Abstract
Global population growth generates problems relating to increasing demand for sustainable energy and waste treatment. Proper solid waste management promotes material reuse, maximizes recovery and reduces anthropological pressure on natural resources. Anaerobic digestion (AD) is an alternative method of stabilizing organic substrates and generating biogas as a source of environmentally friendly energy. In addition, digestate is not only a waste product of that process but also a renewable resource with many potential applications. The circular economy concept encourages the use of digestate as a source of nutrients that promotes plant growth and improves soil properties. However, the stabilized substrates often contain various contaminants, including heavy metals (HMs) and antibiotics that are also detected in digestate. Therefore, the agricultural use of digestate obtained by AD could increase the pool of these pollutants in soil and water environments and contribute to their circulation in these ecosystems. Moreover, digestate may also increase the co-selection of genes determining resistance to HMs and antibiotics in environmental microorganisms. This article comprehensively reviews published data on the residues of various HMs and antimicrobial substances in different digestates around the world and maps the scope of the problem. Moreover, the potential risk of residual levels of these contaminants in digestate has also been evaluated. The review highlights the lack of legal standards regulating the concentrations of drugs introduced into the soil with digestate. The results of the ecological risk assessment indicate that the presence of medically important antimicrobials in digestate products, especially those used in agriculture, should be limited.
1. Introduction
Due to the intensification of agriculture, progressing urbanization, and human population growth, the volumes of waste generated worldwide have become a significant burden on the natural environment [1]. The global production of various types of organic waste, including agricultural residues, animal manure, food waste, and sewage sludge, is estimated at 2, 120, 1.3, and 16.4 billion tons per year, respectively [2]. The global volume of organic waste, as well as the consumption and demand for energy, continues to increase. Organic biomass is biodegradable and can be used for energy generation under anaerobic conditions. During anaerobic digestion (AD), various organic wastes are converted into biogas—an environmentally friendly and renewable energy source that can be used to produce vehicle fuel, electricity, or heat. In recent years, AD of waste from agriculture, industry, and municipal facilities has become one of the most promising pathways for renewable energy generation [3]. Sustainable management of organic waste plays an important role in the circular economy by promoting material reuse and maximizing recovery. Energy recovery from organic waste provides environmental benefits by decreasing the following: (1) greenhouse gas emissions; (2) fossil fuel use; (3) water pollution; and (4) the volume of landfilled waste. Moreover, the AD process offers an additional advantage by promoting the simultaneous recovery of material in the form of digestate [4,5]. While biogas as a renewable energy source is discussed intensively, digestate still plays a minor role in political and scientific debates.
Digestate is a stable and valuable by-product of the AD process that is rich in nutrients. It includes a balanced mixture of micro- and macronutrients which are essential for plant growth [6]. Digestate generated by AD can be returned to farms that supply substrates to biogas plants, sold on the market or recirculated [7]. The use of digestate as fertilizer or soil improver offers one of the simplest solutions for managing this by-product, improving the economic sustainability of biogas production and minimizing its negative environmental impacts. Digestate obtained by AD is more stable, hygienic, and abundant in bioavailable nitrogen (in the form of N-NH4) than undigested organic biomass. It is suitable for agricultural use due to specific properties that can improve the chemical and physical properties of soil, including soil structure, moisture retention, microbial activity, and organic carbon content. Moreover, the recovery of nutrients (such as nitrogen and phosphorus) limits soil erosion. Ultimately, digestate enhances soil quality and fertility. The agricultural use of digestate offers an excellent alternative to reducing the use of chemical fertilizers [5,8].
However, despite these advantages, the introduction of digestate into the soil environment entails some risks. In Europe, agricultural residues and animal manure are the dominant feedstocks for AD. Due to various waste management options and the legal restrictions related to the climate and energy policy, all types of organic waste and by-products are being increasingly used as substrates in biogas production. For this reason, sewage sludge, biowaste, and industrial waste are also commonly processed by AD [6]. It should be emphasized that the specific properties of the obtained digestate are influenced mainly by the type of substrates used in the AD process. Digestate composition and quality are determined by the composition and quality of the feedstock, as well as the efficiency of the AD process [9]. The substrate should be free of contaminants to prevent undesirable substances from reaching the digestate. There are concerns regarding the presence of hazardous compounds, especially heavy metals (HMs) and antibiotics, in digestates obtained from animal, household, or industrial wastes [10,11,12]. These types of pollutants are most widely encountered in animal manure and sewage sludge [13].
Heavy metals are widely distributed in wastewater and animal feed, and they are present in sewage sludge and manure, respectively [14,15,16,17]. In addition, these micropollutants are also detected in the waste stream [18]. Heavy metals are toxic and non-biodegradable, and they pose a serious environmental risk. Antibiotics also undermine the quality of digestate. According to estimates, up to 80% of ingested drugs can be excreted with feces and urine in unchanged form. The antibiotics used in human medicine reach wastewater treatment plants and accumulate in sewage sludge, while veterinary drugs have been detected in manure and slurry [1]. Antimicrobial substances reach anaerobic bioreactors and are released into the environment, posing a threat to the soil, water ecosystems, and plants. Even low concentrations of HMs and antibiotics in various ecosystems can have adverse consequences for the environment [12,19]. The agricultural use of digestate containing HMs and antibiotics leads to the accumulation of these contaminants in the soil, posing a risk for both the environment and public health [1,19,20]. Moreover, the use of contaminated digestate in agricultural land and crop production can exert selective pressure on bacteria, lead to the spread of HMs and promote antibiotic resistance in microorganisms [1,21]. It should be emphasized that, in Europe, the quality and potential applications of digestate are regulated by legal acts pertaining to fertilizers, waste, and soil protection, or a combination of these laws. Although HM concentrations are strictly regulated in digestate that is intended for use as fertilizer or soil improver, statutory limits on drug concentrations have not been introduced to date [13]. The lack of appropriate risk assessment for the safe management of digestate has also been highlighted by other authors [4].
The main objective of this study was to review the current knowledge on the presence of HMs and antibiotics in digestate derived from various feedstocks. The article describes the key reservoirs of these micropollutants and the role of the AD process in their release to the environment. The growing levels of microbial resistance to HMs and antibiotics pose a serious public health concern and an environmental issue worldwide, which is why the potential effects of micropollutant residues in digestate have also been discussed. An ecological risk assessment of HMs and drugs has been performed to determine the severity of the risk resulting from the introduction of digestate into the soil environment. However, the current study has several limitations. Firstly, despite a large number of scientific papers on the presence of HMs and antibiotics in potential AD substrates, including raw animal manure, agricultural residues, sewage sludge, and the organic fraction of municipal solid waste or mixed wastes [22,23,24,25,26,27,28,29,30,31,32], the concentrations of these micropollutants in the resulting digestates have been analyzed by very few authors. Secondly, most studies investigating the fate of HMs and antibiotics during AD included feedstock supplementation before the process, making it impossible to assess the real, environmental concentrations of these pollutants in digestates [33,34,35,36,37]. Moreover, many scientific papers examining the impact of soil fertilization with digestate focused only on the concentrations of HMs and antibiotics in soil samples, while disregarding AD by-products [38,39,40]. In addition, the solid−liquid separation of AD products has also received little attention in the existing literature. To the best of our knowledge, there is a general scarcity of comprehensive review studies addressing the levels of HM and antibiotic residues in various anaerobically stabilized organic wastes. The number of reviewed papers from various regions of the world is presented in Figure 1. The authors were unable to find any studies presenting data on the content of HMs and/or antibiotics in digestates from Africa and South America. The only published studies addressing digestate contamination on the Asian continent came from China (11 articles). This issue was most often studied by research teams from European countries (22 articles), but many of these articles present selective data. Residual contamination is a broad and complex area of research involving different types of feedstocks, AD conditions, digestate fractions, and their environmental impacts, which is why further research is needed to fill these knowledge gaps.
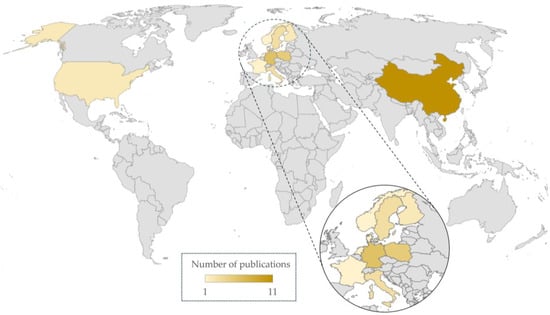
Figure 1.
Map showing the regions of the world where the reviewed studies were conducted (including the number of articles from each country).
2. Materials and Methods
2.1. Data Sources
In compliance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [41], the articles were selected according to the following four criteria: (i) identification; (ii) screening studies; (iii) eligibility; and (iv) inclusion. PubMed and Google Scholar scientific literature databases were surveyed to find papers published between 1 January 2004 and 30 November 2024.
