Hydrochemical Formation Mechanisms and Source Apportionment in Multi-Aquifer Systems of Coastal Cities: A Case Study of Qingdao City, China
Abstract
1. Introduction
2. Materials and Methods
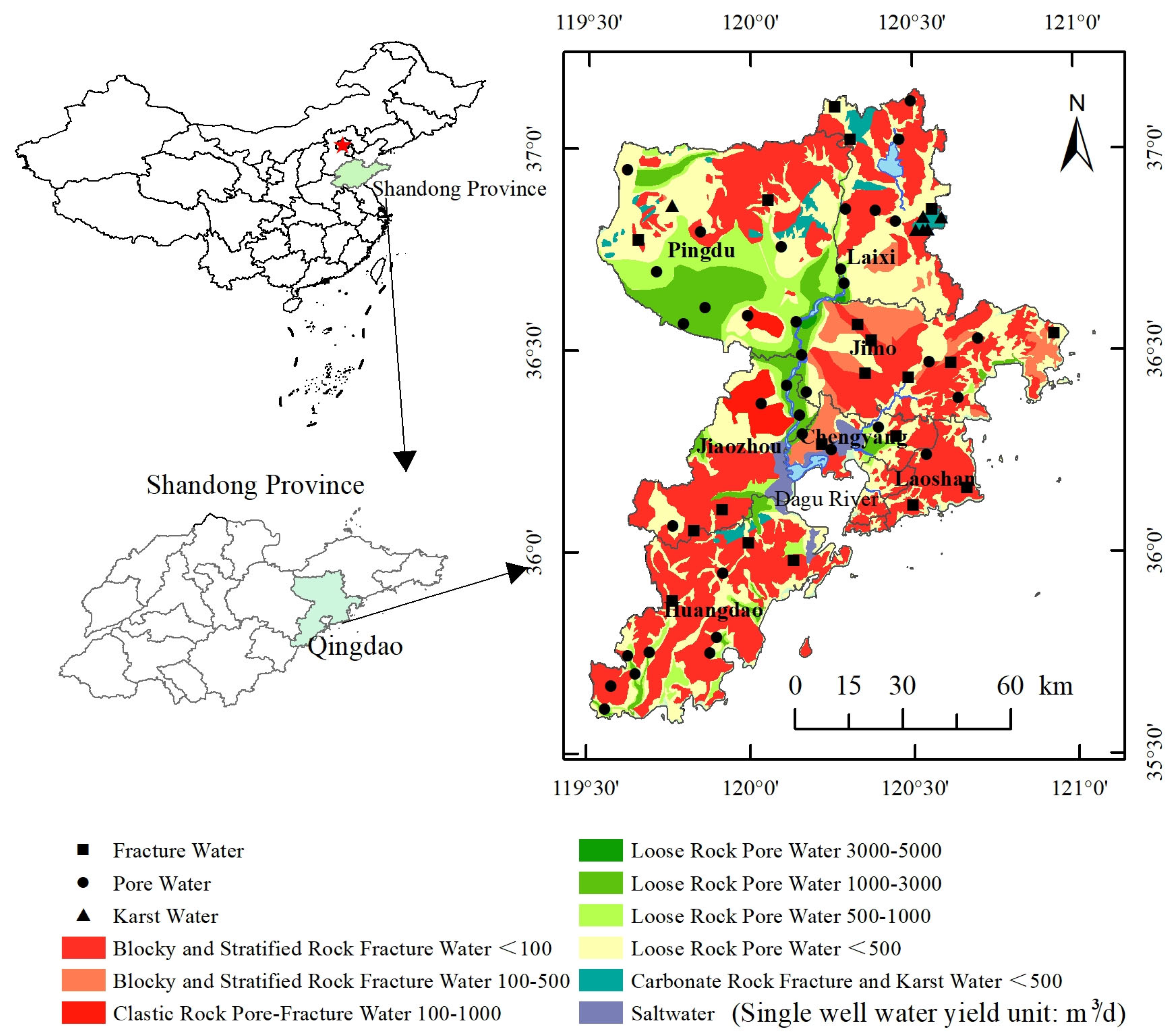
2.1. Study Area
2.2. Sample Collection and Testing Analysis
2.3. Research Methods
3. Results and Discussion
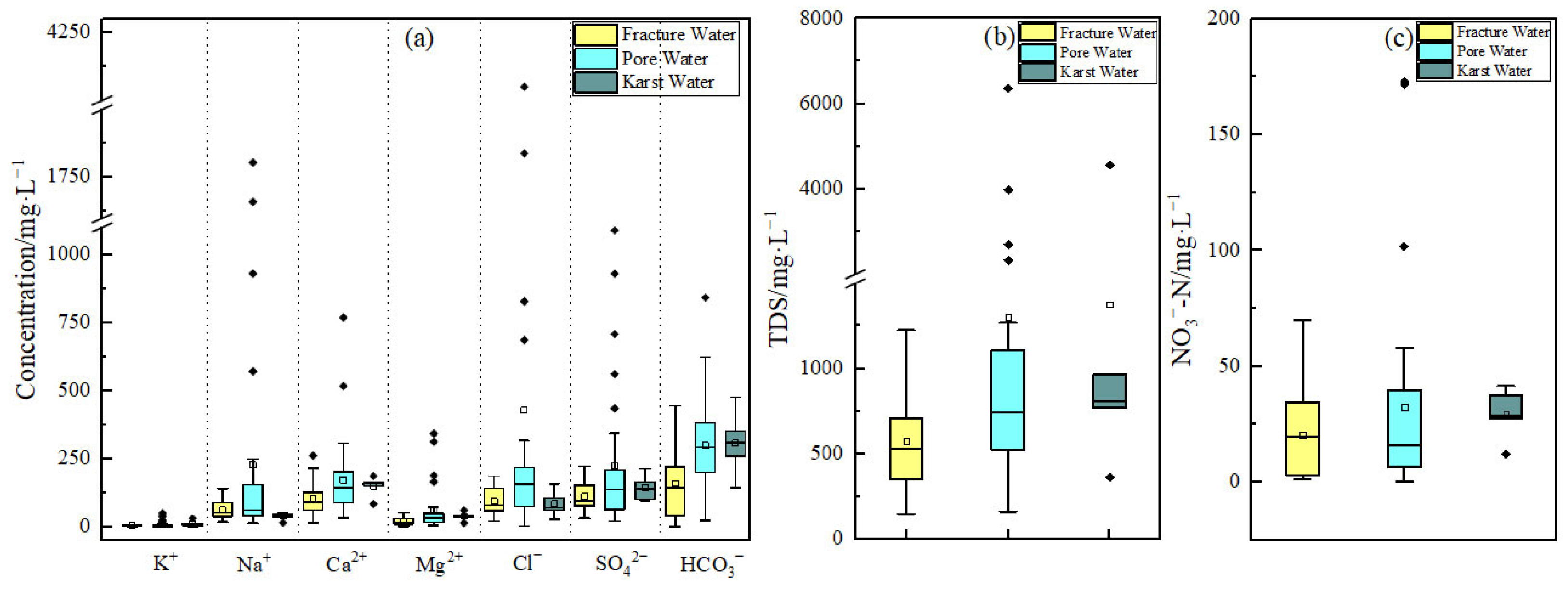
3.1. Hydrochemical Characteristics of Groundwater
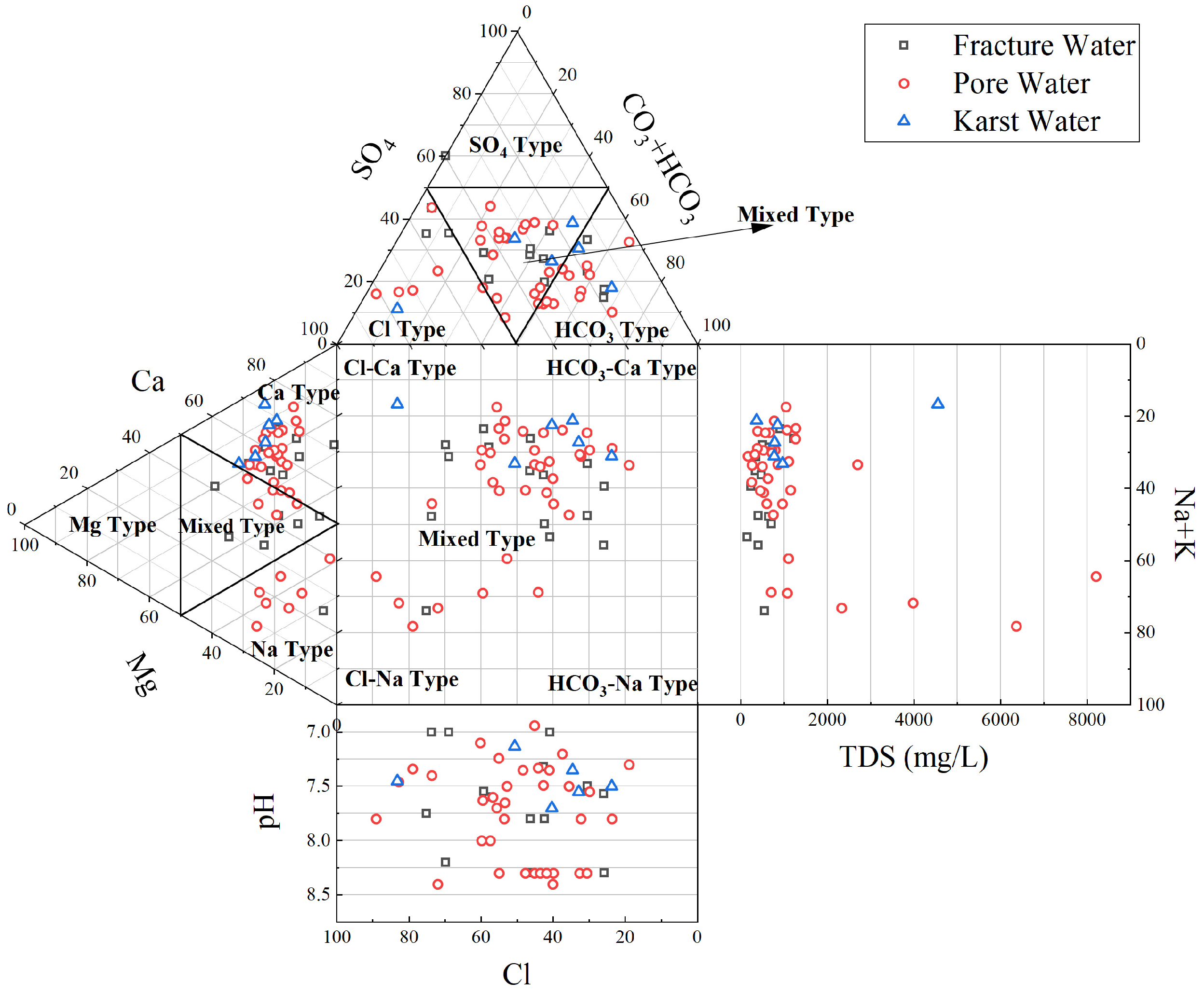
