Research on Thermochemical and Gas Emissions Analysis for the Sustainable Co-Combustion of Petroleum Oily Sludge and High-Alkali Lignite
Abstract
1. Introduction
2. Materials and Methodology
2.1. Sample Preparation
2.2. Analysis of the Combustion Process
2.2.1. TG-MS Analysis
2.2.2. Combustion Characteristic Parameters
- (1)
- Ignition temperature (Ti)
- (2)
- Burnout temperature (Tf)
- (3)
- Maximum combustion rate (DTGmax)
- (4)
- Maximum combustion rate temperature (Tmax)
- (5)
- Average burning rate (DTGmean)
- (6)
- Comprehensive combustion performance parameters (S)
2.3. Sample Property Testing
2.4. Analysis of Co-Combustion Interaction
2.5. Kinematic Analysis and Calculation Method
3. Results and Discussion
3.1. Chemical Property Analysis
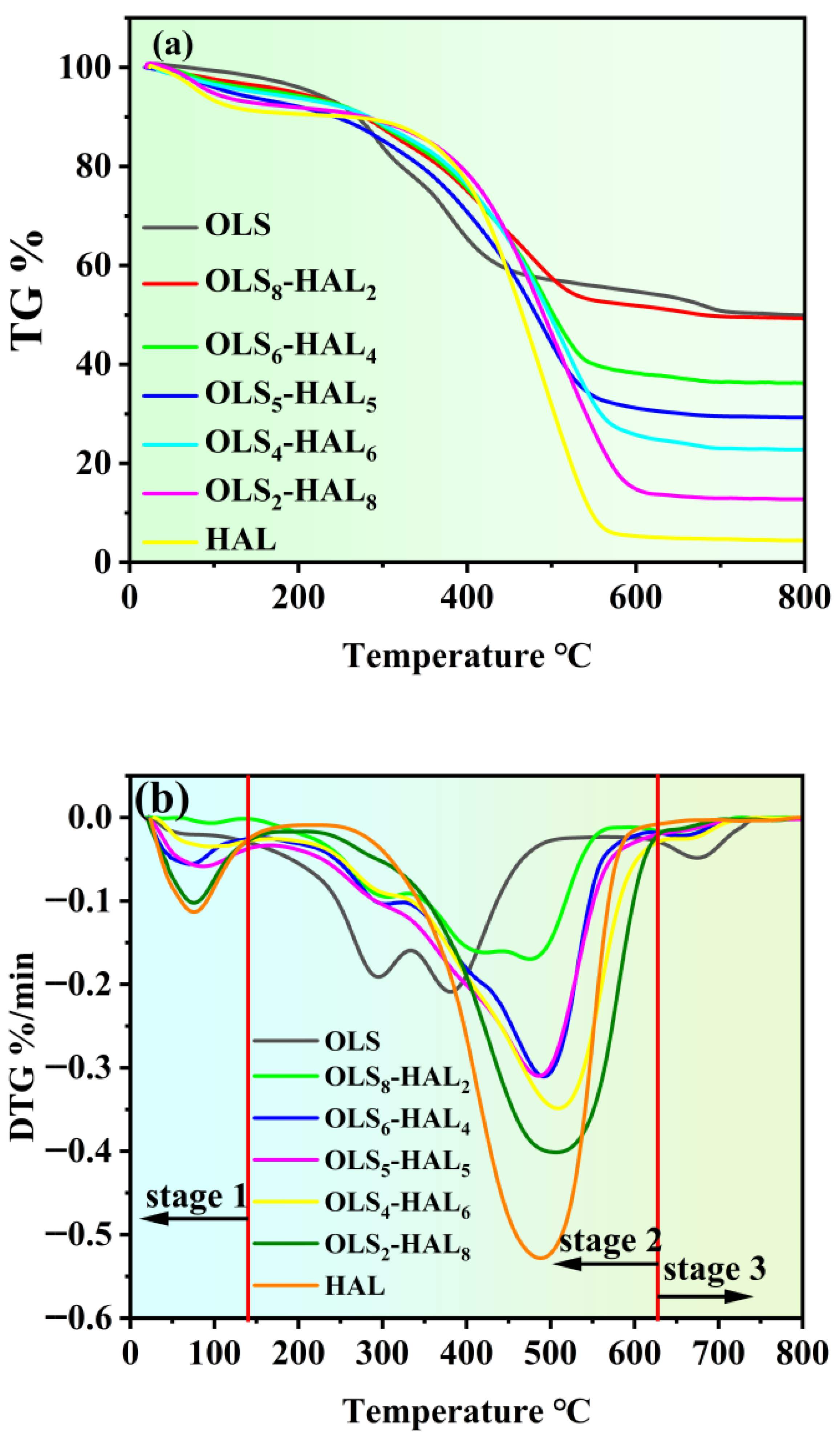
3.2. Co-Combustion Performance Analysis
3.3. Interaction Analysis
3.4. Analysis of Combustion Kinetics
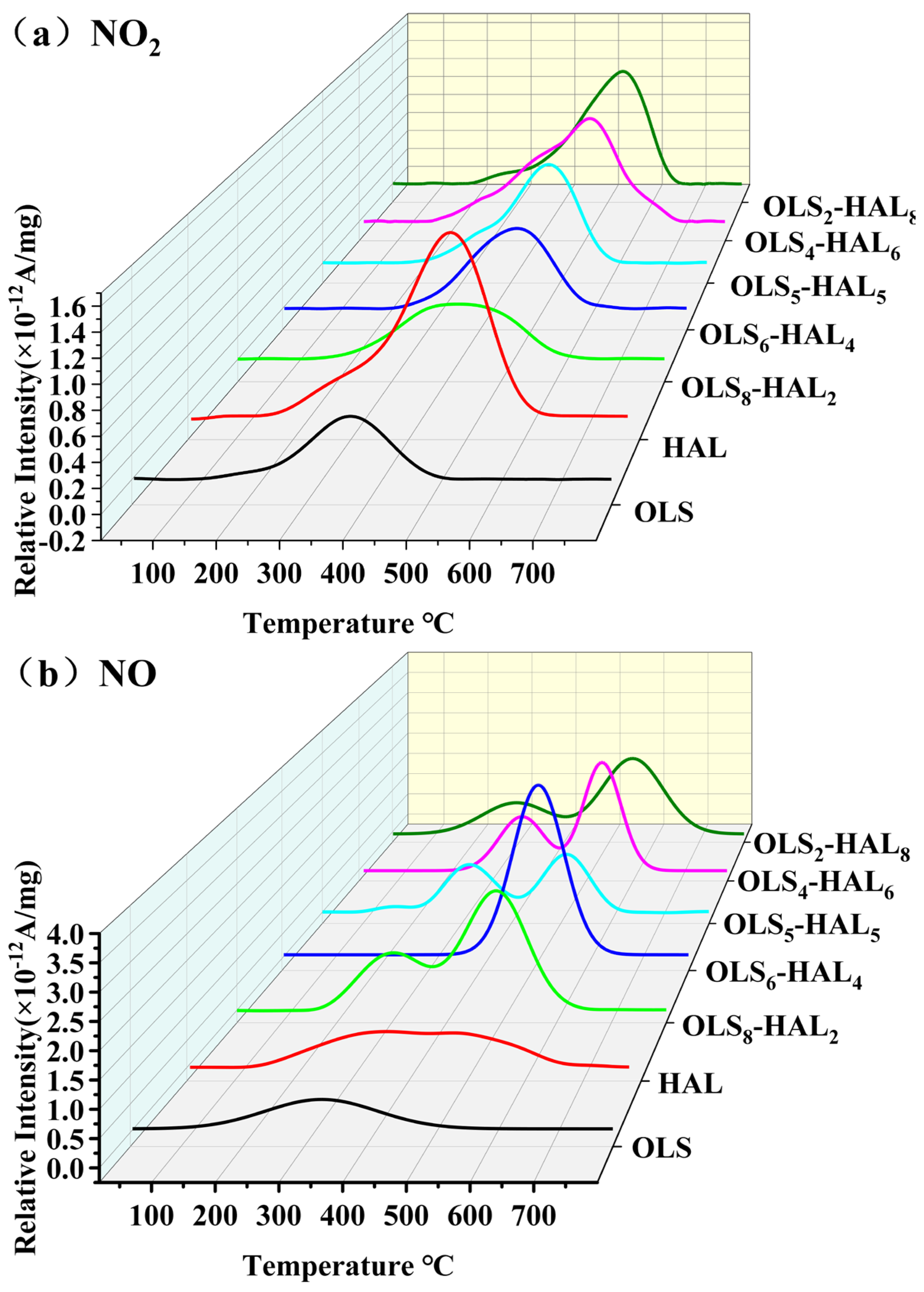
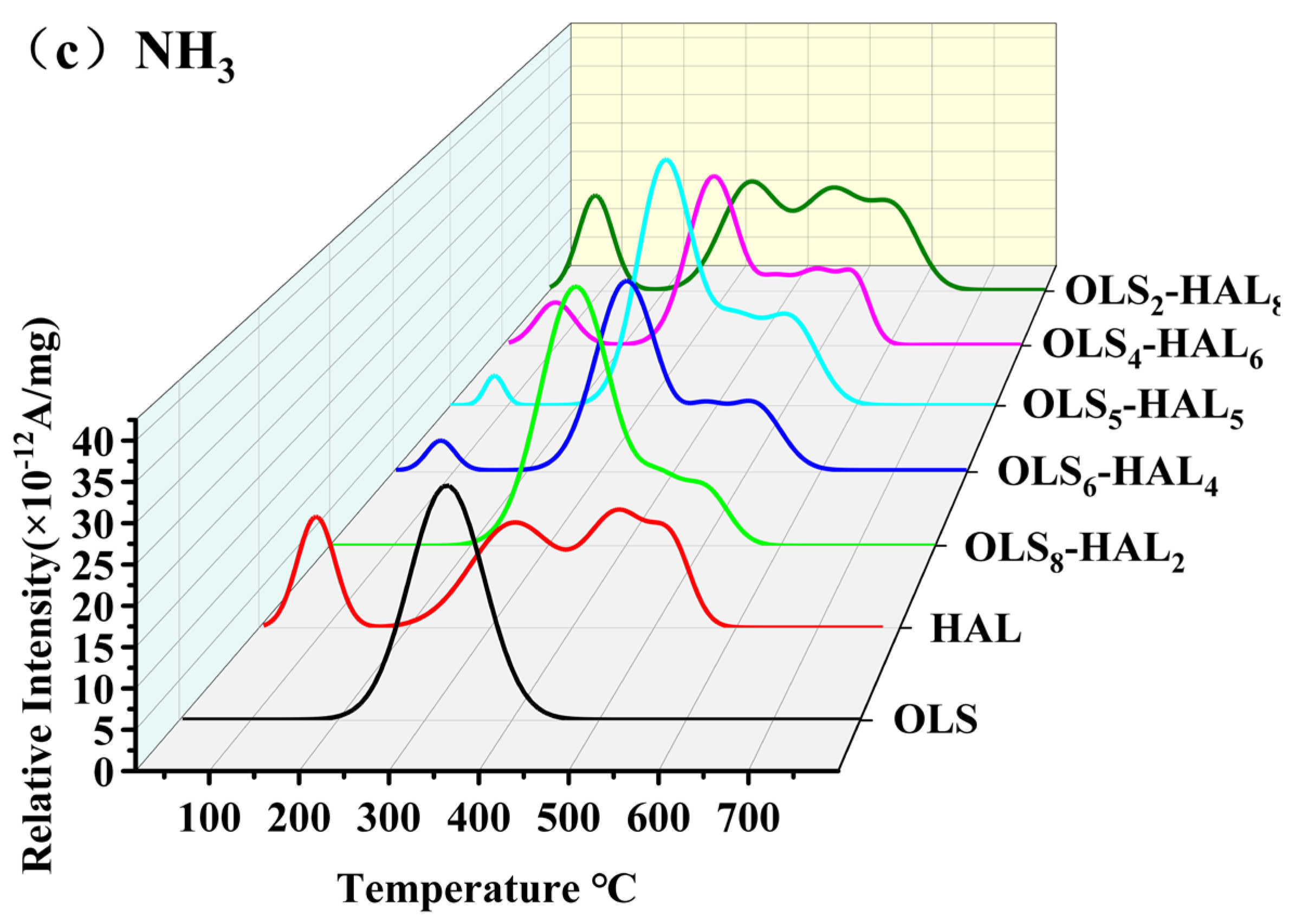
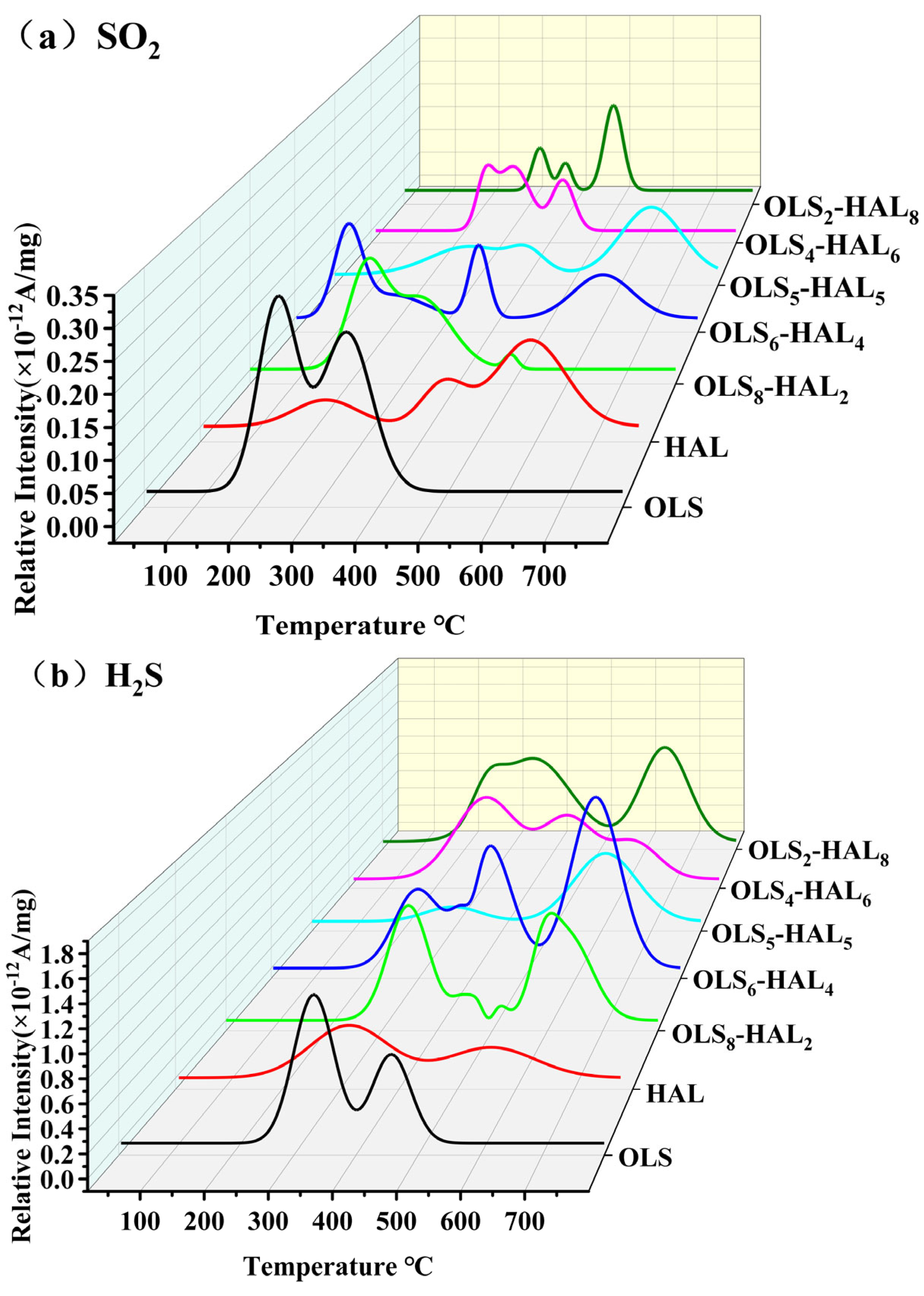
3.5. Release Behavior for Typical Gaseous Pollutants
3.5.1. Emission Characteristics of Carbon-Containing Gaseous Pollutants

3.5.2. Emission Characteristics of Nitrogen-Containing Gaseous Pollutants
3.5.3. Emission Characteristics of Sulfur-Containing Gaseous Pollutants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Nie, C.; Li, W.; Ao, Z.; Wang, S.; An, T. Oily sludge derived carbons as peroxymonosulfate activators for removing aqueous organic pollutants: Performances and the key role of carbonyl groups in electron-transfer mechanism. J. Hazard. Mater. 2021, 414, 125552. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Li, J.; Zeng, G. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, J.; Liu, X.; Tang, X.; Li, T.; Zhu, Y. Optimization and mechanism of oily sludge treatment by a synergistic approach of high-speed stirring cleaning integrated with thermal-activated Na2S2O8 oxidation. J. Environ. Manag. 2025, 381, 125230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, J.; He, C.; Tian, Y.; He, L.; Sui, H.; Li, X. Universal low-carbon strategy for deep-resourcilization of industrial oily sludges. J. Clean. Prod. 2025, 496, 145097. [Google Scholar] [CrossRef]