2.2. Search Strategy
The search strategy is presented in Figure S1 in Supplementary Materials. The following keywords were used in the search strategy: ((anaerobic digestion) AND (digestate) AND ((antibiotic) OR (antimicrobial) OR (drug) OR (heavy metal)) AND ((residue) OR (degradation) OR (concentration) OR (content))) AND ((anaerobic digestion) AND (digestate) AND ((antibiotic) OR (antimicrobial) OR (drug) OR (heavy metal)) AND ((residue) OR (degradation) OR (concentration) OR (content))). These keywords were tailored to each database.
A preliminary search of published scientific articles relating to the subject of this review was conducted to identify the keywords for the advanced search process. The selected keywords are presented in Figure 2. In addition, a reference list of articles was checked manually to find adequate scientific publications for the review. After filtering the literature, 131 scientific publications were selected for this review article.
Figure 2.
A co-occurrence map of scientific papers containing the following keywords: anaerobic digestion, digestate, antibiotic/antimicrobial/drug/heavy metal, and residue/degradation/concentration/ content. The frame size represents the frequency of the keyword’s co-occurrence, and the color of clusters denotes the publication date. The map was created in VOSviewer (v1.6.16; 2020; Center for Science and Technology Studies, Leiden University, the Netherlands).
2.3. Ecological Risk Assessment
The indicators related to the levels of pollution with HMs and pharmaceuticals were computed to evaluate the influence of digestates on the soil ecosystem and the associated hazards. The geo-accumulation index and ecological risk factors were calculated for HM contamination of digestates. The risk quotient (RQ) method was used to assess the effect of pharmaceutical contaminants in digestates on the soil ecosystem, including co-selection for antimicrobial resistance.
2.3.1. Geo-Accumulation Index
The geo-accumulation index (Igeo) measures the level of pollution with inorganic or organic trace substances and the presence of bioelements in sediment or soil. This index is commonly used to assess HM pollution, and it was calculated by comparing the concentrations of HMs in samples with the natural background levels of metals in soils with the use of the following equation [42]:
where
Cn is the concentration of the analyzed heavy metal in digestate (mg∙kg−1);
Bn is the geochemical background value of the analyzed heavy metal in soil (mg∙kg−1). Background values were derived from the elemental concentrations in the upper continental crust [43].
The values of the Igeo index were classified on a 5-point scale: <0, practically unpolluted; 0–1, unpolluted to moderately polluted; 1–2, moderately polluted; 2–3, moderately to strongly polluted; 3–4, strongly polluted; 4–5, strongly to extremely polluted; and >5, extremely polluted.
2.3.2. Potential Ecological Risk Index
The ecological risk index (Er) was applied to evaluate the ecological risk posed by each HM individually. This index was calculated using the following formula [44]:
where
Tr is the toxicity factor of the analyzed HM; the following values were adopted: cadmium = 30, chromium = 2, mercury = 40, nickel = 5, lead = 5, zinc = 1, copper = 5, arsenic = 10, iron = 1, manganese = 1, cobalt = 5, molybdenum = 15;
Cf is the pollution factor.
The toxic response factors for manganese (Mn) and cobalt (Co) were not provided by [44]; therefore, the present study relied on the relevant values computed by other researchers [45,46,47]. The Tr values for silver (Ag) and aluminum (Al) were not available; therefore, the Er values for these elements were not calculated.
The contamination factor (Cf) is the ratio of metal contamination levels to pre-industrial levels that are commonly found in the Earth’s crust. This factor was calculated using the following formula:
where
Cn is the concentration of the analyzed HM in digestate (mg∙kg−1);
Cob is the concentration of the analyzed HM in the soil (mg∙kg−1). Background values were derived from the elemental concentrations found in the upper continental crust [43].
The Er values were classified on the following scale: very high risk (Er > 320), high risk (160 < Er < 320), considerable risk (80 < Er < 160), moderate risk (40 < Er < 80), and low risk (Er < 40).
2.3.3. Predicted Environmental Concentrations (PECs)
To assess the influence of antibiotic residues in digestates on the soil ecosystem, the predicted environmental concentrations (PECs) of antibiotics in fertilizer-amended soil (PEC(soil)) were calculated using the prescribed methodology [48] and the following equation:
where
Co(soil) is the background concentration of the analyzed antibiotic in soil before the application of digestate (assumed to be zero in this study);
MEC(digestate) is the concentration of the analyzed antibiotic in digestate (μg kg−1);
APP (digestate) is the typical rate of sludge application in soil, usually 0.5 kg m−2 for agricultural applications [49];
DEPTH(soil) is the mixing depth, usually set at 0.2 m for agricultural soil;
RHO(soil) is the bulk density of wet soil (1700 kg m−3) [49].
The calculated PEC(soil) values were compared with the known predicted no-effect concentrations (PNECs) for resistance selection in the environment (PNECMIC) and ecotoxicity (PNECENV) used in antibiotic environmental risk assessments for individual antibiotics detected in digestates. This analysis involved only antimicrobial substances with known values of PNECMIC/PNECENV [50,51].
The risk assessment based on PNECMIC/PNECENV values was conducted with the use of the RQ method according to the following formula:
RQenv values were classified on the following scale: RQ > 1—high potential risk for soil-living organisms; 0.1 < RQ < 1—moderate potential risk; and RQ < 0.1—low potential risk. PEC(soil) values exceeding the PNECMIC for specific antibiotics (RQmic > 1) were regarded as contributors to selection for antibiotic resistance in soil.
3. Results
3.1. Heavy Metal Content of Various Digestates
The use of digestate as soil fertilizer or improver is an environmentally friendly and cost-effective method of managing AD by-products that enhances soil capability for agriculture, supplies plants with nutrients and promotes waste recycling. However, HM concentrations in various digestates raise serious concerns. Although AD has the potential to reduce microbial loads and antibiotics in the processed substrate, it is not effective in eliminating HMs that are highly stable [52]. Heavy metals can be introduced into the soil when agricultural land is amended with contaminated fertilizers. Although some HMs, including copper (Cu) and zinc (Zn), are essential for the growth of various organisms, high concentrations of these elements can be toxic and can cause damage to nucleic acids and the cell membrane [53,54].
Heavy metal concentrations can differ considerably in various digestates. The origin of green waste or biowaste feedstocks is a significant consideration because aerial deposition of HMs is higher in urban than in rural environments. Moreover, the season of substrate collection also plays an important role due to the peak deposition of HMs in winter [55,56]. However, these variations are related mainly to the type of feedstock processed by AD. In intensive livestock production systems, HMs (mainly Cu, Zn, and arsenic (As)) are used as feed additives to prevent disease, increase weight gains or boost egg production in poultry farming [57]. Even more than 80% of dietary Zn and Cu is excreted in active form by humans and animals, which leads to the accumulation of these HMs in various feedstocks that are processed by AD [24]. The above leads to an increase in the HM content of animal by-products, which is stabilized during the AD process. Animal manure is recognized as a valuable fertilizer, but it is also the main source of HMs [57,58]. Sewage sludge, yet another popular substrate for AD, is an equally important source of HMs [52]. The HMs that are most frequently identified in sewage sludge include Cu, Zn, Co, iron (Fe), chromium (Cr), lead (Pb), mercury (Hg), nickel (Ni), cadmium (Cd), and the highly toxic As and selenium (Se). Heavy metals can occur in sewage sludge in dissolved or precipitated form, and they can also be associated with solid particles [52]. Industrial development increases the concentrations of HMs in wastewater, directly leading to sewage sludge contamination [59]. Surface runoffs and industrial wastewater are the main sources of these micropollutants in sewage sludge [60]. It should be noted that HM concentrations in sewage sludge rarely exceed the regulatory limits, but prolonged application of sewage sludge in agriculture contributes to the accumulation of HMs in soil [61,62,63]. Soil fertilization with both digested manure and sewage sludge increases HM levels in soil, which poses serious environmental risks. As previously mentioned, HM concentrations in raw substrates for AD and in fertilized soil have been extensively researched. However, very few studies have examined the HM content of digestates. The concentrations of HMs in the by-products of AD of various feedstocks are presented in Table 1.

Table 1.
Heavy metal concentrations in digestates derived from various feedstock sources.