3.1.1. Characteristics of the Hydrochemical Composition of Groundwater
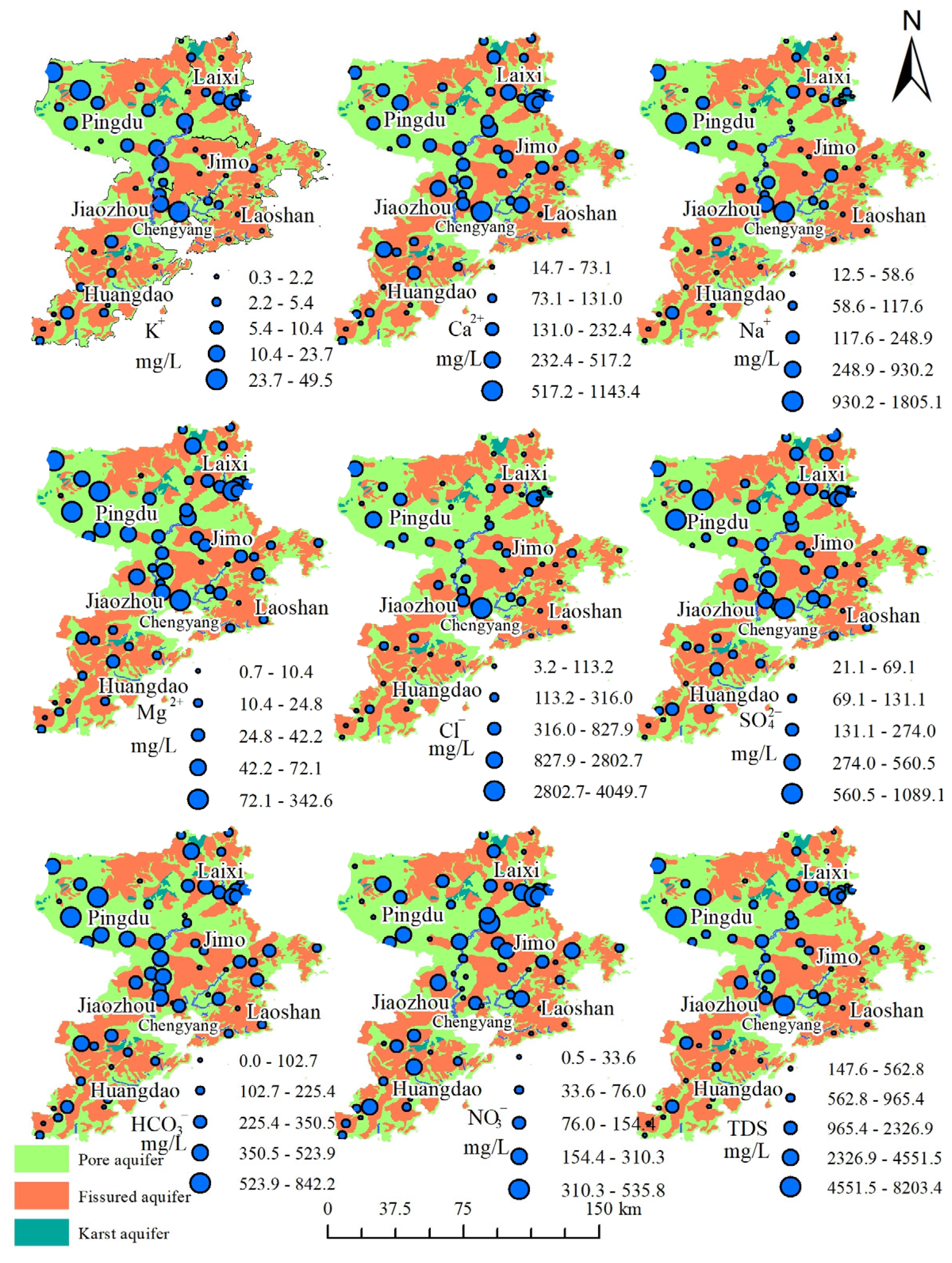
3.1.2. Distribution Characteristics of Groundwater Hydrochemistry
3.2. Analysis of Natural Sources of Hydrochemical Components in Different Groundwater Types
3.2.1. Water–Rock Model Analysis
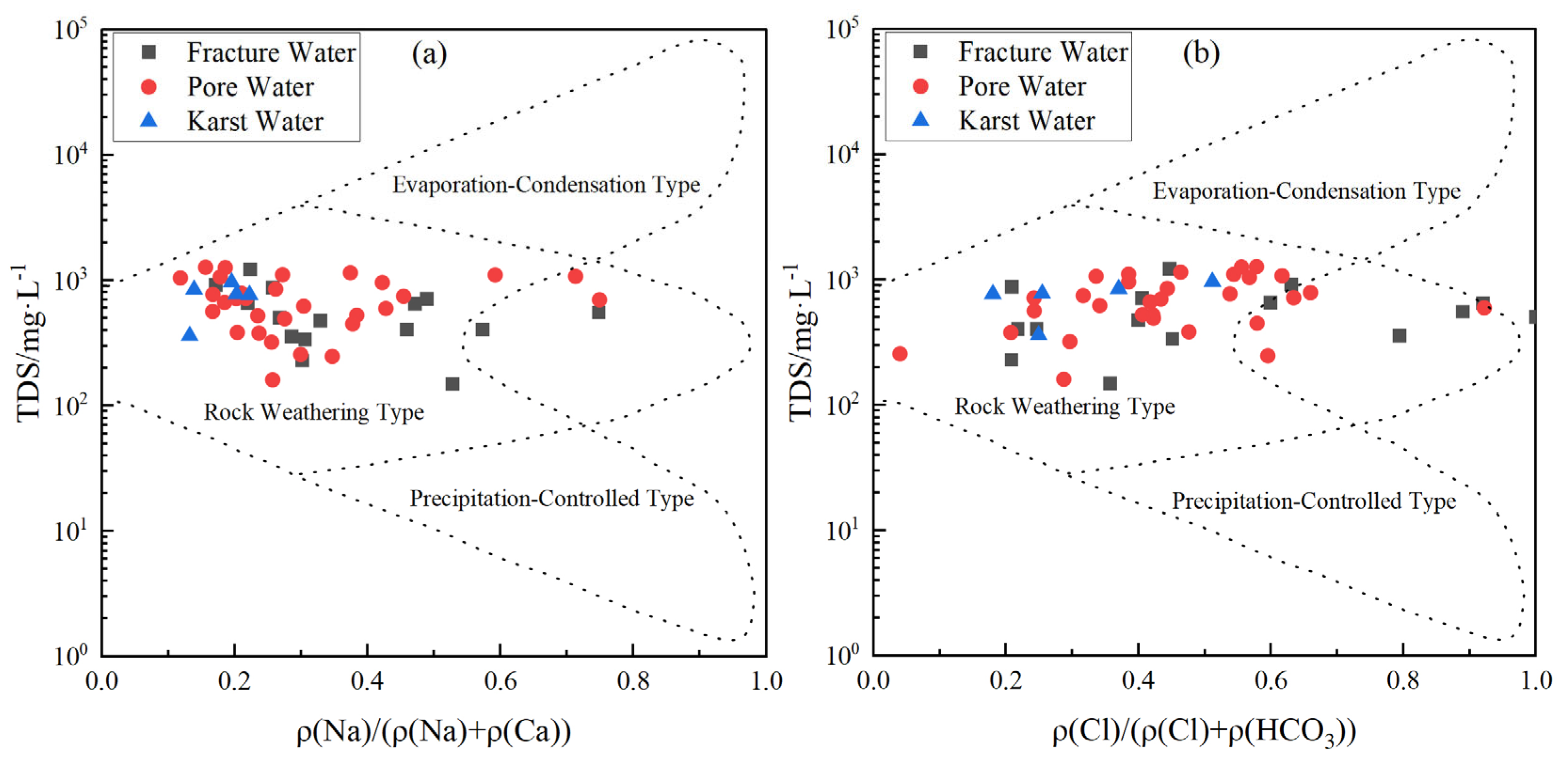
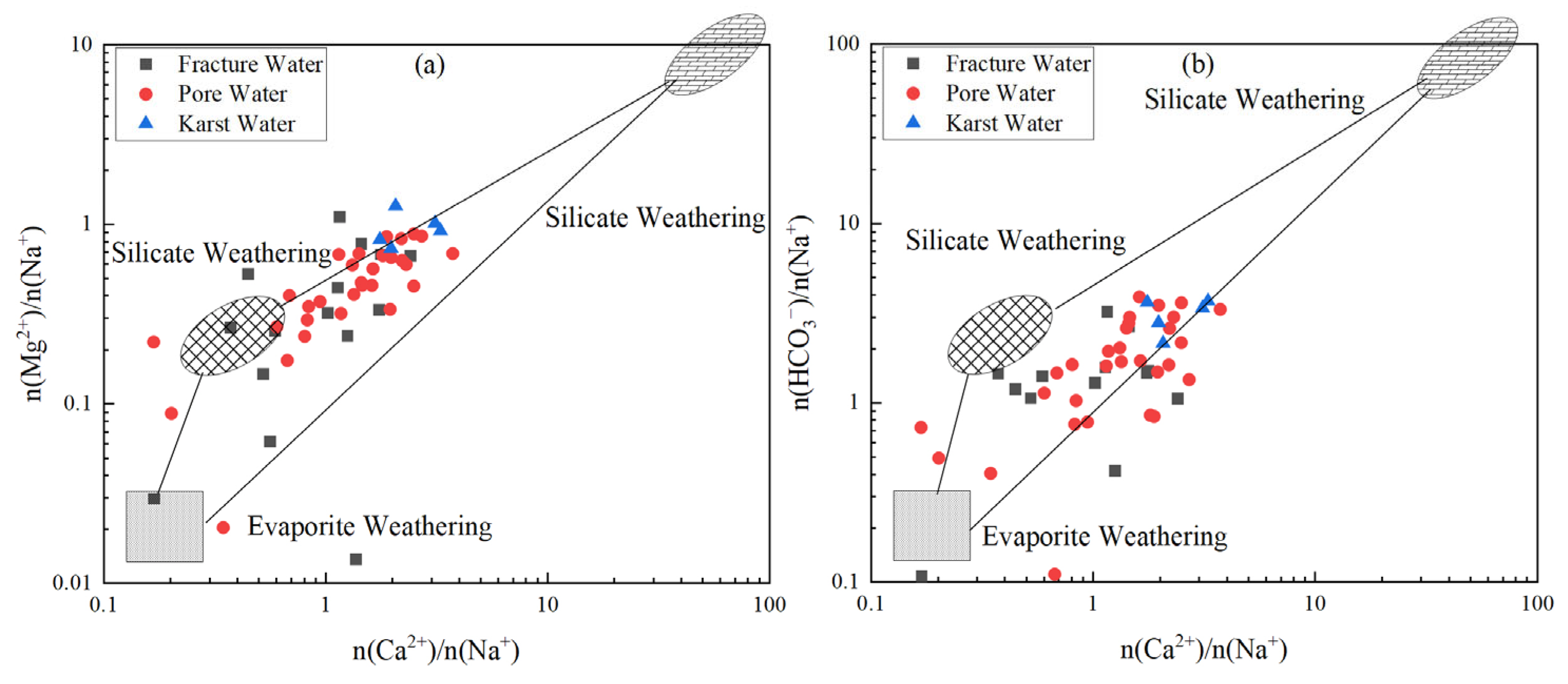
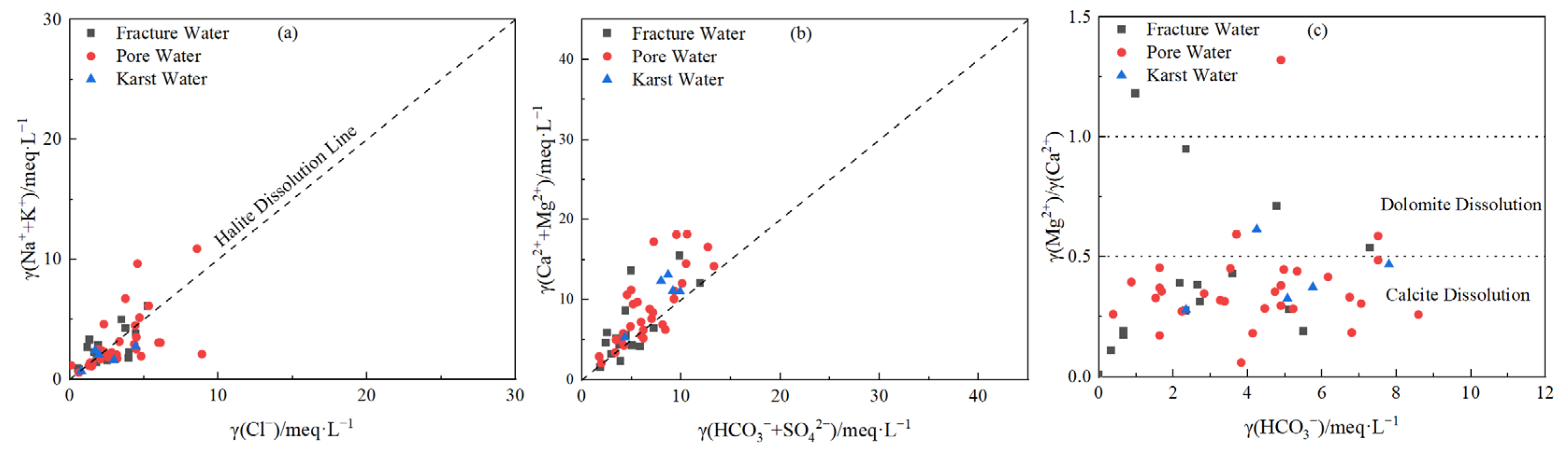
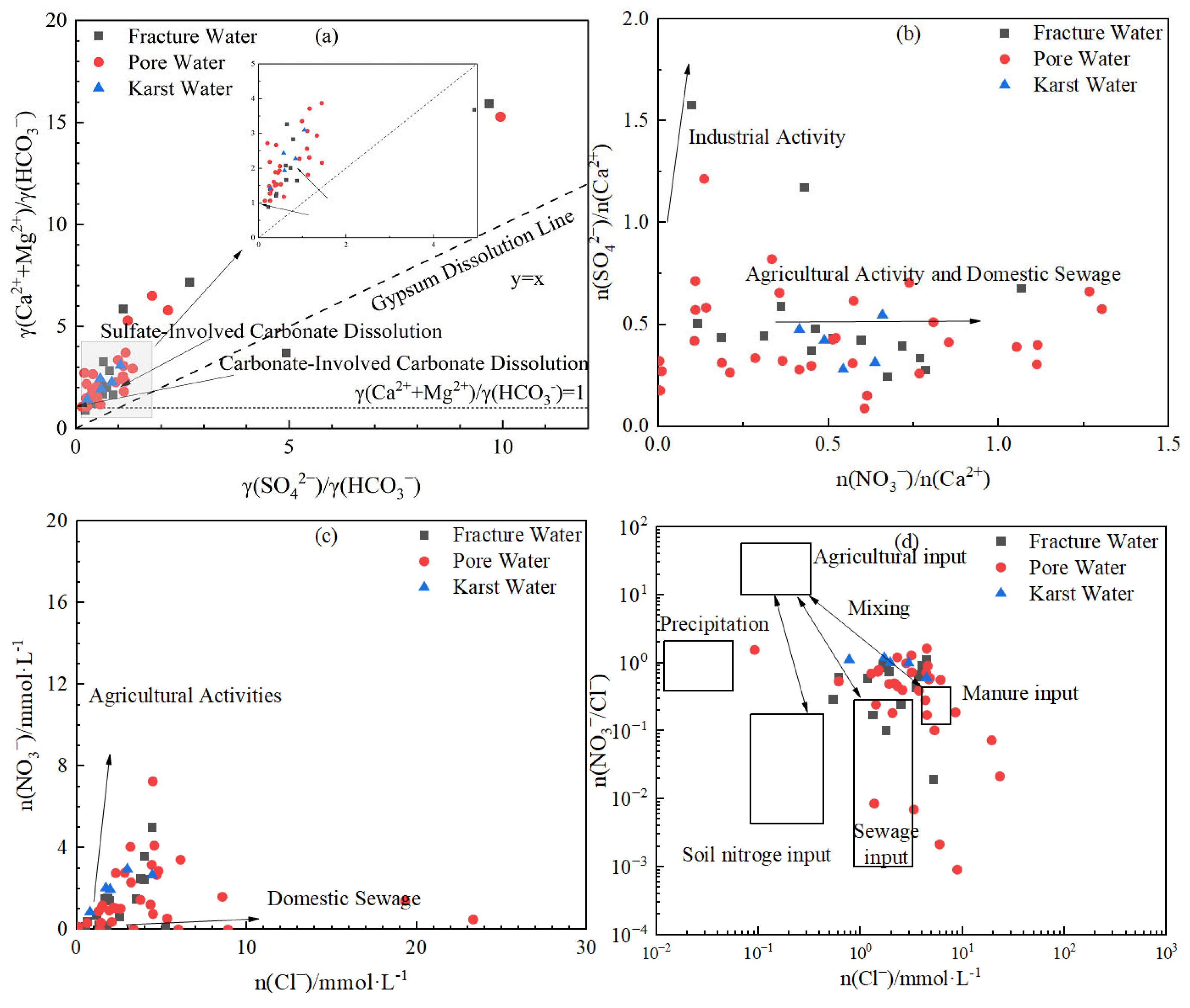
