Water and Soil Physico-Chemical Characteristics in Ibex Reserve: An Environmental Case Study of Houta Bani Tamim
Abstract
1. Introduction
2. Materials and Methods
2.1. Environmental Conditions
2.2. Plant Species
2.3. Sampling Mechanism
2.4. Heavy Metals Determination Through Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
2.5. Soil Analysis (pH, TDS, EC, Anions, Cations, and CaCO3)
2.6. Water Analysis (Physical Parameters pH, TDS, EC, and Turbidity)
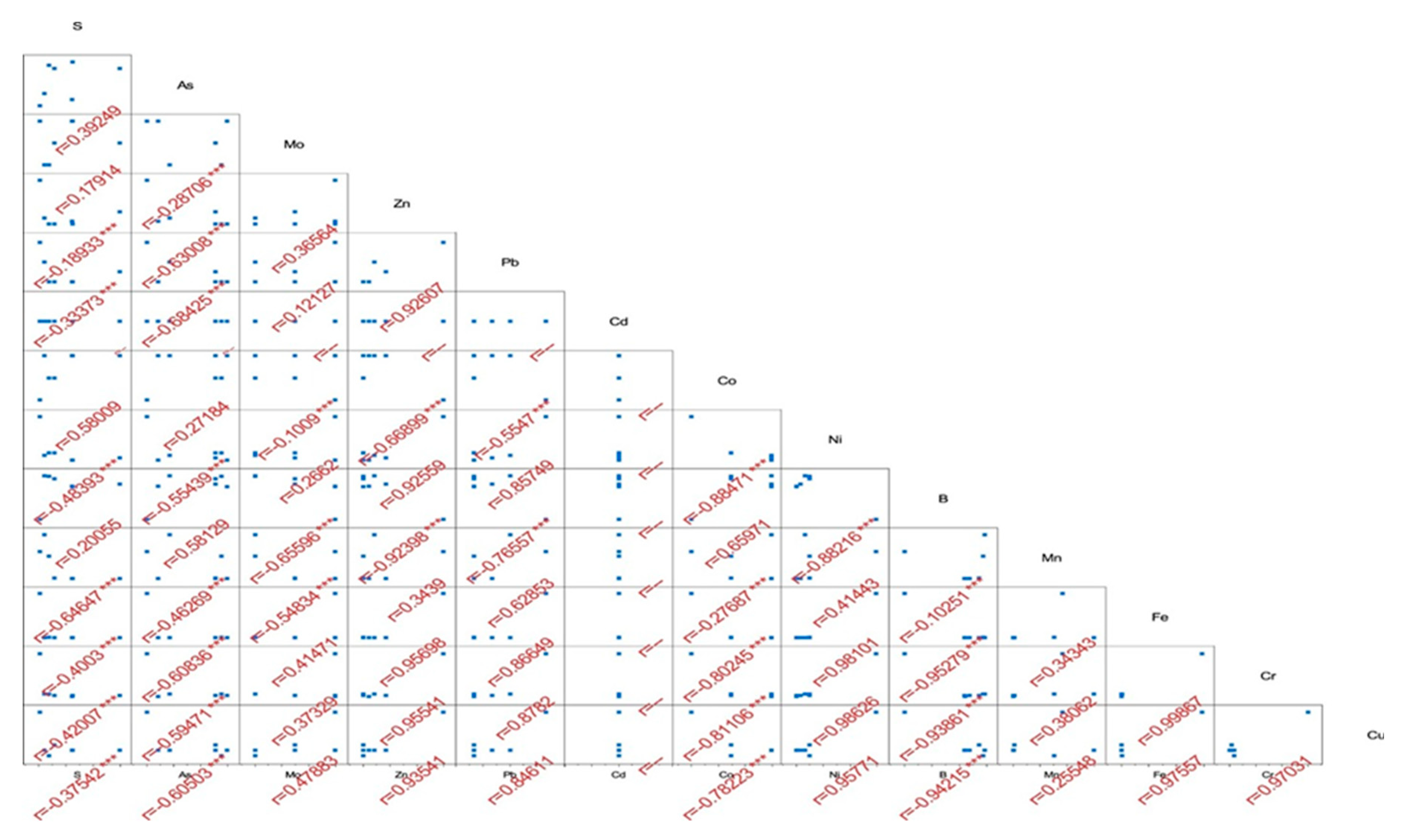
2.7. Statistical Analysis
3. Results & Discussion
3.1. Soil Properties (pH, TDS, EC, Anions and Cations)
3.2. Soil Organic Matter (OM%), Total Phosphorus (TP), Total Nitrogen (TN), and Calcium Carbonate
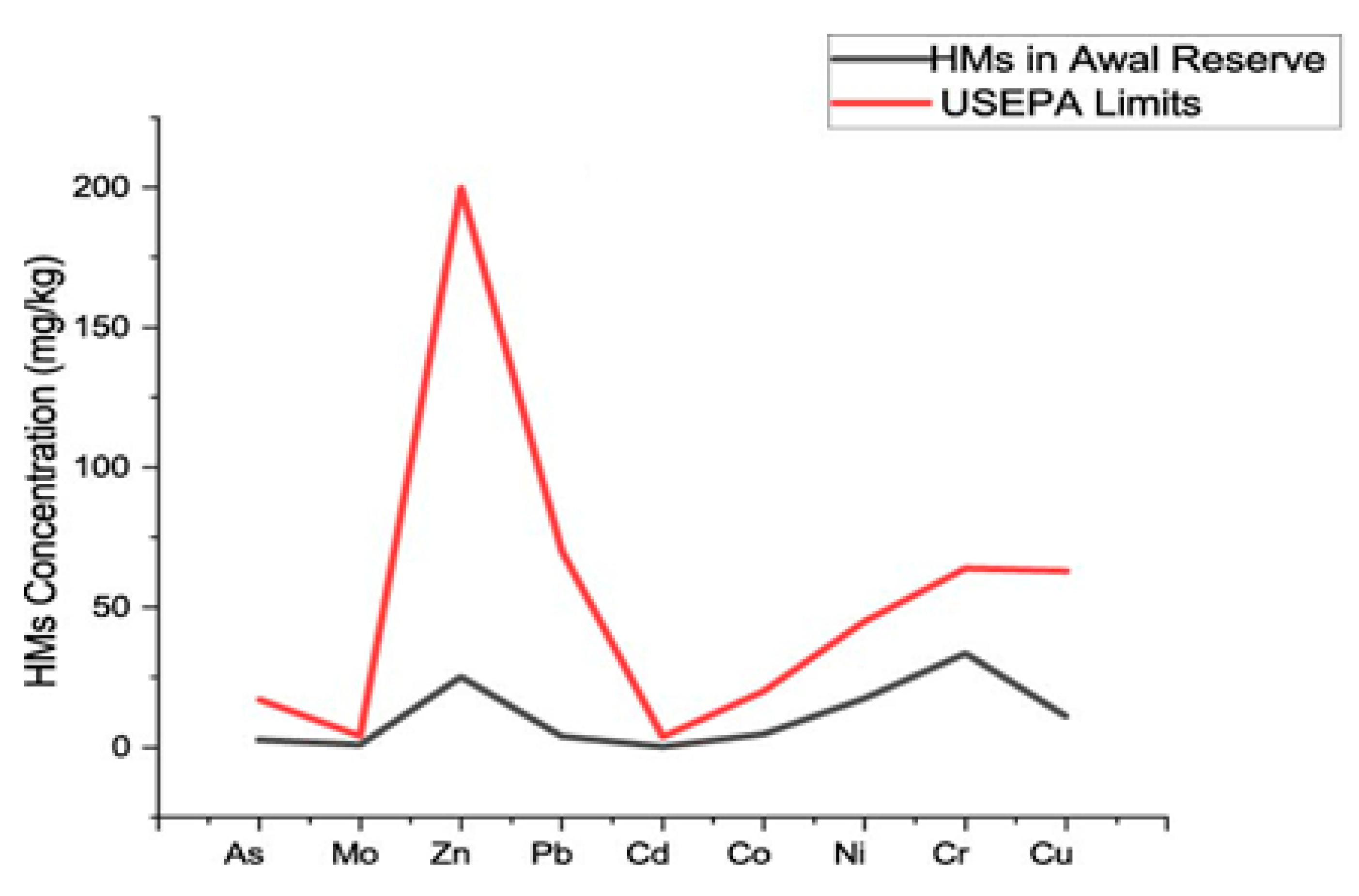
3.3. Heavy Metals in Soil Samples
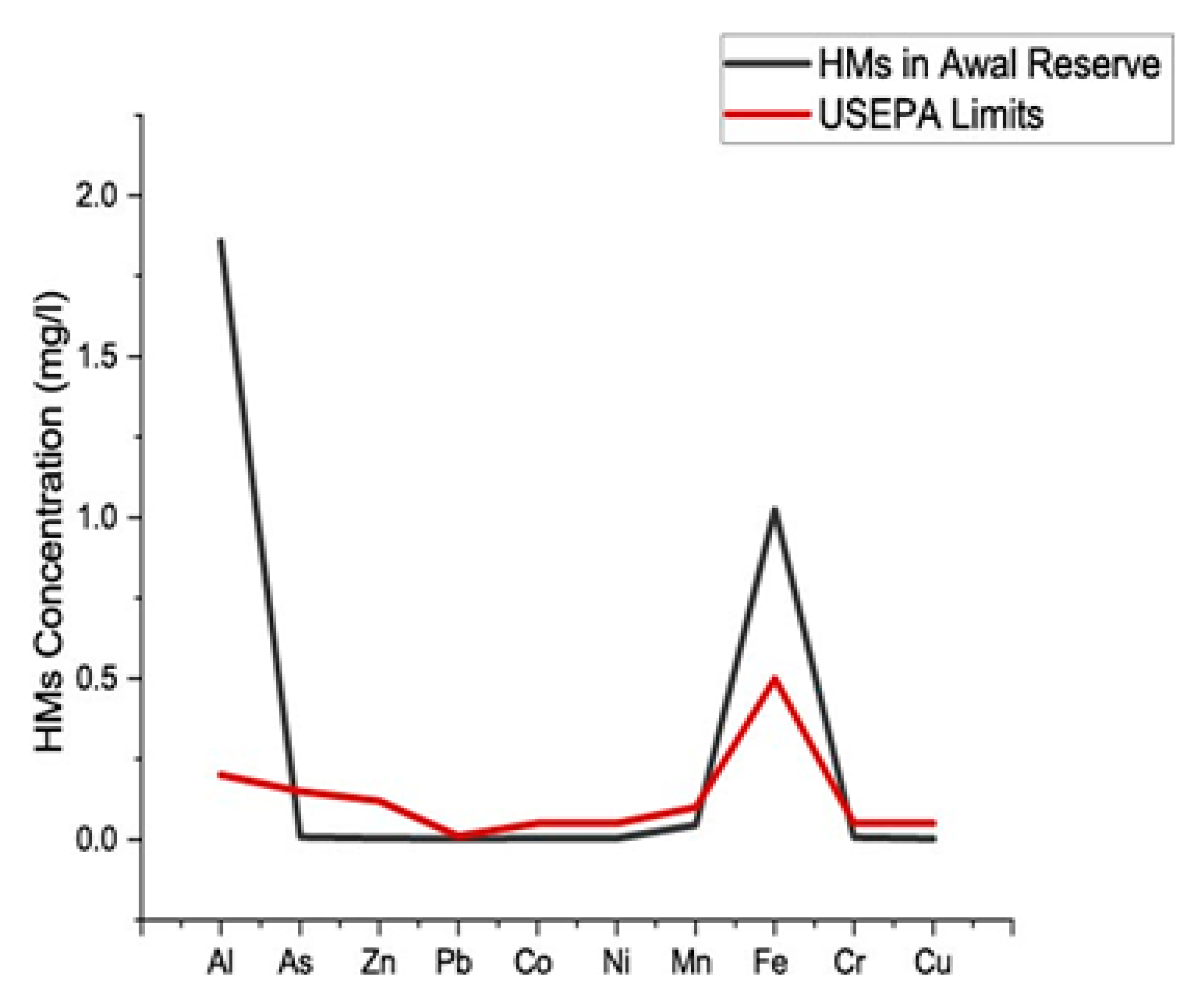
3.4. Water Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Azam, W. Natural resource scarcity, fossil fuel energy consumption, and total greenhouse gas emissions in top emitting countries. Geosci. Front. 2024, 15, 101757. [Google Scholar] [CrossRef]
- Tinazzi, I. Water Scarcity, Migrations and Climate Change: An Assessment of their Nexus. 2024; in press. [Google Scholar]
- Kalfas, D.; Kalogiannidis, S.; Papaevangelou, O.; Chatzitheodoridis, F. Assessing the connection between land use planning, water resources, and global climate change. Water 2024, 16, 333. [Google Scholar] [CrossRef]
