Abstract
The application of biochar is extensively recognized as an effective strategy to enhance soil ecosystem services. However, its combined effect with beneficial microorganisms, such as Trichoderma, still requires further investigation to understand its impact on soil microbiota and nutrient cycling processes. To address this gap, this study aimed to evaluate the effect of biochar produced from on-farm winery waste, specifically grape stalks (GSB) and grape fermentation residues (GFB), generated after wine production, when co-applied with Trichoderma aureoviride URM 5158 and Trichoderma hamatum URM 6656 in soil cultivated with Malbec grapevines. Our findings reveal that both types of biochar and Trichoderma promoted changes in soil properties. The application of GSB biochar combined with T. hamatum increased grape productivity, while GFB biochar enhanced soil enzymatic activities, particularly those expressed per unit of microbial biomass carbon. Additionally, biochar applications increased pH, phosphorus, potassium, organic carbon, and microbial biomass carbon of the soil. Soils treated with the GFB + T. hamatum treatment exhibited an increase of 569.23% in microbial biomass carbon compared to the control. The results of this study provide substantial evidence that biochar and Trichoderma can be used to improve the chemical and biological properties of vineyard soils, increasing nutrient availability, especially carbon. These effects may contribute to soil fertility by promoting a more favorable environment for microbiota development and grapevine growth. This is the first field study to investigate the impact of on-farm winery waste transformed into biochar, combined with Trichoderma isolates, on Malbec grapevines.
1. Introduction
Viticulture is one of the oldest, most widespread, and economically valuable agricultural practices worldwide. However, it faces significant challenges due to the intensive and prolonged use of unsustainable practices, such as excessive application of fertilizers and pesticides [1]. These practices can disrupt soil biodiversity and negatively impact essential ecosystem functions [2], ultimately reducing soil resilience and long-term sustainability. Soil health is a key factor in sustainable viticulture, directly influencing both grape yield and wine quality. Therefore, it is essential to develop alternative strategies that minimize environmental impacts while enhancing soil quality to support vine growth and productivity. In this context, biochar—a carbon-rich material derived from the pyrolysis of plant residues—emerges as a promising solution for improving soil quality, increasing fertility, and mitigating environmental impacts [3].
Biochar serves as a soil conditioner and plays a pivotal role in climate change mitigation by facilitating carbon sequestration and reducing greenhouse gas emissions [4,5]. Its effectiveness in this process is attributed to its highly stable carbonaceous structure, which decomposes slowly in the environment, acting as a long-term carbon reservoir and thereby limiting the release of gases such as carbon dioxide (CO2) into the atmosphere [6]. In recent years, biochar addition in agricultural soils has been widely investigated as a promising strategy to minimize organic waste disposal while simultaneously enhancing soil quality and mitigating environmental impacts through multiple mechanisms. Its porous structure increases soil aeration and water retention while providing habitat for beneficial microorganisms. Additionally, biochar has a high cation exchange capacity (CEC), which enhances nutrient retention and reduces leaching. This approach positions biochar as a valuable tool for fostering more resilient and sustainable agricultural systems. Its incorporation into the soil has been shown to regulate pH levels [7], enhance water retention capacity [8], and improve soil fertility by providing a steady supply of macro- and micronutrients due to its rich nutrient composition [9]. Overall, these physical, chemical, and biological improvements contribute to better soil structure, nutrient availability, and microbial functioning—key components of soil quality.
Biochar applications can exert various direct and indirect effects on soil microbial communities due to their sensitivity to environmental changes [10]. Depending on its physicochemical properties, the application of biochar into the soil has the potential to enhance microbial community structure by modulating biochemical processes and microbial functions [11]. However, the physicochemical characteristics of biochar can vary significantly based on pyrolysis temperature, feedstock type, and production methods [12,13], all of which are critical determinants of its stability in soil. A recent study has explored interactions between biochar and beneficial soil microorganisms, highlighting this combination as a promising strategy to promote plant development and improve soil properties [14]. However, associations with the Trichoderma genus and their effects on soil dynamics remain largely understudied, necessitating further investigations to elucidate their potential benefits and mechanisms of action.
In this sense, Trichoderma is a genus of filamentous fungi widely recognized for its role in protecting plants against various soilborne phytopathogens [15,16]. Notably, Trichoderma serves as the primary active component in numerous commercially available biocontrol formulations, officially recognized as plant protection products [17]. However, its potential extends beyond biocontrol, as Trichoderma also functions as a plant growth promoter by enhancing nutrient uptake and activating natural defense mechanisms. Recent studies [18,19,20] have highlighted its positive effects on plant development, reinforcing its potential as a key contributor to sustainable agriculture.
The plant growth-promoting mechanisms of Trichoderma involve the release of indole-3-acetic acid (IAA) and other auxin-related compounds, which play an essential role in root development during the early stages of plant growth [21]. In addition to stimulating root system expansion, these compounds regulate various physiological processes, leading to improved nutrient uptake and enhanced resistance to environmental stress. Although numerous studies have highlighted the beneficial effects of microorganism-biochar interactions [14,22,23], the specific impacts of the co-application of biochar and Trichoderma spp. in vineyard soils remain largely unexplored. Further investigation is needed to develop a sustainable approach that enables producers to repurpose agricultural waste into a value-added product, thereby reducing reliance on external inputs. We hypothesize that different types of biochar will amplify the beneficial effects of Trichoderma on vineyard productivity while enhancing soil chemical properties, as well as absolute and specific enzymatic activities. To address this, we evaluated the potential of biochar derived from different vineyard residues, combined with Trichoderma spp., on Malbec grapevines’ productivity and soil properties.
2. Materials and Methods
2.1. Biochar Production and Characterization
Biochar was produced through pyrolysis using grape stalks (GSB) and grape fermentation residues (GFB), both generated as byproducts of wine production. These residues were obtained from a winery in the semiarid region of Brazil, where the field experiment was conducted (Figure 1). The pyrolysis process was performed at 400 °C for 12 h under oxygen-limited conditions.
Figure 1.
Map showing the location of the winery that supplied the residues for biochar production and the site where the field experiment was conducted with Malbec grapevines.
