Energy Cane Ash: Property Assessment for Its Valorization in Sustainable Cementing Systems
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Soil Chemical Analysis
3.2. Productivity Evaluations
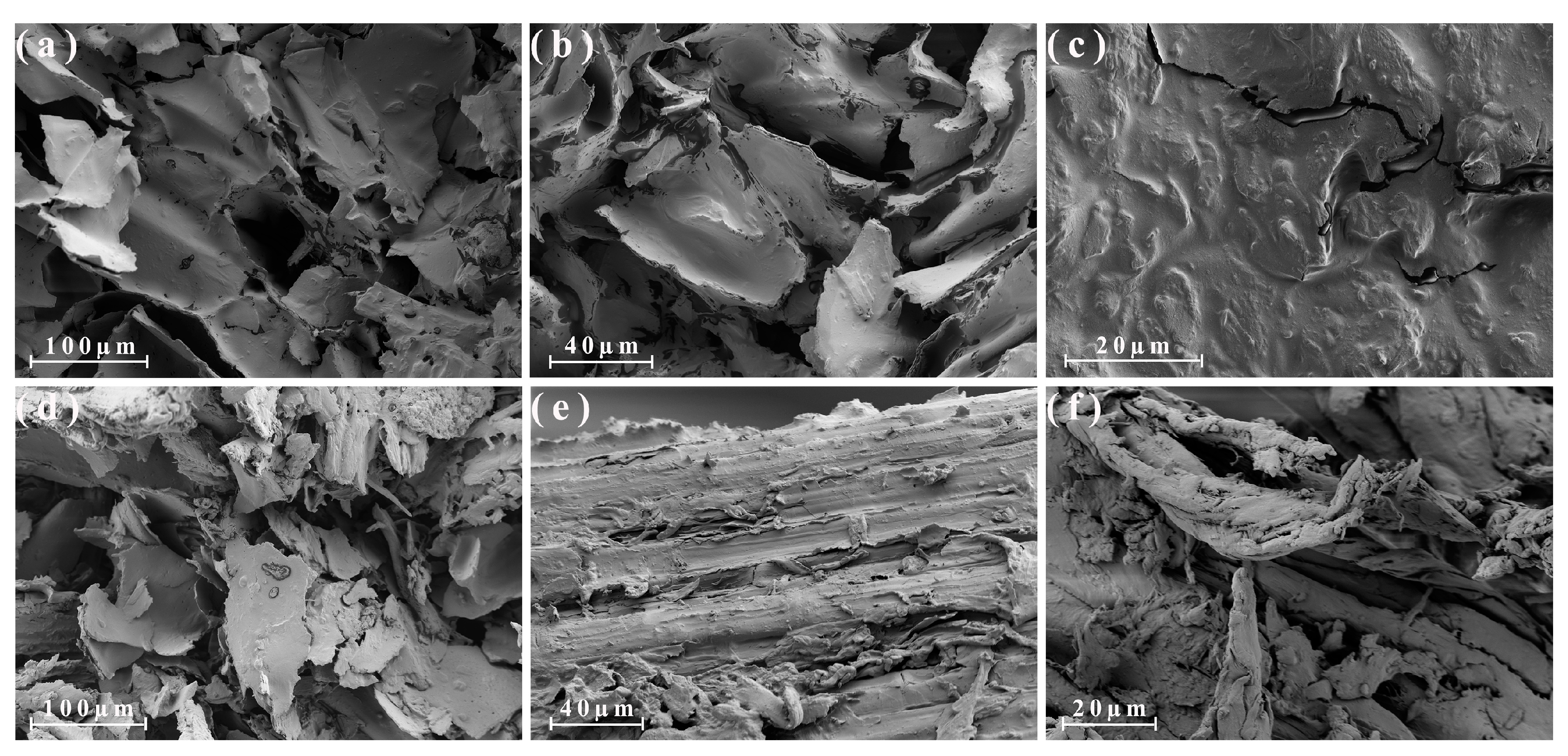
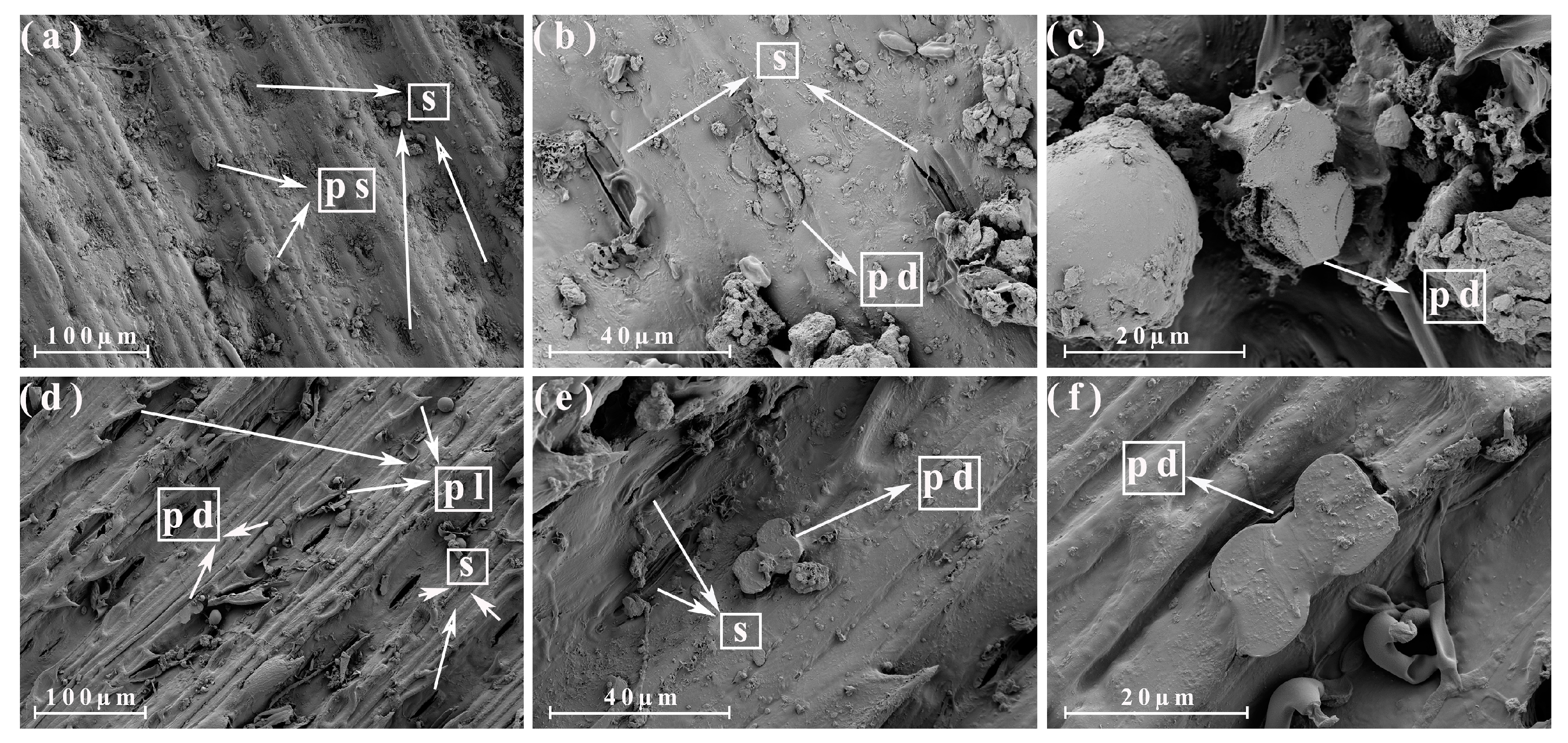
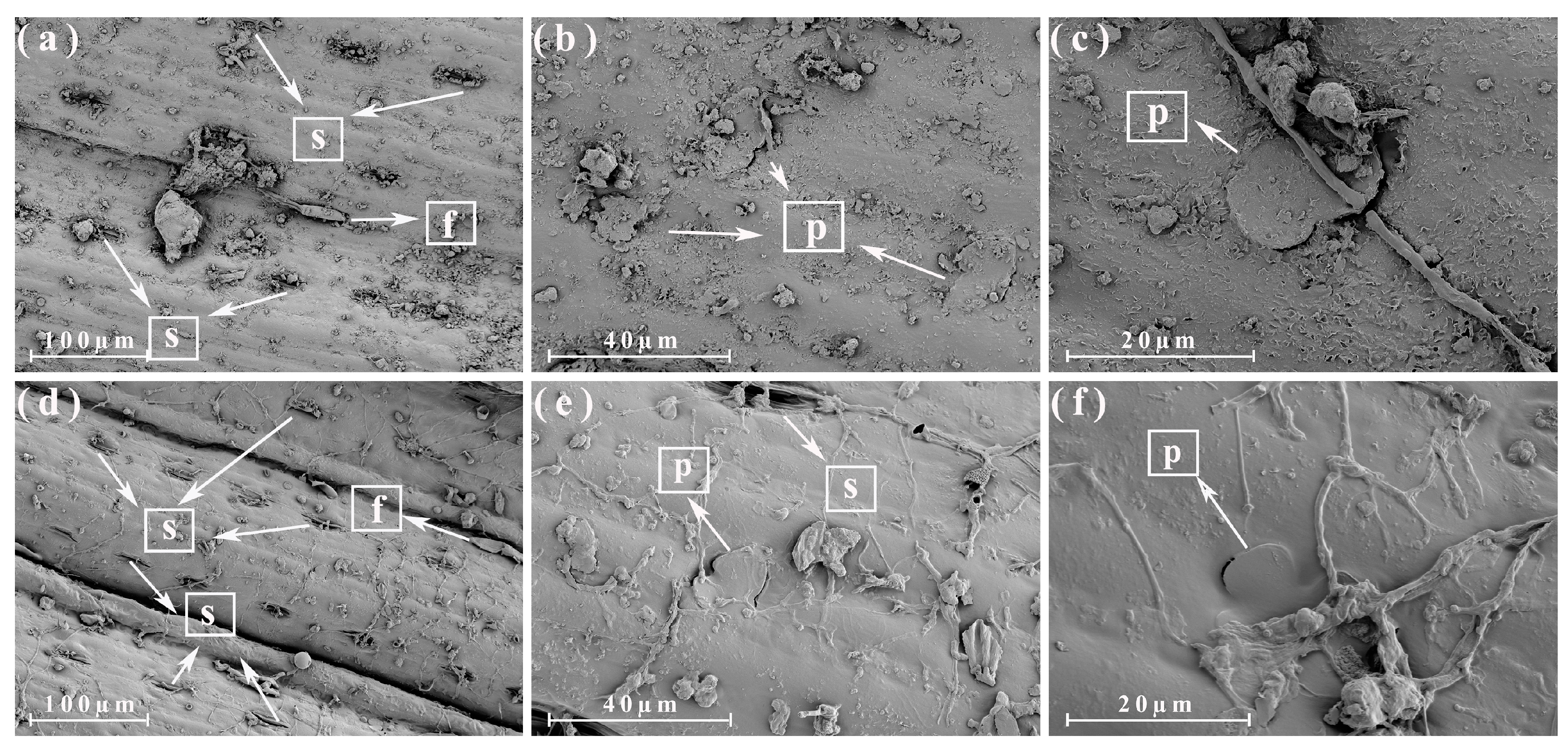
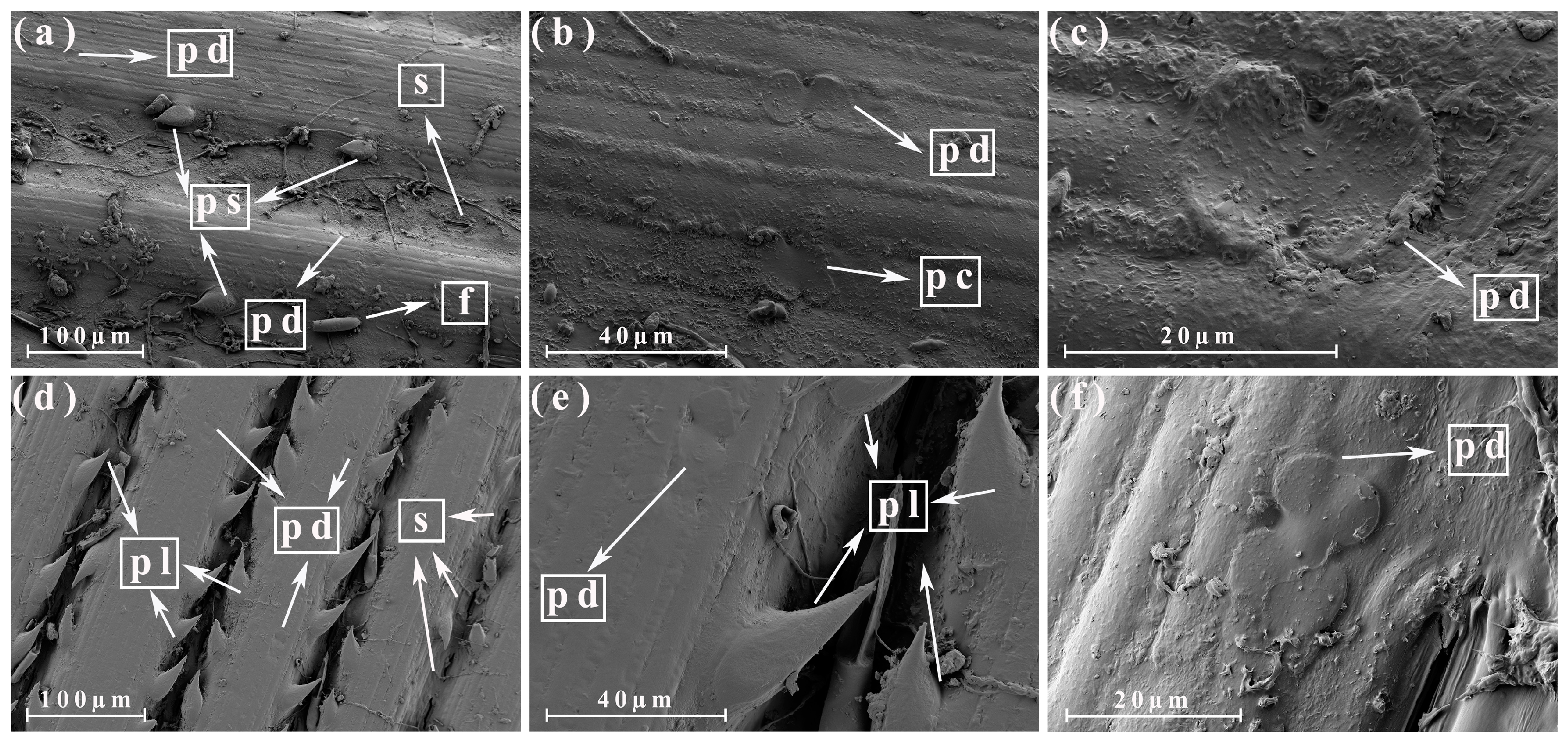
3.3. Morphological Analysis of the Different Parts of the Energy Cane and Sugarcane
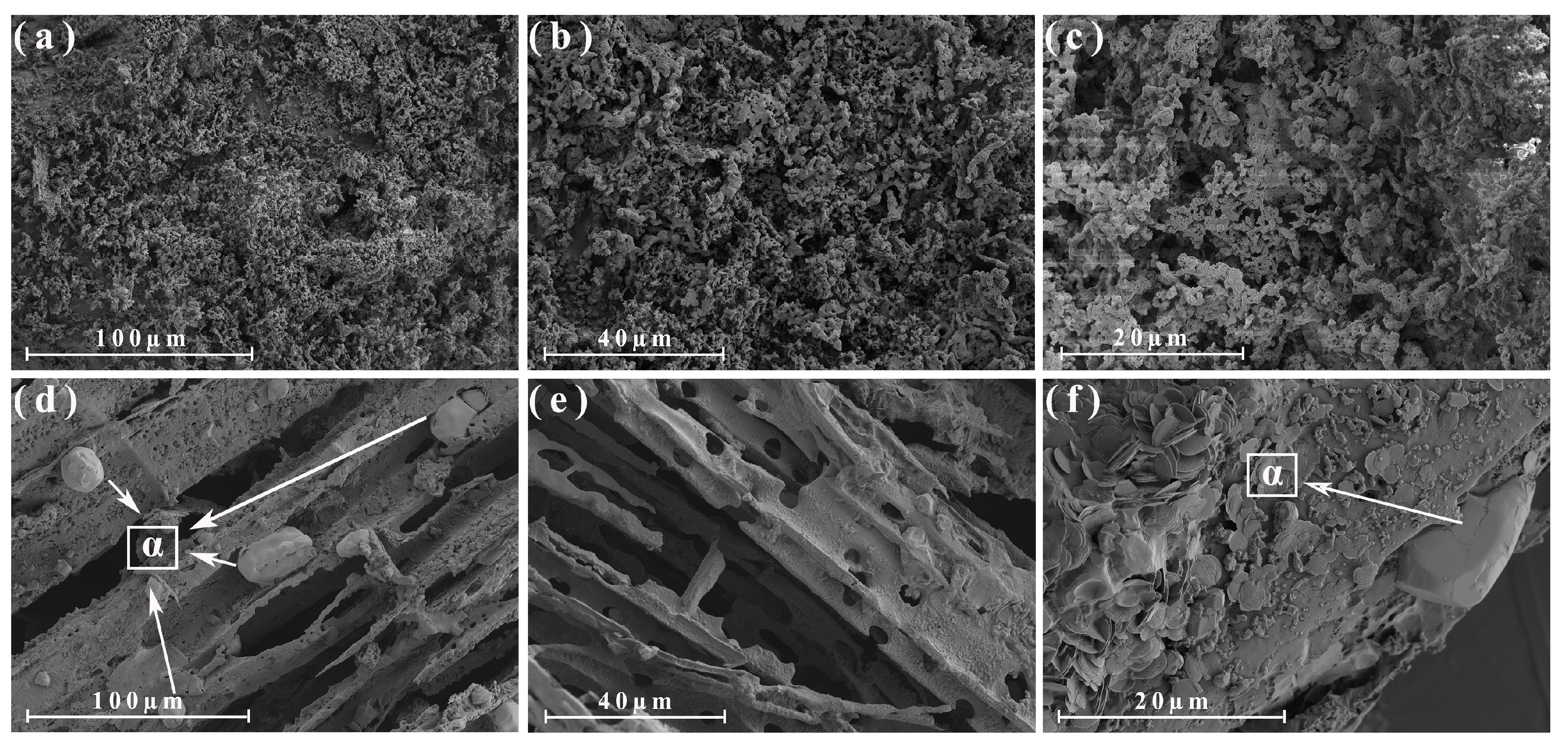
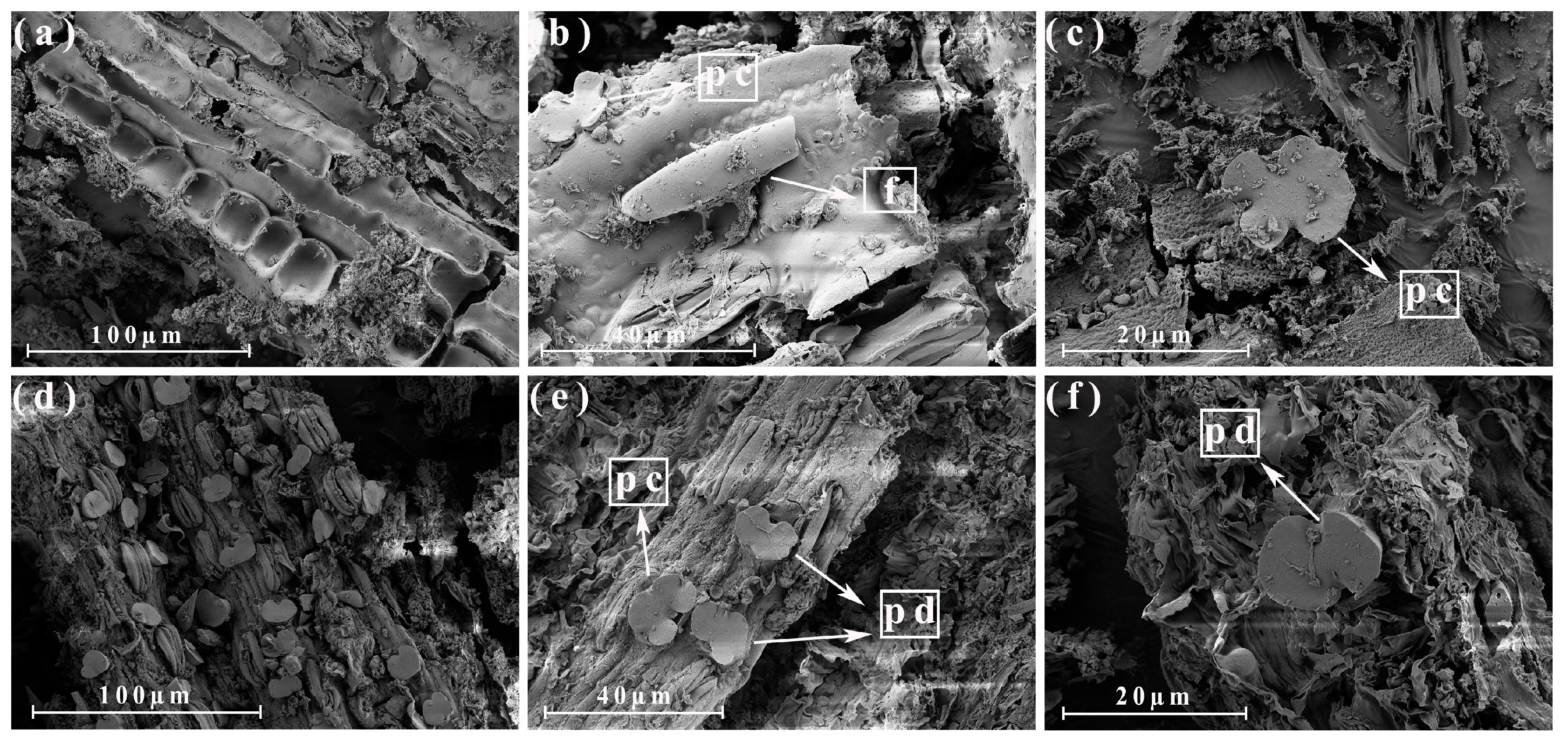
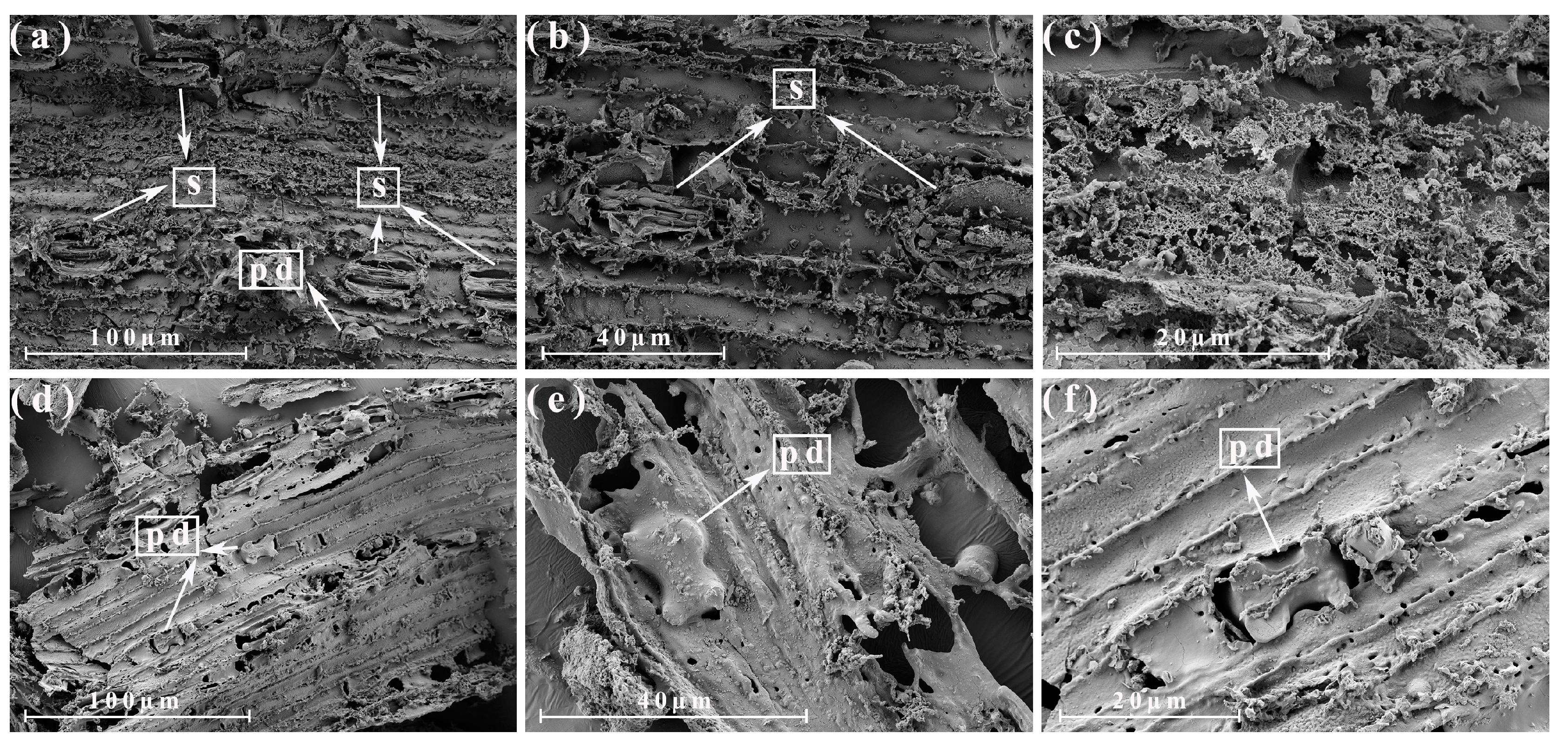
