Circular Model for the Valorization of Black Grape Pomace for Producing Pasteurized Red Must Enriched in Health-Promoting Phenolic Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Cultivation
2.2. Obtaining Phenolic Extracts from Grape Pomace
2.3. Obtaining Pasteurized Grape Must with Enhanced Health-Promoting Properties
2.4. Must Analysis
2.5. Extraction Analysis
2.6. Circular Model for the Valorization of Grape Pomace
- Grape pomace was obtained through the vinification of grape varieties Fetească Neagră, Cabernet Sauvignon, Blauer Zweigelt, and Arcaș from the 2023 harvest.
- Polyphenol extractions were performed using hydroalcoholic solutions at concentrations of 25%, 50%, and 75%.
- Obtaining fresh must: Grapes from the varieties Fetească Neagră, Cabernet Sauvignon, Blauer Zweigelt, and Arcaș were harvested, then subjected to destemming and crushing, followed by gentle pressing. The resulting must was pasteurized at 80–85 °C for 3–5 min.
- Extraction of phenolic compounds from pomace: Pomace obtained from each of the four grape varieties was extracted using a 50% hydroalcoholic solution, at a solid-to-liquid ratio of 200 g pomace to 800 mL solvent (as illustrated in Figure 1).
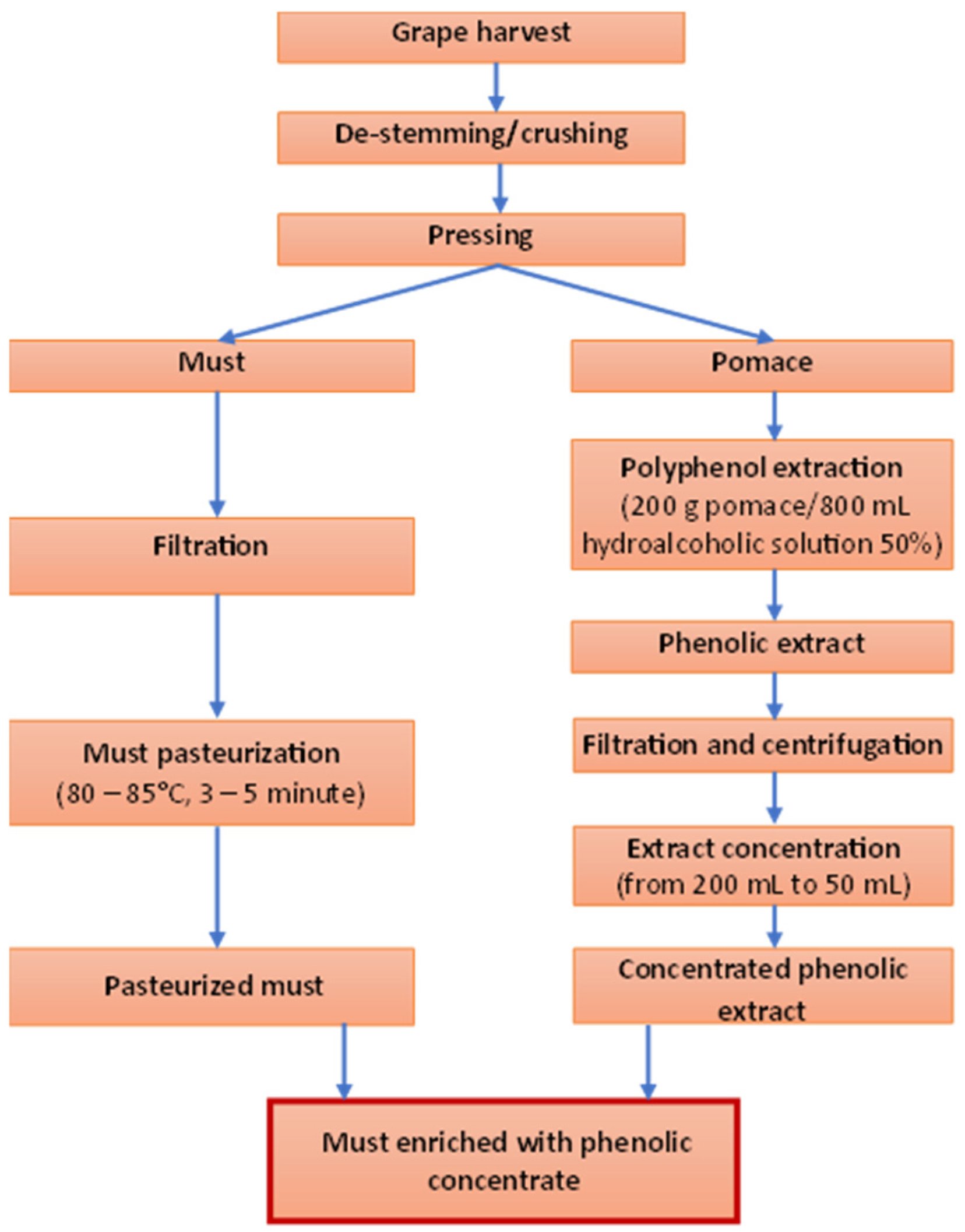
- Concentration of the phenolic extract: The obtained extract was concentrated using a rotary evaporator, reducing the volume from 200 mL to approximately 50 mL.
