Abstract
Polycyclic aromatic hydrocarbons (PAHs) in biochar, as opposed to those in pyrolysis liquid products that exit the reactor without adhering to the solid product, are particularly undesirable due to their environmental persistence and potential toxicity. When applied as a soil amendment, biochar containing PAHs poses risks to soil ecosystems and human health. Their formation during pyrolysis presents a significant challenge in biochar production, requiring the optimization of pyrolysis process parameters to minimize PAH content for safe soil amendment applications. This study explored the effects of particle size and heating rate on PAH formation during corn cob pyrolysis. Thermogravimetric analysis (TGA) was employed to heat corn cob powder of varying sample masses from ambient temperature to 550 °C at heating rates of 5, 10, and 20 °C/min. Simultaneously, the Chemical Reaction Engineering and Chemical Kinetics (CRECK) model simulated the pyrolysis of spherical corn cob biomass particles with a radius ranging from 1 to 40 mm, using feedstock chemical compositions as inputs. Tar species generated from the solid biomass model were introduced into a gas-phase batch reactor model to evaluate PAH formation. The results demonstrate that the particle size and heating rate significantly affect PAH formation, shedding light on the complex dynamics of biomass pyrolysis. A single spherical particle with a radius close to 1 mm approximates ideal TGA conditions by minimizing temperature and mass transfer limitations. The CRECK model suggested that a particle radius of 5–10 mm, combined with a low heating rate of 5 °C/min, optimally reduces PAH formation. Future research should focus on using thermogravimetric analysis coupled with gas chromatography–mass spectrometry (TGA-GC-MS) to comprehensively quantify PAH species formation.
Keywords:
biomass; pyrolysis; heating rate; particle size; polycyclic aromatic hydrocarbons; biochar 1. Introduction
The utilization of renewable biomass resources has garnered increasing attention in recent years due to growing concerns over climate change and the depletion of fossil fuel reserves [1]. Converting biomass waste into carbon-rich materials through up-recycling is gaining significant interest for its ability to produce valuable products while mitigating environmental pollution [2]. During waste pyrolysis, a thermochemical process, organic materials are decomposed at high temperatures in an oxygen-limited environment, yielding three primary products: solid (biochar), liquid (bio-oil), and gaseous (syngas) products [3,4]. The composition and yield of these products—bio-oil for liquid fuel, biochar for soil enhancement or carbon sequestration, and syngas for energy generation—depend on factors like temperature, heating rate, and feedstock type [5,6,7,8,9].
Biochar has emerged as a promising tool with which to mitigate climate change by sequestering carbon, enhancing agricultural productivity, and facilitating effective waste management. Its multifaceted applications span industries and bolster national economies, serving as a sorbent, fuel source, reducing agent in metallurgy, and a component in coal coke mixtures, bio-composites, explosive modifications, and fertilizers, among other uses. The specific application of biochar hinges on its quality and properties influenced by the type of raw material, its chemical composition, and the pyrolysis condition. These factors determine the suitability of biochar for various industrial and economic purposes [10].
Biochar’s large surface area, high porosity, and abundant functional groups render it valuable for applications in agriculture and environmental management [11,12,13]. Biochar pyrolysis product yield and efficiency are strongly dependent on biomass properties, particularly particle size, which significantly influences pyrolysis kinetics and product distribution, as well as moisture content and chemical composition [14,15]. As a soil additive, biochar enhances soil characteristics by boosting fertility, improving water retention, increasing aeration, and elevating nutrient availability, which promotes healthier plant growth and higher crop yields. As a nutrient delivery system, biochar supplies vital elements and can be modified for slow-release fertilizers, minimizing environmental harm and fostering sustainable farming practices [2].
Corn cob, an abundant agricultural residue generated during corn harvesting, has attracted interest as a potential feedstock for biochar production. Its composition—rich in cellulose, hemicellulose, and lignin—along with favorable physicochemical properties, positions it as a promising candidate for pyrolysis, offering benefits for soil health and environmental sustainability [16,17,18,19].
However, a significant challenge in biochar production is the formation of PAHs—organic compounds with carcinogenic and mutagenic properties that pose substantial environmental and health risks. The presence of PAHs in biochar restricts its use in agriculture and carbon sequestration, prompting regulatory limits from bodies such as the European Biochar Certificate (EBC) and the International Biochar Initiative (IBI) for soil-applied biochar [20]. PAHs in biochar, as opposed to those in pyrolysis liquid products that exit the reactor without adhering to the solid product, are particularly undesirable due to their negative impacts on soil ecosystems, including the inhibition of bacterial colonization and the resulting loss of beneficial effects. Consequently, developing efficient pyrolysis techniques to minimize PAH formation remains a critical research priority.
Pyrolysis product composition and yield depend on multiple factors, including biomass type, heating rate, vapor and solid residence times, temperature, reactor design, sample mass, and particle size and shape [21,22,23]. PAH content in biochar is similarly influenced by these parameters [24]. The formation of PAHs during pyrolysis is governed by intricate chemical pathways that are highly sensitive to both the characteristics of the feedstock and the operational conditions of the process. Among the most critical factors influencing PAH formation are particle size and heating rate, which directly affect internal heat and mass transfer within biomass particles. These internal dynamics determine the extent of both primary decomposition and the secondary reactions responsible for PAH generation and transformation. The devolatilization stage of pyrolysis, during which volatile compounds are released from the biomass, plays a pivotal role in PAH formation, as these volatiles can undergo secondary reactions to produce PAHs [25,26]. Understanding the factors affecting devolatilization is essential for reducing PAH generation.
Particle size significantly impacts devolatilization behavior by influencing pyrolysis kinetics, including accessibility to biomass components, chemical reactivity, and thermal resistance [27]. The size of biomass particles significantly impacts the characteristics of the pyrolysis process, particularly in large-scale applications. For smaller particles, the chemical reaction rates (kinetics) primarily control the process, benefiting from efficient heat and mass transfer. However, as the particle size increases, the movement of gases within the particle (gas diffusion) becomes the dominant controlling factor. This shift towards diffusion control in larger particles tends to enhance the yield of biochar [28]. While very small particles (e.g., under 1 mm) offer advantages in heat and mass transfer rates, the significant energy required to grind biomass compounded by its fibrous structure, to such fine sizes often makes this approach economically inefficient [29]. Adjusting particle size can thus tailor the final product distribution [30]. Similarly, the heating rate affects pyrolysis kinetics and product outcomes. High heating rates favor rapid gas decomposition and polymerization, yielding more liquid products than biochar, while slow heating rates and extended residence times enhance secondary pyrolysis, increasing biochar yields [31,32]. In TGA, the heating rate is a critical parameter influencing thermal decomposition, necessitating experiments at varied rates to address potential compensation effects [33]. Thus, elucidating the interplay between heating rate and devolatilization is vital for optimizing pyrolysis processes, especially when targeting PAH formation.
Research into the role of particle size has revealed (Table 1) a complex and often contradictory influence on PAH yields, with variations depending on the type of feedstock, pyrolysis conditions, and specifically the phase from which PAHs are measured. For example, in fluidized bed pyrolysis systems, larger biomass particles have been found to increase PAH formation. This effect, as reported in modeling studies [34], is attributed to lower effective devolatilization temperatures and higher yields of lignin-derived precursors such as synapyl aldehyde—though these are not directly predictive of the 16 priority PAHs identified by the U.S. Environmental Protection Agency (USEPA).
Similarly, experimental work conducted in a fluidized-bed reactor at temperatures ranging from 500 °C to 900 °C with particle sizes from 6 to 25 mm [35] demonstrated that PAH yields, measured via integrated mass spectral intensities of key aromatic compounds, increased with particle size, particularly in the 700–800 °C range. This trend was linked to enhanced secondary reactions within larger particles, driven by longer residence times of volatiles and internal temperature gradients. In the context of hazardous waste pyrolysis, studies using a continuous tubular reactor [36] have shown that increasing particle size from 1 to 3 cm led to higher PAH concentrations in both the condensed oil (121–29,440 mg/L) and char (223–1610 mg/kg), peaking at 2 cm. This again was attributed to more extensive secondary reactions within larger particles, facilitated by longer retention and higher internal temperatures. However, this trend does not seem to be consistent and universal.
In contrast, the pyrolysis of sewage sludge at 850 °C in a horizontal quartz tube reactor [37] revealed that smaller particles (0.075 mm) generated the highest PAH yields, with a decline observed as particle size increased up to 2 mm. This inverse relationship suggests that finer particles enable more efficient heat transfer and rapid devolatilization, thus intensifying primary reactions that favor PAH formation. A similar non-linear relationship was observed in the pyrolysis of straw powder [38], where particle sizes ranging from 9.31 to 101.9 μm were carbonized at 350 °C. The total concentration of 27 PAHs increased with particle size up to 60.77 μm but then declined, reaching a minimum at 101.9 μm. This non-monotonic trend highlights the possibility of an optimal particle size range where PAH formation is minimized due to a balance between heat transfer efficiency and volatile retention.

Table 1.
A literature review on the effect of biomass particle size (PS) on PAHs.
Table 1.
A literature review on the effect of biomass particle size (PS) on PAHs.
| No. | Title and Year of Publication | Methods Used | Conclusions and Corresponding Reference |
|---|---|---|---|
| 1. EXP | PAH concentration in straw biochar with different particle sizes (2016). | Straw powder of PS (9.31 to 101.9 μm) carbonized in a muffle furnace (350 °C, 50 °Cmin−1 and 6 h). PAHs in biochar analyzed by GC-MS. | PAH levels peak at 60.77 μm (166.52 ng/g) and drop to 14.63 ng/g at 101.90 μm, with low- and medium-molecular-weight PAHs rising then falling as PS increases from 9.31 μm to 101.9 μm [38]. |
| 2. EXP | Biomass Pyrolysis and Gasification of Varying PS in a Fluidized-Bed Reactor (2011). | White oak (PS 6–25 mm) was pyrolyzed (500–900 °C) in a fluidized bed gasifier, with tar species quantified by molecular beam mass spectrometry. | PAHs increase with larger PSs due to secondary reactions and rise significantly between 700 and 800 °C, but statistical analysis shows no significant PS effect [35]. |
| 3. EXP | Investigation into PS influence on PAH formation during dry sewage sludge pyrolysis: TG-FTIR analysis and batch scale research (2015). | Sewage sludge (0.075–2 mm) was pyrolyzed (850 °C) in a quartz tube reactor, with gases captured by XAD-2 resin and dichloromethane solutions in ice baths, and 16 USEPA PAHs analyzed via GC-MS. | The smallest PS (0.075 mm) produce the highest PAH concentrations, mainly naphthalene, fluorene, and phenanthrene (80%), while larger PS (2 mm) show a decrease in higher molar weight PAHs from 15.87% to 10.21% [37]. |
| 4. EXP | PAH formation during the fast pyrolysis of hazardous health-care waste (2019). | Waste was pyrolyzed in a continuous tubular fast pyrolysis reactor (300–700 °C, 100–190 S and PS 1–3 cm), and 16 US EPA PAHs in oil and char were quantified using a GC-FID system. | PAHs in pyrolytic oil and char rise with higher temperatures, longer residence times, and larger PS, peaking in char at 1–2 cm but slightly dropping at 2–3 cm, with 121–29,440 mg/L in oil and 223–1610 mg/kg in char [36]. |
| 5. EXP | Pear Wood Pyrolysis Influences Quality and Levels of PAH in Liquid Smoke (2024). | Pear wood (0.8–12.5 mm) were pyrolyzed (<250 °C, 250–350 °C, >350 °C), with the gas smoke condensed, and analyzed for PAHs using GC-MS. | PS significantly affected 13 PAHs, but not acenaphthene, benzo[a]pyrene, and benzo[g,h,i] perylene, with low-ring PAHs most prevalent at 1.6 mm, influencing the distribution [39]. |
| 6. Model | Quantification of the influence of particle diameter on PAH formation in fluidized bed biomass Pyrolysis (2017). | A model for biomass devolatilization in a fluidized bed reactor, tested with white oak PS 6–25 mm at 500–900 °C, measured tar compounds using a MS, validated by experimental data. | Larger particle diameters increase PAH formation during high-temperature pyrolysis due to lower devolatilization temperatures, boosting synapyl aldehyde in tar and thus PAH yields [34]. |
| 7. EXP | Fast Pyrolysis of MGW in an Auger Reactor: Effects of Residence Time and PS on the Yield and characteristic- s of Produced Oil (2024). | Municipal green waste (1–10 mm) was pyrolyzed at 500 °C to produce biochar and bio-oil, with the bio-oil’s compounds analyzed by GC-MS for ≥80% similarity and significant peak areas. | Increasing PS causes slight compound-specific fluctuations in PAH peak areas, with fluorene decreasing (0.99 to 0.87) and 1-methylnaphtha-lene increasing (0.51 to 0.56), indicating a modest and inconsistent effect [15]. |
| 8. EXP | The study of the effect of operating parameters on PAH formation during the combustion of coconut shell in a fluidised bed (2003). | Experiments at 675 °C and 750 °C with PS (1–1.5 mm and 2.5–3.15 mm) measured O2, CO, CO2, and PAHs in flue gas calculated based on peak height and computer-based data recorded. | Among the combustion parameters studied, which included temperature, excess air, fuel PS, and fuel moisture content, only excess air proved to have significant influence on PAH formation [40]. |
Exp: Experimental.
