Municipal Solid Waste Gasification: Technologies, Process Parameters, and Sustainable Valorization of By-Products in a Circular Economy
Abstract
1. Introduction
2. MSW Gasification: Process Description and Comparative Analysis with Pyrolysis and Incineration
3. Key Factors Influencing the Gasification Performance
3.1. Temperature
3.2. Pressure
3.3. Waste Moisture Content
3.4. Waste Particle Size and Shape
3.5. Ash Content and Inorganic Composition of MSW
3.6. Gasification Agents
3.7. The Equivalence Ratio
3.8. Residence Time
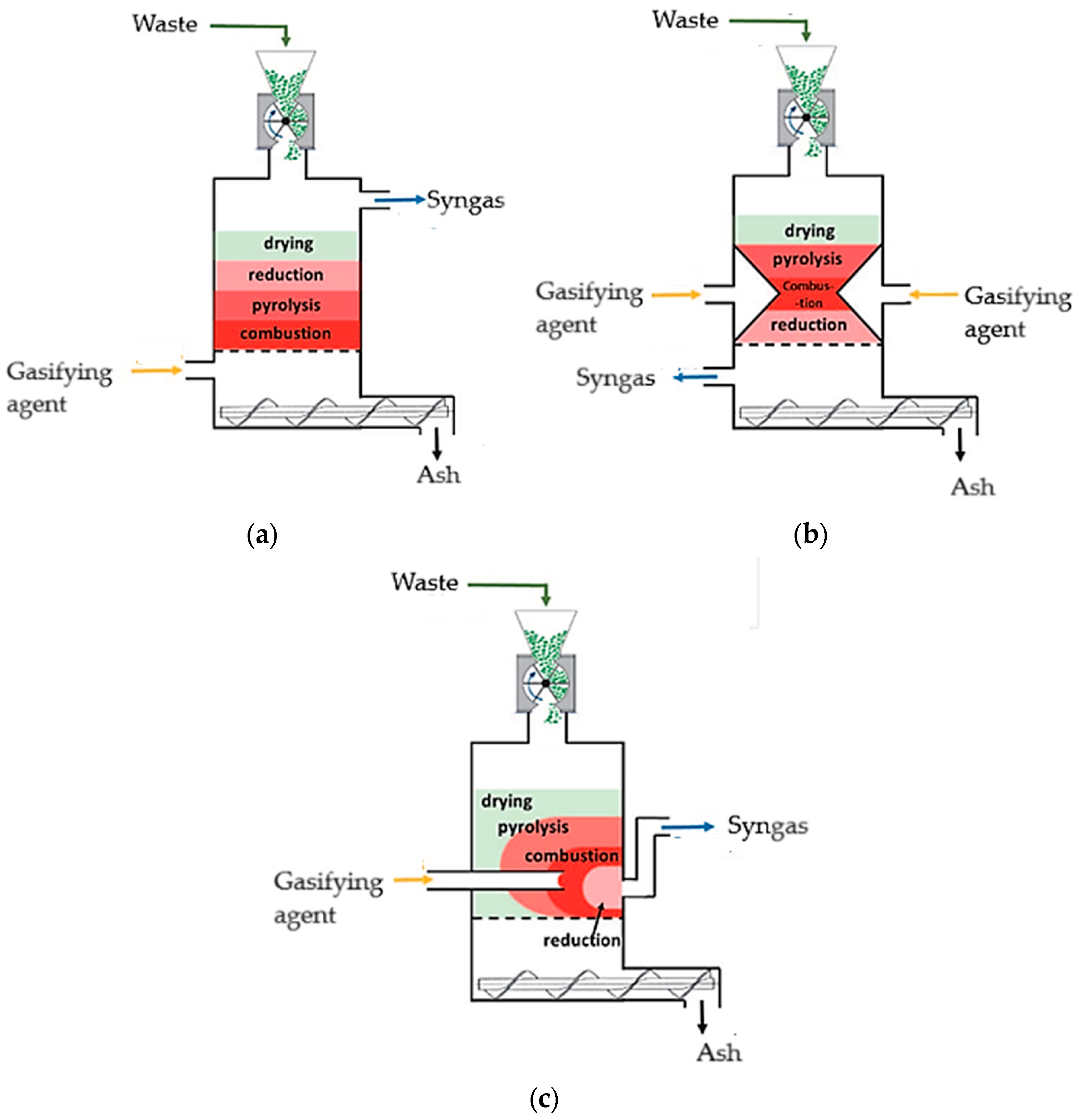
3.9. Gasifier Configuration
4. Technologies for Municipal Solid Waste Gasification
4.1. Fixed Bed Gasification