- Enrichment of pasteurized must: The pasteurized must was enriched with the concentrated phenolic extract by adding 50 mL of concentrated extract to every 950 mL of must.
2.7. HPLC Analysis of Phenolic Compounds
2.8. Statistical Analysis
3. Results and Discussion
3.1. Recovery of Phenolic Compounds from Grape Pomace
- Arcaș: 851 mg/100 g (highest overall).
- Cabernet Sauvignon: 722 mg/100 g.
- Fetească Neagră: 616 mg/100 g.
- Blauer Zweigelt 302 mg/100 g.
3.2. Correlation of Phenolic Parameters Obtained in Hydroalcoholic Extractions
3.3. Quality Parameters of Control and Phenolic-Enriched Musts
3.4. Individual Phenolic Compounds in Control and Phenolic-Enriched Musts
- Blauer Zweigelt > Fetească Neagră > Cabernet Sauvignon > Arcaș.
- In contrast, for the phenolic-enriched musts, the hierarchy was
- Fetească Neagră > Blauer Zweigelt > Arcaș > Cabernet Sauvignon.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, M.; Silva, R.; Ferreira, P. Circular economy and sustainable development: A comprehensive overview. Environ. Sci. Policy 2020, 112, 27–35. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, H.; Li, S. The role of circular economy in reducing environmental impact and enhancing resource efficiency. J. Clean. Prod. 2023, 272, 122679. [Google Scholar]
- Manca, M.L.; Casula, E.; Marongiu, F.; Bacchetta, G.; Sarais, G.; Zaru, M.; Escribano-Ferrer, E.; Peris, J.E.; Usach, I.; Fais, S.; et al. From Waste to Health: Sustainable exploitation of grape pomace seed extract to manufacture antioxidant, regenerative and prebiotic nanovesicles within circular economy. Sci. Rep. 2020, 10, 14184–14197. [Google Scholar] [CrossRef]
- Perra, M.; Bacchetta, G.; Muntoni, A.; De Gioannis, G.; Castangia, I.; Rajha, H.N.; Manca, M.L.; Manconi, M. An outlook on modern and sustainable approaches to the management of grape pomace by integrating green processes, biotechnologies and advanced biomedical approaches. J. Funct. Foods 2022, 98, 105276–105285. [Google Scholar] [CrossRef]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.P.; Gando-Ferreira, L.M.; Quina, M.J. Increasing value of winery residues through integrated biorefinery processes: A review. Molecules 2022, 27, 4709. [Google Scholar] [CrossRef]
- Oliveira, M.; Duarte, E. Integrated approach to winery waste: Waste generation and data consolidation. Front. Environ. Sci. Eng. 2016, 10, 168–176. [Google Scholar] [CrossRef]
- Kwiatkowski, J.; Nowak, A.; Matuszak, M. Grape pomace: Composition, properties, and utilization in food and non-food applications. J. Agric. Food Chem. 2019, 67, 1–9. [Google Scholar]
- Castellanos-Gallo, L.; Ballinas-Casarrubias, L.; Espinoza-Hicks, J.C.; Hernández-Ochoa, L.R.; Muñoz-Castellanos, L.N.; Zermeño-Ortega, M.R.; Borrego-Loya, A.; Salas, E. Grape pomace valorization by extraction of phenolic polymeric pigments: A review. Processes 2022, 10, 469. [Google Scholar] [CrossRef]
- Wimalasiri, S.; Pereira, C.; Silva, R. Bioactive compounds from grape pomace and their potential health benefits: A review. J. Food Sci. 2022, 87, 3422–3435. [Google Scholar]