Further complexities are evident in studies involving pear wood particles [39], where pyrolysis at various temperature ranges and particle sizes (0.8 to 12.5 mm) revealed that 1.6 mm particles yielded the highest levels of low-molecular-weight (LMW) PAHs in liquid smoke. Interestingly, certain high-molecular-weight PAHs such as acenaphthene, benzo[a]pyrene, and benzo[g,h,i] perylene remained unaffected by particle size, indicating compound-specific behavior.
Adding another layer to this variability, a study on municipal green waste (MGW) pyrolyzed at 500 °C in an auger reactor [15] found only minor differences in PAH concentrations across particle sizes (1–10 mm), with no clear trend emerging. In this case, compounds like naphthalene and fluorene showed minimal variation, suggesting that under certain process conditions, particle size may play a less dominant role. The inconsistency continues in combustion studies [40], where biomass particles of 1–3.15 mm were subjected to bed temperatures of 675 °C and 750 °C. PAH concentrations measured in flue gas showed no significant dependence on particle size, indicating that in combustion environments, other factors such as bed temperature and amount of excess air may overshadow the influence of particle size.
Collectively, these findings underscore that the effect of particle size on PAH formation is highly context-dependent, shaped by the material matrix, reaction environment (pyrolysis, gasification, or combustion), temperature regimes, and the product phase from which the individual PAH species are measured (gas, liquid, or solid). Notably, most existing research has predominantly examined fast pyrolysis, gasification, and combustion conditions, often at extreme temperatures (either at 850 °C or 350 °C), as well as primarily PAH measurements focused on the gas phase at the reactor outlet, not at the formation stage. This highlights a significant gap in our understanding of PAH dynamics in slow-pyrolysis systems and biochar-centric applications, where low heating rates and longer feedstock residence times may alter PAH behavior in yet unexplored ways using a predictive model, concerning which no specific study has been reported to predict the effect of particle size on PAH during formation stage.
While previous studies have extensively investigated the effects of feedstock characteristics and operational conditions on biomass pyrolysis products (biochar, tar, and syngas) [41,42], a notable research gap persists regarding the optimal biomass particle size and heating rate for minimizing individual PAH species formation during devolatilization. This study seeks to fill this void by comprehensively examining the relationships among feedstock properties, heating rates, and PAH species formation. To achieve this, the CRECK model was chosen for its proven ability to simulate the intricate chemical reaction networks in biomass pyrolysis, including PAH species formation [43]. Its detailed kinetic framework, encompassing a broad range of primary and secondary reactions, offers a more thorough understanding of PAH-influencing factors than simpler models. Its novelty lies in combining physical process parameters with a detailed kinetic model to address PAH species formation from a widely available biomass feedstock [29,44]. The CRECK model outperforms simpler models like Single First-Order Reaction (SFOR) and the Distributed Activation Energy Model (DAEM) in resolving PAH formation pathways due to its comprehensive reaction network, though it is computationally intensive [45]. Compared to Chemical Percolation Devolatilization (CPD) and Functional Group-Depolymerization, Vaporization, Crosslinking (FG-DVC), CRECK offers superior detail in gas-phase reactions, making it ideal for PAH species formation studies [46]. Moreover, its capacity to illuminate critical reaction pathways—validated by successful applications in past pyrolysis studies—reinforces its suitability for predicting individual PAH species formation [29]. Additionally, TGA was used to measure the mass loss of corn cob biomass during pyrolysis, and these results were compared with predictions from the CRECK model.
In summary, the novelty of the present work targets the following:
- To enhance our understanding on the effect of particle size on PAH formation (in totality encompassing all product phases, i.e., gases, liquid, and solid) under slow-pyrolysis conditions while segregating individual PAH species.
- To look into the ambiguities present to resolve the trade-offs likely involved up to different levels.
- To employ a comprehensive predictive model (CRECK) to mimic the effect of particle size on PAHs (16 USEPA-PAHs) specifically under slow pyrolysis, including validation.
This integrated approach will enhance our understanding of influencing factors for PAH formation and chalk out mitigation strategies for these harmful byproducts, supporting the development of sustainable biochar production for soil amendment practices.
2. Materials and Methods
2.1. Feedstock Characterization (Proximate, Ultimate, and Chemical Analysis)
This study utilized corn cob as the feedstock, collected from Omo Nada district in Jimma, Ethiopia. The rationale for selecting corn cob as the feedstock stems from its abundance as an agricultural byproduct in Ethiopia, particularly in the Jimma region, making it a readily available and sustainable resource, coupled with its potential for high lignocellulosic content suitable for thermochemical conversion processes like pyrolysis [47]. The corn cobs were gathered directly from local farms in the Omo Nada district, ensuring a fresh and representative sample of the regional agricultural residue. The characteristics of the corn cob feedstock were assessed through proximate and elemental analysis, and its thermal decompositions behavior was evaluated using TGA. To prepare the feedstock, the corn cobs were chopped into smaller pieces using a mechanical chopper to facilitate handling and ensure uniform processing. The particle sizes used in the study were 355 , achieved by grinding and sieving the air-dried corn cobs to maintain consistency across the proximate, elemental, and TGA experiments.
The corncob feedstock samples were subjected to proximate analysis to evaluate key parameters such as moisture content, volatile matter content, ash content, and fixed carbon, following the ASTM D3172-13 [48] standard method (Table 2). The detailed analysis process was carried out at the Geological Institute of Ethiopia [49].

Table 2.
Characterization of corn cob used for the study.
The analysis of carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) was carried out following the DIN EN ISO 16948 [50] standard protocol using elemental analysis. The detailed analysis took place at the DBFZ German Biomass Research Center in Germany (Table 2). Following the determination of C, H, N, and S content, the oxygen (O) content was subsequently calculated by the principle of difference [51].
The chemical compositions of the biomass samples, including cellulose (CELL), hemicellulose (HCE), lignin-c (LIGC), lignin-h (LIGH), lignin-o (LIGO), tannins (TANN), and triglycerides (TGL), were estimated for the feedstock. These estimations were conducted as a reference species utilizing an extended biomass characterization method, aimed at providing a comprehensive analysis of the diverse components present in the biomass materials [52]. A detailed understanding of the chemical makeup and potential applications of the feedstock can be gained, contributing valuable insights to biomass utilization and processing strategies [53]. The results obtained from this comprehensive analysis provide valuable insights into the composition and characteristics of the corncob feedstock, aiding in better understanding its potential applications and properties within the context of biomass utilization and biochar production.
2.2. Thermo-Gravimetric (TG) and Derivative Thermogram (DTG) Analysis
Thermal decomposition analysis was conducted using a thermogravimetric analyzer–differential thermal analyzer (TGA-DTA) instrument at the Department of Materials Science and Engineering, Adama Science and Technology University, Ethiopia. This instrument allows for the simultaneous measurement of weight loss (TGA) and heat flow (DTA) during controlled heating, providing valuable insights into the thermal decomposition behavior of the materials under investigation [54,55]. The reliability of the TGA data was ensured through rigorous calibration procedures performed before each analysis. The instrument used was a Shimadzu DTG-60H (Shimadzu Corporation, based in Kyoto, Japan), with the following specifications: temperature range, room temperature to 1500 °C; weight readability, 0.1 μg; measurable range (weight), ±500 mg; measurement accuracy (weight), ±1 %; measurable range (DTA), ±1 to ±1000 μV; heating rate, 0.1–99.9 °C/min; and maximum sample size (gross weight), 1 g. These specifications reflect the instrument’s inherent precision and reproducibility.
Corn cob powder with bulk density of 419.6 kg/m3, placed in a sample holder of platinum crucible in different sample sizes (Weight, mg), was heated from room temperature to 550 °C at a heating rate of 5, 10 and 20 °C/min with a nitrogen flow rate of 50 mL/min. The selection of the sample mass and heating rate range was based on their ability to balance experimental precision with real-world applicability: smaller sample mass sizes ensure uniform heat transfer and reaction consistency, while a range of heating rates provides a comprehensive understanding of thermal behavior, aiding in the optimization of pyrolysis processes for biochar production from corn cob feedstock.
2.3. CRECK Single-Particle Solid Biomass and Batch Reactor Gas-Phase Model
The CRECK-S-B (solid biomass model, total analysis) kinetic mechanism describing biomass devolatilization consisting of 32 reactions and 58 species (29 solid species) was used [29,56,57]. The chemical compositions of feedstock were applied as a solid input of 419.6 kg/m3 density at a spherical radius of 1, 5, 10, 15, 20, 25, 30, 35, and 40 mm with heating rates of 5, 10, and 20 °C/min−1. The tar species product from the solid biomass model was used as an input for the gas-phase batch reactor model at a temperature of 550 °C, with a maximum residence time of 12,000 s. The gas-phase kinetic mechanism encompassed both low- and high-temperature reactions, featuring 623 species and approximately 27,831 reactions, including polycyclic aromatic hydrocarbon (PAH) formation up to C18. This study focused on the early stages of PAH growth, where individual species were explicitly tracked rather than grouped into pseudo-species. The equivalence ratio was maintained at zero, ensuring that the initial and final mass fractions of nitrogen remained equal (inert).
The model assumes biomass decomposition occurs via a set of lumped, multi-step reactions for each reference component (cellulose, hemicellulose, lignin). The solid-phase kinetics are governed by first-order reaction rate equations, expressed for a given solid species Si (e.g., cellulose, hemicellulose, or lignin). Here, the rate of mass loss due to devolatilization (the main control equation) is
where
= mass of species Si (kg);
= reaction rate constant (s−1), following the Arrhenius form:
Ai = pre-exponential factor (s−1)
Ei = activation energy (J/mol)
R = universal gas constant (8.314 J/mol·K)
T = temperature (K)
where
intrinsic density of the solid material (kg/m3);
total particle volume (m3);
solid volume fraction.
During pyrolysis, the mass of biomass decreases due to devolatilization, and and change due to shrinkage and porosity evolution. To model shrinkage, it was assumed that intrinsic solid density remains relatively constant (a common simplification, though it may decrease slightly due to charification), and the volume shrinks as mass is lost, while increases. The volume shrinkage model, where the volume change is proportional to mass loss, adjusted by a shrinkage factor, was introduced [58,59]. The normalized volume is expressed as
where
= initial particle volume (m3);
= initial biomass weight (kg);
β = shrinkage factor (0 ≤ β ≤ 1), calibrated experimentally.
The model typically includes multiple parallel reactions per component, producing volatile species (gases and tars) and solid residues (char). This equation, coupled with energy and gas-phase reaction balances, governs the core process of biomass pyrolysis in the model.
Figure 1’s block diagram compares the experimental and modeled thermal decomposition of corn cob biomass, focusing on mass loss and PAH species. Experimentally, corn cob powder underwent TGA (ambient to 550 °C, 5–20 °C/min heating rates) to measure mass loss, while PAH data were collected separately. The CRECK model used spherical biomass (1–40 mm radius) to predict mass loss and volatiles/tar species at the same conditions, followed by a gas-phase BATCH reactor model for PAH prediction. The experimental mass loss and PAH data were then compared with the model’s outputs to validate its accuracy in simulating both decomposition and PAH formation.
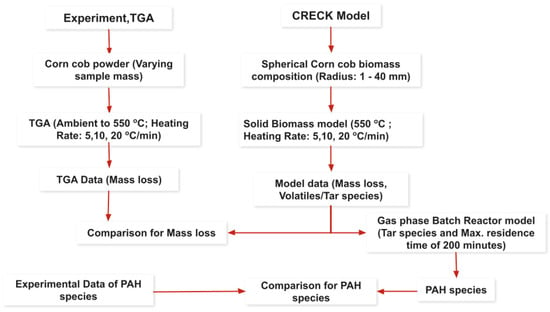
Figure 1.
The block diagram of the research.
2.4. Optimization and Validation Techniques
In this study, validation with other experimental data was systematically conducted to ensure the accuracy and reliability of the results obtained. In the context of predictive modeling, external validation serves as a critical benchmark for evaluating the model’s performance in real-world applications. By validating the model with external data, the researcher can verify its predictive accuracy, identify potential biases or overfitting issues, and enhance the model’s reliability. This technique plays a vital role in promoting transparency, rigor, and reproducibility in research practices, ultimately contributing to the advancement of knowledge and the development of reliable predictive models in various scientific disciplines. Comparable experimental data available from earlier works [60] were utilized to validate the model’s findings regarding the impact of pyrolysis process parameters on the generation of PAH species. To validate the predictive capabilities of the CRECK model, it is important to compare the model’s output with experimental data [15,36,39] obtained from the literature on the influence of feedstock particle size on PAH formation during devolatilization. By simulating the devolatilization process for these different particle sizes using the CRECK model, the researchers were able to examine the model’s ability to capture the trends and magnitudes of PAH formation as a function of the feedstock particle size.
In addition to direct comparisons, predictions were made for the experimental data (derived from coffee husk experiments) using the coffee husk chemical compositions presented in Table 3—where biochar production was conducted at Jimma University College of Agriculture, and PAH analysis was performed at Germany’s DBFZ German Biomass Research Center—to validate the model.

Table 3.
Parameters considered during experimental work and used for prediction as well.
The data extracted from the CRECK model were exported to Design Expert 13.0 software to generate surface plot diagrams and identify optimal conditions for minimizing the PAH fraction. The optimization of PAH species concentrations prioritized three key pyrolysis process parameters: particle size, heating rate, and residence time.