The total concentrations of phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sodium (Na), silicon (Si), iron (Fe), copper (Cu), manganese (Mn), zinc (Zn), and boron (B) were determined following the protocol described by Nair et al. [24]. Approximately 30 mg of biochar from each sample was incinerated in a muffle furnace at 500 °C for a minimum of 8 h. After cooling to room temperature, 1.5 mL of nitric acid (HNO3) diluted in ultrapure water (1:3 v/v) was added to each sample. Subsequently, 13.5 mL of ultrapure water was added, and the solutions were thoroughly mixed. The resulting suspensions were filtered through 0.45 μm pore size membranes before analysis. The concentrations of all elements were quantified using inductively coupled plasma optical emission spectrometry (ICP). Biochar CEC was determined by the modified ammonium acetate compulsory displacement method, adapted for biochar [25]. Nitrogen was determined by sulfuric digestion, using the Kjeldahl method, and total soil organic carbon was determined by the dry combustion method, using a muffle furnace, according to Teixeira [26]. The chemical characterization of the two types of biochar is shown in Table 1.

Table 1.
Chemical characterization of biochar from grape stalk biochar (GSB) and biochar from grape fermentation (GFB).
2.2. Inoculum Production
We used two strains: Trichoderma aureoviride URM 5158 (Ta) and Trichoderma hamatum URM 6656 (Th). Both strains were obtained from the URM Culture Collection https://www.ufpe.br/micoteca/ (accessed on 4 July 2024). Previous studies demonstrated that two strains were capable of producing chitinase and were efficient against different pathogens [27,28].
The Trichoderma inocula were grown in Erlenmeyer flasks (125 mL) containing 25 mL of potato dextrose broth and were incubated in an incubator for 7 days at 30 °C. The inocula were prepared according to the method described by [28] and adjusted to maintain a concentration of 106 spores mL−1.
2.3. Field Experiment
The study was conducted at Vale das Colinas Winery, located in Garanhuns, Pernambuco State, Brazil (8°56′19′′ S, 36°31′21′′ W) at 800 m above sea level (Figure 1). The climate is humid and classified as “As” (Köppen). The region has an average annual temperature of 20 °C and an average annual rainfall of 1300 mm [29]. The soil has been classified as a dystrophic Red-Yellow Argisol with a sandy loam texture, according to the Brazilian Soil Classification System [30]. The chemical properties of the experimental soil are in Table 2.

Table 2.
Soil chemical properties of the area planted with Malbec grapevines.
The samples were air-dried, crushed, homogenized, and sieved (2 mm mesh) for the following analyses: C, organic matter, pH in water (1:2.5), and P, Na+, K+, Ca2+, and Mg2+ contents, according to Teixeira et al. [26]. The O.M. was determined by the dry combustion method, using a muffle furnace, according to Teixeira [26]. P, K+, and Na+ were extracted with Mehlich 1 solution, based on ion exchange with 0.0125 mol L−1 H2SO4 + 0.05 mol L−1 HCl solution. P was determined in a spectrophotometer after molybdate reduction with ascorbic acid, while K+ and Na+ were measured by flame photometry. The Ca2+ and Mg2+ cations were extracted with 1.0 mol L−1 KCl and determined by atomic absorption spectrophotometry, using strontium chloride as a suppressor of interferents.
The experiment was conducted using a randomized block design with nine treatments: (1) control (Ctrl); (2) grape stalk biochar (GSB); (3) grape fermentation biochar (GFB); (4) Trichoderma aureoviride URM 5158 (Ta); (5) Trichoderma hamatum URM 6656 (Th); (6) GSB combined with T. aureoviride (GSB.Ta); (7) GSB combined with T. hamatum (GSB.Th); (8) GFB combined with T. aureoviride (GFB.Ta); and (9) GFB combined with T. hamatum (GFB.Th). Each treatment was applied in four replicates.
Each biochar was individually inoculated with 100 mL TRI spore solution containing 9.73 × 106 spores mL−1. The biochar was inoculated with the TRI strains 20 days before the experiment and applied near the plant roots. The viability of the microorganisms incorporated into biochar was assessed using the serial dilution method. Serial dilutions ranging from 10−2 to 10−5; were spread onto potato dextrose agar (PDA) plates. After inoculation, the plates were incubated in a BOD at 28 °C. The findings indicated that the Trichoderma inoculum retained its viability throughout the assessment period. The present study was carried out in an area cultivated with Malbec grapevines, which were used as planting materials. The vineyard was managed following standard agricultural practices commonly adopted by grape producers in the region’s wineries.
We evaluated the number of grape bunches (NGB) and the weight of grape bunches (WGB) as productivity.
We sampled soil (0–10 cm) near the plants’ roots. The soil chemical parameters determined were pH (1:2.5), available P, K, and Na [26]. The total organic carbon (TOC) content was determined using the method described by [31]. Microbial biomass carbon (MBC) was measured using the irradiation method [32,33].
The activities of β-glucosidase, acid, alkaline phosphatases, and arylsulfatase were determined using standard methods. Briefly, β-glucosidase activity was measured with ρ-nitrophenyl β-glucopyranoside as the substrate, incubated for 1 h at 37 °C, and the resulting ρ-nitrophenol was quantified spectrophotometrically at 400 nm [34]. Acid and alkaline phosphatase activity were assessed using disodium ρ-nitrophenyl phosphate as the substrate, incubated for 1 h at 37 °C, and the ρ-nitrophenol produced was measured at 420 nm [35]. Urease activity was determined using urea as the substrate, incubated for 2 h at 37 °C, and the ammonium produced was measured spectrophotometrically at 660 nm [36]. Each absolute enzyme activity was divided by TOC to determine the activities of specific enzymes per TOC unit. Data on all the enzyme activities were divided by MBC to set the specific enzyme activities per MBC unit.
