Abstract
Conservation agriculture is increasingly recognized as a sustainable alternative to conventional farming in temperate regions due to its benefits in terms of reducing soil erosion, enhancing water retention, and mitigating climate change. Despite these benefits, these practices are not broadly adopted, partially due to perceived weed management challenges in conservation systems. This paper explores how a conservation system that uses cover crops and non-inversion tillage (chiselling) influences the weed flora abundance and evolution before cover crop termination and over a complete rotation cycle (sunflower–winter wheat–maize–sunflower) in southeastern Romania when compared to conventional tillage (ploughing). Overall, the conservation system significantly reduced weed density by 31%, preserving a higher diversity and evenness (H′ = 0.75, E = 0.46) by the end of the rotation cycle and an evenly distributed weed community compared to the conventional system, where the opportunistic species Veronica hederifolia exhibited dominance.
1. Introduction
The adoption of practices specific to conservation agriculture are increasing [1,2]. Prestele et al. (2018) [3] suggest that the area of arable land globally utilising these practices ranges between 122 and 215 million hectares, i.e., between 9% and 15% of the arable land globally. Projections indicate a potential expansion to 38–81% (533–1130 million hectares) of global arable land, most likely amid aridization, an effect of climate change, with conservation agriculture being an effective way to mitigate its impact while ensuring sufficient food resources and sustainable management of soil resources [4].
The practice of conservation agriculture is one of the most important strategies to combat the phenomena associated with soil degradation, favouring better organic matter content, higher fertility, and increased soil moisture retention efficiency [5,6,7,8,9]. Moreover, it also contributes to the optimization of fertiliser inputs [10], carbon sequestration, greenhouse gas reduction [11,12,13], and the reduction of weed seed reserves in the soil profile [14]. In Romania, previous research focused on the adoption of reduced tillage as a conservation agriculture practice applied in classical rotation systems, without the use of cover crops [8,15,16].
Conventional tillage is an effective short-term weed control practice because it mechanically suppresses weeds by disturbing their root systems and buries seeds outside their optimal germination zone, thereby preventing emergence. Ploughing, however, is often associated with soil degradation and soil fertility depletion and can cause reccuring weed emergence issues [17,18]. In contrast, conservation agriculture aims to develop a more sustainable agroecosystem and reduce weed pressure in the long term, by reducing the soil profile containing a seed weed reservoir at variying depths [14,19]. However, adopting reduced tillage systems poses a challenge in terms of favouring small-seeded weed species, with seeds that germinate readily on the soil surface and weeds that reproduce via perennial propagules [18,20,21,22]. Effective weed control in conservation agriculture may involve the long-term use of a narrow range of herbicide modes of action without a mechnical alternative, potentially shifting weed populations toward more resilient or herbicide-resistant species [23]. Cover crops have proven to be effective suppressors [21,24,25]; in addition to weed control, they also contribute to reducing pesticide residues in soil by stimulating microbial degradation [26].
There have been several recent studies investigating the integration of cover crops as a conservation agriculture practice in combination with minimum tillage to improve fertility and weed control, demonstrating clear tangible benefits for weed control [27,28]. Cover crops and their plant residues can influence weed population dynamics, especially in no-till systems, either chemically through allelopathic processes [29,30] or physically by preventing their emergence [31,32]. According to Gerhards and Schappert (2020) [33], cover crops can suppress the emergence of weeds by up to 90% between two main crops. The research of Zannopoulos et al. (2024) [34] showed that legume cover crops were able to reduce weed biomass by 50%. In southwest Germany, Weber et al. (2017) [17] found that cover crops reduced weed density by 51 plants m−2 in a no-tillage organic soybean system. Schappert et al. (2019) [35] tested different winter cover crops and cover crop mixtures for weed control and found an efficiency ranging of 57% for multi-species cover crop mixtures, 72% for a straight Avena strigosa Schreb. (black oat) mixtures, and 83% for a straight Raphanus sativus var. oleiformis Pers. (oilseed radish) mixture. Similarly, Leskovšek et al. (2025) [32] found that cover crops can reduce weed biomass by up to 85%, depending on cover crop species, with oilseed radish being the most effective. Conversely, Baraibar et al. (2018) [24] found that legume cover crops are less suppressive when compared to grass cover crops and mixtures, emphasizing the importance of species selections. However, the authors also state that cover crops are a suitable tool in managing weed reserves, reducing the weed pressure for future cash crops. In a maize cropping system in Switzerland, Wittwer et al. (2017) [36] found no differences in weed infestation between different cover crop treatments, i.e., legume, non-legume, and mixtures. If species within a cover crop mixture are selected carefully for their environment, this can aid their management. Büchi et al. (2020) [37], in a four-year study in western Switzerland, found that frost-killed cover crops reduce weed cover by up to 10%, depending on the species. The authors also highlight that the effectiveness of cover crops in reducing weed coverage varied across years. In a review study, Kocira et al. (2020) [27] provide comprehensive evidence that legume cover crop efficacy in reducing weed density ranges from 10% to 96%, depending on the species, agricultural practices, and field conditions.