3. Results and Discussions
3.1. Feedstock Characterization
The proximate analysis parameters are fundamental in characterizing the composition of biomass. The ranges for each proximate analysis parameter in biomass can vary depending on the type of biomass and its specific composition.
The volatile matter content of 75.23% in Table 2 falls within the reported range [61]. The volatile matter content of biomass plays a crucial role in determining the balance between tar and char production during pyrolysis. Higher volatile matter content generally leads to increased tar production and reduced char yield, whereas lower volatile matter content results in decreased tar formation and higher char yields [62]. Similarly, biomass with high moisture content can lead to increased tar production during pyrolysis. This is because the presence of moisture can lower the pyrolysis temperature and promote the formation of volatile organic compounds, which can condense into tar. Because the energy required to drive off moisture can lower the overall pyrolysis temperature, affecting the formation of char [63], biomass with a higher fixed carbon content tends to produce less tar during pyrolysis. This is because fixed carbon is more stable, and a major component of char that is less likely to break down into volatile compounds that contribute to tar formation [64,65]. Even if biomass with low ash content can lead to increased tar production during pyrolysis [66], ash components can catalyze tar formation by promoting secondary reactions that generate tar compounds. The presence of ash can interfere with char formation by agglomerating and blocking the pores in the char structure.
The hydrogen-to-carbon ratio (H/C) and oxygen-to-carbon ratio (O/C) of biomass are crucial factors that influence the products generated during pyrolysis, including tar and biochar. A higher H/C and O/C ratio in biomass typically lead to increased tar generation during pyrolysis. This is because hydrogen-rich compounds are more likely to form volatile products that can condense into tar. Similarly, oxygen-rich functional groups in biomass can contribute to the formation of volatile compounds that can condense into tar. Lower H/C and O/C ratios mean a higher degree of carbonization and biochar stability [66,67].
The relative proportions of cellulose, hemicellulose, and lignin in biomass significantly influence the yield and composition of tar and char during thermochemical conversion. Understanding these relationships is crucial for optimizing process conditions and selecting appropriate biomass feedstocks for specific applications. Hemicellulose decomposes in a temperature range of 200–300 °C and produces a complex mixture of volatile compounds, including acetic acid, Furfural, and various ketones, which can contribute to tar formation. Whereas cellulose tends to decompose relatively within a temperature range of (250–350 °C) and primarily yields levoglucosan and other anhydro sugars, which are major precursors to tar formation, lignin is the most complex and thermally stable part, decomposing over a wide range of temperatures (250–500 °C). Its decomposition yields a diverse array of phenolic compounds, few of which contribute to tar formation. However, due to its highly aromatic structure, lignin shows the highest char yield among the three components. Its complex, cross-linked structure provides high thermal stability and promotes char formation during thermal decomposition [68,69]. Lignin can be considered a weighted combination of three units, LIG-C (C15H14O4), LIG-H (C22H28O9), and LIG-O (C20H22O10), which have decreasing ratios of (C/O)molar [70].
3.2. Effect of Particle Size and Heating Rate on Temperature Profile
During pyrolysis, the temperature distribution within a feedstock particle is not uniform, especially for larger particles. This non-uniformity arises due to the interplay of heat transfer from the external source to the particle surface and heat conduction within the particle itself. The temperature distribution significantly influences the reaction rates at various locations within the particle, ultimately affecting the overall product distribution and the properties of the resulting biochar.
A steep drop in the TGA curve shows a rapid mass loss event, like a major decomposition step. While the furnace temperature increases linearly as shown in Figure 2a, in a temperature–time graph, the sample temperature does not follow the same linear trend during this rapid mass loss. This is due to the thermal effects of the decomposition reaction itself, coupled with the limitations of heat transfer. Higher heating rates shift the decomposition temperatures to higher levels. This is because the sample does not have enough time to reach equilibrium at each temperature step, and the decomposition reactions occur at higher temperatures than they would under slower heating rates [60]. But on the same graph with a sample mass of 4.6 mg, no steep drop in the TGA curve indicating the sample temperature follows linear trends all the time. As shown in Figure 2b, smaller particles have a larger surface area-to-volume ratio. This facilitates rapid and relatively uniform heating throughout the particle. The temperature difference between the surface and the core is minimal, leading to a more homogenous reaction environment. Larger particles pose a greater challenge for heat transfer. Heat takes longer to penetrate the core of the particle [71,72,73]. Consequently, significant temperature gradients develop, with the surface reaching higher temperatures than the core. This temperature difference can lead to different reactions occurring simultaneously at different locations within the particle. The outer layers might experience rapid pyrolysis and char formation, while the inner core might undergo slower decomposition or even remain unreacted [74]. The maximum temperature difference between the inner core and the outer surface was seen to increase with the particle size for all heating rates considered [75,76]. For a particle with a 1 mm radius, the maximum temperature difference is 0.173 °C, 0.338 °C, and 0.628 °C at a heating rate of 5, 10, and 20 °C/min, respectively. In contrast, for a 40 mm particle, the temperature difference increases significantly to 135 °C, 203 °C, and 270 °C at the same heating rates.
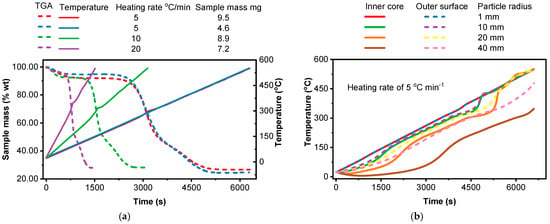
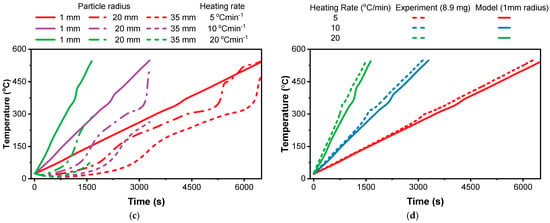
Figure 2.
Effect of sample mass (experimental) and spherical particle of different radius (CRECK model) at different heating rates on temperature profile: (a) influence of sample mass with the same particle size, experimental; (b) outer vs. core temperature, CRECK; (c) temperature profile, CRECK model; (d) model (spherical particle of 1 mm radius) vs. experimental (sample mass of 8.9 mg) comparison.
At later stages of decomposition for particle sizes of 1 to 30 mm at 5 °C/min, 1 to 15 mm at 10 °C/min, and 1 to 10 mm at 20 °C/min, the inner core temperature is seen to exceed that of the surface. This may be due to the interplay of self-heating (exothermic reactions) and reduced heat transfer pathways. As the sample heats up, decomposition begins at the surface, where the temperature is highest. If the decomposition is strongly exothermic, the heat released can raise the temperature of the material inside the sample faster than heat can be conducted away to the surface and then to the surrounding environment. This self-heating effect can become more pronounced at later stages when the decomposition rate is high, and a significant portion of the sample is reacting. The rate of heat transfer into the particle must be sufficient to overcome the heat absorbed by the endothermic pyrolysis reaction for the reaction to proceed. This dynamic interplay determines the temperature of the particle and how quickly the pyrolysis reaction progresses [77]. Particle size influenced both heating rate and pyrolysis temperature, with smaller particles heating faster and reaching higher temperatures, as illustrated in Figure 2c.
As shown in Figure 2d, a single spherical particle model with a radius of 1 mm approaches the ideal conditions of TGA due to minimized temperature and mass transfer limitations. The model allows for better comparison with experimental TGA data obtained using small sample masses. Larger particles, however, exhibit significant deviations from TGA behavior due to pronounced temperature gradients and mass transfer limitations. Therefore, it is the smaller end of the 1–40 mm range that provides a reasonable approximation of TGA conditions, not the larger end. So, in a single spherical particle model, a particle radius of less than 1 mm is suggested [78]. In summary, the particle size has great influence on the pyrolysis time of the particles during devolatilization.
3.3. Effect of Particle Size and Heating Rate on Thermal Decomposition
The relationship between the heating rate and particle size significantly influences the mass loss decomposition profiles observed during the TGA of the biomass. At high heating rates, the time available for heat transfer is limited. The surface temperature rises quickly, but the core does not have enough time to catch up. If the heating duration was extended, the core temperature would eventually approach the surface temperature, but at high heating rates (Figure 3b), this equilibrium is not reached within the typical timescale of the test [15,79].
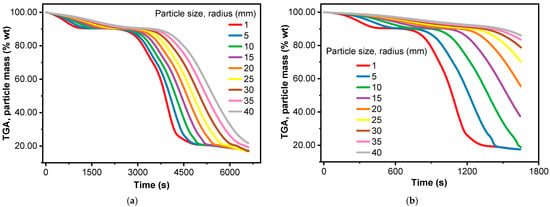
Figure 3.
Effect of particle size at different heating rates using the CRECK model: (a) heating rate of 5 °C/min; (b) heating rate of 20 °C/min.
As shown in Figure 3, larger particles require more time for heat to penetrate the core. This means that even at the same heating rate, the core of a larger particle reaches the decomposition temperature later than the core of a smaller particle [80]. This leads to a longer overall decomposition time for larger particles. Furthermore, the escape of volatiles from larger particles takes longer due to increased diffusion pathways, further extending the decomposition time. Smaller particles heat up more uniformly and quickly, reducing the time required to reach decomposition temperatures throughout the particle [79]. The shorter diffusion pathways also allow for faster escape of volatiles, resulting in a shorter overall decomposition time [27,75].
Conventional thermogravimetric analysis (TGA) typically employs ~2–50 mg samples, where heat and mass transfer limitations are generally moderate [81]. While temperature gradients within the sample can occur, particularly at elevated heating rates, these gradients are less pronounced than those observed in macro-TGA, which uses larger sample sizes ranging from grams to tens of grams [69]. So, as shown in Figure 4a, TGA results with a larger sample mass might show a delayed decomposition process compared to those with a smaller sample mass. This is because the biomass requires a certain amount of energy to decompose, and at higher heating rates, this energy is supplied more quickly, leading to later decomposition onset. The interplay of the sample mass and heating rate affects the DTA curve by influencing the rate and extent of both primary and secondary reactions. At higher heating rates (Figure 4b–d), the rapid decomposition results in steeper mass loss curves in TGA and sharper, longer peaks in DTA, shifted to higher temperatures. An increase in sample mass from 3.6 to 9.5 mg resulted in a corresponding increase in the height of the DTA peaks, as depicted in Figure 4b–d.
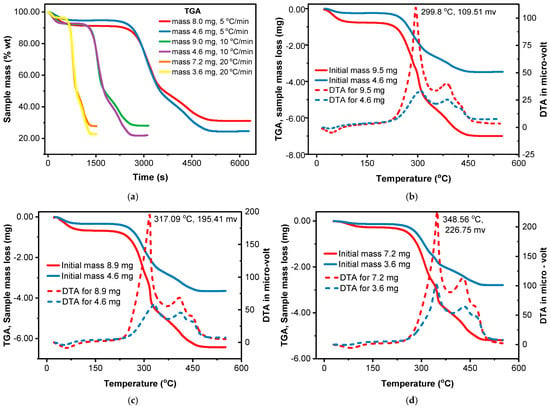
Figure 4.
Effect of sample mass and heating rate on weight loss and differential thermal analysis, experimentally: (a) different sample masses and heating rates; (b) heating rate of 5 °C/min; (c) heating rate of 10 °C/min; (d) heating rate of 20 °C/min.
Figure 5a illustrates that shrinkage commences almost immediately across all particle sizes, marked by a swift initial reduction in both volume and diameter. A minor decrease in diameter results in a more substantial volume drop, indicating the rapid onset of pyrolysis. This is likely triggered by quick heating and the start of devolatilization reactions. Among the sizes, 1 mm particles exhibit the most rapid initial shrinkage, followed by 10 mm, 20 mm, and 30 mm particles. This pattern stems from smaller particles with a higher surface-area-to-volume ratio, which facilitates faster heat penetration and reaction initiation. The 1 mm and 10 mm particles achieve a lower final volume fraction, implying more complete conversion and greater mass loss compared to the 30 mm particles, which retain more unreacted material. Smaller particles experience more uniform heating, leading to consistent reactions as assumed in the model. Additionally, volatiles escape more readily from smaller particles, minimizing internal pressure buildup and enhancing shrinkage. In contrast, larger particles develop temperature gradients, with the core reacting later than the surface, and volatiles take longer to escape, potentially creating internal pressure that counteracts shrinkage.
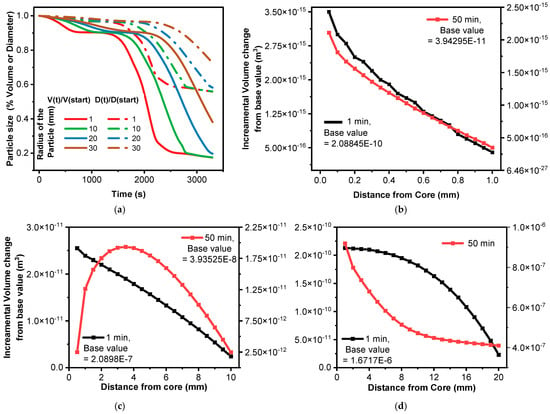
Figure 5.