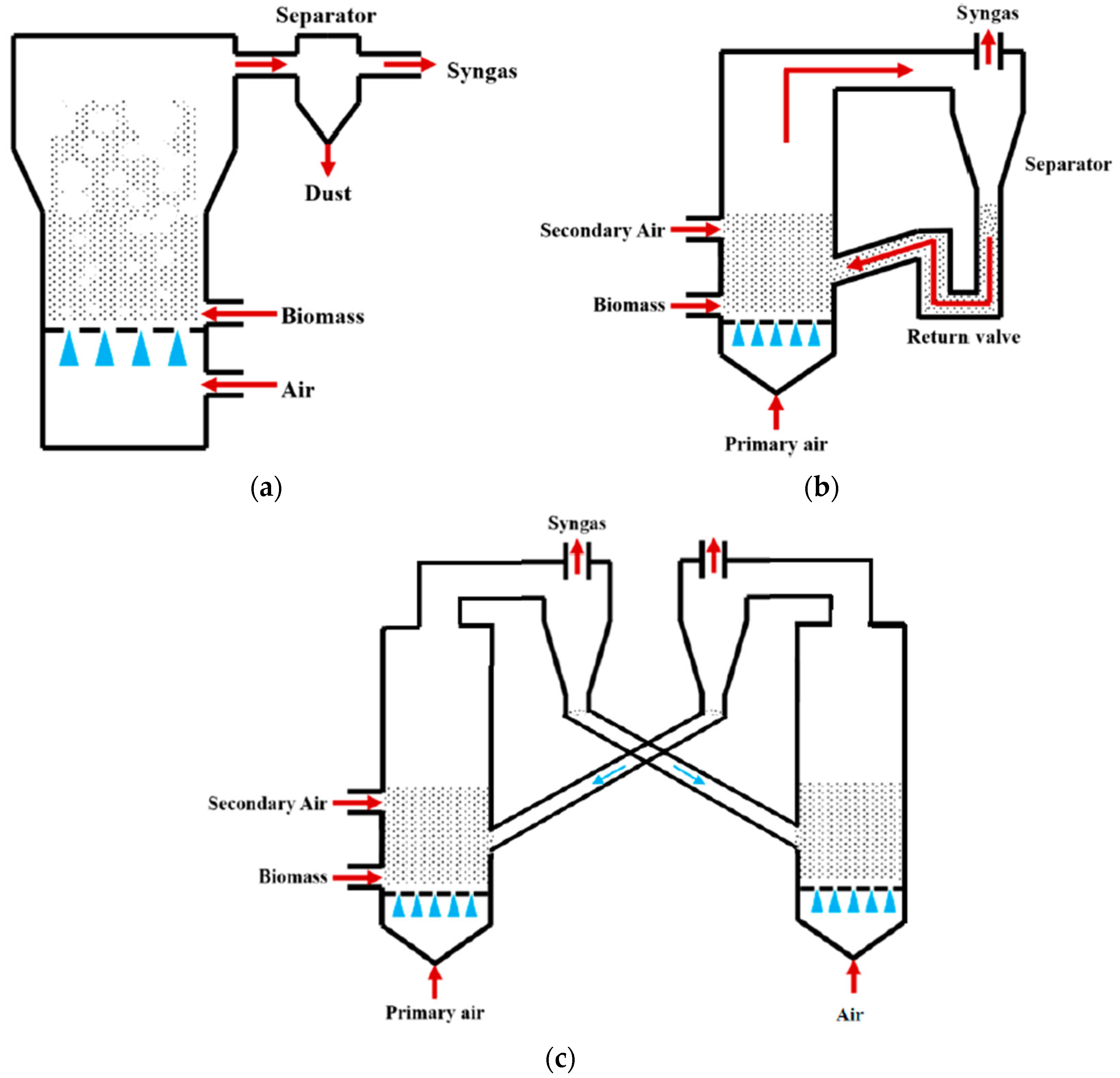
4.2. Fluidized Bed Gasification
4.3. Entrained Flow Gasification
4.4. Plasma Gasification
4.5. Supercritical Water Gasification
4.6. Microwave-Assisted Gasification
4.7. High-Temperature Steam Gasification
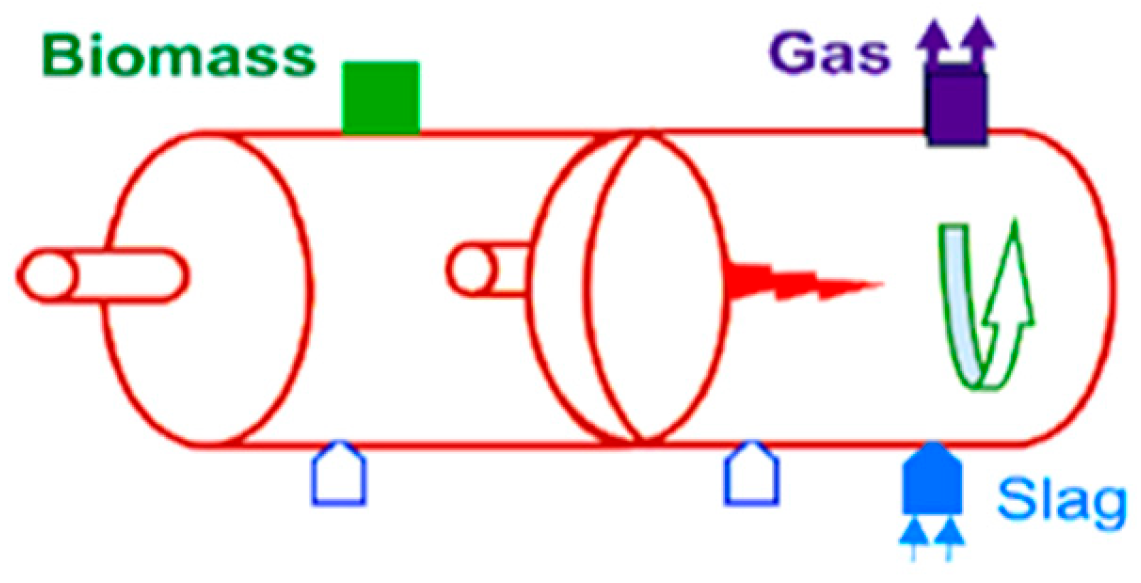
4.8. Rotary Kiln Gasification
4.9. Synthetic Comparison of Gasification Technologies
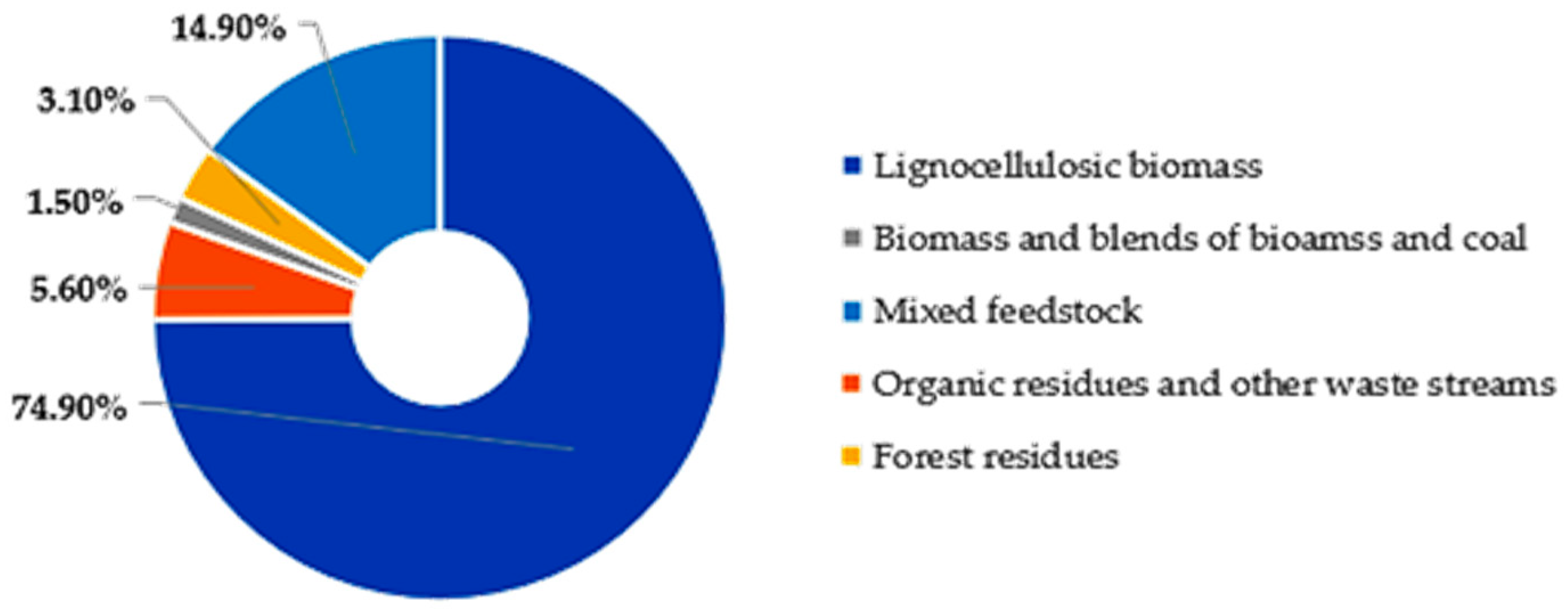
5. Potential Applications of the Gasification Products
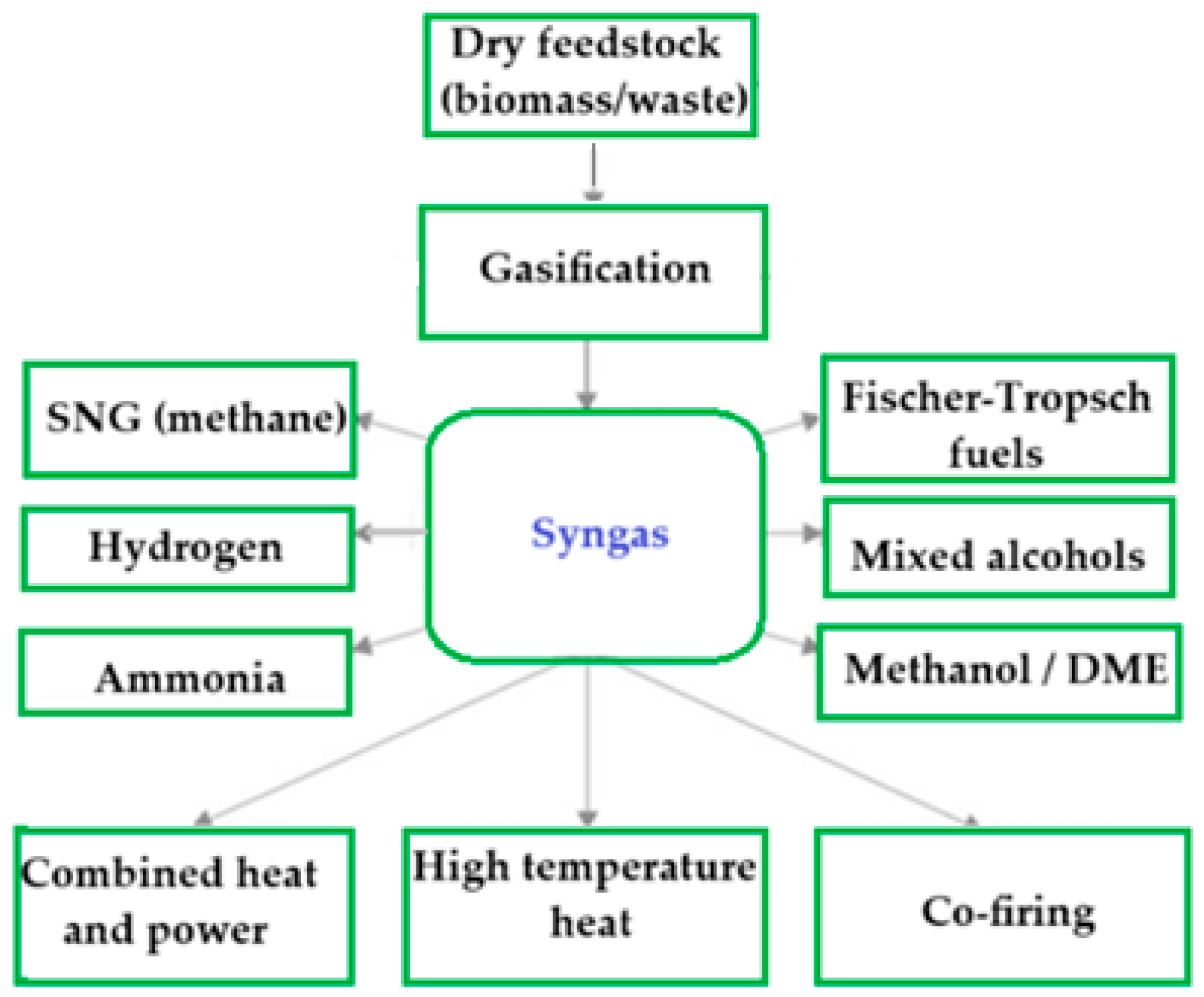
5.1. Syngas
5.2. Ash
5.3. Tar
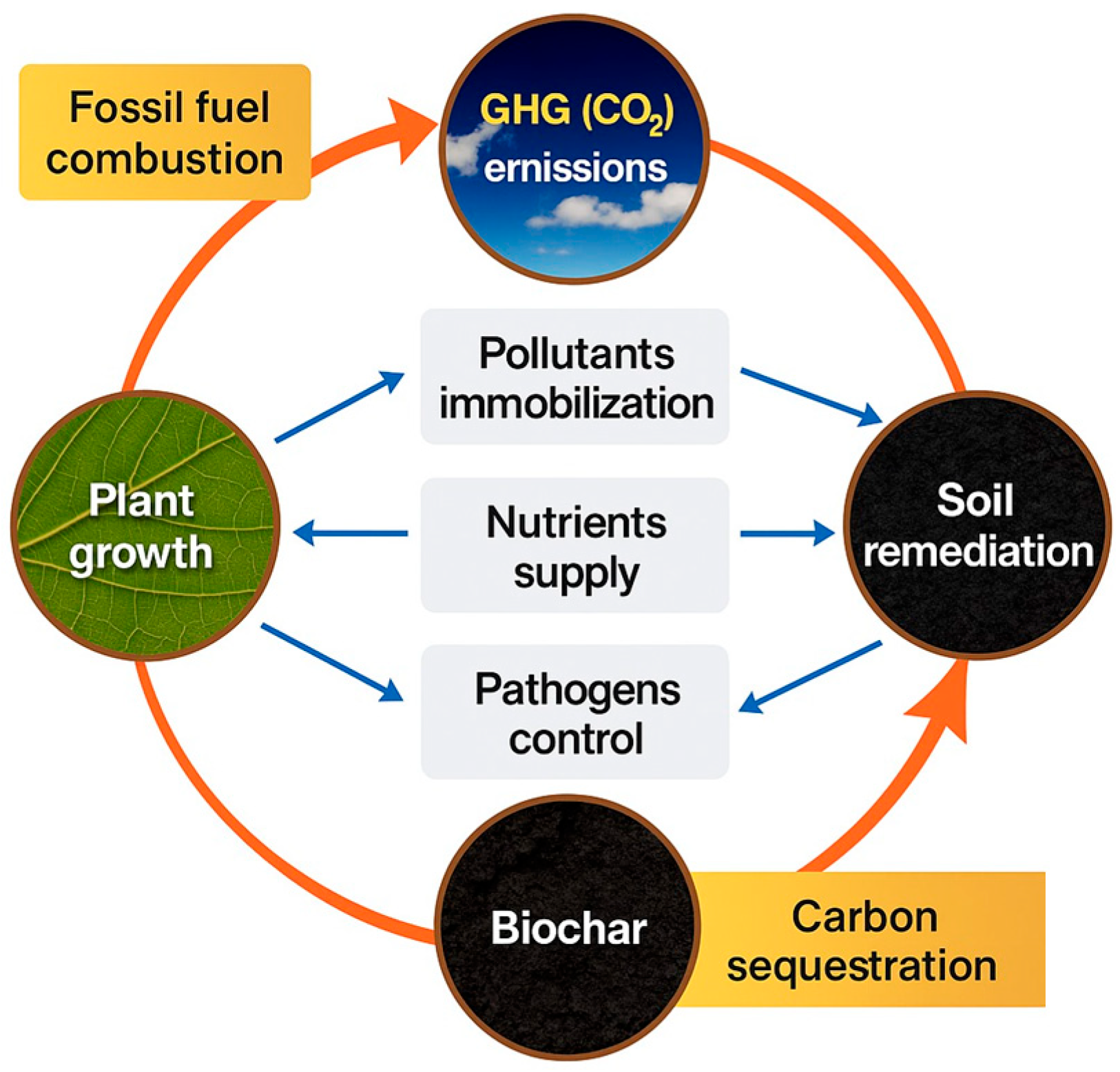
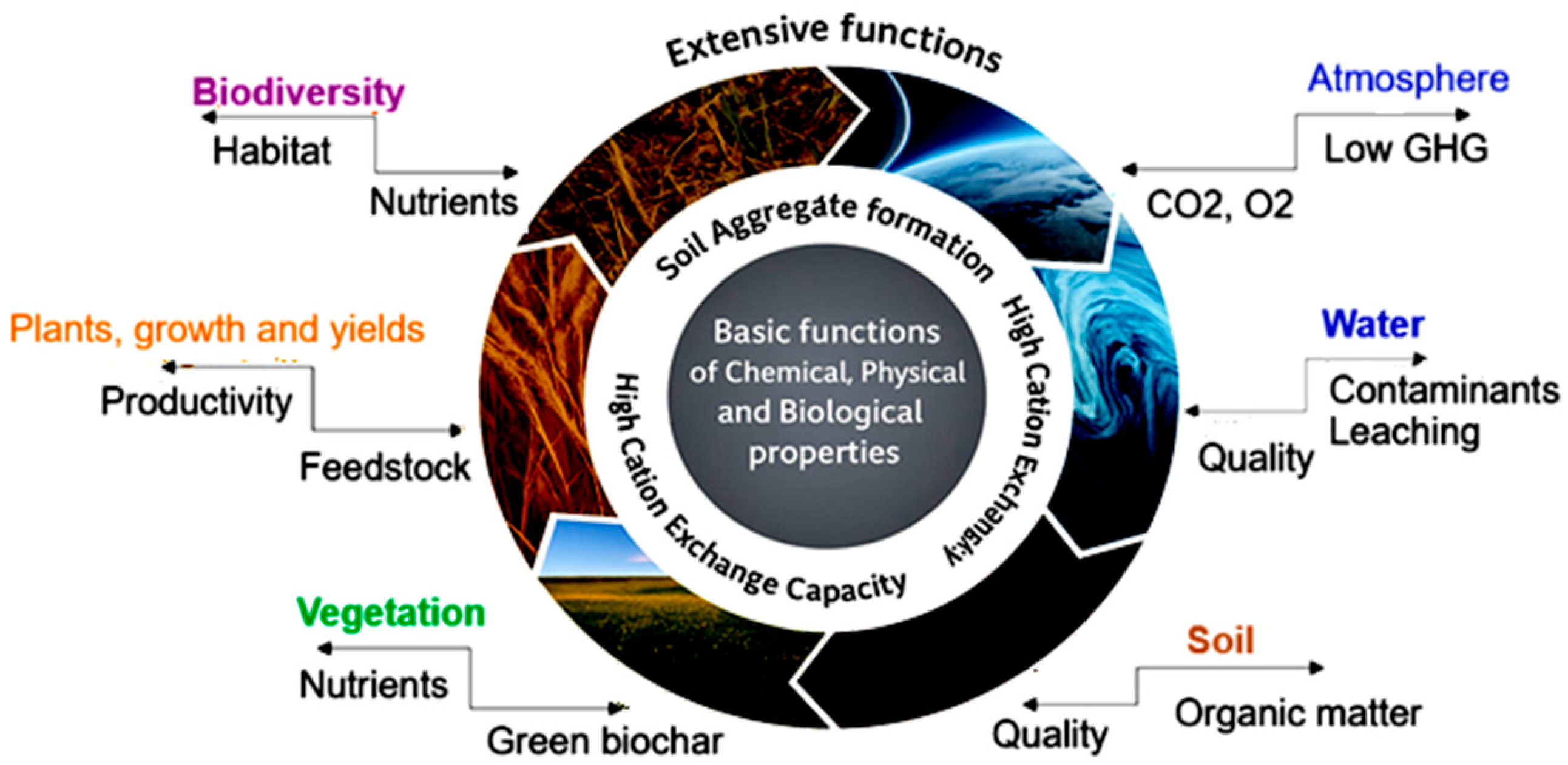
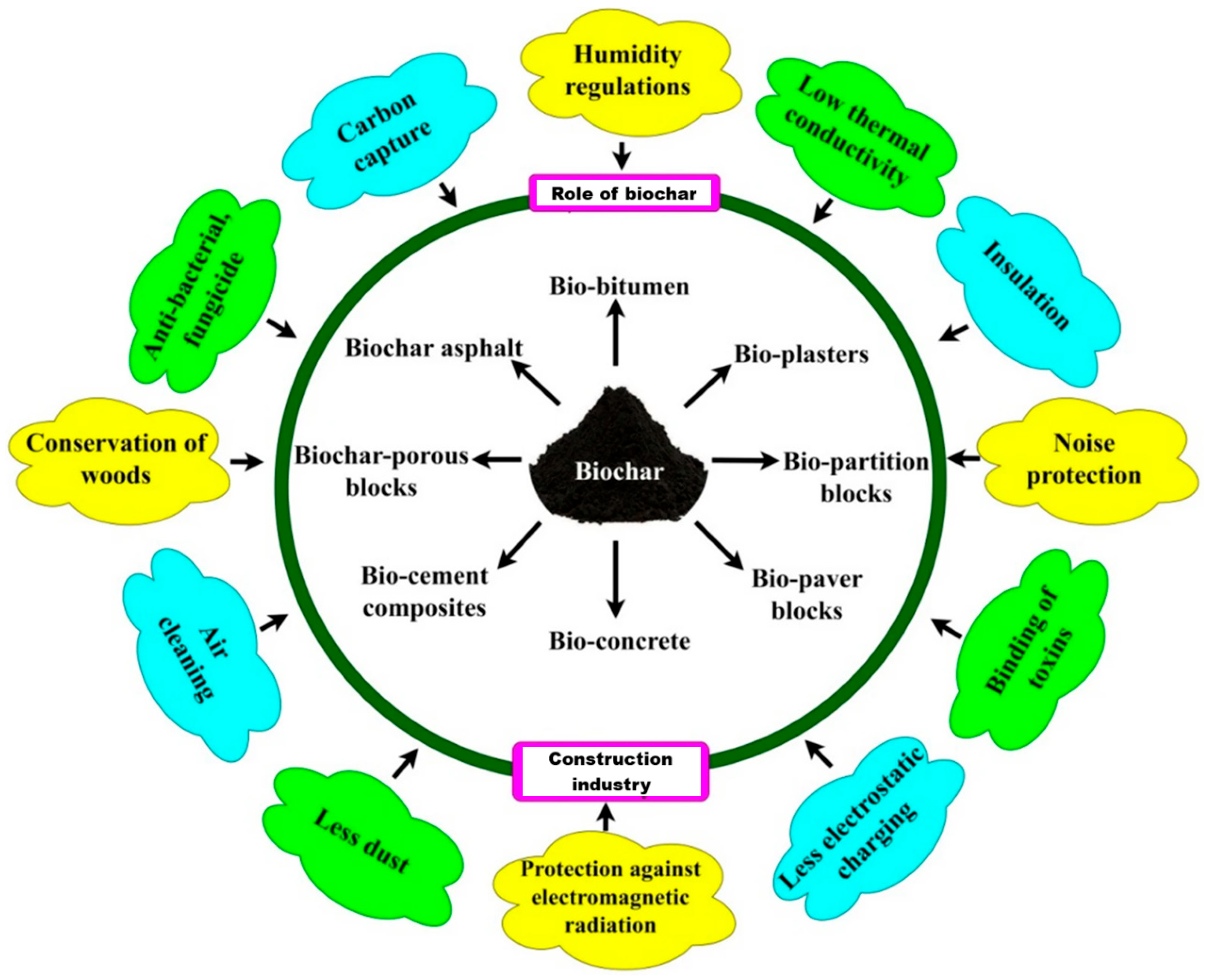
5.4. Biochar
- Agriculture
- Additive in animal feed