- Gavrilescu, M. Water, soil, and plants interactions in a threatened environment. Water 2021, 13, 2746. [Google Scholar] [CrossRef]
- Bayabil, H.K.; Teshome, F.T.; Li, Y.C. Emerging contaminants in soil and water. Front. Environ. Sci. 2022, 10, 873499. [Google Scholar] [CrossRef]
- Pepper, I.L.; Brusseau, M.L. Physical-chemical characteristics of soils and the subsurface. Environ. Pollut. Sci. 2019, 1, 9–22. [Google Scholar]
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various natural and anthropogenic factors responsible for water quality degradation: A review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- USEPA. Available online: https://www.epa.gov/ (accessed on 20 October 2024).
- Finlayson, C.M.; Arthington, A.H.; Pittock, J. Freshwater Ecosystems in Protected Areas; Taylor & Francis: London, UK, 2018. [Google Scholar]
- Rodríguez-Rodríguez, D.; Martínez-Vega, J. Effectiveness of Protected Areas in Conserving Biodiversity; A Worldwide Review; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Vimal, R.; Navarro, L.M.; Jones, Y.; Wolf, F.; Le Moguédec, G.; Réjou-Méchain, M. The global distribution of protected areas management strategies and their complementarity for biodiversity conservation. Biol. Conserv. 2021, 256, 109014. [Google Scholar] [CrossRef]