According to the literature, digestates are most abundant in Zn, followed by Cu, Mn, and As [79]. Although the content of As, Cd, Cr, and Pb in various digestates is much lower relative to the commonly present Cu and Zn, these HMs are regarded as hazardous and highly persistent microelements [80]. The concentrations of HMs in digestates often exceed the values noted in the feedstock. Several studies reported on increasing concentrations of HMs after anaerobic treatment [64,81,82]. The above can be attributed to the production of methane during the AD of organic matter. This process leads to a decrease in the weight and volume of the substrate [83,84], which ultimately increases HM concentrations. Heavy metals are often closely bound to insoluble solids, which is why their concentrations are considerably higher in solid fractions after anaerobic treatment [82]. These micropollutants are present in the liquid fraction, and they are deposited and adsorbed by solid particles or colloids to form precipitates at the end of the treatment [78]. In addition, HM solubilization may be limited under alkaline conditions [71]. Due to the complexity of AD, HMs can be involved in many physical and/or chemical processes, including adsorption on the solid fraction and precipitation as carbonates, sulfides, and hydroxides [85]. The observed increase in HM concentrations in digestate indicates that the impact of this by-product on the soil environment should be examined, especially if digestate is used as a crop fertilizer. It should be emphasized that HM residues in soil, even at low concentrations, can be absorbed by roots and can accumulate in edible plant parts [52,86].
Digestate is a significant source of potential environmental contamination, which is why permissible HM levels for biomass incorporated into the soil are set by national and international laws. In Europe, the relevant limits have been prescribed by the EU Fertilizing Products Regulation (2019/1009). This regulation classifies the materials introduced into the soil into various categories. Digestate has been classified in four categories as organic fertilizer, soil improver, growing medium, and plant biostimulant. The permissible limits of HMs in each of these categories are presented in Table 2. The EU Fertilizing Products Regulation (EU 2019/1009, Table 2) lists eight HMs, and some of the AD by-products listed in Table 1 do not meet the European agricultural standards for fertilizers. In some studies, Ni, Zn, and Cu concentrations in the examined digestates, in particular digested animal manure, significantly exceeded the regulatory limits. The reported HM content of various digestates further highlights the environmental risks associated with the accumulation of these micropollutants in soils. However, some authors found that the application of digestate derived from animal manure and/or plant material can reduce the mobility and bioavailability of HM in soil through complexation, adsorption, and precipitation [28].

Table 2.
Permissible levels of HMs in digestates classified as fertilizers (EU 2019/1009).
3.2. Antimicrobial Residue Levels in Digestates
Intensive use of antimicrobial substances in agriculture, industry, human, and veterinary medicine has led to an increase in the concentrations of antibiotic residues in various types of organic wastes that may be applied as feedstocks for AD [24,87]. The continuous release of antibiotics into the environment and their accumulation in organic biomass pose a great concern. Most drugs are not completely metabolized by humans and animals, and a significant percentage of administered antibiotics enter anaerobic bioreactors with the processed feedstock, including agricultural residues, animal manure, and sewage sludge [1]. The concentrations of antimicrobial residues in raw substrates and digestates vary significantly and range from nanograms to micrograms per kg or mL. Although antibiotics can be degraded during AD, drug removal rates vary considerably due to differences in treatment parameters, such as temperature, organic load rate, or hydraulic retention time [87,88]. Moreover, antibiotic degradation rates during AD are influenced by the chemical structure of the drug, as well as the type and characteristic parameters of the feedstock. The reported rates of drug degradation differ between studies [24,81,89]. The efficiency of drug removal is determined by process conditions and the type of processed raw material, and it has been estimated at 7–98% for tetracyclines, 36–95% for MLS antibiotics, and 20–83% for fluoroquinolones [22,37,90]. Antibiotics persist in AD by-products, which can potentially lead to the emergence, persistence, or accumulation of these micropollutants in the environment when digestate is applied to soil [87,91]. The persistence and transformation of antibiotic residues in soil are influenced by numerous processes. Various antibiotic degradation rates in soil have been reported in the literature [87,92]. Digestate can be applied as a soil conditioner to promote sustainable biogas production and the circular economy, which is why the fate of antibiotics in substrates processed by AD should be analyzed in greater detail. The risks associated with drug residues in AD by-products have not been sufficiently investigated to date, which highlights the need for further research [93]. Therefore, the types and concentrations of antimicrobials identified in digestates derived from various feedstocks are presented in Table 3.

Table 3.
Types and concentrations of antimicrobial substances detected in digestates derived from the AD of various substrates.
The role of digestate as a source of antibiotics in soil ecosystems needs to be clarified. As indicated in Table 3, the concentrations of antimicrobial substances differ widely in various digestates. Antibiotic levels in digestates are determined mainly by the quality of the substrate. Sewage sludge and animal manure are more likely to be contaminated with drugs than agricultural residues. It has been reported that sulfonamide and fluoroquinolone antimicrobial agents are most prevalent in animal manure and sewage sludge [12,49,109]. Research has also shown that tetracycline and sulfonamide drugs can persist after the AD process [91]. As shown in Table 3, fluoroquinolone, sulfonamide, and tetracycline antibiotics have been most frequently detected in various AD by-products. However, the influence of AD on antibiotic concentrations in the substrates processed in anaerobic bioreactors remains unclear [12]. According to the literature, antimicrobial agents are degraded during anaerobic treatment, but this process has not been specifically designed to remove drugs [110]. As mentioned previously, the efficiency of antibiotic degradation depends on many factors. In some studies, antibiotics were not effectively removed during the AD process [104,111]. In turn, other authors have reported very high drug removal rates during AD and, consequently, very low drug concentrations in the obtained digestates [112].
The efficiency of drug removal and the concentrations of drug residues in digestates are also highly dependent on the chemical structure and specificity of antibiotics. Some antimicrobial substances are removed effectively, but their removal rates are very low (such as chlortetracycline and oxytetracycline that are characterized by high adsorption rates due to the presence of chlorine atoms and hydroxyl groups). Fluoroquinolones have a lower removal rate due to the presence of a fluorine atom [110]. In turn, β-lactams contain highly unstable β-lactam rings that are degraded by microbial β-lactamases during AD [113]. Moreover, antibiotic removal is considerably influenced by AD conditions. It has been reported that drug removal efficiency is strictly dependent on the total content of solids in the feedstock and the temperature of the anaerobic process [110]. Low drug removal rates are responsible for high concentrations of drug residues in AD by-products. The levels of ciprofloxacin, enrofloxacin, sulfadiazine, and oxytetracycline residues were particularly high in various digestates (Table 3). Due to their high stability and high adsorption potential, these antibiotics can persist in soil for long periods of time, thus posing a serious threat to the environment.
The lack of legal regulations concerning safe concentrations of drugs introduced into the soil with digestate gives serious cause for concern. Although the potential of digestate as an organic fertilizer has been studied to determine contamination with pathogens and HMs, as well as nutrient levels, the risks associated with antibiotic residues in digestate have been overlooked [21]. In Europe, the use of organic fertilizers (including digestate) is promoted by the EU Fertilizing Products Regulation (EU 2019/1009) which establishes the threshold values for total nitrogen, phosphorous, organic carbon, pathogens, and HMs that affect fertilizer quality. However, antibiotic residue levels have not been regulated to date [12]. Legal regulations concerning the permissible levels of antibiotics in digestates intended for soil fertilization should be urgently introduced. To the best of our knowledge, the presence of some antimicrobials should be banned, whereas the concentrations of other antibiotics should be restricted in fertilizer products (by defining limiting concentrations, as in the case of HMs). These measures are urgently needed to prevent the accumulation of pharmaceuticals in the environment and mitigate their negative effects on public health. At the beginning of 2024, the World Health Organization (WHO) published an updated list of medically important antimicrobials (MIAs) [114]. These drugs have been listed based on their importance in medicine, risk of antimicrobial resistance, and potential implications for public health resulting from inappropriate use, particularly in livestock farming. We believe that digestates containing third- and fourth-generation cephalosporins, quinolones, and polymyxins, i.e., drugs considered “the highest priority critically important antimicrobials”, should be banned from agricultural use. In addition, maximum permissible levels should be introduced for antimicrobials classified as “critically important” and “highly important”, especially aminoglycosides, macrolides, lincosamides, streptogramins, penicillins, pleuromutilins, sulfonamides, and tetracyclines, which were often detected in various digestates (Table 3).