3.4. Morphological Analysis of the Different Parts of the Energy Cane Ash and Sugarcane Ash
3.5. Chemical Determination of the Ash
3.6. Particle Size Distribution of the Ash
3.7. Specific Mass of the Ash
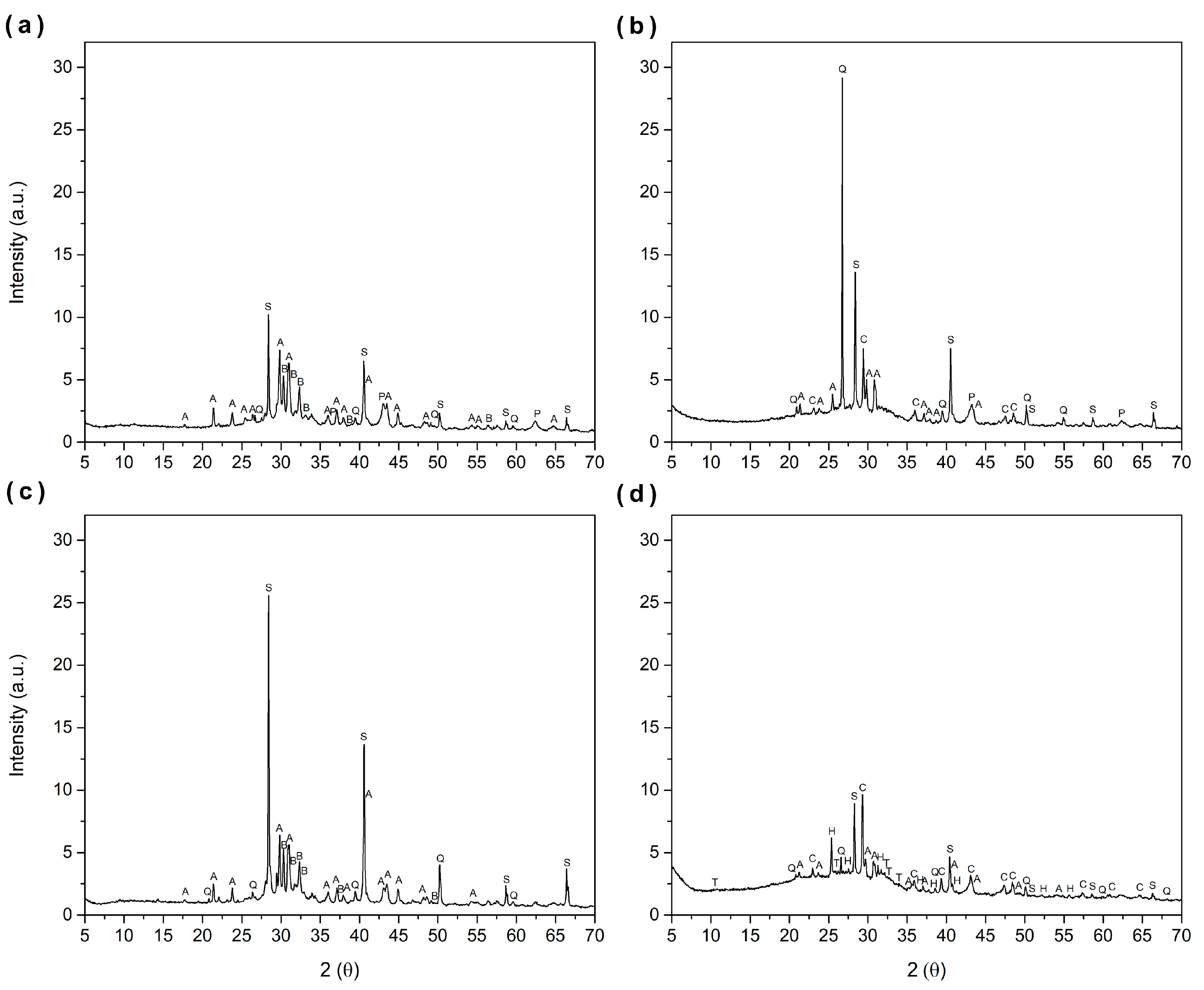
3.8. Mineralogical Composition Analysis of the Ash
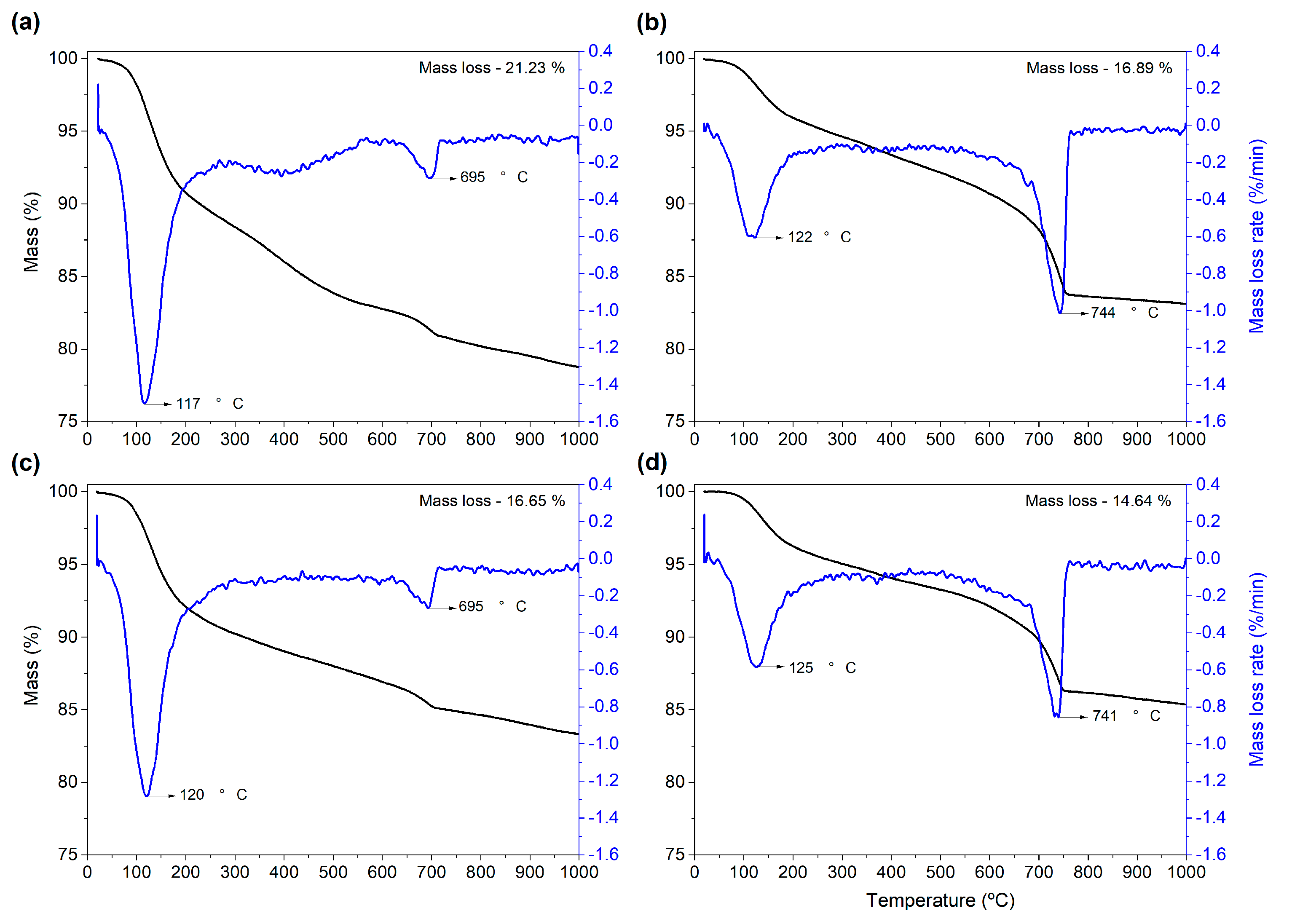
3.9. Thermal Analyses of the Ash
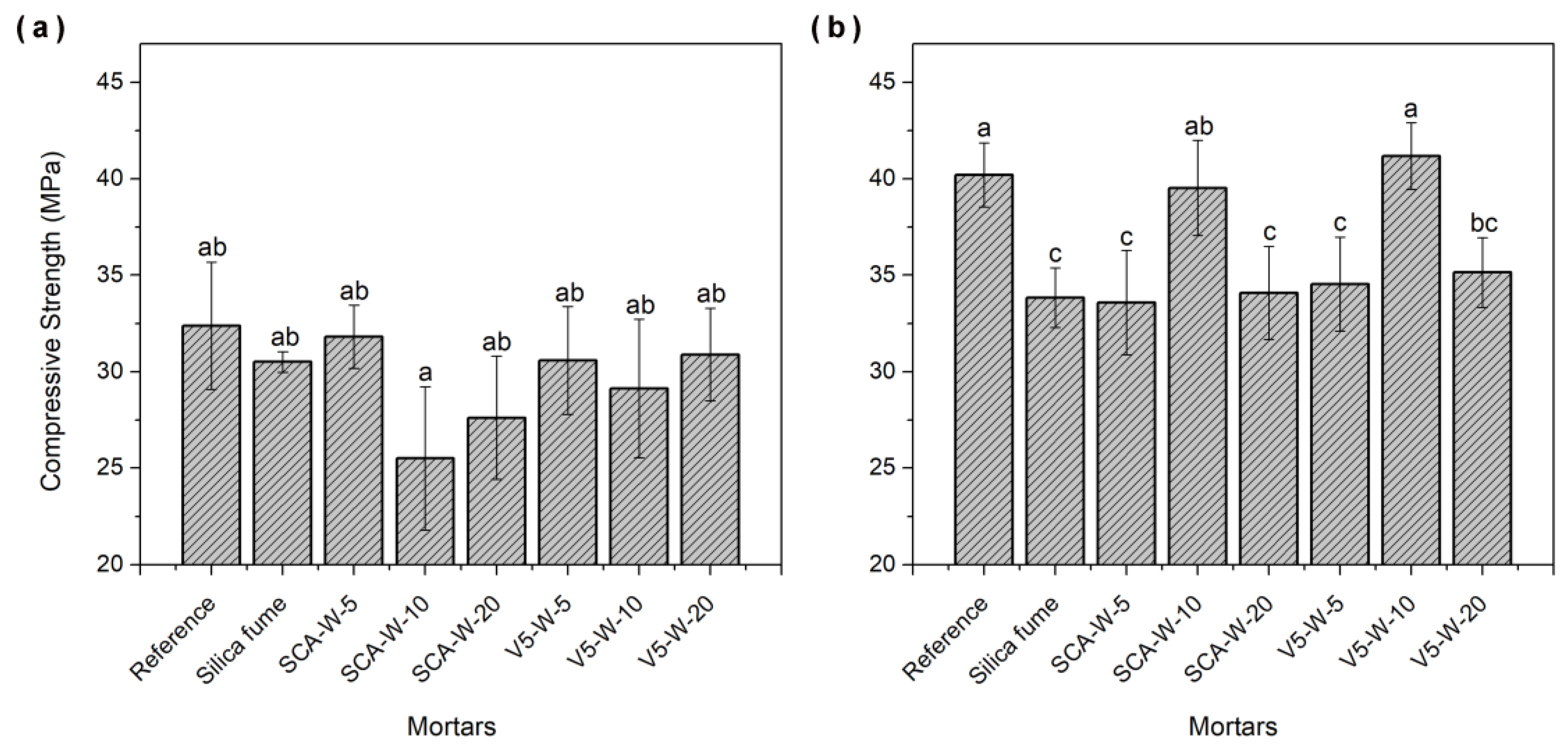
3.10. Compressive Strength of Mortars
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richard, E.P.; Anderson, W.F. Sugarcane, Energy Cane and Napier Grass. In Cellulosic Energy Cropping