Bioconversion of Seasonal Vegetable By-Products into Nutrient-Rich Biomass Using Black Soldier Fly Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Agro-Industrial By-Products Used for BSFL Feeding
2.3. Rearing of BSFL
2.4. Extraction of Total Lipids and Fatty Acid Profiling in Diets and BSFL
2.5. Amino Acid Determination in Diet and BSFL
2.6. Carotenoid Determination in Diets and BSFL
2.7. Tocopherol Determination in Diets and BSFL
2.8. Statistical Analysis
3. Results
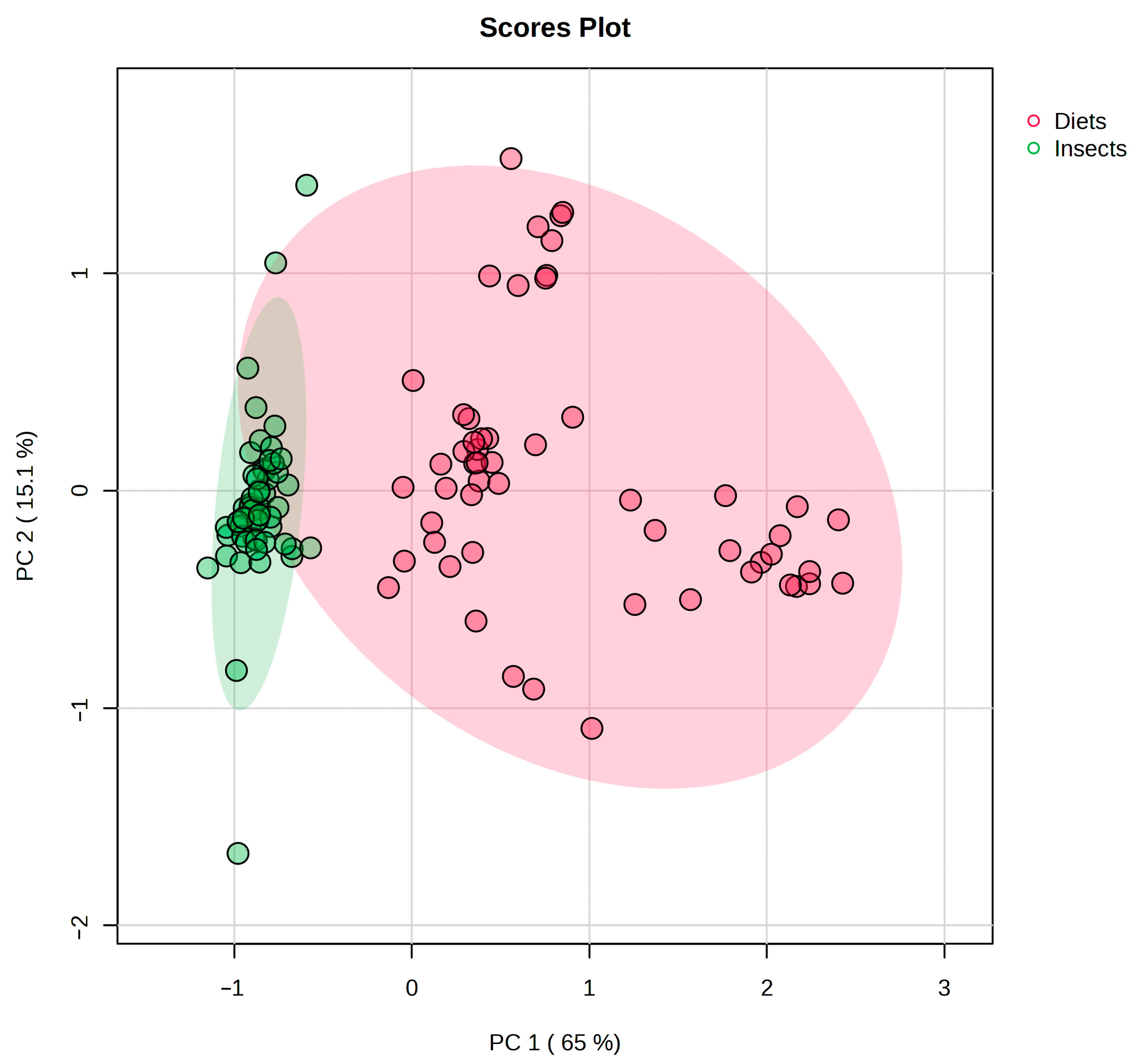
3.1. Overall Characterization of Diets and BSFL
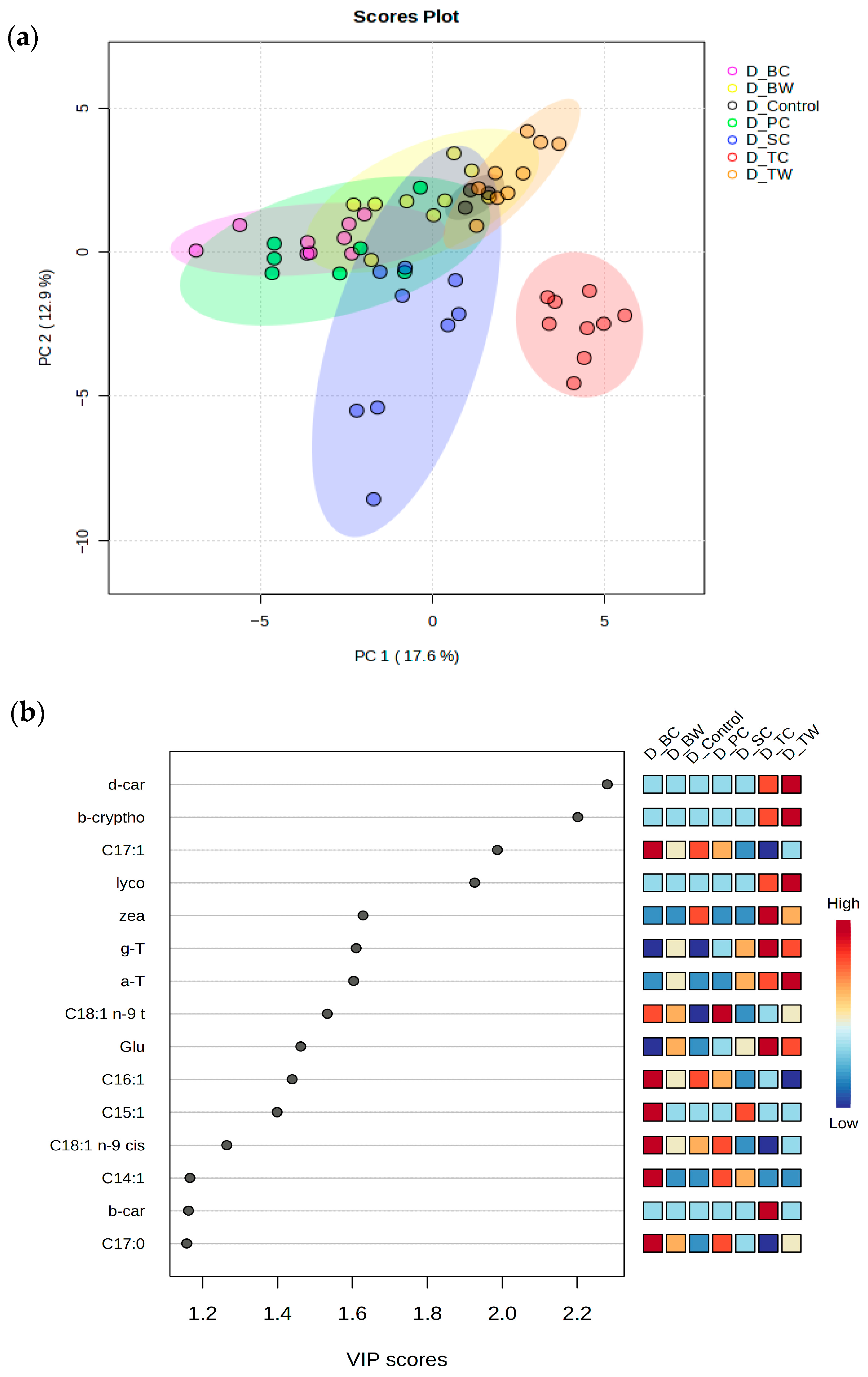
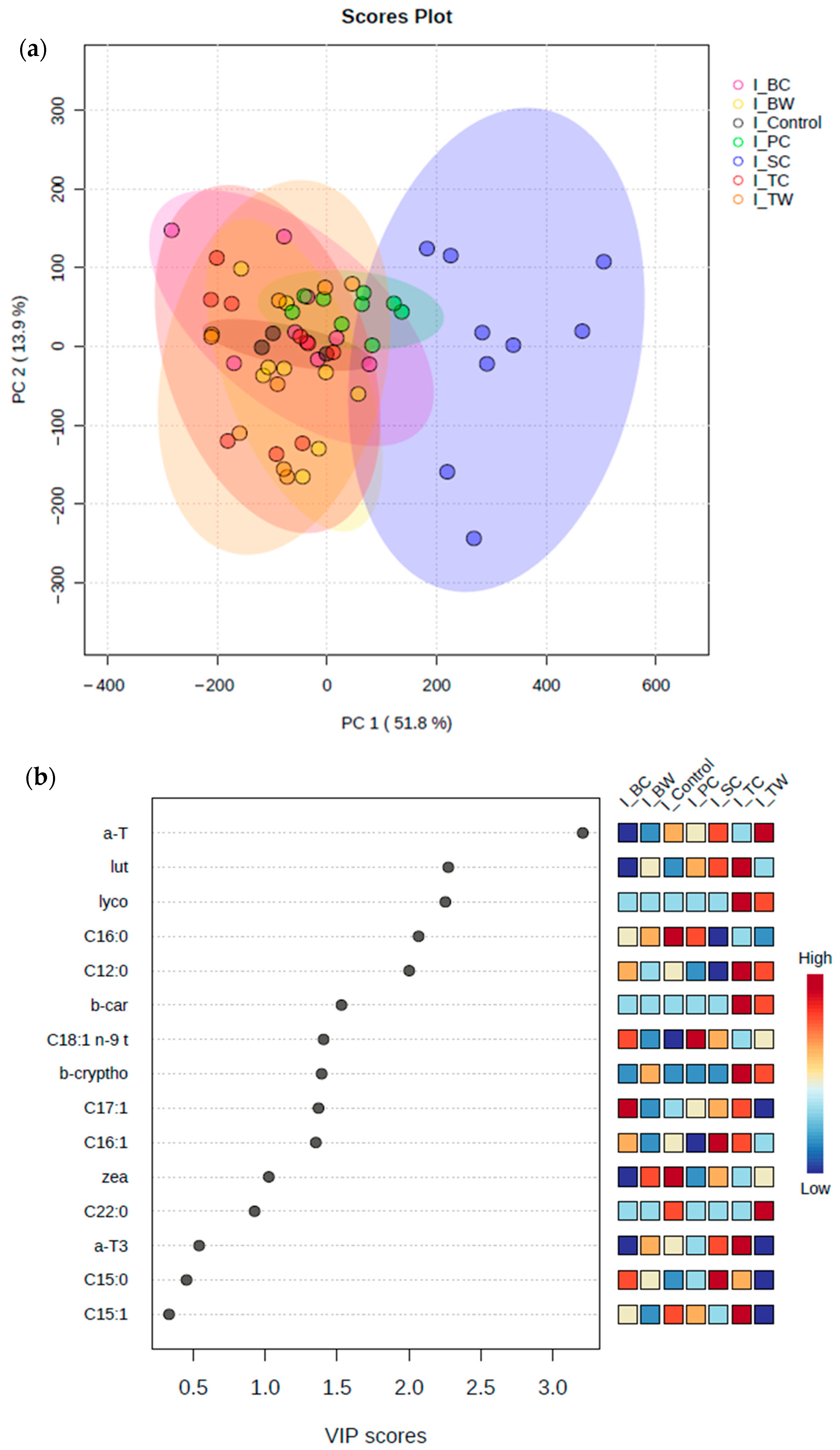
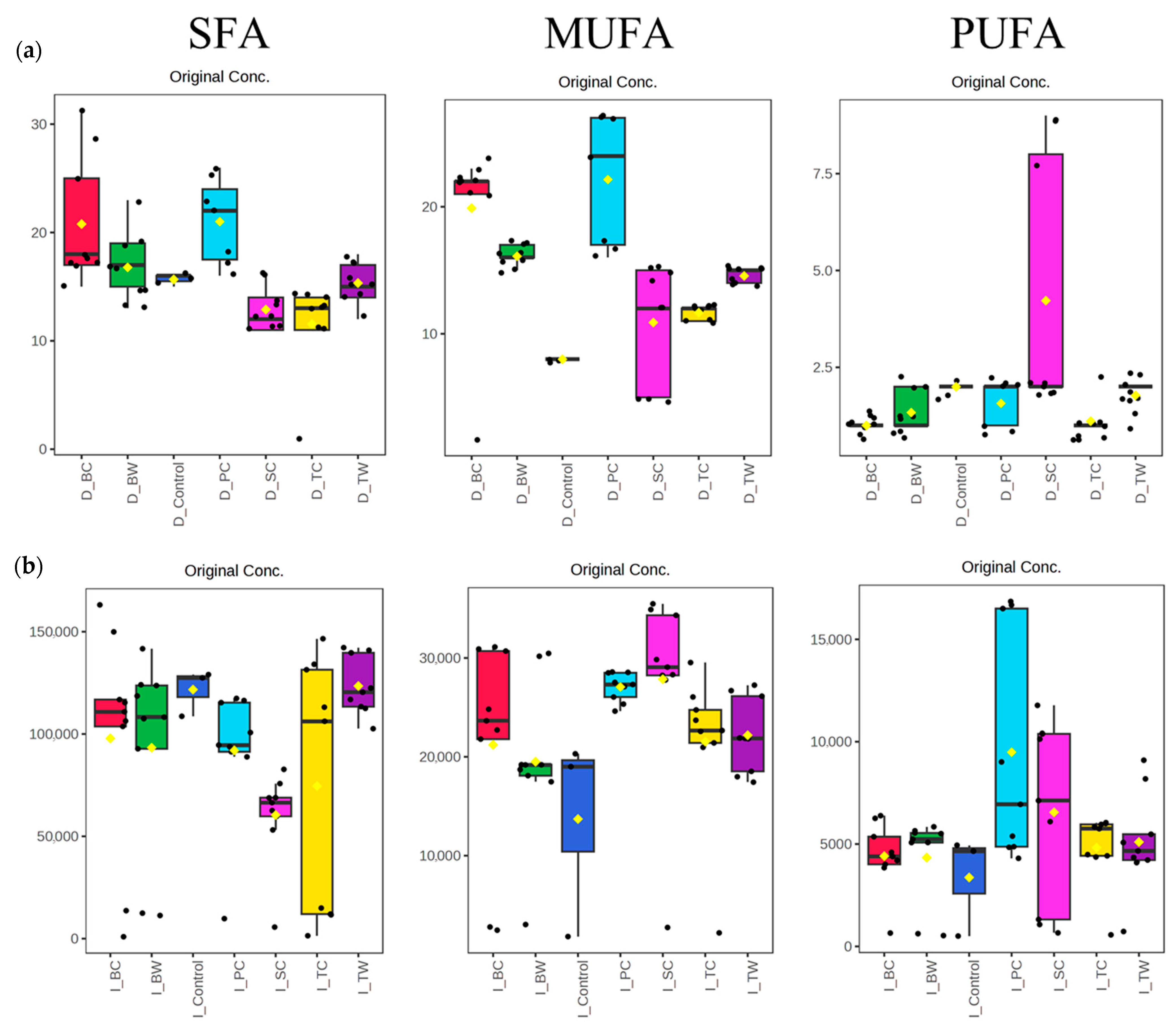
3.2. Fatty Acid Composition
3.3. Amino Acids Profile
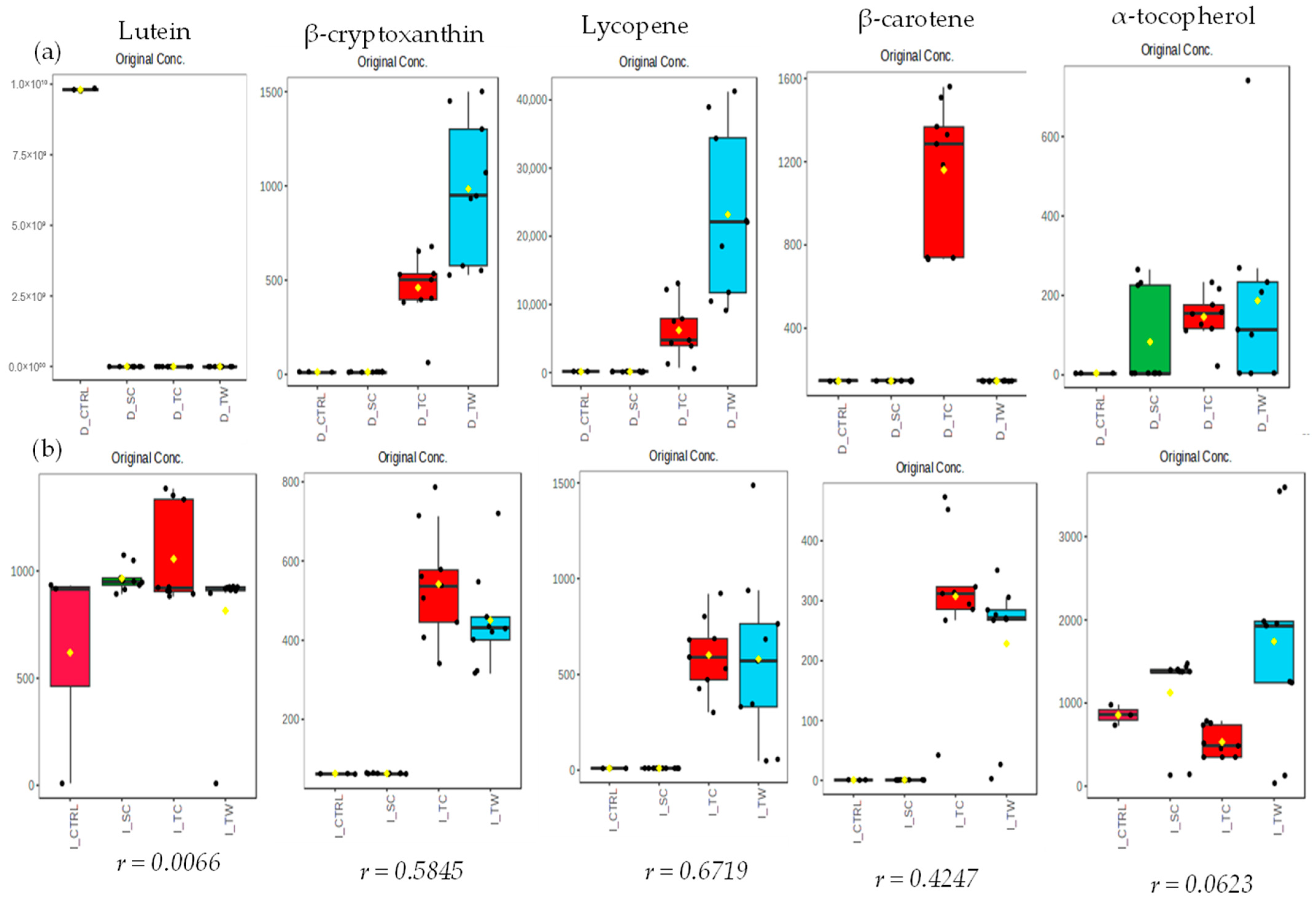
3.4. Carotenoids and Tocopherols
4. Discussion
4.1. Fatty Acid Profile
4.2. Amino Acid Composition
4.3. Carotenoid and Tocopherol Profile
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Pilar Vaquero, M. Foods for Plant-Based Diets: Challenges and Innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef]
- Pedretti, E.F.; Duca, D.; Ballarini, M.; Boakye-Yiadom, K.A.; Ilari, A. Environmental Impact Assessment of Producing Frozen Spinach in Central Italy. Resour. Environ. Sustain. 2023, 12, 100110. [Google Scholar] [CrossRef]
- Grover, Y.; Negi, P.S. Recent Developments in Freezing of Fruits and Vegetables: Striving for Controlled Ice Nucleation and Crystallization with Enhanced Freezing Rates. J. Food Sci. 2023, 88, 4799–4826. [Google Scholar] [CrossRef] [PubMed]
- Ilari, A.; Duca, D.; Toscano, G.; Pedretti, E.F. Evaluation of Cradle to Gate Environmental Impact of Frozen Green Bean Production by Means of Life Cycle Assessment. J. Clean. Prod. 2019, 236, 117638. [Google Scholar] [CrossRef]
- Vitez, T.; Dokulilova, T.; Vitezova, M.; Elbl, J.; Kintl, A.; Kynicky, J.; Hladky, J.; Brtnicky, M. The Digestion of Waste from Vegetables and Maize Processing. Waste Biomass Valorization 2020, 11, 2467–2473. [Google Scholar] [CrossRef]
- Azman, A.T.; Isa, N.S.M.; Zin, Z.M.; Abdullah, M.A.A.; Aidat, O.; Zainol, M.K. Protein Hydrolysate from Underutilized Legumes: Unleashing the Potential for Future Functional Foods. Prev. Nutr. Food Sci. 2023, 28, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Nartea, A.; Kuhalskaya, A.; Fanesi, B.; Orhotohwo, O.L.; Susek, K.; Rocchetti, L.; Di Vittori, V.; Bitocchi, E.; Pacetti, D.; Papa, R. Legume Byproducts as Ingredients for Food Applications: Preparation, Nutrition, Bioactivity, and Techno-Functional Properties. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1953–1985. [Google Scholar] [CrossRef]
- Santos, D.; Lopes da Silva, J.A.; Pintado, M. Fruit and Vegetable By-Products’ Flours as Ingredients: A Review on Production Process, Health Benefits and Technological Functionalities. LWT 2022, 154, 112707. [Google Scholar] [CrossRef]
- Chung, R.W.S.; Leanderson, P.; Gustafsson, N.; Jonasson, L. Liberation of Lutein from Spinach: Effects of Heating Time, Microwave-Reheating and Liquefaction. Food Chem. 2019, 277, 573–578. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone, E. Valorization of Tomato Waste as a Source of Carotenoids. Molecules 2021, 26, 5062. [Google Scholar] [CrossRef]
- Lange, K.W.; Nakamura, Y. Edible Insects as Future Food: Chances and Challenges. J. Future Foods 2021, 1, 38–46. [Google Scholar] [CrossRef]
- van Huis, A. Edible Insects Contributing to Food Security? Agric. Food Secur. 2015, 4, 20. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Bormon, C.C.; Ataher, M.S.; Hossain, M.; Dutta, A.; Mahfuz, S. Insects as Sustainable Source of Feed for Poultry. JSFA Rep. 2025, 5, 370–383. [Google Scholar] [CrossRef]
- Chang, C.L.; Chang, S.C.; Lin, L.J.; Chang, J.S.; Lin, M.J.; Lee, T.T. Assessment of Hermetia illucens Pupal Exuviae Fermented by Bacillus amyloliquefaciens as Functional Feed Additive for Red Feather Native Chickens. Poult. Sci. 2025, 104, 105584. [Google Scholar] [CrossRef] [PubMed]