- Dudley, N.; Shadie, P.; Stolton, S. Guidelines for Applying Protected Area Management Categories, 2nd ed.; IUCN: Gland, Switzerland, 2016. [Google Scholar]
- Shirdam, R.; Modarres-Tehrani, Z.; Dastgoshadeh, F. Microwave assisted digestion of soil, sludge and sediment for determination of heavy metals with ICP-OES and FAAS. Rasayan J. Chem. 2008, 1, 757–765. [Google Scholar]
- Horváth, B.; Opara-Nadi, O.; Beese, F. A simple method for measuring the carbonate content of soils. Soil Sci. Soc. Am. J. 2005, 69, 1066–1068. [Google Scholar] [CrossRef]
- EPA. Guidelines for Ensuring and Maximizing the Quality, Objectivity, Utility, and Integrity of Information Disseminated by the Environmental Protection Agency; Office of Environmental Information: Washington, DC, USA, 2002. [Google Scholar]
- World Health Organization. Recommendations Incorporating the First and Second Addenda. In Guidelines for Drinking-Water Quality, 3rd ed.; WHO: Geneva, Switzerland, 2008; Volume 1. [Google Scholar]
- U.S. Department of Agriculture. Soil Survey Field and Laboratory Methods Manual, Soil Survey Investigations Report; U.S. Department of Agriculture: Washington, DC, USA, 2014; No. 51 Version 2. [Google Scholar]
- ISO 10390; Soil Quality, Subcommittee SC 3, Chemical Methods and Soil Characteristics. ISO: Geneva, Switzerland, 2005.