- Domingues Neto, F.J.; Tecchio, M.A.; Junior, A.P.; Vedoato, B.T.F.; Lima, G.P.P.; Roberto, S.R. Effect of ABA on Colour of Berries, Anthocyanin Accumulation and Total Phenolic Compounds of “Rubi” Table Grape (Vitis vinifera). Aust. J. Crop Sci. 2017, 11, 199–205. Available online: https://www.cropj.com/neto_11_2_2017_199_205.pdf (accessed on 26 May 2025). [CrossRef]
- Souleman, S.I.; Hammad, A.H. Utilization of grape pomace for bioactive compounds extraction: A sustainable approach for food and pharmaceutical industries. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Caponio, G.R.; Noviello, M.; Calabrese, F.M.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of Grape Pomace Polyphenols and In Vitro Gastrointestinal Digestion on Antimicrobial Activity: Recovery of Bioactive Compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef]
- Canalejo, J.; Herrera, R. The contribution of phenolic compounds from grape pomace to improving the aromatic profile of wine. Food Chem. 2024, 405, 125032. [Google Scholar]
- Bulgărea, S.A.; Salvatore, M.; Vasilescu, I. Grape pomace as a functional ingredient: From waste to health-promoting food additives. Antioxidants 2021, 10, 1445. [Google Scholar] [CrossRef]
- Vural, N.; Algan Cavuldak, Ö.; Anlı, R.E. Multi Response Optimisation of Polyphenol Extraction Conditions from Grape Seeds by Using Ultrasound Assisted Extraction (UAE). Sep. Sci. Technol. 2018, 53, 1540–1551. [Google Scholar] [CrossRef]
- Blasi, F.; Trovarelli, V.; Mangiapelo, L.; Ianni, F.; Cossignani, L. Grape pomace for feed enrichment to improve the quality of animal-based foods. Foods 2024, 13, 3541. [Google Scholar] [CrossRef]
- Kokkonen, A.A.; Tiecher, T.L.; Loss, A.; Toselli, M.; Garlet, L.P.; Marques, A.L.L.; Papalia, D.G.; Schemmer, S.; Costa, V.F.; Moura-Bueno, J.M.; et al. The use of grape pomace residues as a nutrient source in subtropical viticulture. Agronomy 2025, 15, 1010. [Google Scholar] [CrossRef]
- Paradelo, R.; Moldes, A.B.; Barral, M.T. Carbon and nitrogen mineralization in a vineyard soil amended with grape marc vermicompost. Waste Manag. Res. 2011, 29, 1177–1184. [Google Scholar] [CrossRef]
- Prata, C.; Zalambani, C.; Rossi, F.; Rossello, S.; Cerchiara, T.; Cappadone, C.; Malucelli, E. Nutrients and Nutraceuticals from Vitis vinifera L. Pomace: Biological Activities, Valorization, and Potential Applications. Nutrients 2025, 17, 583. [Google Scholar] [CrossRef]
- Gerardi, C.; Pinto, L.; Baruzzi, F.; Giovinazzo, G. Comparison of Antibacterial and Antioxidant Properties of Red (Cv. Negramaro) and White (Cv. Fiano) Skin Pomace Extracts. Molecules 2021, 26, 5918. [Google Scholar] [CrossRef]
- Tolve, R.; Simonato, B.; Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G. Wheat bread fortification by grape pomace powder: Nutritional, technological, antioxidant, and sensory properties. Foods 2021, 10, 75. [Google Scholar] [CrossRef]
- Carpentieri, S.; Larrea-Wachtendorff, D.; Donsì, F.; Ferrari, G. Functionalization of pasta through the incorporation of bioactive compounds from agri-food by-products: Fundamentals, opportunities, and drawbacks. Trends Food Sci. Technol. 2022, 122, 49–65. [Google Scholar] [CrossRef]