- Xia, P.; Jing, S.; Zhao, Z.; Dou, T.; Gao, J.; Guo, Z.; Xu, L.; Guo, H.; Zhang, M.; Qiao, W. Enhancement of dewatering performance and effective degradation of petroleum hydrocarbons in biological oily sludge using atmospheric pressure plasma jet. Bioresour. Technol. 2025, 418, 131974. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Chen, L.; Zhang, Q.; Guo, D.; Yin, Q.; Nie, M.; Wang, L.; Guo, Y. Study on the bacteria consortium for treating tank cleaning oily sludge and its synergistic degradation mechanism. J. Environ. Chem. Eng. 2023, 11, 110803. [Google Scholar] [CrossRef]
- Teng, Q.; Zhang, D.; Yang, C. A review of the application of different treatment processes for oily sludge. Environ. Sci. Pollut. Res. 2021, 28, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Zheng, G.; Tang, J.; Tao, C.; Liu, R.; Liu, Z. Electric field efficiently enhanced thermochemical cleaning for oil recovery from oily sludge. Chem. Eng. Process.-Process Intensif. 2023, 185, 109314. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Z.; He, C.; Liu, J.; Zhang, R.; Chen, Q. Oily sludge treatment in subcritical and supercritical water: A review. J. Hazard. Mater. 2022, 433, 128761. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Lv, Y.; Li, S.; He, C.; Gong, Z.; Wen, X.; Dan, J.; Li, X. Study on thermal behavior and gas pollutant emission control during the co-combustion of waste tire-modified oily sludge and coal. Fuel 2025, 391, 134758. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Lin, F.; Zeng, M.; Li, R.; Yan, B.; Chen, G. Pyrolysis/combustion potential and heavy metal risk of oily sludge and derived products in industrial scale. Fuel 2023, 344, 128044. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Liang, H.; Chen, B.; Liu, Y.; Ma, Z.; Wang, Z. Characterization and treatment of oily sludge: A systematic review. Environ. Pollut. 2024, 344, 123245. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, Q.; Wang, F. Co-combustion of municipal solid waste and coal for carbon emission reduction: A bi-level multi-objective programming approach. J. Clean. Prod. 2020, 272, 121923. [Google Scholar] [CrossRef]
