Innovative Approaches to Mitigating Microplastic Pollution in Effluents and Soils
Abstract
1. Introduction
2. Microplastic Occurrence and Impacts
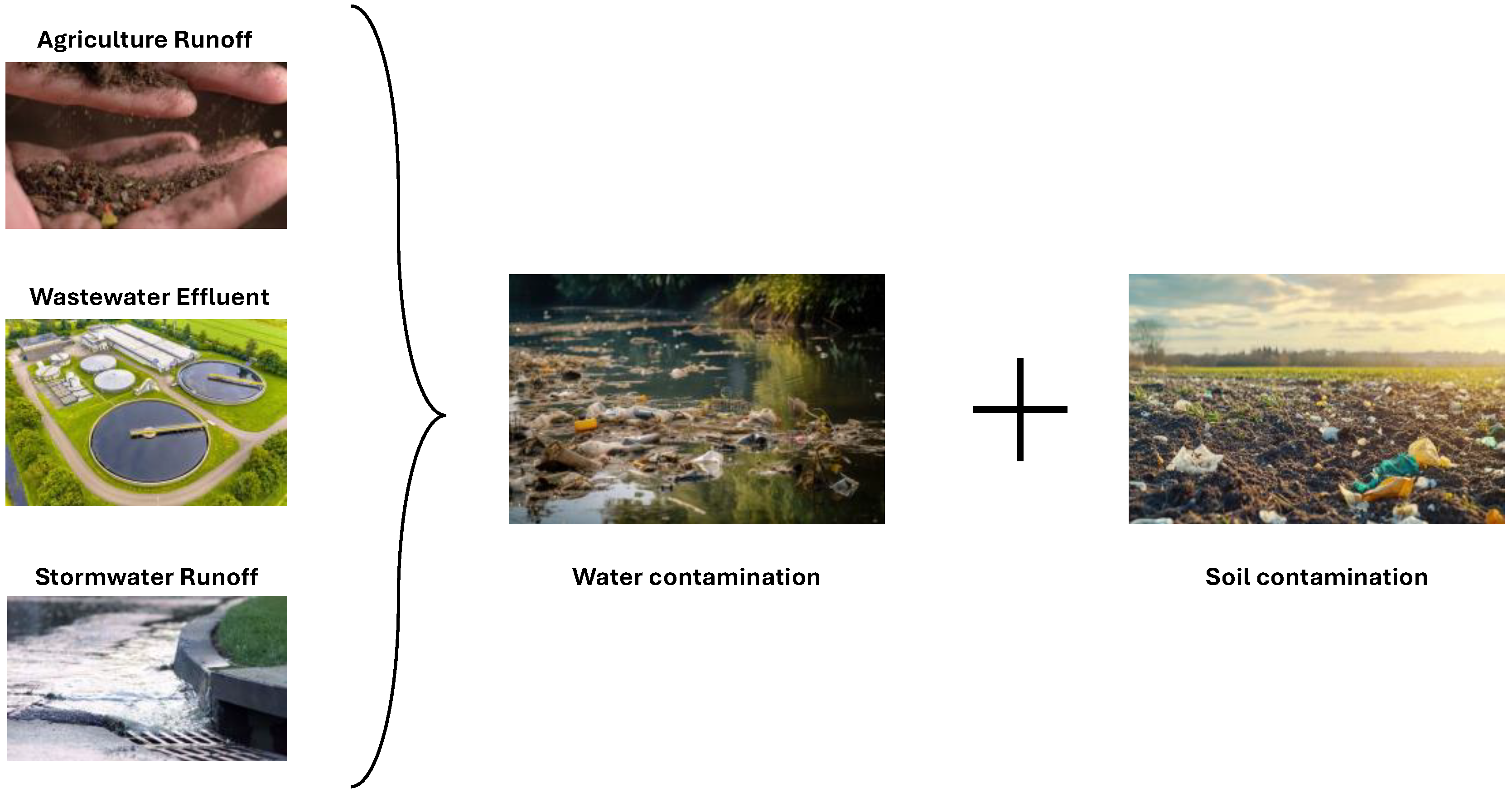
2.1. Microplastics in Aqueous Effluents
2.2. Microplastics in Soils
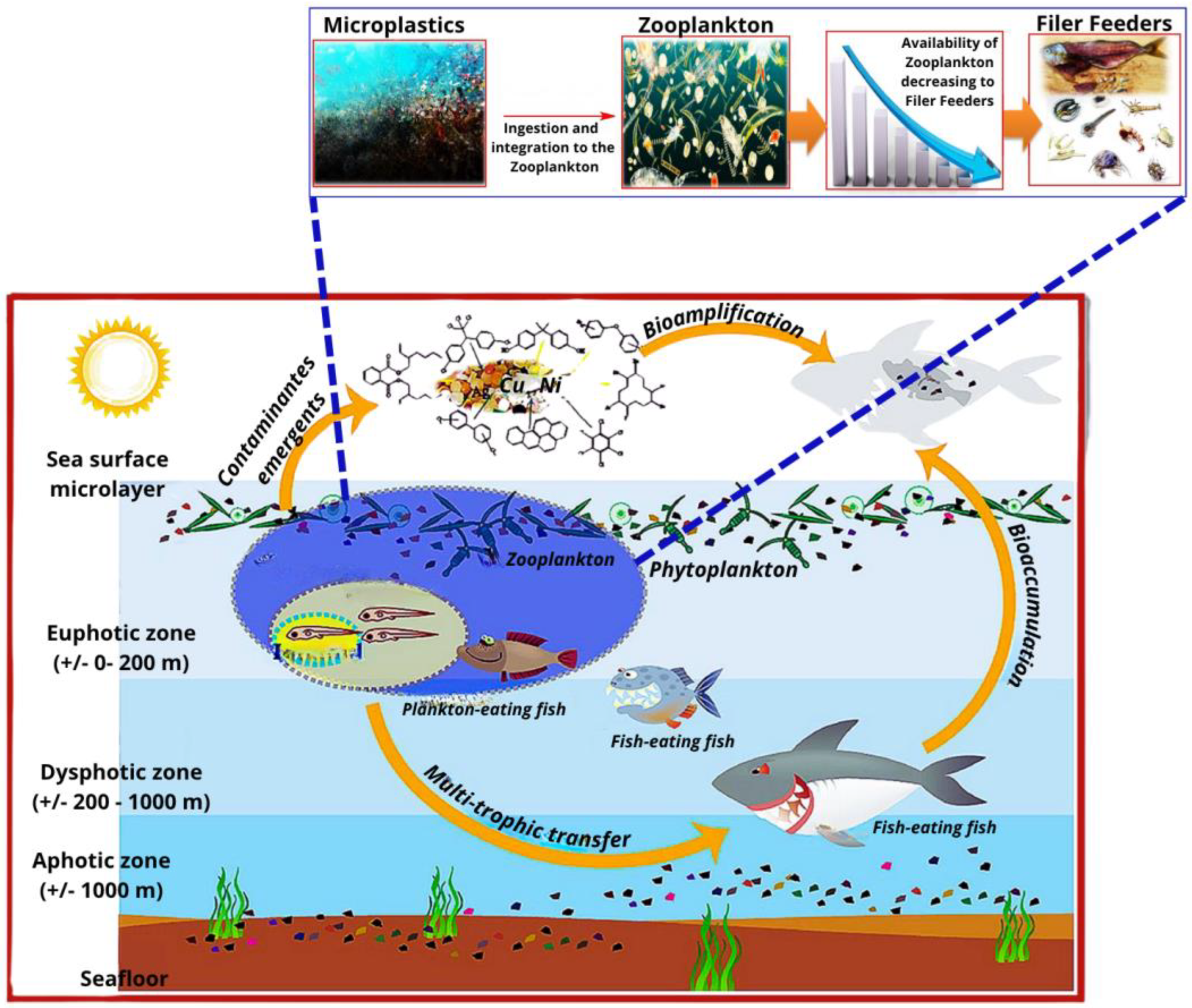
2.3. Environmental and Health Implications
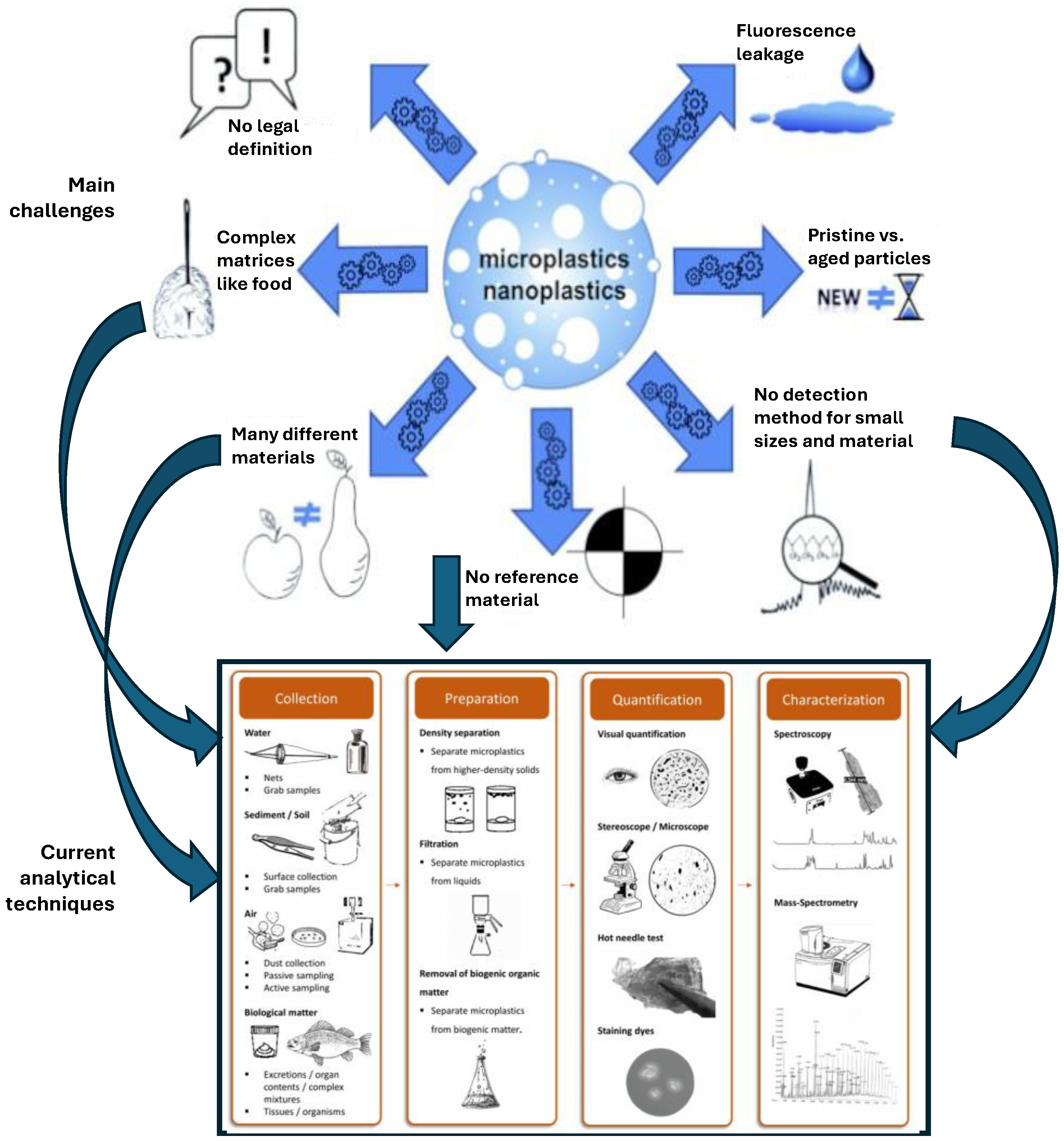
3. Analytical and Regulatory Challenges
4. Methods for Microplastic Removal in Effluents
4.1. Filtration
4.2. Flotation
4.3. Chemical Coagulation
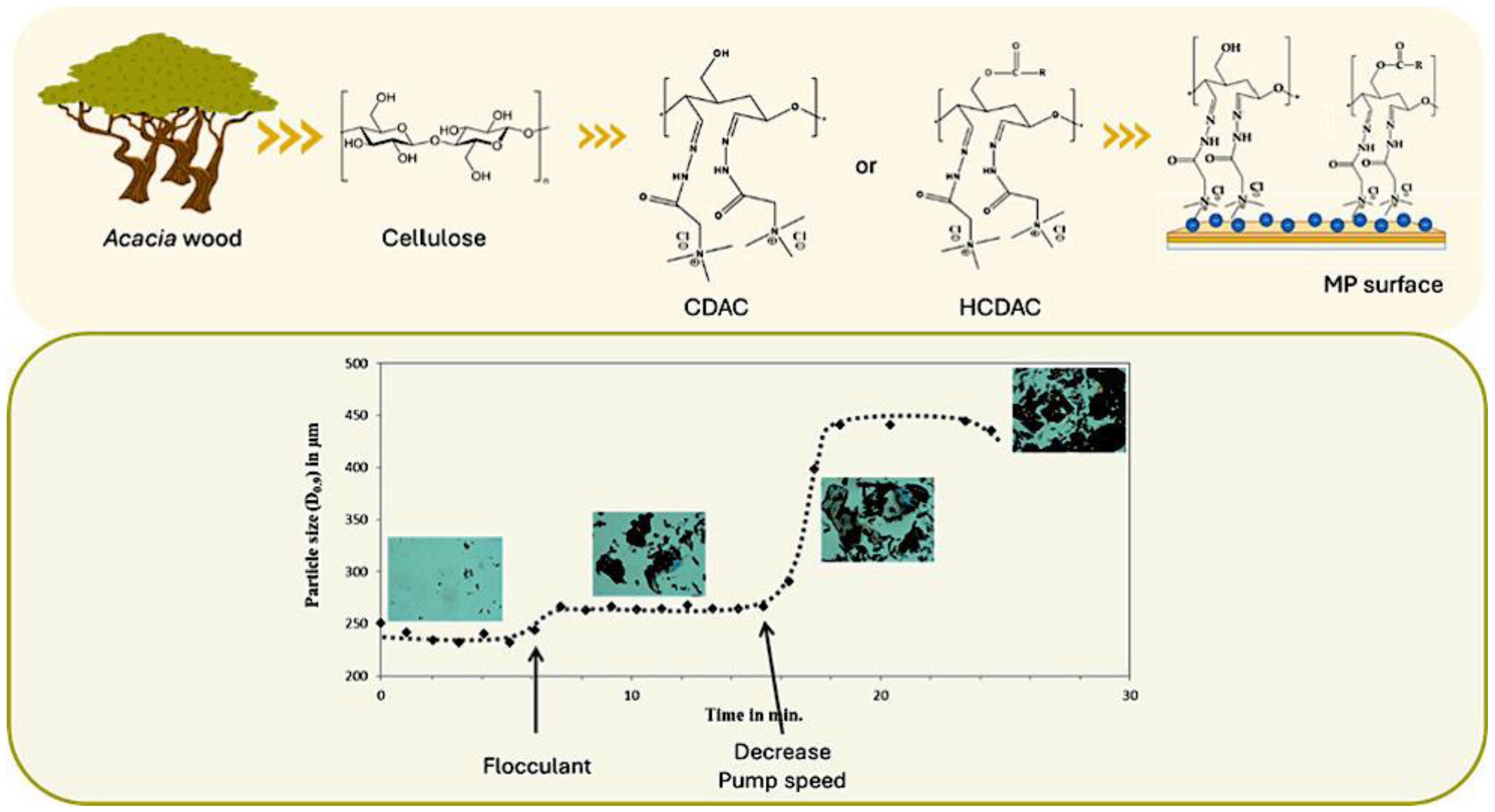
4.4. Flocculation
4.5. Advanced Oxidation Processes
4.6. Bioremediation
4.7. Integrated and Hybrid Systems
4.8. Limitations and Challenges
5. Methods for Microplastic Removal in Soils
5.1. Mechanical Separation
5.2. Density Separation
5.3. Bioremediation
5.4. Phytoremediation
5.5. Chemical and Enzymatic Approaches
5.6. Integrated and Hybrid Approaches
5.7. Limitations and Challenges
6. Overview of Microplastic Removal Processes
6.1. Particle Size and Diversity
6.2. Cost, Reusability, and Scalability
6.3. Environmental and Health Risks
6.4. Lack of Standardization and Monitoring
7. Microplastic Characterization
- Physical Characterization
- Chemical Characterization
- Advances and Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pottinger, A.S.; Geyer, R.; Biyani, N.; Martinez, C.C.; Nathan, N.; Morse, M.R.; Liu, C.; Hu, S.; de Bruyn, M.; Boettiger, C.; et al. Pathways to reduce global plastic waste mismanagement and greenhouse gas emissions by 2050. Science 2024, 386, 1168–1173. [Google Scholar] [CrossRef]
- Dokl, M.; Copot, A.; Krajnc, D.; Fan, Y.V.; Vujanović, A.; Aviso, K.B.; Tan, R.R.; Kravanja, Z.; Čuček, L. Global projections of plastic use, end-of-life fate and potential changes in consumption, reduction, recycling and replacement with bioplastics to 2050. Sustain. Prod. Consum. 2024, 51, 498–518. [Google Scholar] [CrossRef]
- Magalhães, S.; Alves, L.; Medronho, B.; Romano, A.; Rasteiro, M.d.G. Microplastics in Ecosystems: From Current Trends to Bio-Based Removal Strategies. Molecules 2020, 25, 3954. [Google Scholar] [CrossRef] [PubMed]
- Hasan Anik, A.; Hossain, S.; Alam, M.; Binte Sultan, M.; Hasnine, M.D.T.; Rahman, M.M. Microplastics pollution: A comprehensive review on the sources, fates, effects, and potential remediation. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100530. [Google Scholar] [CrossRef]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.S.; Wu, Y.S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef]
- Dey, T.K.; Uddin, M.E.; Jamal, M. Detection and removal of microplastics in wastewater: Evolution and impact. Environ. Sci. Pollut. Res. 2021, 28, 16925–16947. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, A.; Kundu, P.; Bhattacharya, S.; Dutta, N. Microplastic contamination in wastewater: Sources, distribution, detection and remediation through physical and chemical-biological methods. Sci. Total Environ. 2024, 916, 170254. [Google Scholar] [CrossRef]
- Wang, J.; Bucci, K.; Helm, P.A.; Hoellein, T.; Hoffman, M.J.; Rooney, R.; Rochman, C.M.; Liu, Y. Runoff and discharge pathways of microplastics into freshwater ecosystems: A systematic review and meta-analysis. FACETS 2022, 7, 1473–1492. [Google Scholar] [CrossRef]
- Li, W.; Zou, H.; Zheng, Y.; Zhang, G.; Xiang, Y.; Zhi, D.; Zhou, Y. Microplastics in aquatic environments: Detection, abundance, characteristics, and toxicological studies. Environ. Monit. Assess. 2025, 197, 150. [Google Scholar] [CrossRef]