2.4. Statistical Analyses
All analyses were performed using R v.4.3.1 [37], through the RStudio interface (RStudio Team, 2023). The assumptions of data normality and variance homogeneity were evaluated using the Shapiro–Wilk test and Levene’s test, respectively. The statistical approach adopted was a completely randomized design, followed by analysis of variance and Skott–Knott post-hoc test in cases of significant differences, both at a 5% significance level. Non-parametric data were subjected to the Kruskal–Wallis test followed by Dunn’s post-hoc test at a 5% level of significance. Principal Component Analysis (PCA) was conducted to study the variance explained by the soil and plant variables simultaneously, identifying the clustering related to the factorial treatments. Modeling was performed using the resources of the factoextra package, and all graphs were created using the ggplot2 package, both under R version 4.5.0.3. Results
The soil properties differed between the Trichoderma and biochar applied (Figure 2 and Figure 3). The application of biochar GSB + T. hamatum increased the soil pH by 12.22% (Figure 2a). The application of GSB led to a 4.68% increase in phosphorus and an 86.70% increase in potassium content. Additionally, total organic carbon content increased by 60.73% compared to the control (Figure 2).
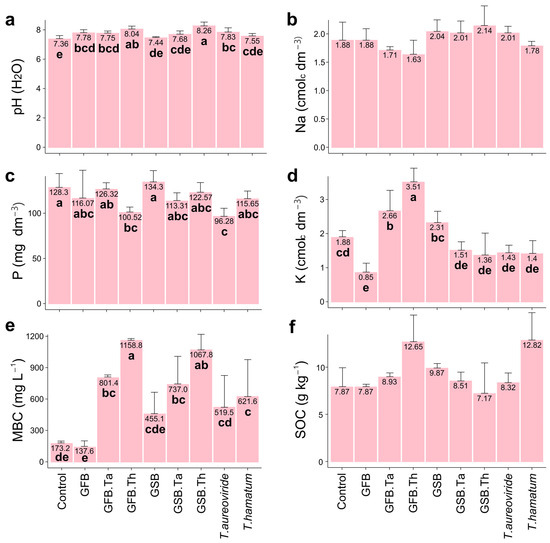
Figure 2.
Chemical and biological attributes of soil subjected to different biochar sources and Trichoderma isolates and cultivated with grape plants of the Malbec cultivar. (a) pH, (b) Na, (c) P, (d) K, (e) Microbial biomass carbon (MBC), (f) Soil organic carbon (SOC). Error bars represent the standard error (SE) based on four experimental replicates. Treatments marked with distinct lowercase letters differ significantly (p < 0.05). Treatments: control (Ctrl); grape stalk biochar (GSB); grape fermentation biochar (GFB); Trichoderma aureoviride URM 5158 (Ta); Trichoderma hamatum URM 6656 (Th); GSB combined with T. aureoviride (GSB.Ta); GSB combined with T. hamatum (GSB.Th); GFB combined with T. aureoviride (GFB.Ta); and GFB combined with T. hamatum (GFB.Th).
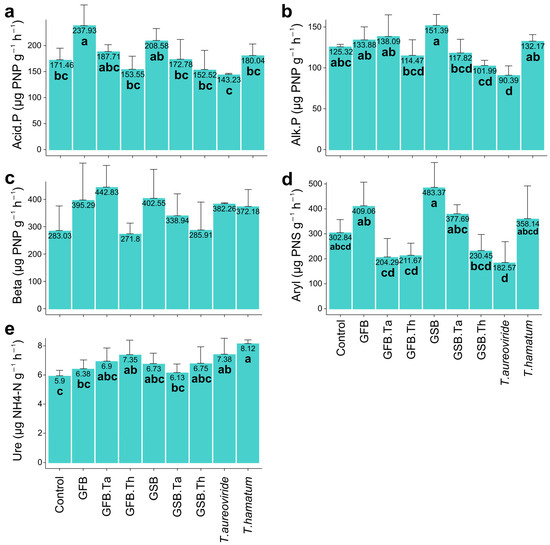
Figure 3.
Absolute enzymatic activities of soil subjected to different biochar sources and Trichoderma isolates and cultivated with grape plants of the Malbec cultivar. (a) Acid phosphatase, (b) Alkaline phosphatase, (c) Beta-glucosidase, (d) Arylsulfatase, (e) Urease. Error bars represent the standard error (SE) based on four experimental replicates. Treatments marked with distinct lowercase letters differ significantly (p < 0.05). Treatments: control (Ctrl); grape stalk biochar (GSB); grape fermentation biochar (GFB); Trichoderma aureoviride URM 5158 (Ta); Trichoderma hamatum URM 6656 (Th); GSB combined with T. aureoviride (GSB.Ta); GSB combined with T. hamatum (GSB.Th); GFB combined with T. aureoviride (GFB.Ta); and GFB combined with T. hamatum (GFB.Th).
The biochar application, alone or in combination with Trichoderma, also significantly affected microbial biomass carbon. Soils treated with the GFB + T. hamatum treatment exhibited an increase of 569.23% in microbial biomass carbon compared to the control.
Absolute soil enzymatic activities responded differently to different biochar sources and the presence of Trichoderma. The application of GFB significantly increased acid phosphatase activity by 38.76% (Figure 3a), while GSB promoted increases of 20.80% in alkaline phosphatase activity (Figure 3b) and 59.61% in arylsulfatase activity (Figure 3d). For β-glucosidase, the highest increase (56.46%) was observed with the combination of GFB and T. aureoviride (Figure 3c). Additionally, urease activity showed a 37.62% increase compared to the control (Figure 3e).
The soil treated with GFB biochar and cultivated with Malbec grapes exhibited a significant increase in specific enzymatic activities per unit of microbial biomass carbon (Figure 4). This finding suggests that the application of biochar derived from wine fermentation residues can enhance soil microbial activity, improving the efficiency of organic matter decomposition and nutrient cycling.
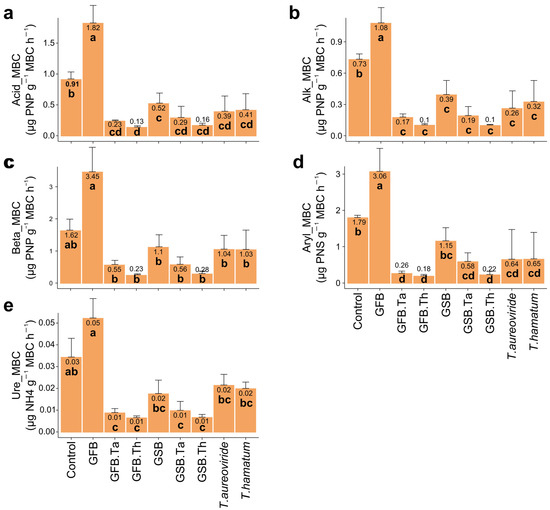
Figure 4.