Systems; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 91–108. [Google Scholar]
- de Abreu, L.G.F.; Silva, N.V.; Ferrari, A.J.R.; de Carvalho, L.M.; Fiamenghi, M.B.; Carazzolle, M.F.; Fill, T.P.; Pilau, E.J.; Pereira, G.A.G.; Grassi, M.C.B. Metabolite Profiles of Energy Cane and Sugarcane Reveal Different Strategies during the Axillary Bud Outgrowth. Plant Physiol. Biochem. 2021, 167, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Viator, R.P.; Richard, E.P. Sugar and Energy Cane Date of Planting Effects on Cane, Sucrose, and Fiber Yields. Biomass Bioenergy 2012, 40, 82–85. [Google Scholar] [CrossRef]
- Alexander, A.G. Sugar Cane Physiology: Comprehensive Study of the Saccharum Source-to-Sink System; Elsevier: Amsterdam, The Netherlands, 1973; ISBN 0444410163. [Google Scholar]
- Byrt, C.S.; Grof, C.P.L.; Furbank, R.T. C4 Plants as Biofuel Feedstocks: Optimising Biomass Production and Feedstock Quality from a Lignocellulosic Perspective. J. Integr. Plant Biol. 2011, 53, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Kennedy, A.J.; dos Santos, E.G.D.; Tomazela, A.L.; Rubio, L.C.S. Energy Cane: Its Concept, Development, Characteristics, and Prospects. Adv. Bot. 2014, 2014, 597275. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2020; FAO: Rome, Italy, 2020; ISBN 9789251333945. [Google Scholar]
- Companhia Nacional de Abastecimento (CONAB) Produção de Cana-de-Açúcar Na Safra 2023/24 Chega a 713,2 Milhões de Toneladas, a Maior da Série Histórica. Available online: https://www.conab.gov.br/ultimas-noticias/5489-producao-de-cana-de-acucar-na-safra-2023-24-chega-a-713-2-milhoes-de-toneladas-a-maior-da-serie-historica#:~:text=Nestecenário%2Caproduçãototal,delitrosdeetanolhidratado (accessed on 22 August 2024).