- Liu, L.; Memon, M.Z.; Xie, Y.; Gao, S.; Guo, Y.; Dong, J.; Gao, Y.; Li, A.; Ji, G. Recent advances of research in coal and biomass co-firing for electricity and heat generation. Circ. Econ. 2023, 2, 100063. [Google Scholar] [CrossRef]
- Majeed, A.; Kanwal, S.; Batool, S.A.; Chaudhry, M.N.; Zeb, H.; Abbasi, G.H.; Malik, Z.; Munir, A. Physio-chemical evaluation and co-combustion efficiency of different biomass waste fractions with indigenous coal blends for utilization as alternative fuel. Glob. NEST J. 2022, 24, 97–104. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Lv, Y.; Jiang, W.; He, C.; Hou, S.; Ma, W.; Dan, J. Study on thermal behavior and gas pollutant emission control during the co-combustion of rice straw-modified oily sludge and coal. Renew. Energy 2024, 230, 120857. [Google Scholar] [CrossRef]
- Dong, L.; Huang, X.; Ren, J.; Deng, L.; Da, Y. Thermogravimetric assessment and differential thermal analysis of blended fuels of coal, biomass and oil sludge. Appl. Sci. 2023, 13, 11058. [Google Scholar] [CrossRef]
- Liu, P.; Le, J.; Zhang, D.; Wang, S.; Pan, T. Free radical reaction mechanism on improving tar yield and quality derived from lignite after hydrothermal treatment. Fuel 2017, 207, 244–252. [Google Scholar] [CrossRef]
- Kułażyński, M.; Jabłoński, S.; Kaczmarczyk, J.; Świątek, Ł.; Pstrowska, K.; Łukaszewicz, M. Technological aspects of sunflower biomass and brown coal co-firing. J. Energy Inst. 2018, 91, 668–675. [Google Scholar] [CrossRef]
- Khan, A.; Iqbal, N.; Noor, T.; Hassan, M.; Akhter, J. Synergistic production of fuels from co-pyrolysis of lignite coal and waste plastic. J. Energy Inst. 2024, 113, 101527. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, L.; Chen, X.; Liu, Y.; Zhai, X.; Xue, Y.; Wang, W.; Liu, J.; Xu, K. Emissions characteristics of hazardous air pollutants from the incineration of sacrificial offerings. Atmosphere 2019, 10, 332. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Sun, J.; Wang, Y.; Sun, Z.; Ma, K.; Wei, L. Investigation of oxygen-enriched atmosphere combustion of oily sludge: Performance, mechanism, emission of the S/N-containing compound, and residue characteristics. J. Clean. Prod. 2022, 378, 134233. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, M.; Sharma, A.K.; Sharma, M.; Sharma, M. Mapping the impact of environmental pollutants on human health and environment: A systematic review and meta-analysis. J. Geochem. Explor. 2023, 255, 107325. [Google Scholar] [CrossRef]
