scholar] [CrossRef]
- Li, Z.; Okamoto, K.; Ohki, Y.; Tanaka, T. The role of nano and micro particles on partial discharge and breakdown strength in epoxy composites. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 675–681. [Google Scholar] [CrossRef]
- Nelson, J.K.; Fothergill, J.C. Internal charge behaviour of nanocomposites. Nanotechnology 2004, 15, 586. [Google Scholar] [CrossRef]
- Angalane, S.K.; Kasinathan, E. A review on polymeric insulation for high-voltage application under various stress conditions. Polym. Compos. 2022, 43, 4803–4834. [Google Scholar] [CrossRef]
- Wong, A.C.-Y.; Lam, F. Study of selected thermal characteristics of polypropylene/polyethylene binary blends using DSC and TGA. Polym. Test. 2002, 21, 691–696. [Google Scholar] [CrossRef]
- Kazemi, Y.; Ramezani Kakroodi, A.; Rodrigue, D. Compatibilization Efficiency in Post-Consumer Recycled Polyethylene/Polypropylene Blends: Effect of Contamination. Polym. Eng. Sci. 2015, 55, 2368–2376. [Google Scholar] [CrossRef]
- Ahmed Dabbak, S.Z.; Illias, H.A.; Ang, B.C.; Abdul Latiff, N.A.; Makmud, M.Z. Electrical Properties of Polyethylene/Polypropylene Compounds for High-Voltage Insulation. Energies 2018, 11, 1448. [Google Scholar] [CrossRef]






| Sample/Element | C | O | Ti | Ca | Si | Al | S | Cl | K | Mg | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rHDPE | 96.49 | 1.91 | 0.88 | 0.23 | 0.1 | 0.22 | 0.05 | 0.04 | 0.08 | - | - |
| r(PE-PP) | 97.47 | 1.51 | 0.39 | 0.25 | 0.08 | 0.27 | - | 0.04 | - | - | - |
| HDPE | 98.09 | 1.06 | - | - | 0.71 | 0.14 | - | - | - | - | - |
| Sample | wt% of Virgin HDPE | wt% of Recycled rHDPE | wt% of Recycled r(PE-PP) |
|---|---|---|---|
| HDPE | 100 | 0 | 0 |
| r(PE-PP)-100% | 0 | 0 | 100 |
| r(PE-PP)-85% | 15 | 0 | 85 |
| r(PE-PP)-70% | 30 | 0 | 70 |
| r(PE-PP)-50% | 50 | 0 | 50 |
| r(PE-PP)-30% | 70 | 0 | 30 |
| r(PE-PP)-15% | 85 | 0 | 15 |
| rHDPE-100% | 0 | 100 | 0 |
| rHDPE-85% | 15 | 85 | 0 |
| rHDPE-70% | 30 | 70 | 0 |
| rHDPE-50% | 50 | 50 | 0 |
| rHDPE-30% | 70 | 30 | 0 |
| rHDPE-15% | 85 | 15 | 0 |
| Polymer Blend | ΔHPE | ΔHtotal | PP% | ||||
|---|---|---|---|---|---|---|---|
| HDPE | 133 | - | 120 | - | 233.2 | 233.2 | 0 |
| r(PE-PP)-30% | 132 | 161 | 121 | - | 178.2 | 190.2 | 7 |
| r(PE-PP)-50% | 132 | 161 | 121 | - | 145.1 | 167.3 | 13 |
| r(PE-PP)-70% | 131 | 160 | 121 | 123 | 121.1 | 156.5 | 22 |
| r(PE-PP)-100% | 130 | 161 | 119 | 124 | 69.6 | 119 | 43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirzaei Sani, I.; Demarquette, N.R.; David, E. Enhancing the Dielectric Properties of Recycled Polyolefin Streams Through Blending. Sustainability 2025, 17, 4123. https://doi.org/10.3390/su17094123
Shirzaei Sani I, Demarquette NR, David E. Enhancing the Dielectric Properties of Recycled Polyolefin Streams Through Blending. Sustainability. 2025; 17(9):4123. https://doi.org/10.3390/su17094123
Chicago/Turabian StyleShirzaei Sani, Iman, Nicole R. Demarquette, and Eric David. 2025. "Enhancing the Dielectric Properties of Recycled Polyolefin Streams Through Blending" Sustainability 17, no. 9: 4123. https://doi.org/10.3390/su17094123
APA StyleShirzaei Sani, I., Demarquette, N. R., & David, E. (2025). Enhancing the Dielectric Properties of Recycled Polyolefin Streams Through Blending. Sustainability, 17(9), 4123. https://doi.org/10.3390/su17094123