- Environment
- Industry
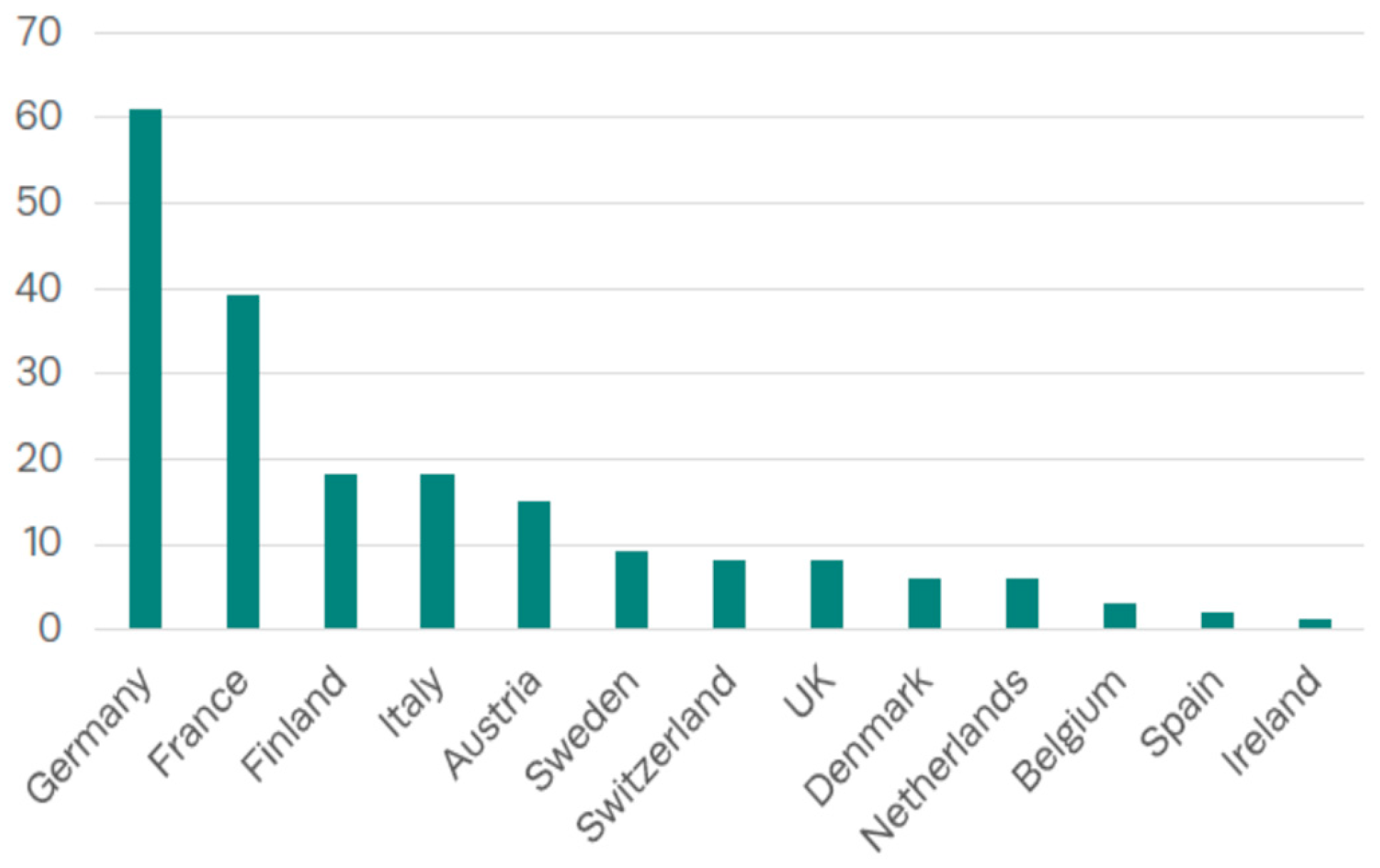
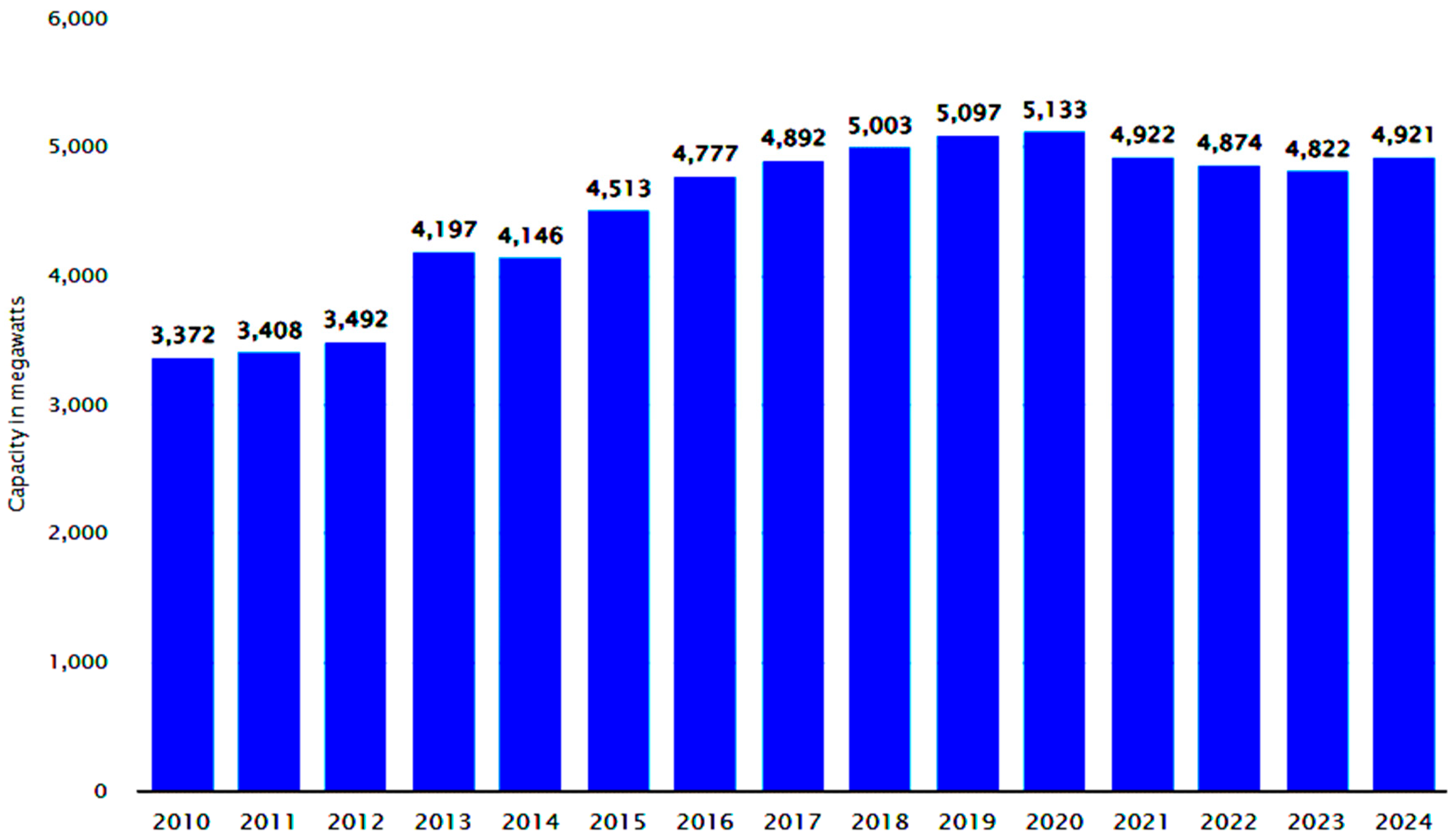
6. Current Deployment of Waste Gasification and Waste-to-Energy Technologies in Europe
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Iqbal, A.; Liu, X.; Chen, G.H. Municipal solid waste: Review of best practices in application of life cycle assessment and sustainable management technique. Sci. Total Environ. 2020, 729, 138622. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environmental Program. Global Waste Management Outlook 2024. 2024. Available online: https://www.unep.org/ietc/resources/report/global-waste-management-outlook-2024 (accessed on 13 June 2025).