- Dillard, R.B.; Jones, M.K.; Davis, A.J. Assessing Dried Black Soldier Fly Larva as a Feed Component for Poultry Production. J. Appl. Poult. Res. 2025, 34, 100570. [Google Scholar] [CrossRef]
- Younas, W.; Zuberi, A.; Lodhi, F.A.; Zaman, M.; Noorullah, M.; Ali, M. Potential Beneficial Effects of Dietary Replacement of Fish Meal with Black Soldier Fly (Hermetia illucens) Larvae Meal on Growth, General Physiology, and Microanatomy of Nile Tilapia (Oreochromis niloticus). Fish. Physiol. Biochem. 2025, 51, 1–29. [Google Scholar] [CrossRef]
- Copelotti, E.; Sogari, G.; Andreani, G.; Fronte, B.; Moruzzo, R.; Sangiacomo, C.; Zanzot, A.; Serra, A.; Parisi, G.; Tucciarone, I.; et al. Insect Fish—The Use of Insect Meal in the Fish Sector in Creating Farm-to-Fork Value: Chemical and Quality Characteristics of Sparus aurata Fillets Fed Hermetia illucens Larvae-Based Feed. Foods 2025, 14, 3107. [Google Scholar] [CrossRef] [PubMed]
- Sangiacomo, C.; Trombetta, L.; Susini, F.; Brogi, L.; Licitra, R.; Machese, M.; Falabella, P.; Franco, A.; Scieuzo, C.; Del Vecchio, G.; et al. Hermetia illucens Meal from Different Substrates for Replacing Fishmeal: Study on Zebrafish as Fish Model. Aquac. Rep. 2025, 44, 103043. [Google Scholar] [CrossRef]
- Yordanova, G.; Nedeva, R.D.; Apostolov, A.P.; Mansbridge, S.C.; Whiting, I.M.; Mackenzie, A.M.; Nikolova, G.D.; Karamalakova, Y.D.; Pirgozliev, V.R. Partial Replacement of Soyabean Meal with Defatted Black Soldier Fly (Hermetia illucens L.) Larvae Meal Influences Blood Biochemistry and Modulate Oxidative Stress, but Not Growth Performance of Pigs. Animals 2025, 15, 1077. [Google Scholar] [CrossRef]
- Buffoni, M.; Viveen, M.; De Visser, J.A.G.; Mughini-Gras, L.; Willems, R.J.; Schürch, A.C.; Kers, J.G. Effect of Black Soldier Fly (Hermetia illucens) Larvae Oil Feed Inclusion on Growth Performance and Fecal Microbiome Development in Post-Weaning Pigs. Livest. Sci. 2025, 301, 105819. [Google Scholar] [CrossRef]
- Magee, K.; Halstead, J.; Small, R.; Young, I. Valorisation of Organic Waste By-Products Using Black Soldier Fly (Hermetia illucens) as a Bio-Convertor. Sustainability 2021, 13, 8345. [Google Scholar] [CrossRef]
- Romano, N.; Fischer, H.; Kumar, V.; Francis, S.A.; Sinha, A.K. Productivity, Conversion Ability, and Biochemical Composition of Black Soldier Fly (Hermetia illucens) Larvae Fed with Sweet Potato, Spent Coffee or Dough. Int. J. Trop. Insect Sci. 2021, 1, 3. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Shafi, M.E.; Alghamdi, W.Y.; Abdelnour, S.A.; Shehata, A.M.; Noreldin, A.E.; Ashour, E.A.; Swelum, A.A.; Al-sagan, A.A.; Alkhateeb, M.; et al. Black Soldier Fly (Hermetia illucens) Meal as a Promising Feed Ingredient for Poultry: A Comprehensive Review. Agriculture 2020, 10, 339. [Google Scholar] [CrossRef]
- Müller, A.; Wolf, D.; Gutzeit, H.O. The Black Soldier Fly, Hermetia illucens—A Promising Source for Sustainable Production of Proteins, Lipids and Bioactive Substances. Z. Naturforschung -Sect. C J. Biosci. 2017, 72, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Ratti, S.; Zarantoniello, M.; Chemello, G.; Giammarino, M.; Palermo, F.A.; Cocci, P.; Mosconi, G.; Tignani, M.V.; Pascon, G.; Cardinaletti, G.; et al. Spirulina-Enriched Substrate to Rear Black Soldier Fly (Hermetia illucens) Prepupae as Alternative Aquafeed Ingredient for Rainbow Trout (Oncorhynchus mykiss) Diets: Possible Effects on Zootechnical Performances, Gut and Liver Health Status, and Fillet Quality. Animals 2023, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- European Commission Regulation-2017/893-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2017/893/oj/eng?utm (accessed on 4 November 2025).
- Sogari, G.; Bellezza Oddon, S.; Gasco, L.; van Huis, A.; Spranghers, T.; Mancini, S. Review: Recent Advances in Insect-Based Feeds: From Animal Farming to the Acceptance of Consumers and Stakeholders. Animals 2023, 17, 100904. [Google Scholar] [CrossRef]