Specific enzymatic activities per unit of microbial biomass in soil subjected to different biochar sources and Trichoderma isolates and cultivated with grape plants of the Malbec cultivar. (a) Acid phosphatase/ microbial biomass carbon (MBC), (b) Alkaline phosphatase/MBC, (c) Beta-glucosidase/MBC, (d) Arylsulfatase/MBC, (e) Urease/MBC. Error bars represent the standard error (SE) based on four experimental replicates. Treatments marked with distinct lowercase letters differ significantly (p < 0.05). Treatments: control (Ctrl); grape stalk biochar (GSB); grape fermentation biochar (GFB); Trichoderma aureoviride URM 5158 (Ta); Trichoderma hamatum URM 6656 (Th); GSB combined with T. aureoviride (GSB.Ta); GSB combined with T. hamatum (GSB.Th); GFB combined with T. aureoviride (GFB.Ta); and GFB combined with T. hamatum (GFB.Th).
On the other hand, specific enzymatic activities per unit of soil organic carbon were more pronounced in soils treated with GSB biochar and Trichoderma hamatum URM 6656 (Figure 5). This effect may be attributed to the chemical composition of GSB biochar, which likely influenced nutrient retention and the stability of soil organic carbon, creating a favorable environment for enzymatic activity.
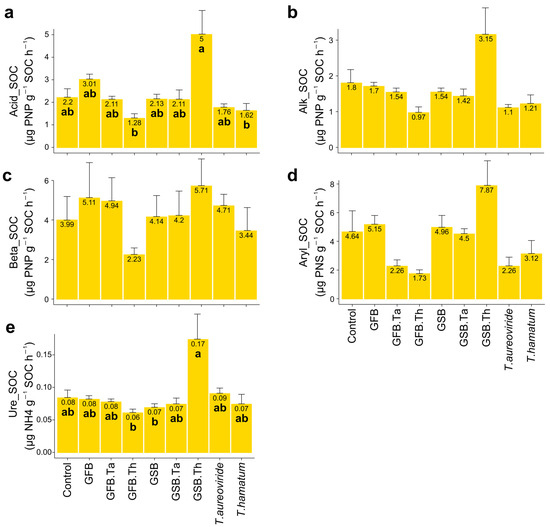
Figure 5.
Specific enzymatic activities per unit of soil organic carbon (SOC) in soil subjected to different biochar sources and Trichoderma isolates and cultivated with grape plants of the Malbec cultivar. (a) Acid phosphatase/SOC, (b) Alkaline phosphatase/SOC, (c) Beta-glucosidase/SOC, (d) Arylsulfatase/SOC, (e) Urease/SOC. Error bars represent the standard error (SE) based on four experimental replicates. Treatments marked with distinct lowercase letters differ significantly (p < 0.05). Treatments: control (Ctrl); grape stalk biochar (GSB); grape fermentation biochar (GFB); Trichoderma aureoviride URM 5158 (Ta); Trichoderma hamatum URM 6656 (Th); GSB combined with T. aureoviride (GSB.Ta); GSB combined with T. hamatum (GSB.Th); GFB combined with T. aureoviride (GFB.Ta); and GFB combined with T. hamatum (GFB.Th).
The combination of GSB + T. aureoviride led to a reduction in both the number of grape bunches and the total bunch weight per plant. In contrast, the highest contribution to productivity was observed with the isolated application of T. hamatum, which increased the number of grape bunches by 51.20% and the total grape bunch weight by 121.14% compared to the control (Figure 6).
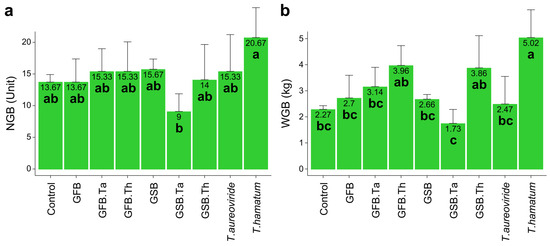
Figure 6.
Productivity of Malbec grapevines grown in soil treated with different biochar sources and Trichoderma isolates. (a) Number of grape bunches; (b) Total bunch weight per plant. Error bars represent the standard error (SE) based on four experimental replicates. Treatments marked with distinct lowercase letters differ significantly (p < 0.05). Treatments: control (Ctrl); grape stalk biochar (GSB); grape fermentation biochar (GFB); Trichoderma aureoviride URM 5158 (Ta); Trichoderma hamatum URM 6656 (Th); GSB combined with T. aureoviride (GSB.Ta); GSB combined with T. hamatum (GSB.Th); GFB combined with T. aureoviride (GFB.Ta); and GFB combined with T. hamatum (GFB.Th).
Principal Component Analysis (PCA) revealed a clear separation of the influence of the different biochar types and Trichoderma on grape productivity variables as well as the chemical and biological attributes of the soil (Figure 7). The principal components (PC1 and PC2) accounted for 29.15% and 21.9% of the total variance, respectively (Figure 7a). Overall, the effects of the treatments were distinctly grouped into different clusters compared to the control treatment. However, soils that received the GSB.th treatment exhibited the highest correlation with grape productivity variables, positioning themselves in the same quadrant as soil microbial biomass. Conversely, soils treated with GFB showed stronger correlations with the most sensitive variables, aligning with all specific enzyme activities per unit of microbial biomass carbon. The enzyme activities expressed per unit of microbial biomass carbon were the most sensitive in explaining the data variability (Figure 7b,c).
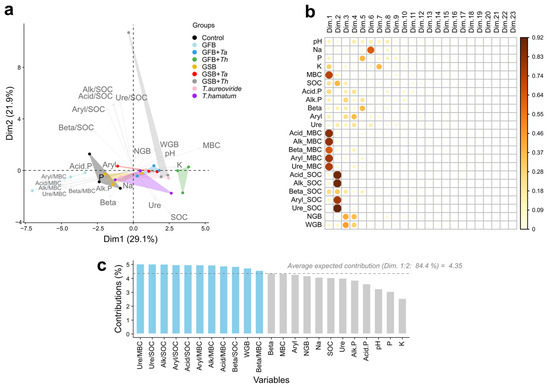
Figure 7.
