Towards Zoo Sustainability: Assessment of Indoor and Outdoor Bacterial Air Contamination Levels and Their Correlations with Microclimate Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Zoo Management
2.2. Data Collection
2.3. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Air Protection Act. Off. J. Repub. Croat. 2019, 127, 2553. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2019_12_127_2553.html (accessed on 15 April 2025). (In Croatian).
- Zhao, Y.; Aarnink, A.J.A.; De Jong, M.C.M.; Groot Koerkamp, P.W.G. Airborne microorganisms from livestock production systems and their relation to dust. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1071–1128. [Google Scholar] [CrossRef]
- David, B.; Mejdell, C.; Michel, V.; Lund, V.; Moe, R.O. Air quality in alternative housing systems may have an impact on laying hen welfare. Part II—Ammonia. Animals 2015, 5, 886–896. [Google Scholar] [CrossRef]
- Clauß, M. Emission of Bioaerosols from Livestock Facilities: Methods and Results from Available Bioaerosol Investigations in and Around Agricultural Livestock Farming; Thünen Working Paper, No. 138a; Johann Heinrich von Thünen-Institut: Braunschweig, Germany, 2020. [Google Scholar] [CrossRef]
- Chmielowiec-Korzeniowska, A.; Trawińska, B.; Tymczyna, L.; Bis-Wencel, H.; Matuszewski, Ł. Microbial contamination of the air in livestock buildings as a threat to human and animal health—A review. Ann. Anim. Sci. 2021, 21, 417–431. [Google Scholar] [CrossRef]
- Seedorf, J.; Hartung, J.; Schröder, M.; Linkert, K.H.; Phillips, V.R.; Holden, M.R.; Sneath, R.W.; Short, J.L.; White, R.P.; Pedersen, S.; et al. Concentrations and emissions of airborne endotoxins and microorganisms in livestock buildings in northern Europe. J. Agric. Eng. Res. 1998, 70, 97–109. [Google Scholar] [CrossRef]
- Radon, K.; Danuser, B.; Iversen, M.; Monso, E.; Weber, C.; Hartung, J.; Donham, K.J.; Palmgren, U.; Nowak, D. Air contaminants in different European farming environments. Ann. Agric. Environ. Med. 2002, 9, 41–48. [Google Scholar]
- Matković, K.; Vučemilo, M.; Vinković, B. Airborne fungi in dwellings for dairy cows and laying hens. Arh. Hig. Rada Toksikol. 2009, 60, 395–399. [Google Scholar] [CrossRef][Green Version]
- Millner, P.D. Bioaerosols associated with animal production operations. Bioresour. Technol. 2009, 100, 5379–5385. [Google Scholar] [CrossRef]
- Mostafa, E. Air-polluted with particulate matters from livestock buildings. In Air Quality—New Perspective; Lopez Badilla, G., Valdez, B., Schorr, M., Eds.; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef][Green Version]
- Douglas, P.; Robertson, S.; Gay, R.; Hansell, A.L.; Gant, T.W. A systematic review of the public health risks of bioaerosols from intensive farming. Int. J. Hyg. Environ. Health 2018, 221, 134–173. [Google Scholar] [CrossRef]
- Buoio, E.; Cialini, C.; Costa, A. Air quality assessment in pig farming: The Italian Classyfarm. Animals 2023, 13, 2297. [Google Scholar] [CrossRef]
- Rivas, A.E.; Dykstra, M.J.; Kranz, K.; Bronson, E. Environmental fungal loads in an indoor-outdoor African penguin (Spheniscus demersus) exhibit. J. Zoo Wildl. Med. 2018, 49, 542–555. [Google Scholar] [CrossRef]
- Grzyb, J.; Lenart-Boroń, A. Bacterial bioaerosol concentration and size distribution in the selected animal premises in a zoological garden. Aerobiologia 2019, 35, 253–268. [Google Scholar] [CrossRef]
- Grzyb, J.; Lenart-Boroń, A. Size distribution and concentration of fungal aerosol in animal premises of a zoological garden. Aerobiologia 2020, 36, 233–248. [Google Scholar] [CrossRef]