- Meijer, N.; Safitri, R.A.; Tao, W.; Hoek-Van den Hil, E.F. Review: European Union Legislation and Regulatory Framework for Edible Insect Production—Safety Issues. Animals 2025, 19, 101468. [Google Scholar] [CrossRef]
- Mohamad, A.; Tan, C.K.; Shah, N.N.A.K.; Nayan, N.; Ibrahim, A.; Abdi, G.; Aadil, R.M. Insect Protein: A Pathway to Sustainable Protein Supply Chains, Challenges, and Prospects. J. Agric. Food Res. 2025, 19, 101678. [Google Scholar] [CrossRef]
- Borel, P.; Hammaz, F.; Morand-Laffargue, L.; Creton, B.; Halimi, C.; Sabatier, D.; Desmarchelier, C. Using Black Soldier Fly Larvae Reared on Fruits and Vegetables Waste as a Sustainable Dietary Source of Provitamin a Carotenoids. Food Chem. 2021, 359, 129911. [Google Scholar] [CrossRef]
- Leni, G.; Maistrello, L.; Pinotti, G.; Sforza, S.; Caligiani, A. Production of Carotenoid-Rich Hermetia illucens Larvae Using Specific Agri-Food by-Products. J. Insects Food Feed. 2022, 9, 171–182. [Google Scholar] [CrossRef]
- Durge, S.M.; Das, A.; Saha, S.K.; Pande, A.; Thakuria, D.; Saxena, A.; Bhardvaj, Y.; Verma, A.K. Dietary Lutein Supplementation Improves Immunity and Antioxidant Status of Captive Indian Leopards (Panthera fusca). Zoo. Biol. 2022, 41, 328–339. [Google Scholar] [CrossRef]
- Schalch Junior, F.J.; Polizel, G.H.G.; Cançado, F.A.C.Q.; Fernandes, A.C.; Mortari, I.; Pires, P.R.L.; Fukumasu, H.; Santana, M.H.d.A.; Saran Netto, A. Prenatal Supplementation in Beef Cattle and Its Effects on Plasma Metabolome of Dams and Calves. Metabolites 2022, 12, 347. [Google Scholar] [CrossRef]
- Hogsette, J.A. New Diets for Production of House Flies and Stable Flies (Diptera: Muscidae) in the Laboratory. J. Econ. Entomol. 1992, 85, 2291–2294. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of Rearing Substrate on Growth Performance, Waste Reduction Efficiency and Chemical Composition of Black Soldier Fly (Hermetia illucens) Larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Sideris, V.F.; Tsagkarakis, A.E. Immature Development Time of Hermetia illucens L. in Different Varieties of Feed. Adv. Entomol. 2017, 5, 109–114. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional Composition of Black Soldier Fly (Hermetia illucens) Prepupae Reared on Different Organic Waste Substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional Value of the Black Soldier Fly (Hermetia Illucens L.) and Its Suitability as Animal Feed—A Review. J. Insects Food Feed. 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Cattaneo, A.; Belperio, S.; Sardi, L.; Martelli, G.; Nannoni, E.; Dabbou, S.; Meneguz, M. Black Soldier Fly Larvae’s Optimal Feed Intake and Rearing Density: A Welfare Perspective (Part II). Insects 2025, 16, 5. [Google Scholar] [CrossRef]
- Ismaiel, L.; Rizzo, V.; Di Mattia, C.; Fanesi, B.; Lucci, P.; D’Alessio, G.; Pacetti, D.; Pittia, P. Oil-in-Water Emulsions Made of Pistachio Oil: Physical and Chemical Properties and Stability. Foods 2024, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Nartea, A.; Ismaiel, L.; Frapiccini, E.; Falcone, P.M.; Pacetti, D.; Frega, N.G.; Lucci, P.; Colella, S. Impact of Modern Oven Treatments on Lipid Oxidation and Vitamin E Content of Fillets from Sardine (Sardina pilchardus) at Different Reproductive Cycle Phases. Antioxidants 2023, 12, 1312. [Google Scholar] [CrossRef] [PubMed]
- Nartea, A.; Fanesi, B.; Falcone, P.M.; Pacetti, D.; Frega, N.G.; Lucci, P. Impact of Mild Oven Cooking Treatments on Carotenoids and Tocopherols of Cheddar and Depurple Cauliflower (Brassica oleracea L. var. botrytis). Antioxidants 2021, 10, 196. [Google Scholar] [CrossRef]
- Yordanova, G.; Nedeva, R.D.; Apostolov, A.P.; Whiting, I.M.; Mansbridge, S.C.; Rose, S.P.; Pirgozliev, V.R. Estimation of the Digestible Energy Value of Fat Obtained from Black Soldier Fly Larvae (Hermetia illucens) for Growing Pigs. Arch. Anim. Nutr. 2024, 78, 315–324. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Carvalho, P.L.P.F.D.; Flint, C.; Miranda, C.; Tomberlin, J.K. Evaluation of Lauric Acid Enhancement of Black Soldier Fly Larvae from Coconut. J. Econ. Entomol. 2024, 117, 1235–1241. [Google Scholar] [CrossRef]