- Shetty, S.S.; Deepthi, D.; Harshitha, S.; Sonkusare, S.; Naik, P.B.; Madhyastha, H. Environmental pollutants and their effects on human health. Heliyon 2023, 9, e19496. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Shang, J.; Zhao, D. The roles of heterogeneous chemical processes in the formation of an air pollution complex and gray haze. Sci. China Chem. 2011, 54, 145–153. [Google Scholar] [CrossRef]
- Deng, S.; Wang, X.; Tan, H.; Mikulčić, H.; Yang, F.; Li, Z.; Duić, N. Thermogravimetric study on the co-combustion characteristics of oily sludge with plant biomass. Thermochim. Acta 2016, 633, 69–76. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Adeosun, A.; Li, Y.; Vujanović, M.; Tan, H.; Duić, N. Effect of potassium-doping and oxygen concentration on soot oxidation in O2/CO2 atmosphere: A kinetics study by thermogravimetric analysis. Energy Convers. Manag. 2017, 149, 686–697. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, C.; Zhang, Y.; Zhou, L.; Guo, Z.; Miao, Z.; Zhao, X.; Wu, J.; Guo, F. Comparative study on the structure characteristics, combustion reactivity, and potential environmental impacts of coal gasification fine slag with different particle size fractions. Fuel 2022, 311, 122493. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, F.; Zhou, L.; Guo, Z.; Miao, Z.; Liu, H.; Zhang, X.; Wu, J.; Zhang, Y. Investigation on co-combustion of coal gasification fine slag residual carbon and sawdust char blends: Physiochemical properties, combustion characteristic and kinetic behavior. Fuel 2021, 292, 120387. [Google Scholar] [CrossRef]
- GB/T 212–2008; Method for Proximate Analysis of Coal. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- GB/T 31391–2015; Ultimate Analysis of Coal. Standardization Administration of the People’s Republic of China: Beijing, China, 2015.
- GB/T 213–2008; Determination of Calorific Value of Coal. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- ASTM D4326–2013; Standard Test Method for Major and Minor Elements in Coal and Coke by X-Ray Fluorescence. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2013.
