The Potential of Hydroxyapatite for the Remediation of Lead-Contaminated Territories: A Case Study of Soils in Primorsky Krai
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sampling and Preparation of Soils
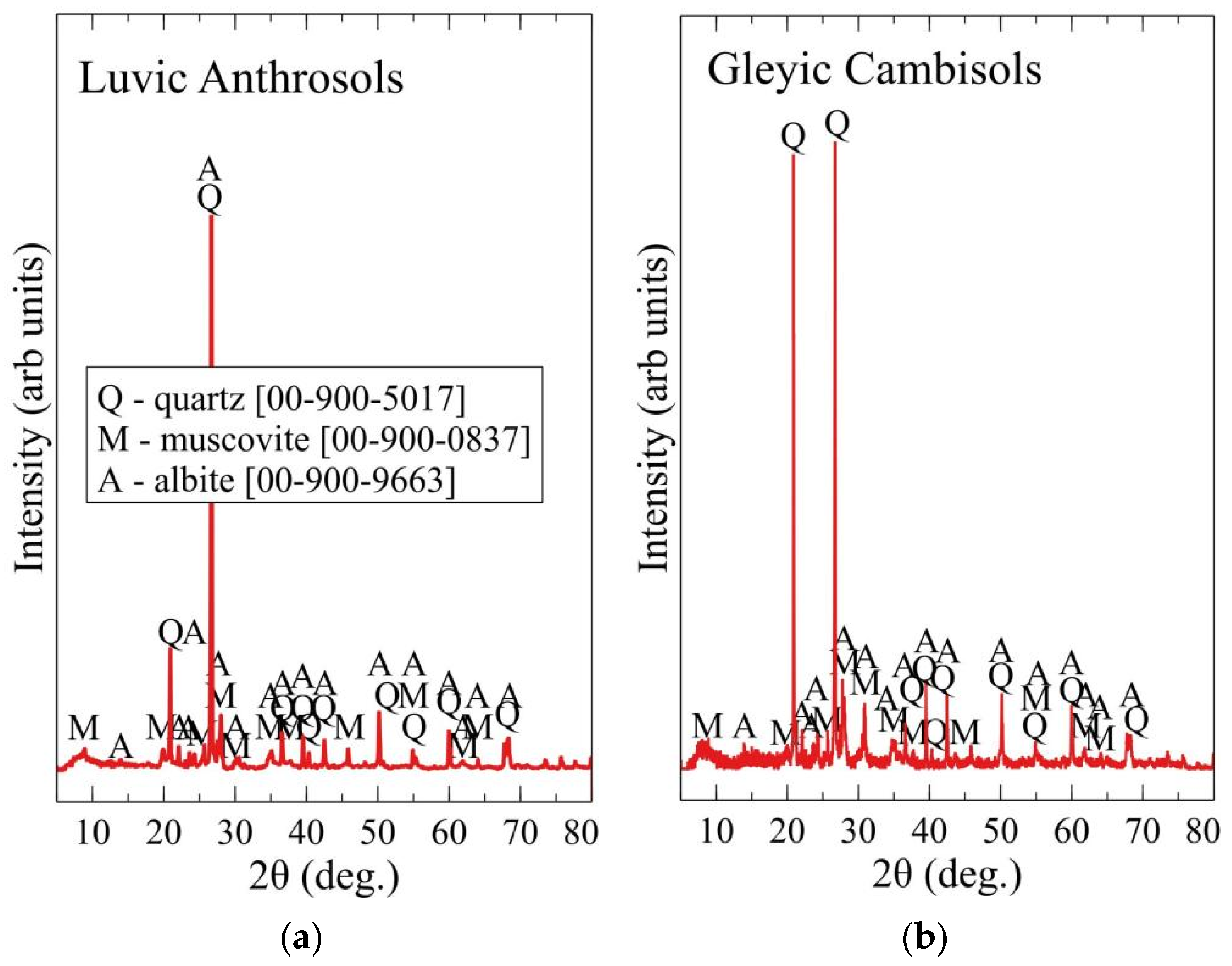
2.3. Evaluation of the Main Soil Characteristics
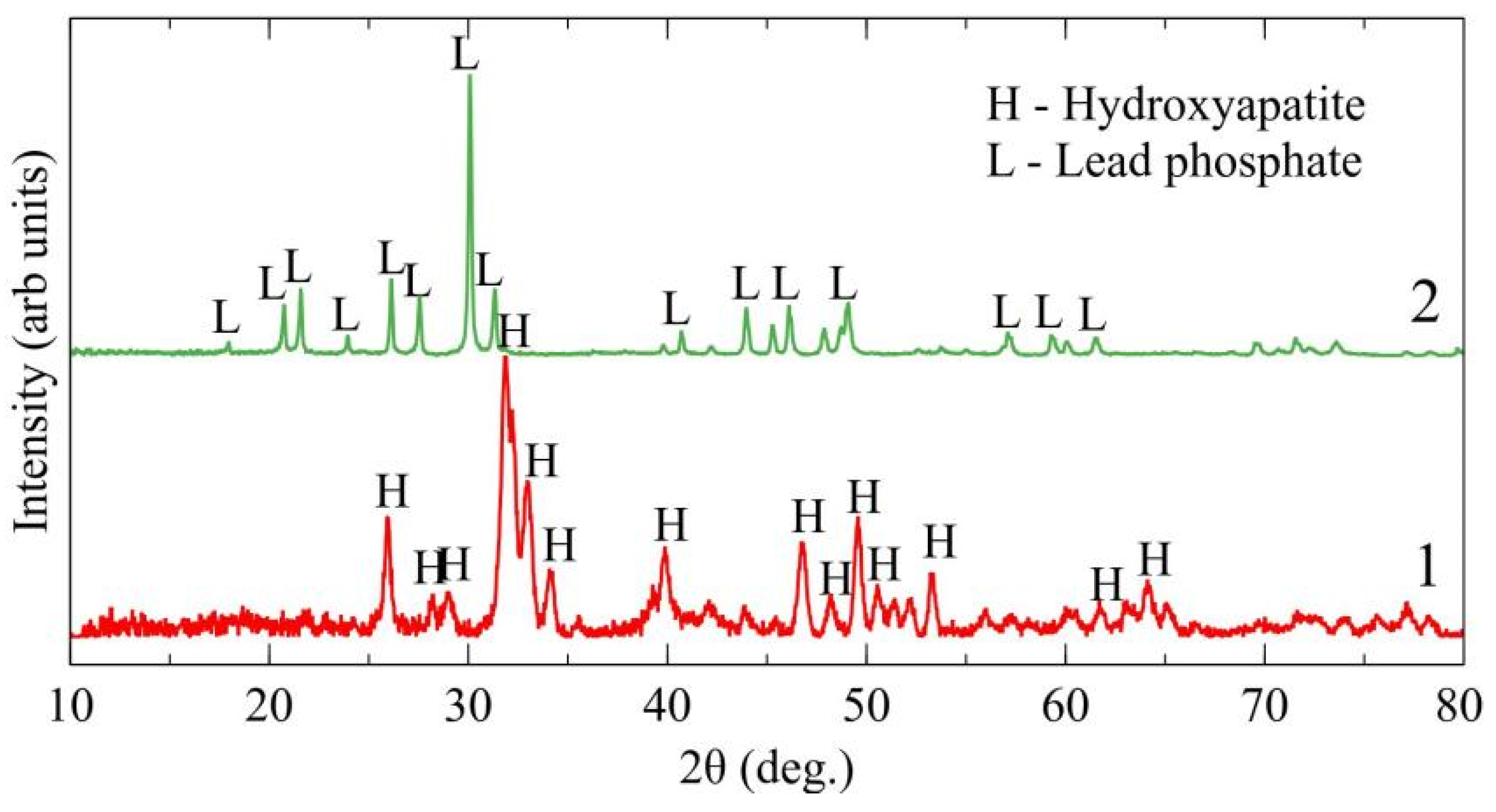
2.4. Synthesis of Hydroxyapatite
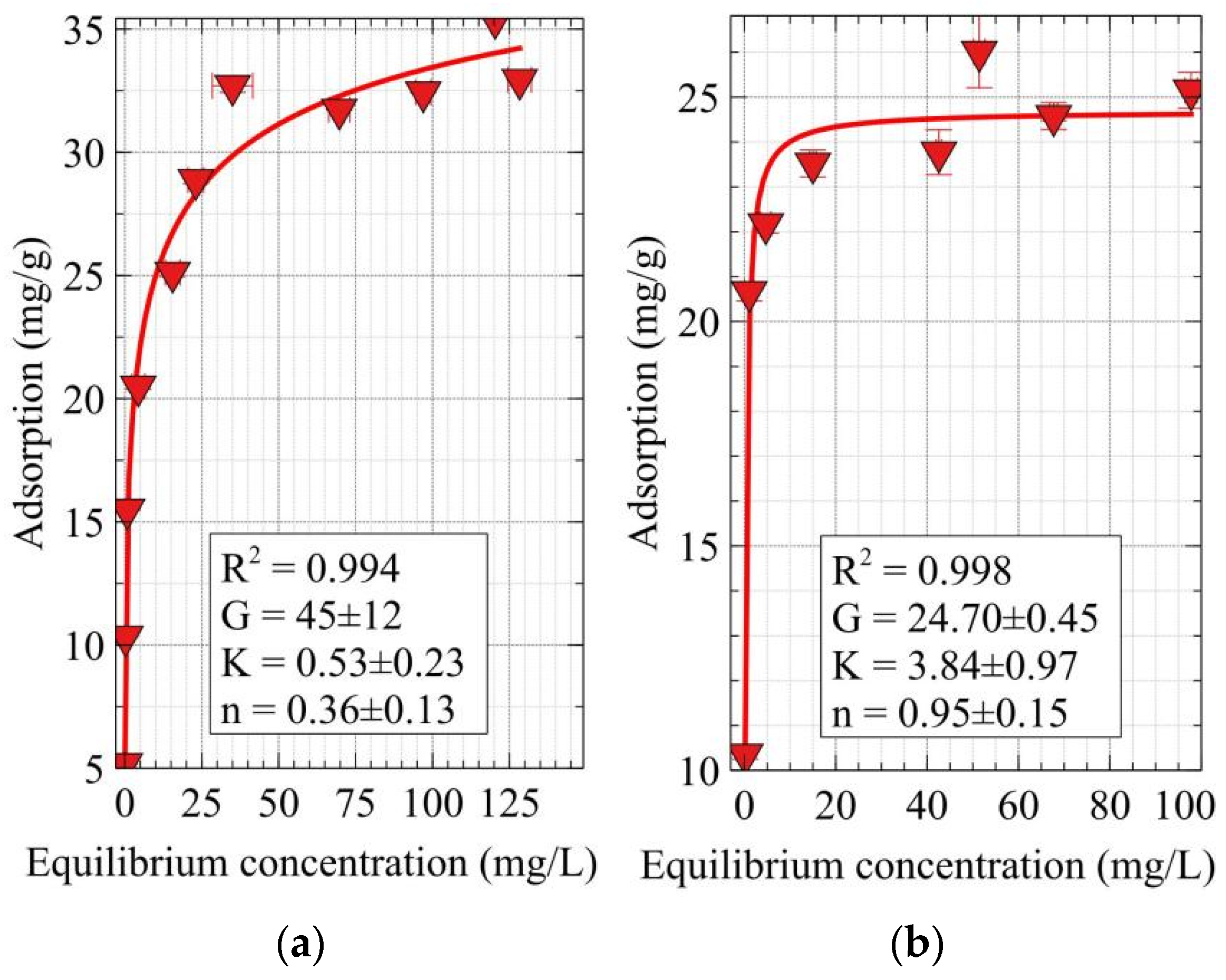
2.5. Adsorption Experiments
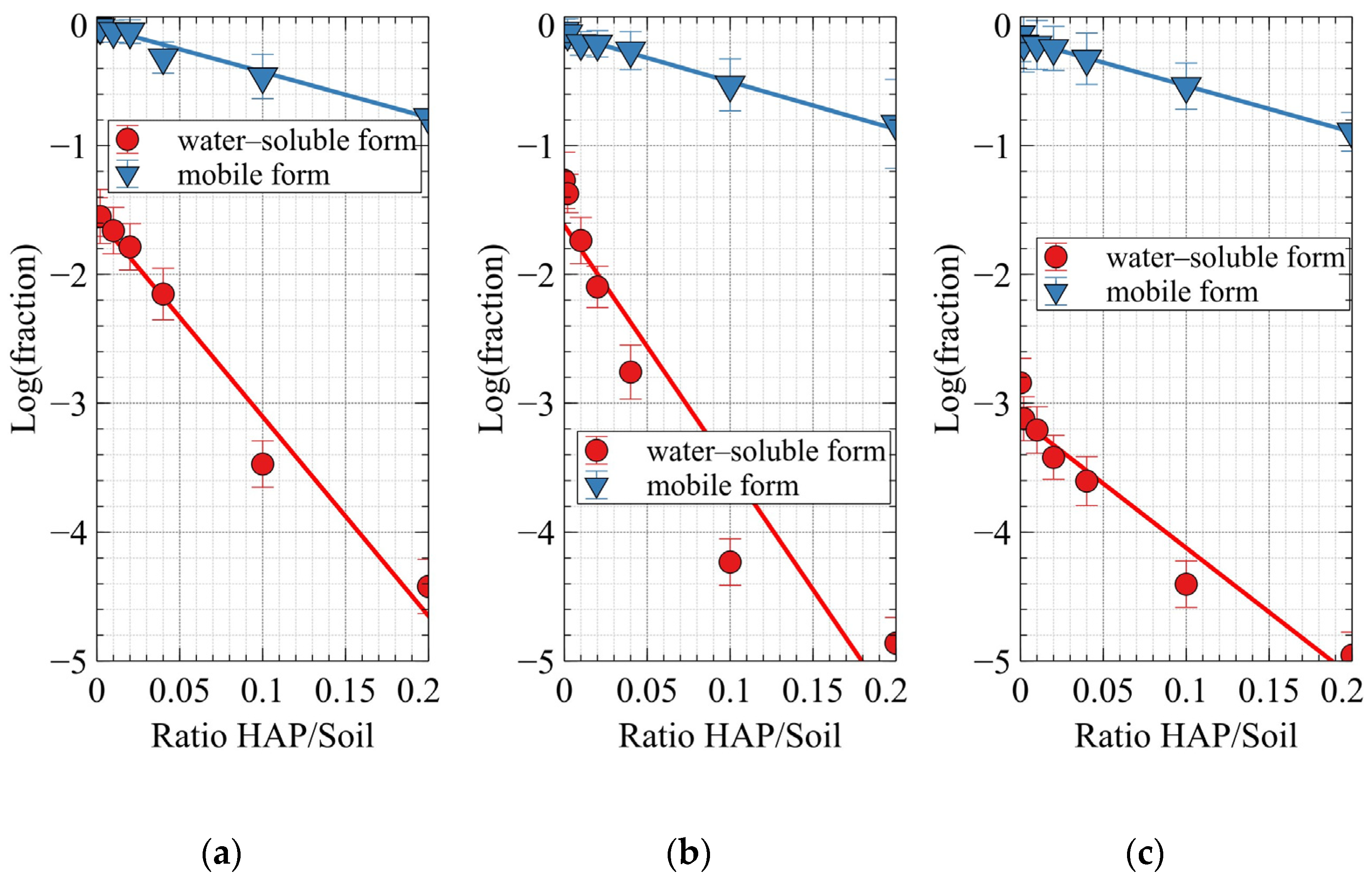
2.6. Determination of Pb Forms in Soil Samples
2.7. Biological Testing
2.8. Equipment and Software
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.-S.; Beiyuan, J.; Tsang, D.C.W.; Wang, L.; Poon, C.S.; Li, X.-D.; Fendorf, S. Arsenic-Containing Soil from Geogenic Source in Hong Kong: Leaching Characteristics and Stabilization/Solidification. Chemosphere 2017, 182, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhao, Y.; Li, J.; Beiyuan, J.; Tsang, D.C.W.; Poon, C.; Chan, T.; Wang, W.; Li, X. Speciation, Mobilization, and Bioaccessibility of Arsenic in Geogenic Soil Profile from Hong Kong. Environ. Pollut. 2018, 232, 375–384. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar Application for the Remediation of Heavy Metal Polluted Land: A Review of in Situ Field Trials. Sci. Total Environ. 2018, 619–620, 815–826. [Google Scholar] [CrossRef]
- Hou, D.; Li, F. Complexities Surrounding China’s Soil Action Plan. Land Degrad. Dev. 2017, 28, 2315–2320. [Google Scholar] [CrossRef]
- Luo, X.; Yu, S.; Zhu, Y.; Li, X. Trace Metal Contamination in Urban Soils of China. Sci. Total Environ. 2012, 421–422, 17–30. [Google Scholar] [CrossRef]
- Davis, H.T.; Marjorie Aelion, C.; McDermott, S.; Lawson, A.B. Identifying Natural and Anthropogenic Sources of Metals in Urban and Rural Soils Using GIS-Based Data, PCA, and Spatial Interpolation. Environ. Pollut. 2009, 157, 2378–2385. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Li, X.; Shi, W.; Cheung, S.C.; Thornton, I. Metal Contamination in Urban, Suburban, and Country Park Soils of Hong Kong: A Study Based on GIS and Multivariate Statistics. Sci. Total Environ. 2006, 356, 45–61. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A Review of Soil Heavy Metal Pollution from Industrial and Agricultural Regions in China: Pollution and Risk Assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Tsadilas, C.D.; Rinklebe, J. Immobilization of Soil Copper Using Organic and Inorganic Amendments. J. Plant Nutr. Soil Sci. 2015, 178, 112–117. [Google Scholar] [CrossRef]