- Omar, S.; Jalaludin, F.A.; Yee, J.M.; Kamarudin, Z.; Jayaseelan, K.; Khlubi, A.N.M.; Madaki, Y.L.; Hassan, H.; Ramli, M.N.; Topani, R.; et al. Mycological isolation from animal enclosures and environments in National Wildlife Rescue Centre and National Zoo, Malaysia. J. Vet. Med. Sci. 2020, 82, 1236–1242. [Google Scholar] [CrossRef]
- Grzyb, J.; Pawlak, K. Impact of bacterial aerosol, particulate matter, and microclimatic parameters on animal welfare in Chorzów (Poland) zoological garden. Environ. Sci. Pollut. Res. Int. 2021, 28, 3318–3330. [Google Scholar] [CrossRef]
- Grzyb, J.; Pawlak, K. Staphylococci and fecal bacteria as bioaerosol components in animal housing facilities in the Zoological Garden in Chorzów. Environ. Sci. Pollut. Res. Int. 2021, 28, 56615–56627. [Google Scholar] [CrossRef]
- Álvarez-Pérez, S.; García, M.E.; Martínez-Nevado, E.; Blanco, J.L. Presence of Aspergillus fumigatus with the TR34/L98H Cyp51A mutation and other azole-resistant aspergilli in the air of a zoological park. Res. Vet. Sci. 2023, 164, 104993. [Google Scholar] [CrossRef]
- Plewa-Tutaj, K.; Krzyściak, P.; Dobrzycka, A. Mycological air contamination level and biodiversity of airborne fungi isolated from the zoological garden air—Preliminary research. Environ. Sci. Pollut. Res. Int. 2024, 31, 43066–43079. [Google Scholar] [CrossRef]
- Plewa-Tutaj, K.; Twarużek, M.; Kosicki, R.; Soszczyńska, E. Analysis of mycotoxins and cytotoxicity of airborne molds isolated from the zoological garden–screening research. Pathogens 2024, 13, 294. [Google Scholar] [CrossRef]
- Jesse Joel, T.; Gomez, P.L.A.; Gautam, S.; Likhith, B.; Mary, C.R.D.; Upadhyay, R.; Abhilash, P. Unveiling the microbial symphony: Exploring emerging contaminants in zoological environments for enhanced animal welfare. Aerosol Sci. Eng. 2025, 9, 308–319. [Google Scholar] [CrossRef]
- Walsh, M.T.; Pelton, C.A. Chapter 34—Air Quality and Zoo Health Management. Fowler’s Zoo Wild Anim. Med. Curr. Ther. 2023, 10, 223–230. [Google Scholar] [CrossRef]
- Building a Sustainable NEW Zoo. Available online: https://newzoo.org/wp-content/uploads/2013/08/building-a-sustainable-new-zoo.pdf (accessed on 7 November 2025).
- Association of Zoos and Aquariums (AZA). AZA Green Guide: Building and Measuring Zoo & Aquarium Sustainability Plans; AZA: Silver Spring, MD, USA, 2013; Volume 2, Available online: https://assets.speakcdn.com/assets/2332/aza_green_guide_volume_2.pdf (accessed on 24 October 2025).
- Rodriguez, J. One Health ethics and the ethics of zoonoses: A silent call for global action. Vet. Sci. 2024, 11, 394. [Google Scholar] [CrossRef]
- Wang, F.; Xiang, L.; Sze-Yin Leung, K.; Elsner, M.; Zhang, Y.; Guo, Y.; Pan, B.; Sun, H.; An, T.; Ying, G.; et al. Emerging contaminants: A One Health perspective. Innovation 2024, 5, 100612. [Google Scholar] [CrossRef]
- Correia, G.; Calheiros, D.; Rosa, N.; Rodrigues, L.; Cunha, S.; Santiago, L.M.; Costa, J.; Gameiro da Silva, M.; Gonçalves, T. Indoor air quality and airborne transmission under the One Health lens: A scoping review. One Health 2025, 21, 101160. [Google Scholar] [CrossRef] [PubMed]
- Gębarowska, E.; Pusz, W.; Kucińska, J.; Kita, W. Comparative analysis of airborne bacteria and fungi in two salt mines in Poland. Aerobiologia 2018, 34, 127–138. [Google Scholar] [CrossRef]
- Zoo Zagreb. Available online: https://zoo.hr/about/ (accessed on 22 April 2025).