- dos Santos, K.M.O.; de Oliveira, I.C.; Lopes, M.A.C.; Cruz, A.P.G.; Buriti, F.C.A.; Cabral, L.M. Addition of grape pomace extract to probiotic fermented goat milk: The effect on phenolic content, probiotic viability and sensory acceptability. J. Sci. Food Agric. 2017, 97, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Barbaccia, P.; Busetta, G.; Barbera, M.; Alfonzo, A.; Garofalo, G.; Francesca, N.; Moscarelli, A.; Moschetti, G.; Settanni, L.; Gaglio, R. Effect of grape pomace from red cultivar ‘Nero d’Avola’ on the microbiological, physicochemical, phenolic profile and sensory aspects of ovine Vastedda-like stretched cheese. J. Appl. Microbiol. 2022, 133, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gambacorta, G.; Caponio, F. Physico-chemical, sensory and volatile profiles of biscuits enriched with grape marc extract. Food Res. Int. 2014, 65, 385–393. [Google Scholar] [CrossRef]
- Difonzo, G.; Troilo, M.; Allegretta, I.; Pasqualone, A.; Caponio, F. Grape Skin and Seed Flours as Functional Ingredients of Pizza: Potential and drawbacks related to nutritional, physicochemical and sensory attributes. LWT 2023, 175, 114494. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Pasqualone, A.; Caponio, F. Grape pomace as innovative flour for the formulation of functional muffins: How particle size affects the nutritional, textural and sensory properties. Foods 2022, 11, 1799. [Google Scholar] [CrossRef]
- Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G.; Simonato, B. Breadstick fortification with red grape pomace: Effect on nutritional, technological and sensory properties. J. Sci. Food Agric. 2022, 102, 2545–2552. [Google Scholar] [CrossRef]
- Bianchi, F.; Lomuscio, E.; Rizzi, C.; Simonato, B. Predicted shelf-life, thermodynamic study and antioxidant capacity of breadsticks fortified with grape pomace powders. Foods 2021, 10, 2815. [Google Scholar] [CrossRef]
- Panić, M.; Dragović, U. Development and pilot-scale validation of anthocyanin extraction from grape pomace for use in various industries. J. Food Eng. 2019, 120, 99–108. [Google Scholar] [CrossRef]
- Vargas, M.; Fernández-García, E. Phenolic compounds from grape pomace: Extraction, composition, and health benefits in functional foods. J. Funct. Foods 2020, 65, 103743. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Kostić, A.Ž.; Gašić, U.M.; Lević, S.; Stanojević, S.P.; Barać, M.B.; Tešić, Ž.L.; Nedović, V.; Pešić, M.B. Skimmed goat’s milk powder enriched with grape pomace seed extract: Phenolics and protein characterization and antioxidant properties. Biomolecules 2021, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Ratu, R.N.; Usturoi, M.G.; Radu Rusu, R.M.; Velescu, I.D.; Lipsa, F.D.; Arsenoaia, V.N.; Postolache, A.N.; Crivei, I.C.; Carlescu, P.M. Effect of grape skin powder addition on chemical, nutritional and technological properties of cheese. J. Appl. Life Sci. Environ. 2023, 56, 41–58. [Google Scholar] [CrossRef]
- Wimalasiri, A.; Lee, H.J. Applications of grape pomace in food fortification: A review of health benefits and innovative uses. Food Res. Int. 2022, 162, 112003. [Google Scholar]
