Spatiotemporal Variations in Soil Organic Carbon and Microbial Drivers in the Yellow River Delta Wetland, China
Abstract
1. Introduction
2. Materials and Methods
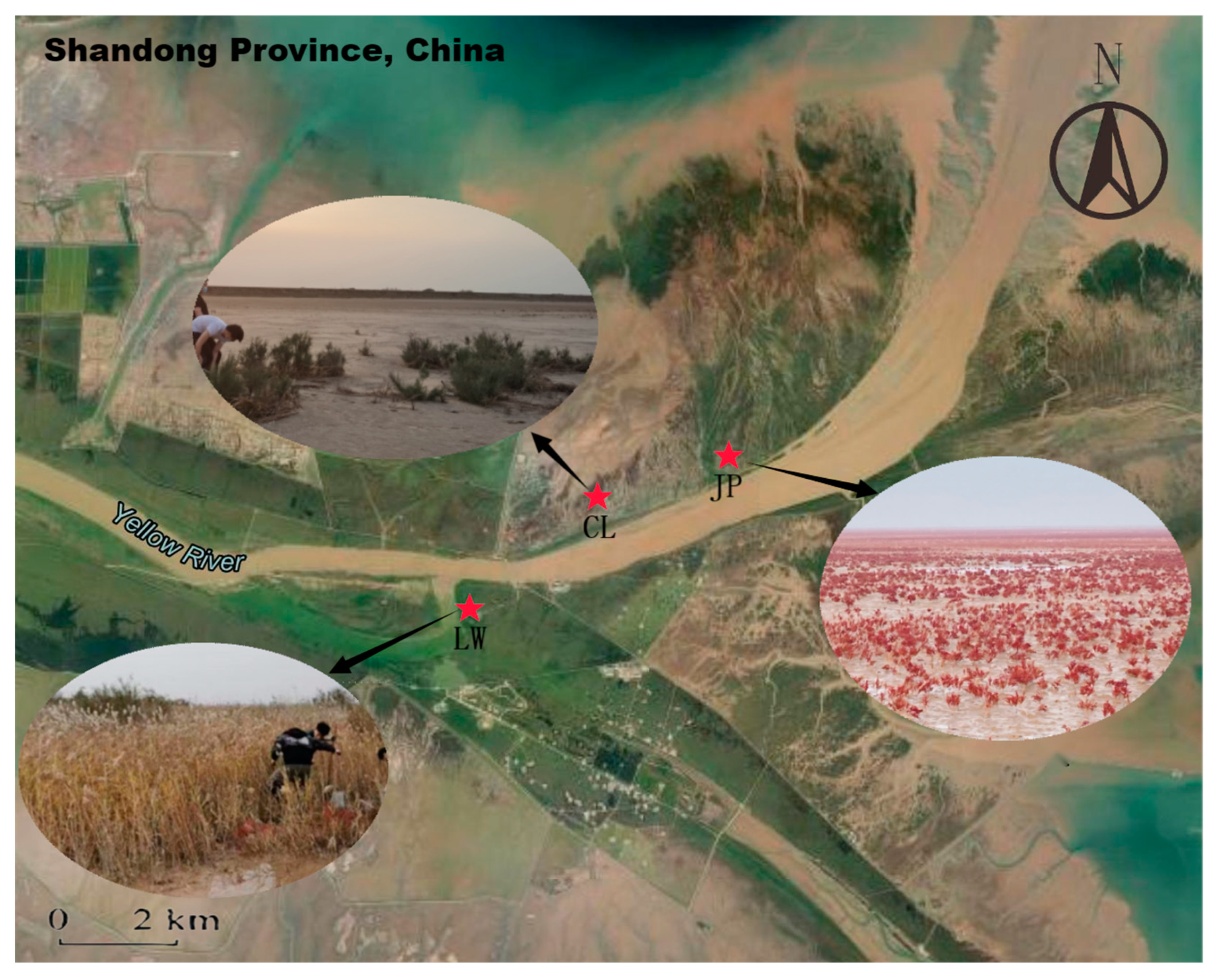
2.1. Study Area
2.2. Soil Sampling and Processing
2.3. Laboratory Analysis
2.4. DNA Extraction and Sequence Analyses
2.5. Statistical Analyses
3. Results
3.1. Soil Characteristics, Carbon Pool, and Carbon Mineralization
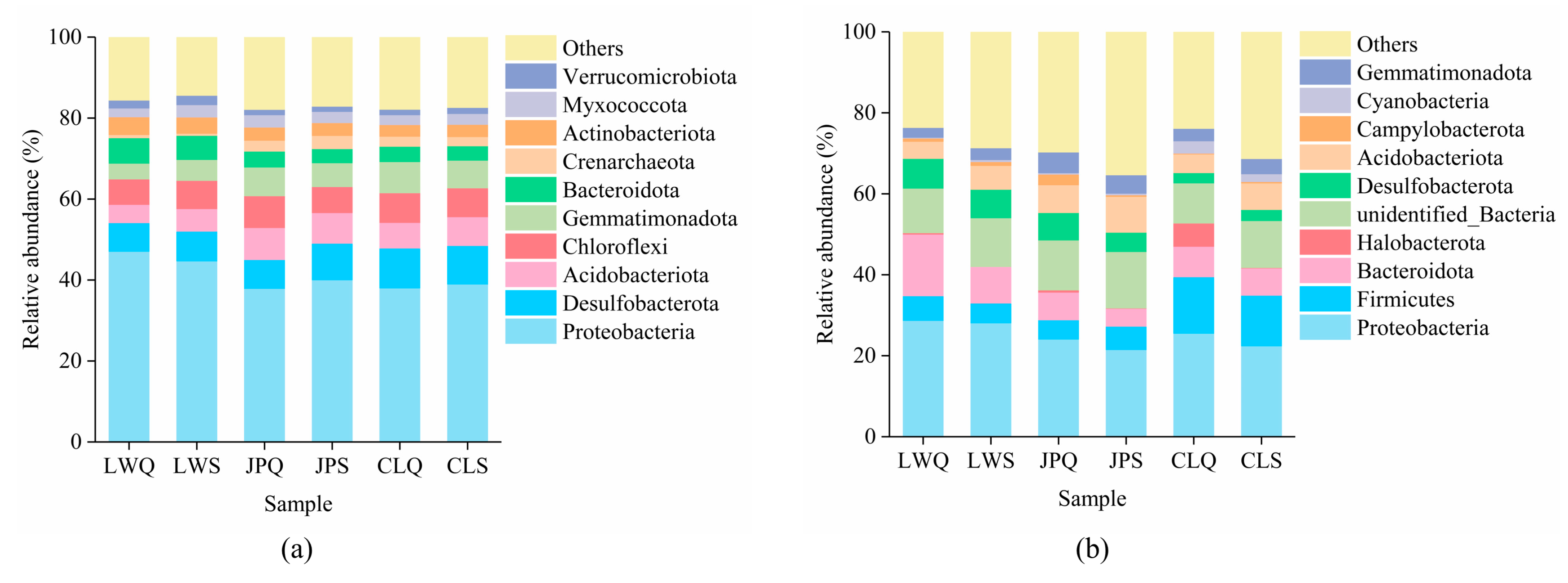
3.2. Variations in Soil Microbial Community Diversity, Composition, and Structure
3.3. Relationships Between the Soil Environment and the Microbial Communities
4. Discussion
4.1. Spatiotemporal Distribution Patterns of Wetland SOC Fractions
4.2. Effects of Soil Microbial Communities on Wetland SOC
4.3. Interactions Among Plants, Soil Environment, and Soil Microbes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertram, C.; Quaas, M.; Reusch, T.B.; Vafeidis, A.T.; Wolff, C.; Rickels, W.J. The blue carbon wealth of nations. Nat. Clim. Change 2021, 11, 704–709. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, Ü.; Zhang, L.; Anderson, C.J.; Jørgensen, S.E.; Brix, H.J. Wetlands, carbon, and climate change. Landsc. Ecol. 2013, 28, 583–597. [Google Scholar] [CrossRef]
- Wang, F.; Sanders, C.J.; Santos, I.R.; Tang, J.; Schuerch, M.; Kirwan, M.L.; Kopp, R.E.; Zhu, K.; Li, X.; Yuan, J.J.; et al. Global blue carbon accumulation in tidal wetlands increases with climate change. Natl. Sci. Rev. 2021, 8, nwaa296. [Google Scholar] [CrossRef] [PubMed]
- Erwin, K.L. Wetlands and global climate change: The role of wetland restoration in a changing world. Wetl. Ecol. Manag. 2009, 17, 71–84. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Z.; Li, P.; Xu, G.; Gao, H.; Cheng, Y.; Chang, E.; Yuan, S.; Zhang, Y.; Feng, Z. Spatial distribution of soil organic carbon and its influencing factors under the condition of ecological construction in a hilly-gully watershed of the Loess Plateau, China. Geoderma 2017, 296, 10–17. [Google Scholar] [CrossRef]
- Moomaw, W.R.; Chmura, G.; Davies, G.T.; Finlayson, C.; Middleton, B.A.; Natali, S.M.; Perry, J.; Roulet, N.; Sutton-Grier, A.E.J.W. Wetlands in a changing climate: Science, policy and management. Wetlands 2018, 38, 183–205. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, K.; Yang, Y.; Gao, B.; Zheng, H. Effects of biochar on the accumulation of necromass-derived carbon, the physical protection and microbial mineralization of soil organic carbon. Crit. Rev. Environ. Sci. Technol. 2024, 54, 39–67. [Google Scholar] [CrossRef]
- Kayranli, B.; Scholz, M.; Mustafa, A.; Hedmark, Å. Carbon storage and fluxes within freshwater wetlands: A critical review. Wetlands 2010, 30, 111–124. [Google Scholar] [CrossRef]
- Loisel, J.; Gallego-Sala, A.V.; Amesbury, M.; Magnan, G.; Anshari, G.; Beilman, D.; Benavides, J.; Blewett, J.; Camill, P.; Charman, D.J.; et al. Expert assessment of future vulnerability of the global peatland carbon sink. Nat. Clim. Change 2021, 11, 70–77. [Google Scholar] [CrossRef]