- Thacharodi, A.; Meenatchi, R.; Hassan, S.; Hussain, N.; Bhat, M.A.; Arockiaraj, J.; Ngo, H.H.; Le, Q.H.; Pugazhendhi, A. Microplastics in the environment: A critical overview on its fate, toxicity, implications, management, and bioremediation strategies. J. Environ. Manag. 2024, 349, 119433. [Google Scholar] [CrossRef]
- Islam, T.; Cheng, H. Existence and fate of microplastics in terrestrial environment: A global fretfulness and abatement strategies. Sci. Total Environ. 2024, 953, 176163. [Google Scholar] [CrossRef]
- Safdar, A.; Ismail, F.; Imran, M. Characterization of Detergent-Compatible Lipases from Candida albicans and Acremonium sclerotigenum under Solid-State Fermentation. ACS Omega 2023, 8, 32740–32751. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Wu, B.; Yu, H.; Lei, P.; He, J.; Yi, J.; Wu, W.; Wang, H.; Yang, Q.; Zeng, G.; Sun, D. Microplastics in aquatic ecosystems: Detection, source tracing, and sustainable management strategies. Ecotoxicol. Environ. Saf. 2025, 291, 117883. [Google Scholar] [CrossRef]
- Uwamungu, J.Y.; Wang, Y.; Shi, G.; Pan, S.; Wang, Z.; Wang, L.; Yang, S. Microplastic contamination in soil agro-ecosystems: A review. Environ. Adv. 2022, 9, 100273. [Google Scholar] [CrossRef]
- Shi, W.; Wu, N.; Zhang, Z.; Liu, Y.; Chen, J.; Li, J. A global review on the abundance and threats of microplastics in soils to terrestrial ecosystem and human health. Sci. Total Environ. 2024, 912, 169469. [Google Scholar] [CrossRef] [PubMed]
- Begum, M.; Vaishnavi, G.; Muralidaran, Y.; Mishra, P. Chapter 22—Chemical, physical, and biological techniques to remove microplastics. In Microplastics; Singh, B., Upadhyay, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 509–530. [Google Scholar]
- Miranda Zoppas, F.; Sacco, N.; Soffietti, J.; Devard, A.; Akhter, F.; Marchesini, F.A. Catalytic approaches for the removal of microplastics from water: Recent advances and future opportunities. Chem. Eng. J. Adv. 2023, 16, 100529. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, D.; Dao, G.; Shi, Q.; Yu, T.; Guo, F.; Wu, G. Environmental impact of the effluents discharging from full-scale wastewater treatment plants evaluated by a hybrid fuzzy approach. Sci. Total Environ. 2021, 790, 148212. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef]
- Pirc, U.; Vidmar, M.; Mozer, A.; Kržan, A. Emissions of microplastic fibers from microfiber fleece during domestic washing. Environ. Sci. Pollut. Res. Int. 2016, 23, 22206–22211. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- Prata, J.C. Microplastics in wastewater: State of the knowledge on sources, fate and solutions. Mar. Pollut. Bull. 2018, 129, 262–265. [Google Scholar] [CrossRef]
- Luzi, B.; Carnevale Miino, M.; Rada, E.C.; Zullo, R.; Baltrocchi, A.P.D.; Torretta, V.; Galafassi, S. Critical review of microfiber release from textiles: Results, comparative challenges, mitigation strategies, and legislative perspectives. Chemosphere 2025, 378, 144394. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Islam, N.; Tasannum, N.; Mehjabin, A.; Momtahin, A.; Chowdhury, A.A.; Almomani, F.; Mofijur, M. Microplastic removal and management strategies for wastewater treatment plants. Chemosphere 2024, 347, 140648. [Google Scholar] [CrossRef]
- Liao, Z.; Ji, X.; Ma, Y.; Lv, B.; Huang, W.; Zhu, X.; Fang, M.; Wang, Q.; Wang, X.; Dahlgren, R.; et al. Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China. J. Hazard. Mater. 2021, 417, 126007. [Google Scholar] [CrossRef]
- Canha, N.; Jafarova, M.; Grifoni, L.; Gamelas, C.A.; Alves, L.C.; Almeida, S.M.; Loppi, S. Microplastic contamination of lettuces grown in urban vegetable gardens in Lisbon (Portugal). Sci. Rep. 2023, 13, 14278. [Google Scholar] [CrossRef]