- Nicholson, F.A.; Smith, S.R.; Alloway, B.J.; Carlton-Smith, C.; Chambers, B.J. An Inventory of Heavy Metals Inputs to Agricultural Soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar] [CrossRef]
- Luo, W.; Lu, Y.; Giesy, J.P.; Wang, T.; Shi, Y.; Wang, G.; Xing, Y. Effects of Land Use on Concentrations of Metals in Surface Soils and Ecological Risk around Guanting Reservoir, China. Environ. Geochem. Health 2007, 29, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, M.B.; Ali, S.; Farid, M.; Farooq, M.A.; Tauqeer, H.M.; Iftikhar, U.; Hannan, F.; Bharwana, S.A. Heavy Metal Pollution, a Global Problem and Its Remediation by Chemically Enhanced Phytoremediation: A Review. J. Biodivers. Environ. Sci. 2013, 3, 12–20. [Google Scholar]
- Vodyanitskiy, Y.N. Modern trends in soil pollution by heavy metals. Agrochemistry 2013, 88–96. Available online: https://www.elibrary.ru/item.asp?id=20250698 (accessed on 14 February 2025). (In Russian).
- Rastanina, N.K. Negative Environmental Impact Of Man–Made Facilities of a Closed Tin Ore Enterprise in Primorsky Krai on the Example of Khrustalnensky Gok. Eur. Sci. Forum Collect. Artic. III Int. Sci. Pract. Conf. Petrozavodsk 2020, 205–208. Available online: https://www.elibrary.ru/item.asp?id=42593195 (accessed on 14 February 2025). (In Russian).
- Khanchuk, A.I.; Krupskaya, L.T.; Zvereva, V.P. Ecological Problems of Development of Tin Ore Resources in Primorie and Priamurie. Geogr. Nat. Resour. 2012, 33, 45–49. [Google Scholar] [CrossRef]
- Yakunina, G.A.; Salomatova, L.P.; Kostenko, M.Y.; Shiyanova, E.A.; Kravtsova, V.M. Hygienic assessment of the soil condition in the territories of the Dalnegorsk urban district and the Terneysky municipal district. Health Med. Ecol. Nauka 2015, 62, 100–105. (In Russian) [Google Scholar]
- Krupskaya, L.T.; Zvereva, V.P.; Volobueva, N.G. Providing Stable Development of Technogenic Systems Formed during Mineral Exploration in the Far Eastern Federal District. Appl. Mech. Mater. 2014, 644–650, 5379–5382. [Google Scholar] [CrossRef]