- ASTM D3174–12; Standard Test Method for Ash in Analysis Samples of Coal and Coke. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2012.
- Liu, X.; Hu, J.; Zhao, S.; Wang, W.; Zhang, Q.; Yan, X. Chemical looping co-gasification of wheat straw and lignite with calcium-enhanced iron-based oxygen carrier for syngas production. Fuel Process. Technol. 2022, 227, 107108. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, D.; Liu, X.; Wu, J.; Guo, F.; Zhang, Y.; Yuan, S.; Du, C.; Lin, M.; Liu, J. Insights into the co-combustion attributes and mutual effects of high-alkali lignite and flotation carbon-rich fractions from coal gasification fine slag. Results Eng. 2025, 26, 105310. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, J.; Guo, F.; Zhang, Y.; Yuan, S.; Du, C.; Chen, J.; Lin, M. Recycling of flotation residual carbon from coal gasification fine slag through thermal combustion reaction: Insight into the spatial and temporal multi-scale evolution of typical heavy metals. Fuel 2025, 381, 133378. [Google Scholar] [CrossRef]
- Cheng, S.; Takahashi, F.; Gao, N.; Yoshikawa, K.; Li, A. Evaluation of oil sludge ash as a solid heat carrier in the pyrolysis process of oil sludge for oil production. Energy Fuels 2016, 30, 5970–5979. [Google Scholar] [CrossRef]
- Baqain, M.; Neshumayev, D.; Konist, A. TG-MS analysis and kinetic study of co-combustion of ca-rich oil shale with biomass in air and oxy-like conditions. Carbon Capture Sci. Technol. 2024, 10, 100162. [Google Scholar] [CrossRef]
- Liu, C.; Gong, Z.; Zhang, H.; Wang, Z.; Chu, Z.; Liu, L.; Li, X.; Guo, Y.; Zhang, J.; Li, G.; et al. Combustion characteristics and kinetic analysis of oil sludge with CaO additive. J. Environ. Sci. Health Part A 2021, 56, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.-Q.; Wu, X.; De Girolamo, A.; Zhang, L. Inhibition of lignite ash slagging and fouling upon the use of a silica-based additive in an industrial pulverised coal-fired boiler. Part 1. Changes on the properties of ash deposits along the furnace. Fuel 2015, 139, 720–732. [Google Scholar] [CrossRef]