3.2.2. Major Weathering Processes and Hydrochemical Evolution
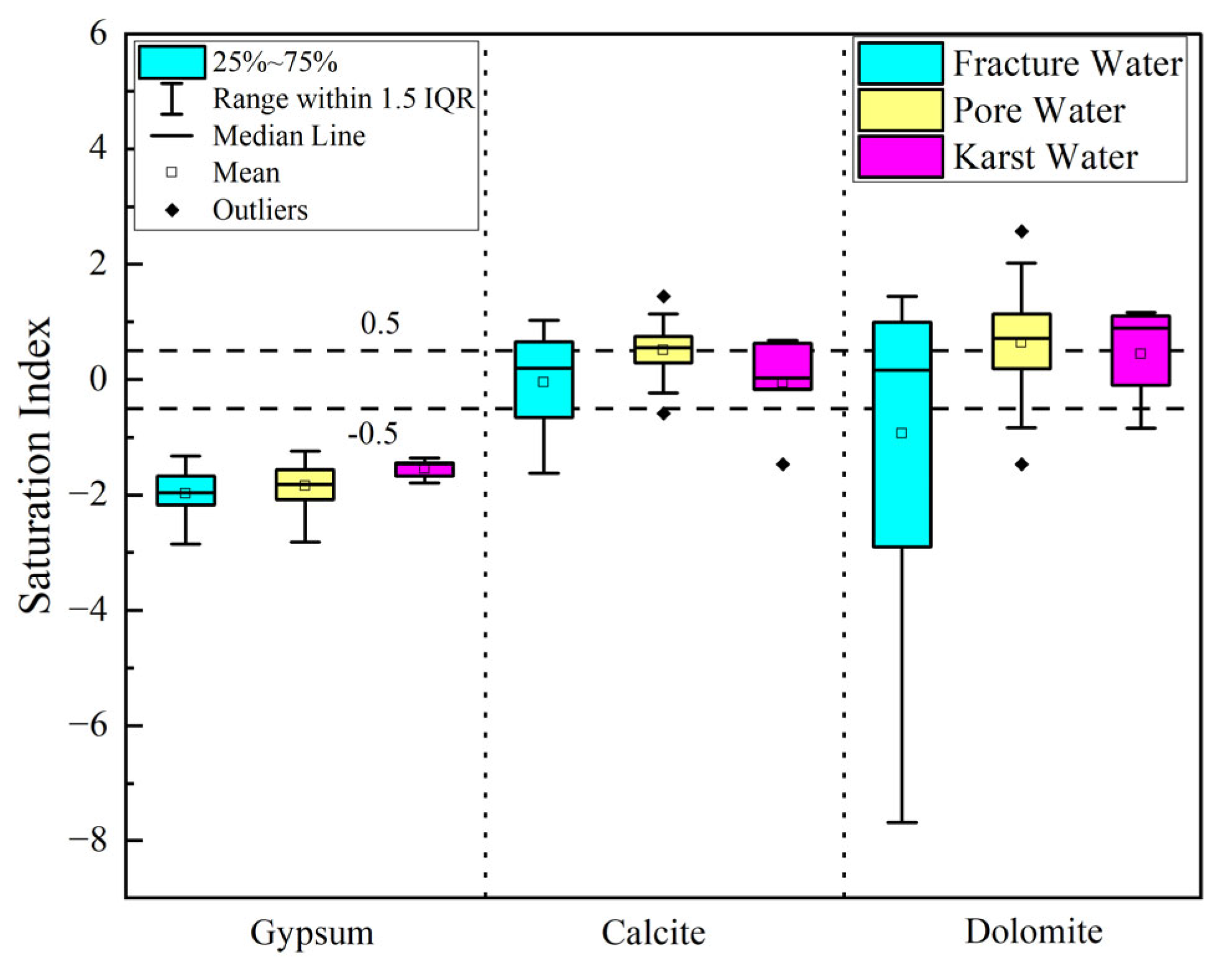
3.2.3. Mineral Dissolution Equilibrium
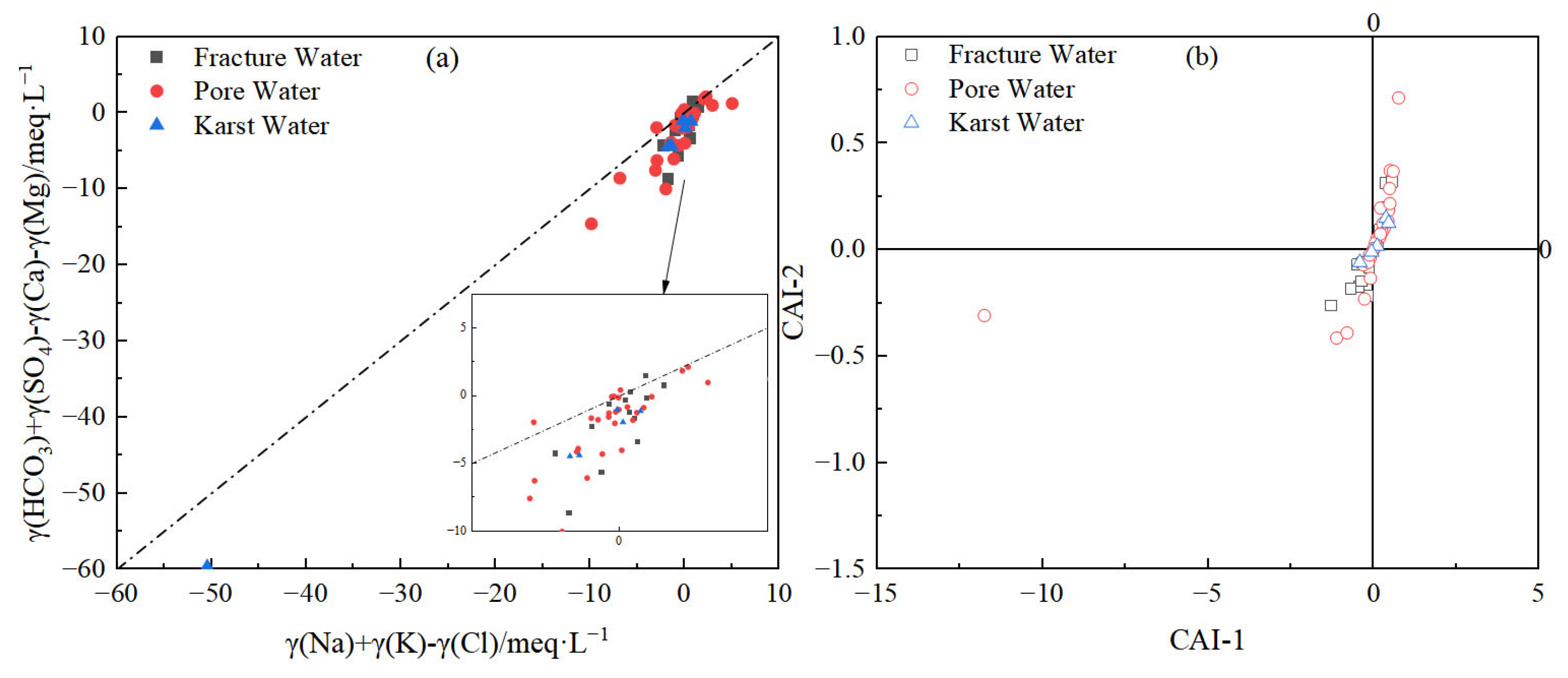
3.2.4. Cation Exchange Processes
3.3. Analysis of Anthropogenic Sources of Hydrochemical Components in Different Groundwater Types
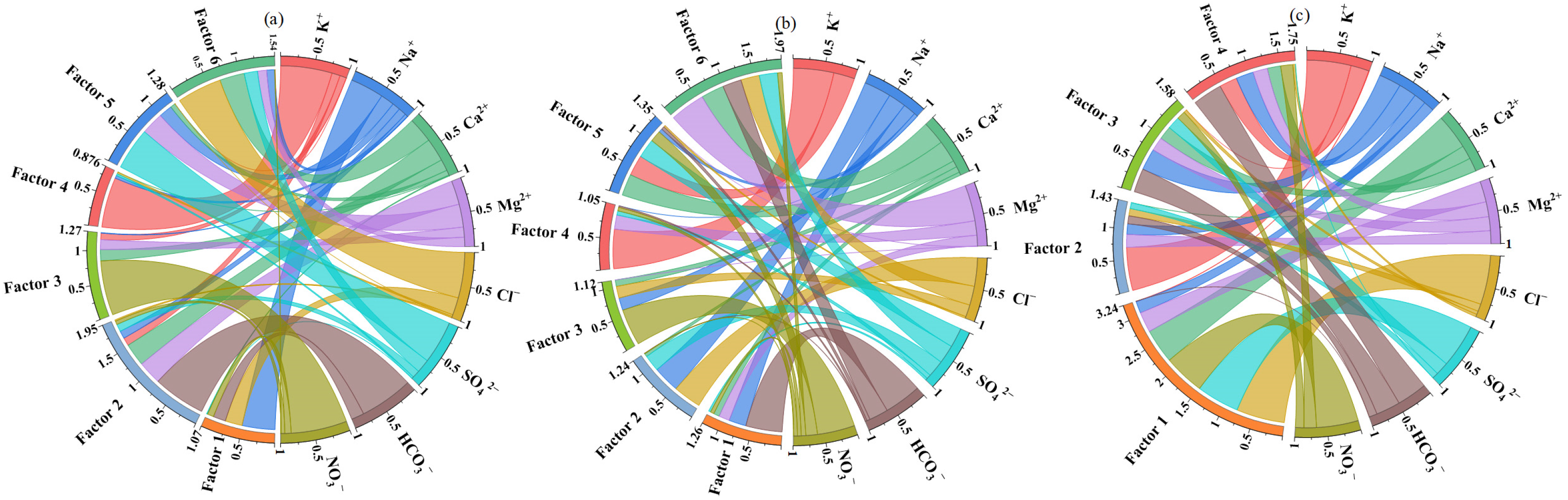
3.4. Source Apportionment of Hydrochemical Components Through PMF Analysis
3.5. Multi-Source Interaction Mechanisms in Regional Hydrochemical Formation
4. Conclusions
- (1)
- Groundwater in Qingdao is weakly alkaline (pH 7.2–8.4), with Ca2+ and Mg2+ as dominant cations and HCO3− as the primary anion. Its chemical composition is co-controlled by natural geogenic processes (carbonate/silicate weathering, evaporation–concentration) and anthropogenic activities, exhibiting marked spatial heterogeneity. Along the Dagu River basin to its estuary, porous groundwater transitions from HCO3-Ca to SO4-Ca and Cl-Na types, with a mean TDS of 716.6 mg/L. In coastal areas (e.g., Dagu River estuary) and the Pingdu depression cone, seawater intrusion and evaporation elevate TDS to over 4000 mg/L. Fractured groundwater (mean TDS: 562.2 mg/L) is predominantly HCO3-Ca type, yet shows HCO3·SO4-Ca·Na type in human-impacted Jimo District. Karst groundwater (mean TDS: 743.5 mg/L) remains homogeneous, dominated by HCO3-Ca type due to carbonate weathering.