- Krupskaya, L.T.; Zvereva, V.P.; Kirienko, O.A.; Imranova, E.L.; Volobueva, N.G. Using an Comprehensive Method for Evaluation of Environment State in a Zone of Influence of a Tailing Dam. Russ. J. Gen. Chem. 2016, 86, 3012–3014. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Wang, W.; Li, T.; He, Z.; Yang, X. Current Status of Agricultural Soil Pollution by Heavy Metals in China: A Meta-Analysis. Sci. Total Environ. 2019, 651, 3034–3042. [Google Scholar] [CrossRef]
- Arefieva, O.D.; Tregubova, V.G.; Gruschakova, N.V.; Starozhilov, V.T. Properties of Soils of Abandoned Coal Mine Industrial Areas (Primorsky Krai, Russia). J. Geosci. Environ. Prot. 2018, 6, 78. [Google Scholar] [CrossRef]
- Lopatina, A.A. Influence of Lead and Cadmium on Soybean Yield and Quality; Bulletin of the Krasnoyarsk State Agrarian University: Krasnoyarsk, Russia, 2017; pp. 160–166. (In Russian) [Google Scholar]
- Zharikova, E.A. Heavy metals in urban soils: Assessment of content and environmental risk // Proceedings of Tomsk Polytechnic University. Georesour. Eng. 2021, 332, 164–173. (In Russian) [Google Scholar]
- Soboleva, E.; Kovekovdova, L. Lead in soils and plants of the city of Ussurisk and the Ussurisky District. Res. Russ. 2003, 6, 2188–2195. (In Russian) [Google Scholar]
- Gao, C.; Lin, Q.; Bao, K.; Zhao, H.; Zhang, Z.; Xing, W.; Lu, X.; Wang, G. Historical Variation and Recent Ecological Risk of Heavy Metals in Wetland Sediments along Wusuli River, Northeast China. Environ. Earth Sci. 2014, 72, 4345–4355. [Google Scholar] [CrossRef]
- Shulkin, V.; Bogdanova, N. Concentration of Metals in the Rivers of the South Part of Primorski Krai, Russia. In Proceedings of the Climate Variability and Human Activities in Relation to Notheast Asia and Their Land-Ocean Interactions and Their Implications for Coastal Zone Management, Nanjing, China, 4–8 December 2004; pp. 28–36. [Google Scholar]
- Government of Primorsky Krai. Reports on The Environmental Situation in the Region. Available online: https://primorsky.ru/upload/Экoлoгический%20дoклад%20за%202022%20-публикация.pdf (accessed on 14 February 2025).