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Black, A.P.; Hannah, S.A. Measurement of low turbidities. J. Am. Water Works Assoc. 1965, 57, 901–916. [Google Scholar] [CrossRef]
- Endou, M.; Asakura, Y.; Watanabe, A.; Sakagami, M.; Uchida, S.; Nagase, M.; Ohsumi, K. Water Quality Control Method, and Method and Apparatus for Measuring Electrical Conductivity Used in the Water Quality Control. U.S. Patent 4853638, 1 August 1989. [Google Scholar]
- FAO; ITPS. Status of the World’s Soil Resources (SWSR)–Main Report; Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils: Rome, Italy, 2015. [Google Scholar]
- Panagos, P.; Imeson, A.; Meusburger, K.; Borrelli, P.; Poesen, J.; Alewell, C. Soil conservation in Europe: Wish or reality? Land Degrad. Dev. 2016, 27, 1547–1551. [Google Scholar] [CrossRef]
- Keesstra, S.D.; Bouma, J.; Wallinga, J.; Tittonell, P.; Smith, P.; Cerdà, A.; Montanarella, L.; Quinton, J.; Pachepsky, Y.; Van Der Putten, W.H.; et al. FORUM paper: The significance of soils and soil science towards realization of the UN sustainable development goals (SDGs). Soil Discuss. 2016, 2, 111–128. [Google Scholar] [CrossRef]
- Abdel-Satar, A.M.; Al-Khabbas, M.H.; Alahmad, W.R.; Yousef, W.M.; Alsomadi, R.H.; Iqbal, T. Quality assessment of groundwater and agricultural soil in Hail region, Saudi Arabia. Egypt. J. Aquat. Res. 2017, 43, 55–64. [Google Scholar] [CrossRef]
- Masoud, A.A.; Aal, A.K.A. Three-dimensional geotechnical modeling of the soils in Riyadh city, KSA. Bull. Eng. Geol. Environ. 2019, 78, 1–17. [Google Scholar] [CrossRef]
- Al-Barakah, F.N.; Alnohait, F.A.S.; Sohaib, M.; Ramzan, M.; Radwan, S. Microbial diversity in the rhizosphere of some wild plants in Riyadh Region, Saudi Arabia. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3518–3534. [Google Scholar] [CrossRef]
- Oumenskou, H.; El Baghdadi, M.; Barakat, A.; Aquit, M.; Ennaji, W.; Karroum, L.A.; Aadraoui, M. Multivariate statistical analysis for spatial evaluation of physicochemical properties of agricultural soils from Beni-Amir irrigated perimeter, Tadla plain, Morocco. Geol. Ecol. Landsc. 2018, 3, 83–94. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Pereira, M.E.; Duarte, A.C.; Römkens, P.F.A.M. Heavy metal mobility and bioavailability in soils: A case study from Portugal. Environ. Pollut. 2008, 155, 524–532. [Google Scholar] [CrossRef]
- Sherene, T. Mobility and transport of heavy metals in polluted soil environment. Biol. Forum Int. J. 2010, 2, 112–121. [Google Scholar]
- Kim, R.Y.; Yoon, J.K.; Kim, T.S.; Yang, J.E.; Owens, G.; Kim, K.R. Bioavailability of heavy metals in soils: Definitions and practical implementation—A critical review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef] [PubMed]
- Timpano, A.J.; Zipper, C.E.; Soucek, D.J.; Schoenholtz, S.H. Seasonal pattern of anthropogenic salinization in temperate forested headwater streams. Water Res. 2018, 133, 8–18. [Google Scholar] [CrossRef]
- Bob, M.; Abd Rahman, N.; Taher, S.; Elamin, A. Multi-objective assessment of groundwater quality in Madinah City, Saudi Arabia. Water Qual. Expo. Health 2014, 7, 53–66. [Google Scholar] [CrossRef]
- Al-Ahmadi, M.E. Groundwater quality assessment in Wadi Fayd, Western Saudi Arabia. Arab. J. Geosci. 2013, 6, 247–258. [Google Scholar] [CrossRef]
- Krull, E.S.; Skjemstad, J.O.; Baldock, J.A. Functions of Soil Organic Matter and the Effect on Soil Properties; Cooperative Research Centre for Greenhouse Accounting: Canberra, Australia, 2004. [Google Scholar]
- Obalum, S.E.; Okpara, I.M.; Obi, M.E.; Wakatsuki, T. Short term effects of tillage-mulch practices under sorghum and soybean on organic carbon and eutrophic status of a degraded Ultisol in southeastern Nigeria. Trop. Subtrop. Agroecosyst. 2011, 14, 393–403. [Google Scholar]
- Spaccini, R.; Piccolo, A.; Mbagwu, J.S.; Zena Teshale, A.; Igwe, C.A. Influence of the addition of organic residues on carbohydrate content and structural stability of some highland soils in Ethiopia. Soil Use Manag. 2002, 18, 404–411. [Google Scholar] [CrossRef]
- Standardization and Metrology Organization for the Gulf Cooperation Council Countries#GS 149/193; Unbottled Drinking Water Standards. G.C.C.S. (Gulf Cooperation Council Standard): Riyadh, Saudi Arabia, 1993.