Principal Component Analysis (PCA) of plant and soil variables treated with different biochar sources and Trichoderma isolates in Malbec grapevines. (a) Biplot of the principal components; (b) Correlation of variables with the principal components; (c) Contribution of variables in explaining the observed differences. Treatments: control (Ctrl); grape stalk biochar (GSB); grape fermentation biochar (GFB); Trichoderma aureoviride URM 5158 (Ta); Trichoderma hamatum URM 6656 (Th); GSB combined with T. aureoviride (GSB.Ta); GSB combined with T. hamatum (GSB.Th); GFB combined with T. aureoviride (GFB.Ta); and GFB combined with T. hamatum (GFB.Th). Microbial biomass carbon (MBC), Soil organic carbon (SOC), Absolute enzymatic activities: Acid phosphatase (Acid.P), Alkaline phosphatase (Alk.P), Beta-glucosidase (Beta), Arylsulfatase (Ary), Urease (Ure), Number of grape bunches (NGB), and Total bunch weight (WGB) per plant.
3. Discussion
The present study investigated the potential of applying biochar and Trichoderma on the chemical and biological properties of a soil cultivated with Malbec grapes. Here, we used winery waste for biochar production, a porous material widely used in agriculture [38], to provide a sustainable solution to reduce reliance on external inputs. The biochar types studied, derived from wine production waste, effectively improved soil quality and the productivity of Malbec grapes. The results showed that GSB.Th biochar contributed to higher grape productivity, while GFB biochar enhanced the most sensitive soil variables, specifically enzymatic activities per unit of microbial biomass. These findings align with the main hypothesis that biochar produced from on-farm waste is effective in enhancing soil productivity and soil attributes, confirming the potential of biochar to solve problems in agriculture and the environment [14,39,40], as it reuses waste that could be considered environmental liabilities and provides a sustainable destination.
The application of both biochar types, with or without Trichoderma, as expected, increased the pH of soils cultivated with Malbec grapes, consistent with previous studies [41,42,43]. In particular, the increase in soil pH following biochar application is attributed to the alkaline nature of most biochar types, whose reactive surface undergoes protonation, leading to the accumulation of positive charges [44]. Similarly, the Na, P, and K contents in the soil tended to increase with biochar applications. The increase in these elements in the soil following biochar application has been attributed both to the composition of the biochar itself, which contains these nutrients within its structure, and to its high cation exchange capacity, which enhances the retention and release of these elements in the soil [45].
Furthermore, the porous structure of biochar can act as a nutrient reservoir, reducing leaching losses and enhancing nutrient availability for plants over time [22]. The findings of this study confirm that biochar application is an effective strategy for improving soil fertility and increasing the availability of essential nutrients for plant growth. Additionally, it serves as a climate-smart agricultural practice, promoting sustainable farming while helping to reduce greenhouse gas emissions [46]. However, the magnitude of these effects may vary depending on the application rate, the type of biomass used in biochar production, and soil characteristics [47].
In this study, the application of biochar combined with Trichoderma isolates resulted in a rise in total soil organic carbon (SOC). This rise in SOC played a crucial role in enhancing soil biological properties by providing a more stable carbon source for microbiota, thereby stimulating enzymatic activity and microbial metabolism [7].
Extracellular enzymes serve as sensitivity indicators of the potential mineralization of carbon (β-glucosidase), nitrogen (urease), phosphorus (acid phosphatase), and sulfur (arylsulfatase), providing insights into possible alterations in the soil’s biochemical status [45]. They also rapidly reflect changes in soil fertility and biological functioning. Enzymes are primarily derived from microbial biomass and catalyze biochemical reactions that promote nutrient cycling and organic matter decomposition [48]. Here, the application of both biochar types combined with Trichoderma isolates stimulated soil enzymatic activity. This effect can be explained by the input of carbon and other nutrients present in the biochar composition, leading to increased activity, particularly of β-glucosidase, one of the key enzymes involved in the carbon cycle [49]. Previous studies have reported that the application of biochar to the soil increases the availability of labile carbon or releases organic compounds that may serve as a substrate to enhance the activity of these enzymes in the soil [27,50].
The activity of acid and alkaline phosphatase, as well as urease, was influenced by the type of biochar and Trichoderma isolate. This effect can be explained by the ability of biochar to adsorb soluble phosphates in the soil, reducing their availability to plants and microorganisms. In response, there is an increase in the production of phosphatases, enzymes responsible for releasing phosphorus from organic matter. Additionally, the high surface area of biochar may enhance this effect by increasing phosphorus retention and, consequently, stimulating enzymatic activity in the soil to compensate for this limitation [51].
As expected, our study demonstrated that the use of biochar, with or without Trichoderma isolates, increased all enzymatic activities per unit of microbial biomass and certain enzymatic activities per unit of soil organic carbon, consistent with previous studies [52]. Specific enzymatic activities are considered more sensitive indicators as they reflect the contribution of microorganisms and soil organic carbon (SOC) to enzyme activity [52,53]. Compared to absolute enzyme activities, specific enzymatic activities offer advantages, as they eliminate strong covariance with SOC and microbial biomass carbon (MBC), standardize differences in SOC and MBC content, and express microbial characteristics based on organic substances [54]. Notably, the multivariate analysis showed that specific enzyme activities were more sensitive to detecting changes in soil properties and plant productivity (Figure 7). This suggests a beneficial impact of this interaction as it brings together the advantages of Trichoderma with biochar.
Furthermore, when comparing T. hamatum with or without biochar, we observed that the isolated application of T. hamatum was more effective in increasing grape productivity. This effect can be attributed to Trichoderma’s ability to act as a plant growth promoter, stimulating plant development through the production and regulation of phytohormones. In addition to influencing the plant’s hormone synthesis, Trichoderma can directly secrete hormones or provide precursors for the biosynthesis of essential phytohormones, as part of the beneficial mechanisms of the Trichoderma-plant interaction [55]. Another key factor is Trichoderma’s positive impact on the root system, promoting greater root volume and density, which enhances water and nutrient uptake from the soil [56]. The data emphasize the capability of T. hamatum as an efficient bio-stimulant for improving grape productivity and quality, reinforcing its applicability in sustainable agricultural systems.