- Abubakar, I.R.; Maniruzzaman, K.M.; Dano, U.L.; AlShihri, F.S.; AlShammari, M.S.; Ahmed, S.M.S.; Al-Gehlani, W.A.G.; Alrawaf, T.I. Environmental sustainability impacts of solid waste management practices in the Global South. Int. J. Env. Res. Public Health 2022, 19, 12717. [Google Scholar] [CrossRef] [PubMed]
- Saif, S.; Razia, E.T.; Khan, M.; Hatshan, M.R.; Adil, S.F. A comprehensive introduction to solid waste issues. Waste Deriv. Carbon Nanomater. 2025, 1, 1–16. [Google Scholar]
- European Confederation of Waste-to-Energy Plants—CEWEP. Latest Eurostat Figures: Municipal Waste Treatment. 2024. Available online: https://www.cewep.eu/municipal-waste-treatment-2022/ (accessed on 13 June 2025).
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0. A Global Snapshot of Solid Waste Management to 2050. Urban Development; International Bank for Reconstruction and Development; The World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Batista, M.; Gusmao Caiado, R.G.; Goncalves Quelhas, O.L.; Alves Lima, G.B.; Leal Filho, W.; Rocha Yparraguirre, I.T. A framework for sustainable and integrated municipal solid waste management: Barriers and critical factors to developing countries. J. Clean. Prod. 2021, 312, 127516. [Google Scholar] [CrossRef]
- Islam, N.F.; Gagoi, B.; Saikia, R.; Ypusaf, B.; Narayan, M.; Sarma, H. Encouraging circular economy and sustainable environmental practices by addressing waste management and biomass energy production. Reg. Sust. 2024, 5, 100174. [Google Scholar] [CrossRef]
- Paiva e Silva Muller, L.N.; Delai, I.; Chicarelli Alcantara, R.L. Circular value chain practices for developing resource value retention options. J. Clean. Prod. 2022, 359, 131925. [Google Scholar] [CrossRef]
- Ungureanu, N.; Rusănescu, C.O. Biomass and bioenergy. Energies 2025, 18, 2233. [Google Scholar] [CrossRef]
- Paraschiv, G.; Dincă, N.M.; Ungureanu, N.; Moiceanu, G.; Toma, M.L. Waste Recycling Installations; Politehnica Press Publishing House: Bucharest, Romania, 2017. [Google Scholar]
- Singh, S.K.; Tiwari, A.K. Solar-powered hydrogen production: Advancements, challenges, and the path to net-zero emissions. Int. J. Hydrogen Energy 2024, 84, 549–579. [Google Scholar] [CrossRef]
- Ma, W.; Han, W.; Liu, Q.; Song, X.; Li, J.; Zhang, N.; Xu, G. Hydrogen generation system with zero carbon emission based on synergistic conversion of methane and solar energy. Energy 2025, 316, 134609. [Google Scholar] [CrossRef]
- Boddula, R.; Lee, Y.Y.; Masimukku, S.; Chang-Chien, G.P.; Pothu, R.; Srivastava, R.K.; Sarangi, P.K.; Selvaraj, M.; Basumatary, S.; Al-Qahtani, N. Sustainable hydrogen production: Solar-powered biomass conversion explored through (photo)electrochemical advancements. Process Saf. Environ. Prot. 2024, 186, 1149–1168. [Google Scholar] [CrossRef]
- Xue, X.; Li, G.; Wang, Y.; Han, W.; Liu, C.; Jiao, F. Proposal and evaluation of a near-zero carbon emissions hydrogen production system coupled with photovoltaic, photothermal and coal gasification. Appl. Energy 2025, 377, 124400. [Google Scholar] [CrossRef]
- Ungureanu, N. Biomass-Based Energy Systems (Part II). Gasification Technology and by-Products Valorization; Course for Specialization of Informatics Applied to Environmental Engineering; Faculty of Biotechnical Systems Engineering, National University of Science and Technology Politehnica: Bucharest, Romania, 2023. Available online: https://curs.upb.ro/2024/course/view.php?id=8900 (accessed on 13 June 2025).
- Guan, G.; Kaewpanha, M.; Hao, X.; Abudula, A. Catalytic steam reforming of biomass tar: Prospects and challenges. Renew. Sustain. Energy Rev. 2016, 58, 450–461. [Google Scholar] [CrossRef]
- Montouris, A.; Voutsas, E.; Tassios, D. Solid waste plasma gasification: Equilibrium model development and exergy analysis. Energy Convers. Manag. 2006, 47, 1723–1737. [Google Scholar] [CrossRef]
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef]
- Perry, R.H. Perry’s Chemical Engineers’ Handbook, 7th ed.; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Smith, J.M.; Van Ness, H.C.; Abbott, M.M.; Swihart, M.T. Introduction to Chemical Engineering Thermodynamics, 8th ed.; McGraw-Hill: New York, NY, USA, 2018. [Google Scholar]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenerg. 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Peng, M.-Q.; Chen, T.-H.; Jin, T.; Su, Y.-C.; Luo, S.-T.; Xu, H. A novel first-order kinetic model for simultaneous anaerobic–aerobic degradation of municipal solid waste in landfills. Processes 2024, 12, 2225. [Google Scholar] [CrossRef]
- Alouani, Y.; Saifaoui, D.; Alouani, A.; Alouani, M.A. Municipal solid waste gasification to produce hydrogen: Integrated simulation model and performance analysis. Int. J. Energy Res. 2022, 46, 20068–20078. [Google Scholar] [CrossRef]
- Singh, D.; Yadav, S.; Bharadwaj, N.; Verma, R. Low temperature steam gasification to produce hydrogen rich gas from kitchen food waste: Influence of steam flow rate and temperature. Int. J. Hydrogen Energy 2020, 45, 20843–20850. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, M.; Raheem, A.; Wang, F.; Wei, J.; Xu, D.; Song, X.; Bao, W.; Huang, A.; Zhang, S.; et al. Syngas production from biomass gasification: Influences of feedstock properties, reactor type, and reaction parameters. ACS Omega 2023, 8, 31620–31631. [Google Scholar] [CrossRef]
- Wilk, V.; Hofbauer, H. Influence of fuel particle size on gasification in a dual fluidized bed steam gasifier. Fuel Process. Technol. 2013, 115, 139–151. [Google Scholar] [CrossRef]
- Vlăduțoiu, L.C.; Cristea, M.; Nenciu, F.; Vlăduț, V.; Olan, M.; Grigore, I.; Sorică, C.; Vanghele, N.; Cristea, O.D. Innovative pyrolysis reactor design for enhanced performance and superior biochar quality. INMATEH Agric. Eng. 2025, 75, 414–422. [Google Scholar] [CrossRef]
- Lopes Motta, I.; Toscano Miranda, N.; Maciel Filho, R.; Wolf Maciel, M.R. Biomass gasification in fluidized beds: A review of biomass moisture content and operating pressure effects. Renew. Sustain. Energy Rev. 2018, 94, 998–1023. [Google Scholar] [CrossRef]
- Stapf, D.; Ciceri, G.; Johansson, I.; Whitty, K. Biomass pre-treatment for bioenergy. In Case Study 3—Pretreatment of Municipal Solid Waste (MSW) for Gasification; IEA Bioenergy: Ottawa, ON, Canada, 2019. [Google Scholar]
- Mishra, S.; Upadhyay, R.K. Review on biomass gasification: Gasifiers, gasifying mediums, and operational parameters. Mater. Sci. Energy Technol. 2021, 4, 329–340. [Google Scholar] [CrossRef]
- Brandenberger, M.; Matzenberger, J.; Vogel, F.; Ludwig, C. Producing synthetic natural gas from microalgae via supercritical water gasification: A technoeconomic sensitivity analysis. Biomass Bioenerg. 2013, 51, 26–34. [Google Scholar] [CrossRef]
- Heberlein, S.; Chan, W.P.; Veksha, A.; Giannis, A.; Hupa, L.; Lisak, G. High temperature slagging gasification of municipal solid waste with biomass charcoal. J. Hazard. Mater. 2021, 423, 127057. [Google Scholar] [CrossRef]
- Dunayevska, N.; Shendrik, T.; Fataiev, A. The approach for reducing of slagging and corrosion properties of fuels with a high content of alkali and alkaline earth metals. Fuel 2025, 381, 133359. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield—A review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Wu, W. Design, optimization and energetic efficiency of producing hydrogen-rich gas from biomass steam gasification. Energies 2015, 8, 94–110. [Google Scholar] [CrossRef]
- Inayat, M.; Sulaiman, S.A.; Shahbaz, M.; Bhayo, B.A. Application of response surface methodology in catalytic co-gasification of palm wastes for bioenergy conversion using mineral catalysts. Biomass Bioenerg. 2020, 132, 105418. [Google Scholar] [CrossRef]
- Lourinho, G.; Alves, O.; Garcia, B.; Rijo, B.; Brito, P.; Nobre, C. Costs of gasification technologies for energy and fuel production: Overview, analysis, and numerical estimation. Recycling 2023, 8, 49. [Google Scholar] [CrossRef]
- Weiland, F.; Lundström, S.; Ögren, Y. Oxygen-blown gasification of pulp mill bark residues for synthetic fuel production. Processes 2021, 9, 163. [Google Scholar] [CrossRef]
- Kun, U.H.; Ksepko, E. Advancing municipal solid waste management through gasification technology. Processes 2025, 13, 2000. [Google Scholar] [CrossRef]
- Ofuani, N.; Bhoi, P. Carbon dioxide gasification of biochar: A sustainable way of utilizing captured CO2 to mitigate greenhouse gas emission. Sustainability 2024, 16, 5044. [Google Scholar] [CrossRef]
- Sher, F.; Hameed, S.; Smjecanin Omerbegovic, N.; Chupin, A.; Ul Hai, I.; Wang, B.; Teoh, Y.H.; Yildiz, M.J. Cutting-edge biomass gasification technologies for renewable energy generation and achieving net zero emissions. Energy Convers. Manag. 2025, 323, 119213. [Google Scholar] [CrossRef]
- Andrew, R.; Gokak, D.T.; Sharma, P.; Sharma, J.; Somkuwar, N.; Gupta, S. Practical achievements on biomass steam gasification in a rotary tubular coiled-downdraft reactor. Procedia Environ. Sci. 2016, 35, 818–825. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, T.; Hou, B.; Yang, H.; Hu, N.; Zhang, M. A comprehensive review of biomass gasification characteristics in fluidized bed reactors: Progress, challenges, and future directions. Fluids 2025, 10, 147. [Google Scholar] [CrossRef]
- Rupesh, S.; Muraleedharan, C.; Arun, P. Influence of residence time on syngas composition in CaO enhanced air–steam gasification of biomass. Environ. Dev. Sustain. 2021, 24, 8363–8377. [Google Scholar] [CrossRef]
- Begum, S.; Rasul, M.G.; Akbar, D.; Ramzan, N. Performance analysis of an integrated fixed bed gasifier model for different biomass feedstocks. Energies 2013, 6, 6508–6524. [Google Scholar] [CrossRef]
- Kaneesamkandi, Z.; Rehman, A.U.; Usmani, Y.S.; Sayeed, A.; Alabi, H.S. Methodology for selecting an ideal thermal gasification technique for municipal solid waste using multi-criteria decision analysis. Appl. Sci. 2023, 13, 12675. [Google Scholar] [CrossRef]