- Nounou, R.S.A.; Shaban, I.A.; Labeeb Ali Altarawneh, M.; Elgendi, M. Kinetic and thermodynamic analysis of the PET-date seed mixture co-pyrolysis using coats-redfern method. Renew. Energy 2024, 237, 121524. [Google Scholar] [CrossRef]
- Raza, M.; Abu-Jdayil, B.; Al-Marzouqi, A.H.; Inayat, A. Kinetic and thermodynamic analyses of date palm surface fibers pyrolysis using coats-redfern method. Renew. Energy 2022, 183, 67–77. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, J.; Jia, W.; Guo, F.; Qiu, G.; Wang, R.; Zhang, Y.; Dai, B. Evaluation of the thermal behavior, synergistic catalysis, and pollutant emissions during the co-combustion of sewage sludge and coal gasification fine slag residual carbon. Catalysts 2021, 11, 1142. [Google Scholar] [CrossRef]
- Zou, H.; Liu, C.; Evrendilek, F.; He, Y.; Liu, J. Evaluation of reaction mechanisms and emissions of oily sludge and coal co-combustions in O2/CO2 and O2/N2 atmospheres. Renew. Energy 2021, 171, 1327–1343. [Google Scholar] [CrossRef]
- Mohsin, I.U.; Hofmann, A.; Ziebert, C. Exploring the reactivity of Na3V2(PO4)3/C and hard carbon electrodes in sodium-ion batteries at various charge states. Electrochim. Acta 2024, 487, 144197. [Google Scholar] [CrossRef]
- Zhao, R.; Qin, J.; Chen, T.; Wang, L.; Wu, J. Experimental study on co-combustion of low rank coal semicoke and oil sludge by TG-FTIR. Waste Manag. 2020, 116, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chang, K.; Yao, J.; Guo, X.; Shen, C.; Yan, S. Effect of fluoroalcohol chain extension modified HTPB binder on the combustion performance of aluminized propellants. Crystals 2024, 14, 258. [Google Scholar] [CrossRef]
- Dai, M.; Gu, B.; Su, P.; Zhou, Y.; Meng, Q.; Li, D.; Zhu, M.; Chun, T. Examining low nitrogen oxides combustion in iron ore sintering: Utilization of reductants. Renew. Sustain. Energy Rev. 2024, 205, 114864. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Li, K.; Lin, F.; Tian, W.; Che, L.; Yan, B.; Ma, W.; Song, Y. Nitrogen, sulfur, chlorine containing pollutants releasing characteristics during pyrolysis and combustion of oily sludge. Fuel 2020, 273, 117772. [Google Scholar] [CrossRef]