- Rodrigues, D.; Antunes, J.; Pais, J.; Pequeno, J.; Caetano, P.S.; Rocha, F.; Sobral, P.; Costa, M.H. Distribution patterns of microplastics in subtidal sediments from the Sado river estuary and the Arrábida marine park, Portugal. Front. Environ. Sci. 2022, 10, 998513. [Google Scholar] [CrossRef]
- Godoy, V.; Prata, J.C.; Pérez, A.; da Costa, J.P.; Rocha-Santos, T.; Duarte, A.C. Microplastics in Sediments from a Sandy Beach in Costa Nova (Aveiro, Portugal). Sustainability 2023, 15, 6186. [Google Scholar] [CrossRef]
- Gorito, A.M.; Ribeiro, A.R.L.; Ramos, S.; Silva, A.M.T.; Almeida, C.M.R. Occurrence of micropollutants in surface waters: Monitoring of Portuguese Lima and Douro River estuaries and interconnecting northwest coast. Mar. Pollut. Bull. 2024, 209, 117140. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.C.G.; Barbosa, M.O.; Ribeiro, A.R.L.; Ratola, N.; Pereira, M.F.R.; Silva, A.M.T. Distribution of micropollutants in estuarine and sea water along the Portuguese coast. Mar. Pollut. Bull. 2020, 154, 111120. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.W.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as pollutants in agricultural soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef]
- Moeck, C.; Davies, G.; Krause, S.; Schneidewind, U. Microplastics and nanoplastics in agriculture—A potential source of soil and groundwater contamination? Grundwasser 2023, 28, 23–35. [Google Scholar] [CrossRef]
- Xu, Z.; Deng, X.; Lin, Z.; Wang, L.; Lin, L.; Wu, X.; Wang, Y.; Li, H.; Shen, J.; Sun, W. Microplastics in agricultural soil: Unveiling their role in shaping soil properties and driving greenhouse gas emissions. Sci. Total Environ. 2025, 958, 177875. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Q.; Adams, C.A.; Sun, Y.; Zhang, S. Effects of microplastics on soil properties: Current knowledge and future perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ye, Z.; Zhao, Y.; Lu, Q.; Shen, B.; Zhang, X.; Zhang, W.; Chen, S.-C.; Li, Y. Effects of different microplastic types on soil physicochemical properties, enzyme activities, and bacterial communities. Ecotoxicol. Environ. Saf. 2024, 286, 117219. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Bolan, N.; Li, Y.; Ding, S.; Atugoda, T.; Vithanage, M.; Sarkar, B.; Tsang, D.C.W.; Kirkham, M.B. Weathering of microplastics and interaction with other coexisting constituents in terrestrial and aquatic environments. Water Res. 2021, 196, 117011. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Liu, X.; Zhang, D.; Yuan, X. Adsorption of three bivalent metals by four chemical distinct microplastics. Chemosphere 2020, 248, 126064. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Joos, L.; De Tender, C. Soil under stress: The importance of soil life and how it is influenced by (micro)plastic pollution. Comput. Struct. Biotechnol. J. 2022, 20, 1554–1566. [Google Scholar] [CrossRef]
- Desforges, J.-P.W.; Galbraith, M.; Ross, P.S. Ingestion of Microplastics by Zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contam. Toxicol. 2015, 69, 320–330. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Li, J.; Shi, H. Microplastics in Food: Health Risks. In Microplastics in Terrestrial Environments: Emerging Contaminants and Major Challenges; He, D., Luo, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 343–356. [Google Scholar]
- Mishra, S.; Kumar, R.; Kumar, M. Use of treated sewage or wastewater as an irrigation water for agricultural purposes- Environmental, health, and economic impacts. Total Environ. Res. Themes 2023, 6, 100051. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Aguilar-Trigueros, C.A.; Onandia, G.; Maaß, S.; Zhao, T.; Rillig, M.C. Effects of microplastics and drought on soil ecosystem functions and multifunctionality. J. Appl. Ecol. 2021, 58, 988–996. [Google Scholar] [CrossRef]