- Al-Turki, A.L. Assessment of well drinking water quality in Hail Region of North Central Saudi Arabia. J. Agric. Vet. Sci. 2010, 2, 101–110. [Google Scholar]
- Toumi, N.; Hussein, B.H.; Rafrafi, S.; El Kassas, N. Groundwater quality and hydrochemical properties of Al-Ula region, Saudi Arabia. Environ. Monit. Assess. 2015, 187, 84. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, A.A.; Tadesse, Y.; Adgaba, N.; Alghamdi, A.G. Soil degradation and restoration in southwestern Saudi Arabia through investigation of soil physiochemical characteristics and nutrient status as indicators. Sustainability 2021, 13, 9169. [Google Scholar] [CrossRef]
- Dotsika, E.; Poutoukis, D.; Kloppmann, W.; Raco, B.; Psomiadis, D. Distribution and origin of boron in fresh and thermal waters in different areas of Greece. In Water Security in the Mediterranean Region: An International Evaluation of Management, Control, and Governance Approaches; Stournaras, G., Zerefos, C., Lalas, D., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 209–228. [Google Scholar] [CrossRef]
- Brown, J.R. Soil Testing: Sampling, Correlation, Calibration, and Interpretation; SSSA Special Publication Number 21; Soil Science Society of America, Inc.: Madison, WI, USA, 1987. [Google Scholar]
- Aly, A.; Alomran, A.; Alwabel, M.; Almahaini, A.; Alamari, M. Hydrochemical and quality of water resources in Saudi Arabia groundwater: A comparative study of Riyadh and Al-Ahsa Regions. Proc. Int. Acad. Ecol. Environ. Sci. 2013, 3, 42–51. [Google Scholar]
- Cannon, H.L.; Connally, G.G.; Epstein, J.B.; Parker, J.G.; Thornton, I.; Rocks, W.G. Geological sources of most trace elements. Geochem. Environ. 1978, 3, 17–31. [Google Scholar]
- Obeng-Gyasi, E. Sources of lead exposure in various countries. Rev. Environ. Health 2019, 34, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Wasiullah; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Fendorf, S.; Nico, P.S.; Kocar, B.D.; Masue, Y.; Tufano, K.J. Arsenic chemistry in soils and sediments. Dev. Soil Sci. 2010, 34, 357–378. [Google Scholar] [CrossRef]
- Sharaf, M.A.; Hussein, M.T. Groundwater quality in the Saq aquifer, Saudi Arabia. Hydrol. Sci. J. 1996, 41, 683–696. [Google Scholar] [CrossRef]
- UN-ESCWA (United Nations Economic and Social Commission for Western Asia); BGR (Bundesanstalt für Geowissenschaften und Rohstoffe). Inventory of Shared Water Resources in Western Asia; UN-ESCWA: Beirut, Lebanon, 2013. [Google Scholar]
- Al-Omran, A.; Al-Barakah, F.; Altuquq, A.; Aly, A.; Nadeem, M. Drinking water quality assessment and water quality index of Riyadh, Saudi Arabia. Water Qual. Res. J. Can. 2015, 50, 287–296. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Q. Risk assessment and seasonal variations of dissolved trace elements and heavy metals in the Upper Han River, China. J. Hazard. Mater. 2010, 181, 1051–1058. [Google Scholar] [CrossRef]
- Karki, B.K.; Lamichhane, K.; Joshi, L.; Raj, K.C.; Sah, M.K.; Pathak, M.; Karki, K.R. Risk assessment of heavy metals in the major surface water system of Nepal with potential remediation technologies. Environ. Chall. 2024, 8, 100865. [Google Scholar] [CrossRef]