- Srisuksai, K.; Limudomporn, P.; Kovitvadhi, U.; Thongsuwan, K.; Imaram, W.; Lertchaiyongphanit, R.; Sareepoch, T.; Kovitvadhi, A.; Fungfuang, W. Physicochemical Properties and Fatty Acid Profile of Oil Extracted from Black Soldier Fly Larvae (Hermetia illucens). Vet. World 2024, 17, 518. [Google Scholar] [CrossRef] [PubMed]
- Zandi-Sohani, N.; Tomberlin, J.K. Comparison of Growth and Composition of Black Soldier Fly (Hermetia illucens L.) Larvae Reared on Sugarcane By-Products and Other Substrates. Insects 2024, 15, 771. [Google Scholar] [CrossRef]
- Leong, S.Y.; Kutty, S.R.M.; Tan, C.K.; Tey, L.H. Comparative Study on the Effect of Organic Waste on Lauric Acid Produced by Hermetia illucens Larvae via Bioconversion. J. Eng. Sci. Technol. Spec. Issue ACEE 2015, 8, 52–63. [Google Scholar]
- He, Y.; Peng, H.; Jin, M.; Wang, J.; Li, S.; Li, M.; Zhu, T.; Zhang, L.; Chen, X.; Zhou, Q. Application Evaluation of Black Soldier Fly (Hermetia illucens) Larvae Oil in Shrimp Feed: Effects on Growth Performance, Antioxidant Capacity and Lipid Metabolism. Aquac. Rep. 2024, 36, 102174. [Google Scholar] [CrossRef]
- Papin, M.; Sabran, C.; Morand-Laffargue, L.; Sabatier, D.; Sefah, A.; Engel, E.; Planche, C.; Borel, P. Concentrations of Fat-Soluble Vitamins and Carotenoids in Black Soldier Fly Larvae (Hermetia illucens) Fed with Fermented Authorized and Unauthorized Biowaste in Europe. Future Foods 2025, 11, 100614. [Google Scholar] [CrossRef]
- Morand-Laffargue, L.; Vairo, D.; Halimi, C.; Chiarello, E.; Creton, B.; Sabatier, D.; Borel, P. Ability of Black Soldier Fly Larvae to Bioaccumulate Tocopherols from Different Substrates and Measurement of Larval Tocopherol Bioavailability in Vitro. J. Insects Food Feed. 2023, 10, 795–807. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, T.; Jin, M.; Li, X.; Xie, S.; Cui, Y.; Zhou, Q. Black Soldier Fly Larvae Oil Can Partially Replace Fish Oil in the Diet of the Juvenile Mud Crab (Scylla paramamosain). Anim. Nutr. 2025, 20, 469. [Google Scholar] [CrossRef] [PubMed]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty Acid Composition of Black Soldier Fly Larvae (Hermetia illucens)—Possibilities and Limitations for Modification through Diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Su, H.; Zhang, B.; Shi, J.; He, S.; Dai, S.; Zhao, Z.; Wu, D.; Li, J. Black Soldier Fly Larvae as a Novel Protein Feed Resource Promoting Circular Economy in Agriculture. Insects 2025, 16, 830. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, M.G.; Jeong, K.; Yun, E.Y.; Goo, T.W. Medium-Chain Fatty Acids Extracted from Black Soldier Fly (Hermetia illucens) Larvae Prevents High-Fat Diet-Induced Obesity In Vivo in C57BL/6J Mice. Animals 2025, 15, 1384. [Google Scholar] [CrossRef]
- Nekrasov, R.V.; Ivanov, G.A.; Chabaev, M.G.; Zelenchenkova, A.A.; Bogolyubova, N.V.; Nikanova, D.A.; Sermyagin, A.A.; Bibikov, S.O.; Shapovalov, S.O. Effect of Black Soldier Fly (Hermetia illucens L.) Fat on Health and Productivity Performance of Dairy Cows. Animals 2022, 12, 2118. [Google Scholar] [CrossRef]
- Finke, M.D. Complete Nutrient Content of Four Species of Feeder Insects. Zoo. Biol. 2013, 32, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B.; Finke, M.D. Nutritional Value of Insects and Ways to Manipulate Their Composition. J. Insects Food Feed. 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Danieli, P.P.; Lussiana, C.; Gasco, L.; Amici, A.; Ronchi, B. The Effects of Diet Formulation on the Yield, Proximate Composition, and Fatty Acid Profile of the Black Soldier Fly (Hermetia illucens L.) Prepupae Intended for Animal Feed. Animals 2019, 9, 178. [Google Scholar] [CrossRef]
- Barroso, F.G.; Sánchez-Muros, M.J.; Segura, M.; Morote, E.; Torres, A.; Ramos, R.; Guil, J.L. Insects as Food: Enrichment of Larvae of Hermetia illucens with Omega 3 Fatty Acids by Means of Dietary Modifications. J. Food Compos. Anal. 2017, 62, 8–13. [Google Scholar] [CrossRef]