- Gasiński, A.; Kawa-Rygielska, J.; Mikulski, D.; Kłosowski, G.; Głowacki, A. Application of white grape pomace in the brewing technology and its impact on the concentration of esters and alcohols, physicochemical parameters and antioxidative properties of the beer. Food Chem. 2022, 367, 130646. [Google Scholar] [CrossRef]
- Karastergiou, A.; Gancel, A.-L.; Jourdes, M.; Teissedre, P.-L. Valorization of Grape Pomace: A Review of Phenolic Composition, Bioactivity, and Therapeutic Potential. Antioxidants 2024, 13, 1131. [Google Scholar] [CrossRef]
- Aguilar, T.; De Bruijn, J.; Loyola, C.; Bustamante, L.; Vergara, C.; Von Baer, D.; Mardones, C.; Serra, I. Characterization of an Antioxidant-Enriched Beverage from Grape Musts and Extracts of Winery and Grapevine By-Products. Beverages 2018, 4, 4. [Google Scholar] [CrossRef]
- Błaszak, M.; Lachowicz-Wiśniewska, S.; Kapusta, I.; Szewczuk, M.; Ochmian, I. Enhanced Extraction of Polyphenols, Physicochemical Properties, and Microbial Control in Vitis vinifera L. Juice Using Ultrasound-Assisted Maceration. Molecules 2025, 30, 587. [Google Scholar] [CrossRef]
- Margean, A.; Lupu, M.I.; Alexa, E.; Padureanu, V.; Canja, C.M.; Cocan, I.; Negrea, M.; Calefariu, C.; Poiana, M.A. An Overview of Effects Induced by Pasteurization and High-Power Ultrasound Treatment on the Quality of Red Grape Juice. Molecules 2020, 25, 1669. [Google Scholar] [CrossRef]
- Saraiva, A.; Presumido, P.; Silvestre, J.; Feliciano, M.; Rodrigues, G.; Oliveira e Silva, P.; Damasio, M.; Ribeiro, A.; Ramôa, S.; Ferreira, L.; et al. Water footprint sustainability as a tool to address climate change in the vitivinicultural sector: A methodological approach applied to a Portuguese case study. Atmosphere 2020, 11, 934. [Google Scholar] [CrossRef]
- Bonamente, E.; Scrucca, F.; Asdrubali, F.; Cotana, F.; Presciutti, A. Water footprint of the wine industry: Implementation of an assessment methodology and application in a case study. Sustainability 2015, 7, 12190–12208. [Google Scholar] [CrossRef]
- Russo, V.; Strever, A.E.; Ponstein, H.J. Exploring sustainability potentials in vineyards through LCA: Evidence from farming practices in South Africa. Int. J. Life Cycle Assess. 2021, 26, 1374–1390. [Google Scholar] [CrossRef]
- Antoce, A.O.; Cojocaru, G. Characterization of quality potential of Feteasca neagra grapes cultivated in different Romanian wine region. In Proceeding of the Conference Agriculture for Life, Life for Agriculture, Bucharest, Romania, 7–9 June 2018; pp. 238–243. [Google Scholar]
- Dubois, A.; Lemoine, M.; Guillaume, J. Cabernet Sauvignon: Providing a Distinctive Aromatic Profile in European Viticulture. Rev. Française D’oenologie 2019, 74, 345–350. [Google Scholar]
- Weiß, S. Blauer Zweigelt: The potential of red grape varieties in modern viticulture. Oenology Sci. J. 2020, 62, 111–118. [Google Scholar]
- Dăscălescu, C.; Vlad, I.; Rusu, A. Romanian indigenous varieties: Arcaș, a promising cultivar in local viticulture. Rom. J. Vitic. Winemak. 2017, 35, 98–103. [Google Scholar]