- Fang, T.; Wang, H.; Liang, Y.; Cui, K.; Yang, K.; Lu, W.; Li, J.; Zhao, X.; Gao, N.; Yu, Q.; et al. Source tracing with cadmium isotope and risk assessment of heavy metals in sediment of an urban river, China. Environ. Pollut. 2022, 305, 119325. [Google Scholar] [CrossRef] [PubMed]

| Species | Growth Form | Ecological Role | Habitat Preference |
|---|---|---|---|
| Acacia tortilis | Tree | Keystone species: soil stabilization, fodder, shade | Terraces, alluvial plains |
| Panicum turgidum | Perennial grass | Dune stabilizer; fodder resource | Sandy soils, dunes |
| Cenchrus ciliaris | Perennial grass | Drought-tolerant forage grass | Alluvial soils, plains |
| Stipagrostis spp. | Perennial grass | Soil binder; adapted to arid soils | Rocky slopes, sandy soils |
| Haloxylon salicornicum | Shrub | Salt-tolerant; prevents desertification | Saline/alkaline soils |
| Sample ID | meq/L | EC ds/m | Cl− meq/L | meq/L | meq/L | meq/L | meq/L | meq/L | meq/L | meq/L | EC ds/m |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Faraa | 1.46 | 0.66 | 0.31 | 0 | 0.23 | 1.28 | 0.05 | 0.75 | 0.0319 | 159 | 7.1 |
| Alzalaq-1 | 0.51 | 0.25 | 0.15 | 0 | 0.15 | 0.54 | 0.02 | 0.25 | 0.0124 | 66.3 | 7.75 |
| Hashwan-1 | 3.62 | 2 | 0.93 | 0 | 1.06 | 3.7 | 0.21 | 2.14 | 0.1073 | 538 | 7.4 |
| Hashwan-2 | 35.43 | 3.46 | 3.66 | 0.32 | 11.2 | 26.5 | 2.56 | 4.35 | 0.412 | 2060 | 6.72 |
| Shoaib Al-aswad | 2.97 | 1.32 | 0.5 | 0 | 0.95 | 2.26 | 0.16 | 1.37 | 0.0681 | 344 | 7.15 |
| Aldakhil-1 | 0.51 | 0.25 | 0.15 | 0 | 0.15 | 0.54 | 0.02 | 0.25 | 0.0121 | 60.7 | 7.73 |
| Aldakhil-2 | 33.06 | 3.81 | 2.77 | 0.28 | 5.92 | 16.4 | 7.62 | 12.96 | 0.4 | 2000 | 6.9 |
| Shabak Mateam-1 | 0.49 | 0.29 | 0.13 | 0 | 0.11 | 0.49 | 0.02 | 0.3 | 0.0124 | 62.4 | 7.57 |
| Shabak Mateam-2 | 0.84 | 0.39 | 0.19 | 0 | 0.18 | 0.78 | 0.03 | 0.42 | 0.0192 | 96 | 7.92 |
| Albuyitlar-1 | 1.5 | 0.73 | 0.41 | 0 | 0.43 | 1.49 | 0.1 | 0.9 | 0.0377 | 189 | 7.82 |
| Albuyitlar-2 | 4.12 | 2.21 | 0.95 | 0.01 | 1.3 | 3.83 | 0.24 | 2.28 | 0.0789 | 393 | 7.16 |
| Hamit-1 | 0.76 | 0.35 | 0.16 | 0 | 0.16 | 0.62 | 0.03 | 0.41 | 0.016 | 80 | 7.81 |
| Hamit-2 | 3.14 | 1.12 | 0.54 | 0 | 0.41 | 2.73 | 0.09 | 1.42 | 0.0658 | 329 | 7.5 |
| Ras Alruhl | 7.56 | 2.56 | 0.8 | 0 | 1.92 | 3.25 | 0.72 | 4.48 | 0.1454 | 728 | 7.05 |
| Alwakf-1 | 1.98 | 0.95 | 0.51 | 0 | 0.65 | 1.63 | 0.11 | 0.9 | 0.0436 | 219 | 7.14 |
| p value | 0.011 | 0.292 | 0.058 | 0.001 | 0.017 | 0.022 | 0.037 | 0.021 | 0.021 | 0.595 | |
| Max | 35.43 | 3.81 | 3.66 | 0.32 | 11.2 | 26.5 | 7.62 | 12.96 | 0.412 | 2060 | 7.92 |
| Min | 1.98 | 0.25 | 0.13 | 0 | 0.11 | 0.49 | 0.02 | 0.25 | 0.0121 | 60.7 | 6.72 |
| Mean | 6.53 | 1.357 | 0.811 | 0.041 | 1.655 | 4.403 | 0.799 | 2.212 | 0.098 | 488.293 | 7.381 |
| Sample ID | Organic Matter (OM) % | Total Phosphorus (TP) mg/kg | Total Nitrogen (TN) % | Calcium Carbonate % |
|---|---|---|---|---|
| Al-Faraa | 0.37 | <LOD | 0.16 | <LOD |
| Alzalaq 1 | 0.15 | 0.29 | <LOR | <LOD |
| Hashwan 1 | 0.07 | 0.43 | 0.24 | <LOD |
| Hashwan 2 | 0.053 | 0.33 | 0.31 | <LOD |