- Truzzi, C.; Giorgini, E.; Annibaldi, A.; Antonucci, M.; Illuminati, S.; Scarponi, G.; Riolo, P.; Isidoro, N.; Conti, C.; Zarantoniello, M.; et al. Fatty Acids Profile of Black Soldier Fly (Hermetia illucens): Influence of Feeding Substrate Based on Coffee-Waste Silverskin Enriched with Microalgae. Anim. Feed. Sci. Technol. 2020, 259, 114309. [Google Scholar] [CrossRef]
- Tognocchi, M.; Abenaim, L.; Adamaki-Sotiraki, C.; Athanassiou, G.C.; Rumbos, I.C.; Mele, M.; Conti, B.; Conte, G. Effect of Different Diet Composition on the Fat Profile of Two Different Black Soldier Fly Larvae Populations. Animals 2024, 18, 101205. [Google Scholar] [CrossRef]
- Quigley, J.D.; Zontini, A.; Schroeder, G.F.; Roman-Garcia, Y.; Houbiers, L.; Bach, A. Nutritional Value of Black Soldier Fly Larvae Oil in Calf Milk Replacers. J. Dairy. Sci. 2025, 108, 2481–2488. [Google Scholar] [CrossRef]
- Ramzy, R.R.; Goenka, V.; El-Dakar, M.A.; Lee, J.S.H. Assessing the Environmental Impacts of the Black Soldier Fly-Based Circular Economy and Decentralized System in Singapore: A Case Study. Sustainability 2025, 17, 6115. [Google Scholar] [CrossRef]
- Somparn, N.; Pootthachaya, P.; Puangsap, W.; Pintaphrom, N.; Srikha, T.; Tengjaroenkul, B.; Cherdthong, A.; Wongtangtintharn, S. Evaluation of Black Soldier Fly Larvae Oil as a Feed Ingredient for Broiler Chickens: Effects on Performance, Carcass Traits, Meat Characteristics, and Blood Parameters. Front. Anim. Sci. 2024, 5, 1496763. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Zeiri, A.; Asif Shah, M. Insect Lipids as Novel Source for Future Applications: Chemical Composition and Industry Applications—A Comprehensive Review. Food Sci. Nutr. 2025, 13, e70553. [Google Scholar] [CrossRef] [PubMed]
- Fawole, F.J.; Labh, S.N.; Hossain, M.S.; Overturf, K.; Small, B.C.; Welker, T.L.; Hardy, R.W.; Kumar, V. Insect (Black Soldier Fly Larvae) Oil as a Potential Substitute for Fish or Soy Oil in the Fish Meal-Based Diet of Juvenile Rainbow Trout (Oncorhynchus mykiss). Anim. Nutr. 2021, 7, 1360. [Google Scholar] [CrossRef]
- Rodrigues, D.P.; Ameixa, O.M.C.C.; Vázquez, J.A.; Calado, R. Improving the Lipid Profile of Black Soldier Fly (Hermetia illucens) Larvae for Marine Aquafeeds: Current State of Knowledge. Sustainability 2022, 14, 6472. [Google Scholar] [CrossRef]
- Fuso, A.; Barbi, S.; Macavei, L.I.; Luparelli, A.V.; Maistrello, L.; Montorsi, M.; Sforza, S.; Caligiani, A. Effect of the Rearing Substrate on Total Protein and Amino Acid Composition in Black Soldier Fly. Foods 2021, 10, 1773. [Google Scholar] [CrossRef]
- Cappellozza, S.; Leonardi, M.G.; Savoldelli, S.; Carminati, D.; Rizzolo, A.; Cortellino, G.; Terova, G.; Moretto, E.; Badaile, A.; Concheri, G.; et al. A First Attempt to Produce Proteins from Insects by Means of a Circular Economy. Animals 2019, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.; Zare, F.; Pletch, A. Pulse Proteins: Processing, Characterization, Functional Properties and Applications in Food and Feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Cui, S.W.; Li, Y.; Xu, S.; Wu, Y.; Wang, J.; Bai, Z.; et al. Nutrients, Phytochemicals and Antioxidant Activities of 26 Kidney Bean Cultivars. Food Chem. Toxicol. 2017, 108, 467–477. [Google Scholar] [CrossRef]
- Eggink, K.M.; Lund, I.; Pedersen, P.B.; Hansen, B.W.; Dalsgaard, J. Biowaste and By-Products as Rearing Substrates for Black Soldier Fly (Hermetia illucens) Larvae: Effects on Larval Body Composition and Performance. PLoS ONE 2022, 17, e0275213. [Google Scholar] [CrossRef]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.M.; Subramanian, S.; Ekesi, S.; van Huis, A.; Borgemeister, C. The Nutritive Value of Black Soldier Fly Larvae Reared on Common Organic Waste Streams in Kenya. Sci. Rep. 2019, 9, 10110. [Google Scholar] [CrossRef]
- Schiavone, A.; De Marco, M.; Martínez, S.; Dabbou, S.; Renna, M.; Madrid, J.; Hernandez, F.; Rotolo, L.; Costa, P.; Gai, F.; et al. Nutritional Value of a Partially Defatted and a Highly Defatted Black Soldier Fly Larvae (Hermetia illucens L.) Meal for Broiler Chickens: Apparent Nutrient Digestibility, Apparent Metabolizable Energy and Apparent Ileal Amino Acid Digestibility. J. Anim. Sci. Biotechnol. 2017, 8, 51. [Google Scholar] [CrossRef]