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Gurajala, H.K.; Rashid, M.S.; He, Z.; Yang, X. Efficiency of Lime, Biochar, Fe Containing Biochar and Composite Amendments for Cd and Pb Immobilization in a Co-Contaminated Alluvial Soil. Environ. Pollut. 2020, 257, 113609. [Google Scholar] [CrossRef]
- Lim, J.E.; Ahmad, M.; Lee, S.S.; Shope, C.L.; Hashimoto, Y.; Kim, K.-R.; Usman, A.R.A.; Yang, J.E.; Ok, Y.S. Effects of Lime-Based Waste Materials on Immobilization and Phytoavailability of Cadmium and Lead in Contaminated Soil. CLEAN-Soil Air Water 2013, 41, 1235–1241. [Google Scholar] [CrossRef]
- Holland, J.E.; Bennett, A.E.; Newton, A.C.; White, P.J.; McKenzie, B.M.; George, T.S.; Pakeman, R.J.; Bailey, J.S.; Fornara, D.A.; Hayes, R.C. Liming Impacts on Soils, Crops and Biodiversity in the UK: A Review. Sci. Total Environ. 2018, 610–611, 316–332. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Guo, Z.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Shen, F.; Li, R.; Zhang, Z. Impact of CaO, Fly Ash, Sulfur and Na2S on the (Im)Mobilization and Phytoavailability of Cd, Cu and Pb in Contaminated Soil. Ecotoxicol. Environ. Saf. 2016, 134, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.M.; Rinklebe, J. Impact of Emerging and Low Cost Alternative Amendments on the (Im)Mobilization and Phytoavailability of Cd and Pb in a Contaminated Floodplain Soil. Ecol. Eng. 2015, 74, 319–326. [Google Scholar] [CrossRef]
- Gu, H.-H.; Qiu, H.; Tian, T.; Zhan, S.-S.; Deng, T.-H.-B.; Chaney, R.L.; Wang, S.-Z.; Tang, Y.-T.; Morel, J.-L.; Qiu, R.-L. Mitigation Effects of Silicon Rich Amendments on Heavy Metal Accumulation in Rice (Oryza Sativa L.) Planted on Multi-Metal Contaminated Acidic Soil. Chemosphere 2011, 83, 1234–1240. [Google Scholar] [CrossRef]
- Ma, W.; Tai, L.; Qiao, Z.; Zhong, L.; Wang, Z.; Fu, K.; Chen, G. Contamination Source Apportionment and Health Risk Assessment of Heavy Metals in Soil around Municipal Solid Waste Incinerator: A Case Study in North China. Sci. Total Environ. 2018, 631–632, 348–357. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Rinklebe, J.; Selim, M.H. Impact of Various Amendments on Immobilization and Phytoavailability of Nickel and Zinc in a Contaminated Floodplain Soil. Int. J. Environ. Sci. Technol. 2015, 12, 2765–2776. [Google Scholar] [CrossRef]
- Ram, L.C.; Masto, R.E. Fly Ash for Soil Amelioration: A Review on the Influence of Ash Blending with Inorganic and Organic Amendments. Earth-Sci. Rev. 2014, 128, 52–74. [Google Scholar] [CrossRef]
- Milojković, J.; Pezo, L.; Stojanović, M.; Mihajlović, M.; Lopičić, Z.; Petrović, J.; Stanojević, M.; Kragović, M. Selected Heavy Metal Biosorption by Compost of Myriophyllum Spicatum—A Chemometric Approach. Ecol. Eng. 2016, 93, 112–119. [Google Scholar] [CrossRef]
- Katoh, M.; Kitahara, W.; Yagi, R.; Sato, T. Suitable Chemical Properties of Animal Manure Compost to Facilitate Pb Immobilization in Soil. Soil Sediment Contam. Int. J. 2014, 23, 523–539. [Google Scholar] [CrossRef]
- Mehmood, T.; Bibi, I.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Wang, H.; Ok, Y.S.; Sarkar, B.; Javed, M.T.; Murtaza, G. Effect of Compost Addition on Arsenic Uptake, Morphological and Physiological Attributes of Maize Plants Grown in Contrasting Soils. J. Geochem. Explor. 2017, 178, 83–91. [Google Scholar] [CrossRef]
- Ahmad, M.; Ok, Y.S.; Kim, B.-Y.; Ahn, J.-H.; Lee, Y.H.; Zhang, M.; Moon, D.H.; Al-Wabel, M.I.; Lee, S.S. Impact of Soybean Stover- and Pine Needle-Derived Biochars on Pb and As Mobility, Microbial Community, and Carbon Stability in a Contaminated Agricultural Soil. J. Environ. Manag. 2016, 166, 131–139. [Google Scholar] [CrossRef]
- Ahmad, M.; Ok, Y.S.; Rajapaksha, A.U.; Lim, J.E.; Kim, B.-Y.; Ahn, J.-H.; Lee, Y.H.; Al-Wabel, M.I.; Lee, S.-E.; Lee, S.S. Lead and Copper Immobilization in a Shooting Range Soil Using Soybean Stover- and Pine Needle-Derived Biochars: Chemical, Microbial and Spectroscopic Assessments. J. Hazard. Mater. 2016, 301, 179–186. [Google Scholar] [CrossRef]
- Moon, D.H.; Park, J.-W.; Chang, Y.-Y.; Ok, Y.S.; Lee, S.S.; Ahmad, M.; Koutsospyros, A.; Park, J.-H.; Baek, K. Immobilization of Lead in Contaminated Firing Range Soil Using Biochar. Environ. Sci. Pollut. Res. 2013, 20, 8464–8471. [Google Scholar] [CrossRef]
- Herath, I.; Iqbal, M.C.M.; Al-Wabel, M.I.; Abduljabbar, A.; Ahmad, M.; Usman, A.R.A.; Sik Ok, Y.; Vithanage, M. Bioenergy-Derived Waste Biochar for Reducing Mobility, Bioavailability, and Phytotoxicity of Chromium in Anthropized Tannery Soil. J. Soils Sediments 2017, 17, 731–740. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J. Effect of Ageing on Biochar Properties and Pollutant Management. Chemosphere 2022, 292, 133427. [Google Scholar] [CrossRef]
- Wang, L.; O’Connor, D.; Rinklebe, J.; Ok, Y.S.; Tsang, D.C.W.; Shen, Z.; Hou, D. Biochar Aging: Mechanisms, Physicochemical Changes, Assessment, And Implications for Field Applications. Environ. Sci. Technol. 2020, 54, 14797–14814. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.R.A.; Kuzyakov, Y.; Lorenz, K.; Stahr, K. Remediation of a Soil Contaminated with Heavy Metals by Immobilizing Compounds. J. Plant Nutr. Soil Sci. 2006, 169, 205–212. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, X.; Liu, F.; Hu, X.; Zhao, X.; Wang, L.; Gao, P.; Li, X.; Ji, P. Potential of Using a New Aluminosilicate Amendment for the Remediation of Paddy Soil Co-Contaminated with Cd and Pb. Environ. Pollut. 2021, 269, 116198. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Song, B.; Chen, Z.; Liu, Z.; Yu, L.; Zhi, Z.; Zhao, Y.; Wei, H.; Song, M. Immobilization of Heavy Metals in Ceramsite Prepared Using Contaminated Soils: Effectiveness and Potential Mechanisms. Chemosphere 2023, 310, 136846. [Google Scholar] [CrossRef]
- Houben, D.; Pircar, J.; Sonnet, P. Heavy Metal Immobilization by Cost-Effective Amendments in a Contaminated Soil: Effects on Metal Leaching and Phytoavailability. J. Geochem. Explor. 2012, 123, 87–94. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Ahmad, M.; Vithanage, M.; Kim, K.-R.; Chang, J.Y.; Lee, S.S.; Ok, Y.S. The Role of Biochar, Natural Iron Oxides, and Nanomaterials as Soil Amendments for Immobilizing Metals in Shooting Range Soil. Environ. Geochem. Health 2015, 37, 931–942. [Google Scholar] [CrossRef]