- Limpens, J.; Berendse, F.; Blodau, C.; Canadell, J.; Freeman, C.; Holden, J.; Roulet, N.; Rydin, H.; Schaepman-Strub, G. Peatlands and the carbon cycle: From local processes to global implications—A synthesis. Biogeosciences 2008, 5, 1475–1491. [Google Scholar] [CrossRef]
- Zhao, Q.; Bai, J.; Lu, Q.; Zhang, G. Effects of salinity on dynamics of soil carbon in degraded coastal wetlands: Implications on wetland restoration. Phys. Chem. Earth Parts A/B/C 2017, 97, 12–18. [Google Scholar] [CrossRef]
- Zhao, Q.; Bai, J.; Zhang, G.; Jia, J.; Wang, W.; Wang, X. Effects of water and salinity regulation measures on soil carbon sequestration in coastal wetlands of the Yellow River Delta. Geoderma 2018, 319, 219–229. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Zhang, L.; Ji, Z.; Shao, Y.; Zhou, H.; Bao, Y.; Qu, Y.; Liu, L. Comparison of rhizosphere bacterial communities of reed and Suaeda in Shuangtaizi River Estuary, Northeast China. Mar. Pollut. Bull. 2019, 140, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q. Methane emissions from wetlands: Biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Change Biol. 2013, 19, 1325–1346. [Google Scholar] [CrossRef]
- Hu, S.; Feng, W.; Shen, Y.; Jin, X.; Miao, Y.; Hou, S.; Cui, H.; Zhu, H. Greenhouse gases emissions and carbon budget estimation in horizontal subsurface flow constructed wetlands with different plant species. Sci. Total. Environ. 2024, 927, 172296. [Google Scholar] [CrossRef]
- Chomel, M.; Guittonny-Larchevêque, M.; Fernandez, C.; Gallet, C.; DesRochers, A.; Paré, D.; Jackson, B.G.; Baldy, V. Plant secondary metabolites: A key driver of litter decomposition and soil nutrient cycling. J. Ecol. 2016, 104, 1527–1541. [Google Scholar] [CrossRef]
- Yang, J.; Xie, B.; Zhang, D.; Tao, W. Climate and land use change impacts on water yield ecosystem service in the Yellow River Basin, China. Environ. Earth Sci. 2021, 80, 72. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Guggenberger, G.; Kleber, M.; Kandeler, E.; Kalbitz, K.; Scheu, S.; Eusterhues, K.; Leinweber, P. Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry. J. Plant Nutr. Soil Sci. 2008, 171, 61–82. [Google Scholar] [CrossRef]
- Ma, H.; Mo, L.; Crowther, T.W.; Maynard, D.S.; van den Hoogen, J.; Stocker, B.D.; Terrer, C.; Zohner, C.M. The global distribution and environmental drivers of aboveground versus belowground plant biomass. Nat. Ecol. Evol. 2021, 5, 1110–1122. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.; Parra-Saldívar, R. Soil carbon sequestration–An interplay between soil microbial community and soil organic matter dynamics. Sci. Total. Environ. 2022, 815, 152928. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, L.; Zhu, P.; Yang, S.; Guo, W.; Yu, X. Patterns and dynamics of the soil microbial community with gradual vegetation succession in the Yellow River Delta, China. Wetlands 2021, 41, 9. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, X.; Li, X.; Zhang, Y.; Jiang, L.; Li, J.; Guan, Z.; Cai, Y.; Liao, X. The rhizospheric microbial community structure and diversity of deciduous and evergreen forests in Taihu Lake area, China. PLoS ONE 2017, 12, e0174411. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Li, Q.; Zhao, C.; Feng, Y. Influence of plants and environmental variables on the diversity of soil microbial communities in the Yellow River Delta Wetland, China. Chemosphere 2021, 274, 129967. [Google Scholar] [CrossRef]
- Li, D.; Ning, Z.; Chen, G.; Li, Y.n.; Cui, B.; Wang, Q.; Xie, T. The effect of land use and land cover on soil carbon storage in the Yellow River Delta, China: Implications for wetland restoration and adaptive management. J. Environ. Manag. 2024, 367, 122097. [Google Scholar] [CrossRef] [PubMed]
- Baustian, M.M.; Stagg, C.L.; Perry, C.L.; Moss, L.C.; Carruthers, T.J. Long-term carbon sinks in marsh soils of coastal Louisiana are at risk to wetland loss. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG005832. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Qin, G.; Zhang, J.; Zhou, J.; Wu, J.; Zhang, L.; Thapa, P.; Sanders, C.J.; Santos, I.R.; et al. Coastal blue carbon in China as a nature-based solution toward carbon neutrality. Innovation 2023, 4, 100481. [Google Scholar] [CrossRef]
- Durán-Viseras, A.; Sánchez-Porro, C.; Viver, T.; Konstantinidis, K.T.; Ventosa, A. Discovery of the streamlined haloarchaeon Halorutilus salinus, comprising a new order widespread in hypersaline environments across the world. mSystems 2023, 8, e01198-22. [Google Scholar] [CrossRef]
- Duan, Q.; Zhu, Z.; Wang, B.; Chen, M. Recent progress on the salt tolerance mechanisms and application of tamarisk. Int. J. Mol. Sci. 2022, 23, 3325. [Google Scholar] [CrossRef]
- Yoo, Y.; Lee, H.; Lee, J.; Khim, J.S.; Kim, J.-J. Insights into saline adaptation strategies through a novel halophilic bacterium isolated from solar saltern of Yellow sea. Front. Mar. Sci. 2023, 10, 1229444. [Google Scholar] [CrossRef]
- Li, X.; Chen, K.; Zhang, Q.; Zhang, X.; Wang, X.; Zhao, M.; Li, P.; Xie, B.; Han, G.; Song, W.; et al. The response of soil carbon mineralization losses to changes in rainfall frequency is seasonally dependent in an estuarine saltmarsh. Soil Biol. Biochem. 2024, 197, 109538. [Google Scholar] [CrossRef]
- Song, J.; Liang, Z.; Li, X.; Wang, X.; Chu, X.; Zhao, M.; Zhang, X.; Li, P.; Song, W.; Huang, W.; et al. Precipitation changes alter plant dominant species and functional groups by changing soil salinity in a coastal salt marsh. J. Environ. Manag. 2024, 368, 122235. [Google Scholar] [CrossRef]