3.3. Ecological Risks Associated with HM and Antibiotic Contamination
All HMs and some antimicrobial agents are considered persistent pollutants in agricultural ecosystems because long-term accumulation of these substances in the soil not only exerts highly toxic effects on various organisms and plants, but also contributes to the spread of microbial resistance [110]. When digestates contaminated with HMs or/and antibiotics are used as agricultural fertilizers, these substances pollute the environment and exert selective pressure on soil microorganisms [21,88]. Digestates and soil are colonized by diverse microorganisms, where bacteria harboring antibiotic resistance genes (ARGs) and heavy metal resistance genes (MRGs) pose a particular threat. Due to the widespread use of antibiotics in recent decades, ARGs have been classified as a new source of pollution that poses a threat to public health and safety [115]. High consumption of antibiotics promotes the emergence and persistence of antimicrobial resistance. Drugs exert selective pressure on ARGs [116]. In addition, HMs cannot be biodegraded; therefore, the selection pressure exerted on microorganisms in their presence is long-standing. It has been noted that high concentrations of HMs induce metal tolerance in communities of soil-dwelling bacteria [117]. Moreover, HMs also contribute to the spread of antimicrobial resistance. Research has shown that HM contamination can also promote the dissemination of ARGs among bacteria. At the molecular level, these phenomena can be interpreted as co-selection [118]. The presence of HMs in ecosystems can accelerate the development and propagation of ARGs [119], and the emergence of some ARGs may be directly associated with the presence of HMs in the environment [120]. There is scientific evidence to suggest that the release of HMs and antimicrobial substances into the environment is correlated with the presence of MRGs and ARGs. The co-selection of ARGs and MRGs has been reported in various environments [120,121,122,123]. In the context of digestate contamination, the impact of HMs and drugs on the soil environment is most meaningful because anaerobically stabilized organic matter is used primarily as a soil conditioner and fertilizer [124,125,126,127]. In one of the reviewed studies, the abundance of ARGs in the soil increased after exposure to HMs, and the observed increase was proportional to the rise in HM concentrations [128]. In another study, the spread of ARGs increased after HMs was released into antibiotic-contaminated soils [129]. However, HMs and antimicrobials may exert synergistic or antagonistic effects in the soil environment, which affects the correlation between ARG abundance and antibiotic levels [130]. Digestate-based fertilizers can release HMs and antimicrobial substances, as well as promote the spread of MRGs and ARGs in cultivated fields. These micropollutants may be transferred from the soil to groundwater and crops, ultimately reaching humans and animals [131].
In the present study, the specific environmental risks associated with HMs and drugs were analyzed to determine the potential hazards resulting from the introduction of digestates into the soil. The levels of HM contamination were estimated by calculating the geo-accumulation index (Igeo) and the ecological risk factor (Er) which indicate the extent to which the analyzed digestates induce changes in HM concentrations in soil and exert toxic effects on the ecosystem (Figure 3A,B, respectively). The values of Igeo and Er calculated for Cd, Cu, Hg, Mo, and Ni were indicative of high pollution levels and a high ecological risk. Approximately 25% to 33% of the analyzed digestates were extremely contaminated with Cd and Hg (Igeo > 5), and approximately 45% to 50% of the digestates were characterized as posing a very high ecological risk (Er > 320). Contamination with Mo was analyzed in only one publication, and the examined digestate was characterized by the highest level of pollution which exerted a potential risk for the ecosystem. None of the analyzed digestates were contaminated with Co, Fe, or Mn (Igeo < 1), and the concentrations of these HMs were indicative of low ecological risk (Er < 40). In turn, the RQ method was used to assess the extent to which the presence of drug residues in various digestates can affect selection for antibiotic resistance in the environment (RQmic; Figure 3C) and ecotoxicity (RQenv; Figure 3D) of fertilizer-amended soil. In most digestates (63–100%), the RQmic values for β-lactams (amoxicillin), fluoroquinolones (ciprofloxacin, enrofloxacin, and sparfloxacin), and tetracyclines (oxytetracycline) were considerably higher than 1, which points to a high risk of selection for antimicrobial resistance in soil. Drugs representing the same antibiotic classes (β-lactams—amoxicillin; fluoroquinolones—ciprofloxacin and enrofloxacin; tetracyclines—oxytetracycline and tetracycline) posed a high risk for soil-dwelling organisms (RQenv > 1 in 33–100% of digestate samples). In turn, all RQmic values for clindamycin, erythromycin, and sulfamethoxazole (in the range of 0.0001–0.91), as well as RQenv values for clindamycin, norfloxacin, and sulfamethoxazole (0.0006–0.37), were indicative of no risk or low risk for soils fertilized with various digestates.
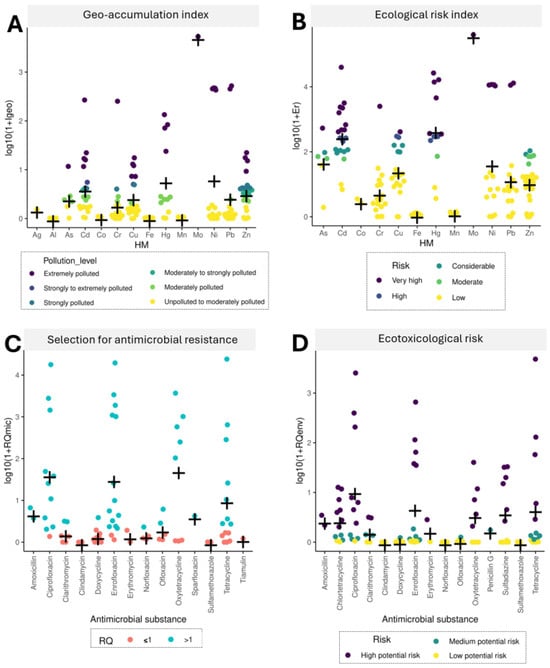
Figure 3.
Ecological risk assessment of HMs and antibiotics detected in digestate based on the values of the geo-accumulation index (A), environmental risk factor (B), risk quotient (RQ) for selection for drug resistance in the environment (C), and ecotoxicity (D). The cross symbol indicates the average value.
The environmental risk assessment demonstrated that most digestates containing HMs and antibiotics could pose a high risk to the ecosystem and promote the spread of antimicrobial resistance if introduced into the soil. Moreover, the antibiotic classes considered critically important (quinolones) and highly important (β-lactams and tetracyclines) in the WHO MIA List tend to be present in high concentrations in various digestates and may exceed the alert limit as regards the risk of selection for drug resistance. The above reiterates the need for the establishment of legal limits concerning the maximum admissible concentrations of individual drugs in anaerobically stabilized organic matter that can be released into the soil environment. Considering the alarming levels of anthropogenic pollution, the fact that soil contamination with HMs and antimicrobials can exert long-term effects and promote widespread co-selection pressure for microbial resistance is of particular concern. Therefore, the close links between ARGs, MRGs and their co-transfer, especially in the presence of HMs and antibiotics, pose the greatest challenge for research in the field of environmental microbiology. The role of digestate in the environmental transfer of HMs and antibiotics is extremely important; therefore, the residual levels of these micropollutants in AD by-products should be systematically monitored and analyzed.
4. Conclusions and Future Perspectives
This review article analyzes the global scope of digestate contamination with HMs and antibiotics. Based on the results of the analysis, it can be concluded that the AD process and the resulting digestates directly contribute to the presence of HMs and antibiotics in the environment. The absence of effective methods for removing these micropollutants during anaerobic treatment promotes their further transfer into the ecosystem. Although digestates generally meet quality standards, their application in farming could pose a threat to the soil and water environment and, subsequently, to public health. This review demonstrated that HM and antibiotic residues in digestates pose a risk for soil-dwelling organisms and contribute to selection for microbial resistance in soil ecosystems. The presence of antimicrobial substances in digestates intended for agricultural use should be urgently addressed by legal regulations. We believe that a better understanding of the role of digestate as a source of anthropogenic micropollutants and the risks associated with its introduction into the soil environment will encourage a scientific debate and lead to the implementation of dedicated legislative initiatives.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17020416/s1, Figure S1: PRISMA flowchart showing the results of the literature search and the screening process for this review.