| Shoaib Al-aswad | 0.03 | 0.37 | <LOR | <LOD |
| Aldakhil 1 | 0.15 | 0.29 | <LOR | <LOD |
| Aldakhil 2 | 0.145 | 0.52 | 0.37 | <LOD |
| Shabak Mateam 1 | 0.25 | 0.26 | 0.07 | <LOD |
| Shabak Mateam 2 | 0.15 | 0.04 | 0.05 | <LOD |
| Albuyitlar 1 | 0.34 | 0.62 | 0.16 | <LOD |
| Albuyitlar 2 | 0.17 | 0.83 | <LOR | <LOD |
| Hamit 1 | 0.22 | 0.63 | 0.09 | <LOD |
| Hamit 2 | 0.32 | 0.41 | 0.24 | <LOD |
| Ras Alruhl | 0.024 | 0.41 | 0.19 | <LOD |
| Alwakf 1 | 0.37 | 0.13 | 0.17 | <LOD |
| Maximum | 0.37 | 0.83 | 0.37 | |
| Minimum | 0.024 | 0.04 | 0.05 | |
| Average | 0.187 | 0.397 | 0.186 |
| Sample ID | Al mg/kg | As mg/kg | Mo mg/kg | Zn mg/kg | Pb mg/kg | Cd mg/kg | Co mg/kg | Ni mg/kg | Mn mg/kg | Fe mg/kg | Cr mg/kg | Cu mg/kg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Faraa | 3363 | 0.21 | 0.79 | 12.62 | 3.23 | 0.146 | 0.753 | 8.791 | 93.11 | 5604 | 15.898 | 5.258 |
| Alzalaq 1 | 2480 | 0.48 | 1.2 | 12.61 | 1.74 | 0.281 | 0.247 | 6.001 | 77.52 | 4629 | 13.571 | 5.391 |
| Hashwan 1 | 2743 | 1.24 | 1.05 | 13.77 | 1.9 | 0.1 | 0.441 | 6.066 | 83.98 | 5206 | 13.446 | 4.483 |
| Hashwan 2 | 4548 | 2.61 | 0.55 | 19.55 | 2.69 | 0.09 | 1.942 | 13.672 | 112.24 | 6840 | 22.272 | 9.052 |
| Shoaib Al-aswad | 4849 | 2.04 | 1.03 | 17.08 | 3.1 | 0.096 | 2.166 | 15.423 | 148.58 | 9169 | 28.878 | 8.965 |
| Aldakhil 1 | 5832 | 1.56 | 0.83 | 41.7 | 4.33 | 0.899 | 4.652 | 30.113 | 228.24 | 13648 | 43.821 | 13.502 |
| Aldakhil 2 | 5941 | 3.13 | 0.97 | 24.97 | 4.62 | 0.138 | 3.847 | 26.383 | 170.73 | 10884 | 41.184 | 12.011 |
| Shabak Mateam 1 | 6855 | 3.14 | 1.68 | 27.21 | 7.73 | 0.052 | 12.206 | 21.012 | 324.89 | 38808 | 68.015 | 14.689 |
| Shabak Mateam 2 | 5126 | 3.08 | 1.19 | 34.83 | 5.3 | 0.161 | 1.973 | 14.385 | 146.03 | 8344 | 23.409 | 20.517 |
| Albuyitlar 1 | 6898 | 4.34 | 1.09 | 28.69 | 8.52 | 0.02 | 10.96 | 18.583 | 435.86 | 41279 | 60.859 | 13.339 |
| Albuyitlar 2 | 7283 | 4.94 | 0.8 | 58.93 | 8.26 | 0.111 | 9.812 | 51.628 | 413.03 | 24122 | 76.29 | 20.908 |
| Hamit 1 | 6556 | 3.95 | 1.22 | 24.61 | 2.65 | 0.063 | 2.891 | 22.414 | 157.95 | 9936 | 35.9 | 9.413 |
| Hamit 2 | 7882 | 3.3 | 0.05 | 57.46 | 2.84 | 0.038 | 16.855 | 27.145 | 711.07 | 28063 | 57.495 | 25.648 |
| Ras Alruhl | 20 | 2.53 | 0.15 | 2.48 | 0.56 | 0.045 | 0.757 | 0.136 | 0.761 | 11.68 | 1.033 | 0.515 |
| Alwakf 1 | 2.36 | 2.75 | 0.2 | 0.09 | 0.05 | 0.023 | 0.82 | 0.111 | 0.001 | 1.79 | 0.143 | 0.153 |
| p value | 0.103 | 0.042 | 0.295 | 0.517 | 0.170 | 0.087 | 0.595 | 0.786 | 0.175 | 0.825 | 0.591 | 0.305 |
| Limit | - | 17 | 4 | 200 | 70 | 3.8 | 20 | 45 | - | - | 64 | 63 |
| Maximum | 7882 | 4.94 | 1.68 | 58.93 | 8.52 | 0.899 | 16.855 | 51.628 | 711.07 | 41279 | 76.29 | 25.648 |
| Minimum | 2.36 | 0.21 | 0.05 | 0.09 | 0.05 | 0.02 | 0.247 | 0.111 | 0.001 | 1.79 | 0.143 | 0.153 |
| Average | 4691.891 | 2.62 | 0.853333 | 25.10667 | 3.834667 | 0.150867 | 4.688133 | 17.45753 | 206.9328 | 13769.7 | 33.48093 | 10.92293 |
| Sample ID | pH | TDS | EC | Turb. | Ca | Mg | Na |
|---|---|---|---|---|---|---|---|
| Aldakhil 1 | 7.81 | 330 | 659 | 0.8 | 23.646 | 16.636 | 79.62 |