- Van der Stel, A.-X.; Wösten, M.M. Regulation of respiratory pathways in Campylobacterota: A review. Front. Microbiol. 2019, 10, 1719. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, L.; Bucci, A.; Maiuro, L.; Bulgarelli, D.; Naclerio, G. Unraveling the composition of the root-associated bacterial microbiota of Phragmites australis and Typha latifolia. Front. Microbiol. 2018, 9, 1650. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zheng, W.; Shi, H.; Wang, Y.; Ding, D. Spatiotemporal heterogeneity of coastal wetland ecosystem services in the Yellow River Delta and their response to multiple drivers. Remote. Sens. 2023, 15, 1866. [Google Scholar] [CrossRef]
- Creamer, C.; Waldrop, M.; Stagg, C.; Manies, K.L.; Baustian, M.M.; Laurenzano, C.; Aw, T.G.; Haw, M.; Merino, S.; Schoolmaster, D.R., Jr.; et al. Vegetation loss following vertical drowning of Mississippi River deltaic wetlands leads to faster microbial decomposition and decreases in soil carbon. J. Geophys. Res. Biogeosci. 2024, 129, e2023JG007832. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, S.; Wang, J. Examining the influences of urbanization on carbon dioxide emissions in the Yangtze River Delta, China: Kuznets curve relationship. Sci. Total. Environ. 2019, 675, 472–482. [Google Scholar] [CrossRef]
- Ma, Z.; Duan, X.; Wang, L.; Wang, Y.; Kang, J.; Yun, R. A Scenario Simulation Study on the Impact of Urban Expansion on Terrestrial Carbon Storage in the Yangtze River Delta, China. Land 2023, 12, 297. [Google Scholar] [CrossRef]
- Su, Y.Z.; Wang, X.F.; Yang, R.; Lee, J. Effects of sandy desertified land rehabilitation on soil carbon sequestration and aggregation in an arid region in China. J. Environ. Manag. 2010, 91, 2109–2116. [Google Scholar] [CrossRef]
- Ye, S.; Laws, E.; Yuknis, N.; Ding, X.; Yuan, H.; Zhao, G.; Wang, J.; Yu, X.; Pei, S.; DeLaune, R.D. Carbon sequestration and soil accretion in coastal wetland communities of the Yellow River Delta and Liaohe Delta, China. Estuaries Coasts 2015, 38, 1885–1897. [Google Scholar] [CrossRef]
- Krull, E.S.; Baldock, J.A.; Skjemstad, J.O. Importance of mechanisms and processes of the stabilisation of soil organic matter for modelling carbon turnover. Funct. Plant Biol. 2003, 30, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Xinwei, Z.; Yunchao, Z.; Qiulan, F. Main influencing factors of soil particle distribution in the karst basin. Catena 2023, 224, 107002. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.; Yu, Y.; Mitchell, M.J.; Zhang, L. Soil organic carbon of degraded wetlands treated with freshwater in the Yellow River Delta, China. J. Environ. Manag. 2011, 92, 2628–2633. [Google Scholar] [CrossRef]
- Gill, R.; Burke, I.C.; Milchunas, D.G.; Lauenroth, W.K. Relationship between root biomass and soil organic matter pools in the shortgrass steppe of eastern Colorado. Ecosystems 1999, 2, 226–236. [Google Scholar]