- Balkrishna, A.; Kaushik, P.; Singh, S.; Agrahari, P.; Kumar, B.; Kumar, P.; Arya, V.P. Potential use of sewage sludge as fertilizer in organic farming. Clean. Waste Syst. 2025, 10, 100245. [Google Scholar] [CrossRef]
- Jamali, M.K.; Kazi, T.G.; Arain, M.B.; Afridi, H.I.; Memon, A.R.; Jalbani, N.; Shah, A. Use of Sewage Sludge After Liming as Fertilizer for Maize Growth. Pedosphere 2008, 18, 203–213. [Google Scholar] [CrossRef]
- DECREE LAW No. 102-D/2020. 102-D/2020 2020, 25-(22) a 25-(269). Available online: https://diariodarepublica.pt/dr/en/detail/decree-law/102-d-2020-150908012 (accessed on 10 June 2025).
- Hanif, M.N.; Aijaz, N.; Azam, K.; Akhtar, M.; Laftah, W.A.; Babur, M.; Abbood, N.K.; Benitez, I.B. Impact of microplastics on soil (physical and chemical) properties, soil biological properties/soil biota, and response of plants to it: A review. Int. J. Environ. Sci. Technol. 2024, 21, 10277–10318. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Ali, N.; Khan, M.H.; Ali, M.; Sidra; Ahmad, S.; Khan, A.; Nabi, G.; Ali, F.; Bououdina, M.; Kyzas, G.Z. Insight into microplastics in the aquatic ecosystem: Properties, sources, threats and mitigation strategies. Sci. Total Environ. 2024, 913, 169489. [Google Scholar] [CrossRef]
- Strojny, W.; Gruca-Rokosz, R.; Cieśla, M. Microplastics in Water Resources: Threats and Challenges. Appl. Sci. 2025, 15, 4118. [Google Scholar] [CrossRef]
- Issac, M.N.; Kandasubramanian, B. Effect of microplastics in water and aquatic systems. Environ. Sci. Pollut. Res. 2021, 28, 19544–19562. [Google Scholar] [CrossRef]
- Luo, H.; Tu, C.; He, D.; Zhang, A.; Sun, J.; Li, J.; Xu, J.; Pan, X. Interactions between microplastics and contaminants: A review focusing on the effect of aging process. Sci. Total Environ. 2023, 899, 165615. [Google Scholar] [CrossRef]
- Rafa, N.; Ahmed, B.; Zohora, F.; Bakya, J.; Ahmed, S.; Ahmed, S.F.; Mofijur, M.; Chowdhury, A.A.; Almomani, F. Microplastics as carriers of toxic pollutants: Source, transport, and toxicological effects. Environ. Pollut. 2024, 343, 123190. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, B.; Zhao, T.; Yin, W.; Zhao, X.; Xie, Q.; Khan, K.Y.; Zhao, X.; Nazar, M.; Li, G.; Du, D. Impacts of soil microplastics on crops: A review. Appl. Soil Ecol. 2023, 181, 104680. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in Aquatic Ecosystems. Angew. Chem. Int. Ed. 2017, 56, 1720–1739. [Google Scholar] [CrossRef]
- Shim, W.J.; Hong, S.H.; Eo, S.E. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Käppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef]
- Gigault, J.; Halle, A.t.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Prata, J.C.; Padrão, J.; Khan, M.T.; Walker, T.R. Do’s and don’ts of microplastic research: A comprehensive guide. Water Emerg. Contam. Nanoplastics 2024, 3, 8. [Google Scholar] [CrossRef]
- Dongo, D.; Penna, A.A.D. Microplastics, the First Restrictions in the Old Continent in a Mini-Reform of the REACH Regulation; Food Times: Rome, Italy, 2023. [Google Scholar]
- Cowger, W.; Booth, A.M.; Hamilton, B.M.; Thaysen, C.; Primpke, S. Reporting Guidelines to Increase the Reproducibility and Comparability of Research on Microplastics. Appl. Spectrosc. 2020, 74, 1066–1077. [Google Scholar] [CrossRef]
- 2023/2055,C.R.E. Commission Regulation (EU) 2023/2055—Restriction of microplastics intentionally added to products. 2023. Available online: https://eur-lex.europa.eu/eli/reg/2023/2055/oj (accessed on 5 June 2025).