- Ribéreau-Gayon, P. Chimie du Vin—Stabilisation et Traitements, 7th ed.; Dunod: Paris, France, 2000. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Geana, E.-I.; Ciucure, C.T.; Tamaian, R.; Marinas, I.C.; Gaboreanu, D.M.; Stan, M.; Chitescu, C.L. Antioxidant and wound healing bioactive potential of extracts obtained from bark and needles of softwood species. Antioxidants 2023, 12, 7. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef] [PubMed]
- Grosu, A.C.; Diguta, F.C.; Pristavu, M.C.; Popa, A.; Badea, F.; Dragoi, M.C.; Ortan, A.; Dopcea, I.; Babeanu, N. Exploring the phytochemical profiles, and antioxidant and antimicrobial activities of the hydroethanolic grape pomace extracts from two Romanian indigenous varieties. Fermentation 2024, 10, 470. [Google Scholar] [CrossRef]
- Crupi, P.; Alba, V.; Gentilesco, G.; Gasparro, M.; Ferrara, G.; Mazzeo, A.; Coletta, A. Viticultural climate indexes and their role in the prediction of anthocyanins and other flavonoids content in seedless table grapes. Horticulturae 2024, 10, 28. [Google Scholar] [CrossRef]
- Phajon, Y.; Tan, H.; Liu, B.; Zhang, Y.; Ju, Y.; Shen, T.; Xu, M.; Fang, Y. Effect of terroir on phenolic content and aroma properties of grapes and wines. Foods 2025, 14, 1409. [Google Scholar] [CrossRef] [PubMed]

| Wine Region | Vineyard | Variety | Planting Year | Coordinates | Planting Distance (m) | Planting Density (Vines/Ha) |
|---|---|---|---|---|---|---|
| Hills of Dobrogea | Murfatlar | Feteasca neagra | 2007 | 44°10′27.57″ N; 28°25′41.38″ E | 2.2 × 1.1 | 4132 |
| Hills of Vallachia and Oltenia | Dealu Mare | Cabernet Sauvignon | 1998 | 44°58′0.75″ N; 26°08′55.77″ E | 2.0 × 1.0 | 4805 |
| Stefanesti Arges | Blauer Zweigelt | 1985 | 44°48′ N; 25°08′ NE; | 2.2 × 1.0 | 4500 | |
| Hills of Moldova | Iasi | Arcas | 2009 | 47°12′30.91″ N 27°32′01.03″ E | 2.2 × 1.2 | 3787 |
| Polyphenol Parameters of the Extracts | Grape Variety/Ethanolic Solution Used for Extraction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Feteasca Neagra | Cabernet Sauvignon | Blauer Zweigelt | Arcas | |||||||
| 25% | 50% | 75% | 25% | 50% | 75% | 50% | 25% | 50% | 75% | |
| Total polyphenols (GAE g.L−1), TP | 14.3 ± 2.0 ef | 18.3 ± 2.8 cde | 16.8 ± 2.4 de | 11.7 ± 1.8 f | 24.8 ± 3.1 a | 19.2 ± 2.5 bcd | - | 17.3 ± 1.6 de | 22.0 ± 2.5 abc | 23.1 ± 2.7 ab |
| Anthocyanins (mg/100 g pomace), AN | 406 ± 20 e | 616 ± 31 d | 622 ± 34 d | 296 ± 18 f | 722 ± 39 c | 572 ± 28 d | 302 ± 22 f | 603 ± 29 d | 851 ± 40 a | 791 ± 32 b |
| Color intensity, CI | 6.7 ± 1.2 f | 17.1 ± 2.1 d | 18.6 ± 3.2 cd | 12.3 ± 1.8 e | 24.4 ± 3.1 b | 22.6 ± 2.9 bc | - | 21.5 ± 1.6 bc | 29.9 ± 2.7 a | 22.8 ± 2.2 bc |
| Antioxidant activity (%), AA | 86.1 ± 6.7 a | 88.3 ± 7.2 a | 89.8 ± 7.6 a | - | - | - | 86.9 ± 8.0 a | 91.6 ± 7.8 a | 92.2 ± 8.0 a | 92.9 ± 8.0 a |
| Folin Ciocalteau index, 750 nm, FCI | 10.6 ± 1.8 e | 24.5 ± 2.0 a | 20.2 ± 2.0 b | 11.4 ± 1.5 e | 23.5 ± 1.9 a | 18.5 ± 1.7 b | - | 13.0 ± 1.0 de | 15.6 ± 1.5 bc | 17.4 ± 1.7 bc |
| Total polyphenol index, 280 nm, TPI | 85.4 ± 9.2 g | 277.4 ± 17.5 ab | 228 ± 15.2 d | 116.0 ± 10.9 f | 261.7 ± 18.0 bc | 238.3 ± 16.5 cd | 173.8 ± 14.2 e | 221.2 ± 20.1 d | 291.8 ± 21.2 ab | 302.5 ± 25.8 a |
| Chemical Parameters of the Extracts | Grape Variety/Type o Must | |||||||