- Ghimire, R.; Thapa, V.R.; Cano, A.; Acosta-Martinez, V. Soil organic matter and microbial community responses to semiarid croplands and grasslands management. Appl. Soil Ecol. 2019, 141, 30–37. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, F.; Huang, Y.; Tang, J. Variability of dissolved organic matter in two coastal wetlands along the Changjiang River Estuary: Responses to tidal cycles, seasons, and degradation processes. Sci. Total. Environ. 2022, 807, 150993. [Google Scholar] [CrossRef]
- Jin, X.; He, Y.; Kirumba, G.; Hassan, Y.; Li, J. Phosphorus fractions and phosphate sorption-release characteristics of the sediment in the Yangtze River estuary reservoir. Ecol. Eng. 2013, 55, 62–66. [Google Scholar] [CrossRef]
- Zhang, L.; Gałka, M.; Kumar, A.; Liu, M.; Knorr, K.-H.; Yu, Z.-G. Plant succession and geochemical indices in immature peatlands in the Changbai Mountains, northeastern region of China: Implications for climate change and peatland development. Sci. Total. Environ. 2021, 773, 143776. [Google Scholar] [CrossRef]
- Kalbitz, K.; Solinger, S.; Park, J.-H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Yu, L.; Zhuang, T.; Bai, J.; Wang, J.; Yu, Z.; Wang, X.; Zhang, G. Effects of water and salinity on soil labile organic carbon in estuarine wetlands of the Yellow River Delta, China. Ecohydrol. Hydrobiol. 2020, 20, 556–569. [Google Scholar] [CrossRef]
- Ansola, G.; Arroyo, P.; de Miera, L.E.S. Characterisation of the soil bacterial community structure and composition of natural and constructed wetlands. Sci. Total. Environ. 2014, 473, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ravenschlag, K.; Sahm, K.; Pernthaler, J.; Amann, R. High bacterial diversity in permanently cold marine sediments. Appl. Environ. Microbiol. 1999, 65, 3982–3989. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Zhang, G.; Li, S.; Zhang, W.; Chen, X.; Sun, L.; Zhang, B.; Liu, G.; Chen, T. The diversity and biogeography of the communities of Actinobacteria in the forelands of glaciers at a continental scale. Environ. Res. Lett. 2016, 11, 054012. [Google Scholar] [CrossRef]
- Chen, X.; Wei, W.; Wang, J.; Li, H.; Sun, J.; Ma, R.; Jiao, N.; Zhang, R. Tide driven microbial dynamics through virus-host interactions in the estuarine ecosystem. Water Res. 2019, 160, 118–129. [Google Scholar] [CrossRef]
- Zada, S.; Rafiq, M.; Sajjad, W.; Afzal, M.; Su, Z.; Lihua, L. Isolation and characterization of ureolytic calcifying bacteria from methane hydrate-bearing marine sediments for bio-cementation application. Phys. Chem. Earth Parts A/B/C 2025, 137, 103808. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Y.; Shi, T.; Zhang, X.; Huang, G.; Gong, J. Intertidal zonation affects diversity and functional potentials of bacteria in surface sediments: A case study of the Golden Bay mangrove, China. Appl. Soil Ecol. 2018, 130, 159–168. [Google Scholar] [CrossRef]