These findings are intriguing because they combine the well-known benefits of biochar, which is already widely used in sustainable agriculture, with the advantages of Trichoderma fungi. This combination can help preserve essential microbial functions, such as pathogen suppression and plant growth promotion, through different mechanisms. As is widely recognized, maintaining microbial functionality directly impacts plant performance. Therefore, repurposing farm-generated waste into a biochar-based solution presents a sustainable alternative for producers, reducing dependency on external inputs while enhancing soil health and crop productivity.
4. Conclusions
This study demonstrates that the co-application of biochar, derived from on-farm residues, and Trichoderma spp. improved Malbec grape productivity and soil health. The highest contribution to productivity was with the application of T. hamatum, which increased the number of grape bunches by 51.20% and the total grape bunch weight by 121.14%. The biochar derived from grape fermentation residues promoted higher enzymatic activities, particularly specific enzyme activities expressed per unit of microbial biomass. Both types of biochar revealed notable effectiveness in improving soil fertility, microbial activity, and nutrient availability. Additionally, biochar application improved soil chemical properties such as pH, phosphorus, potassium, organic carbon, and microbial biomass carbon. Soils treated with the GFB + T. hamatum exhibited an increase of 569.23% in microbial biomass carbon compared to the control. This finding is particularly relevant for viticulture, as it offers a sustainable alternative to enhance soil health and crop productivity while reducing reliance on external inputs. Additionally, the synergistic effects between biochar and Trichoderma highlight their potential as a climate-smart agricultural strategy. This is one of the first studies to evaluate biochar derived from winery waste in combination with Trichoderma for improving soil quality and grapevine productivity. However, further research is needed to explore different biochar compositions, assess long-term impacts on soil microbial dynamics, and evaluate economic feasibility in field-scale applications.
Author Contributions
Data curation, D.P.d.C. and E.M.d.L.; funding acquisition, E.V.d.M.; investigation, R.F.d.F. and M.M.d.S.; methodology, G.P.D. and J.R.d.S.L.; project administration, E.V.d.M.; resources, J.A.d.B. and A.P.M.F.; visualization, writing—review and editing; A.S.F.A. and E.V.d.M. All authors have read and agreed to the published version of the manuscript.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (313421/2021-8, 313174/2018-0; 426497/2018-0; 307335/2017-8; 304107/2020-4; ONDACBC:465764/2014-2 and NEXUS: 441305/2017-2), and Fundação de Amparo a Ciência e Tecnologia de Pernambuco (FACEPE) (APQ-1747-5.01/22; APQ-1464-5.01/22; APQ-0223-5.01/15; APQ-0419-5.01/15; APQ-0431-5.01/17; APQ-0498-3.07/17). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES 88887.736369/2017-00 and Finance Code 001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data from this study are available from the corresponding author upon request.
Acknowledgments
The authors thank the Vale das Colinas winery in Garanhuns-PE-Brazil for providing the material and all the infrastructure for the research.
Conflicts of Interest
The authors declare no competing interests.
Abbreviations
The following abbreviations are used in this manuscript:
| GSB | grape stalks biochar |
| GFB | grape fermentation residues |
| Ctrl | control |
| Ta | Trichoderma aureoviride URM 5158 |
| Th | Trichoderma hamatum URM 6656 |
| MBC | Microbial biomass carbon |
| SOC | Soil organic carbon |
| NGB | Number of grape bunches |
| WGB | Total bunch weight per plant |
| Acid.P | Acid phosphatase |
| Alk.P | Alkaline phosphatase |
| Beta | beta-glucosidase |
| Ary | Arylsulfatase |
| Ure | Urease |
References
- Giffard, B.; Winter, S.; Guidoni, S.; Nicolai, A.; Castaldini, M.; Cluzeau, D.; Leyer, I. Vineyard management and its impacts on soil biodiversity, functions, and ecosystem services. Front. Ecol. Evol. 2022, 10, 850272. [Google Scholar] [CrossRef]
- Teixeira, A.; Martins, V.; Manso, J.; Correia, S.; Ferreira, A.R.; Fontes, N.; Gerós, H. Exploring the influence of cover crops with native plant species on soil and berry microbiota in a Moscatel Galego vineyard: Implications for sustainable viticulture. Agric. Ecosyst. Environ. 2025, 380, 109384. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Liang, X.; Goh, H.H.; Gikas, P.; Chong, K.K.; Chew, K.W. Challenges and opportunities for biochar to promote circular economy and carbon neutrality. J. Environ. Manage. 2023, 332, 117429. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Z.; Li, D.; Wang, Y.; Yang, J.; Han, W.; Li, S. Effects of aged biochar additions at different addition ratios on soil greenhouse gas emissions. Sci. Total Environ. 2024, 955, 176914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, Y.; Wang, J.; Hu, J.; Qi, S.; Jiang, Z.; Yang, S. Impact of biochar on the antibiotic resistome and associated microbial functions in the rhizosphere and bulk soil of rice fields under water-saving and flooded irrigation. Environ. Pollut. 2024, 342, 123026. [Google Scholar] [CrossRef]
- Marques, C.A.; Silva, D.A.D.; Apresentação, M.D.J.F.D.; Nakashima, G.T.; Yamaji, F.M. Biochar production with sugarcane straw (Saccharum sp.). Res. Soc. Dev. 2022, 11, e31211124675. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Zou, C.; Yu, N.; Ma, C.; Li, B.; Liu, S. Effects of pyrolysis temperature and atmosphere on grinding properties of semicoke prepared from Shenmu low-rank coal. J. Anal. Appl. Pyrolysis 2023, 173, 106059. [Google Scholar] [CrossRef]