- Feng, X.H.; Zhai, L.M.; Tan, W.F.; Liu, F.; He, J.Z. Adsorption and Redox Reactions of Heavy Metals on Synthesized Mn Oxide Minerals. Environ. Pollut. 2007, 147, 366–373. [Google Scholar] [CrossRef]
- Dong, D.; Nelson, Y.M.; Lion, L.W.; Shuler, M.L.; Ghiorse, W.C. Adsorption of Pb and Cd onto Metal Oxides and Organic Material in Natural Surface Coatings as Determined by Selective Extractions: New Evidence for the Importance of Mn and Fe Oxides. Water Res. 2000, 34, 427–436. [Google Scholar] [CrossRef]
- Tournassat, C.; Charlet, L.; Bosbach, D.; Manceau, A. Arsenic(III) Oxidation by Birnessite and Precipitation of Manganese(II) Arsenate. Environ. Sci. Technol. 2002, 36, 493–500. [Google Scholar] [CrossRef]
- Tang, Y.; Webb, S.M.; Estes, E.R.; Hansel, C.M. Chromium(III) Oxidation by Biogenic Manganese Oxides with Varying Structural Ripening. Environ. Sci. Process. Impacts 2014, 16, 2127–2136. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.Q.; Chen, M.; Singh, S.P.; Harris, W.G. Impacts of Phosphate Amendments on Lead Biogeochemistry at a Contaminated Site. Environ. Sci. Technol 2002, 36, 5296–5304. [Google Scholar] [CrossRef] [PubMed]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in Soil Using Amendments—A Review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Matsufuru, H.; Takaoka, M.; Tanida, H.; Sato, T. Impacts of Chemical Amendment and Plant Growth on Lead Speciation and Enzyme Activities in a Shooting Range Soil: An X-Ray Absorption Fine Structure Investigation. J. Environ. Qual. 2009, 38, 1420–1428. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Shams, M.S.; Khalifa, M.R.; El-Dali, M.A.; Rinklebe, J. Various Soil Amendments and Environmental Wastes Affect the (Im)Mobilization and Phytoavailability of Potentially Toxic Elements in a Sewage Effluent Irrigated Sandy Soil. Ecotoxicol. Environ. Safety 2017, 142, 375–387. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Shi, H.; Zhao, X.; Yu, Y.; Qu, B. Immobilization of Pb and Cd in Contaminated Soil Using Nano-Crystallite Hydroxyapatite. Procedia Environ. Sci. 2013, 18, 657–665. [Google Scholar] [CrossRef]
- Sghaier, R.B.; Labidi, A.; Abdallah, M.A.; Latrous, L.; Megriche, A. Green Magnetic Snail Shell Hydroxyapatite Sorbent for Reliable Solid-Phase Extraction of Pesticides from Water Samples. J. Sep. Sci. 2023, 46, 2300290. [Google Scholar] [CrossRef] [PubMed]
- Alali, A.F.; Almojil, S.F.; Almohana, A.I.; Anqi, A.E.; Rajhi, A.A.; Alamri, S.; Dhahad, H.A. Hydroxyapatite@Mn–Fe Composite as a Reusable Sorbent for Removal of Nile Blue Dye and Cr(VI) from Polluted Water. Environ. Sci. Pollut. Res. 2023, 30, 18419–18437. [Google Scholar] [CrossRef]
- Rosskopfová, O.; Viglašová, E.; Galamboš, M.; Daňo, M.; Tóthová, D. The Removal of Pertechnetate from Aqueous Solution by Synthetic Hydroxyapatite: The Role of Reduction Reagents and Organic Ligands. Int. J. Environ. Res. Public Health 2023, 20, 3227. [Google Scholar] [CrossRef]
- Dong, L.; Zhu, Z.; Qiu, Y.; Zhao, J. Removal of Lead from Aqueous Solution by Hydroxyapatite/Magnetite Composite Adsorbent. Chem. Eng. J. 2010, 165, 827–834. [Google Scholar] [CrossRef]
- Long, Y.; Jiang, J.; Hu, J.; Hu, X.; Yang, Q.; Zhou, S. Removal of Pb(Ⅱ) from Aqueous Solution by Hydroxyapatite/Carbon Composite: Preparation and Adsorption Behavior. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 471–479. [Google Scholar] [CrossRef]
- Zuo, W.; Song, B.; Shi, Y.; Zupanic, A.; Guo, S.; Huang, H.; Jiang, L.; Yu, Y. Using Bacillus Thuringiensis HM-311@hydroxyapatite@biochar Beads to Remediate Pb and Cd Contaminated Farmland Soil. Chemosphere 2022, 307, 135797. [Google Scholar] [CrossRef]
- Ahmed, W.; Xu, T.; Mahmood, M.; Núñez-Delgado, A.; Ali, S.; Shakoor, A.; Qaswar, M.; Zhao, H.; Liu, W.; Li, W.; et al. Nano-Hydroxyapatite Modified Biochar: Insights into the Dynamic Adsorption and Performance of Lead (II) Removal from Aqueous Solution. Environ. Res. 2022, 214, 113827. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Hu, R.; Chen, G. Micro-Nano-Engineered Nitrogenous Bone Biochar Developed with a Ball-Milling Technique for High-Efficiency Removal of Aquatic Cd(II), Cu(II) and Pb(II). J. Hazard. Mater. 2020, 387, 121980. [Google Scholar] [CrossRef]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K.L. Latest Trends in Heavy Metal Removal from Wastewater by Biochar Based Sorbents. J. Water Process Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Zhang, D.; Ding, A.; Li, T.; Wu, X.; Liu, Y.; Naidu, R. Immobilization of Cd and Pb in a Contaminated Acidic Soil Amended with Hydroxyapatite, Bentonite, and Biochar. J. Soils Sediments 2021, 21, 2262–2272. [Google Scholar] [CrossRef]
- Zhang, D.; Li, T.; Ding, A.; Wu, X. Effects of an Additive (Hydroxyapatite–Bentonite–Biochar) on Cd and Pb Stabilization and Microbial Community Composition in Contaminated Vegetable Soil. RSC Adv. 2021, 11, 12200–12208. [Google Scholar] [CrossRef]
- Matei, E.; Predescu, A.M.; Râpă, M.; Țurcanu, A.A.; Mateș, I.; Constantin, N.; Predescu, C. Natural Polymers and Their Nanocomposites Used for Environmental Applications. Nanomaterials 2022, 12, 1707. [Google Scholar] [CrossRef]
- Liao, Q.; He, L.; Tu, G.; Yang, Z.; Yang, W.; Tang, J.; Cao, W.; Wang, H. Simultaneous Immobilization of Pb, Cd and As in Soil by Hybrid Iron-, Sulfate- and Phosphate-Based Bio-Nanocomposite: Effectiveness, Long-Term Stability and Bioavailablity/Bioaccessibility Evaluation. Chemosphere 2021, 266, 128960. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, L.; Yang, W.; Shi, W.; Tong, Y.; Chai, L.; Gao, S.; Liao, Q. Simultaneous Immobilization of Cadmium and Lead in Contaminated Soils by Hybrid Bio-Nanocomposites of Fungal Hyphae and Nano-Hydroxyapatites. Environ. Sci. Pollut. Res. 2018, 25, 11970–11980. [Google Scholar] [CrossRef]
- Arabyarmohammadi, H.; Darban, A.K.; van der Zee, S.E.A.T.M.; Abdollahy, M.; Ayati, B. Fractionation and Leaching of Heavy Metals in Soils Amended with a New Biochar Nanocomposite. Environ. Sci. Pollut. Res. 2018, 25, 6826–6837. [Google Scholar] [CrossRef]
- Soil Maps and Databases|FAO SOILS PORTAL|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/en/ (accessed on 14 February 2025).