- Johnson, A.; Nyman, A.; Åström, M.; Dopson, M. Regional variation in Swedish acid sulfate soil microbial communities is influenced by temperature and geochemistry. Eur. J. Soil Sci. 2024, 75, e13452. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, D.; Tian, X.; Bi, X.; Zhou, Z.; Luo, F.; Ning, R.; Li, J. Exploring the factors influencing the carbon sink function of coastal wetlands in the Yellow River Delta. Sci. Rep. 2024, 14, 28938. [Google Scholar] [CrossRef]
- Plugge, C.M.; Zhang, W.; Scholten, J.C.; Stams, A.J.M. Metabolic flexibility of sulfate-reducing bacteria. Front. Microbiol. 2011, 2, 81. [Google Scholar] [CrossRef]
- Zhen-Yu, W.; Yuan-Zheng, X.; Dong-Mei, G.; Feng-Min, L.; Bao-Shan, X. Microbial community characteristics in a degraded wetland of the Yellow River Delta. Pedosphere 2010, 20, 466–478. [Google Scholar]
- Miki, T.; Ushio, M.; Fukui, S.; Kondoh, M. Functional diversity of microbial decomposers facilitates plant coexistence in a plant–microbe–soil feedback model. Proc. Natl. Acad. Sci. USA 2010, 107, 14251–14256. [Google Scholar] [CrossRef] [PubMed]
- Miki, T. Microbe-mediated plant–soil feedback and its roles in a changing world. Ecol. Res. 2012, 27, 509–520. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Creamer, C.; Whitaker, J. Deconstructing the microbial necromass continuum to inform soil carbon sequestration. Funct. Ecol. 2022, 36, 1396–1410. [Google Scholar] [CrossRef]
- Ferlauto, M.; Schmitt, L.; Burghardt, K. Legacy effects of long-term autumn leaf litter removal slow decomposition rates and reduce soil carbon in suburban yards. Plants People Planet 2024, 6, 875–884. [Google Scholar] [CrossRef]
- Afzal, M.; Muhammad, S.; Tan, D.; Kaleem, S.; Khattak, A.A.; Wang, X.; Chen, X.; Ma, L.; Mo, J.; Muhammad, N.; et al. The effects of heavy metal pollution on soil nitrogen transformation and rice volatile organic compounds under different water management practices. Plants 2024, 13, 871. [Google Scholar] [CrossRef]
- Chen, D.; Adjei, L.A.; Samwini, A.M.-N.; Addo, F.G.; Baah, W.A.; Bofah-Buoh, R.; Manirakiza, B. Effects of Cadmium Exposure on Epiphytic Bacterial Communities and Water Quality in Vegetated Mesocosmic Wetlands; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Afzal, M.; Tang, C.; Yu, M.; Muhammad, N.; Zhao, H.; Xu, J. Water regime is important to determine cadmium toxicity on rice growth and rhizospheric nitrifier communities in contaminated paddy soils. Plant Soil 2022, 472, 609–628. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, H.; Gao, Y.; Zheng, L.; Wang, J.; Bai, J. Alterations of bacterial and archaeal communities by freshwater input in coastal wetlands of the Yellow River Delta, China. Appl. Soil Ecol. 2020, 153, 103581. [Google Scholar] [CrossRef]
- Canfora, L.; Bacci, G.; Pinzari, F.; Lo Papa, G.; Dazzi, C.; Benedetti, A. Salinity and bacterial diversity: To what extent does the concentration of salt affect the bacterial community in a saline soil? PLoS ONE 2014, 9, e106662. [Google Scholar] [CrossRef]