- Wang, C.; Chen, D.; Shen, J.; Yuan, Q.; Fan, F.; Wei, W.; Li, Y.; Wu, J. Biochar alters soil microbial communities and potential functions 3–4 years after amendment in a double rice cropping system. Agric. Ecosyst. Environ. 2021, 311, 107291. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Palem, R.R.; Al-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod. 2021, 297, 126645. [Google Scholar] [CrossRef]
- Ruan, G.; Yang, Y.; Peng, X.; Wang, J.; Guo, Y.; Hu, W.; Lin, D. A review of the current status of nitrogen self-doped biochar applications. J. Environ. Chem. Eng. 2025, 13, 115291. [Google Scholar] [CrossRef]
- Araujo, I.D.S.; Filho, A.P.M.; da Costa, D.P.; Silva, A.O.; da França, R.F.; Junior, M.D.A.L.; Duda, G.P.; Lima, J.R.D.S.; da Silva, M.M.; Araujo, A.S.F.; et al. Biochar and plant growth-promoting bacteria boost chemical and biological properties of semiarid soil in cowpea. Soil Syst. 2025, 9, 19. [Google Scholar] [CrossRef]
- Poveda, J.; Millen, M.R.; Bailey, A.M. Analysis of Trichoderma as an effective biological control agent against the honey fungus (Armillaria spp.). Biol. Control 2024, 188, 105424. [Google Scholar] [CrossRef]
- Kredics, L.; Büchner, R.; Balázs, D.; Allaga, H.; Kedves, O.; Racić, G.; Sipos, G. Recent advances in the use of Trichoderma-containing multicomponent microbial inoculants for pathogen control and plant growth promotion. World J. Microbiol. Biotechnol. 2024, 40, 162. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ji, S.; Zhang, H.; Wang, Y.; Liu, Z. Isolation of Trichoderma in the rhizosphere soil of Syringa oblata from Harbin and their biocontrol and growth promotion function. Microbiol. Res. 2020, 235, 126445. [Google Scholar] [CrossRef]
- Asghar, W.; Kataoka, R. Effect of co-application of Trichoderma spp. with organic composts on plant growth enhancement, soil enzymes and fungal community in soil. Arch. Microbiol. 2021, 203, 4281–4291. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Schmoll, M.; Esquivel-Ayala, B.A.; González-Esquivel, C.E.; Rocha-Ramírez, V.; Larsen, J. Mechanisms for plant growth promotion activated by Trichoderma in natural and managed terrestrial ecosystems. Res. Microbiol. 2024, 281, 127621. [Google Scholar] [CrossRef]
- Illescas, M.; Pedrero-Méndez, A.; Pitorini-Bovolini, M.; Hermosa, R.; Monte, E. Phytohormone production profiles in Trichoderma species and their relationship to wheat plant responses to water stress. Pathogens 2021, 10, 991. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Liu, X.; He, Y.; Xu, X.; Wu, Z.; Yu, K.; Zheng, X. Biochar inoculated with Pseudomonas putida improves grape (Vitis vinifera L.) fruit quality and alters bacterial diversity. Rhizosphere 2020, 16, 100261. [Google Scholar] [CrossRef]
- de Medeiros, E.V.; da Costa, D.P.; Silva, E.L.D.; de França, A.F.; Lima, J.R.D.S.; Hammecker, C.; Mendes, L.W.; Pereira, A.P.D.A.; Araujo, A.S.F. Biochar and Trichoderma as an eco-friendly and low-cost alternative to improve soil chemical and biological properties. Waste Biomass Valorization 2023, 15, 1439–1450. [Google Scholar] [CrossRef]
- Nair, R.R.; Schaate, A.; Klepzig, L.F.; Turcios, A.E.; Lecinski, J.; Shamsuyeva, M.; Endres, H.-J.; Papenbrock, J.; Behrens, P.; Weichgrebe, D. Physico-chemical characterization of walnut shell biochar from uncontrolled pyrolysis in a garden oven and surface modification by ex-situ chemical magnetization. Clean Technol. Environ. Policy 2023, 25, 2727–2746. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; Melo, I.C.N.D.; Melo, L.C.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo; EMBRAPA: Brasília, Brazil, 2017; 15p. [Google Scholar]
- da Silva, J.A.T.; de Medeiros, E.V.; Tenório, D.D.A.; Moreira, K.A.; Nascimento, T.C.E.d.S.; Souza-Motta, C. Trichoderma aureoviride URM 5158 and Trichoderma hamatum URM 6656 are biocontrol agents that act against cassava root rot through different mechanisms. J. Phytopathol. 2016, 164, 1003–1011. [Google Scholar] [CrossRef]
- da Silva, J.S.A.; de Medeiros, E.V.; da Costa, D.P.; de Souza, C.A.F.; de Oliveira, J.B.; da França, R.F.; Souza-Motta, C.M.; Lima, J.R.d.S.; Hammecker, C. Biochar and Trichoderma aureoviride URM 5158 as alternatives for the management of cassava root rot. Appl. Soil Ecol. 2022, 172, 104353. [Google Scholar] [CrossRef]
- Pernambuco Agency of Water and Climate (APAC). APAC Portal [Internet]. Available online: https://www.apac.pe.gov.br/#tabs (accessed on 3 April 2025).
- dos Santos, H.D.; Jacomine, P.T.; Dos Anjos, L.H.C.; De Oliveira, V.Á.; Lumbreras, J.F.; Coelho, M.R.; De Almeida, J.A.; de Araujo Filho, J.C.; De Oliveira, J.B.; Cunha, T.J.F. Brazilian Soil Classification System; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil. Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Mendonça, E.D.S.; Matos, E.D.S. Soil Organic Matter: Methods of Analysis. 2005. Available online: https://www.ciodaterra.com.br/materia-organica-do-solo-metodos-de-analises?srsltid=AfmBOopx79zLFMoNfIK9asAjqq3VppRf1-zeBjAgFGwhB2Q7dwu0jiSS (accessed on 3 April 2025). (In Portuguese).
- Bartlett, R.J.; Ross, D.S. Colorimetric Determination of Oxidizable Carbon in Acid Soil Solutions. Soil Sci. Soc. Am. J. 1988, 52, 1191–1192. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fert. Soils. 1988, 6, 68–72. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 24 January 2025).