- Liu, H.; Yi, L.; Zhang, Q.; Hu, H.; Lu, G.; Li, A.; Yao, H. Co-production of clean syngas and ash adsorbent during sewage sludge gasification: Synergistic effect of Fenton peroxidation and CaO conditioning. Appl. Energy 2016, 179, 1062–1068. [Google Scholar] [CrossRef]
- Ng, K.H.; Liew, S.C.; Zhang, S. Thermodynamic analysis of CaS production from various Ca-based precursors: A prequel to SO2 reduction mediated by CaS/CaSO4 redox agents. Process Saf. Environ. Prot. 2021, 147, 900–911. [Google Scholar] [CrossRef]
- Garcıía-Calzada, M.; Marbán, G.; Fuertes, A.B. Decomposition of CaS particles at ambient conditions. Chem. Eng. Sci. 2000, 55, 1661–1674. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Kinetic analysis of complex chemical reactions by coupling model-free and model-fitting analysis. Thermochim. Acta 2023, 719, 179416. [Google Scholar] [CrossRef]
- Qin, C.; Dang, M.; Meng, Y.; Zhao, D. Fusion calorimetric experiments and QSPR modeling to inversely explore the accuracy of thermodynamic methods: A case study of the apparent activation energies of 18 aromatic nitro compounds. Process Saf. Environ. Prot. 2023, 172, 659–680. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Hu, J.; Zhang, Y.; Chen, W.; Chang, C.; Pang, S. Research on the kinetics of catalyst coke formation during biomass catalytic pyrolysis: A mini review. J. Energy Inst. 2023, 110, 101315. [Google Scholar] [CrossRef]

| Samples | Oily Sludge Content (wt%) | Lignite Content (wt%) |
|---|---|---|
| OLS | 100 | 0 |
| OLS8-HAL2 | 80 | 20 |
| OLS6-HAL4 | 60 | 40 |
| OLS5-HAL5 | 50 | 50 |
| OLS4-HAL6 | 40 | 60 |
| OLS2-HAL8 | 20 | 80 |
| HAL | 0 | 100 |
| Samples | Proximate Analysis (wt%) | Ultimate Analysis (wt%) | HHV (ad, MJ/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vd | FCd | Cd | Hd | Od | Nd | Sd | ||
| OLS | 21.10 | 53.19 | 42.72 | 4.09 | 35.89 | 4.48 | 13.97 | 0.61 | 1.43 | 16.21 |
| HAL | 21.35 | 7.64 | 38.16 | 54.2 | 71.89 | 3.60 | 15.55 | 0.89 | 0.43 | 21.31 |
| Compositions wt% | ||||||||
|---|---|---|---|---|---|---|---|---|
| Samples | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | K2O | MgO | Others |
| OLS | 24.38 | 19.58 | 20.63 | 18.87 | 5.32 | 1.03 | 5.68 | 4.51 |
| HAL | 17.25 | 19.64 | 8.38 | 23.59 | 10.63 | 6.75 | 11.28 | 2.48 |