- Meng, X.; Yuan, J.; Huang, Q.; Liu, R.; Yang, Y.; Yang, X.; Wang, K. A Review of Sources, Hazards, and Removal Methods of Microplastics in the Environment. Water 2025, 17, 102. [Google Scholar] [CrossRef]
- Lu, Y.; Li, M.-C.; Lee, J.; Liu, C.; Mei, C. Microplastic remediation technologies in water and wastewater treatment processes: Current status and future perspectives. Sci. Total Environ. 2023, 868, 161618. [Google Scholar] [CrossRef]
- Kurt, Z.; Özdemir, I.; James R, A.M. Effectiveness of microplastics removal in wastewater treatment plants: A critical analysis of wastewater treatment processes. J. Environ. Chem. Eng. 2022, 10, 107831. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Hadibarata, T. Microplastics removal through water treatment plants: Its feasibility, efficiency, future prospects and enhancement by proper waste management. Environ. Chall. 2021, 5, 100264. [Google Scholar] [CrossRef]
- Acarer, S. Microplastics in wastewater treatment plants: Sources, properties, removal efficiency, removal mechanisms, and interactions with pollutants. Water Sci. Technol. 2023, 87, 685–710. [Google Scholar] [CrossRef]
- Wakeman, R. The influence of particle properties on filtration. Sep. Purif. Technol. 2007, 58, 234–241. [Google Scholar] [CrossRef]
- Raza, A.; Hassan, J.Z.; Mahmood, A.; Nabgan, W.; Ikram, M. Recent advances in membrane-enabled water desalination by 2D frameworks: Graphene and beyond. Desalination 2022, 531, 115684. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef]
- Le, L.-T.; Bui, X.-B.; Tran, C.-S.; Chiemchaisri, C.; Pandey, A. Chapter 9—Membrane and filtration processes for microplastic removal. In Current Developments in Biotechnology and Bioengineering; Bui, X.-T., Guo, W., Chiemchaisri, C., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 203–220. [Google Scholar]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef]
- Jiang, H.; Bu, J.; Bian, K.; Su, J.; Wang, Z.; Sun, H.; Wang, H.; Zhang, Y.; Wang, C. Surface change of microplastics in aquatic environment and the removal by froth flotation assisted with cationic and anionic surfactants. Water Res. 2023, 233, 119794. [Google Scholar] [CrossRef]
- Jia, M.; Farid, M.U.; Ho, Y.-W.; Ma, X.; Wong, P.W.; Nah, T.; He, Y.; Boey, M.W.; Lu, G.; Fang, J.K.-H.; et al. Advanced nanobubble flotation for enhanced removal of sub-10 µm microplastics from wastewater. Nat. Commun. 2024, 15, 9079. [Google Scholar] [CrossRef]
- Tang, W.; Li, H.; Fei, L.; Wei, B.; Zhou, T.; Zhang, H. The removal of microplastics from water by coagulation: A comprehensive review. Sci. Total Environ. 2022, 851, 158224. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Chen, C.; Huang, Z. Sedimentation of nanoplastics from water with Ca/Al dual flocculants: Characterization, interface reaction, effects of pH and ion ratios. Chemosphere 2020, 252, 126450. [Google Scholar] [CrossRef]
- Misra, A.; Zambrzycki, C.; Kloker, G.; Kotyrba, A.; Anjass, M.H.; Franco Castillo, I.; Mitchell, S.G.; Güttel, R.; Streb, C. Water Purification and Microplastics Removal Using Magnetic Polyoxometalate-Supported Ionic Liquid Phases (magPOM-SILPs). Angew. Chem. Int. Ed. 2020, 59, 1601–1605. [Google Scholar] [CrossRef]
- Wang, L.; Kaeppler, A.; Fischer, D.; Simmchen, J. Photocatalytic TiO2 Micromotors for Removal of Microplastics and Suspended Matter. ACS Appl. Mater. Interfaces 2019, 11, 32937–32944. [Google Scholar] [CrossRef]
- Lourenço, A.; Reis, M.S.; Arnold, J.; Rasteiro, M.G. Data-Driven Modelling of the Complex Interaction between Flocculant Properties and Floc Size and Structure. Processes 2020, 8, 349. [Google Scholar] [CrossRef]
- Magalhães, S.; Paciência, D.; Rodrigues, J.M.M.; Lindman, B.; Alves, L.; Medronho, B.; Rasteiro, M.d.G. Insights on Microplastic Contamination from Municipal and Textile Industry Effluents and Their Removal Using a Cellulose-Based Approach. Polymers 2024, 16, 2803. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, S.; Norgren, M.; Alves, L.; Medronho, B.; da Graça Rasteiro, M. Tailored cellulose-based flocculants for microplastics removal: Mechanistic insights, pH influence, and efficiency optimization. Powder Technol. 2025, 456, 120838. [Google Scholar] [CrossRef]
- Magalhães, S.; Aliaño-González, M.J.; Cruz, P.F.; Rosenberg, R.; Haffke, D.; Norgren, M.; Alves, L.; Medronho, B.; da Graça Rasteiro, M. Customising Sustainable Bio-Based Polyelectrolytes: Introduction of Charged and Hydrophobic Groups in Cellulose. Polymers 2024, 16, 3105. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Busquets, R.; Campos, L.C. Enhancing microplastic removal from natural water using coagulant aids. Chemosphere 2024, 364, 143145. [Google Scholar] [CrossRef]
- Jeong, Y.; Gong, G.; Lee, H.-J.; Seong, J.; Hong, S.W.; Lee, C. Transformation of microplastics by oxidative water and wastewater treatment processes: A critical review. J. Hazard. Mater. 2023, 443, 130313. [Google Scholar] [CrossRef]
- Bule Možar, K.; Miloloža, M.; Martinjak, V.; Cvetnić, M.; Kušić, H.; Bolanča, T.; Kučić Grgić, D.; Ukić, Š. Potential of Advanced Oxidation as Pretreatment for Microplastics Biodegradation. Separations 2023, 10, 132. [Google Scholar] [CrossRef]
- He, Y.; Rehman, A.U.; Xu, M.; Not, C.A.; Ng, A.M.C.; Djurišić, A.B. Photocatalytic degradation of different types of microplastics by TiOx/ZnO tetrapod photocatalysts. Heliyon 2023, 9, e22562. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Xiangyu, B.; Chao, L.; Shilong, H.; Jiping, Z.; Hu, J. Combining advanced oxidation processes with biological processes in organic wastewater treatment: Recent developments, trends, and advances. Desalination Water Treat. 2025, 323, 101263. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Zhang, G. Degradation of microplastic in water by advanced oxidation processes. Chemosphere 2024, 357, 141939. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zhou, C.; Hu, T.; Zeng, G.; Zhang, Y. Advanced oxidation processes for the elimination of microplastics from aqueous systems: Assessment of efficiency, perspectives and limitations. Sci. Total Environ. 2022, 842, 156723. [Google Scholar] [CrossRef]