- Animal Protection Act. Off. J. Repub. Croat. 2017, 102, 2342. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2017_10_102_2342.html (accessed on 22 April 2025). (In Croatian).
- Ordinance on the Requirements for the Establishment and Operation of Zoos. Off. J. Repub. Croat. 2005, 67, 1334. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2005_06_67_1334.html (accessed on 22 April 2025). (In Croatian).
- Commission of the European Communities (CEC). Indoor Air Quality & Its Impact on Man. Biological Particles in Indoor Environments, Report No. 12; CEC: Luxembourg, 1993; Available online: https://www.aivc.org/sites/default/files/members_area/medias/pdf/Inive/ECA/ECA_Report12.pdf (accessed on 27 October 2025).
- World Health Organization (WHO). WHO Guidelines for Indoor Air Quality: Dampness and Mould; Heseltine, E., Rosen, J., Eds.; WHO: Geneva, Switzerland, 2009; ISBN 978 92 890 4168 3. [Google Scholar]
- Occupational Safety and Health Administration (OSHA). Indoor Air Quality Investigation. In OSHA Technical Manual (OTM); Section III: Chapter 2; Occupational Safety and Health Administration (OSHA): Washington, DC, USA, 2023. Available online: https://www.osha.gov/otm/section-3-health-hazards/chapter-2 (accessed on 27 October 2025).
- Jerez, S.B.; Cheng, Y.; Bray, J. Exposure of workers to dust and bioaerosol on a poultry farm. J. Appl. Poult. Res. 2014, 23, 7–14. [Google Scholar] [CrossRef]
- Mentese, S.; Rad, A.Y.; Arısoy, M.; Güllü, G. Multiple comparisons of organic, microbial, and fine particulate pollutants in typical indoor environments: Diurnal and seasonal variations. J. Air Waste Manag. Assoc. 2012, 62, 1380–1393. [Google Scholar] [CrossRef] [PubMed]
- Van Leuken, J.P.G.; Swart, A.N.; Havelaar, A.H.; Van Pul, A.; Van der Hoek, W.; Heederik, D. Atmospheric dispersion modelling of bioaerosols that are pathogenic to humans and livestock—A review to inform risk assessment studies. Microb. Risk Anal. 2016, 1, 19–39. [Google Scholar] [CrossRef]
- Almatawah, Q.A.; Al-Khalaifah, H.S.; Aldameer, A.S.; Ali, A.K.; Benhaji, A.H.; Varghese, J.S. Microbiological indoor and outdoor air quality in chicken fattening houses. J. Environ. Public Health 2023, 2023, 3512328. [Google Scholar] [CrossRef] [PubMed]
- Gržinić, G.; Piotrowicz-Cieślak, A.; Klimkowicz-Pawlas, A.; Górny, R.L.; Ławniczek-Wałczyk, A.; Piechowicz, L.; Olkowska, E.; Potrykus, M.; Tankiewicz, M.; Krupka, M.; et al. Intensive poultry farming: A review of the impact on the environment and human health. Sci. Total Environ. 2023, 858, 160014. [Google Scholar] [CrossRef] [PubMed]