- Ongen, A.; Kurtulus Ozcan, H.; Ozbas, E.E. Gasification of biomass and treatment sludge in a fixed bed gasifier. Int. J. Hydrogen Energy 2016, 41, 8146–8153. [Google Scholar] [CrossRef]
- Jothiprakash, G.; Balasubramaniam, P.; Sundaram, S.; Ramesh, D. Catalytic biomass gasification for syngas production: Recent progress in tar reduction and future perspectives. Biomass 2025, 5, 37. [Google Scholar] [CrossRef]
- Mai, T.P.; Nguyen, D.Q. Gasification of Biomass. In Gasification for Low-Grade Feedstock; Basu, P., Ed.; IntechOpen: London, UK, 2020; pp. 1–13. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory, 4th ed.; Academic Press, Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- IEA Bioenergy. Status Report on Thermal Gasification of Biomass and Waste 2021. IEA Bioenergy Task 33. 2022. Available online: https://www.ieabioenergy.com/wp-content/uploads/2022/03/Status-Report2021_final.pdf (accessed on 24 April 2025).
- European Biogas Association. Gasification. Diversification of Biomass Processing and Waste Utilization; European Biogas Association: Brussels, Belgium, 2024. Available online: https://www.europeanbiogas.eu/wp-content/uploads/2024/12/EBA-Gasification_December-2024.pdf (accessed on 24 April 2025).
- Arena, U.; Di Gregorio, F.; Zaccariello, L. Fluidized bed gasification of solid recovered fuels: Performance and syngas quality assessment. Waste Manag. 2019, 84, 151–162. [Google Scholar] [CrossRef]
- Vargas-Salgado, C.; Hurtado-Pérez, E.; Alfonso-Solar, D.; Malmquist, A. Empirical design, construction, and experimental test of a small-scale bubbling fluidized bed reactor. Sustainability 2021, 13, 1061. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Z. Thermochemical conversion of sewage sludge: Progress in pyrolysis and gasification. Water 2025, 17, 1833. [Google Scholar] [CrossRef]
- Ewald, A.; Spliethoff, H.; Fendt, S. Entrained flow gasification of sewage sludge-performance parameters and the fate of phosphorus, potassium, sulfur, and heavy metals. Energy Fuels 2025, 39, 2616–2629. [Google Scholar] [CrossRef]
- Dammann, M.; Santo, U.; Boning, D.; Knoch, H.; Eberhard, M.; Kolb, T. Entrained flow gasification: Pilot-scale experimental, balancing and equilibrium data for model validation. Fuel 2025, 382, 132809. [Google Scholar] [CrossRef]
- Mower, G. Plasma Gasification: Revolutionizing Waste Management. Alliance for Innovation and Infrastructure. Policy Blog, Innovation and Technology, Energy. 2024. Available online: https://www.aii.org/plasma-gasification-revolutionizing-waste-management/ (accessed on 14 June 2025).
- Kaushal, R.; Rohit; Dhaka, A.K. A comprehensive review of the application of plasma gasification technology in circumventing the medical waste in a post-COVID-19 scenario. Biomass Convers. Biorefin. 2024, 14, 1427–1442. [Google Scholar] [CrossRef]
- Cai, X.; Wei, X.; Du, C. Thermal plasma treatment and coprocessing of sludge for utilization of energy and material. Energy Fuel 2020, 34, 7775–7805. [Google Scholar] [CrossRef]
- Gabbar, H.A.; Darda, S.A.; Damideh, V.; Hassen, I.; Aboughaly, M.; Lisi, D. Comparative study of atmospheric pressure DC, RF, and microwave thermal plasma torches for waste to energy applications. Sustain. Energy Technol. Assess. 2021, 47, 101447. [Google Scholar] [CrossRef]
- Yaliwal, V.S.; Banapurmath, N.R.; Gireesh, N.M.; Tewari, P.G. Production and utilization of renewable and sustainable gaseous fuel for power generation applications: A review of literature. Renew. Sustain. Energy Rev. 2014, 34, 608–627. [Google Scholar] [CrossRef]
- Erdogan, A.A.; Yilmazoglu, M.Z. Plasma gasification of the medical waste. Int. J. Hydrogen Energy 2021, 46, 29108–29125. [Google Scholar] [CrossRef]
- Tagliaferri, C.; Evangelisti, S.; Clift, R.; Lettieri, P.; Chapman, C.; Taylor, R. Life cycle assessment of conventional and advanced two-stage energy-from-waste technologies for methane production. J. Clean. Prod. 2016, 129, 144–158. [Google Scholar] [CrossRef]
- Materazzi, M.; Lettieri, P.; Mazzei, L.; Taylor, R.; Chapman, C. Thermodynamic modelling and evaluation of a two-stage thermal process for waste gasification. Fuel 2013, 108, 356–369. [Google Scholar] [CrossRef]
- Tamošiūnas, A.; Milieška, M.; Gimžauskaitė, D.; Aikas, M.; Uscila, R.; Zakarauskas, K.; Fendt, S.; Bastek, S.; Spliethoff, H. Plasma gasification of medical plastic waste to syngas in a greenhouse gas (CO2) Environment. Sustainability 2025, 17, 2040. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; Gil, M.V.; Gómez, X. Syngas fermentation: Cleaning of syngas as a critical stage in fermentation performance. Fermentation 2023, 9, 898. [Google Scholar] [CrossRef]
- Munir, M.T.; Mardon, I.; Al-Zuhair, S.; Shawabkeh, A.; Saqib, N.U. Plasma gasification of municipal solid waste for waste-to-value processing. Renew. Sustain. Energy Rev. 2019, 116, 109461. [Google Scholar] [CrossRef]
- Zhang, Y.; Matsumura, Y. Recent advances in supercritical water gasification for hydrogen production from biomass. Renew. Sustain. Energy Rev. 2021, 137, 110489. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Tanvir Alam, M.; Krishna, B.B.; Bhaskar, T.; Perkins, G. A review on the production of renewable aviation fuels from the gasification of biomass and residual wastes. Bioresour. Technol. 2020, 312, 123596. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; He, M. A review on supercritical water gasification for CO2 utilization and hydrogen production. Int. J. Hydrogen Energ. 2022, 47, 8012–8031. [Google Scholar] [CrossRef]
- Du, X.; Lei, J.; Zhu, C.; Sima, J.; Sun, C.; Wang, J.; Song, K.; Huang, L.; Huang, Q.; Yan, J. Microwave-assisted gasification of plastics for tunable syngas production via bauxite residue-based catalytic oxygen carriers. Chem. Eng. J. 2025, 510, 161606. [Google Scholar] [CrossRef]
- Arpia, A.A.; Nguyen, B.; Chen, W.H.; Dong, C.D.; Ok, Y.S. Microwave-assisted gasification of biomass for sustainable and energy-efficient biohydrogen and biosyngas production: A state-of-the-art review. Chemosphere 2022, 287, 132014. [Google Scholar] [CrossRef]
- Che, Y.; Yan, B.; Li, J.; Zhao, Z.; Gao, X.; Chen, G.; Cravotto, G.; Sun, Y.; Zhao, J. Microwave applied to the thermochemical conversion of biomass: A review. Renew. Sustain. Energy Rev. 2025, 216, 115674. [Google Scholar] [CrossRef]
- Ayuso-Díaz, I.; Perez-Gil, S.; Lopez, G.; Santamaria, L.; Antoñanzas-González, F.J. Progress on waste plastics gasification process: A review of operating conditions, reactors and catalysts for clean syngas production and tar abatement. Int. J. Hydrogen Energy 2025, 148, 150000. [Google Scholar] [CrossRef]
- Arregi, A.; Lopez, G.; Amutio, M.; Bilbao, J. High-temperature steam gasification of plastic waste: Effect on syngas composition and hydrogen production. J. Clean. Prod. 2022, 363, 132635. [Google Scholar] [CrossRef]
- Nipattummakul, N.; Ahmed, I.; Kerdsuwan, S.; Gupta, A.K. High temperature steam gasification of wastewater sludge. Appl. Energy 2010, 87, 3729–3734. [Google Scholar] [CrossRef]
- Lee, U.; Chung, J.N.; Ingley, H.A. High-temperature steam gasification of municipal solid waste, rubber, plastic and wood. Energy Fuels 2014, 28, 4573–4587. [Google Scholar] [CrossRef]
- Mungyeko Bisulandu, B.J.R.; Huchet, F. Rotary kiln process: An overview of physical mechanisms, models and applications. Appl. Therm. Eng. 2022, 221, 119637. [Google Scholar] [CrossRef]
- Freda, C.; Catizzone, E.; Villone, A.; Cornacchia, G. Biomass gasification in rotary kiln integrated with a producer gas thermal cleaning unit: An experimental investigation. Results Eng. 2024, 21, 101763. [Google Scholar] [CrossRef]
- Shi, H.; Si, W.; Li, X. The concept, design and performance of a novel rotary kiln type air-staged biomass gasifier. Energies 2016, 9, 67. [Google Scholar] [CrossRef]
- Abouemara, K.; Shahbaz, M.; Mckay, G.; Al-Ansari, T. The review of power generation from integrated biomass gasification and solid oxide fuel cells: Current status and future directions. Fuel 2024, 360, 130511. [Google Scholar] [CrossRef]
- Situmorang, Y.A.; Zhao, Z.; Yoshida, A.; Abudula, A.; Guan, G. Small-scale biomass gasification systems for power generation (<200 kW class): A review. Renew. Sustain. Energy Rev. 2020, 117, 109486. [Google Scholar] [CrossRef]
- Courson, C.; Gallucci, K. 8—Gas cleaning for waste applications (syngas cleaning for catalytic synthetic natural gas synthesis). In Substitute Natural Gas from Waste: Technical Assessment and Industrial Applications of Biochemical and Thermochemical Processes; Materazzi, M., Foscolo, P.U., Eds.; Academic Press, Elsevier: Amsterdam, The Netherlands, 2019; pp. 161–220. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, S.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939. [Google Scholar] [CrossRef]
- Valderrama Rios, M.L.; Gonzalez, A.M.; Lora, E.E.S.; Almazan del Olmo, O.A. Reduction of tar generated during biomass gasification: A review. Biomass Bioenerg. 2018, 108, 345–370. [Google Scholar] [CrossRef]
- Castagnoli, A.; Salem, A.M.; Desideri, U.; Pecorini, I. Environmental assessment of gasification and green hydrogen potential role in waste management decarbonization. J. Clean. Prod. 2024, 482, 144174. [Google Scholar] [CrossRef]
- Yan, B.; Jia, X.; Li, J.; Li, Z.; Che, Y.; Zhou, Z.; Zhao, J.; Zhou, S.; Chen, G. In-situ elimination of biomass gasification tar based on the understanding of tar formation process: A review. J. Energy Inst. 2024, 112, 101477. [Google Scholar] [CrossRef]
- Shahbaz, M.; AlNouss, A.; Ghiat, I.; Mckay, G.; Mackey, H.; Elkhalifa, S.; Al-Ansari, T. A comprehensive review of biomass based thermochemical conversion technologies integrated with CO2 capture and utilisation within BECCS networks. Resour. Conserv. Recycl. 2021, 17, 105734. [Google Scholar] [CrossRef]
- IEA Bioenergy. Valuable (by-)Products of Gasification. IEA Bioenergy Task 33. 2022. Available online: https://www.ieabioenergy.com/wp-content/uploads/2022/12/WS_Report-final1.pdf (accessed on 13 March 2025).