Effect of devolatilization on particle shrinkage (volume\diameter) with respect to heating time and radius: (a) particle size change vs. heating time (V(t)/Vstart is the ratio of the particle volume at time (t) to its initial volume at the start of pyrolysis; similarly, D(t)/Dstart is the ratio of the particle diameter at time (t) to its initial diameter); (b) volume change with respect to distance (from core to the surface within the discretized 20 layers) for 1 mm radius particle; (c) the same as in (b) for a 10 mm radius particle; (d) the same as in (b) for a 20 mm radius particle.
As depicted in Figure 5b, after 1 min, the volume decreases slightly from the core (0 mm) to the surface (1 mm) of a 1 mm radius particle. The core volume is approximately 2.08848 × 10−10 m3, decreasing to about 2.08845 × 10−10 m3 near the surface—a reduction difference of roughly 3.0 × 10−15 m3. This suggests that the outer layers undergo more pyrolysis than the inner layers due to faster heat penetration at the surface, though the small volume change indicates the reaction is still in its early stages with minimal mass loss and shrinkage. After 50 min, the volume reduction becomes far more pronounced: the core volume drops to around 3.943161 × 10−11 m3, and the surface volume falls to approximately 3.942985 × 10−11 m3—a reduction difference of 1.76 × 10−15 m3. This reflects significant mass loss and shrinkage in the outer layers, with the core reacting to a lesser degree. The steeper core-to-surface gradient at 50 min compared to 1 min suggests a more defined reaction front or gradient in reaction extent.
Figure 5c shows that at 50 min, a peak is found around 2 mm from the core, as well as a sharp drop towards the surface, increasing the volume reduction. Similarly, Figure 5d indicates that for a 20 mm radius particle, the volume decreases from 1.671913 × 10−6 m3 at the core to 1.671723 × 10−6 m3 at the surface after 1 min—a reduction difference of 1.9 × 10−10 m3. This minor change suggests the reaction has just begun, particularly in the core. After 50 min, however, the volume falls dramatically from 9.1874440 × 10−7 m3 at the core to 4.1024590 × 10−7 m3 at the surface reduction difference of 5.0849850 × 10−7 m3. The outer layers exhibit far greater reaction extent, with a steeper gradient than at 1 min, reflecting slower heat transfer in larger particles. The volume reduction is more pronounced near the surface, and the core of the 20 mm particle remains largely unaffected due to limited heat penetration.
These findings highlight significant dimensional changes during pyrolysis, with particle size playing a critical role in shrinkage patterns [82]. While non-linear behavior is observed in the model assumptions for larger particles, focusing on conditions where heat and mass transfer limitations are minimal—typically with smaller particles—is recommended [83].
3.4. Experimental vs. Model Comparison on Thermal Decomposition Because of Particle Size and Heating Rate
Comparing experimental and model predictions for the effects of particle size and heating rate on thermal decomposition in biomass pyrolysis is crucial for validating models and understanding the underlying mechanisms.
As shown in Figure 6, the model qualitatively agrees with the experimental trends regarding the effects of particle size and heating rate on overall decomposition. The discrepancies between the model predictions and experimental data can be attributed to small sample sizes in TGA minimizing heat and mass transfer limitations within the sample, enabling accurate measurement of intrinsic kinetic parameters. However, one-dimensional models assume uniform temperature and concentration profiles within the particle, which might not be accurate, especially for larger particles. The assumption of spherical particles might not be representative of all biomass types. So, the sample sizes used in TGA, even up to 9.5 mg, are still considered small and may not exhibit significant particle size effects compared to the 1 mm particle size modeled in CRECK. Similarly, it is reasonable for deviations to occur when conditions differ, especially in a complex process like thermal decomposition. Matching conditions more closely would probably reduce the gap between experiment and simulation, but practical challenges (e.g., particle size distribution) and model limitations mean some differences might remain.
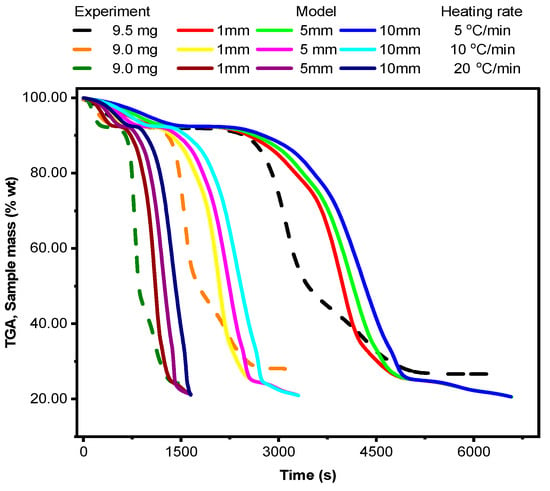
Figure 6.
Experimental and model comparison on the effect of particle size and heating rate.
3.5. Effect of Particle Size and Heating Rate on Tar Fraction
The heating rate and biomass particle size significantly influence the generation of tar species during pyrolysis [84]. As shown in Figure 7a,b, some of the tar species fractions are increasing while others are decreasing with the heating rate. Higher heating rates favor faster reaction pathways. This leads to a rapid decomposition of biomass, promoting the formation of volatiles such as light oxygenates (CH2OHCHO acetic acid, CH3CHO Acetaldehyde, C2H5CHO Propionaldehyde, CHOCHO Glyoxal, CH3OH Methanol, CH2OHCH2CHO Propionic Acid), Furans (C6H6O3 Hydroxymethyl furfural, Furfural C4H3OCHO) and anhydro-sugars (C6H10O5 levoglucosan, C5H8O4 Xylofuranose) [85,86]. At lower heating rates, slower reactions, including those leading to char formation, become more competitive. Most of the tar species (C8H8O3 Vanillin, C6H5OCH3 Anisole, C7H8O Cresol) derived from lignin decomposition decrease with the heating rate [87]. Similarly, as shown in Figure 7c, it decreases with particle size, especially for the highest heating rate of 20 °C/min. A plausible rationale for this trend is that when the particle size is low, all pore openings remain unobstructed, enabling vapors to escape without becoming trapped [88].
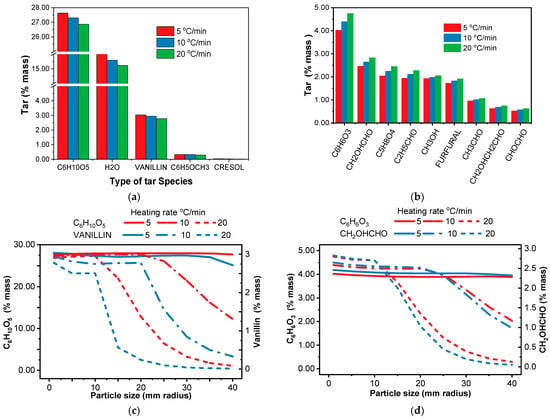
Figure 7.
(a) Tar species that decrease with a heating rate within the particle size considered; (b) Tar fraction vs. type of tar species, tar species that increase with the heating rate for the particle size 1 to 10 mm radius; (c) the fraction of C6H10O5 and Vanillin at different heating rates with spherical particle size increment; (d) the fraction of C6H6O3 and CH2OHCHO at different heating rates with spherical particle size increment.
Figure 7d illustrates the impact of particle size on the tar species fraction of the other group (Figure 7b). So, the highest tar fraction is observed for particles with a radius in between 1 and 10 mm at a heating rate of 20 °C/min, dropping off sharply for larger particles (10–25 mm radius). With a slower heating rate (10 °C/min), the tar fraction decreases more gradually up to a 20 mm radius before declining sharply. This is likely due to temperature gradients within larger particles, where slower core heating promotes char formation while faster surface heating favors volatiles like light oxygenates and anhydro-sugars. Secondary reactions can further decompose primary products like anhydro-sugars into lighter compounds or char. Furthermore, as depicted in Figure 7a, levoglucosan (C6H10O5), a key product of cellulose pyrolysis [89,90], constitutes the largest portion of the tar fraction of 27.9%.
As shown in Figure 8, a slow heating rate reduced the total tar fraction favoring the formation of smaller molecules. Secondary reactions break down larger tar molecules (like C24H28O4 heavy lignin) into smaller species like CH3OH (Methanol), CH3CHO (Acetaldehyde), CH2OHCHO (acetic acid), C5H4O2 (Furfural), etc. As shown in Figure 8a, a large particle size (e.g., >10 mm radius) increases the residence time of volatiles released from the particle core, promoting secondary reactions that decrease overall tar yield and increase the production of smaller, secondary products like gases and light liquids in the inner core. This is analogous to the effect of the heating rate: fast heating preserves primary products, while slow heating, similar to larger particle sizes due to hindered heat transfer, promotes secondary product formation through tar cracking [91,92].
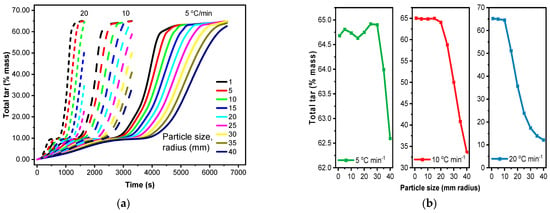
Figure 8.
Effect of particle size at different heating rates on total tar fraction (sum of tar species), CRECK model. (a) Total tar fraction of the species at various heating rates and particle sizes over time; (b) Total tar fraction of the species under the following conditions: 6600 s at 5 °C/min, 3300 s at 10 °C/min, and 1650 s at 20 °C/min, in relation to particle size.
The combination of large particles and low heating rates is generally unfavorable for tar generation. Slow heating and long residence times promote secondary reactions, leading to reduced tar yield and increased char and gas formation. Additionally, larger particles cause uneven heating, which leads to an incomplete breakdown and a reduced bio-oil output [15]. Similarly (Figure 8b), in small particles with low heating rates, while the uniform heating of small particles is beneficial, the low heating rate still allows for some secondary reactions to occur, potentially reducing tar yield compared to high heating rates. The combination of small particles and high heating rates, like 20 °C/min, favors tar generation. The rapid, uniform heating and short diffusion pathways minimize secondary reactions, maximizing the release of volatiles, whereas the scenario of large particles and high heating rates is more complex. While rapid heating promotes primary pyrolysis, the temperature gradients and hindered mass transfer lower the overall conversion due to incomplete conversion with little tar formation in the outer layers. Smaller particles and high heating rates generally favor tar production [93,94].
Understanding the interplay of heat and mass transfer with reaction kinetics is crucial for controlling the pyrolysis process and maximizing the desired product yields. As discussed before, a faster heating rate leads to a higher temperature gradient within larger particles. The surface heats up quickly, while the core lags. This can lead to increased tar formation in the outer layers but also increased secondary char-forming reactions in the core due to the eventual higher temperatures reached. Essentially, the amount and speed of particle fragments released are influenced by the particle’s internal structure (porosity) and size. Larger particles tend to have higher internal resistance to transport, leading to lower tar production [46].
Smaller particles experience less severe temperature gradients due to their faster heating and shorter diffusion pathways. The core temperature of a large biomass particle lags the surface temperature at high heating rates due to the low thermal conductivity of biomass, its significant heat capacity, and the limited time available for heat transfer. This temperature difference affects the decomposition process and the resulting product distribution. Using smaller particles minimizes these effects and allows for more uniform and controlled pyrolysis. Secondary reactions are crucial in biomass pyrolysis as they significantly influence the final product distribution.
For particles of certain sizes (0.85–3.15 mm), increased tar yields were reported [22], suggesting more extensive internal reactions during pyrolysis. This particle size dependency highlights the complex interplay between heat and mass transfer within the biomass during slow pyrolysis, where particle sizes typically range from 0.075 to 19 mm, significantly influencing both energy transfer efficiency and product composition. Others reported [95] that the concentration of levoglucosan, a key cellulose pyrolysis product, representing a substantial portion of the bio-oil (26.5 wt%) at 450 °C, decreases significantly (to 9.1 wt%) at 650 °C due to thermal cracking. This degradation of levoglucosan at higher temperatures underscores the importance of optimizing pyrolysis conditions to maximize the yield of desired product components.
3.6. Effect of Particle Size and Heating Rate on PAH Fraction
The formation of specific PAH species (e.g., naphthalene (C10H8), acenaphthylene (C12H8), biphenyl (C12H10), fluorene (C13H10), phenanthrene (C14H10), and pyrene (C16H10)) is significantly influenced by process parameters such as particle size and heating rate. Understanding these influences is crucial for optimizing pyrolysis conditions to minimize PAH formation and environmental impact. Particle size significantly affects the rates of heat and mass transfer within the biomass particle. Smaller particles have a larger surface area-to-volume ratio, facilitating faster heat transfer into the particle and quicker release of volatile products. This rapid heating and volatile release influence the pathways of PAH formation. Rapid heating and volatile release in smaller particles can lead to a higher yield of lighter PAHs, such as naphthalene and acenaphthylene. The shorter residence time of volatiles within the particle reduces the likelihood of secondary reactions that form larger PAHs, whereas the heating rate directly influences the kinetics of pyrolysis reactions. Higher heating rates accelerate the decomposition process, leading to the rapid formation and release of volatiles. Lower heating rates favor slower, more controlled pyrolysis reactions. This can lead to a higher yield of char and a lower yield of volatiles, including PAHs. Higher heating rates promote rapid devolatilization, leading to a higher yield of volatiles [96].
The 3D surface plot in Figure 9a shows the predicted yield of naphthalene (C10H8) as a function of time and particle size. The highest naphthalene yield is observed at intermediate residence times (up to 2000 s) and smaller particle sizes (1–5 mm). Naphthalene formation increases rapidly, reaches a peak, and then decreases. This suggests that naphthalene is formed during the initial stages of pyrolysis and then either decomposes or is converted to other species at longer times.