|---|---|---|---|---|---|---|---|---|
| Feteasca Neagra | Cabernet Sauvignon | Blauer Zweigelt | Arcas | |||||
| Mc | Mep | Mc | Mep | Mc | Mep | Mc | Mep | |
| Sugar (g.L−1) | 232.2 ± 10.1 ab | 227.5 ± 12.4 ab | 245.5 ± 14.8 a | 230.0 ± 11.8 ab | 230.9 ± 11.2 ab | 222.1 ± 10.9 b | 229.8 ± 11.0 ab | 218.1 ± 9.5 b |
| Total acidity (g.L−1) as tartaric acid | 4.94 ± 0.4 b | 4.51 ± 0.3 b | 4.96 ± 0.4 b | 4.51 ± 0.2 b | 5.13 ± 0.5 b | 4.62 ± 0.3 b | 6.31 ± 0.5 a | 6.23 ± 0.5 a |
| pH | 3.89 ± 0.2 a | 3.89 ± 0.1 a | 3.94 ± 0.1 a | 3.92 ± 0.2 a | 3.73 ± 0.3 ab | 3.74 ± 0.3 ab | 3.32 ± 0.2 b | 3.32 ± 0.3 b |
| Polyphenols (g.L−1) | 4.69 ± 1.3 a | 5.59 ± 0.6 a | 2.01 ± 0.1 b | 2.27 ± 0.1 b | 5.44 ± 1.4 a | 5.72 ± 1.3 a | 1.91 ± 0.9 b | 4.02 ± 1.0 a |
| Feteasca Neagra | Cabernet Sauvignon | Blauer Zweigelt | Arcas | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mc | Mep | Mc | Mep | Mc | Mep | Mc | Mep | ||
| Phenolic acids (mg·L−1) | gallic acid | 0.63 ± 0.03 e | 10.25 ± 0.54 a | 0.81 ± 0.04 e | 0.96 ± 0.05 e | 2.12 ± 0.21 d | 5.53 ± 0.30 b | 0.15 ± 0.01 f | 4.01 ± 0.32 c |
| 3,4-dihydroxybenzoic acid | 0.34 ± 0.02 f | 3.00 ± 0.15 a | 0.68 ± 0.03 e | 0.70 ± 0.03 e | 0.83 ± 0.05 d | 2.05 ± 0.10 b | 0.24 ± 0.01 f | 1.12 ± 0.06 c | |
| 2,5-dihydroxybenzoic acid | 0.09 ± 0.01 c | 0.14 ± 0.01 b | 0.11 ± 0.01 c | 0.16 ± 0.01 b | 0.42 ± 0.03 a | 0.40 ± 0.02 a | 0.05 ± 0.01 d | 0.06 ± 0.01 d | |
| 4-dihydroxybenzoic acid | 0.62 ± 0.05 b | 0.26 ± 0.02 e | 0.45 ± 0.03 c | 0.37 ± 0.02 d | 0.42 ± 0.03 cd | 0.68 ± 0.05 a | 0.14 ± 0.01 f | 0.19 ± 0.01 f | |
| caffeic acid | 0.66 ± 0.05 g | 0.44 ± 0.03 h | 3.07 ± 0.15 a | 2.27 ± 0.11 d | 2.50 ± 0.12 c | 2.85 ± 0.15 b | 1.60 ± 0.08 e | 0.92 ± 0.04 f | |
| syringic acid | 2.26 ± 0.12 f | 58.71 ± 2.12 a | 7.76 ± 0.45 e | 9.34 ± 0.58 e | 11.53 ± 0.56 d | 26.65 ± 1.10 c | 2.46 ± 0.10 f | 37.88 ± 1.30 b | |
| p-coumaric acid | 0.86 ± 0.04 d | 1.18 ± 0.06 c | 0.39 ± 0.02 e | 0.39 ± 0.02 e | 1.65 ± 0.07 a | 1.48 ± 0.04 b | 0.21 ± 0.01 f | 0.36 ± 0.02 e | |
| chlorogenic acid | 2.93 ± 0.10 d | 6.36 ± 0.22 a | 4.90 ± 0.25 b | 3.82 ± 0.15 c | 1.89 ± 0.09 e | 3.19 ± 0.19 d | 1.62 ± 0.08 f | 0.75 ± 0.04 g | |
| ellagic acid | 0.19 ± 0.01 bc | 0.56 ± 0.03 a | 0.17 ± 0.01 bc | 0.15 ± 0.01 cd | 0.59 ± 0.04 a | 0.58 ± 0.05 a | 0.12 ± 0.01 d | 0.21 ± 0.02 b | |
| abscisic acid | 0.53 ± 0.03 c | 0.51 ± 0.04 c | 0.55 ± 0.03 bc | 0.54 ± 0.02 bc | 0.61 ± 0.04 b | 0.61 ± 0.03 b | 0.51 ± 0.02 c | 1.00 ± 0.07 a | |
| Flavanols (mg·L−1) | catechins | 8.07 ± 0.42 e | 9.69 ± 0.51 d | 4.52 ± 0.20 f | 3.21 ± 0.18 g | 15.51 ± 0.56 a | 14.30 ± 0.54 b | 1.29 ± 0.10 h | 12.57 ± 0.62 c |
| epicatechin | 2.25 ± 0.12 d | 3.37 ± 0.14 b | 0.64 ± 0.04 e | 0.46 ± 0.04 e | 2.34 ± 0.10 d | 2.83 ± 0.14 c | 0.02 ± 0.01 f | 6.66 ± 0.32 a | |
| Flavones (mg·L−1) | taxifolin | 0.13 ± 0.01 c | 0.17 ± 0.01 b | 0.05 ± 0.01 d | 0.12 ± 0.01 c | 0.02 ± 0.01 e | 0.39 ± 0.02 a | 0.03 ± 0.01 de | 0.13 ± 0.01 c |
| vitexin | 0.09 ± 0.01 e | 0.55 ± 0.03 b | 0.05 ± 0.01 fg | 0.06 ± 0.01 ef | 0.15 ± 0.01 d | 0.24 ± 0.02 c | 0.02 ± 0.301 g | 0.91 ± 0.03 a | |