Author Contributions
Conceptualization, M.C.; methodology, M.C. and D.R.; validation, M.C.; formal analysis, M.C.; investigation, M.C.; data curation, M.C.; writing—original draft preparation, M.C. and D.R.; writing—review and editing, E.K. and M.H.; visualization, M.C.; supervision, E.K. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Czatzkowska, M.; Wolak, I.; Harnisz, M.; Korzeniewska, E. Impact of Anthropogenic Activities on the Dissemination of ARGs in the Environment—A Review. Int. J. Environ. Res. Public Health 2022, 19, 12853. [Google Scholar] [CrossRef]
- Khan, N.; Bolan, N.; Joseph, S.; Anh, M.T.L.; Meier, S.; Kookana, R.; Borchard, N.; Sánchez-Monedero, M.A.; Jindo, K.; Solaiman, Z.M.; et al. Chapter One—Complementing compost with biochar for agriculture, soil remediation and climate mitigation. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 179, pp. 1–90. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Samoraj, M.; Mironiuk, M.; Izydorczyk, G.; Witek-Krowiak, A.; Szopa, D.; Moustakes, K.; Chojnacka, K. The challenges and perspectives for anaerobic digestion of animal waste and fertilizer application of the digestate. Chemosphere 2022, 295, 133799. [Google Scholar] [CrossRef]
- Lamolinara, B.; Pérez-Martínez, A.; Guardado-Yordi, E.; Fiallos, C.G.; Diéguez-Santana, K.; Ruiz-Mercado, G.J. Anaerobic digestate management, environmental impacts, and techno-economic challenges. Waste Manag. 2022, 140, 14–30. [Google Scholar] [CrossRef]
- Czekała, W.; Lewicki, A.; Pochwatka, P.; Czekała, A.; Wojcieszak, D.; Jóźwiakowski, K.; Waliszewska, H. Digestate management in polish farms as an element of the nutrient cycle. J. Clean. Prod. 2020, 242, 118454. [Google Scholar] [CrossRef]
- Stürmer, B.; Pfundtner, E.; Kirchmeyr, F.; Uschnig, S. Legal requirements for digestate as fertilizer in Austria and the European Union compared to actual technical parameters. J. Environ. Manag. 2020, 253, 109756. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, R.; Valboa, G.; Lagomarsino, A.; Fabiani, A.; Simoncini, S.; Zaghi, M.; Vignozzi, N. Recycling Biogas Digestate from Energy Crops: Effects on Soil Properties and Crop Productivity. Appl. Sci. 2021, 11, 750. [Google Scholar] [CrossRef]
- Czatzkowska, M.; Wolak, I.; Harnisz, M.; Korzeniewska, E. Microbial diversity and biosafety judgment of digestates derived from different biogas plants for agricultural applications. J. Environ. Manag. 2024, 371, 123329. [Google Scholar] [CrossRef] [PubMed]
- Witorożec-piechnik, A.; Kopiński, J.; Markowska-strzemska, E.; Woźniak, M. Environmental safety aspects of using the digestate from an agricultural biogas plant. Pol. J. Agron. 2023, 52, 54–61. [Google Scholar] [CrossRef]
- Wolak, I.; Bajkacz, S.; Harnisz, M.; Stando, K.; Męcik, M.; Korzeniewska, E. Digestate from Agricultural Biogas Plants as a Reservoir of Antimicrobials and Antibiotic Resistance Genes—Implications for the Environment. Int. J. Environ. Res. Public Health 2023, 20, 2672. [Google Scholar] [CrossRef]
- Visca, A.; Rauseo, J.; Spataro, F.; Patrolecco, L.; Grenni, P.; Massini, G.; Mazzurco Miritana, V.; Barra Caracciolo, A. Antibiotics and antibiotic resistance genes in anaerobic digesters and predicted concentrations in agroecosystems. J. Environ. Manag. 2022, 301, 113891. [Google Scholar] [CrossRef]
- Al Seadi, T.; Drosg, B.; Fuchs, W.; Rutz, D.; Janssen, R. 12—Biogas digestate quality and utilization. In The Biogas Handbook; Wellinger, A., Murphy, J., Baxter, D., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Cambridge, UK, 2013; pp. 267–301. [Google Scholar] [CrossRef]
- Shamuyarira, K.K.; Gumbo, J.R. Assessment of Heavy Metals in Municipal Sewage Sludge: A Case Study of Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2014, 11, 2569–2579. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Buta, M.; Korzeniewska, E.; Harnisz, M.; Hubeny, J.; Zieliński, W.; Rolbiecki, R.; Bajkacz, S.; Felis, E.; Kokoszka, K. Microbial and chemical pollutants on the manure-crops pathway in the perspective of ‘One Health’ holistic approach. Sci. Total Environ. 2021, 785, 147411. [Google Scholar] [CrossRef]
- Ji, X.; Shen, Q.; Liu, F.; Ma, J.; Xu, G.; Wang, Y.; Wu, M. Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai; China. J. Hazard. Mater. 2012, 235–236, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Essien, J.P.; Ikpe, D.I.; Inam, E.D.; Okon, A.O.; Ebong, G.A.; Benson, N.U. Occurrence and spatial distribution of heavy metals in landfill leachates and impacted freshwater ecosystem: An environmental and human health threat. PLoS ONE 2022, 17, e0263279. [Google Scholar] [CrossRef]
- Caracciolo, A.B.; Visca, A.; Rauseo, J.; Spataro, F.; Garbini, G.L.; Grenni, P.; Mariani, L.; Mazzurco Miritana, V.; Massini, G.; Patrolecco, L. Bioaccumulation of antibiotics and resistance genes in lettuce following cattle manure and digestate fertilization and their effects on soil and phyllosphere microbial communities. Environ. Pollut. 2022, 315, 120413. [Google Scholar] [CrossRef]
- Catenacci, A.; Boniardi, G.; Mainardis, M.; Gievers, F.; Farru, G.; Asunis, F.; Malpei, F.; Goi, D.; Cappai, G.; Canziani, R. Processes, applications and legislative framework for carbonized anaerobic digestate: Opportunities and bottlenecks. A critical review. Energy Convers. Manag. 2022, 263, 115691. [Google Scholar] [CrossRef]
- Yusuf, H.H.; Pan, X.; Cai, G.; Cai, J.; Huang, X.; Ye, Z.L. Semi-solid anaerobic co-digestion of source-separated fecal slag and food waste: Focusing on methane production, ecological risk assessment, and quality evaluation as fertilizer. Environ. Sci. Pollut. Res. 2022, 29, 66578–66590. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Li, Z.; Zhu, R.; Wang, J.; Liu, X.; Liu, X. The Antibiotics Degradation and Its Mechanisms during the Livestock Manure Anaerobic Digestion. Molecules 2023, 28, 4090. [Google Scholar] [CrossRef] [PubMed]
- Tytła, M. Assessment of Heavy Metal Pollution and Potential Ecological Risk in Sewage Sludge from Municipal Wastewater Treatment Plant Located in the Most Industrialized Region in Poland-Case Study. Int. J. Environ. Res. Public Health 2019, 16, 2430. [Google Scholar] [CrossRef] [PubMed]
- Marutescu, L.G.; Jaga, M.; Postolache, C.; Barbuceanu, F.; Milita, N.M.; Romascu, L.M.; Schmitt, H.; de Roda Husman, A.M.; Sefeedpari, P.; Glaeser, S.; et al. Insights into the impact of manure on the environmental antibiotic residues and resistance pool. Front. Microbiol. 2022, 13, 965132. [Google Scholar] [CrossRef]
- Yin, F.; Dong, H.; Ji, C.; Tao, X.; Chen, Y. Effects of anaerobic digestion on chlortetracycline and oxytetracycline degradation efficiency for swine manure. Waste Manag. 2016, 56, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Cheng, G.; Iqbal, Z.; Ai, X.; Hussain, H.I.; Huang, L.; Dai, M.; Wang, Y.; Liu, Z.; Yuan, Z. Benefits and risks of antimicrobial use in food-producing animals. Front. Microbiol. 2014, 5, 288. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Tian, Y.; Zhang, H.; Chai, Y. Copper stressed anaerobic fermentation: Biogas properties, process stability, biodegradation and enzyme responses. Biodegradation 2017, 28, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.; Malik, A.H.; Shaukat, S.; Mushtaq, S.; Ashraf, M. Characterization of Heavy Metals in Livestock Manures. Pol. J. Environ. Stud. 2013, 22, 1257–1262. [Google Scholar]
- Adegbeye, M.J.; Adetuyi, B.O.; Igirigi, A.I.; Adisa, A.; Palangi, V.; Aiyedun, S.; Alvarado-Ramírez, E.R.; Elghandour, M.M.M.Y.; Márquez Molina, O.; Oladipo, A.A.; et al. Comprehensive insights into antibiotic residues in livestock products: Distribution, factors, challenges, opportunities, and implications for food safety and public health. Food Control 2024, 163, 110545. [Google Scholar] [CrossRef]
- Liu, W.-R.; Zeng, D.; She, L.; Su, W.-X.; He, D.-C.; Wu, G.-Y.; Ma, X.-R.; Jiang, S.; Jiang, C.-H.; Ying, G.-G. Comparisons of pollution characteristics, emission situations, and mass loads for heavy metals in the manures of different livestock and poultry in China. Sci. Total Environ. 2020, 734, 139023. [Google Scholar] [CrossRef]