- Paiu, M.; Lutic, D.; Favier, L.; Gavrilescu, M. Heterogeneous Photocatalysis for Advanced Water Treatment: Materials, Mechanisms, Reactor Configurations, and Emerging Applications. Appl. Sci. 2025, 15, 5681. [Google Scholar] [CrossRef]
- Shahwar, D.; Ibrahim, P.M.S.N.M.; Ali, S.M.B.; Khan, Z. Chapter 9—Remediation of heavy metals contaminated wastewaters through microbes: Recent progress and future prospects. In Bio-Organic Amendments for Heavy Metal Remediation; Husen, A., Iqbal, M., Ditta, A., Mehmood, S., Imtiaz, M., Tu, M.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 135–153. [Google Scholar]
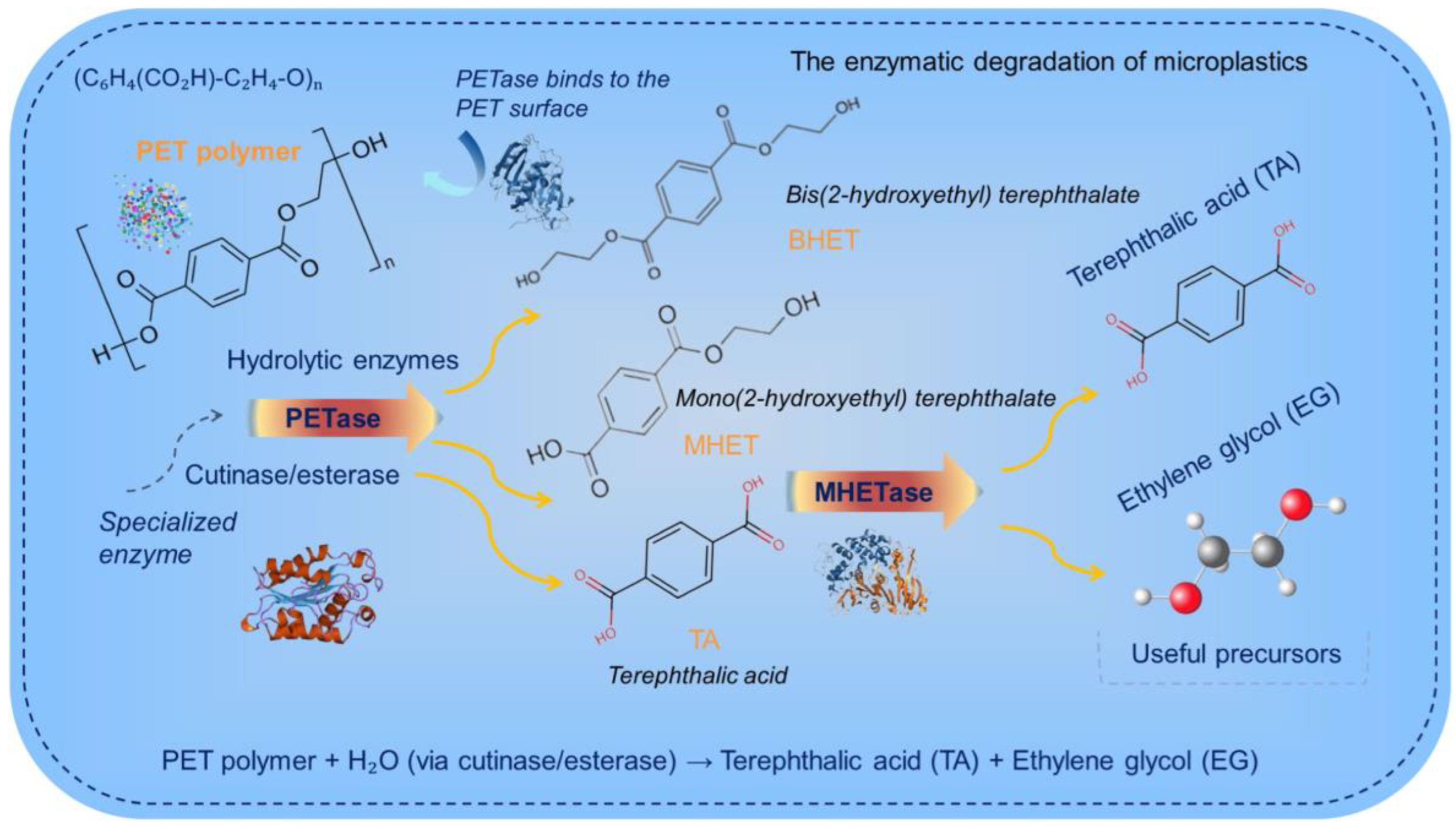
- Yoshida, S.; Hiraga, K.; Taniguchi, I.; Oda, K. Chapter Nine—Ideonella sakaiensis, PETase, and MHETase: From identification of microbial PET degradation to enzyme characterization. In Methods in Enzymology; Weber, G., Bornscheuer, U.T., Wei, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 648, pp. 187–205. [Google Scholar]
- Tao, H.; Zhou, L.; Yu, D.; Chen, Y.; Luo, Y.; Lin, T. Effects of polystyrene microplastics on the metabolic level of Pseudomonas aeruginosa. Sci. Total Environ. 2024, 922, 171335. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Ismail, F.; Iftikhar, H.; Majid Khokhar, A.; Javed, A.; Imran, M.; Safdar, B. Determination of Biodegradation Potential of Aspergillus niger, Candida albicans, and Acremonium sclerotigenum on Polyethylene, Polyethylene Terephthalate, and Polystyrene Microplastics. Int. J. Microbiol. 2024, 2024, 7682762. [Google Scholar] [CrossRef]
- Ferreira-Filipe, D.A.; Oliveira, L.; Paço, A.; Fernandes, A.J.S.; Costa, F.M.; Duarte, A.C.; Rocha-Santos, T.; Patrício Silva, A.L. Biodegradation of e-waste microplastics by Penicillium brevicompactum. Sci. Total Environ. 2024, 935, 173334. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Neal, B.; White, B.; Nelson, B.; Lai, J.; Long, B.; Arreola-Vargas, J.; Yu, J.; Banik, M.T.; Dai, S.Y. Microplastics removal in the aquatic environment via fungal pelletization. Bioresour. Technol. Rep. 2023, 23, 101545. [Google Scholar] [CrossRef]
- Lagarde, F.; Olivier, O.; Zanella, M.; Daniel, P.; Hiard, S.; Caruso, A. Microplastic interactions with freshwater microalgae: Hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ. Pollut. (Barking Essex 1987) 2016, 215, 331–339. [Google Scholar] [CrossRef]
- Sturm, M.T.; Schuhen, K.; Horn, H. Method for rapid biofilm cultivation on microplastics and investigation of its effect on the agglomeration and removal of microplastics using organosilanes. Sci. Total Environ. 2022, 806, 151388. [Google Scholar] [CrossRef]
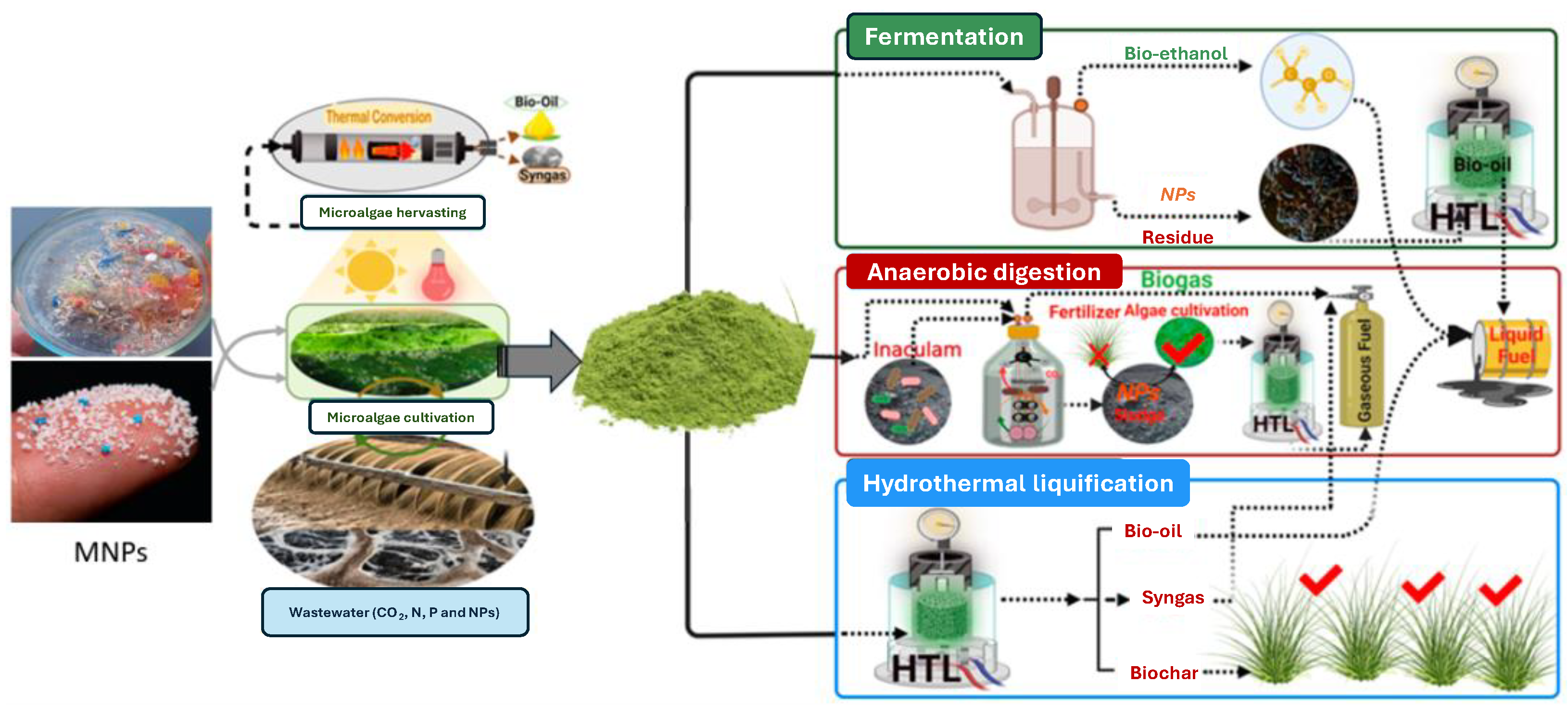
- Abomohra, A.; Hanelt, D. Recent Advances in Micro-/Nanoplastic (MNPs) Removal by Microalgae and Possible Integrated Routes of Energy Recovery. Microorganisms 2022, 10, 2400. [Google Scholar] [CrossRef] [PubMed]
- Bodzek, M.; Bodzek, P. Remediation of Micro- and Nanoplastics by Membrane Technologies. Membranes 2025, 15, 82. [Google Scholar] [CrossRef]
- Lin, Z.; Hu, X.; Lin, H.; Yu, G.; Shen, L.; Yu, W.; Li, B.; Zhao, L.; Ying, M. Membrane technology for microplastic removal: Microplastic occurrence, challenges, and innovations of process and materials. Chem. Eng. J. 2025, 520, 166183. [Google Scholar] [CrossRef]
- Xue, W.; Tabucanon, A.S.; Amarakoon, A.M.S.N.; Xiao, K.; Huang, X. Recent advances in membrane and electrochemical hybrid technologies for emerging contaminants removal. Water Cycle 2025, 6, 176–194. [Google Scholar] [CrossRef]
- Inoue, T.; Asai, K.; Morisawa, T.; Tamaue, K. New method for extracting microplastics from sediments using a hydrocyclone and sieve. Results Eng. 2024, 24, 103232. [Google Scholar] [CrossRef]
- Chen, Y.; Junaid, M.; Yin, K.; Li, X.; Wang, X.; Wang, S.; Zhou, H. Development and application of an efficient microplastics extraction method based on glycerol flotation for environmental soil samples. Gondwana Res. 2025, 143, 226–238. [Google Scholar] [CrossRef]
- Zeng, L.; Li, L.; Xiao, J.; Zhou, P.; Han, X.; Shen, B.; Dai, L. Microplastics in the Environment: A Review Linking Pathways to Sustainable Separation Techniques. Separations 2025, 12, 82. [Google Scholar] [CrossRef]
- Hu, H.; Qiang, L.; Xu, J.; Li, G.; Cheng, J.; Zhong, X.; Zhang, R. Enhanced microplastic retrieval efficiency from cultivated soil samples through optimized pre-treatment in density-based extraction. Soil Tillage Res. 2024, 242, 106134. [Google Scholar] [CrossRef]
- Othman, A.R.; Hasan, H.A.; Muhamad, M.H.; Ismail, N.I.; Abdullah, S.R.S. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021, 19, 3057–3073. [Google Scholar] [CrossRef]
- Pan, I.; Umapathy, S.; Issac, P.K.; Rahman, M.M.; Guru, A.; Arockiaraj, J. The bioaccessibility of adsorped heavy metals on biofilm-coated microplastics and their implication for the progression of neurodegenerative diseases. Environ. Monit. Assess. 2023, 195, 1264. [Google Scholar] [CrossRef]
- Rahman, M.M.; Chowdhury, F.N. Evidence on Potential Bioremediation of Microplastics from Soil Environment around the World. In Bioremediation: Removing Microplastics from Soil; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2023; Volume 1459, pp. 99–124. [Google Scholar]
- Wu, F.; Guo, Z.; Cui, K.; Dong, D.; Yang, X.; Li, J.; Wu, Z.; Li, L.; Dai, Y.; Pan, T. Insights into characteristics of white rot fungus during environmental plastics adhesion and degradation mechanism of plastics. J. Hazard. Mater. 2023, 448, 130878. [Google Scholar] [CrossRef]
- Khurana, S.; Ali, S.; Srivastava, A.K.; Singh, A.; Agarwal, H.; Chauhan, R.; Joshi, N.C.; Dufossé, L.; Chauhan, A. Bioremediation of microplastic pollution: A systematic review on mechanism, analytical methods, innovations, and omics approaches. J. Hazard. Mater. Adv. 2025, 19, 100777. [Google Scholar] [CrossRef]