| Alfahili2 | 7.57 | 121.5 | 243 | 2 | 21.284 | 7.357 | 13.552 |
| Hashwan | 7.64 | 181 | 362 | 1.3 | 21.903 | 12.672 | 51.802 |
| Aldakhila2 | 7.99 | 162 | 322 | 1.2 | 18.695 | 9.518 | 22.619 |
| Alfahil 1 | 7.53 | 133 | 265 | 21.1 | 21.449 | 6.316 | 9.993 |
| Tumir Hamayd1 | 7.24 | 421 | 843 | 0.6 | 21.855 | 12.656 | 50.968 |
| Tumir Hamayd2 | 7.81 | 627 | 1246 | 0.2 | 24.167 | 4.198 | 10.94 |
| p Value | 0.904 | 0.509 | 0.501 | 0.025 | 0.592 | 0.812 | 0.663 |
| Maximum | 7.99 | 627 | 1246 | 21.1 | 24.167 | 16.636 | 79.62 |
| Minimum | 7.24 | 121.5 | 243 | 0.2 | 18.695 | 4.198 | 9.993 |
| Average | 7.655714 | 282.2143 | 562.8571 | 3.885714 | 21.857 | 9.907571 | 34.21343 |
| Sample ID | Al | S | As | Mo | Zn | Pb | Cd | Co | Ni | B | Mn | Fe | Cr | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldakhil-1 | 0.019 | 242 | 0.012 | 0.001 | 0.004 | 0.002 | 0 | 0.004 | 0.001 | 0.923 | 0.001 | 0.008 | 0.001 | 0.001 |
| Alfahili-2 | 0.07 | 30.02 | 0.013 | 0 | 0 | 0.001 | 0 | 0.003 | 0.003 | 1.121 | 0.074 | 0.062 | 0.002 | 0 |
| Hashwan | 0.029 | 100.39 | 0.014 | 0.002 | 0 | 0.001 | 0 | 0.004 | 0 | 0.856 | 0 | 0.007 | 0.001 | 0.001 |
| Aldakhila-2 | 0.045 | 46.388 | 0.012 | 0.001 | 0 | 0.001 | 0 | 0.003 | 0.003 | 1.059 | 0.002 | 0.001 | 0.001 | 0.002 |
| Alfahil 1 | 0.131 | 16.463 | 0.004 | 0 | 0.002 | 0.003 | 0 | 0.004 | 0.002 | 1.138 | 0.146 | 0.032 | 0.002 | 0.001 |
| Tumir-hamayd-1 | 0.021 | 99.667 | 0.002 | 0.002 | 0.001 | 0.001 | 0 | 0.004 | 0 | 0.862 | 0 | 0.01 | 0 | 0.001 |
| Tumir-Hamayd-2 | 12.681 | 2.797 | 0 | 0.002 | 0.014 | 0.005 | 0 | 0.002 | 0.018 | 0.001 | 0.09 | 7.055 | 0.033 | 0.008 |
| p value | 0.955 | 0.544 | 0.165 | - | 0.943 | - | 0.939 | 0.975 | 0.872 | 0.677 | 0.954 | 0.65 | 0.945 | |
| Limit | 0.2 | 0.15 | 0.12 | 0.01 | 0.000025 | 0.05 | 0.05 | 0.1 | 0.5 | 0.05 | 0.05 | |||
| Maximum | 12.681 | 242 | 0.014 | 0.002 | 0.014 | 0.005 | 0 | 0.004 | 0.018 | 1.138 | 0.146 | 7.055 | 0.033 | 0.008 |
| Average | 1.856 | 76.81 | 0.008 | 0.0011 | 0.003 | 0.002 | 0 | 0.0034 | 0.0038 | 0.8514 | 0.0447 | 1.025 | 0.0057 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, A.J.; Redwan, O.K.; Chieb, M.; El-Saeid, M.H. Water and Soil Physico-Chemical Characteristics in Ibex Reserve: An Environmental Case Study of Houta Bani Tamim. Sustainability 2025, 17, 10151. https://doi.org/10.3390/su172210151
Alzahrani AJ, Redwan OK, Chieb M, El-Saeid MH. Water and Soil Physico-Chemical Characteristics in Ibex Reserve: An Environmental Case Study of Houta Bani Tamim. Sustainability. 2025; 17(22):10151. https://doi.org/10.3390/su172210151
Chicago/Turabian StyleAlzahrani, Abdulhakim J., Osama Khled Redwan, Maha Chieb, and Mohamed H. El-Saeid. 2025. "Water and Soil Physico-Chemical Characteristics in Ibex Reserve: An Environmental Case Study of Houta Bani Tamim" Sustainability 17, no. 22: 10151. https://doi.org/10.3390/su172210151
APA StyleAlzahrani, A. J., Redwan, O. K., Chieb, M., & El-Saeid, M. H. (2025). Water and Soil Physico-Chemical Characteristics in Ibex Reserve: An Environmental Case Study of Houta Bani Tamim. Sustainability, 17(22), 10151. https://doi.org/10.3390/su172210151