- Eduard, W. Exposure to non-infectious microorganisms and endotoxins in agriculture. Ann. Agric. Environ. Med. 1997, 4, 179–186. [Google Scholar]
- Matković, K.; Vučemilo, M.; Vinković, B. Influence of microclimate on dust and on concentration of microorganisms in the air of poultry houses for laying hens. Stočarstvo 2009, 63, 57–63. (In Croatian) [Google Scholar]
- Górny, R.L.; Gołofit-Szymczak, M.; Cyprowski, M.; Ławniczek-Wałczyk, A.; Stobnicka-Kupiec, A.; Wolska, L.A. Poultry house as point source of intense bioaerosol emission. Ann. Agric. Environ. Med. 2023, 30, 432–454. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Oliveira, J.; Reygaert, W.C. Gram-negative bacteria. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Macesic, N.; Uhlemann, A.-C.; Peleg, A.Y. Multidrug-resistant Gram-negative bacterial infections. Lancet 2025, 405, 257–272. [Google Scholar] [CrossRef]
- Bakutis, B.; Monstviliene, E.; Januskeviciene, G. Analyses of airborne contamination with bacteria, endotoxins and dust in livestock barns and poultry houses. Acta Vet. Brno 2004, 73, 283–289. [Google Scholar] [CrossRef][Green Version]
- Hađina, S.; Pinter, L.J.; Uhitil, S.; Vučemilo, M.; Jakšić, S. The assessment of gram-negative bacteria in the air of two swine nursery buildings. Vet. arhiv 2009, 79, 219–227. [Google Scholar]
- Dutkiewicz, J.; Pomorski, Z.J.H.; Sitkowska, J.; Krysińska-Traczyk, E.; Skórska, C.; Prażmo, Z.; Cholewa, G.; Wójtowicz, H. Airborne microorganisms and endotoxin in animal houses. Grana 1994, 33, 85–90. [Google Scholar] [CrossRef]
- Zucker, B.A.; Trojan, S.; Müller, W. Airborne gram-negative bacterial flora in animal houses. J. Vet. Med. B Infect. Dis. Vet. Public Health 2000, 47, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Clemente, L.; Fernandes, T.L.; Barahona, M.J.; Bernardino, R.; Botelho, A. Confirmation by PCR of Coxiella burnetii infection in animals at a zoo in Lisbon, Portugal. Vet. Rec. 2008, 163, 221–222. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, D.; Ruiz-Fons, F. Coxiella burnetii in wild mammals: A systematic review. Transbound. Emerg. Dis. 2019, 66, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Um, J.; Kim, J.; Cho, S.-J.; Park, M.-H.; Cho, H.-C.; Park, Y.-J.; Choi, K.-S. Identification of zoonotic pathogens in zoo animals in the Republic of Korea. Int. J. Parasitol. Parasites Wildl. 2025, 27, 101067. [Google Scholar] [CrossRef]