- Interstate Standard (GOST) 5180-2015. Soils. Laboratory Methods for Determination of Physical Characteristics. Interstate Counc. Stand. Metrol. Certif. (ISC). 2015. Available online: https://docs.cntd.ru/document/1200126371 (accessed on 14 February 2025). (In Russian).
- Interstate standard (GOST) 25100-2011. Soils. Classification. Interstate Counc. Stand. Metrol. Certif. (ISC). 2013. Available online: https://docs.cntd.ru/document/1200095052 (accessed on 14 February 2025). (In Russian).
- Burt, R.; United States, National Soil Survey Center (U.S.); National Soil Survey Laboratory (U.S.). Soil Survey Investigations Report No. 42, Version 4.0. In Soil Survey Laboratory Methods Manual; Natural Resources Conservation Service: Washington, DC, USA, 2004. Available online: https://www.nrcs.usda.gov/sites/default/files/2023-01/SSIR42.pdf (accessed on 14 February 2025). (In Russian)
- Interstate Standard (GOST) 26423-85. Soils. Methods for Determination of Specific Electric Conductivity, pH and Solid Residue of Water Extract. Interstate Counc. Stand. Metrol. Certif. (ISC). 1985. Available online: https://docs.cntd.ru/document/1200023484 (accessed on 14 February 2025). (In Russian).
- Bakina, L.G.; Orlova, N.E. Special features of humus acids extraction from soils by sodium pyrophosphate solutions of different alkalinity. Eurasian Soil Sci. 2012, 45, 392–398. [Google Scholar] [CrossRef]
- Walkley, A. An Examination of Methods for Determining Organic Carbon and Nitrogen in Soils. J. Agric. Sci. 1935, 25, 598–609. [Google Scholar] [CrossRef]
- Vysotskaja, E.V.; Tarasevich, Y.I.; Klimova, G.M.; Kuzmenko, L.N. Synthesis of Hydroxyapatite and Use for Removal of Heavy Metals from Aqueous Solutions. J. Chem. Technol. Water 2002, 24, 535–546. [Google Scholar]
- Guidance Document RD 52.18.286-91. Methodological Guidelines. The Method of Measuring the Mass Fraction of Water-Soluble Forms Of Metals (Copper, Lead, Zinc, Nickel, Cadmium, Cobalt, Chromium, Manganese) in Soil Samples by Atomic Absorption Analysis. USSR State Committee for Hydrometeorology. 1991. Available online: https://docs.cntd.ru/document/1200048597 (accessed on 14 February 2025). (In Russian).
- Guidance Document RD 52.18.289–2022. Mass Fraction of Mobile Forms of Metals in Soil and Soil Samples. Measurement Procedure by Atomic Absorption Spectrometry. Ministry of Natural Resources and Ecology of the Russian Federation Federal. Federal Service for Hydrometeorology and Environmental Monitoring 2023. Available online: http://mgmtmo.ru/edumat/rd/52.18.289_2022.pdf (accessed on 14 February 2025). (In Russian).
- National Standard of The Russian Federation GOST R ISO 18763-2019. Soil Quality. Determination of the Toxic Effects of Pollutants on Germination and Early Growth of Higher Plants. Fed. Agency Tech. Regul. Metrol. Russ. Fed. 2019. Available online: https://docs.cntd.ru/document/1200166928 (accessed on 14 February 2025). (In Russian).
- Guidelines for the Determination of Heavy Metals In Farmland Soils And Crop Production (2nd Edition, Revised and Supplemented). Minist. Agric. Russ. Fed. Cent. Inst. Agric. Agrochem. Serv. 1992. Available online: https://docs.cntd.ru/document/1200078918 (accessed on 14 February 2025). (In Russian).