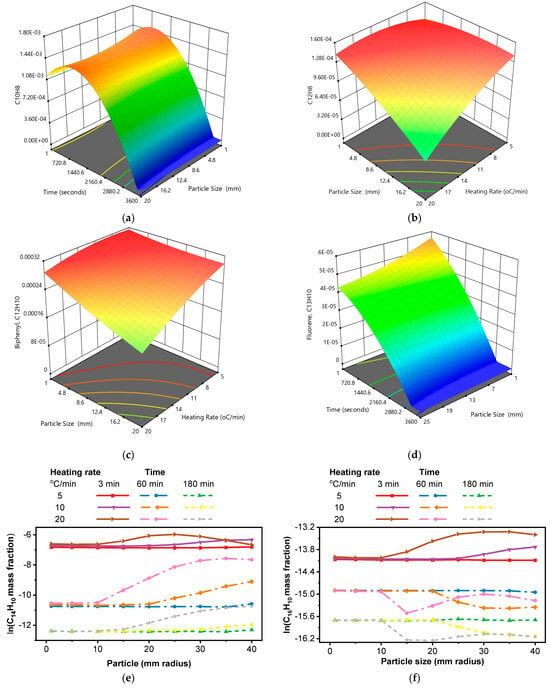
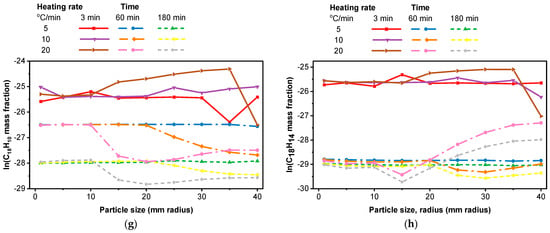
Figure 9.
Effect of particle size at different heating rates and residence times, CRECK model. (a) C10H8, heating rate of 15 °C/min; (b) C12H8, at 3000 s; (c) C12H10, at 3000 s; (d) C13H10, heating rate of 6 °C/min; (e) C14H10; (f) C16H10; (g) C18H10; (h) C18H14.
Analogous to the behavior observed with naphthalene, smaller particle sizes markedly enhance the formation of acenaphthylene (C12H8), as evidenced in Figure 9b. The peak acenaphthylene yield manifests at a particle size of 1 mm across the evaluated heating rates, with a similar trend observed at an elevated heating rate of 20 °C/min across the particle size range under consideration. This phenomenon arises from the superior heat transfer in smaller particles, which undergo rapid heating, thereby accelerating the release of volatile species. Slower heating rates provide sufficient time for these reactions to occur while minimizing the decomposition of acenaphthylene or its conversion to other species. Likewise, the maximum biphenyl formation, depicted in Figure 9c, aligns with conditions favoring acenaphthylene—namely, a particle size of 1 mm. Smaller particles consistently promote biphenyl production, with fractions dropping sharply as particle size increases, underscoring the critical role of particle size in reaction kinetics. Similarly, Figure 9d reveals that the highest fluorene formation occurs at shorter initial residence times and a particle size of 1 mm. However, the fraction of fluorene diminishes as both particle size and residence time increase, suggesting sensitivity to prolonged thermal exposure and reduced heat transfer efficiency in larger particles.
In the case of Figure 9e, higher particle sizes and heating rates contribute to an increased formation of PAHs during the pyrolysis process of corn cob feedstock. At higher heating rates and with larger particles, the pyrolysis reactions can be more rapid and intense. This can lead to increased decomposition of the feedstock, potentially resulting in higher PAH formation as more volatile compounds are released and recombined to form PAHs. Despite the challenges posed by larger particles in terms of mass transfer, at higher heating rates, the increased energy input can help overcome diffusion limitations. Also, it can enhance the release of volatile compounds from the surface of the particles, contributing to PAH formation. For higher-molecular-weight compounds C16H10 and C18H10 (Figure 9f,g), the PAH fraction generally decreases with increasing heating rate for particle sizes beyond a 10 mm radius, except for the 3 min residence time. In the case of C18H14 (Figure 9h), the effect of changing the residence time from 60 to 180 min is less noticeable. However, for particle sizes beyond a 15 mm radius, the PAH fraction increases at a heating rate of 20 °C/min in both residence time cases (60 and 180 min), in Figure 9h. Overall, the highest PAH fraction is observed at a heating rate of 20 °C/min with a 5 min residence time across the particle sizes considered. Across all species (naphthalene, acenaphthylene, biphenyl, and fluorene), smaller particle sizes consistently lead to higher yields. This is attributed to enhanced heat and mass transfer, promoting rapid heating and volatile release [29]. They are the primary PAHs commonly produced. However, the reduction in heavier PAH levels over time is attributed to their conversion into denser byproducts, particularly soot [97].
Based on the literature [37], the particle size range (0.075 mm to 2 mm) has a notable impact on PAH formation. This influence is likely due to the rapid release of volatile matter, increasing the potential for PAH formation. The distribution of PAHs can be elucidated by the preferential decomposition of PAHs with higher molar weights than those with lower molar weights. According to Mika et al. [98], a particle size smaller than 0.2 cm is recommended for optimal heat transfer, as it ensures uniform temperature distribution within the particles, facilitating chemical reactions throughout their structure. However, this size also maximizes the formation of PAH species. Research reports generally indicate that the concentration of PAHs is lower in biochar produced through slow pyrolysis compared to fast pyrolysis. And within plant-derived biochar, the PAH content diminishes with escalating pyrolysis temperatures ranging from 450 to 600 °C [24].
3.7. Validation and Optimization Results
The accurate prediction and modeling of PAH formation during biomass pyrolysis are crucial for understanding the impact of this process. One key parameter that influences PAH formation is the particle size of the biomass feedstock. The CRECK model, a comprehensive chemical kinetic model, was employed to investigate the influence of particle size during the devolatilization of corn cob, a commonly used agricultural residue.
In the context of the experimental findings, it was observed that the highest proportion of low-molecular-weight PAHs was recorded around a 2 mm particle size. This outcome indicates a clear influence of particle size on the distribution of ring numbers within PAHs during pyrolysis. Based on Figure 10a, from the experimental data [39], researchers observed that with an increase in particle size, the 16PAH content exhibited an initial increase followed by a decrease, which was noted. Similarly, based on Figure 10b–d, with an increase in particle size, the PAH fraction decreases. The CRECK model was able to capture the general trend of decreased PAH formation with increasing particle size, which is consistent with the experimental observations. This trend can be attributed to the differences in heat and mass transfer dynamics within the larger particles, leading to variations in the pyrolysis conditions and the resulting PAH concentrations. As shown in Figure 10e,f, with an increase in residence time, the PAH fraction decreases. Longer residence times allow more time for secondary reactions, and PAHs are intermediate; their decline at longer residence times aligns with their role in forming stable end-products.
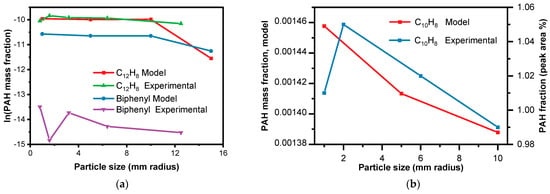
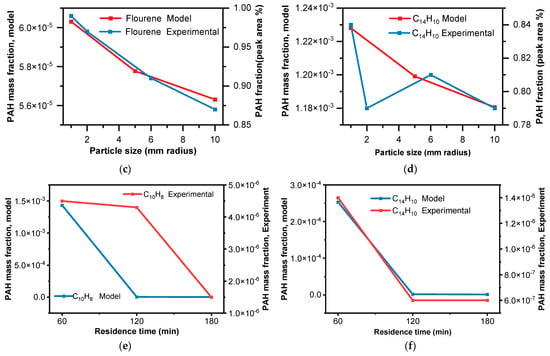
Figure 10.
Model comparison with experimental results, showing the trend of PAH species generation with particle size. (a) Under the same condition (20 °C·min−1) [39]; (b–d) under the same conditions of 10 °C·min−1, 500 °C, and 3 min but the experimental data were found in terms of peak area % [15]; (e) from experimental data in Table 3; (f) from experimental data in Table 3.
The discrepancies between the model predictions and the experimental data could be due to the inherent complexities of the pyrolysis process, including the influence of specific reactor configurations, the presence of secondary reactions, and the potential for condensation effects that are not accounted for in the model. The assumption of spherical particles in the CRECK model is a simplification and can have potential impact on the accuracy of the model predictions because of mass and heat transfer problems in real-world applications. In the case of TGA experiments, where the sample biomass used in the experiments was ground to a relatively uniform size and shape, the assumption might be more reasonable. So, through continuous refinement incorporating shape factors to model more complex particle shapes and the validation of the model against diverse experimental datasets, researchers can enhance our understanding of the complex pyrolysis process and its environmental implications.
Table 4 illustrates that the model’s F-value of 285.22, along with p-values less than 0.05, indicates that the model terms are significant. In particular, terms A, B, C, AB, AC, BC, A2, B2, and C2 are recognized as significant contributors to the model. The predicted R2 of 0.877 is in good agreement with the adjusted R2 of 0.881, with their difference being less than 0.2.

Table 4.
ANOVA for quadratic model, C12H8 species.
As demonstrated in Table 5, the regression equations from the model establish a clear connection between pyrolysis factors—such as particle size, heating rate, and residence time—and PAH levels, allowing for accurate predictions without the need for exhaustive experimentation. These equations play a crucial role in identifying optimal conditions to minimize toxic PAHs in biochar, effectively balancing yield and quality. Also, they reveal how these factors interact to influence PAH formation, providing essential insights for practical process control in real-world applications. Furthermore, they ensure compliance with regulatory standards by keeping PAH contamination low, a critical requirement for biochar intended for agricultural use. By offering a reproducible framework, the equations facilitate scaling the process from laboratory to field settings. Ultimately, they guide practical decisions, such as adjusting particle size, heating rate, and residence time, to produce safe and sustainable biochar from biomass like corn cobs.

Table 5.
Equations generated for PAH species fraction prediction within given factor range.
As shown in Figure 11, the optimal conditions minimizing PAH species formation from the model have been identified. A slow heating rate provides sufficient time for heat to penetrate the particle core, which helps reduce temperature gradients (i.e., preventing a cold core and hot surface). Smaller particles heat up too quickly, while larger particles, beyond the optimized size, risk incomplete pyrolysis. Under these conditions, particles release volatiles (precursors to PAHs) more gradually, which diminishes the chances of localized hot spots that can trigger secondary reactions forming PAHs. A slow heating rate also restricts the rapid release of volatiles into the gas phase, thereby avoiding conditions conducive to PAH synthesis, such as high-temperature radical recombination. This allows volatiles to remain within the particle, where lower temperatures (compared to rapid pyrolysis) inhibit gas-phase reactions that generate PAHs. It was reported [35] that as the diameter of spherical particles increases from 6 to 25 mm, PAH formation rises due to incomplete pyrolysis caused by shorter residence times. Conversely, a particle size of 1 cm was reported [99] to enhance the conversion efficiency of biomass feedstock.
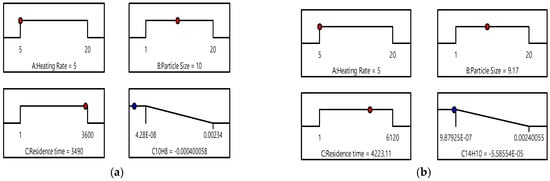
Figure 11.
Optimized condition at which the PAH species became the minimum: (a) C10H8; (b) C14H10. The red dot indicates the parameter value at which the PAH species concentration reaches its minimum. The blue dot represents the corresponding PAH species concentration that aligns with the position of the red dot.
Building on these findings, it can be concluded that a spherical biomass particle size with a radius of approximately 5 to 10 mm is optimal for minimizing PAH formation at a low heating rate of 5 °C/min. This size range ensures adequate heat penetration to the particle core while preventing rapid volatilization that could overload the system with tar precursors.
Overall, the comparison and validation of the CRECK model against the experimental data on the influence of corn cob particle size on PAH formation provided valuable insights into the model’s capabilities and limitations. The findings from this study serve as a foundation for further improving the model’s predictive accuracy and expanding its applicability to a broader range of biomass feedstocks and pyrolysis conditions.
Strategically controlling PAH formation during biochar production requires careful management of several key pyrolysis parameters. Temperature plays a crucial role, with an optimal range of 500–600 °C, often cited as balancing effective carbonization with PAH suppression [100]. Going beyond this range can lead to increased PAH formation, potentially due to biochar contamination through condensation and deposition in post-pyrolysis zones [101], as evidenced by lower total PAHs in tar oil at 500 °C compared to 400 °C and 600 °C [102]. Coupled with temperature control, a slow heating rate (≤10 °C/min) is essential. This minimizes the thermal cracking of volatiles, thereby limiting the secondary reactions that contribute to PAH formation and promoting gradual devolatilization for enhanced biochar stability. Extending the residence time beyond 50 min further ensures sufficient time for volatile PAH precursors, such as phenols and furans, to escape before condensing into aromatic structures.