- Zha, Y.; Li, Q.; Liu, H.; Ge, Y.; Wei, Y.; Wang, H.; Zhang, L.; Fan, J.; Chen, Y.; Zhang, C.; et al. Occurrence and ecological risk assessment of antibiotics in manure and the surrounding soil from typical chicken farms in Hangzhou, China. Front. Environ. Sci. 2023, 11, 1241405. [Google Scholar] [CrossRef]
- do Carmo Precci Lopes, A.; Ebner, C.; Gerke, F.; Wehner, M.; Robra, S.; Hupfauf, S.; Bockreis, A. Residual municipal solid waste as co-substrate at wastewater treatment plants: An assessment of methane yield, dewatering potential and microbial diversity. Sci. Total Environ. 2022, 804, 149936. [Google Scholar] [CrossRef]
- Shi, J.C.; Liao, X.D.; Wu, Y.B.; Liang, J.B. Effect of antibiotics on methane arising from anaerobic digestion of pig manure. Anim. Feed Sci. Technol. 2011, 166–167, 457–463. [Google Scholar] [CrossRef]
- Turker, G.; Akyol, Ç.; Ince, O.; Aydin, S.; Ince, B. Operating conditions influence microbial community structures, elimination of the antibiotic resistance genes and metabolites during anaerobic digestion of cow manure in the presence of oxytetracycline. Ecotoxicol. Environ. Saf. 2018, 147, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, J.A.; Otero, L.; Lema, J.M.; Omil, F. The effect and fate of antibiotics during the anaerobic digestion of pig manure. Bioresour. Technol. 2010, 101, 8581–8586. [Google Scholar] [CrossRef]
- Coban, H.; Ertekin, E.; Ince, O.; Turker, G.; Akyol, Ç.; Ince, B. Degradation of oxytetracycline and its impacts on biogas-producing microbial community structure. Bioprocess Biosyst. Eng. 2016, 39, 1051–1060. [Google Scholar] [CrossRef]
- Czatzkowska, M.; Harnisz, M.; Korzeniewska, E.; Rusanowska, P.; Bajkacz, S.; Felis, E.; Jastrzębski, J.P.; Paukszto, Ł.; Koniuszewska, I. The impact of antimicrobials on the efficiency of methane fermentation of sewage sludge, changes in microbial biodiversity and the spread of antibiotic resistance. J. Hazard. Mater. 2021, 416, 125773. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, Y.; Wan, Y.; Wang, Q.; Luo, Z.; Qiao, Y.; Su, D.; Li, H. Effects of continuous fertilization on bioavailability and fractionation of cadmium in soil and its uptake by rice (Oryza sativa L.). J. Environ. Manag. 2018, 215, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Adsorption and degradation of five selected antibiotics in agricultural soil. Sci. Total Environ. 2016, 545–546, 48–56. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Muller, G. Index of geoaccumulation in sediments of the rhine river. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Reimann, C.; Caritat, P. Chemical Elements in the Environment; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar] [CrossRef]
- Håkanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, C.; Yang, Y.; Niu, J.; Shen, Z. Risk assessment of sedimentary metals in the Yangtze Estuary: New evidence of the relationships between two typical index methods. J. Hazard. Mater. 2012, 241–242, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Z.; Chen, Z.; Ou, X.; Chen, J. Calculation of Toxicity Coefficient of Potential Ecological Risk Assessment of Rare Earth Elements. Bull. Environ. Contam. Toxicol. 2020, 104, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J.; Larsson, D.G.J. Concentrations of antibiotics predicted to select for resistant bacteria: Proposed limits for environmental regulation. Environ. Int. 2016, 86, 140–149. [Google Scholar] [CrossRef] [PubMed]
- EMA. Committee for Medicinal Products for Human Use (CHMP) Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use. 2024. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-environmental-risk-assessment-medicinal-products-human-use-revision-1_en.pdf (accessed on 9 October 2024).
- Ajibola, A.S.; Zwiener, C. Occurrence and Risk Assessment of Antibiotic Residues in Sewage Sludge of Two Nigerian Hospital Wastewater Treatment Plants. Water Air Soil Pollut. 2022, 233, 405. [Google Scholar] [CrossRef]
- Vestel, J.; Caldwell, D.J.; Tell, J.; Constantine, L.; Häner, A.; Hellstern, J.; Journel, R.; Ryan, J.J.; Swenson, T.; Xei, W. Default predicted no-effect target concentrations for antibiotics in the absence of data for the protection against antibiotic resistance and environmental toxicity. Integr. Environ. Assess. Manag. 2022, 18, 863–867. [Google Scholar] [CrossRef] [PubMed]
- AMR Industry Alliance. AMR Alliance Science-Based PNEC Targets for Risk Assessments. 2024. Available online: https://www.amrindustryalliance.org/wp-content/uploads/2023/02/AMR-Table-1-Update-20230222_corrected.pdf (accessed on 7 October 2024).
- Buta, M.; Hubeny, J.; Zieliński, W.; Harnisz, M.; Korzeniewska, E. Sewage sludge in agriculture—The effects of selected chemical pollutants and emerging genetic resistance determinants on the quality of soil and crops—A review. Ecotoxicol. Environ. Saf. 2021, 214, 112070. [Google Scholar] [CrossRef]
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef]
- Kang, W.; Zhang, Y.-J.; Shi, X.; He, J.-Z.; Hu, H.-W. Short-term copper exposure as a selection pressure for antibiotic resistance and metal resistance in an agricultural soil. Environ. Sci. Pollut. Res. 2018, 25, 29314–29324. [Google Scholar] [CrossRef] [PubMed]
- Kovačić, Đ.; Lončarić, Z.; Jović, J.; Samac, D.; Popović, B.; Tišma, M. Digestate Management and Processing Practices: A Review. Appl. Sci. 2022, 12, 9216. [Google Scholar] [CrossRef]
- Kupper, T.; Bürge, D.; Bachmann, H.J.; Güsewell, S.; Mayer, J. Heavy metals in source-separated compost and digestates. Waste Manag. 2014, 34, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Liu, J.; Zhuang, Z.; Wang, Q.; Li, H. Heavy Metals in Agricultural Soils: Sources, Influencing Factors, and Remediation Strategies. Toxics 2024, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhen, H.; Jia, L.; Huang, C.; Qiao, Y.; Li, J.; Li, H.; Chen, Q.; Wan, Y. Long-term effects of intensive application of manure on heavy metal pollution risk in protected-field vegetable production. Environ. Pollut. 2020, 263, 114552. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Wu, L.; Huang, Y.; Luo, Y.; Christie, P. Total concentrations of heavy metals and occurrence of antibiotics in sewage sludges from cities throughout China. J. Soils Sediments 2014, 14, 1123–1135. [Google Scholar] [CrossRef]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminations in sewage sludge—The current situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.; Marguí, E.; Camps, F.; Hidalgo, M. Extractability and crop transfer of potentially toxic elements from mediterranean agricultural soils following long-term sewage sludge applications as a fertilizer replacement to barley and maize crops. Waste Manag. 2018, 75, 312–318. [Google Scholar] [CrossRef]
- Kelepertzis, E. Accumulation of heavy metals in agricultural soils of Mediterranean: Insights from Argolida basin, Peloponnese, Greece. Geoderma 2014, 221–222, 82–90. [Google Scholar] [CrossRef]
- Liu, C.; Tong, Q.; Li, Y.; Wang, N.; Liu, B.; Zhang, X. Biogas production and metal passivation analysis during anaerobic digestion of pig manure: Effects of a magnetic Fe3O4/FA composite supplement. RSC Adv. 2019, 9, 4488–4498. [Google Scholar] [CrossRef] [PubMed]
- Derehajło, S.; Tymińska, M.; Skibko, Z.; Borusiewicz, A.; Romaniuk, W.; Kuboń, M.; Olech, E.; Koszel, M. Heavy Metal Content in Substrates in Agricultural Biogas Plants. Agric. Eng. 2023, 27, 315–329. [Google Scholar] [CrossRef]
- Golovko, O.; Ahrens, L.; Schelin, J.; Sörengård, M.; Bergstrand, K.-J.; Asp, H.; Hultberg, M.; Wiberg, K. Organic micropollutants, heavy metals and pathogens in anaerobic digestate based on food waste. J. Environ. Manag. 2022, 313, 114997. [Google Scholar] [CrossRef]
- Guo, Q.; Ji, J.; Ling, Z.; Zhang, K.; Xu, R.; Leng, X.; Mao, C.; Zhou, T.; Wang, H.; Liu, P.; et al. Bioaugmentation improves the anaerobic co-digestion of cadmium-containing plant residues and cow manure. Environ. Pollut. 2021, 289, 117885. [Google Scholar] [CrossRef] [PubMed]