- Rossi, G.; Psarianos, M.; Ojha, S.; Schlüter, O.K. Review: Insects as a Novel Feed Ingredient: Processing Technologies, Quality and Safety Considerations. Animals 2025, 19, 101495. [Google Scholar] [CrossRef]
- Lu, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; Thongpea, S.; Paengkoum, S.; Purba, R.A.P.; et al. Nutritional Composition of Black Soldier Fly Larvae (Hermetia illucens L.) and Its Potential Uses as Alternative Protein Sources in Animal Diets: A Review. Insects 2022, 13, 831. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbø, R.; Krogdahl, Å.; Lock, E.J. Black Soldier Fly Larvae Meal Can Replace Fish Meal in Diets of Sea-Water Phase Atlantic Salmon (Salmo salar). Aquaculture 2019, 503, 609–619. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, M.; Yuan, B.; Jin, X.; Zhang, X.; Xie, G.; Wang, Z.; Lv, Y.; Wang, W.; Huang, Y. Growth Performance and Meat Quality of Growing Pigs Fed with Black Soldier Fly (Hermetia illucens) Larvae as Alternative Protein Source. Processes 2022, 10, 1498. [Google Scholar] [CrossRef]
- Onsongo, V.O.; Osuga, I.M.; Gachuiri, C.K.; Wachira, A.M.; Miano, D.M.; Tanga, C.M.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Insects for Income Generation Through Animal Feed: Effect of Dietary Replacement of Soybean and Fish Meal With Black Soldier Fly Meal on Broiler Growth and Economic Performance. J. Econ. Entomol. 2018, 111, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Lemme, A.; Klüber, P. Rethinking Amino Acid Nutrition of Black Soldier Fly Larvae (Hermetia illucens) Based on Insights from an Amino Acid Reduction Trial. Insect 2024, 15, 862. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Uehara, T.; Shimoda, M. Enhancing Amino Acid Productivity and Profile in Black Soldier Fly Larvae through NAT Transporter Suppression in the Excretion System. bioRxiv 2024. bioRxiv:2024.03.24.586506. [Google Scholar] [CrossRef]
- Tanga, C.M.; Mokaya, H.O.; Kasiera, W.; Subramanian, S. Potential of Insect Life Stages as Functional Ingredients for Improved Nutrition and Health. Insects 2023, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Brai, A.; Pasqualini, C.; Poggialini, F.; Vagaggini, C.; Dreassi, E. Insects as Source of Nutraceuticals with Antioxidant, Antihypertensive, and Antidiabetic Properties: Focus on the Species Approved in Europe up to 2024. Foods 2025, 14, 1383. [Google Scholar] [CrossRef]
- Boykin, K.L.; Mitchell, M.A. Evaluation of Vitamin A Gut Loading in Black Soldier Fly Larvae (Hermetia illucens). Zoo. Biol. 2021, 40, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, M.; Mansour, A.; Park, I.; Lee, D.W.; Seo, Y.S. Insect-Based Agri-Food Waste Valorization: Agricultural Applications and Roles of Insect Gut Microbiota. Environ. Sci. Ecotechnol. 2024, 17, 100287. [Google Scholar] [CrossRef]
- Hamam, M.; D’Amico, M.; Di Vita, G. Advances in the Insect Industry within a Circular Bioeconomy Context: A Research Agenda. Env. Sci. Eur. 2024, 36, 29. [Google Scholar] [CrossRef]
- Lisboa, H.M.; Nascimento, A.; Arruda, A.; Sarinho, A.; Lima, J.; Batista, L.; Dantas, M.F.; Andrade, R. Unlocking the Potential of Insect-Based Proteins: Sustainable Solutions for Global Food Security and Nutrition. Foods 2024, 13, 1846. [Google Scholar] [CrossRef]
- Tepper, K.; Edwards, O.; Sunna, A.; Paulsen, I.T.; Maselko, M. Diverting Organic Waste from Landfills via Insect Biomanufacturing Using Engineered Black Soldier Flies (Hermetia illucens). Commun. Biol. 2024, 7, 862. [Google Scholar] [CrossRef]
- Albarki, H.R.; Suntara, C.; Wongtangtintharn, S.; Iwai, C.B.; Jayanegara, A.; Cherdthong, A. Sustainable Animal Nutrition and Feeding Strategies for Reducing Methane Emissions and Enhancing Feed Digestibility with Encapsulated Black Soldier Fly Larvae Oil. Sustainability 2025, 17, 3155. [Google Scholar] [CrossRef]
| Samples | Diets (By-Products) | Insects (BSFL) |