Feedstock characteristics and physical properties also significantly influence PAH yields. Study [103] reveals that PAH formation is driven by incomplete combustion and varies with fuel type and temperature. Biomass (wheat straw and corn straw) generates higher PAH emissions than coal during pyrolysis and combustion, despite coal’s higher initial PAH content. Lignin-rich woody biomass typically produces lower PAH concentrations compared to herbaceous or straw-based feedstocks due to differences in the reactivity of cellulose and hemicellulose [104]. Particle size optimization (5–10 mm radius) is crucial for uniform heat transfer, preventing localized overheating that can drive PAH synthesis. Smaller particles risk rapid volatilization and incomplete combustion, while larger particles may retain PAH-laden tars within their pores. Pre-processing techniques like pelletization can improve thermal consistency and reduce PAH retention.
Furthermore, using CO2 or steam as carrier gases promotes oxidative cracking of PAH precursors, reducing their accumulation in the biochar. Closed-loop reactor designs that facilitate rapid evacuation of volatiles minimize secondary reactions and PAH retention, aligning with standards like the European Biochar Certificate (EBC) [105]. Optimizing gas flow and reactor design to efficiently remove volatiles from the solid phase is crucial in this regard. Ultimately, a holistic approach that considers the interplay of heating rate (<10 °C/min), residence time (>50 min with volatile removal), temperature (500–600 °C), particle size (<10 mm radius), and feedstock characteristics is essential for effectively minimizing PAH formation during slow-pyrolysis biochar production.
4. Conclusions
By integrating thermogravimetric analysis (TGA) data with the Chemical Reaction Engineering and Chemical Kinetics (CRECK) model, this study elucidated the pivotal role of pyrolysis parameters in governing polycyclic aromatic hydrocarbon (PAH) formation during corn cob devolatilization. A single spherical particle with a radius near 1 mm closely aligns with ideal TGA conditions, as it minimizes temperature gradients and mass transfer limitations, enabling better alignment with experimental TGA data obtained from small sample masses. In contrast, larger particles exhibit significant deviations from TGA behavior due to pronounced temperature gradients and mass transfer constraints. At high heating rates or during later decomposition stages, the inner particle temperature may exceed the surface temperature, driven by self-heating, reduced heat transfer pathways, and potential accumulation of gaseous products. These findings highlight the critical influence of particle size, residence time, and heating rate on the mechanisms of PAH formation in biomass pyrolysis. The CRECK model identified an optimal spherical particle radius of 5–10 mm, paired with a low heating rate of 5 °C/min, as effective in minimizing PAH formation. This size range ensures adequate heat penetration to the particle core while preventing rapid volatilization that could overload the system with tar precursors.
Although optimizing pyrolysis conditions markedly reduces PAH formation, uncertainties persist in both experimental and modeling approaches. Experimental PAH measurements relied on gas chromatography–mass spectrometry (GC-MS) analysis of tar components condensed on the biochar surface, whereas the CRECK model predicts PAH species formation during pyrolysis. A more direct comparison would require TGA-GC-MS, which captures PAH species in real time before they escape the reactor or condense. By deepening our understanding of how pyrolysis parameters influence PAH formation, this research lays the groundwork for improved strategies to mitigate environmental risks associated with biomass pyrolysis and advance sustainable biochar production practices.
5. Alignment of Proposed Solution with Sustainable Development Principles
The proposed solution aligns with the principles of sustainable development by addressing the environmental, economic, and social dimensions of biochar production for soil amendment practices. This ensures compliance with prevailing standards for tolerable PAH magnitudes, such as those set by the International Biochar Initiative (IBI) and the European Biochar Certificate (EBC), which specify maximum allowable total PAH concentrations (6 mg/kg for IBI and 4–6 mg/kg for EBC, depending on biochar grade) to minimize risks of soil contamination and human exposure. By identifying an optimal particle size range at a low heating rate, the solution reduces the formation of harmful PAHs, which are toxic environmental pollutants, thereby aligning with environmental and health regulations and mitigating the risk of soil and water contamination when biochar is applied as a soil amendment, promoting environmental sustainability. These standards guide acceptable PAH levels in biochar to prevent adverse ecological and health impacts, and the research’s focus on reducing PAH formation through optimized pyrolysis conditions directly supports compliance with these regulatory frameworks. Furthermore, the use of agricultural residue as a feedstock supports a circular economy by valorizing waste biomass, reducing reliance on non-renewable resources, and minimizing waste disposal challenges. The optimized pyrolysis process enhances biochar quality, improving its efficacy as a soil amendment to enhance soil fertility, water retention, and carbon sequestration, which contribute to long-term agricultural productivity and climate change mitigation.
Economically, the proposed solution supports sustainable development by enabling cost-effective biochar production that adheres to IBI and EBC standards for safe PAH levels, ensuring market acceptance and regulatory compliance. The CRECK model’s recommendation of specific particle sizes and heating rates can be implemented using existing pyrolysis technologies, such as fixed-bed or fluidized-bed reactors commonly used in biochar production, without requiring significant modifications or investments in specialized equipment, thus reducing upfront costs and operational complexity for producers, particularly for small-scale or regional agricultural operations. By reducing PAH formation to levels within the IBI and EBC thresholds, the solution also lowers costs associated with environmental remediation and compliance with stringent regulatory standards for biochar safety, further enhancing economic viability. The potential to reduce inorganic fertilizers substantially through enhanced biochar quality, which improves soil fertility and nutrient retention, further lowers input costs for farmers, increasing economic benefits and encouraging the adoption of sustainable practices.
Additionally, the improved biochar quality increases its market value as a soil amendment, providing economic incentives for farmers and producers. Socially, the approach promotes safer agricultural practices by producing cleaner biochar with PAH concentrations compliant with international standards, reducing health risks associated with PAH exposure for farmers, soils, and communities as such. Future refinements such as integrating TGA-GC-MS for real-time PAH monitoring and incorporating shape factors for diverse biomass types are recommended for further work. This will further enhance the scalability and adaptability of this solution, ensuring its applicability across various agricultural contexts while maintaining alignment with global sustainable development goals and regulatory standards for safe biochar application.
6. Limitations on the Practical Application of the Research and Scope for Further Work
The application of this research, which optimizes pyrolysis conditions to minimize PAH formation during corn cob devolatilization, is constrained by a few limitations. This study primarily focuses on corn cob as a feedstock, limiting its generalizability to other biomass types with varying chemical compositions and structural properties, which may influence PAH formation differently. The reliance on the CRECK model, while effective for spherical particles, does not fully account for complex particle geometries or irregular shapes common in real-world biomass, potentially reducing the model’s predictive accuracy for other geometrical shapes. Additionally, the experimental validation carried out in this study while employing other experimental data derived from GC-MS for PAH detection on condensed tar, which may not capture volatile PAHs escaping the reactor, suggests that TGA-GC-MS could provide more comprehensive data, but it was not employed in this study. The proposed conditions (5–10 mm particle radius and 5 °C/min heating rate) were optimized for specific pyrolysis setups, and their scalability to industrial systems or compatibility with different reactor types remains untested. These limitations highlight the need for further refinement to enhance the model’s applicability across varied pyrolysis conditions and biomass types.
Future research should prioritize TGA-GC-MS for comprehensive PAH quantification and refine the CRECK Solid Biomass model by coupling it with a gas-phase model and tar condensation mechanisms. Furthermore, integrating shape factors to account for complex particle geometries would enhance the model’s predictive accuracy across diverse pyrolysis conditions and biomass types. Conducting experiments with varied biomass types under controlled conditions (e.g., particle size, temperature, heating rate, residence time, and reactor type) remains the most reliable approach with which to validate these findings.
Author Contributions
Conceptualization, T.T.M. and V.R.A.; methodology, T.T.M.; data curation, T.T.M.; writing—original draft preparation, T.T.M.; writing—review and editing, V.R.A., A.N., M.M.A. and R.B.; visualization, V.R.A., A.N. and M.M.A.; supervision, V.R.A. All authors have read and agreed to the published version of the manuscript.
Funding
For the study, financial support was received from Jimma University, Office of Research Council and Jimma Institute of Technology, Center of Excellence. The APC was funded by the German Federal Ministry of Education and Research through its Project Management Agency PtDLR.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data that supports the findings of this study are available in the manuscript, but additional data shall be made available by the corresponding author upon request.
Acknowledgments
The authors are very grateful to Jimma University, Office of Research Council and Jimma Institute of Technology, Center of Excellence for providing the grant to conduct this study. They are also grateful to the CRECK Modeling Group for providing OpenSMOKE++ software. Authors acknowledge the financial support from the German Federal Ministry of Education and Research through its Project Management Agency PtDLR under project C-Cook-Mali-BMBF.
Conflicts of Interest
The authors declare no conflicts of interest in submitting to and publishing in this journal.
References
- Amjith, L.R.; Bavanish, B. A review on biomass and wind as renewable energy for sustainable environment. Chemosphere 2022, 293, 133579. [Google Scholar] [CrossRef] [PubMed]
- Pstrowska, K.; Łużny, R.; Fałtynowicz, H.; Jaroszewska, K.; Postawa, K.; Pyshyev, S.; Witek-Krowiak, A. Unlocking Sustainability: A Comprehensive Review of Up-Recycling Biomass Waste into Biochar for Environmental Solutions. Chem. Chem. Technol. 2024, 18, 211–231. [Google Scholar] [CrossRef]