- Setayesh Gar, P.; Suresh, N.; Bindiganavile, V. Sugar Cane Bagasse Ash as a Pozzolanic Admixture in Concrete for Resistance to Sustained Elevated Temperatures. Constr. Build. Mater. 2017, 153, 929–936. [Google Scholar] [CrossRef]
- Eggleston, G.; Lima, I. Sustainability Issues and Opportunities in the Sugar and Sugar-Bioproduct Industries. Sustainability 2015, 7, 12209–12235. [Google Scholar] [CrossRef]
- Empresa de Pesquisa Energética (EPE). National Energy Balance—BEN 2023; Empresa de Pesquisa Energética (EPE): Rio de Janeiro, Brazil, 2023. [Google Scholar]
- Grassi, M.C.B.; Pereira, G.A.G. Energy-Cane and RenovaBio: Brazilian Vectors to Boost the Development of Biofuels. Ind. Crops Prod. 2019, 129, 201–205. [Google Scholar] [CrossRef]
- Pesonen, J.; Kuokkanen, V.; Kuokkanen, T.; Illikainen, M. Co-Granulation of Bio-Ash with Sewage Sludge and Lime for Fertilizer Use. J. Environ. Chem. Eng. 2016, 4, 4817–4821. [Google Scholar] [CrossRef]
- Joshaghani, A.; Moeini, M.A. Evaluating the Effects of Sugar Cane Bagasse Ash (SCBA) and Nanosilica on the Mechanical and Durability Properties of Mortar. Constr. Build. Mater. 2017, 152, 818–831. [Google Scholar] [CrossRef]
- Frías, M.; Villar, E.; Savastano, H. Brazilian Sugar Cane Bagasse Ashes from the Cogeneration Industry as Active Pozzolans for Cement Manufacture. Cem. Concr. Compos. 2011, 33, 490–496. [Google Scholar] [CrossRef]
- Cordeiro, G.C.; Toledo Filho, R.D.; Fairbairn, E.D.M.R. Characterization of Sugar Cane Bagasse Ash for Use as Pozzolan in Cementitious Materials. Quim. Nova 2009, 32, 82–86. [Google Scholar] [CrossRef]
- Matsuoka, S.; Rubio, L.; Santos, E. The Evolution of Pro-Alcohol; Agroanalysis: São Paulo, Brazil, 2016; pp. 29–30. [Google Scholar]
- Salassi, M.E.; Brown, K.; Hilbun, B.M.; Deliberto, M.A.; Gravois, K.A.; Mark, T.B.; Falconer, L.L. Farm-Scale Cost of Producing Perennial Energy Cane as a Biofuel Feedstock. Bioenergy Res. 2014, 7, 609–619. [Google Scholar] [CrossRef]
- Santchurn, D.; Badaloo, M.G.H.; Zhou, M.; Labuschagne, M.T. Contribution of Sugarcane Crop Wild Relatives in the Creation of Improved Varieties in Mauritius. Plant Genet. Resour. Characterisation Util. 2019, 17, 151–163. [Google Scholar] [CrossRef]
- Shields, S.; Boopathy, R. Ethanol Production from Lignocellulosic Biomass of Energy Cane. Int. Biodeterior. Biodegrad. 2011, 65, 142–146. [Google Scholar] [CrossRef]
- Suhardi, V.S.H.; Prasai, B.; Samaha, D.; Boopathy, R. Evaluation of Pretreatment Methods for Lignocellulosic Ethanol Production from Energy Cane Variety L 79-1002. Int. Biodeterior. Biodegrad. 2013, 85, 683–687. [Google Scholar] [CrossRef]
- Thammasittirong, S.N.R.; Chatwachirawong, P.; Chamduang, T.; Thammasittirong, A. Evaluation of Ethanol Production from Sugar and Lignocellulosic Part of Energy Cane. Ind. Crops Prod. 2017, 108, 598–603. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, V.; Biriș, S.-Ș. Sustainable Valorization of Waste and By-Products from Sugarcane Processing. Sustainability 2022, 14, 11089. [Google Scholar] [CrossRef]
- da Silva, J.A. The Importance of the Wild Cane Saccharum Spontaneum for Bioenergy Genetic Breeding. Sugar Tech 2017, 19, 229–240. [Google Scholar] [CrossRef]
- Kell, D.B. Breeding Crop Plants with Deep Roots: Their Role in Sustainable Carbon, Nutrient and Water Sequestration. Ann. Bot. 2011, 108, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Netto, O.V.; Bressiani, J.A.; Soriano, H.L.; Fiori, C.S.; Santos, J.M.; Barbosa, G.V.; Xavier, M.A.; Landell, M.G.; Pereira, G.A. The Potential of the Energy Cane as the Main Biomass Crop for the Cellulosic Industry. Chem. Biol. Technol. Agric. 2014, 1, 20. [Google Scholar] [CrossRef]
- de Abreu, L.G.F.; Grassi, M.C.B.; de Carvalho, L.M.; da Silva, J.J.B.; Oliveira, J.V.C.; Bressiani, J.A.; Pereira, G.A.G. Energy Cane vs Sugarcane: Watching the Race in Plant Development. Ind. Crops Prod. 2020, 156, 112868. [Google Scholar] [CrossRef]
- Fanelli, A.; Reinhardt, L.; Matsuoka, S.; Ferraz, A.; da Franca Silva, T.; Hatfield, R.D.; Romanel, E. Biomass Composition of Two New Energy Cane Cultivars Compared with Their Ancestral Saccharum Spontaneum during Internode Development. Biomass Bioenergy 2020, 141, 105696. [Google Scholar] [CrossRef]
- Kim, M.; Day, D.F. Composition of Sugar Cane, Energy Cane, and Sweet Sorghum Suitable for Ethanol Production at Louisiana Sugar Mills. J. Ind. Microbiol. Biotechnol. 2011, 38, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Gravois, K.A.; Milligan, S.B. Genetic Relationship between Fiber and Sugarcane Yield Components. Crop Sci. 1992, 32, 62–687. [Google Scholar] [CrossRef]
- Wang, L.; Czedik-Eysenberg, A.; Mertz, R.A.; Si, Y.; Tohge, T.; Nunes-Nesi, A.; Arrivault, S.; Dedow, L.K.; Bryant, D.W.; Zhou, W.; et al. Comparative Analyses of C4 and C3 Photosynthesis in Developing Leaves of Maize and Rice. Nat. Biotechnol. 2014, 32, 1158–1164. [Google Scholar] [CrossRef]
- Matsuoka, S.; Bressiani, J.; Maccheroni, W.; Fouto, I. Sugarcane Bioenergy. In Sugarcane; Santos, F., Borém, A., Caldas, C., Eds.; Academic Press: Cambridge, MA, USA, 2012; ISBN 978-85-60249-39-8. [Google Scholar]
- Carvalho, V.S.d.; Tannous, K. Thermal Decomposition Kinetics Modeling of Energy Cane Saccharum Robustum. Thermochim. Acta 2017, 657, 56–65. [Google Scholar] [CrossRef]
- Sagastume Gutiérrez, A.; Cabello Eras, J.J.; Huisingh, D.; Vandecasteele, C.; Hens, L. The Current Potential of Low-Carbon Economy and Biomass-Based Electricity in Cuba. The Case of Sugarcane, Energy Cane and Marabu (Dichrostachys Cinerea) as Biomass Sources. J. Clean. Prod. 2018, 172, 2108–2122. [Google Scholar] [CrossRef]
- Farinha, C.B.; Silvestre, J.D.; de Brito, J.; Veiga, M.d.R. Life Cycle Assessment of Mortars with Incorporation of Industrial Wastes. Fibers 2019, 7, 59. [Google Scholar] [CrossRef]
- Mehta, P.K. Pozzolanic and Cementitious By-Products in Concrete—Another Look; American Concrete Institute: Farmington Hills, MI, USA, 1989. [Google Scholar]