|---|---|---|
| Control | D_CTRL | I_CTRL |
| Spinach: Chickpea | D_SC | I_SC |
| Tomato: Chickpea | D_TC | I_TC |
| Tomato: Wheat | D_TW | I_TW |
| Pea: Chickpea | D_PC | I_PC |
| Green bean: Chickpea | D_BC | I_BC |
| Green bean: Wheat | D_BW | I_BW |
| D_CTRL | D_PC | D_TC | D_SC | D_BC | D_BW | D_TW | |
|---|---|---|---|---|---|---|---|
| C16:0 | 0.16 ± 0.00 ab | 0.17 ± 0.02 ab | 0.11 ± 0.02 d | 0.11 ± 0.01 cd | 0.17 ± 0.04 a | 0.15 ± 0.02 ab | 0.14 ± 0.01 ac |
| C18:0 | ND | 0.03 ± 0.01 b | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.02 ± 0.01 ab | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| C18:1 | 0.09 ± 0.00 a | 0.23 ± 0.05 d | 0.12 ± 0.00 ab | 0.12 ± 0.05 ab | 0.22 ± 0.01 d | 0.17 ± 0.01 c | 0.16 ± 0.01 bc |
| C20:3 | 0.02 ± 0.00 ab | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.04 ± 0.03 b | 0.01 ± 0.00 a | 0.02 ± 0.01 a | 0.02 ± 0.00 a |
| ΣSFA | 0.17 ± 0.00 abc | 0.23 ± 0.04 a | 0.13 ± 0.01 b | 0.15 ± 0.02 bc | 0.23 ± 0.06 a | 0.18 ± 0.03 abc | 0.19 ± 0.03 ac |
| ΣMUFA | 0.10 ± 0.00 a | 0.24 ± 0.06 d | 0.13 ± 0.00 ab | 0.12 ± 0.05 ab | 0.24 ± 0.01 d | 0.18 ± 0.01 c | 0.16 ± 0.01 bc |
| ΣPUFA | 0.02 ± 0.00 ab | 0.02 ± 0.00 a | 0.01 ± 0.00 a | 0.04 ± 0.03 b | 0.01 ± 0.00 a | 0.02 ± 0.01 a | 0.02 ± 0.00 a |
| I_CTRL | I_PC | I_TC | I_SC | I_BC | I_BW | I_TW | |
|---|---|---|---|---|---|---|---|
| C10:0 | 10.02 ± 0.99 b | 12.72 ± 6.24 b | 11.23 ± 1.59 b | 1.73 ± 1.16 a | 11.61 ± 2.04 b | 10.60 ± 1.61 b | 12.81 ± 0.95 b |
| C12:0 | 826.80 ± 94.86 ab | 692.98 ± 95.94 b | 894.15 ± 108.41 a | 383.48 ± 52.70 c | 842.30 ± 165.60 ab | 825.92 ± 93.85 ab | 882.24 ± 124.73 a |
| C14:0 | 128.42 ± 12.87 ab | 101.63 ± 14.63 bc | 131.84 ± 21.18 a | 86.16 ± 15.80 c | 125.84 ± 26.57 ab | 130.23 ± 18.14 a | 114.45 ± 20.32 abc |
| C14:1 | 3.52 ± 1.09 | 2.94 ± 0.34 | 3.52 ± 1.28 | 3.41 ± 0.76 | 3.29 ± 0.85 | 2.59 ± 0.45 | 2.64 ± 0.53 |
| C15:0 | 7.02 ± 1.70 | 9.94 ± 1.47 | 9.74 ± 2.33 | 14.45 ± 16.47 | 12.17 ± 2.48 | 9.12 ± 6.99 | 8.65 ± 1.02 |
| C15:1 | 5.44 ± 1.36 ab | 4.82 ± 0.80 ab | 6.25 ± 3.27 a | 3.08 ± 1.11 b | 5.03 ± 0.73 ab | 0.66 ± 0.99 c | 0.34 ± 0.51 c |
| C16:0 | 202.65 ± 6.73 bc | 169.21 ± 7.83 bc | 200.55 ± 22.33 c | 126.50 ± 26.88 a | 186.11 ± 37.74 bc | 162.84 ± 18.00 b | 183.38 ± 13.91 bc |
| C16:1 | 46.52 ± 5.91 ab | 45.49 ± 1.40 ab | 52.50 ± 11.21 ab | 57.16 ± 11.61 a | 46.72 ± 13.12 ab | 41.10 ± 6.76 b | 48.11 ± 7.43 ab |
| C17:0 | 5.75 ± 0.40 ab | 6.15 ± 1.38 ab | 6.96 ± 1.00 a | 3.47 ± 0.63 b | 6.69 ± 1.14 a | 6.04 ± 4.14 ab | 6.75 ± 1.04 a |
| C17:1 | 10.45 ± 1.13 ab | 13.76 ± 1.82 ab | 13.82 ± 2.89 ab | 16.78 ± 0.81 ab | 20.84 ± 7.58 a | 11.81 ± 11.31 b | 11.63 ± 2.64 b |
| C18:0 | 23.37 ± 0.93 ab | 22.39 ± 1.00 ab | 24.89 ± 2.21 b | 18.43 ± 3.38 a | 24.29 ± 5.22 b | 21.42 ± 3.59 ab | 25.36 ± 4.53 b |
| C18:1 | 140.30 ± 4.93 c | 222.30 ± 13.49 a | 175.67 ± 12.84 bc | 243.81 ± 25.66 a | 209.20 ± 28.97 ab | 179.64 ± 38.86 bc | 178.25 ± 33.25 bc |
| C18:2 | 35.06 ± 2.19 abc | 83.14 ± 54.19 a | 42.08 ± 6.50 c | 81.27 ± 20.37 ab | 39.99 ± 9.07 c | 42.47 ± 3.50 c | 46.17 ± 18.76 bc |
| C20:3 | 13.76 ± 0.50 ab | 11.74 ± 1.62 ab | 11.86 ± 0.98 ab | 14.71 ± 4.65 b | 10.63 ± 2.75 a | 12.46 ± 1.16 ab | 12.08 ± 0.56 ab |
| C20:0 | 22.98 ± 4.28 ab | 15.38 ± 0.55 b | 19.28 ± 4.27 ab | 28.07 ± 4.01 a | 17.67 ± 7.58 b | 15.78 ± 10.74 b | 11.14 ± 7.33 b |
| C20:1 | 2.97 ± 2.58 a | nd | 6.09 ± 1.75 a | 5.57 ± 8.59 a | nd | 6.40 ± 6.06 a | nd |
| ΣSFA | 1227.15 ± 114.38 ab | 1030.40 ± 120.77 b | 1298.64 ± 156.57 a | 662.28 ± 93.22 c | 1226.66 ± 237.68 ab | 1181.95 ± 139.87 ab | 1247.37 ± 143.03 ab |
| ΣMUFA | 209.21 ± 12.49 b | 289.31 ± 15.49 ab | 257.84 ± 30.72 b | 329.81 ± 46.68 a | 285.08 ± 42.71 ab | 242.18 ± 62.24 b | 240.98 ± 42.36 b |
| ΣPUFA | 48.82 ± 2.05 abc | 94.88 ± 55.73 ab | 53.93 ± 7.36 c | 95.98 ± 24.38 a | 50.62 ± 10.81 c | 54.93 ± 3.77 c | 58.26 ± 18.75 bc |
| Saturated FAs | C12:0 | C14:0 | C16:0 | C17:0 | C18:0 | C20:0 | ΣSFA |
| (r) | −0.05 | 0.56 | 0.93 | 0.66 | 0.62 | 0.40 | 0.35 |