| Samples | Ti (°C) | Tf (°C) | Tmax (°C) | DTGmax (%/min) | DTGmean (%/min) | S (×10−7, %2/(min2 °C3)) |
|---|---|---|---|---|---|---|
| OLS | 238.47 | 699.31 | 380.81 | 2.09 | 0.92 | 0.484 |
| OLS8-HAL2 | 273.33 | 667.45 | 475.00 | 1.84 | 1.02 | 0.376 |
| OLS6-HAL4 | 341.63 | 638.27 | 491.77 | 3.11 | 1.56 | 0.651 |
| OLS5-HAL5 | 324.65 | 620.31 | 486.31 | 3.14 | 1.57 | 0.754 |
| OLS4-HAL6 | 357.59 | 646.59 | 509.09 | 3.49 | 2.02 | 0.853 |
| OLS2-HAL8 | 366.15 | 607.60 | 506.60 | 4.02 | 2.88 | 1.421 |
| HAL | 370.94 | 568.81 | 488.31 | 5.28 | 3.87 | 2.611 |
| Samples | Models | y = ax + b | R2 | E (KJ/mol) |
|---|---|---|---|---|
| HAL | D1 | y = −6687.8x−5.58 | 0.958 | 55.6 |
| OLS | D3 | y = −6039.91x−8.96 | 0.991 | 50.2 |
| OLS8-HAL2 | D1 | y = −3607.69x−9.16 | 0.985 | 30.0 |
| OLS6-HAL4 | D1 | y = −3939.83x−8.98 | 0.972 | 32.7 |
| OLS5-HAL5 | D2 | y = −3912.74x−9.31 | 0.962 | 32.5 |
| OLS4-HAL6 | D1 | y = −4751.92x−8.17 | 0.981 | 39.5 |
| OLS2-HAL8 | D1 | y = −5185.07x−7.76 | 0.953 | 43.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zheng, J.; Wang, D.; Zhang, P.; Zhang, Y.; Lin, M.; Yuan, S. Research on Thermochemical and Gas Emissions Analysis for the Sustainable Co-Combustion of Petroleum Oily Sludge and High-Alkali Lignite. Sustainability 2025, 17, 6703. https://doi.org/10.3390/su17156703
Guo Y, Zheng J, Wang D, Zhang P, Zhang Y, Lin M, Yuan S. Research on Thermochemical and Gas Emissions Analysis for the Sustainable Co-Combustion of Petroleum Oily Sludge and High-Alkali Lignite. Sustainability. 2025; 17(15):6703. https://doi.org/10.3390/su17156703
Chicago/Turabian StyleGuo, Yang, Jie Zheng, Demian Wang, Pengtu Zhang, Yixin Zhang, Meng Lin, and Shiling Yuan. 2025. "Research on Thermochemical and Gas Emissions Analysis for the Sustainable Co-Combustion of Petroleum Oily Sludge and High-Alkali Lignite" Sustainability 17, no. 15: 6703. https://doi.org/10.3390/su17156703
APA StyleGuo, Y., Zheng, J., Wang, D., Zhang, P., Zhang, Y., Lin, M., & Yuan, S. (2025). Research on Thermochemical and Gas Emissions Analysis for the Sustainable Co-Combustion of Petroleum Oily Sludge and High-Alkali Lignite. Sustainability, 17(15), 6703. https://doi.org/10.3390/su17156703