| rutin | ≤0.023 | 0.10 ± 0.01 c | 0.93 ± 0.05 a | 0.96 ± 0.05 a | 0.03 ± 0.01 d | ≤0.023 | 0.02 ± 0.01 d | 0.30 ± 0.02 b | |
| kaempferol | 0.20 ± 0.02 d | 0.34 ± 0.03 c | 0.16 ± 0.01 de | 0.10 ± 0.01 ef | 1.70 ± 0.08 b | 1.80 ± 0.09 a | 0.03 ± 0.01 f | 0.04 ± 0.01 f | |
| quercitin | 0.19 ± 0.01 b | 0.56 ± 0.03 a | 0.17 ± 0.01 bc | 0.15 ± 0.01 cd | 0.59 ± 0.02 a | 0.58 ± 0.03 a | 0.12 ± 0.01 d | 0.20 ± 0.02 b | |
| Dihydrocalcones (mg·L−1) | phlorizin | 0.68 ± 0.04 c | 0.61 ± 0.04 d | 0.41 ± 0.03 e | 0.44 ± 0.03 e | 1.55 ± 0.07 a | 1.37 ± 0.05 b | 0.23 ± 0.01 g | 0.32 ± 0.02 f |
| phloretin | 0.01 ± 0.01 b | 0.03 ± 0.01 a | ≤0.008 | ≤0.008 | 0.02 ± 0.01 ab | 0.03 ± 0.01 a | 0.01 ± 0.01 b | 0.01 ± 0.01 b | |
| Flavonoids (mg·L−1) | apigenin | 0.09 ± 0.01 a | 0.03 ± 0.01 b | 0.03 ± 0.01 b | 0.03 ± 0.01 b | ≤0.024 | 0.04 ± 0.01 b | 0.03 ± 0.01 b | ≤0.024 |
| pinocembrin | 0.03 ± 0.01 b | ≤0.024 | ≤0.024 | ≤0.024 | ≤0.024 | ≤0.024 | 0.05 ± 0.01 a | ≤0.024 | |
| crisin | 0.16 ± 0.01 b | 0.03 ± 0.01 c | 0.03 ± 0.01 c | ≤0.025 | ≤0.025 | ≤0.025 | 0.27 ± 0.02 a | ≤0.025 | |
| galangin | 0.05 ± 0.01 b | ≤0.024 | ≤0.024 | ≤0.024 | ≤0.024 | ≤0.024 | 0.12 ± 0.02 a | ≤0.024 | |
| Stilbens (mg·L−1) | trans-resferatrol | 0.03 ± 0.01 e | 0.03 ± 0.01 e | 0.03 ± 0.01 e | 0.38 ± 0.02 c | 0.64 ± 0.04 b | 1.40 ± 0.06 a | 0.03 ± 0.01 e | 0.27 ± 0.02 d |
| polydatin | 0.40 ± 0.02 c | 0.30 ± 0.02 d | 0.17 ± 0.01 e | 0.10 ± 0.01 f | 0.93 ± 0.06 b | 1.11 ± 0.05 a | 0.14 ± 0.01 ef | 0.18 ± 0.02 e | |
| Anthocyanins (mg·L−1) | Oenin (malvidin-3-O-glucozide) | 63.33 ± 3.10 a | 37.19 ± 1.74 d | 8.12 ± 0.41 f | 5.72 ± 0.24 gh | 52.84 ± 2.43 b | 41.46 ± 1.89 c | 3.81 ± 0.15 g | 12.44 ± 0.70 e |
| TOTAL phenolic compounds (mg·L−1) | 84.78 ± 3.20 d | 134.37 ± 5.26 a | 34.01 ± 1.5 e | 30.40 ± 1.78 e | 98.90 ± 4.50 c | 109.54 ± 4.96 b | 13.21 ± 0.60 f | 80.62 ± 3.78 d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artem, V.; Antoce, A.O.; Geana, E.I.; Nechita, A.; Tudor, G.; Onache, P.A.; Ranca, A. Circular Model for the Valorization of Black Grape Pomace for Producing Pasteurized Red Must Enriched in Health-Promoting Phenolic Compounds. Sustainability 2025, 17, 6633. https://doi.org/10.3390/su17146633
Artem V, Antoce AO, Geana EI, Nechita A, Tudor G, Onache PA, Ranca A. Circular Model for the Valorization of Black Grape Pomace for Producing Pasteurized Red Must Enriched in Health-Promoting Phenolic Compounds. Sustainability. 2025; 17(14):6633. https://doi.org/10.3390/su17146633
Chicago/Turabian StyleArtem, Victoria, Arina Oana Antoce, Elisabeta Irina Geana, Ancuta Nechita, Georgeta Tudor, Petronela Anca Onache, and Aurora Ranca. 2025. "Circular Model for the Valorization of Black Grape Pomace for Producing Pasteurized Red Must Enriched in Health-Promoting Phenolic Compounds" Sustainability 17, no. 14: 6633. https://doi.org/10.3390/su17146633
APA StyleArtem, V., Antoce, A. O., Geana, E. I., Nechita, A., Tudor, G., Onache, P. A., & Ranca, A. (2025). Circular Model for the Valorization of Black Grape Pomace for Producing Pasteurized Red Must Enriched in Health-Promoting Phenolic Compounds. Sustainability, 17(14), 6633. https://doi.org/10.3390/su17146633