- Altomare, A.; Corriero, N.; Cuocci, C.; Falcicchio, A.; Moliterni, A.; Rizzi, R. QUALX2.0: A Qualitative Phase Analysis Software Using the Freely Available Database POW_COD. J. Appl. Cryst. 2015, 48, 598–603. [Google Scholar] [CrossRef]
- Shein, E.V.; Zaidelman, F.R.; Karpachevsky, L.O.; Berezin, P.N.; Smagin, A.V.; Zubkova, T.A.; Guber, A.K.; Manucharov, A.S.; Chernomorchenko, N.I.; Sidorova, M.A. Theories and Methods of Soil Physics; “Grif i K”: Moscow, Russia, 2007; ISBN 978-5-8125-0921-7. (In Russian) [Google Scholar]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Angove, M.J. Adsorption of Humic and Fulvic Acids onto a Range of Adsorbents in Aqueous Systems, and Their Effect on the Adsorption of Other Species: A Review. Sep. Purif. Technol. 2020, 247, 116949. [Google Scholar] [CrossRef]
- Ritchie, J.D.; Perdue, E.M. Proton-Binding Study of Standard and Reference Fulvic Acids, Humic Acids, and Natural Organic Matter. Geochim. Cosmochim. Acta 2003, 67, 85–96. [Google Scholar] [CrossRef]
- Lee, S.-Z.; Chang, L.; Yang, H.-H.; Chen, C.-M.; Liu, M.-C. Adsorption Characteristics of Lead onto Soils. J. Hazard. Mater. 1998, 63, 37–49. [Google Scholar] [CrossRef]
- Zhao, Q.; Qiu, Y.; Lan, T.; Li, J.; Li, B.; Wu, Z.; Chen, L.; Liu, R.; Zhou, Y.; Wu, W. Comparison of Lead Adsorption Characteristics onto Soil-Derived Particulate Organic Matter versus Humic Acid. J Soils Sediments 2021, 21, 2589–2603. [Google Scholar] [CrossRef]
- Coutinho, I.B.; Barros de Souza, C.d.; Lima, E.S.A.; García, A.C.; Pereira, M.G.; Valladares, G.S.; Do Amaral Sobrinho, N.M.B. Roles of Soil Organic Matter and Humic Substance Structure in Cu and Pb Adsorption in Histosols. Soil Sediment Contam. Int. J. 2021, 30, 148–162. [Google Scholar] [CrossRef]
- Chardon, E.S.; Bosbach, D.; Bryan, N.D.; Lyon, I.C.; Marquardt, C.; Römer, J.; Schild, D.; Vaughan, D.J.; Wincott, P.L.; Wogelius, R.A.; et al. Reactions of the Feldspar Surface with Metal Ions: Sorption of Pb(II), U(VI) and Np(V), and Surface Analytical Studies of Reaction with Pb(II) and U(VI). Geochim. Cosmochim. Acta 2008, 72, 288–297. [Google Scholar] [CrossRef]
- Olu-Owolabi, B.I.; Diagboya, P.N.; Unuabonah, E.I.; Alabi, A.H.; Düring, R.-A.; Adebowale, K.O. Fractal-like Concepts for Evaluation of Toxic Metals Adsorption Efficiency of Feldspar-Biomass Composites. J. Clean. Prod. 2018, 171, 884–891. [Google Scholar] [CrossRef]
- Farquhar, M.L.; Vaughan, D.J.; Hughes, C.R.; Charnock, J.M.; England, K.E.R. Experimental Studies of the Interaction of Aqueous Metal Cations with Mineral Substrates: Lead, Cadmium, and Copper with Perthitic Feldspar, Muscovite, and Biotite. Geochim. Cosmochim. Acta 1997, 61, 3051–3064. [Google Scholar] [CrossRef]
- Bailliez, S.; Nzihou, A.; Bèche, E.; Flamant, G. Removal of Lead (Pb) by Hydroxyapatite Sorbent. Process Saf. Environ. Prot. 2004, 82, 175–180. [Google Scholar] [CrossRef]
- Mavropoulos, E.; Rossi, A.M.; Costa, A.M.; Perez, C.A.C.; Moreira, J.C.; Saldanha, M. Studies on the Mechanisms of Lead Immobilization by Hydroxyapatite. Environ. Sci. Technol. 2002, 36, 1625–1629. [Google Scholar] [CrossRef]


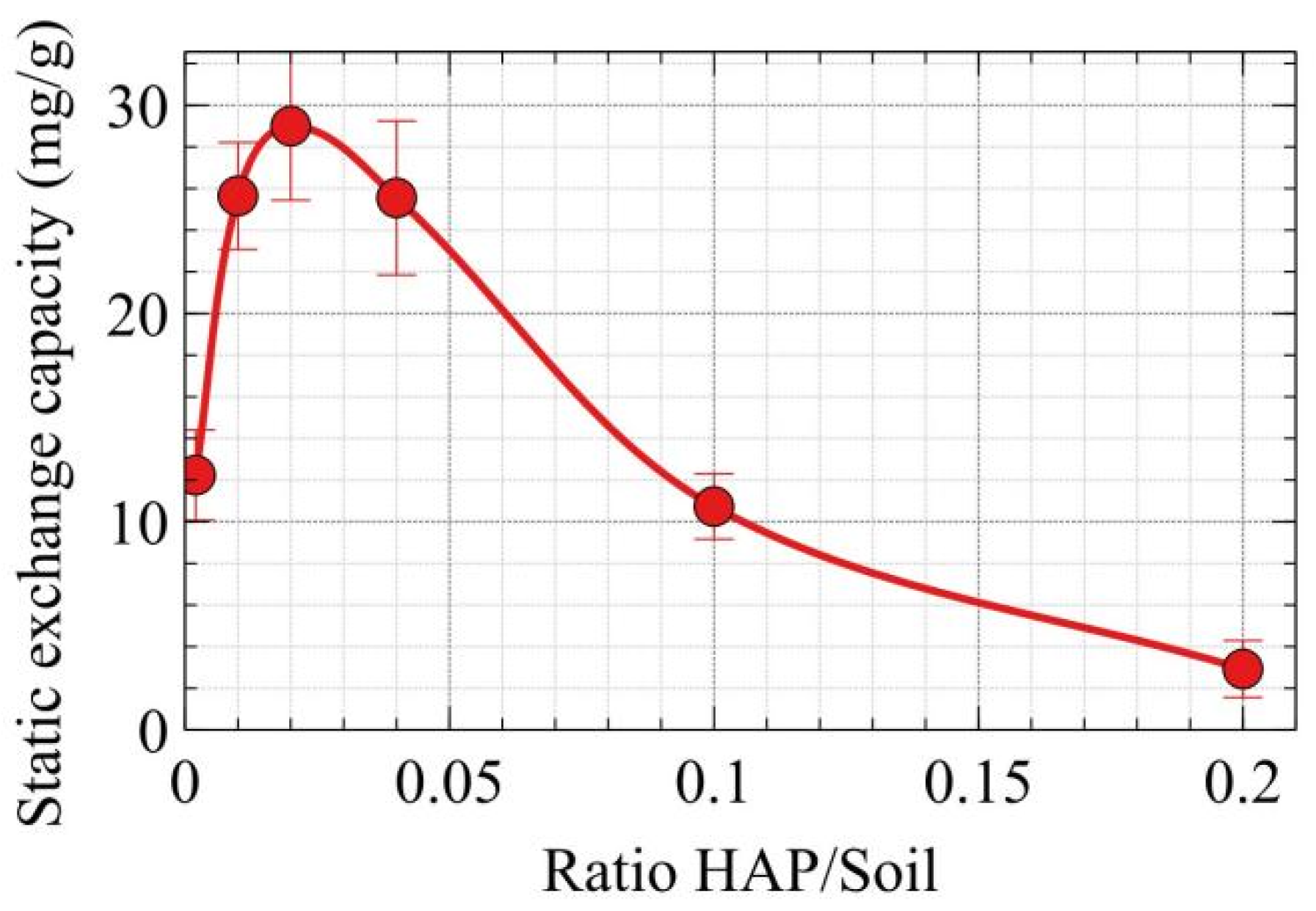