- Varkolu, M.; Gundekari, S.; Chandra, V.; Palla, S.; Kumar, P.; Bhattacharjee, S.; Vinodkumar, T. Recent Advances in Biochar Production, Characterization, and Environmental Applications. Catalysts 2025, 15, 243. [Google Scholar] [CrossRef]
- Amalina, F.; Syukor, A.; Razak, A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Journal of Hazardous Materials Advances Biochar production techniques utilizing biomass waste-derived materials and environmental applications—A review. J. Hazard. Mater. Adv. 2022, 7, 100134. [Google Scholar] [CrossRef]
- Durak, H. Comprehensive assessment of thermochemical processes for sustainable waste management and resource recovery. Processes 2023, 11, 2092. [Google Scholar] [CrossRef]
- Lee, D.; Nam, H.; Won Seo, M.; Hoon Lee, S.; Tokmurzin, D.; Wang, S.; Park, Y.-K. Recent progress in the catalytic thermochemical conversion process of biomass for biofuels. Chem. Eng. J. 2022, 447, 137501. [Google Scholar] [CrossRef]
- Joshi, N.C.; Sinha, S.; Bhatnagar, P.; Nath, Y.; Negi, B.; Kumar, V.; Gururani, P. A concise review on waste biomass valorization through thermochemical conversion. Curr. Res. Microb. Sci. 2024, 6, 100237. [Google Scholar] [CrossRef]
- Egbosiuba, T.C. Biochar and bio-oil fuel properties from nickel nanoparticles assisted pyrolysis of cassava peel. Heliyon 2022, 8, e10114. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Miroshnichenko, D.; Zhylina, M.; Shmeltser, K. Modern Use of Biochar in Various Technologies and Industries. A Review. Chem. Chem. Technol. 2024, 18, 232–243. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Yue, X.; Zhang, K.; Zhang, Z.; Zheng, H.; Zhao, X.; Li, H.; Zhou, P.; Wu, F.; et al. Advances in biochar composites for environmental sustainability. Adv. Compos. Hybrid Mater. 2025, 8, 74. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.A.; Hashem, A.; Abd_Allah, E.F.; et al. Biochar Production and Characteristics, Its Impacts on Soil Health, Crop Production, and Yield Enhancement: A Review. Plants 2024, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Rambhatla, N.; Panicker, T.F.; Mishra, R.K.; Manjeshwar, S.K.; Sharma, A. Biomass pyrolysis for biochar production: Study of kinetics parameters and effect of temperature on biochar yield and its physicochemical properties. Results Eng. 2025, 25, 103679. [Google Scholar] [CrossRef]
- Perez-Martinez, B.B.; Lopez-Urionabarrenechea, A.; Serras-Malillos, A.; Acha, E.; Caballero, B.M.; Asueta, A.; Arnaiz, S. Influence of Feedstock Particle Size on the Certain Determination of Chlorine and Bromine in Pyrolysis Oils from Waste Electrical and Electronic Equipment Plastics. ACS Omega 2024, 9, 32593–32603. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rasul, M.G.; Jahirul, M.I.; Khan, M.M.K. Fast Pyrolysis of Municipal Green Waste in an Auger Reactor: Effects of Residence Time and Particle Size on the Yield and Characteristics of Produced Oil. Energies 2024, 17, 2914. [Google Scholar] [CrossRef]
- Drané, M.; Zbair, M.; Hajjar-Garreau, S.; Josien, L.; Michelin, L.; Bennici, S.; Limousy, L. Unveiling the Potential of Corn Cob Biochar: Analysis of Microstructure and Composition with Emphasis on Interaction with NO2. Materials 2024, 17, 159. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, Y.; Zhang, Y.; Liu, C.; Wang, Y.; Xie, Y.; Ji, G.; Li, A. Fast pyrolysis of biomass with diverse properties to produce liquid hydrogen storage molecules. Carbon Capture Sci. Technol. 2024, 12, 100230. [Google Scholar] [CrossRef]
- Manka’abusi, D.; Zongo, N.; Lompo, D.J.P.; Steiner, C.; Marschner, B.; Buerkert, A. Soil properties and agronomic effects of repeated biochar amendment and fertilization in an urban horticultural system of Burkina Faso. Eur. J. Agron. 2024, 159, 127234. [Google Scholar] [CrossRef]
- Abban-Baidoo, E.; Manka’abusi, D.; Apuri, L.; Marschner, B.; Frimpong, K.A. Biochar addition influences C and N dynamics during biochar co-composting and the nutrient content of the biochar co-compost. Sci. Rep. 2024, 14, 23781. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, D.; Scheufele, F.B.; Ro, K.S.; Libra, J.A.; Marzban, N.; Chen, H.; Ribeiro, C.; Jeong, C. Occurrence of Polycyclic Aromatic Hydrocarbons (PAHs) in Pyrochar and Hydrochar during Thermal and Hydrothermal Processes. Agronomy 2024, 14, 2040. [Google Scholar] [CrossRef]
- Tiwari, M.; Dirbeba, M.J.; Lehmusto, J.; Yrjas, P.; Vinu, R. Analytical and applied pyrolysis of challenging biomass feedstocks: Effect of pyrolysis conditions on product yield and composition. J. Anal. Appl. Pyrolysis 2024, 177, 106355. [Google Scholar] [CrossRef]
- Jerzak, W.; Acha, E.; Li, B. Comprehensive Review of Biomass Pyrolysis: Conventional and Advanced Technologies, Reactor Designs, Product Compositions and Yields, and Techno-Economic Analysis. Energies 2024, 17, 5082. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Liu, P.; Wang, Z.; Huhe, T.; Chen, Z.; Wu, Y.; Lei, T. A Study on the Pyrolysis Behavior and Product Evolution of Typical Wood Biomass to Hydrogen-Rich Gas Catalyzed by the Ni-Fe/HZSM-5 Catalyst. Catalysts 2024, 14, 200. [Google Scholar] [CrossRef]
- Soko, A.; Boguszewska-czubara, A.; Zarzycki, R.; Oleszczuk, P.; Gao, Y.; Ko, M. Bioresource Technology Increased oxygen content in biochar lowered bioavailability of polycyclic aromatic hydrocarbons—Related toxicity to various organisms. Bioresour. Technol. 2024, 407, 131110. [Google Scholar] [CrossRef]
- Piazza, V.; Batista, R.; Frassoldati, A.; Lietti, L.; Chiaberge, S.; Gambaro, C.; Siviero, A.; Faravelli, T.; Beretta, A. Detailed speciation of biomass pyrolysis products with a novel TGA-based methodology: The case-study of cellulose. J. Anal. Appl. Pyrolysis 2024, 178, 106413. [Google Scholar] [CrossRef]
- Osman, A.I.; Farghali, M.; Ihara, I.; Elgarahy, A.M.; Ayyad, A.; Mehta, N.; Ng, K.H.; Abd El-Monaem, E.M.; Eltaweil, A.S.; Hosny, M.; et al. Materials, fuels, upgrading, economy, and life cycle assessment of the pyrolysis of algal and lignocellulosic biomass: A review. Environ. Chem. Lett. 2023, 21, 1419–1476. [Google Scholar] [CrossRef]
- Mariyam, S.; Al-Ansari, T.; McKay, G. Particle size impact on pyrolysis of multi-biomass: A solid-state reaction modeling study. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 3681–3691. [Google Scholar] [CrossRef]
- Rozzi, E.; Minuto, F.D.; Lanzini, A.; Leone, P. Green Synthetic Fuels: Renewable Routes for the Conversion of Non-Fossil Feedstocks into Gaseous Fuels and Their End Uses. Energies 2024, 13, 420. [Google Scholar] [CrossRef]
- Afessa, M.M.; Locaspi, A.; Debiagi, P.; Frassoldati, A.; Caraccio, R.; Ramayya, A.V.; Faravelli, T. Pyrolysis of large biomass particles: Model validation and application to coffee husks valorization. J. Anal. Appl. Pyrolysis 2025, 188, 107028. [Google Scholar] [CrossRef]
- Fernández, I.; Pérez, S.F.; Fernández-Ferreras, J.; Llano, T. Microwave-Assisted Pyrolysis of Forest Biomass. Energies 2024, 17, 4852. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.; Lai, Z.Y.; Rashwan, A.K.; Farghali, M.; Ahmed, A.A.; Liu, Y.; Fang, B.; Chen, Z.; Al-Fatesh, A.; et al. Machine learning and computational chemistry to improve biochar fertilizers: A review. Environ. Chem. Lett. 2023, 21, 3159–3244. [Google Scholar] [CrossRef]
- Aboelela, D.; Saleh, H.; Attia, A.M.; Elhenawy, Y.; Majozi, T.; Bassyouni, M. Recent Advances in Biomass Pyrolysis Processes for Bioenergy Production: Optimization of Operating Conditions. Sustainability 2023, 15, 11238. [Google Scholar] [CrossRef]
- Silva, J.; Teixeira, S.; Teixeira, J. A Review of Biomass Thermal Analysis, Kinetics and Product Distribution for Combustion Modeling: From the Micro to Macro Perspective. Energies 2023, 16, 6705. [Google Scholar] [CrossRef]
- Stark, A.K.; Ghoniem, A.F. Quantification of the influence of particle diameter on Polycyclic Aromatic Hydrocarbon (PAH) formation in fluidized bed biomass pyrolysis. Fuel 2017, 206, 276–288. [Google Scholar] [CrossRef]
- Gaston, K.R.; Jarvis, M.W.; Pepiot, P.; Smith, K.M.; Frederick, W.J.; Nimlos, M.R. Biomass pyrolysis and gasification of varying particle sizes in a fluidized-bed reactor. Energy Fuels 2011, 25, 3747–3757. [Google Scholar] [CrossRef]
- Mohseni-bandpei, A.; Majlesi, M.; Ra, M. Polycyclic aromatic hydrocarbons (PAHs) formation during the fast pyrolysis of hazardous health-care waste. Chemosphere 2019, 227, 277–288. [Google Scholar] [CrossRef]
- Dai, Q.; Jiang, X.; Lv, G.; Ma, X.; Jin, Y.; Wang, F.; Chi, Y.; Yan, J. Investigation into particle size influence on PAH formation during dry sewage sludge pyrolysis: TG-FTIR analysis and batch scale research. J. Anal. Appl. Pyrolysis 2015, 112, 388–393. [Google Scholar] [CrossRef]
- Li, Y.; Liao, Y.; He, Y.; Xia, K.; Qiao, S.; Zhang, Q. Polycyclic Aromatic Hydrocarbons Concentration in Straw Biochar with different Particle Size. Environ. Sci. 2016, 31, 91–97. [Google Scholar] [CrossRef][Green Version]
- Qiao, M.; Wang, F.; Meng, S.; Liu, Y.; Song, L.; Zhao, J.; Ma, Y.; Zhao, G.; Huang, X.; Hai, D. Pear Wood Pyrolysis Influences Quality and Levels of Polycyclic Aromatic Hydrocarbons in Liquid Smoke. J. Food Prot. 2024, 87, 100320. [Google Scholar] [CrossRef]
- Gulyurtlu, I.; Karunaratne, D.G.G.P.; Cabrita, I. The study of the effect of operating parameters on the PAH formation during the combustion of coconut shell in a fluidised bed. Fuel 2003, 82, 215–223. [Google Scholar] [CrossRef]
- Afshar, M.; Mofatteh, S. Biochar for a sustainable future: Environmentally friendly production and diverse applications. Results Eng. 2024, 23, 102433. [Google Scholar] [CrossRef]
- Li, Y.; Gupta, R.; Zhang, Q.; You, S. Review of biochar production via crop residue pyrolysis: Development and perspectives. Bioresour. Technol. 2023, 369, 128423. [Google Scholar] [CrossRef]
- Hafidzal, M.; Mohd, B.; Nakamura, H.; Hasegawa, S.; Tezuka, T.; Maruta, K. Effects of n-butanol addition on sooting tendency and formation of C 1–C 2 primary intermediates of n -heptane / air mixture in a micro flow reactor with a controlled temperature profile. Combust. Sci. Technol. 2018, 190, 2066–2081. [Google Scholar] [CrossRef]
- Drakon, A.V.; Eremin, A.V.; Mikheyeva, E.Y. Influence of chemically active additives on kinetics of acetylene self-decomposition and following soot formation. Combust. Sci. Technol. 2023, 195, 2774–2800. [Google Scholar] [CrossRef]
- Wu, S.; Liu, H.; Duan, Q.; Li, J.; Sun, Q.; Li, Z. A Discrete Distributed Activation Energy Model for Cedar and Polyethylene Fast Heating Pyrolysis Kinetics. Processes 2024, 12, 2618. [Google Scholar] [CrossRef]
- Pielsticker, S.; Debiagi, P.; Cerciello, F.; Hasse, C.; Kneer, R. Comparative analysis of pyrolysis models including SFOR, CRECK, and Bio-CPD to predict reaction kinetics and products from extracted biomass components. Fuel 2024, 371, 131867. [Google Scholar] [CrossRef]
- Assefa, B.T.; Chamberlin, J.; Reidsma, P.; Silva, J.V.; van Ittersum, M.K. Correction to: Unravelling the variability and causes of smallholder maize yield gaps in Ethiopia. Food Secur. 2020, 12, 489–490. [Google Scholar] [CrossRef]
- ASTM D3172-13; Standard Practice for Proximate Analysis of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2013.
- Bizerra, D.; Nunes, J.; Barros, C.; Paixão, R.; Marques, R.; Neto, F.S.; Santos, J.; Melo, R.; Fernandes, B.; Rios, M. Carnauba Straw as Feedstock for Solid Biofuel Production. Adv. Environ. Eng. Res. 2023, 4, 43. [Google Scholar] [CrossRef]
- DIN EN ISO 16948:2015-09; Solid biofuels-Determination of Total Content of Carbon, Hydrogen and Nitrogen. Deutsches Institut für Normung: Berlin, Germany, 2015.