- Gong, X.; Shi, G.; Zou, D.; Wu, Z.; Qin, P.; Yang, Y.; Hu, X.; Zhou, L.; Zhou, Y. Micro- and nano-plastics pollution and its potential remediation pathway by phytoremediation. Planta 2023, 257, 35. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiaowei, W.; Chunting, R.; Niroshika, P.K.; Zhenyu, W.; Chang, S.X. Microplastic pollution: Phytotoxicity, environmental risks, and phytoremediation strategies. Crit. Rev. Environ. Sci. Technol. 2024, 54, 486–507. [Google Scholar] [CrossRef]
- Kumar, D.; Biswas, J.K.; Mulla, S.I.; Singh, R.; Shukla, R.; Ahanger, M.A.; Shekhawat, G.S.; Verma, K.K.; Siddiqui, M.W.; Seth, C.S. Micro and nanoplastics pollution: Sources, distribution, uptake in plants, toxicological effects, and innovative remediation strategies for environmental sustainability. Plant Physiol. Biochem. 2024, 213, 108795. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, A.; Zhao, B.; Ma, F.; Zhang, X.; Zhang, Y.; Duan, K.; Li, Y. Effects of microplastics and cadmium co-contamination on soil properties, maize (Zea mays L.) growth characteristics, and cadmium accumulation in maize in loessial soil-maize systems. Environ. Pollut. 2024, 356, 124363. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, W.; Zeb, A.; Lian, J.; Sun, Y.; Sun, H. Polystyrene microplastic interaction with Oryza sativa: Toxicity and metabolic mechanism. Environ. Sci. Nano 2021, 8, 3699–3710. [Google Scholar] [CrossRef]
- Iqbal, B.; Javed, Q.; Khan, I.; Tariq, M.; Ahmad, N.; Elansary, H.O.; Jalal, A.; Li, G.; Du, D. Influence of soil microplastic contamination and cadmium toxicity on the growth, physiology, and root growth traits of Triticum aestivum L. S. Afr. J. Bot. 2023, 160, 369–375. [Google Scholar] [CrossRef]
- Yuan, W.; Xu, E.G.; Shabaka, S.; Chen, P.; Yang, Y. The power of green: Harnessing phytoremediation to combat micro/nanoplastics. Eco-Environ. Health 2024, 3, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xu, X.; Guo, L.; Jin, R.; Lu, Y. Uptake and transport of micro/nanoplastics in terrestrial plants: Detection, mechanisms, and influencing factors. Sci. Total Environ. 2024, 907, 168155. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Yang, Z.; Bazina, L.; Kharaghani, D.; Sadrieh, F.; Demokritou, P. Mechanisms of ingested polystyrene micro-nanoplastics (MNPs) uptake and translocation in an in vitro tri-culture small intestinal epithelium. J. Hazard. Mater. 2024, 473, 134706. [Google Scholar] [CrossRef]
- Sahoo, A.; Chhotaray, S.P.; Meher, I.; Behera, S.P.; Pal, A.; Meena, M.; Swapnil, P.; Yadav, A.; Bhardwaj, R. Phytoremediation for a sustainable future: Integrating plant based strategies in soil and wastewater remediation. Bioresour. Technol. Rep. 2025, 31, 102266. [Google Scholar] [CrossRef]
- Ford, H.; Garbutt, A.; Ladd, C.; Malarkey, J.; Skov, M.W. Soil stabilization linked to plant diversity and environmental context in coastal wetlands. J. Veg. Sci. Off. Organ Int. Assoc. Veg. Sci. 2016, 27, 259–268. [Google Scholar] [CrossRef]
- Lee, H.; Sam, K.; Coulon, F.; De Gisi, S.; Notarnicola, M.; Labianca, C. Recent developments and prospects of sustainable remediation treatments for major contaminants in soil: A review. Sci. Total Environ. 2024, 912, 168769. [Google Scholar] [CrossRef]
- Adamu, H.; Bello, U.; IbrahimTafida, U.; Garba, Z.N.; Galadima, A.; Lawan, M.M.; Abba, S.I.; Qamar, M. Harnessing bio and (Photo)catalysts for microplastics degradation and remediation in soil environment. J. Environ. Manag. 2024, 370, 122543. [Google Scholar] [CrossRef]
- Nguyen, M.-K.; Rakib, M.R.J.; Hwangbo, M.; Kim, J. Microplastic accumulation in soils: Unlocking the mechanism and biodegradation pathway. J. Hazard. Mater. Adv. 2025, 17, 100629. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Bian, K.; Wang, C.; Xie, X.; Wang, H.; Zhao, H. Is it possible to efficiently and sustainably remove microplastics from sediments using froth flotation? Chem. Eng. J. 2022, 448, 137692. [Google Scholar] [CrossRef]
- Rahman, L.; Gary, M.; Ryan, K.; Halappanavar, S. Microplastics and nanoplastics science: Collecting and characterizing airborne microplastics in fine particulate matter. Nanotoxicology 2021, 15, 1253–1278. [Google Scholar] [CrossRef]
- Sobhani, Z.; Luo, Y.; Gibson, C.T.; Tang, Y.; Naidu, R.; Megharaj, M.; Fang, C. Collecting Microplastics in Gardens: Case Study (i) of Soil. Front. Environ. Sci. 2021, 9, 739775. [Google Scholar] [CrossRef]
- García-Ávila, F.; Zambrano-Jaramillo, A.; Velecela-Garay, C.; Coronel-Sánchez, K.; Valdiviezo-Gonzales, L. Effectiveness of membrane technologies in removing emerging contaminants from wastewater: Reverse Osmosis and Nanofiltration. Water Cycle 2025, 6, 357–373. [Google Scholar] [CrossRef]
- Al Alwan, B.; Ismail, B.; El Jery, A.; Badawi, A.K. State-of-the-art strategies for microplastics mitigation in aquatic environments: Identification, technological innovations, and prospects for advancement. J. Water Process Eng. 2024, 61, 105336. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Recent approaches and advanced wastewater treatment technologies for mitigating emerging microplastics contamination—A critical review. Sci. Total Environ. 2023, 858, 159681. [Google Scholar] [CrossRef] [PubMed]
- Lalrinfela, P.; Vanlalsangi, R.; Lalrinzuali, K.; Babu, P.J. Microplastics: Their effects on the environment, human health, and plant ecosystems. Environ. Pollut. Manag. 2024, 1, 248–259. [Google Scholar] [CrossRef]
- Hoang, V.-H.; Nguyen, M.-K.; Hoang, T.-D.; Ha, M.C.; Huyen, N.T.T.; Bui, V.K.H.; Pham, M.-T.; Nguyen, C.-M.; Chang, S.W.; Nguyen, D.D. Sources, environmental fate, and impacts of microplastic contamination in agricultural soils: A comprehensive review. Sci. Total Environ. 2024, 950, 175276. [Google Scholar] [CrossRef]
- Boctor, J.; Hoyle, F.C.; Farag, M.A.; Ebaid, M.; Walsh, T.; Whiteley, A.S.; Murphy, D.V. Microplastics and nanoplastics: Fate, transport, and governance from agricultural soil to food webs and humans. Environ. Sci. Eur. 2025, 37, 68. [Google Scholar] [CrossRef]
- Ta, A.T.; Promchan, N. Microplastics in wastewater from developing countries: A comprehensive review and methodology suggestions. TrAC Trends Anal. Chem. 2024, 171, 117537. [Google Scholar] [CrossRef]