- Cordeiro, G.C.; Toledo Filho, R.D.; Fairbairn, E.M.R. Effect of Calcination Temperature on the Pozzolanic Activity of Sugar Cane Bagasse Ash. Constr. Build. Mater. 2009, 23, 3301–3303. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.-D. Benefits of Plant Silicon for Crops: A Review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Kazmi, S.M.S.; Munir, M.J.; Patnaikuni, I.; Wu, Y.-F. Pozzolanic Reaction of Sugarcane Bagasse Ash and Its Role in Controlling Alkali Silica Reaction. Constr. Build. Mater. 2017, 148, 231–240. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon Uptake and Accumulation in Higher Plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Piperno, D.R. Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists; AltaMira P.: Oxford, UK, 2006. [Google Scholar]
- Wilding, L.P.; Drees, L.R. Biogenic Opal in Ohio Soils. Soil Sci. Soc. Am. J. 1971, 35, 1004–1010. [Google Scholar] [CrossRef]
- de Siqueira, A.A.; Cordeiro, G.C. Sustainable Cements Containing Sugarcane Bagasse Ash and Limestone: Effects on Compressive Strength and Acid Attack of Mortar. Sustainability 2022, 14, 5683. [Google Scholar] [CrossRef]
- Kolawole, J.T.; Babafemi, A.J.; Fanijo, E.; Chandra Paul, S.; Combrinck, R. State-of-the-Art Review on the Use of Sugarcane Bagasse Ash in Cementitious Materials. Cem. Concr. Compos. 2021, 118, 103975. [Google Scholar] [CrossRef]
- Van Raij, B.; de Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Chemical Analysis to Evaluate the Fertility of Tropical Soils; Instituto Agronômico: Campinas, Brazil, 2001; ISBN 8585564059. [Google Scholar]
- Rossi, M. Pedological Map of the State of São Paulo. Revised and Expanded; Florestal, I., Ed.; Instituto Florestal: São Paulo, Brazil, 2017; Volume 1, ISBN 978-75-64808-16-4. [Google Scholar]
- American Society for Testing and Materials (ASTM). C114: Standard Test Methods for Chemical Analysis of Hydraulic Cement; American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2018; p. 33. [Google Scholar]
- Lyra, G.P.; Borrachero, M.V.; Soriano, L.; Payá, J.; Rossignolo, J.A. Comparison of Original and Washed Pure Sugar Cane Bagasse Ashes as Supplementary Cementing Materials. Constr. Build. Mater. 2021, 272, 122001. [Google Scholar] [CrossRef]
- Teixeira, E.R.; Mateus, R.; Camões, A.; Branco, F.G. Quality and Durability Properties and Life-Cycle Assessment of High Volume Biomass Fly Ash Mortar. Constr. Build. Mater. 2019, 197, 195–207. [Google Scholar] [CrossRef]
- ABNT NBR 5739; Concrete—Compression Test of Cylindrical Specimens. Associação Brasileira de Normas Técnicas: São Paulo, Brazil, 2018; p. 9.
- Roselló, J.; Soriano, L.; Santamarina, M.P.; Akasaki, J.L.; Melges, J.L.P.; Payá, J. Microscopy Characterization of Silica-Rich Agrowastes to Be Used in Cement Binders: Bamboo and Sugarcane Leaves. Microsc. Microanal. 2015, 21, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach, 2nd ed.; Wiley: New York, NY, USA, 1995; ISBN 978-0-471-58747-7. [Google Scholar]
- de Andrade, A.F.; Flores, R.A.; Casaroli, D.; Bueno, A.M.; Pessoa-de-Souza, M.A.; de Lima, F.S.R.; Marques, E.P. K Dynamics in the Soil–Plant System for Sugarcane Crops: A Current Field Experiment Under Tropical Conditions. Sugar Tech 2021, 23, 1247–1257. [Google Scholar] [CrossRef]
- Malavolta, E. Mineral Nutrition Elements of Plants; Ceres, A., Ed.; Sociedade Brasileira de Ciência do Solo: São Paulo, Brazil, 1980. [Google Scholar]
- International Plant Nutrition Institute. Agronomic Information on Plant Nutrients; IPNI: Piracicaba, Brazil, 2020. [Google Scholar]
- Watanabe, K.; Fukuzawa, Y.; Kawasaki, S.I.; Ueno, M.; Kawamitsu, Y. Effects of Potassium Chloride and Potassium Sulfate on Sucrose Concentration in Sugarcane Juice Under Pot Conditions. Sugar Tech 2016, 18, 258–265. [Google Scholar] [CrossRef]
- Diamond, S. A Review of Alkali-Silica Reaction and Expansion Mechanisms 1. Alkalies in Cements and in Concrete Pore Solutions. Cem. Concr. Res. 1975, 5, 329–345. [Google Scholar] [CrossRef]
- John, V.M.; Cincotto, M.A.; Silva, M.G. Ash and Alternative Binders. In Tecnologia e Materiais Alternativos de Construção; Freire, W.J., Beraldo, A.L., Eds.; UNICAMP: Campinas, Brazil, 2003; pp. 145–190. [Google Scholar]
- Marschner, P. Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9780123849052. [Google Scholar]
- Jensen, O.M.; Hansen, P.F.; Coats, A.M.; Glasser, F.P. Chloride Ingress in Cement Paste and Mortar. Cem. Concr. Res. 1999, 29, 1497–1504. [Google Scholar] [CrossRef]
- Mehta, P.K. Durability of Concrete in Marine Environment—A Review. ACI Symp. Publ. 1982, 65, 1–20. [Google Scholar]
- de Castro, A.L.; Pandolfelli, V.C. Review: Concepts of Particle Dispersion and Packing for Special Concretes Production. Cerâmica 2009, 55, 18–32. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers, 8th ed.; Pearson: New York, NY, USA, 2021; ISBN 978-0135033739. [Google Scholar]
- Agarie, S.; Hanaoka, N.; Ueno, O.; Miyazaki, A.; Kubota, F.; Agata, W.; Kaufman, P.B. Effects of Silicon on Tolerance to Water Deficit and Heat Stress in Rice Plants (Oryza sativa L.), Monitored by Electrolyte Leakage. Plant Prod. Sci. 1998, 1, 96–103. [Google Scholar] [CrossRef]
- Yoshida, S.; Ohnishi, Y.; Kitagishi, K. Chemical Forms, Mobility and Deposition of Silicon in Rice Plant. Soil Sci. Plant Nutr. 1962, 8, 15–21. [Google Scholar] [CrossRef]
- Takahashi, E. Uptake Mode and Physiological Functions of Silica. In Science of Rice Plant Physiology; Matsuo, T., Kumazawa, K., Ishii, R., Ishihara, K., Hirata, H., Eds.; Nobunkyo: Tokio, Japan, 1995; pp. 420–433. [Google Scholar]
- Balasta, M.L.F.C.; Perez, C.M.; Juliano, B.O.; Vlllareal, C.P.; Lott, J.N.A.; Roxas, D.B. Effects of Silica Level on Some Properties of Oryza Sativa Straw and Hull. Can. J. Bot. 1989, 67, 2356–2363. [Google Scholar] [CrossRef]
- Moraes, J.C.B.; Cordeiro, G.C.; Akasaki, J.L.; Vieira, A.P.; Payá, J. Improving the Reactivity of a Former Ground Sugarcane Bagasse Ash Produced by Autogenous Combustion through Employment of Two Different Additional Grinding Procedures. Constr. Build. Mater. 2021, 270, 121471. [Google Scholar] [CrossRef]
- ABNT NBR 12653; Pozzolanic Materials—Requirements. Associação Brasileira de Normas Técnicas: São Paulo, Brazil, 2014.