- Saidi, M.; Gohari, M.H.; Ramezani, A.T. Hydrogen production from waste gasification followed by membrane filtration: A review. Environ. Chem. Lett. 2020, 18, 1529–1556. [Google Scholar] [CrossRef]
- Macedo, M.S.; Soria, M.A.; Madeira, L.M. Process intensification for hydrogen production through glycerol steam reforming. Renew. Sustain. Energy Rev. 2021, 146, 111151. [Google Scholar] [CrossRef]
- Maj, I.; Niesporek, K.; Płaza, P.; Maier, J.; Łój, P. Biomass ash: A review of chemical compositions and management trends. Sustainability 2025, 17, 4925. [Google Scholar] [CrossRef]
- Puri, L.; Hu, Y.; Naterer, G. Critical review of the role of ash content and composition in biomass pyrolysis. Front. Fuels 2024, 2, 1378361. [Google Scholar] [CrossRef]
- Vlăduț, N.V.; Atanasov, A.; Ungureanu, N.; Ivașcu, L.V.; Cioca, L.I.; Matei, G.; Boruz, S.; Cerempei, V.; Țîței, V.; Żelaziński, T.; et al. Trends in the development of conservative/ecological agriculture in the context of current climate change—A review. INMATEH Agric. Eng. 2024, 74, 980–1032. [Google Scholar] [CrossRef]
- Shen, Y.; Yoshikawa, K. Recent progresses in catalytic tar elimination during biomass gasification or pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 21, 371–392. [Google Scholar] [CrossRef]
- de Lasa, H.; Torres Brauer, N.; Rojas Chaves, F.; Serrano Rosales, B. Biomass-derived tar conversion via catalytic post-gasification in circulating fluidized beds: A review. Catalysts 2025, 15, 611. [Google Scholar] [CrossRef]
- Santana, H.E.P.; Jesus, M.; Santos, J.; Rodrigues, A.C.; Pires, P.; Ruzene, D.S.; Silva, I.P.; Silva, D.P. Lignocellulosic biomass gasification: Perspectives, challenges, and methods for tar elimination. Sustainability 2025, 17, 1888. [Google Scholar] [CrossRef]
- Anis, S.; Zainal, Z.A. Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: A review. Renew. Sustain. Energy Rev. 2011, 15, 2355–2377. [Google Scholar] [CrossRef]
- Loweski Feliz, M.; Abdelouahed, L.; Taouk, B. Comparative and descriptive study of biomass gasification simulations using Aspen Plus. Energies 2024, 17, 4443. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Ryota, I. Study on the effects of tar reforming and steam gasification of Keyaki Bark in Saitama Prefecture. Sustainability 2025, 17, 2215. [Google Scholar] [CrossRef]
- Iturbides, R.D.; Haza, U.J.; Polaert, I. Recent technological innovations on continuous microwave assisted biomass pyrolysis and perspectives for industrial scale applications. Bioresour. Technol. Rep. 2022, 19, 101202. [Google Scholar] [CrossRef]
- Okebalama, C.B.; Marschner, B. Reapplication of biochar, sewage waste water, and NPK fertilizers afects soil fertility, aggregate stability, and carbon and nitrogen in dry-stable aggregates of semi-arid soil. Sci. Total Environ. 2023, 866, 161203. [Google Scholar] [CrossRef] [PubMed]
- Talwar, P.; Agudelo, M.A.; Nanda, S. Pyrolysis process, reactors, products, and applications: A review. Energies 2025, 18, 2979. [Google Scholar] [CrossRef]
- Ifran, M.F.; Mirara, F. Biochar application in improving soil health and sustainability. Bull. Biol. All. Sci. Res. 2024, 9, 81. [Google Scholar] [CrossRef]
- Zhu, S.; Liang, P.; Yang, L.; Wei, B.; Han, S.; Wu, M.; He, X.; Zeng, W.; He, Z.; Xiao, J.; et al. Effects of biochar-based fertilizers on Fenlong-Ridging soil physical properties, nutrient activation, enzyme activity, bacterial diversity, and sugarcane yield. Agronomy 2025, 15, 1594. [Google Scholar] [CrossRef]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of biochar application to the environment and economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Jatav, H.S.; Rajput, V.D.; Minkina, T.; Singh, S.K.; Chejara, S.; Gorovtsov, A.; Barakhov, A.; Bauer, T.; Sushkova, S.; Mandzhieva, S.; et al. Sustainable approach and safe use of biochar and its possible consequences. Sustainability 2021, 13, 10362. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Nelson, P. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 2010, 78, 1167–1171. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Ibrahim, M.A.; Ditta, A.; Iqbal, R.; Aslam, M.U.; Muhammad, F.; Ali, S.; Çiğ, F.; Ali, B.; Muhammad Ikram, R. Exploring the recuperative potential of brassinosteroids and nano-biochar on growth, physiology, and yield of wheat under drought stress. Sci. Rep. 2023, 13, 15015. [Google Scholar] [CrossRef]
- Edeh, I.G.; Mašek, O.; Buss, W. A meta-analysis on biochar’s effects on soil water properties—New insights and future research challenges. Sci. Total Environ. 2020, 714, 136857. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Bilias, F.; Kalderis, D.; Richardson, C.; Barbayiannis, N.; Gasparatos, D. Biochar application as a soil potassium management strategy: A review. Sci. Total Environ. 2023, 858, 159782. [Google Scholar] [CrossRef]
- Chi, W.; Nan, Q.; Liu, Y.; Dong, D.; Qin, Y.; Li, S.; Wu, W. Stress resistance enhancing with biochar application and promotion on crop growth. Biochar 2024, 6, 43. [Google Scholar] [CrossRef]
- Jatuwong, K.; Aiduang, W.; Kiatsiriroat, T.; Kamopas, W.; Lumyong, S. A review of biochar from biomass and its interaction with microbes: Enhancing soil quality and crop yield in Brassica cultivation. Life 2025, 15, 284. [Google Scholar] [CrossRef] [PubMed]
- Perra, M.; Bacchetta, G.; Muntoni, A.; De Gioannis, G.; Castangia, I.; Rajha, H.N.; Manca, M.L.; Manconi, M. An outlook on modern and sustainable approaches to the management of grape pomace by integrating green processes, biotechnologies and advanced biomedical approaches. J. Funct. Foods 2022, 98, 105276. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, H.; Chu, M.; Zhang, C.; Tang, J.; Chang, S.X.; Mašek, O.; Ok, Y.S. Biochar affects greenhouse gas emissions in various environments: A critical review. Land Degrad. Dev. 2022, 33, 3327–3342. [Google Scholar] [CrossRef]
- Korai, S.K.; Korai, P.K.; Jaffar, M.A.; Qasim, M.; Younas, M.U.; Shabaan, M.; Zulfiqar, U.; Wang, X.; Artyszak, A. Leveraging biochar amendments to enhance food security and plant resilience under climate change. Plants 2025, 14, 1984. [Google Scholar] [CrossRef]
- Dai, W.; Bao, Z.; Meng, J.; Chen, T.; Liang, X. Biochar makes soil organic carbon more labile, but its carbon sequestration potential remains large in an alternate wetting and drying paddy ecosystem. Agronomy 2025, 15, 1547. [Google Scholar] [CrossRef]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, L.; Gusiatin, Z.M.; Kucerik, J.; Hammerschmiedt, T.; Danish, S.; Radziemska, M.; Mravcova, L.; et al. A critical review of the possible adverse effects of biochar in the soil environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar] [CrossRef]
- Pathy, A.; Ray, J.; Paramasivan, B. Biochar amendments and its impact on soil biota for sustainable agriculture. Biochar 2020, 2, 287–305. [Google Scholar] [CrossRef]
- Allohverdi, T.; Mohanty, A.K.; Roy, P.; Misra, M. A review on current status of biochar uses in agriculture. Molecules 2021, 26, 5584. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Laird, D.A.; Ahmedna, M.A.; Niandou, M.A. Short-term CO2 mineralization after additions of biochar and switchgrass to a typic Kandiudult. Geoderma 2010, 154, 281–288. [Google Scholar] [CrossRef]
- Dittmann, M.T.; Baki, C.; Tereanova, M.; Amelchanka, S.L.; Subois, S.; Wiget, A.; Leiber, F.; Krause, H.M.; Baumann, S. The effect of biochar supplementation on feed utilization, milk production and methane emission in lactating dairy cows. Anim. Feed Sci. Technol. 2024, 318, 116127. [Google Scholar] [CrossRef]
- Lind, V.; Sismaz, O.; Demirtas, A.; Sudagidan, M.; Weldon, S.; Budai, A.; O’Toole, A.; Moladinovic, D.D.; Jorgensen, G.M. Biochar effect on sheep feed intake, growth rate and ruminant in vitro and in vivo methane production. Animal 2024, 18, 101195. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.S.N.; Menon, P.S.; Suresh, S.; Sreekanth, A.J.; Sivasabari, K.; Adithya, K.S.; Anuranj, P.R.; Nayana, K.; Parvathy, S.; Chakraborty, S.; et al. Beneficial impacts of biochar as a potential feed additive in animal husbandry. J. Exp. Biol. Agric. Sci. 2023, 11, 479–499. [Google Scholar] [CrossRef]