- Asseng, S.; Spänkuch, D.; Hernandez-Ochoa, I.M.; Laporta, J. The upper temperature thresholds of life. Lancet Planet Health 2021, 5, e378–e385, Erratum in Lancet Planet. Health 2021, 5, e578. https://doi.org/10.1016/S2542-5196(21)00205-9. [Google Scholar] [CrossRef]
- Albelda-Estellés Ness, M.C. Indoor relative humidity: Relevance for health, comfort, and choice of ventilation system. In Proceedings of the 3rd Valencia International Biennial of Research in Architecture, Valencia, Spain, 9–11 November 2022; pp. 218–228. [Google Scholar] [CrossRef]
- Peterková, J.; Michalčíková, M.; Novák, V.; Slávik, R.; Zach, J.; Korjenic, A.; Hodná, J.; Raich, B. The influence of green walls on interior climate conditions and human health. MATEC Web Conf. 2019, 282, 02041. [Google Scholar] [CrossRef]
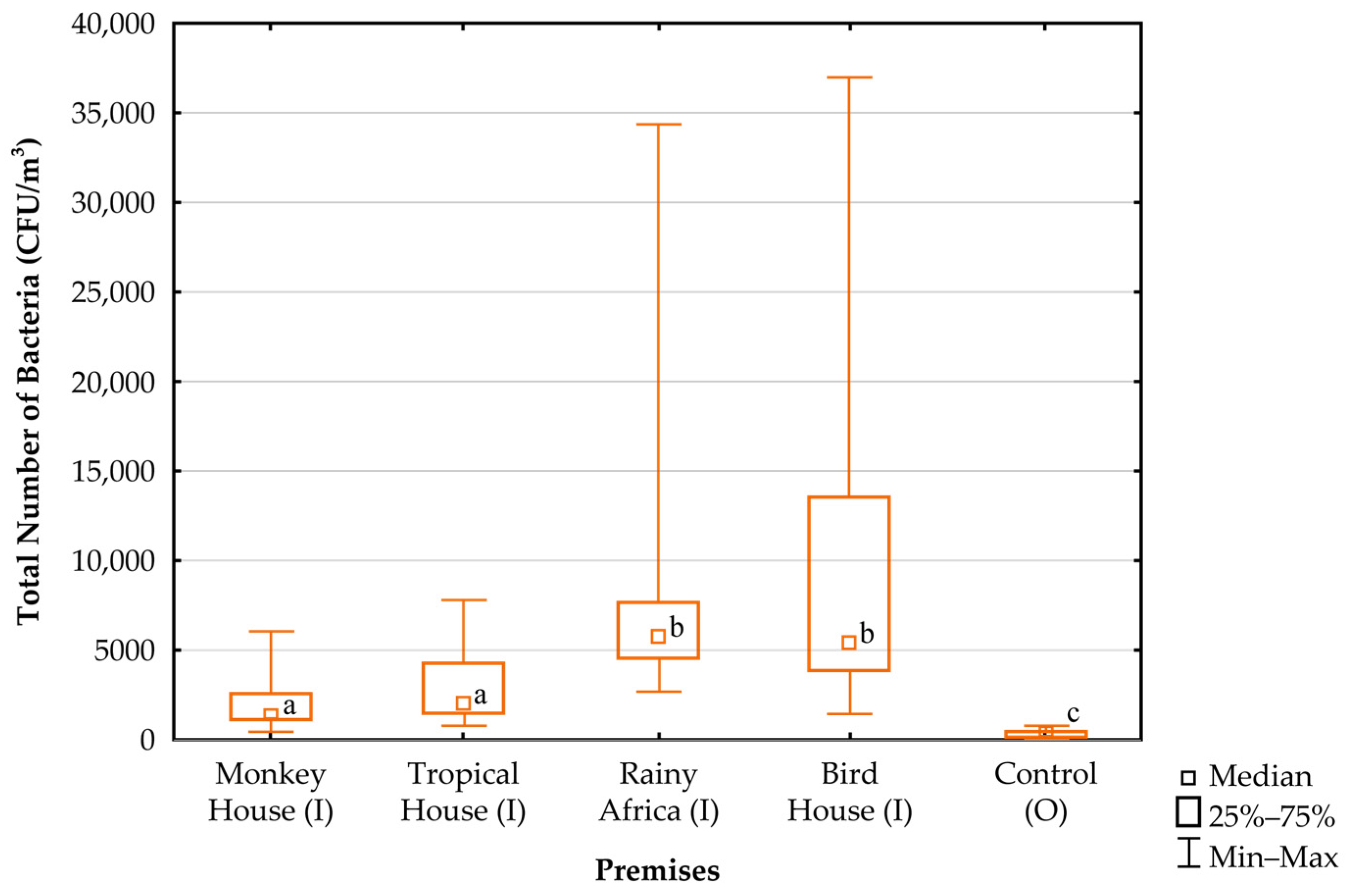

| Parameter | Monkey House | Tropical House | Rainy Africa | Bird House |
|---|---|---|---|---|
| Year of Construction | 1996 renovated | 1974 | 1999 | 1978 |
| Indoor Area for Animals (m2) | 102 | 350 | 162 | 42 |
| Indoor Area for Visitors and Employees (m2) | 32 | 230 | 20 | 44 |
| Animals (n) | 14 | ~358 (crocodiles: 3; caimans: 2; other reptiles: 55; amphibians: 10; fish: ~270; birds: 4; mammals: 14) | 45 (pygmy hippopotamuses: 3; birds: 2; reptiles: 10; fish: 30) | 20 |
| Type of Ventilation | Air conditioning chambers (always on) | Air conditioning chambers (always on) | Air conditioning chambers (always on) | Natural |
| Type of Bedding in Animal Enclosures | No bedding | Shredded bark + sand | Shredded bark No bedding (pygmy hippopotamuses) | No bedding |
| Type of Flooring/Bedding in Area for Visitors and Employees | Ceramic tiles | Concrete + stone | Shredded bark | Concrete |
| Parameter | Total Number of Bacteria (I) (CFU/m3) | Number of Gram-Negative Bacteria (I) (CFU/m3) | Total Number of Bacteria (O) (CFU/m3) | Number of Gram-Negative Bacteria (O) (CFU/m3) |
|---|---|---|---|---|
| Total Number of Bacteria (I) (CFU/m3) | 1.000 | 0.527 * | 0.029 | 0.053 |
| Number of Gram-Negative Bacteria (I) (CFU/m3) | 1.000 | 0.037 | 0.011 | |
| Total Number of Bacteria (O) (CFU/m3) | 1.000 | 0.209 * | ||
| Number of Gram-Negative Bacteria (O) (CFU/m3) | 1.000 |
| Premises | Air Temperature (°C) | Relative Humidity (%) | Airflow Rate (m/s) |
|---|---|---|---|
| Mean ± SD (Min–Max) | |||
| Monkey House (I) | 24.55 a ± 3.00 (19.30–29.00) | 62.18 a ± 6.96 (43.00–74.50) | 0.10 a ± 0.05 (0.01–0.21) |