- Szaja, A.; Montusiewicz, A.; Lebiocka, M. Variability of Micro- and Macro-Elements in Anaerobic Co-Digestion of Municipal Sewage Sludge and Food Industrial By-Products. Int. J. Environ. Res. Public Health 2023, 20, 5405. [Google Scholar] [CrossRef] [PubMed]
- Legros, S.; Levard, C.; Marcato-Romain, C.-E.; Guiresse, M.; Doelsch, E. Anaerobic Digestion Alters Copper and Zinc Speciation. Environ. Sci. Technol. 2017, 51, 10326–10334. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, X.; Wang, Y.; Tuersun, N.; Ismail, M.; Cheng, C.; Li, Z.; Song, Q.; Wang, Y.; Ma, C. Anaerobic co-digestion of textile dyeing sludge: Digestion efficiency and heavy metal stability. Sci. Total Environ. 2021, 801, 149722. [Google Scholar] [CrossRef] [PubMed]
- Knoop, C.; Tietze, M.; Dornack, C.; Raab, T. Fate of nutrients and heavy metals during two-stage digestion and aerobic post-treatment of municipal organic waste. Bioresour. Technol. 2018, 251, 238–248. [Google Scholar] [CrossRef]
- Carchesio, M.; Di Addario, M.; Tatàno, F.; de Rosa, S.; Gambioli, A. Evaluation of the biochemical methane potential of residual organic fraction and mechanically-biologically treated organic outputs intended for landfilling. Waste Manag. 2020, 113, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Cucina, M.; Tacconi, C.; Ricci, A.; Pezzolla, D.; Sordi, S.; Zadra, C.; Gigliotti, G. Evaluation of benefits and risks associated with the agricultural use of organic wastes of pharmaceutical origin. Sci. Total Environ. 2018, 613–614, 773–782. [Google Scholar] [CrossRef]
- Riaz, L.; Wang, Q.; Yang, Q.; Li, X.; Yuan, W. Potential of industrial composting and anaerobic digestion for the removal of antibiotics, antibiotic resistance genes and heavy metals from chicken manure. Sci. Total Environ. 2020, 718, 137414. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Guo, Z.; Xiao, X.; Peng, C.; Zeng, P.; Feng, W.; Xu, W. Feasibility of anaerobic digestion on the release of biogas and heavy metals from rice straw pretreated with sodium hydroxide. Environ. Sci. Pollut. Res. 2019, 26, 19434–19444. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, Y.; Gong, H.; Fang, N.; Tan, Y.; Zhou, W.; Huang, J.; Dai, L.; Dai, X. Variations of heavy metals, nutrients, POPs and particle size distribution during ‘sludge anaerobic digestion-solar drying-land utilization process’: Case study in China. Sci. Total Environ. 2021, 801, 149609. [Google Scholar] [CrossRef]
- Liu, J.; He, X.; Xu, Y.; Zuo, Z.; Lei, P.; Zhang, J.; Yin, Y.; Wei, Y. Fate of mercury and methylmercury in full-scale sludge anaerobic digestion combined with thermal hydrolysis. J. Hazard. Mater. 2021, 406, 124310. [Google Scholar] [CrossRef] [PubMed]
- Montusiewicz, A.; Szaja, A.; Musielewicz, I.; Cydzik-Kwiatkowska, A.; Lebiocka, M. Effect of bioaugmentation on digestate metal concentrations in anaerobic digestion of sewage sludge. PLoS ONE 2020, 15, e0235508. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, Y.; Huang, J.; Du, Z.; Zhouyang, S.; Wang, Y.; Zheng, Y.; Li, Q.; Shen, X. The influence of variables on the bioavailability of heavy metals during the anaerobic digestion of swine manure. Ecotoxicol. Environ. Saf. 2020, 195, 110457. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.M.; Guo, X.J. Sequential extraction of anaerobic digestate sludge for the determination of partitioning of heavy metals. Ecotoxicol. Environ. Saf. 2014, 102, 18–24. [Google Scholar] [CrossRef]
- Raj, D.; Maiti, S.K. Sources, bioaccumulation, health risks and remediation of potentially toxic metal(loid)s (As, Cd, Cr, Pb and Hg): An epitomised review. Environ. Monit. Assess. 2020, 192, 108. [Google Scholar] [CrossRef]
- Kadam, R.; Khanthong, K.; Jang, H.; Lee, J.; Park, J. Occurrence, Fate, and Implications of Heavy Metals during Anaerobic Digestion: A Review. Energies 2022, 15, 8618. [Google Scholar] [CrossRef]
- Jin, H.; Chang, Z. Distribution of Heavy Metal Contents and Chemical Fractions in Anaerobically Digested Manure Slurry. Appl. Biochem. Biotechnol. 2011, 164, 268–282. [Google Scholar] [CrossRef]
- Qi, G.; Pan, Z.; Sugawa, Y.; Andriamanohiarisoamanana, F.J.; Yamashiro, T.; Iwasaki, M.; Kawamoto, K.; Ihara, I.; Umetsu, K. Comparative fertilizer properties of digestates from mesophilic and thermophilic anaerobic digestion of dairy manure: Focusing on plant growth promoting bacteria (PGPB) and environmental risk. J. Mater. Cycles Waste Manag. 2018, 20, 1448–1457. [Google Scholar] [CrossRef]
- Dong, B.; Liu, X.; Dai, L.; Dai, X. Changes of heavy metal speciation during high-solid anaerobic digestion of sewage sludge. Bioresour. Technol. 2013, 131, 152–158. [Google Scholar] [CrossRef]
- Choong, Y.Y.; Norli, I.; Abdullah, A.Z.; Yhaya, M.F. Impacts of trace element supplementation on the performance of anaerobic digestion process: A critical review. Bioresour. Technol. 2016, 209, 369–379. [Google Scholar] [CrossRef]
- Jolly, Y.N.; Islam, A.; Akbar, S. Transfer of metals from soil to vegetables and possible health risk assessment. SpringerPlus 2013, 2, 385. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—Degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Gurmessa, B.; Pedretti, E.F.; Cocco, S.; Cardelli, V.; Corti, G. Manure anaerobic digestion effects and the role of pre- and post-treatments on veterinary antibiotics and antibiotic resistance genes removal efficiency. Sci. Total Environ. 2020, 721, 137532. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Sengar, A.; Basheer, F.; Farooqi, I.H.; Isa, M.H. Anaerobic digestion in the elimination of antibiotics and antibiotic-resistant genes from the environment—A comprehensive review. J. Environ. Chem. Eng. 2022, 10, 106423. [Google Scholar] [CrossRef]
- Koniuszewska, I.; Harnisz, M.; Korzeniewska, E.; Czatzkowska, M.; Jastrzębski, J.P.; Paukszto, Ł.; Bajkacz, S.; Felis, E.; Rusanowska, P. The Effect of Antibiotics on Mesophilic Anaerobic Digestion Process of Cattle Manure. Energies 2021, 14, 1125. [Google Scholar] [CrossRef]
- Kasumba, J.; Appala, K.; Agga, G.E.; Loughrin, J.H.; Conte, E.D. Anaerobic digestion of livestock and poultry manures spiked with tetracycline antibiotics. J. Environ. Sci. Health Part B 2020, 55, 135–147. [Google Scholar] [CrossRef]
- Lu, J.; Xu, S. Post-treatment of food waste digestate towards land application: A review. J. Clean. Prod. 2021, 303, 127033. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ştefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef]
- Nesse, A.S.; Aanrud, S.G.; Lyche, J.L.; Sogn, T.; Kallenborn, R. Confirming the presence of selected antibiotics and steroids in Norwegian biogas digestate. Environ. Sci. Pollut. Res. 2022, 29, 86595–86605. [Google Scholar] [CrossRef]
- Lehmann, L.; Bloem, E. Antibiotic residues in substrates and output materials from biogas plants—Implications for agriculture. Chemosphere 2021, 278, 130425. [Google Scholar] [CrossRef]
- Ratsak, C.; Guhl, B.; Zühlke, S.; Delschen, T. Veterinärantibiotikarückstände in Gülle und Gärresten aus Nordrhein-Westfalen [Veterinary antibiotic residues in manure and digestates in Northrhein-Westfalia]. Environ. Sci. Eur. 2013, 25, 7. [Google Scholar] [CrossRef]
- Sun, C.; Li, W.; Chen, Z.; Qin, W.; Wen, X. Responses of antibiotics, antibiotic resistance genes, and mobile genetic elements in sewage sludge to thermal hydrolysis pre-treatment and various anaerobic digestion conditions. Environ. Int. 2019, 133 Pt A, 105156. [Google Scholar] [CrossRef]
- Yin, F.; Lin, S.; Zhou, X.; Dong, H.; Zhan, Y. Fate of antibiotics during membrane separation followed by physical-chemical treatment processes. Sci. Total Environ. 2021, 759, 143520. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pu, C.; Yu, X.; Sun, Y.; Chen, J. Removal of tetracyclines, sulfonamides, and quinolones by industrial-scale composting and anaerobic digestion processes. Environ. Sci. Pollut. Res. 2018, 25, 35835–35844. [Google Scholar] [CrossRef] [PubMed]
- Patyra, E.; Kwiatek, K.; Nebot, C.; Gavilán, R.E. Quantification of veterinary antibiotics in pig and poultry feces and liquid manure as a non-invasive method to monitor antibiotic usage in livestock by liquid chromatography mass-spectrometry. Molecules 2020, 25, 3265. [Google Scholar] [CrossRef] [PubMed]