| Unsaturated FAs | C18:1 n-9c | C18:1 n-9t | C18:2 | C20:3 | C20:1 | ΣMUFA | ΣPUFA |
| (r) | −0.14 | −0.03 | −0.11 | 0.83 | −0.29 | −0.13 | 0.80 |
| Samples (Diets) | Amino Acid (mg/100 g dw) | Samples (BSFL) | Amino Acid (mg/100 g dw) |
|---|---|---|---|
| D_CTRL | 4.34 ± 1.05 | I_CTRL | 9.31 ± 1.26 |
| D_BC | 5.10 ± 0.41 | I_BC | 9.36 ± 0.87 |
| D_BW | 4.68 ± 0.95 | I_BW | 8.72 ± 1.63 |
| D_PC | 5.23 ± 0.73 | I_PC | 8.90 ± 0.76 |
| D_SC | 5.40 ± 0.96 | I_SC | 9.18 ± 1.03 |
| D_TC | 6.40 ± 0.93 | I_TC | 9.94 ± 0.77 |
| D_TW | 4.45 ± 0.78 | I_TW | 9.76 ± 1.21 |
| D_CTRL | D_BC | D_BW | D_PC | D_SC | D_TC | D_TW | |
|---|---|---|---|---|---|---|---|
| α-tocopherol | nd | nd | 0.25 ± 0.4 b | nd | 0.80 ± 1.2 b | 1.68 ± 0.5 ab | 1.85 ± 2.3 a |
| β-tocopherol | 8.55 ± 0.3 a | 1.67 ± 3.3 b | 7.64 ± 0.2 a | 2.31 ± 3.5 b | nd | 6.43 ± 2.4 a | 6.63 ± 0.3 a |
| γ-tocopherol | nd | nd | 1.73 ± 0.7 bc | 0.68 ± 1.1 c | 2.12 ± 1.7 bc | 15.06 ± 2.6 a | 3.35 ± 0.4 b |
| α-tocotrienol | 1.87 ± 0.8 ab | 0.80 ± 1.2 b | 2.28 ± 0.2 ab | 1.89 ± 2.3 ab | 0.79 ± 0.8 b | 1.16 ± 0.5 ab | 2.60 ± 0.4 a |
| γ-tocotrienol | 4.43 ± 0.2 a | nd | 1.22 ± 0.1 b | nd | nd | nd | 0.43 ± 0.6 c |
| Neoxanthin | nd | nd | nd | nd | 87.05 ± 58.1 a | nd | nd |
| Violaxanthin | nd | nd | nd | nd | 162.01 ± 146.8 a | nd | nd |
| Zeaxanthin | 9.54 ± 0.1 ab | nd | nd | nd | nd | 9.94 ± 0.4 a | 6.06 ± 4.5 b |
| Lutein | 9.81 ± 0.1 b | 9.44 ± 0.3 b | 14.16 ± 3.3 b | 11.33 ± 2.1 b | 31.63 ± 19.9 a | 10.80 ± 0.7 b | 10.40 ± 0.5 b |
| δ-carotene | nd | nd | nd | nd | nd | 41.58 ± 7.4 a | 50.77 ± 14.8 a |
| β-cryptoxanthin | nd | nd | nd | nd | nd | 52.44 ± 11.5 a | 98.45 ± 38.1 b |
| Lycopene | nd | nd | nd | nd | nd | 824 ± 378.3 a | 2318.69 ± 1230.7 b |
| β-carotene | nd | nd | nd | nd | nd | 116.09 ± 33.7 a | nd |
| I_CTRL | I_BC | I_BW | I_PC | I_SC | I_TC | I_TW | |
|---|---|---|---|---|---|---|---|
| α-tocopherol | 8.56 ± 1 bc | nd | 2.99 ± 0.8 c | 7.22 ± 2.6 c | 13.98 ± 0.4 b | 5.31 ± 1.8 c | 22.54 ± 10.2 a |
| α-tocotrienol | 16.76 ± 0.5 ab | nd | 15.15 ± 2.8 ab | 11.34 ± 8.5 b | 15.81 ± 2.6 ab | 19.79 ± 1.2 a | nd |
| Zeaxanthin | 3.86 ± 0.2 a | nd | 3.06 ± 0.2 b | 2.66 ± 0.1 b | 2.99 ± 0.3 b | 3.62 ± 0.8 a | 2.93 ± 0.1 b |
| Lutein | 9.17 ± 0.1 ab | nd | 9.52 ± 0.3 ab | 9.06 ± 0.2 b | 9.65 ± 0.6 ab | 10.56 ± 2.2 a | 9.15 ± 0.1 b |
| β-cryptoxanthin | nd | nd | 3.69 ± 0.4 b | nd | nd | 5.41 ± 1.4 a | 4.51 ± 1.2 ab |
| Lycopene | nd | nd | nd | nd | nd | 6.02 ± 1.9 a | 6.87 ± 3.6 a |
| β-carotene | nd | nd | nd | nd | nd | 3.49 ± 0.7 a | 2.89 ± 0.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orhotohwo, O.L.; Fanesi, B.; Ismaiel, L.; Kuhalskaya, A.; Darko, H.S.O.; Ashim, A.; Corsi, L.; Ruschioni, S.; Ilari, A.; Duca, D.; et al. Bioconversion of Seasonal Vegetable By-Products into Nutrient-Rich Biomass Using Black Soldier Fly Larvae. Sustainability 2025, 17, 10632. https://doi.org/10.3390/su172310632
Orhotohwo OL, Fanesi B, Ismaiel L, Kuhalskaya A, Darko HSO, Ashim A, Corsi L, Ruschioni S, Ilari A, Duca D, et al. Bioconversion of Seasonal Vegetable By-Products into Nutrient-Rich Biomass Using Black Soldier Fly Larvae. Sustainability. 2025; 17(23):10632. https://doi.org/10.3390/su172310632
Chicago/Turabian StyleOrhotohwo, Oghenetega Lois, Benedetta Fanesi, Lama Ismaiel, Anastasiya Kuhalskaya, Helen Stephanie Ofei Darko, Aizhan Ashim, Lorenzo Corsi, Sara Ruschioni, Alessio Ilari, Daniele Duca, and et al. 2025. "Bioconversion of Seasonal Vegetable By-Products into Nutrient-Rich Biomass Using Black Soldier Fly Larvae" Sustainability 17, no. 23: 10632. https://doi.org/10.3390/su172310632
APA StyleOrhotohwo, O. L., Fanesi, B., Ismaiel, L., Kuhalskaya, A., Darko, H. S. O., Ashim, A., Corsi, L., Ruschioni, S., Ilari, A., Duca, D., Foppa Pedretti, E., Pasquini, M., Trombetta, M. F., Pacetti, D., Lucci, P., & Riolo, P. (2025). Bioconversion of Seasonal Vegetable By-Products into Nutrient-Rich Biomass Using Black Soldier Fly Larvae. Sustainability, 17(23), 10632. https://doi.org/10.3390/su172310632