- (2)
- Natural processes govern baseline hydrochemistry: Rock weathering (calcite and dolomite dissolution) serves as the predominant ion source, while cation exchange plays a secondary role in shaping hydrochemical composition Notably, evaporation–concentration significantly elevates TDS in low-lying porous aquifers. Mineral saturation indices (SI) reveal higher dispersion in fractured groundwater (calcite SI: −1.5–1; dolomite SI: 0.5–−4), reflecting complex hydrodynamic controls, whereas porous and karst systems exhibit stable SI distributions (e.g., calcite SI: 0–1), indicating homogeneous chemical conditions.
- (3)
- Human activities alter hydrochemical evolution through dual pathways: Direct pollutant inputs—agricultural non-point sources and domestic wastewater (Cl−, SO42−) are key anthropogenic contributors. Acid deposition (H2SO4, HNO3) enhances carbonate dissolution, elevating γ(SO42−)/γ(HCO3−) to 0.3–0.6 in select samples. Hydrodynamic field modification—overexploitation-induced depression cones intensify inland salt accumulation and coastal seawater intrusion (Cl− up to 4049.7 mg/L), reversing cation exchange direction (CAI > 0 in 67% of samples) and triggering Na+ enrichment with soil Ca2+/Mg2+ depletion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, C.; Zhang, Q. Groundwater Chemical Characteristics and Controlling Factors in a Region of Northern China with Intensive Human Activity. Multidiscip. Digit. Publ. Inst. 2020, 17, 9126. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, H.; Zhang, Q. Assessment of the Evolution of Groundwater Chemistry and Its Controlling Factors in the Huangshui River Basin of Northwestern China, Using Hydrochemistry and Multivariate Statistical Techniques. Int. J. Environ. Res. Public Health 2021, 18, 7551. [Google Scholar] [CrossRef] [PubMed]
- Subba Rao, N.; Marghade, D.; Dinakar, A.; Chandana, I.; Sunitha, B.; Ravindra, B.; Balaji, T. Geochemical characteristics and controlling factors of chemical composition of groundwater in a part of Guntur district, Andhra Pradesh, India. Environ. Earth Sci. 2017, 76, 747. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, S.; Dong, S.; Li, Y.; Sun, B.; Shao, Z. In Influence of Agricultural Activity and Aquifer Intrinsic Vulnerability on Groundwater Quality in the Dagu River Watershed (Qingdao, China). In Proceedings of the International Conference on Bioinformatics & Biomedical Engineering, Granada, Spain, 18–20 June 2010. [Google Scholar]
- Lin, M.-l.; Peng, W.-h.; Gui, H.-r. Hydrochemical characteristics and quality assessment of deep groundwater from the coal-bearing aquifer of the Linhuan coal-mining district, Northern Anhui Province, China. Environ. Monit. Assess. 2016, 188, 202. [Google Scholar] [CrossRef]
- Huang, G.; Liu, C.; Sun, J.; Zhang, M.; Jing, J.; Li, L. A regional scale investigation on factors controlling the groundwater chemistry of various aquifers in a rapidly urbanized area: A case study of the Pearl River Delta. Sci. Total Environ. 2018, 625, 510–518. [Google Scholar] [CrossRef]
- Mohamed, A.; Asmoay, A.; Alshehri, F.; Abdelrady, A.; Othman, A. Hydro-geochemical applications and multivariate analysis to assess the water–rock interaction in arid environments. Appl. Sci. 2022, 12, 6340. [Google Scholar] [CrossRef]
- Subba Rao, N.; Subrahmanyam, A.; Ravi Kumar, S.; Srinivasulu, N.; Babu Rao, G.; Rao, P.S.; Reddy, G.V. Geochemistry and quality of groundwater of Gummanampadu sub-basin, Guntur District, Andhra Pradesh, India. Environ. Earth Sci. 2012, 67, 1451–1471. [Google Scholar] [CrossRef]
- Srinivasamoorthy, K.; Vasanthavigar, M.; Vijayaraghavan, K.; Sarathidasan, R.; Gopinath, S. Hydrochemistry of groundwater in a coastal region of Cuddalore district, Tamilnadu, India: Implication for quality assessment. Arab. J. Geosci. 2013, 6, 441–454. [Google Scholar] [CrossRef]
- Marghade, D.; Malpe, D.B.; Subba Rao, N.; Sunitha, B. Geochemical assessment of fluoride enriched groundwater and health implications from a part of Yavtmal District, India. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 673–694. [Google Scholar] [CrossRef]
- Yao-Qi, Z.; Zhen-Kai, Z.; Wen-Dong, L.; Su, L.; Hui-Wen, Y. Late Mesozoic tectono-magmatic activities and prototype basin restoration in Eastern Shandong Province, China. Earth Sci. Front. 2015, 22, 137. [Google Scholar]
- Luan, G.; Li, A.; Wang, J.; Li, G.; Xie, R.J. The geological origin division of the main sea island in Qingdao area and environment analysis. Period. Ocean Univ. China 2010, 40, 111–116. [Google Scholar]
- GB/T 14848-2017; Standard for Groundwater Quality. Chinese Standard: Beijing, China, 2017.