| Tropical House (I) | 24.46 a,b ± 3.59 (16.50–29.20) | 67.21 b,c ± 7.18 (53.30–82.80) | 0.08 a ± 0.04 (0.01–0.18) |
| Rainy Africa (I) | 23.15 a,b ± 3.83 (16.00–28.30) | 70.05 c ± 6.48 (55.90–83.20) | 0.11 a ± 0.07 (0.01–0.40) |
| Bird House (I) | 24.45 a,b ± 2.87 (16.10–27.70) | 62.41 a ± 7.15 (46.00–74.10) | 0.08 a ± 0.05 (0.01–0.25) |
| Control (O) | 22.24 b ± 7.62 (6.90–32.90) | 65.49 a,b ± 10.01 (49.80–81.40) | 0.44 b ± 0.18 (0.22–1.16) |
| Parameter | Air Temperature (°C) | Relative Humidity (%) | Airflow Rate (m/s) |
|---|---|---|---|
| Total Number of Bacteria (CFU/m3) | −0.141 * | 0.050 | −0.152 * |
| Number of Gram-Negative Bacteria (CFU/m3) | −0.164 * | 0.052 | −0.070 |
| Parameter | Air Temperature (°C) | Relative Humidity (%) | Airflow Rate (m/s) |
|---|---|---|---|
| Total Number of Bacteria (CFU/m3) | 0.413 * | −0.552 * | −0.132 |
| Number of Gram-Negative Bacteria (CFU/m3) | 0.126 | −0.143 | −0.251 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostović, M.; Matković, K.; Ekert Kabalin, A.; Menčik, S.; Pavičić, Ž.; Rudan, N.; Horvatek Tomić, D.; Beneta, D.; Bata, I. Towards Zoo Sustainability: Assessment of Indoor and Outdoor Bacterial Air Contamination Levels and Their Correlations with Microclimate Parameters. Sustainability 2025, 17, 10517. https://doi.org/10.3390/su172310517
Ostović M, Matković K, Ekert Kabalin A, Menčik S, Pavičić Ž, Rudan N, Horvatek Tomić D, Beneta D, Bata I. Towards Zoo Sustainability: Assessment of Indoor and Outdoor Bacterial Air Contamination Levels and Their Correlations with Microclimate Parameters. Sustainability. 2025; 17(23):10517. https://doi.org/10.3390/su172310517
Chicago/Turabian StyleOstović, Mario, Kristina Matković, Anamaria Ekert Kabalin, Sven Menčik, Željko Pavičić, Nevenka Rudan, Danijela Horvatek Tomić, Dijana Beneta, and Ingeborg Bata. 2025. "Towards Zoo Sustainability: Assessment of Indoor and Outdoor Bacterial Air Contamination Levels and Their Correlations with Microclimate Parameters" Sustainability 17, no. 23: 10517. https://doi.org/10.3390/su172310517
APA StyleOstović, M., Matković, K., Ekert Kabalin, A., Menčik, S., Pavičić, Ž., Rudan, N., Horvatek Tomić, D., Beneta, D., & Bata, I. (2025). Towards Zoo Sustainability: Assessment of Indoor and Outdoor Bacterial Air Contamination Levels and Their Correlations with Microclimate Parameters. Sustainability, 17(23), 10517. https://doi.org/10.3390/su172310517