- Miroshnichenko, D.; Zhylina, M.; Shmeltser, K. Modern use of biochar in various technologies and industries. a review. Chem. Chem. Technol. 2024, 18, 232–243. [Google Scholar] [CrossRef]
- Ravindiran, G.; Rajamanickam, S.; Janardhan, G.; Hayder, G.; Alagumalai, A.; Mahian, O.; Lam, S.S.; Sonne, C. Production and modifications of biochar to engineered materials and its application for environmental sustainability: A review. Biochar 2024, 6, 62. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for wastewater treatment—Conversion technologies and applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Boehm, A.B. Escherichia coli removal in biochar-augmented biofilter: Effect of infiltration rate, initial bacterial concentration, biochar particle size, and presence of compost. Environ. Sci. Technol. 2014, 48, 11535–11542. [Google Scholar] [CrossRef]
- Runtti, H.; Tuomikoski, S.; Kangas, T.; Lassi, U.; Kuokkanena, T.; Rämö, J. Chemically activated carbon residue from biomass gasification as a sorbent for iron(II), copper(II) and nickel(II) ions. J. Water Process. Eng. 2014, 4, 12–24. [Google Scholar] [CrossRef]
- Shakya, A.; Agarwal, T. Removal of Cr(VI) from water using pineapple peel derived biochars: Adsorption potential and re-usability assessment. J. Mol. Liq. 2019, 293, 111497. [Google Scholar] [CrossRef]
- Afshar, M.; Mofatteh, S. Biochar for a sustainable future: Environmentally friendly production and diverse applications. Results Eng. 2024, 23, 102433. [Google Scholar] [CrossRef]
- Yang, X.; Han, D.; Zhao, Y.; Li, R.; Wu, Y. Environmental evaluation of a distributed-centralized biomass pyrolysis system: A case study in Shandong. China. Sci. Total Environ. 2020, 716, 136915. [Google Scholar] [CrossRef]
- Yrjälä, K.; Ramakrishnan, M.; Salo, E. Agricultural waste streams as resource in circular economy for biochar production towards carbon neutrality. Curr. Opin. Environ. Sci. Health 2022, 26, 100339. [Google Scholar] [CrossRef]
- Rehman, A.; Nawaz, S.; Alghamdi, H.A.; Alrumman, S.; Yan, W.; Nawaz, M.Z. Effects of manure-based biochar on uptake of nutrients and water holding capacity of different types of soils. Case Stud. Chem. Environ. Eng. 2020, 2, 100036. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, J.; Xue, J.; Zhang, L. Quantifying the effects of biochar application on greenhouse gas emissions from agricultural soils: A global meta-analysis. Sustainability 2020, 12, 3436. [Google Scholar] [CrossRef]
- Sharma, A.; Chhabra, V. A review on the applications of biochar in agricultural farms: A low carbon emission technology. J. Adv. Biol. Biotechnol. 2024, 27, 118181. [Google Scholar] [CrossRef]
- Kaushik, A.; Priyadharshini, P.; Manimegalai, S.; Palaniselvam, V.; Parthiban, K.T. Biochar production from plant residues: A sustainable approach for carbon sequestration and soil fertility improvement. Arch. Curr. Res. Int. 2024, 24, 1–13. [Google Scholar] [CrossRef]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Mosa, A.; Mansour, M.M.; Soliman, E.; El-Ghamry, A.; Alfy, M.E.; Kenawy, A.M.E. Biochar as a soil amendment for restraining greenhouse gases emission and improving soil carbon sink: Current situation and ways forward. Sustainability 2023, 15, 1206. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Xiong, Z.; Liu, P.; Pan, G. Effects of biochar addition on N2O and CO2 emissions from two paddy soils. Biol. Fertil. Soils 2011, 47, 887–896. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R.; Zimmerman, A.R. Effects of biochar and other amendments on the physical properties and greenhouse gas emissions of an artificially degraded soil. Sci. Total Environ. 2014, 487, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Bogomolova, I.; Glaser, B. Biochar stability in soil: Decomposition during eight years and transformation as assessed by compound-specific 14C analysis. Soil. Biol. Biochem. 2014, 70, 229–236. [Google Scholar] [CrossRef]
- Kalu, S.; Kulmala, L.; Zrim, J.; Peltokangas, K.; Tammeorg, P.; Rasa, K.; Kitzler, B.; Pihlatie, M.; Karhu, K. Potential of biochar to reduce greenhouse gas emissions and increase nitrogen use efficiency in boreal arable soils in the long-term. Front. Environ. Sci. 2022, 10, 914766. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.; Huchzermeyer, B.; Bilquees, G.; Khan, M.A. Impact of a biochar or a biochar-compost mixture on water relation, nutrient uptake and photosynthesis of Phragmites karka. Pedosphere 2020, 30, 466–477. [Google Scholar] [CrossRef]
- Adejumo, S.A.; Arowo, D.O.; Ogundiran, M.B.; Srivastava, P. Biochar in combination with compost reduced Pb uptake and enhanced the growth of maize in lead (Pb)-contaminated soil exposed to drought stress. J. Crop Sci. Biotechnol. 2020, 23, 273–288. [Google Scholar] [CrossRef]
- Linville, J.L.; Shen, Y.; Ignacio-de Leon, P.A.; Schoene, R.P.; Urgun-Demirtas, M. In situ biogas upgrading during anaerobic digestion of food waste amended with walnut shell biochar at bench scale. Waste Manag. Res. 2017, 35, 669–679. [Google Scholar] [CrossRef]
- Qu, Y.; Qu, J.; Yan, W.; Yue, T.; Zhang, Q.; Yi, W.; Liu, X.; Sun, Y. Influence of biochar on physico-chemical, microbial community and maturity during biogas residue aerobic composting process. Fermentation 2022, 8, 623. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Sun, J.; Zhang, W.; Huang, R.; Chen, Y. Effects of recycled biochar addition on methane production performance in anaerobic fermentation of pig and cow manure. Fermentation 2025, 11, 372. [Google Scholar] [CrossRef]
- Cara, I.G.; Țopa, D.; Puiu, I.; Jităreanu, G. Biochar a promising strategy for pesticide-contaminated soils. Agriculture 2022, 12, 1579. [Google Scholar] [CrossRef]
- Paula, R.; Carvalho, J.; Júnior, A.; Fagundes, F.; de Lima, R.; Lima, E.; Oliveira, C.; de Oliveira, M.; Bezerra, A.; Ferreira, O.; et al. Analysis of biochar-cement composites by SEM/EDS: Interfacial interactions and effects on mechanical strength. C J. Carbon Res. 2025, 11, 45. [Google Scholar] [CrossRef]
- Gupta, S.; Hua, H.W.; Low, C.Y. Use of biochar as carbon sequestering additive in cement mortar. Cem. Concr. Compos. 2018, 87, 110–129. [Google Scholar] [CrossRef]
- Mensah, R.A.; Shanmugam, V.; Narayanan, S.; Razavi, N.; Ulfberg, A.; Blanksvärd, T.; Sayahi, F.; Simonsson, P.; Reinke, B.; Försth, M.; et al. Biochar-added cementitious materials—A review on mechanical, thermal, and environmental properties. Sustainability 2021, 13, 9336. [Google Scholar] [CrossRef]
- Legan, M.; Gotvajn, A.Z.; Zupan, K. Potential of biochar use in building materials. J. Environ. Manag. 2022, 309, 114704. [Google Scholar] [CrossRef] [PubMed]
- Raja, P.; Murugan, V.; Ravichandran, S.; Behera, L.; Mensah, R.A.; Mani, S.; Kasi, A.K.; Balasubramanian, K.B.N.; Sas, G.; Vahabi, H.; et al. A review of sustainable bio-based insulation materials for energy-efficient buildings. Macromol. Mater. Eng. 2023, 308, 2300086. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Méndez, A.; Gómez, A.; Paz-Ferreiro, J.; Gascó, G. Effects of sewage sludge biochar on plant metal availability after application to a Mediterranean soil. Chemosphere 2013, 89, 1354–1359. [Google Scholar] [CrossRef]
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustain. Energy 2009, 28, 386–396. [Google Scholar] [CrossRef]
- Bosmans, A.; Vanderreydt, I.; Geysen, D.; Helsen, L. The crucial role of waste-to-energy technologies in enhanced landfill mining: A technology review. J. Clean. Prod. 2013, 55, 10–23. [Google Scholar] [CrossRef]
- European Biogas Association. Biogases Towards 2040 and Beyond; European Biogas Association: Brussels, Belgium, 2024. Available online: https://www.europeanbiogas.eu/wp-content/uploads/2024/04/Biogases-towards-2040-and-beyond_FINAL.pdf (accessed on 24 April 2025).
- European Confederation of Waste-to-Energy Plants—CEWEP. Waste-to-Energy Plants in Europe in 2022. 2024. Available online: https://www.cewep.eu/waste-to-energy-plants-in-europe-in-2022/ (accessed on 13 June 2025).
- Statista. Installed Capacity of Municipal Waste Energy in Europe from 2010 to 2024. 2025. Available online: https://www.statista.com/statistics/1122054/europe-waste-to-energy-capacity/ (accessed on 13 March 2025).