- Ma, Z.M.; Luo, Y.Y.; Fang, Y.Z.; Hou, Y.S. Hydrogeochemical mechanism of the petroleum hydrocarbon pollution in Karst Fissure groundwater system. Appl. Mech. 2013, 295, 159–163. [Google Scholar] [CrossRef]
- Kogovšek, J. Impact of chlorides, nitrates, sulfates and phosphates on increased limestone dissolution in the karst vadose zone (Postojna Cave, Slovenia). Acta Carsologica 2011, 40, 319–327. [Google Scholar] [CrossRef]
- Chunxia, M.; Xilai, Z.; Zhenyu, M.A.; Chengjian, W. Assessment on status of Nitrate pollution in rural water supply of Qingdao and its healthy risk. Water Resour. Hydropower Eng. 2014, 9, 24–26+38. [Google Scholar]
- Han, Z.; Ma, H.; Shi, G.; He, L.; Wei, L.; Shi, Q. A review of groundwater contamination near municipal solid waste landfill sites in China. Sci. Total Environ. 2016, 569–570, 1255–1264. [Google Scholar] [CrossRef]
- Gang, S.; Jia, T.; Deng, Y.; Xing, L.; Gao, S. Hydrochemical characteristics and formation mechanism of groundwater in Qingdao City, Shandong Province, China. Water 2023, 15, 1348. [Google Scholar] [CrossRef]
- Keim, D.M.; West, L.J.; Odling, N.E. Convergent Flow in Unsaturated Fractured Chalk. Vadose Zone J. 2012, 11, vzj2011-0146. [Google Scholar] [CrossRef]
- Rozell, D.J.; Reaven, S.J. Water Pollution Risk Associated with Natural Gas Extraction from the Marcellus Shale. Risk Anal. 2012, 32, 1382–1393. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Chandel, S. Identification of hydrogeochemical processes affecting the hydrochemistry of groundwater: A review. J. Nat. Resour. Conserv. Manag. 2023, 4, 15–29. [Google Scholar] [CrossRef]
- Subramani, T.; Rajmohan, N.; Elango, L. Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environ. Monit. Assess. 2010, 162, 123–137. [Google Scholar] [CrossRef]
- Négrel, P.; Millot, R.; Petelet-Giraud, E.; Malcuit, E.; Brenot, A. Impact of Rock Weathering on the Chemical Composition of Groundwater Determined by Inverse Modeling in Large Sedimentary Basins. Procedia Earth Planet. Sci. 2013, 7, 615–619. [Google Scholar] [CrossRef]
- Zaidi, F.K.; Kassem, O.M.; Al-Bassam, A.M.; Al-Humidan, S. Factors governing groundwater chemistry in paleozoic sedimentary aquifers in an arid environment: A case study from Hail Province in Saudi Arabia. Arab. J. Sci. Eng. 2015, 40, 1977–1985. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, F.; Yang, H.; Yu, S. Role of anthropogenic sulfuric and nitric acids in carbonate weathering and associated carbon sink budget in a karst catchment (Guohua), Southwestern China. J. Hydrol. 2021, 599, 126287. [Google Scholar] [CrossRef]
- Huang, Q.B.; Qin, X.Q.; Liu, P.Y.; Zhang, L.K.; Su, C.T. Impact of sulfuric and nitric acids on carbonate dissolution, and the associated deficit of CO2 uptake in the upper-middle reaches of the Wujiang River, China. J. Contam. Hydrol. 2017, 203, 18–27. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Z.; Wang, M.; Li, Y.; Shi, M.; Zhang, H.; Ma, Y. Hydrochemical characteristics and possible controls in the groundwater of the Yarlung Zangbo River Valley, China. Environ. Earth Sci. 2019, 78, 76. [Google Scholar] [CrossRef]
- Szramek, K.; Walter, L.M.; Kandu, T.; Ogrinc, N. Dolomite Versus Calcite Weathering in Hydrogeochemically Diverse Watersheds Established on Bedded Carbonates (Sava and Soča Rivers, Slovenia). Aquat. Geochem. 2011, 17, 357–396. [Google Scholar] [CrossRef]
- Gomo, M. Conceptual hydrogeochemical characteristics of a calcite and dolomite acid mine drainage neutralised circumneutral groundwater system. Water Sci. 2018, 32, 355–361. [Google Scholar] [CrossRef]
- Möller, P.; De Lucia, M. The impact of Mg2+ ions on equilibration of Mg-Ca carbonates in groundwater and brines. Geochemistry 2020, 80, 125611. [Google Scholar] [CrossRef]
- Chidambaram, S.; Karmegam, U.; Sasidhar, P.; Prasanna, M.V.; Manivannan, R.; Arunachalam, S.; Manikandan, S.; Anandhan, P. Significance of saturation index of certain clay minerals in shallow coastal groundwater, in and around Kalpakkam, Tamil Nadu, India. J. Earth Syst. Sci. 2011, 120, 897–909. [Google Scholar] [CrossRef]
- Nagaraju, A.; Sreedhar, Y.; Kumar, K.S.; Thejaswi, A.; Sharifi, Z. Assessment of groundwater quality and evolution of hydrochemical facies around Tummalapalle area, Cuddapah District, Andhra Pradesh, South India. J. Environ. Anal. Chem. 2014, 1, 1000112. [Google Scholar]
- Santhanam, H.; Karthikeyan, A.; Raja, M. Saturation indices of aqueous mineral phases as proxies of seasonal dynamics of a transitional water ecosystem using a geochemical modeling approach. Model. Earth Syst. Environ. Earth Sci. 2021, 7, 1813–1829. [Google Scholar] [CrossRef]
- Yuan, J.; Xu, F.; Deng, G.; Tang, Y.; Li, P. Hydrogeochemistry of shallow groundwater in a karst aquifer system of Bijie City, Guizhou Province. Water 2017, 9, 625. [Google Scholar] [CrossRef]
- Duan, S.; Tong, T.; Zheng, S.; Zhang, X.; Li, S. Achieving low-cost, highly selective nitrate removal with standard anion exchange resin by tuning recycled brine composition. Water Res. 2020, 173, 115571. [Google Scholar] [CrossRef]
- Volkov, V.I.; Chernyak, A.V.; Golubenko, D.V.; Tverskoy, V.A.; Lochin, G.A.; Odjigaeva, E.S.; Yaroslavtsev, A.B. Hydration and diffusion of H+, Li+, Na+, Cs+ ions in cation-exchange membranes based on polyethylene-and sulfonated-grafted polystyrene studied by NMR technique and Ionic conductivity Measurements. Membranes 2020, 10, 272. [Google Scholar] [CrossRef]
- Zaidi, F.K.; Nazzal, Y.; Jafri, M.K.; Naeem, M.; Ahmed, I. Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: A case study from northwestern Saudi Arabia. Environ. Monit. Assess. 2015, 187, 607. [Google Scholar] [CrossRef]
- Smith, C.; Oster, J.; Sposito, G.J.A.W.M. Potassium and magnesium in irrigation water quality assessment. Agric. Water Manag. 2015, 157, 59–64. [Google Scholar] [CrossRef]
- Jiang, Y. The contribution of human activities to dissolved inorganic carbon fluxes in a karst underground river system: Evidence from major elements and δ13CDIC in Nandong, Southwest China. J. Contam. Hydrol. 2013, 152, 1–11. [Google Scholar] [CrossRef]
- Ekklesia, E.; Shanahan, P.; Chua, L.; Eikaas, H. Associations of chemical tracers and faecal indicator bacteria in a tropical urban catchment. Water Res. 2015, 75, 270–281. [Google Scholar] [CrossRef]