- Balasooriya, W.; Denef, K.; Peters, J.; Verhoest, N.; Boeckx, P. Vegetation composition and soil microbial community structural changes along a wetland hydrological gradient. Earth Syst. Sci. 2008, 12, 277–291. [Google Scholar] [CrossRef]
- Guan, B.; Xie, B.; Yang, S.; Hou, A.; Chen, M.; Han, G. Effects of five years’ nitrogen deposition on soil properties and plant growth in a salinized reed wetland of the Yellow River Delta. Ecol. Eng. 2019, 136, 160–166. [Google Scholar] [CrossRef]
- Yu, J.; Zhan, C.; Li, Y.; Zhou, D.; Fu, Y.; Chu, X.; Xing, Q.; Han, G.; Wang, G.; Guan, B.; et al. Distribution of carbon, nitrogen and phosphorus in coastal wetland soil related land use in the Modern Yellow River Delta. Sci. Rep. 2016, 6, 37940. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Rui, J.; Niu, H.; Heděnec, P.; Li, J.; He, Z.; Wang, J.; Cao, W.; Li, X. The differentiation of soil bacterial communities along a precipitation and temperature gradient in the eastern Inner Mongolia steppe. Catena 2017, 152, 47–56. [Google Scholar] [CrossRef]
- Zhang, K.; Delgado-Baquerizo, M.; Zhu, Y.-G.; Chu, H. Space is more important than season when shaping soil microbial communities at a large spatial scale. mSystems 2020, 5. [Google Scholar] [CrossRef]



| Sample | ACE | Chao1 | Simpson | Shannon | OTU | |
|---|---|---|---|---|---|---|
| Spring | LWQ | 2219 | 2209 | 0.976 | 8.47 | 2006 |
| LWS | 4046 | 4026 | 0.993 | 9.42 | 3635 | |
| JPQ | 2507 | 2502 | 0.926 | 8.57 | 2338 | |
| JPS | 3947 | 4198 | 0.976 | 9.06 | 3617 | |
| CLQ | 3776 | 4410 | 0.918 | 8.81 | 2663 | |
| CLS | 2635 | 3206 | 0.896 | 7.12 | 1450 | |
| Autumn | LWQ | 4398 | 4370 | 0.995 | 9.82 | 3687 |
| LWS | 5095 | 6700 | 0.996 | 9.73 | 3896 | |
| JPQ | 3603 | 3506 | 0.982 | 8.18 | 3027 | |
| JPS | 2387 | 2302 | 0.962 | 6.74 | 2010 | |
| CLQ | 4281 | 4170 | 0.988 | 9.05 | 3732 | |
| CLS | 3516 | 3364 | 0.964 | 7.73 | 2897 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, J.; Li, L.; Guo, Y.; Guo, B.; Zhao, C. Spatiotemporal Variations in Soil Organic Carbon and Microbial Drivers in the Yellow River Delta Wetland, China. Sustainability 2025, 17, 5188. https://doi.org/10.3390/su17115188
Wang X, Li J, Li L, Guo Y, Guo B, Zhao C. Spatiotemporal Variations in Soil Organic Carbon and Microbial Drivers in the Yellow River Delta Wetland, China. Sustainability. 2025; 17(11):5188. https://doi.org/10.3390/su17115188
Chicago/Turabian StyleWang, Xinghua, Jun Li, Luzhen Li, Yanke Guo, Beibei Guo, and Changsheng Zhao. 2025. "Spatiotemporal Variations in Soil Organic Carbon and Microbial Drivers in the Yellow River Delta Wetland, China" Sustainability 17, no. 11: 5188. https://doi.org/10.3390/su17115188
APA StyleWang, X., Li, J., Li, L., Guo, Y., Guo, B., & Zhao, C. (2025). Spatiotemporal Variations in Soil Organic Carbon and Microbial Drivers in the Yellow River Delta Wetland, China. Sustainability, 17(11), 5188. https://doi.org/10.3390/su17115188