- Wolters, B.; Widyasari-Mehta, A.; Kreuzig, R.; Smalla, K. Contaminations of organic fertilizers with antibiotic residues, resistance genes, and mobile genetic elements mirroring antibiotic use in livestock? Appl. Microbiol. Biotechnol. 2016, 100, 9343–9353. [Google Scholar] [CrossRef]
- Feng, L.; Casas, M.E.; Ottosen, L.D.M.; Møller, H.B.; Bester, K. Removal of antibiotics during the anaerobic digestion of pig manure. Sci. Total Environ. 2017, 603–604, 219–225. [Google Scholar] [CrossRef]
- Varel, V.H.; Wells, J.E.; Shelver, W.L.; Rice, C.P.; Armstrong, D.L.; Parker, D.B. Effect of anaerobic digestion temperature on odour, coliforms and chlortetracycline in swine manure or monensin in cattle manure. J. Appl. Microbiol. 2012, 112, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Spielmeyer, A.; Ahlborn, J.; Hamscher, G. Simultaneous determination of 14 sulfonamides and tetracyclines in biogas plants by liquid-liquid-extraction and liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 2513–2524. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.S.; Garner, E.; Pruden, A.; Aga, D.S. Occurrence and transformation of veterinary antibiotics and antibiotic resistance genes in dairy manure treated by advanced anaerobic digestion and conventional treatment methods. Environ. Pollut. 2018, 236, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.J.; Clay, S.A.; Zhu, Z.; Wong, K.L.; Porath, L.R.; Spellman, G.M. Effect of antimicrobial compounds tylosin and chlortetracycline during batch anaerobic swine manure digestion. Water Res. 2009, 43, 4740–4750. [Google Scholar] [CrossRef]
- Widyasari-Mehta, A.; Suwito, H.R.K.A.; Kreuzig, R. Laboratory testing on the removal of the veterinary antibiotic doxycycline during long-term liquid pig manure and digestate storage. Chemosphere 2016, 149, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Bousek, J.; Schöpp, T.; Schwaiger, B.; Lesueur, C.; Fuchs, W.; Weissenbacher, N. Behaviour of doxycycline, oxytetracycline, tetracycline and flumequine during manure up-cycling for fertilizer production. J. Environ. Manag. 2018, 223, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Karcı, A.; Balcıoğlu, I.A. Investigation of the tetracycline, sulfonamide, and fluoroquinolone antimicrobial compounds in animal manure and agricultural soils in Turkey. Sci. Total Environ. 2009, 407, 4652–4664. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xie, S.; Yang, M.; Tang, S.; Zhou, L.; Jiang, W.; Zhou, B.; Li, Y.; Si, B. A critical review on retaining antibiotics in liquid digestate: Potential risk and removal technologies. Sci. Total Environ. 2022, 853, 158550. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J.; Frear, C. The effects of the antibiotics ampicillin, florfenicol, sulfamethazine, and tylosin on biogas production and their degradation efficiency during anaerobic digestion. Bioresour. Technol. 2013, 149, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Miritana, V.M.; Massini, G.; Visca, A.; Grenni, P.; Patrolecco, L.; Spataro, F.; Rauseo, J.; Garbini, G.L.; Signorini, A.; Rosa, S.; et al. Effects of Sulfamethoxazole on the Microbial Community Dynamics During the Anaerobic Digestion Process. Front. Microbiol. 2020, 11, 537783. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Jia, Y.; Zhang, H.; Khanal, S.K.; Lu, H. Insights into the Fate and Removal of Antibiotics in Engineered Biological Treatment Systems: A Critical Review. Environ. Sci. Technol. 2019, 53, 7234–7264. [Google Scholar] [CrossRef]
- WHO. WHO’s List of Medically Important Antimicrobials A Risk Management Tool for Mitigating Antimicrobial Resistance Due to Non-Human Use; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Barancheshme, F.; Munir, M. Development of Antibiotic Resistance in Wastewater Treatment Plants. In Antimicrobial Resistance; Kumar, Y., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 5. [Google Scholar] [CrossRef]
- Xiong, W.; Sun, Y.; Ding, X.; Wang, M.; Zeng, Z. Selective pressure of antibiotics on ARGs and bacterial communities in manure-polluted freshwater-sediment microcosms. Front. Microbiol. 2015, 6, 194. [Google Scholar] [CrossRef]
- Santás-Miguel, V.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Díaz-Raviña, M.; Arias-Estévez, M.; Fernández-Calviño, D. Tolerance of soil bacterial community to tetracycline antibiotics induced by As, Cd, Zn, Cu, Ni, Cr, and Pb pollution. Soil 2022, 8, 437–449. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Zhang, H.; Shi, W.; Liu, Y. Bacterial heavy-metal and antibiotic resistance genes in a copper tailing dam area in northern China. Front. Microbiol. 2019, 10, 1916. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Das, K.R.; Naik, M.M. Co-selection of multi-antibiotic resistance in bacterial pathogens in metal and microplastic contaminated environments: An emerging health threat. Chemosphere 2019, 215, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Graham, D.W.; Sreekrishnan, T.R.; Ahammad, S.Z. Effects of heavy metals pollution on the co-selection of metal and antibiotic resistance in urban rivers in UK and India. Environ. Pollut. 2022, 306, 119326. [Google Scholar] [CrossRef] [PubMed]
- Pedro, F.; Espírito Santo, C.; Branco, R.; Francisco, R.; Santos, S.; Hansen, L.; Sorensen, S.; Morais, P.V. Natural Hot Spots for Gain of Multiple Resistances: Arsenic and Antibiotic Resistances in Heterotrophic, Aerobic Bacteria from Marine Hydrothermal Vent Fields. Appl. Environ. Microbiol. 2015, 81, 2534–2543. [Google Scholar] [CrossRef]
- Fang, L.; Li, X.; Li, L.; Li, S.; Liao, X.; Sun, J.; Liu, Y. Co-spread of metal and antibiotic resistance within ST3-IncHI2 plasmids from E. coli isolates of food-producing animals. Sci. Rep. 2016, 6, 25312. [Google Scholar] [CrossRef]
- Edet, U.O.; Bassey, I.U.; Joseph, A.P. Heavy metal co-resistance with antibiotics amongst bacteria isolates from an open dumpsite soil. Heliyon 2023, 9, e13457. [Google Scholar] [CrossRef]
- van Midden, C.; Harris, J.; Shaw, L.; Sizmur, T.; Pawlett, M. The impact of anaerobic digestate on soil life: A review. Appl. Soil Ecol. 2023, 191, 105066. [Google Scholar] [CrossRef]
- Gurmessa, B.; Cocco, S.; Ashworth, A.J.; Udawatta, R.P.; Cardelli, V.; Ilari, A.; Serrani, D.; Fornasier, F.; Del Gatto, A.; Foppa Pedretti, E.; et al. Short term effects of digestate and composted digestate on soil health and crop yield: Implications for sustainable bioWaste Management in the bioenergy sector. Sci. Total Environ. 2024, 906, 167208. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidou, C.; Mola, M.; Papakostas, S.; Aschonitis, V.G.; Monokrousos, N.; Kougias, P.G. The effect of anaerobic digestate as an organic soil fertilizer on the diversity and structure of the indigenous soil microbial and nematode communities. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Doyeni, M.O.; Stulpinaite, U.; Baksinskaite, A.; Suproniene, S.; Tilvikiene, V. The Effectiveness of Digestate Use for Fertilization in an Agricultural Cropping System. Plants 2021, 10, 1734. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, Y.; Dong, H.; Li, J.; Zhang, W.; Shao, Y.; Shao, Y. Mechanisms of the effects of humic acid on antibiotic resistance genes and microbial communities in Cd-contaminated soils. Process Saf. Environ. Prot. 2022, 160, 62–69. [Google Scholar] [CrossRef]
- Guo, H.; Xue, S.; Nasir, M.; Gu, J.; Lv, J. Impacts of cadmium addition on the alteration of microbial community and transport of antibiotic resistance genes in oxytetracycline contaminated soil. J. Environ. Sci. 2021, 99, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhu, Y.; Dong, H.; Li, J.; Zhang, W.; Shao, Y.; Shao, Y. Effects of heavy metals and antibiotics on antibiotic resistance genes and microbial communities in soil. Process Saf. Environ. Prot. 2023, 169, 418–427. [Google Scholar] [CrossRef]
- Xiao, Y.; Raheem, A.; Ding, L.; Chen, W.-H.; Chen, X.; Wang, F.; Lin, S.-L. Pretreatment, modification and applications of sewage sludge-derived biochar for resource recovery—A review. Chemosphere 2022, 287, 131969. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).