- Krekelbergh, N.; Li, J.; Kusumawardani, P.N.; Liu, Y.; Hu, J.; Sleutel, S.; Parakhonskiy, B.; Hoogenboom, R.; De Neve, S.; Skirtach, A. Comparison of Raman and fluorescence microscopy for identification of small (< 2 μm) microplastics in soil. Environ. Pollut. 2025, 374, 126204. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.P.; Sun, C.; Lim, P.E. Chapter 8—Observation and visual identification of microplastics. In Analysis of Microplastics and Nanoplastics; Shi, H., Sun, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 155–182. [Google Scholar]
- Liu, Z.; Wang, W.; Liu, X. Automated characterization and identification of microplastics through spectroscopy and chemical imaging in combination with chemometric: Latest developments and future prospects. TrAC Trends Anal. Chem. 2023, 160, 116956. [Google Scholar] [CrossRef]
- Tirkey, A.; Upadhyay, L.S.B. Microplastics: An overview on separation, identification and characterization of microplastics. Mar. Pollut. Bull. 2021, 170, 112604. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, B.; Wang, H. Analytical methods for microplastics in the environment: A review. Environ. Chem. Lett. 2023, 21, 383–401. [Google Scholar] [CrossRef]
- Veerasingam, S.; Ranjani, M.; Venkatachalapathy, R.; Bagaev, A.; Mukhanov, V.; Litvinyuk, D.; Mugilarasan, M.; Gurumoorthi, K.; Guganathan, L.; Aboobacker, V.M.; et al. Contributions of Fourier transform infrared spectroscopy in microplastic pollution research: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2681–2743. [Google Scholar] [CrossRef]
- Das, R.S.; Agrawal, Y.K. Raman spectroscopy: Recent advancements, techniques and applications. Vib. Spectrosc. 2011, 57, 163–176. [Google Scholar] [CrossRef]
- Picó, Y.; Barceló, D. Pyrolysis gas chromatography-mass spectrometry in environmental analysis: Focus on organic matter and microplastics. TrAC Trends Anal. Chem. 2020, 130, 115964. [Google Scholar] [CrossRef]
- Jiménez-Skrzypek, G.; González-Sálamo, J.; Hernández-Borges, J. Chapter 17—Application of liquid chromatography in studies of microplastics. In Liquid Chromatography, 3rd ed.; Fanali, S., Chankvetadze, B., Haddad, P.R., Poole, C.F., Riekkola, M.-L., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 2, pp. 579–605. [Google Scholar]
- Morgana, S.; Casentini, B.; Tirelli, V.; Grasso, F.; Amalfitano, S. Fluorescence-based detection: A review of current and emerging techniques to unveil micro/ nanoplastics in environmental samples. TrAC Trends Anal. Chem. 2024, 172, 117559. [Google Scholar] [CrossRef]
- Sancataldo, G.; Ferrara, V.; Bonomo, F.P.; Chillura Martino, D.F.; Licciardi, M.; Pignataro, B.G.; Vetri, V. Identification of microplastics using 4-dimethylamino-4′-nitrostilbene solvatochromic fluorescence. Microsc. Res. Tech. 2021, 84, 2820–2831. [Google Scholar] [CrossRef] [PubMed]


| Method | Principle/Process | Advantages | Removal Efficiency | Limitations | Main Applications | Technology Readiness Level (TRL) | Ref. |
|---|---|---|---|---|---|---|---|
| Filtration | Physical size exclusion | Simple, widely implemented | 40–99% | Less effective for <10 µm MPs; clogging | Wastewater treatment plants | 9 (fully implemented in wastewater plants) | [73,75] |
| Flotation | Density-based separation | Effective for larger MPs | ~95% | Use of chemicals; less effective for small/irregular MPs (for 1 to 10 µm MPs, the removal is below 80%) | Water and wastewater treatment | 7–8 (operational, semi-commercial) | [77] |
| Chemical coagulation | Aggregation via coagulants | Removes fine particles | ~95% | MPs above 1 µm; sludge generation; reagent cost; toxicity | Water and wastewater treatment | 8–9 (widely applied in municipal/industrial) | [80,81] |
| Advanced oxidation processes | Chemical oxidation (e.g., ozonation, UV, Fenton) | Degradation of MPs and nanoplastics | ~95% | MPs above 1 µm; high energy/cost; possible secondary pollution | Industrial effluents | 6–7 (pilot set-ups, growing in industry) | [81] |
| Biormeediation | Microbial/enzymatic degradation or adsorption | Sustainable, eco-friendly | 94.5% | MPs from 400 to 1000 µm; slow; limited to certain polymers | Soils, wastewater | 4–6 (laboratory to early field trials) | [103] |
| Density Separation (Soil) | Separation using high-density salt solution | Good for low-density MPs | - | Labor-intensive; waste solution | Soil and sediment analysis | 6 (used for research, limited field tests) | [29] |
| Phytoremediation | Uptake/immobilization by plants | Low-cost, environmentally friendly | - | Limited efficiency; long time needed; risk of food chain transfer | Soil stabilization | 4–6 (mainly lab experiments, some field cases) | [119] |
| Integrated/Hybrid Systems | Combination of above methods | Higher efficiency, broader scope | - | Increased complexity, cost | Advanced wastewater/soil treatment | 5–7 (demonstrated, not fully mature commercially) | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, S.; Alves, L.; Medronho, B.; Svanedal, I.; Norgren, M.; Rasteiro, M.G. Innovative Approaches to Mitigating Microplastic Pollution in Effluents and Soils. Sustainability 2025, 17, 9014. https://doi.org/10.3390/su17209014
Magalhães S, Alves L, Medronho B, Svanedal I, Norgren M, Rasteiro MG. Innovative Approaches to Mitigating Microplastic Pollution in Effluents and Soils. Sustainability. 2025; 17(20):9014. https://doi.org/10.3390/su17209014
Chicago/Turabian StyleMagalhães, Solange, Luís Alves, Bruno Medronho, Ida Svanedal, Magnus Norgren, and Maria Graça Rasteiro. 2025. "Innovative Approaches to Mitigating Microplastic Pollution in Effluents and Soils" Sustainability 17, no. 20: 9014. https://doi.org/10.3390/su17209014
APA StyleMagalhães, S., Alves, L., Medronho, B., Svanedal, I., Norgren, M., & Rasteiro, M. G. (2025). Innovative Approaches to Mitigating Microplastic Pollution in Effluents and Soils. Sustainability, 17(20), 9014. https://doi.org/10.3390/su17209014