- Cabral, A.C.; Stark, J.S.; Kolm, H.E.; Martins, C.C. An integrated evaluation of some faecal indicator bacteria (FIB) and chemical markers as potential tools for monitoring sewage contamination in subtropical estuaries. Environ. Pollut. 2018, 235, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Sameer, V.; Hampannavar, U.; Purandara, B. Assessment of chloride concentration in groundwater: A case study for Belgaum City. Int. J. Environ. Sci. 2011, 2, 271–280. [Google Scholar]
- Yin, H.; Xie, M.; Zhang, L.; Huang, J.; Xu, Z.; Li, H.; Jiang, R.; Wang, R.; Zeng, X. Identification of sewage markers to indicate sources of contamination: Low cost options for misconnected non-stormwater source tracking in stormwater systems. Sci. Total Environ. 2019, 648, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Koh, D.-C.; Kim, Y.S.; Jeen, S.-W.; Lee, J. Stable isotopes of water and nitrate for the identification of groundwater flowpaths: A review. Water 2020, 12, 138. [Google Scholar] [CrossRef]
- Nisi, B.; Raco, B.; Dotsika, E. Groundwater contamination studies by environmental isotopes: A review. In Threats to the Quality of Groundwater Resources; Springer: Berlin/Heidelberg, Germany, 2016; pp. 115–150. [Google Scholar]
- Wang, H.; Zhang, Q. Research advances in identifying sulfate contamination sources of water environment by using stable isotopes. Int. J. Environ. Res. Public Health 2019, 16, 1914. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Chen, J.; Zheng, X.; Sun, X. Reference ion method: A simple and fast method for quantitatively identifying the source of nitrate and denitrification rate in groundwater. Sci. Total Environ. 2021, 769, 144555. [Google Scholar] [CrossRef]
- Ji, X.; Xie, R.; Hao, Y.; Lu, J. Quantitative identification of nitrate pollution sources and uncertainty analysis based on dual isotope approach in an agricultural watershed. Environ. Pollut. 2017, 229, 586–594. [Google Scholar] [CrossRef]
- Minet, E.P.; Goodhue, R.; Meier-Augenstein, W.; Kalin, R.M.; Fenton, O.; Richards, K.G.; Coxon, C. Combining stable isotopes with contamination indicators: A method for improved investigation of nitrate sources and dynamics in aquifers with mixed nitrogen inputs. Water Res. 2017, 124, 85–96. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, X.; Gao, Y.; Xu, Z.; Yang, F.; Wen, X.; Wang, Y. Nitrogen source track and associated isotopic dynamic characteristic in a complex ecosystem: A case study of a subtropical watershed, China. Environ. Pollut. 2018, 236, 177–187. [Google Scholar] [CrossRef]
- Li, J.; Cao, R.; Lao, Q.; Chen, F.; Chen, C.; Zhou, X.; Meng, Y.; Zhu, Q. Assessing seasonal nitrate contamination by nitrate dual isotopes in a monsoon-controlled bay with intensive human activities in South China. Int. J. Environ. Res. Public Health 2020, 17, 1921. [Google Scholar] [CrossRef]
- Mu, D.; Li, P.; De Baets, B.; Li, D.; Li, Z.; He, S. A multi-perspective exploration of the salinization mechanisms of groundwater in the Guanzhong Basin, China. Sci. Total Environ. 2024, 957, 177421. [Google Scholar] [CrossRef]
- Essien, E.E.; Said Abasse, K.; Côté, A.; Mohamed, K.S.; Baig, M.M.F.A.; Habib, M.; Naveed, M.; Yu, X.; Xie, W.; Jinfang, S. Drinking-water nitrate and cancer risk: A systematic review and meta-analysis. Arch. Environ. Occup. Health 2022, 77, 51–67. [Google Scholar] [CrossRef]
- Hosseini, F.; Majdi, M.; Naghshi, S.; Sheikhhossein, F.; Djafarian, K.; Shab-Bidar, S. Nitrate-nitrite exposure through drinking water and diet and risk of colorectal cancer: A systematic review and meta-analysis of observational studies. Clin. Nutr. 2021, 40, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- Stayner, L.T.; Schullehner, J.; Semark, B.D.; Jensen, A.S.; Trabjerg, B.B.; Pedersen, M.; Olsen, J.; Hansen, B.; Ward, M.H.; Jones, R.R. Exposure to nitrate from drinking water and the risk of childhood cancer in Denmark. Environ. Int. 2021, 155, 106613. [Google Scholar] [CrossRef] [PubMed]
| Classification | Project | pH | TDS | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO3−-N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pore water n = 36 | Max | 8.4 | 8203.5 | 49.6 | 1805.1 | 769.2 | 342.7 | 4049.7 | 1089.2 | 842.2 | 101.7 |
| Min | 6.9 | 161.1 | 0.3 | 12.6 | 31.7 | 4.7 | 3.3 | 21.1 | 23.2 | 0.1 | |
| Mean | 7.7 | 716.6 | 4.1 | 69.9 | 134.4 | 27.7 | 123.0 | 122.4 | 256.1 | 23.2 | |
| Std | 0.4 | 1660.9 | 10.5 | 413.3 | 138.9 | 77.1 | 826.3 | 245.2 | 167.5 | 21.9 | |
| CV | 0.06 | 1.29 | 1.6 | 1.99 | 0.82 | 1.41 | 2.08 | 1.16 | 0.58 | 1.06 | |
| Fracture water = 20 | Max | 8.3 | 1224.8 | 7.1 | 139.3 | 261.1 | 50.7 | 186.1 | 221.7 | 444.3 | 70.1 |
| Min | 7.0 | 147.7 | 0.5 | 16.7 | 14.7 | 0.7 | 19.3 | 30.0 | 0.0 | 1.4 | |
| Mean | 7.6 | 562.2 | 2.9 | 61.4 | 99.7 | 18.6 | 90.5 | 106.7 | 167.0 | 20.4 | |
| Std | 0.7 | 275.2 | 1.8 | 33.4 | 65.6 | 12.7 | 50.8 | 55.3 | 126.5 | 19.2 | |
| CV | 0.1 | 0.49 | 0.61 | 0.54 | 0.66 | 0.66 | 0.56 | 0.52 | 0.76 | 0.94 | |
| Karst water = 6 | Max | 7.7 | 4551.5 | 30.3 | 84.6 | 1143.5 | 220.4 | 1934.1 | 382.2 | 475.5 | 121.0 |
| Min | 7.1 | 361.1 | 0.4 | 14.6 | 83.3 | 13.9 | 27.5 | 94.9 | 142.6 | 12.1 | |
| Mean | 7.5 | 743.5 | 10.1 | 45.9 | 314.9 | 68.2 | 392.5 | 182.5 | 335.3 | 44.7 | |
| Std | 0.2 | 1431.3 | 10.1 | 20.9 | 372.0 | 69.4 | 690.6 | 97.8 | 117.8 | 35.4 | |
| CV | 0.02 | 1.04 | 1.00 | 0.46 | 1.18 | 1.02 | 1.76 | 0.54 | 0.35 | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wang, X.; You, J.; Wang, Y.; Zhao, M.; Sun, P.; Fu, J.; Yu, Y.; Mao, K. Hydrochemical Formation Mechanisms and Source Apportionment in Multi-Aquifer Systems of Coastal Cities: A Case Study of Qingdao City, China. Sustainability 2025, 17, 5988. https://doi.org/10.3390/su17135988
Li M, Wang X, You J, Wang Y, Zhao M, Sun P, Fu J, Yu Y, Mao K. Hydrochemical Formation Mechanisms and Source Apportionment in Multi-Aquifer Systems of Coastal Cities: A Case Study of Qingdao City, China. Sustainability. 2025; 17(13):5988. https://doi.org/10.3390/su17135988
Chicago/Turabian StyleLi, Mingming, Xinfeng Wang, Jiangong You, Yueqi Wang, Mingyue Zhao, Ping Sun, Jiani Fu, Yang Yu, and Kuanzhen Mao. 2025. "Hydrochemical Formation Mechanisms and Source Apportionment in Multi-Aquifer Systems of Coastal Cities: A Case Study of Qingdao City, China" Sustainability 17, no. 13: 5988. https://doi.org/10.3390/su17135988
APA StyleLi, M., Wang, X., You, J., Wang, Y., Zhao, M., Sun, P., Fu, J., Yu, Y., & Mao, K. (2025). Hydrochemical Formation Mechanisms and Source Apportionment in Multi-Aquifer Systems of Coastal Cities: A Case Study of Qingdao City, China. Sustainability, 17(13), 5988. https://doi.org/10.3390/su17135988