- Lin, G.; Wang, Y.; Wu, X.; Meng, J.; Ok, Y.S.; Wang, C.H. Enhancing agricultural productivity with biochar: Evaluating feedstock and quality standards. Bioresour. Technol. Rep. 2024, 205, 102059. [Google Scholar] [CrossRef]
- He, M.; Li, L.; Liu, X.; Zhang, H.; Zhang, W.; Wang, J. A critical review on performance indicators for evaluating soil biota and soil health of biochar-amended soils. J. Hazard. Mater. 2021, 414, 125378. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Emenike, E.C.; Omonayin, E.O.; Bamigbola, J.O.; Ojo, H.T.; Awoyale, A.A.; Eletta, O.A.; Adeniyi, A.G. Unlocking the hidden value of pods: A review of thermochemical conversion processes for biochar production. Bioresour. Technol. Rep. 2023, 22, 101488. [Google Scholar] [CrossRef]
- Abhishek, K.; Shrivastava, A.; Vimal, V.; Gupta, A.K.; Bhujbal, S.K.; Biswas, J.K.; Singh, L.; Ghosh, P.; Pandey, A.; Sharma, P.; et al. Biochar application for greenhouse gas mitigation, contaminants immobilization and soil fertility enhancement: A state-of-the-art review. Sci. Total Environ. 2022, 853, 158562. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Q.; Zheng, H.; Li, M.; Liu, Y.; Wang, X.; Xing, B. Biochar as a sustainable tool for improving the health of salt-affected soils. Soil Environ. Health 2023, 1, 100033. [Google Scholar] [CrossRef]
- Jia, A.; Song, X.; Li, S.; Liu, Z.; Liu, X.; Han, Z.; Wang, G. Biochar enhances soil hydrological function by improving the pore structure of saline soil. Agric. Water Manag. 2024, 306, 109170. [Google Scholar] [CrossRef]
- Herrmann, L.; Lesueur, D.; Robin, A.; Robain, H.; Wiriyakitnateekul, W.; Bräu, L. Impact of biochar application dose on soil microbial communities associated with rubber trees in North East Thailand. Sci. Total Environ. 2019, 689, 970–979. [Google Scholar] [CrossRef]
- Fouladidorhani, M.; Shayannejad, M.; Mosaddeghi, M.R.; Shariatmadari, H.; Arthur, E. Biochar, manure and superabsorbent improve the physical quality of saline-sodic soil under greenhouse conditions. Soil Sci. Soc. Am. J. 2023, 87, 1003–1017. [Google Scholar] [CrossRef]
- Nazim, M.; Ghafoor, A.; Hussain, A.; Tabassum, M.; Nawaz, A.; Ahmad, M.; Ali, M. Biochar as a Climate-Smart Agricultural Practice: Reducing Greenhouse Gas Emissions and Promoting Sustainable Farming. Phyton-Int. J. Exp. Bot. 2025, 94, 65–99. [Google Scholar] [CrossRef]
- Costa, R.M.; Araujo, E.M.B.; Silva, D.E.O.; Rocha, S.M.B.; Bonifacio, A.; Sousa, R.S.; Pereira, A.P.d.A.; de Medeiros, E.V.; Sagrilo, E.; de Oliveira Junior, J.O.L.; et al. Seasonal responses of soil microbial biomass C and enzymatic activity comparing no-tillage and integrated crop-livestock systems. Eur. J. Soil Biol. 2024, 121, 103628. [Google Scholar] [CrossRef]
- Barbosa, F.L.A.; Santos, J.M.R.; Mota, J.C.A.; Costa, M.C.G.; Araujo, A.S.F.; Garcia, K.G.V.; Almeida, M.S.; Nascimento, Í.V.; Medeiros, E.V.; Ferreira, O.P.; et al. Potential of biochar to restoration of microbial biomass and enzymatic activity in a highly degraded semiarid soil. Sci. Rep. 2024, 14, 26065. [Google Scholar] [CrossRef]
- Luo, G.; Xue, C.; Jiang, Q.; Xiao, Y.; Zhang, F.; Guo, S.; Shen, Q.; Ling, N. Soil carbon, nitrogen, and phosphorus cycling microbial populations and their resistance to global change depend on soil C: N: P stoichiometry. mSystems 2020, 5, e00162-20. [Google Scholar] [CrossRef] [PubMed]
- Alves de Barros, J.; Martins Filho, P.; Paes da Costa, D.; dos Passos Vieira, A.; Rodrigues Araújo, E.; Henrique Leal Lopes, M.; de Souza Júnior, J.H.; Moura da Silva, M.; Hammecker, C.; Medeiros, E.V. Residual effect of biochar on microbial biomass and enzyme activities of soil cultivated with grape varieties: A two-year field assessment. Rev. Bras. Geogr. Física 2024, 17, 2284–2293. [Google Scholar] [CrossRef]
- Luo, X.; Chen, W.; Liu, Q.; Wang, X.; Miao, J.; Liu, L.; Li, F. Corn straw biochar addition elevated phosphorus availability in a coastal salt-affected soil under the conditions of different halophyte litter input and moisture contents. Sci. Tot. Environ. 2024, 908, 168355. [Google Scholar] [CrossRef]
- Stone, M.M.; DeForest, J.L.; Plante, A.F. Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Lu, B.; Li, P.; Xue, S.; Liu, G. Response of soil specific enzyme activity to vegetation restoration in the Loess hilly region of China. Catena 2020, 191, 104564. [Google Scholar] [CrossRef]
- Raiesi, F.; Beheshti, A. Soil specific enzyme activity shows more clearly soil responses to paddy rice cultivation than absolute enzyme activity in primary forests of northwest Iran. Appl. Soil Ecol. 2014, 75, 63–70. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Porras-Troncoso, M.D.; Olmedo-Monfil, V.; Herrera-Estrella, A. Trichoderma species: Versatile plant symbionts. Phytopathology 2019, 109, 6–16. [Google Scholar] [CrossRef]
- Verma, I.; Soni, S.K.; Singh, P.C. Trichoderma produces methyl jasmonate-rich metabolites in the presence of Fusarium, showing biostimulant activity and wilt resistance in tomatoes. Plant Phys. Bioch. 2024, 215, 108953. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).