- Uddin, M.N. A comprehensive exploration of biomass gasification technologies advancing United Nations sustainable development goals: Part II. John. Matthey Technol. Rev. 2025, 69, 13–23. [Google Scholar] [CrossRef]



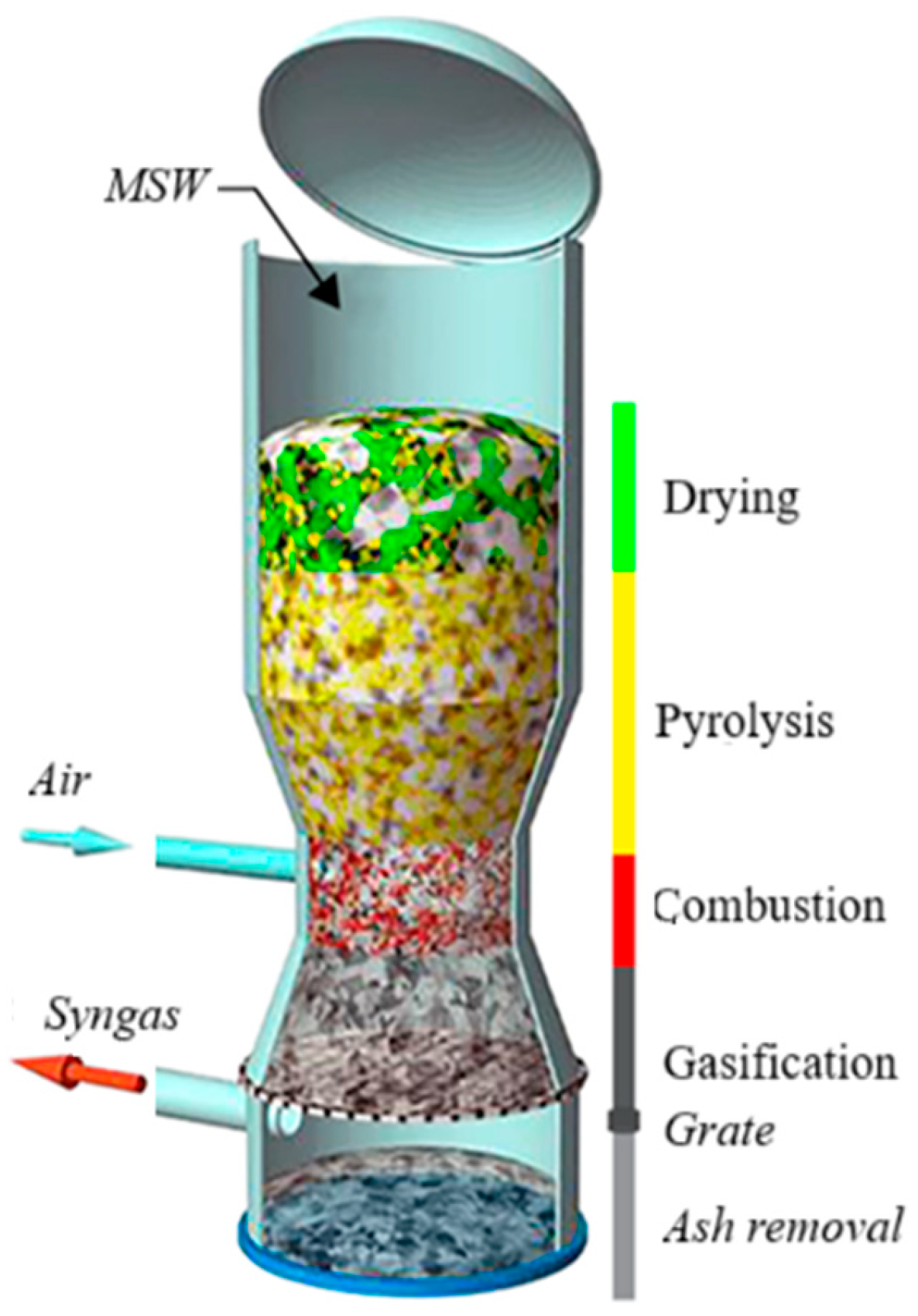
| Stage | Description |
|---|---|
| Drying | MSW and/or biomass, which typically contains a high moisture fraction (especially food waste and green waste), is dried at temperatures of 100–200 °C to reduce moisture content below 5–10%. Efficient drying is essential for thermal stability and improved reaction kinetics in the downstream stages. |
| Pyrolysis (devolatilization) | In the absence or limitation of oxygen, thermochemical decomposition occurs at temperatures between 150–900 °C, breaking down organic fractions of MSW and/or biomass into volatile gases, condensable tars, and a solid carbonaceous residue (char.) This char is the key reactive material for the subsequent gasification reactions. Some volatile compounds condense into a liquid phase upon cooling to room temperature, forming tar, a black, viscous, and corrosive substance composed of complex heavy organic and inorganic molecules. |
| Partial oxidation (combustion) | At temperatures over 750 °C, the fixed carbon (char) reacts exothermically with a limited amount of oxygen or air, generating CO2 and releasing heat. This thermal energy supports the endothermic reduction reactions. Hydrogen from volatile components may also be oxidized to form H2O. |
| Reduction (gasification) | At temperatures between 800–1000 °C, the remaining carbon reacts with CO2 and/or H2O (steam) to produce a combustible gas mixture (syngas) primarily composed of CO and H2, with minor fractions of CH4, CO, CO2, N2, and light hydrocarbons. This is the final and critical stage where energy-rich gas is recovered for downstream applications. |
| Equation | ΔH0 (kJ/mol) | Reaction Type | Reference |
|---|---|---|---|
| (2) | +206 | Endothermic | [20,21] |
| (3) | −41 | Exothermic | [20,21] |
| (4) | +131 | Endothermic | [20,21] |
| (5) | +172 | Endothermic | [20,21] |
| (6) | −221 | Exothermic | [20,21] |
| (7) | −394 | Exothermic | [20,21] |
| (8) | ΔH0 > 0 | Presumed endothermic | [20,21] |
| (2) | +206 | Endothermic | [22] |
| Parameter | Incineration | Pyrolysis | Gasification |
|---|---|---|---|
| Oxygen supply | Excess air (>100%) | None (anaerobic) | Sub-stoichiometric (25–30%) |
| Temperature range (°C) | 850–1100 | 300–700 | 900–1200 |
| Main products | Heat + flue gases (CO2, H2O, NOx, SOx, particles, dioxins etc.) + bottom and fly ash | Char (solid) + condensable vapors (bio-oil or pyrolytic liquid) + non-condensable gases (pyrolysis gas) | Synthesis gas (syngas) + solid residues (slag or ash) + minor amounts of tars and unconverted char |
| Carbon conversion | Complete | Partial (fixed carbon remains) | Near-complete |
| Energy efficiency (% lower heating value) | 15–25 | 35–50 (with valorization) | 60–80 (with syngas utilization) |
| Air pollutants (NOx, SOx, PM) | High (needs advanced control) | Low–moderate | Low (if gas cleanup is applied) |
| Suitability for MSW | Very common | Limited (requires presorting) | Increasingly used, especially RDF/MSW |
| Dioxins and furans | Risk present | Minimal | Very low |
| Ash production | High | Medium | Low–medium (possible vitrified slag) |
| Parameter | Direct Current Transferred | Direct Current Non-Transferred | Radiofrequency |
|---|---|---|---|
| Temperature (°C) | 11,727–19,727 | 9727–13,727 | 2727–7727 |
| Input power (kW) | Maximum 80 | Maximum 80 | 30–35 |
| Electrode erosion | Yes | Yes | No |
| Electrode material | Graphite, copper | Graphite, brass, tungsten | Without electrodes |
| Cooling method | Needed | Not needed | Not needed |
| Plasma gas | N2, Ar, CO2, air | N2, Ar, CO2, H2O, air | Ar, O2, H2 |
| Input waste conductivity | Needed | Not needed | Not needed |
| Thermal efficiency (%) | 70–95 | 70–95 | 40–50 |
| Technology | Optimal Feedstock | Operating Conditions | Advantages | Limitations | TRL (for MSW) |
|---|---|---|---|---|---|
| Fixed bed | Dry biomass, pretreated RDF | 500–1200 °C, 1–100 bar; limited oxygen; 900–1800 s | Robust design, high efficiency, low tar in downdraft mode | Requires dry, homogeneous feedstock; high tar in updraft mode | 5–6 |
| Fluidized bed | MSW, RDF, biomass, sludges | 700–1000 °C, atmospheric pressure; air/steam; 1–10 s | Excellent heat transfer, scalable, suitable for MSW | Risk of bed agglomeration, requires pretreatment, complex control | 7–9 |
| Entrained flow | Fine coal powders, dry RDF, finely milled biomass | 1200–1500 °C, 20–80 bar; oxygen/steam; 1–5 s | High conversion efficiency, low tar, clean syngas | Requires fine, dry feedstock; high equipment cost | 7–9 |
| Plasma | MSW, hazardous waste, sludges, non-recyclables | 3500–6500+ °C (localized); reducing atmosphere | Complete conversion, handles difficult waste, vitrification of residue | High energy consumption, high cost | 4–6 |
| Supercritical water gasification | Wet organic waste, sludges, biodegradable MSW fractions | T > 374 °C, >221 bar; hydrothermal medium | Direct treatment of wet waste, high H2 yield | Corrosive conditions, high pressure, pilot scale only | 4–6 |
| Microwave- assisted gasification | Biomass, plastics, sludges, MBT-treated MSW | 600–900 °C; inert or gasifying atmosphere; 2.45 GHz microwaves | Volumetric heating, reduced tar, suitable for small-scale systems | High capital cost, uneven heating | 3–5 |
| High-temperature steam gasification | Plastics, rubber, mixed MSW, RDF | ~1000 °C; oxygen-free; requires external heat source | High H2 yield, low emissions, clean syngas | Needs external heat, advanced materials | 4–6 |
| Rotary kiln | MSW, RDF, sludges, industrial residues | 300–600 °C; air/steam/oxygen; slow rotation; >60 min residence time | High feedstock flexibility, mechanically simple | Low efficiency, poor syngas quality, high tar | 6–7 |
| Property | Pyrolysis Biochar | Gasification Biochar |
|---|---|---|
| Fixed carbon (%) | 70–85 | 40–60 |
| Ash content (%) | 5–20 | 20–40 |
| Surface area (m2/g) | 200–400 | 10–100 |
| pH | 6–8 | 9–11 |
| Cation exchange capacity (cmol/kg) | 20–30 | 5–15 |
| BET 1 porosity (m2/g) | High | Low–moderate |
| Application suitability | Soil, carbon sequestration | Adsorption, construction |
| Country | Type of Reactor | Examples of Applications/Projects | TRL |
|---|---|---|---|
| Germany | Fixed bed (updraft, downdraft) | Local decentralized initiatives, academic institutions (Fraunhofer IGB) | 7–9 |
| France | Dual fluidized bed, plasma | GAYA Project (ENGIE), CHO Power | 6–8 |
| Finland | Circulating fluidized bed | VTT Technology Centre, Lahti Energia | 8–9 |
| Italy | Bubbling fluidized bed | ENEA pilot plants, research consortia | 7–8 |
| United Kingdom | Plasma arc, moving bed | Tetronics, Advanced Plasma Power | 6–7 |
| Sweden | Circulating fluidized bed, fixed bed hybrid systems | Valmet demo units, Goteborg Energi | 8–9 |
| Austria | Dual fluidized bed | Güssing plant (2001–2011) | 9 (before shutdown) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, N.; Vlăduț, N.-V.; Biriș, S.-Ș.; Ionescu, M.; Gheorghiță, N.-E. Municipal Solid Waste Gasification: Technologies, Process Parameters, and Sustainable Valorization of By-Products in a Circular Economy. Sustainability 2025, 17, 6704. https://doi.org/10.3390/su17156704
Ungureanu N, Vlăduț N-V, Biriș S-Ș, Ionescu M, Gheorghiță N-E. Municipal Solid Waste Gasification: Technologies, Process Parameters, and Sustainable Valorization of By-Products in a Circular Economy. Sustainability. 2025; 17(15):6704. https://doi.org/10.3390/su17156704
Chicago/Turabian StyleUngureanu, Nicoleta, Nicolae-Valentin Vlăduț, Sorin-Ștefan Biriș, Mariana Ionescu, and Neluș-Evelin Gheorghiță. 2025. "Municipal Solid Waste Gasification: Technologies, Process Parameters, and Sustainable Valorization of By-Products in a Circular Economy" Sustainability 17, no. 15: 6704. https://doi.org/10.3390/su17156704
APA StyleUngureanu, N., Vlăduț, N.-V., Biriș, S.-Ș., Ionescu, M., & Gheorghiță, N.-E. (2025). Municipal Solid Waste Gasification: Technologies, Process Parameters, and Sustainable Valorization of By-Products in a Circular Economy. Sustainability, 17(15), 6704. https://doi.org/10.3390/su17156704