- Adam, R.; Pollex, A.; Zeng, T.; Kirsten, C.; Röver, L.; Berger, F.; Lenz, V.; Werner, H. Systematic homogenization of heterogenous biomass batches—Industrial-scale production of solid biofuels in two case studies. Biomass Bioenergy 2023, 173, 106808. [Google Scholar] [CrossRef]
- Debiagi, P.; Gentile, G.; Cuoci, A.; Frassoldati, A.; Ranzi, E.; Faravelli, T. A predictive model of biochar formation and characterization. J. Anal. Appl. Pyrolysis 2018, 134, 326–335. [Google Scholar] [CrossRef]
- Debiagi, P.E.A.; Pecchi, C.; Gentile, G.; Frassoldati, A.; Cuoci, A.; Faravelli, T.; Ranzi, E. Extractives Extend the Applicability of Multistep Kinetic Scheme of Biomass Pyrolysis. Energy Fuels 2015, 29, 6544–6555. [Google Scholar] [CrossRef]
- Wossine, S.E.; Thothadri, G.; Tufa, H.B.; Tucho, W.M.; Murtaza, A.; Edacherian, A.; Sayeed Ahmed, G.M. Isolation and Characterization of Spherical Cellulose Nanocrystals Extracted from the Higher Cellulose Yield of the Jenfokie Plant: Morphological, Structural, and Thermal Properties. Polymers 2024, 16, 1629. [Google Scholar] [CrossRef]
- Afessa, M.M.; Olu, F.E.; Geleta, W.S.; Legese, S.S.; Ramayya, A.V. Unlocking the potential of biochar derived from coffee husk and khat stem for catalytic tar cracking during biomass pyrolysis: Characterization and evaluation. Biomass Convers. Biorefinery 2025, 15, 11011–11026. [Google Scholar] [CrossRef]
- Debiagi, P.; Faravelli, T.; Hasse, C.; Ranzi, E. Kinetic Modeling of Solid, Liquid and Gas Biofuel Formation from Biomass Pyrolysis. In Production of Biofuels and Chemicals with Pyrolysis; Fang, Z., Smith, R.L., Jr., Xu, L., Eds.; Springer: Singapore, 2020; pp. 31–76. ISBN 9789811527319. [Google Scholar]
- Afessa, M.M.; Debiagi, P.; Ferreiro, A.I.; Mendes, M.A.A.; Faravelli, T.; Ramayya, A.V. Experimental and modeling investigation on pyrolysis of agricultural biomass residues: Khat stem and coffee husk for bio-oil application. J. Anal. Appl. Pyrolysis 2022, 162, 105435. [Google Scholar] [CrossRef]
- Gentile, G.; Debiagi, P.E.A.; Cuoci, A.; Frassoldati, A.; Ranzi, E.; Faravelli, T. A computational framework for the pyrolysis of anisotropic biomass particles. Chem. Eng. J. 2017, 321, 458–473. [Google Scholar] [CrossRef]
- Barr, M.R.; Jervis, R.; Zhang, Y.; Bodey, A.J.; Rau, C.; Shearing, P.R.; Brett, D.J.L.; Titirici, M.-M.; Volpe, R. Towards a mechanistic understanding of particle shrinkage during biomass pyrolysis via synchrotron X-ray microtomography and in-situ radiography. Sci. Rep. 2021, 11, 2656. [Google Scholar] [CrossRef]
- Losic, D.; Farivar, F.; Yap, P.L. Refining and Validating Thermogravimetric Analysis (TGA) for Robust Characterization and Quality Assurance of Graphene-Related Two-Dimensional Materials (GR2Ms). C-J. Carbon Res. 2024, 10, 30. [Google Scholar] [CrossRef]
- Kamga, P.L.W.; Vitoussia, T.; Bissoue, A.N.; Nguimbous, E.N.; Dieudjio, D.N.; Bot, B.V.; Njeugna, E. Physical and energetic characteristics of pellets produced from Movingui sawdust, corn spathes, and coconut shells. Energy Rep. 2024, 11, 1291–1301. [Google Scholar] [CrossRef]
- Maulinda, L.; Husin, H.; Rahman, N.A.; Meurah, C. Results in Engineering Effects of temperature and times on the product distribution of bio-oils derived from Typha latifolia pyrolysis as renewable energy. Results Eng. 2023, 18, 101163. [Google Scholar] [CrossRef]
- Shah, H.H.; Amin, M.; Iqbal, A.; Nadeem, I.; Kalin, M.; Soomar, A.M.; Galal, A.M. A review on gasification and pyrolysis of waste plastics. Front. Chem. 2023, 10, 960894. [Google Scholar] [CrossRef]
- Kumar, N.V.; Sawargaonkar, G.; Rani, C.S.; Pasumarthi, R.; Kale, S. Harnessing the potential of pigeonpea and maize feedstock biochar for carbon sequestration, energy generation, and environmental sustainability. Bioresour. Bioprocess. 2024, 11, 5. [Google Scholar] [CrossRef]
- Landrat, M.; Abawalo, M.; Piko, K. Assessing the Potential of Teff Husk for Biochar Production through Slow Pyrolysis: Effect of Pyrolysis Temperature on Biochar Yield. Energies 2024, 17, 1988. [Google Scholar] [CrossRef]
- Vilas-Boas, A.C.M.; Tarelho, L.A.C.; Oliveira, H.S.M.; Silva, F.G.C.S.; Pio, D.T.; Matos, M.A.A. Valorisation of residual biomass by pyrolysis: Influence of process conditions on products. Sustain. Energy Fuels 2023, 8, 379–396. [Google Scholar] [CrossRef]
- Zou, X.; Debiagi, P.; Amjed, M.A.; Zhai, M.; Faravelli, T. Impact of high-temperature biomass pyrolysis on biochar formation and composition. J. Anal. Appl. Pyrolysis 2024, 179, 106463. [Google Scholar] [CrossRef]
- Syguła, E.; Ciolkosz, D.; Białowiec, A. The significance of structural components of lignocellulosic biomass on volatile organic compounds presence on biochar—A review. Wood Sci. Technol. 2024, 58, 859–886. [Google Scholar] [CrossRef]
- Panizio, R.; Castro, C.; Pacheco, N.; Assis, A.C.; Longo, A.; Vilarinho, C.; Teixeira, J.C.; Brito, P.; Gonçalves, M.; Nobre, C. Investigation of biochars derived from waste lignocellulosic biomass and insulation electric cables: A comprehensive TGA and Macro-TGA analysis. Heliyon 2024, 10, e37882. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Gutiérrez-Trashorras, A.J.; Laine-Cuervo, G.; Ríos-Fernández, J.C. The relevant effect of marine salt and epiphytes on Posidonia oceanica waste pyrolysis: Removal of SO2/HCl emissions and promotion of O/HCOOH formation. Waste Manag. 2024, 181, 101–113. [Google Scholar] [CrossRef]
- Gao, X.; Lu, L.; Shahnam, M.; Rogers, W.A.; Smith, K.; Gaston, K.; Robichaud, D.; Brennan Pecha, M.; Crowley, M.; Ciesielski, P.N.; et al. Assessment of a detailed biomass pyrolysis kinetic scheme in multiscale simulations of a single-particle pyrolyzer and a pilot-scale entrained flow pyrolyzer. Chem. Eng. J. 2021, 418, 129347. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, H.; Chu, S.; Zha, Z. Pyrolysis of single large biomass particle: Simulation and experiments. Chin. J. Chem. Eng. 2021, 29, 375–382. [Google Scholar] [CrossRef]
- Karda, D.; Swie, I.W. Modelling thermal behaviour of a single solid particle pyrolysing in a hot gas flow. Energy 2021, 221, 119802. [Google Scholar] [CrossRef]
- Ogunkanmi, J.O.; Kulla, D.M.; Omisanya, N.O.; Sumaila, M.; Obada, D.O. Extraction of bio-oil during pyrolysis of locally sourced palm kernel shells: Effect of process parameters. Case Stud. Therm. Eng. 2018, 12, 711–716. [Google Scholar] [CrossRef]
- Han, F.; Wang, M.; Ma, X.; Yin, L.; Chen, D. Numerical simulation of heat transfer properties of large-sized biomass particles during pyrolysis process. Heliyon 2023, 9, e21255. [Google Scholar] [CrossRef] [PubMed]
- Von Berg, L.; Hochenauer, C.; Scharler, R. Multi-scale modelling of fluidized bed biomass gasification using a 1D particle model coupled to CFD. Fuel 2022, 324, 124677. [Google Scholar] [CrossRef]
- Zhang, F.; Tavakkol, S.; Galeazzo, F.C.C.; Stapf, D. Particle-resolved simulation of the pyrolysis process of a single plastic particle. Heat Mass Transf. 2025, 61, 12. [Google Scholar] [CrossRef]
- Wickramaarachchi, W.A.M.K.P.; Narayana, M. Pyrolysis of single biomass particle using three-dimensional Computational Fluid Dynamics modelling. Renew. Energy 2020, 146, 1153–1165. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Zacharis, A.K. Simulation and Experimental Study of the Isothermal Thermogravimetric Analysis and the Apparent Alterations of the Thermal Stability of Composite Polymers. Polymers 2024, 16, 1454. [Google Scholar] [CrossRef]
- Ahmed, A.; Afolabi, E.A.; Garba, M.U.; Musa, U.; Alhassan, M.; Ishaq, K. Effect of particle size on thermal decomposition and devolatilization kinetics of melon seed shell. Chem. Eng. Commun. 2019, 206, 1228–1240. [Google Scholar] [CrossRef]
- Escalante, J.; Chen, W.H.; Tabatabaei, M.; Hoang, A.T.; Kwon, E.E.; Andrew Lin, K.Y.; Saravanakumar, A. Pyrolysis of lignocellulosic, algal, plastic, and other biomass wastes for biofuel production and circular bioeconomy: A review of thermogravimetric analysis (TGA) approach. Renew. Sustain. Energy Rev. 2022, 169, 112914. [Google Scholar] [CrossRef]
- Caposciutti, G.; Almuina-Villar, H.; Dieguez-Alonso, A.; Gruber, T.; Kelz, J.; Desideri, U.; Hochenauer, C.; Scharler, R.; Anca-Couce, A. Experimental investigation on biomass shrinking and swelling behaviour: Particles pyrolysis and wood logs combustion. Biomass Bioenergy 2019, 123, 1–13. [Google Scholar] [CrossRef]
- Zolghadr, A.; Kelley, M.D.; Sokhansefat, G.; Moradian, M.; Sullins, B.; Ley, T.; Biernacki, J.J. Biomass microspheres—A new method for characterization of biomass pyrolysis and shrinkage. Bioresour. Technol. 2019, 273, 16–24. [Google Scholar] [CrossRef]
- Rony, Z.I.; Rasul, M.G.; Jahirul, M.I.; Hasan, M.M. Optimizing Seaweed (Ascophyllum nodosum) Thermal Pyrolysis for Environmental Sustainability: A Response Surface Methodology Approach and Analysis of Bio-Oil Properties. Energies 2024, 17, 863. [Google Scholar] [CrossRef]
- Paulsen, A.D.; Mettler, M.S.; Dauenhauer, P.J. The role of sample dimension and temperature in cellulose pyrolysis. Energy Fuels 2013, 27, 2126–2134. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. A review on lignin pyrolysis: Pyrolytic behavior, mechanism, and relevant upgrading for improving process efficiency. Biotechnol. Biofuels Bioprod. 2022, 15, 106. [Google Scholar] [CrossRef]
- Quan, C.; Gao, N.; Song, Q. Pyrolysis of biomass components in a TGA and a fixed-bed reactor: Thermochemical behaviors, kinetics, and product characterization. J. Anal. Appl. Pyrolysis 2016, 121, 84–92. [Google Scholar] [CrossRef]
- Pecha, M.B.; Arbelaez, J.I.M.; Garcia-Perez, M.; Chejne, F.; Ciesielski, P.N. Progress in understanding the four dominant intra-particle phenomena of lignocellulose pyrolysis: Chemical reactions, heat transfer, mass transfer, and phase change. Green Chem. 2019, 21, 2868–2898. [Google Scholar] [CrossRef]
- Piazza, V.; Batista da Silva Junior, R.; Frassoldati, A.; Lietti, L.; Gambaro, C.; Rajendran, K.; Chen, D.; Faravelli, T.; Beretta, A. Unravelling the complexity of hemicellulose pyrolysis: Quantitative and detailed product speciation for xylan and glucomannan in TGA and fixed bed reactor. Chem. Eng. J. 2024, 497, 154579. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Zhuang, X.; Gan, Z.; Zhou, J.; Zhang, Y.; Zhang, H. Insight into biomass pyrolysis mechanism based on cellulose, hemicellulose, and lignin: Evolution of volatiles and kinetics, elucidation of reaction pathways, and characterization of gas, biochar and bio-oil. Combust. Flame 2022, 242, 112142. [Google Scholar] [CrossRef]
- Dou, J.; Henrikki, M.; Richards, T. Identifying the primary reactions and products of fast pyrolysis of alkali lignin. J. Anal. Appl. Pyrolysis 2020, 151, 104917. [Google Scholar] [CrossRef]
- Jerzak, W.; Reinmöller, M.; Magdziarz, A. Estimation of the heat required for intermediate pyrolysis of biomass. Clean Technol. Environ. Policy 2022, 24, 3061–3075. [Google Scholar] [CrossRef]
- Atakoohi, S.E.; Spennati, E.; Casazza, A.A.; Riani, P.; Garbarino, G. Investigating the Effect of Operational Variables on the Yield, Characterization, and Properties of End-of-Life Olive Stone Biomass Pyrolysis Products. Molecules 2023, 28, 6516. [Google Scholar] [CrossRef]
- Cortazar, M.; Santamaria, L.; Lopez, G.; Alvarez, J.; Zhang, L.; Wang, R.; Bi, X.; Olazar, M. A comprehensive review of primary strategies for tar removal in biomass gasification. Energy Convers. Manag. 2023, 276, 116496. [Google Scholar] [CrossRef]
- Ochieng, R.; Ceron, A.L.; Konist, A.; Sarker, S. Experimental and modeling studies of intermediate pyrolysis of wood in a laboratory-scale continuous feed retort reactor. Bioresour. Technol. Rep. 2023, 24, 101650. [Google Scholar] [CrossRef]
- Singh, A.; Singh, D.K.; Ojha, D.K. Sustainable Chemistry for Climate Action Chemical engineering perspective of pyrolysis reactor and condenser system for biomass valorisation. Sustain. Chem. Clim. Action 2024, 5, 100046. [Google Scholar] [CrossRef]
- Bensabath, T.; Le, M.D.; Monnier, H.; Glaude, P.; Bensabath, T.; Le, M.D.; Monnier, H.; Polycyclic, P.G.; Réactions, L.; De Lorraine, U.; et al. Polycyclic aromatic hydrocarbon (PAH) formation during acetylene pyrolysis in tubular reactor under low pressure carburizing conditions. Chem. Eng. Sci. 2019, 202, 84–94. [Google Scholar] [CrossRef]
- Pahnila, M.; Koskela, A.; Sulasalmi, P.; Fabritius, T. A Review of Pyrolysis Technologies and the Effect of Process Parameters on Biocarbon Properties. Energies 2023, 16, 6936. [Google Scholar] [CrossRef]
- Elhenawy, Y.; Fouad, K.; Mansi, A.; Bassyouni, M.; Gadalla, M.; Ashour, F.; Majozi, T. Experimental analysis and numerical simulation of biomass pyrolysis. J. Therm. Anal. Calorim. 2024, 149, 10369–10383. [Google Scholar] [CrossRef]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.H.; Cornelissen, G. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef]
- Buss, W.; Hilber, I.; Graham, M.C.; Masěk, O. Composition of PAHs in Biochar and Implications for Biochar Production. ACS Sustain. Chem. Eng. 2022, 10, 6755–6765. [Google Scholar] [CrossRef]
- Andrzej, P.; Onopiuk, A.; Kielar, J.; Chojnacki, J.; Kukiełka, L.; Najser, J.; Mikeska, M.; Knutel, B.; Berner, B. Polycyclic Aromatic Hydrocarbons (PAHs) in Wheat Straw Pyrolysis Products Produced for Energy Purposes. Sustainability 2024, 16, 9639. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, F.; Li, Z.; Tan, H. Formation and emission characteristics of PAHs during pyrolysis and combustion of coal and biomass. Fuel 2024, 378, 132935. [Google Scholar] [CrossRef]
- Buss, W.; Graham, M.C.; MacKinnon, G.; Mašek, O. Strategies for producing biochars with minimum PAH contamination. J. Anal. Appl. Pyrolysis 2016, 119, 24–30. [Google Scholar] [CrossRef]
- Mengesha, T.T.; Ancha, V.R.; Sundar, L.S.; Pollex, A. Review on the Influence of Pyrolysis Process Parameters for Biochar Production with Minimized Polycyclic Aromatic Hydrocarbon Content. J. Anal. Appl. Pyrolysis 2024, 182, 106699. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).