- Imoisili, P.E.; Ukoba, K.O.; Jen, T.C. Synthesis and Characterization of Amorphous Mesoporous Silica from Palm Kernel Shell Ash. Bol. La Soc. Esp. Ceram. Y Vidr. 2020, 59, 159–164. [Google Scholar] [CrossRef]

| Elements | Units | Energy Cane | Sugarcane | Energy Cane | Sugarcane |
|---|---|---|---|---|---|
| 0–20 cm | 20–40 cm | ||||
| pH | CaCl2 | 5.6 | 5.4 | 5.3 | 5.3 |
| P resin | mg∙m−3 | 36 | 16 | 7 | 13 |
| S | 12 | 10 | 34 | 11 | |
| K resin | m molc∙dm−3 | 4 | 2.9 | 2.8 | 2.7 |
| Ca | 38 | 26 | 25 | 23 | |
| Mg | 19 | 6 | 9 | 4 | |
| Al | 0 | 0 | 0 | 0 | |
| H + Al | 26 | 21 | 24 | 20 | |
| MO a | g∙kg−1 | 23 | 16 | 18 | 16 |
| COT b | 13.2 | 9.6 | 10.1 | 9.1 | |
| B | mg∙dm−3 | 0.84 | 0.32 | 0.59 | 0.3 |
| Cu | 8.4 | 0.9 | 1.8 | 1.2 | |
| Fe | 38 | 32 | 23 | 35 | |
| Mn | 16.1 | 1.7 | 2.1 | 3.1 | |
| Zn | 9.3 | 3.9 | 0.3 | 3.5 | |
| SB c | m molc∙dm−3 | 61 | 35 | 37 | 30 |
| CTC d | 87 | 56 | 60 | 50 | |
| V e | % | 70 | 62 | 61 | 59 |
| Mortars | Acronyms |
|---|---|
| Mortar Reference | Reference |
| Mortar with 10% Silica fume | Silica fume |
| Mortar with 5% sugarcane ash | SCA-W-5 |
| Mortar with 10% sugarcane ash | SCA-W-10 |
| Mortar with 20% sugarcane ash | SCA-W-20 |
| Mortar with 5% energy cane ash | V5-W-5 |
| Mortar with 10% energy cane ash | V5-W-10 |
| Mortar with 20% energy cane ash | V5-W-20 |
| Oxides (%) | Stalk | Leaf | Mixed Ash (Stalk + Leaf) | |||
|---|---|---|---|---|---|---|
| Sugarcane | Energy Cane | Sugarcane | Energy Cane | Sugarcane | Energy Cane | |
| MgO | 9.9 | 3.86 | 12.65 | 8.48 | 11.34 | 6.43 |
| SiO2 | 6.34 | 4.46 | 28.47 | 45.73 | 18.82 | 27.11 |
| P2O5 | 6.09 | 5.51 | 2.97 | 2.87 | 4.42 | 4.18 |
| SO3 | 10.17 | 4.21 | 13.08 | 5.56 | 11.93 | 5.01 |
| K2O | 62.87 | 47.48 | 19.07 | 11.37 | 38.29 | 27.75 |
| CaO | 0 | 6.41 | 20.86 | 21.42 | 11.6 | 14.64 |
| TiO2 | 0 | 0 | 0 | 0.21 | 0 | 0.11 |
| MnO | 0 | 0.06 | 0.31 | 0.25 | 0.17 | 0.17 |
| Fe2O3 | 0.06 | 0.12 | 0.42 | 1.13 | 0.27 | 0.67 |
| BaO | 0 | 0 | 0.18 | 0.1 | 0.1 | 0.06 |
| Cl | 4.3 | 27.5 | 1.82 | 2.66 | 2.85 | 13.58 |
| LOI | 0.27 | 0.39 | 0.17 | 0.22 | 0.21 | 0.30 |
| Washed | ||||||
|---|---|---|---|---|---|---|
| Oxides (%) | Stalk | Leaf | Mixed Ash (Stalk + Leaf) | |||
| Sugarcane | Energy Cane | Sugarcane | Energy Cane | Sugarcane | Energy Cane | |
| MgO | 26.96 | 20.15 | 9.93 | 5.12 | 16.02 | 10.75 |
| SiO2 | 34.92 | 40.67 | 60.59 | 65.16 | 51.42 | 58.49 |
| P2O5 | 9.19 | 15.97 | 2.74 | 2.5 | 5.04 | 6.36 |
| SO3 | 0 | 0 | 0.77 | 0.92 | 0.5 | 0.66 |
| K2O | 7.36 | 5.39 | 5.16 | 6.35 | 5.95 | 4.5 |
| CaO | 17.76 | 13.56 | 16.77 | 16.58 | 17.12 | 15.39 |
| TiO2 | 0.27 | 0.75 | 0.42 | 0.43 | 0.37 | 1.2 |
| MnO | 0.62 | 0.23 | 0.5 | 0.22 | 0.54 | 0.25 |
| Fe2O3 | 2.23 | 0.53 | 2.36 | 1.78 | 2.22 | 1.69 |
| BaO | 0 | 0 | 0 | 0 | 0.09 | 0 |
| ZnO | 0 | 0 | 0.27 | 0.13 | 0.32 | 1.44 |
| Cl | 0.17 | 2.29 | 0.41 | 0.46 | 0.1 | 0.04 |
| LOI | 0.25 | 0.33 | 0.35 | 0.47 | 0.31 | 0.43 |
| Ashes | *D10 (μm) | *D50 (μm) | *D90 (μm) | *Dmea (μm) |
|---|---|---|---|---|
| SCA-S | 0.08 | 11.55 | 179.65 | 51.38 |
| SCA-L | 9.95 | 49.64 | 170.97 | 67.04 |
| V5-S | 0.12 | 19.41 | 133.91 | 40.15 |
| V5-L | 9.37 | 47.34 | 164.34 | 65.03 |
| Ashes | Abbreviation | Mineralogical Name | Chemical Formula | PDFcard |
|---|---|---|---|---|
| SCA-S | S | Sylvine | KCl | 411476 |
| A | Arcanite | K2SO4 | 50613 | |
| Q | Quartz | SiO2 | 331161 | |
| B | Potassium oxide | K2O | 270431 | |
| P | Periclase | MgO | 40289 | |
| SCA-L | S | Sylvine | KCl | 411476 |
| A | Arcanite | K2SO4 | 50613 | |
| Q | Quartz | SiO2 | 331161 | |
| P | Periclase | MgO | 40289 | |
| C | Calcite | CaCO3 | 50586 | |
| V5-S | S | Sylvine | KCl | 411476 |
| A | Arcanite | K2SO4 | 50613 | |
| Q | Quartz | SiO2 | 331161 | |
| B | Potassium oxide | K2O | 270431 | |
| V5-L | S | Sylvine | KCl | 411476 |
| A | Arcanite | K2SO4 | 50613 | |
| Q | Quartz | SiO2 | 331161 | |
| C | Calcite | CaCO3 | 50586 | |
| H | Anhydrite | CaSO4 | 371496 | |
| T | Hydroxyapatite | Ca5(PO4)3(OH) | 90432 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyra, G.P.; Brichi, L.; Roselló, J.; Borrachero, M.V.; Soriano, L.; Payá, J.; Rossignolo, J.A. Energy Cane Ash: Property Assessment for Its Valorization in Sustainable Cementing Systems. Sustainability 2025, 17, 803. https://doi.org/10.3390/su17020803
Lyra GP, Brichi L, Roselló J, Borrachero MV, Soriano L, Payá J, Rossignolo JA. Energy Cane Ash: Property Assessment for Its Valorization in Sustainable Cementing Systems. Sustainability. 2025; 17(2):803. https://doi.org/10.3390/su17020803
Chicago/Turabian StyleLyra, Gabriela Pitolli, Lisiane Brichi, Josefa Roselló, María Victoria Borrachero, Lourdes Soriano, Jordi Payá, and João Adriano Rossignolo. 2025. "Energy Cane Ash: Property Assessment for Its Valorization in Sustainable Cementing Systems" Sustainability 17, no. 2: 803. https://doi.org/10.3390/su17020803
APA StyleLyra, G. P., Brichi, L., Roselló, J., Borrachero, M. V., Soriano, L., Payá, J., & Rossignolo, J. A. (2025). Energy Cane Ash: Property Assessment for Its Valorization in Sustainable Cementing Systems. Sustainability, 17(2), 803. https://doi.org/10.3390/su17020803