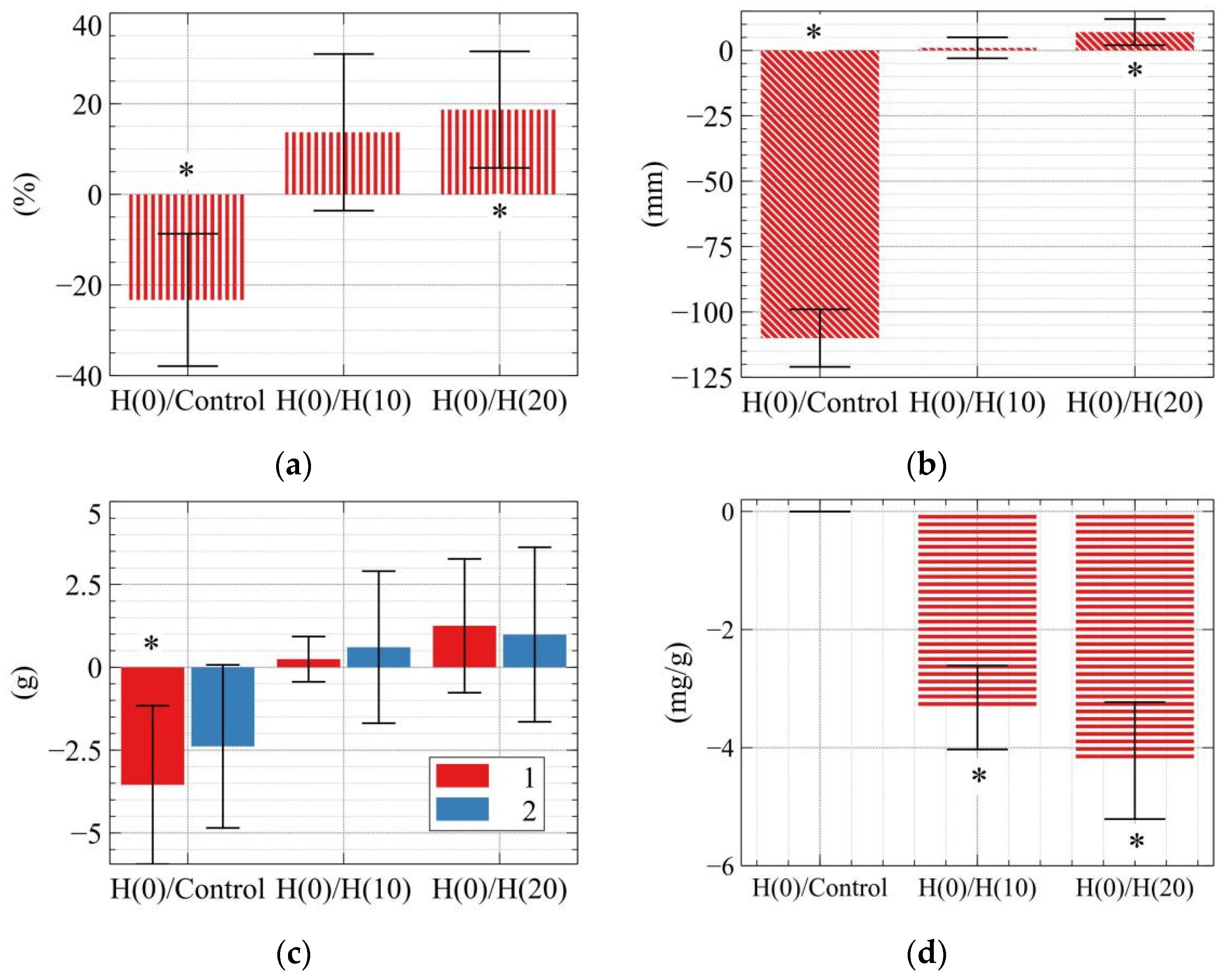
| Legend | Addition of Pb | HAP/Soil Mass Ratio |
|---|---|---|
| Control | No | 0 |
| H(0) | Yes | 0 |
| H(10) | Yes | 0.1 |
| H(20) | Yes | 0.2 |
| Indicator | Soil | ||
|---|---|---|---|
| Luvic Anthrosols | Gleyic Cambisols | ||
| pH(H2O) | 6.27 ± 0.16 | 5.23 ± 0.03 | |
| pH(KCl) | 5.66 ± 0.07 | 4.35 ± 0.16 | |
| Solid phase density (g/cm3) | 2.32 | 2.39 | |
| Content of granulometric fraction (%) | 2–0.05 mm | 23.5 | 46 |
| 0.05–0.002 mm | 63.5 | 45 | |
| <0.002 mm | 13.0 | 9 | |
| Classification of soils based on granulometric composition | Clay | Sandy clay | |
| Humidity (W) | 2.2 ± 0.1 | 1.6 ± 0.2 | |
| Sample | Humus | C(org) | C1 | C(hum) | C(fulvo) |
|---|---|---|---|---|---|
| Luvic Anthrosols | 4.27 ± 0.13 | 2.48 ± 0.08 | 0.83 ± 0.06 | 0.49 ± 0.05 | 0.34 ± 0.01 |
| Gleyic Cambisols | 14.28 ± 0.08 | 8.28 ± 0.04 | 1.72 ± 0.04 | 0.51 ± 0.07 | 1.20 ± 0.14 |
| The Form of Lead in the Soil | Experiment | Parameters of the Linear Equation | ||
|---|---|---|---|---|
| B | A | R2 | ||
| Water-soluble | Luvic Anthrosols, «Experiment A» | −1.6 ± 0.1 | −15 ± 1 | 0.995 |
| Luvic Anthrosols, «Experiment B» | −1.6 ± 0.2 | −18 ± 3 | 0.981 | |
| Gleyic Cambisols | −3.1 ± 0.1 | −10 ± 1 | 0.997 | |
| Mobile | Luvic Anthrosols, «Experiment A» | −0.10 ± 0.02 | −3.5 ± 0.3 | 0.991 |
| Luvic Anthrosols, «Experiment B» | −0.14 ± 0.01 | −3.6 ± 0.2 | 0.996 | |
| Gleyic Cambisols | −0.17 ± 0.01 | −3.6 ± 0.1 | 0.998 | |
| Experiment | Germination Rate (%) | Weight of Roots(g) | Weight of Sprouts (g) | Length of Sprouts (mm) | Lead Content in Tissues (mg/g) |
|---|---|---|---|---|---|
| Control | 49 ± 16 | 6.1 ± 2.6 | 3.8 ± 2.8 | 101.3 ± 10.9 | 0 |
| H(0) | 26 ± 17 | 3.7 ± 2.8 | 0.2 ± 0.2 | 12.1 ± 1.8 | 5.04 ± 1.06 |
| H(10) | 39 ± 21 | 4.3 ± 2.0 | 0.4 ± 0.7 | 19.6 ± 4.3 | 1.71 ± 0.33 |
| H(20) | 44 ± 11 | 4.7 ± 0.5 | 1.5 ± 2.0 | 27.8 ± 6.1 | 0.82 ± 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novikova, S.; Gilev, A.; Brikmans, A.; Priymak, I.; Shlyk, D.; Nesterova, O.; Egorin, A. The Potential of Hydroxyapatite for the Remediation of Lead-Contaminated Territories: A Case Study of Soils in Primorsky Krai. Sustainability 2025, 17, 2369. https://doi.org/10.3390/su17062369
Novikova S, Gilev A, Brikmans A, Priymak I, Shlyk D, Nesterova O, Egorin A. The Potential of Hydroxyapatite for the Remediation of Lead-Contaminated Territories: A Case Study of Soils in Primorsky Krai. Sustainability. 2025; 17(6):2369. https://doi.org/10.3390/su17062369
Chicago/Turabian StyleNovikova, Svetlana, Andrei Gilev, Anastasia Brikmans, Igor Priymak, Daria Shlyk, Olga Nesterova, and Andrei Egorin. 2025. "The Potential of Hydroxyapatite for the Remediation of Lead-Contaminated Territories: A Case Study of Soils in Primorsky Krai" Sustainability 17, no. 6: 2369. https://doi.org/10.3390/su17062369
APA StyleNovikova, S., Gilev, A., Brikmans, A., Priymak, I., Shlyk, D., Nesterova, O., & Egorin, A. (2025). The Potential of Hydroxyapatite for the Remediation of Lead-Contaminated Territories: A Case Study of Soils in Primorsky Krai. Sustainability, 17(6), 2369. https://doi.org/10.3390/su17062369