Single- and mixed-species cover crops can aid weed management within an agricultural system; however, the effects of cover crops on weed control remain contradictory in various studies, highlighting the impact of the environment, field-specific conditions, weed species composition, crop rotation, and the use of different management systems. Effective weed control is one of the two main challenges in adopting conservative practices in Europe, along with cover crop management strategies [38].
This research aimed to determine the efficacy of two management systems, i.e., conservation (minimum tillage and cover crops) and conventional tillage (ploughing without cover crops) systems, on weed flora abundance and species diversity evolution when assessed before cover crop termination, over a complete rotation cycle (i.e., sunflower–winter wheat–maize–sunflower) under southeastern Romanian environmental conditions.
2. Materials and Methods
2.1. Experimental Site, Design, and Crop Management
The study was conducted from 2021 to 2024 at the Moara Domnească Agricultural Research and Development Didactic Station (ARDDS Moara Domnească), southeastern Romania (44°29′36″ N, 26°15′29″ E). The experiment was designed using randomized blocks. Each cash crop was sown per the management system in three replicated blocks (i.e., three blocks × six plots). The soil type was Chromic Luvisol with a clay-loamy texture [39].
The conservation management system (CONS) consisted of non-inversion tillage (chisel plough) and the use of a cover crop mixture. The conventional system (CONV) consisted of ploughing the plots down to a depth of a minimum of 25 cm, without cover crops. Cash crop rotation over the four years was Helianthus annuus–Triticum aestivum–Zea mays–Helianthus annuus (sunflower–wheat–maize–sunflower). Cash crops were managed by standard commercial pratices and technologies applied at the Moara Domnească Agricultural Research and Development Didactic Station. Maize and sunflower plots were fertilized using 36 kg ha−1 of nitrogen and phosphorus before sowing. Weed control in sunflower and maize crops was carried out by a preemergence application of S-metolachlor 312.5 g L−1 + terbuthylazine 187.5 g L−1 (Gardoprim Plus Gold 500 SC, produced by Syngenta, Basel, Switerland), followed by a post-emergence application of mesotrione 75 g L−1 + nicosulfuron 30 g L−1 (Elumis OD, produced by Syngenta) in maize and 40 g L−1 imazamox (Listego SL, produced by BASF, Ludwigshafen, Germany) in sunflower during crops’ vegetative development. Weed control in wheat plots was carried out using fluroxypyr 135 g L−1 + thifensulfuron metil 30 g L−1 + metsulfuron metil 5 g L−1 (Omnera OD, produced by FMC Agricultural Solutions, Philadelphia, PA, USA). Herbicides were applied to maize, sunflower, and wheat crops in both management systems (CONV and CONS) according to the host farm’s management protocol. This approach was used to reflect farm conditions as closely as possible and to keep the outcomes practically relevant in the regional agricultural context. Post-emergence herbicide treatments were applied at BBCH 16–17 for maize and at BBCH 14–16 for sunflower. For wheat, spring treatment with herbicides was conducted during BBCH 29–30.
A cover crop mixture with the following species composition (expressed as percentage by weight) was used: 25% Lolium perenne (perennial ryegrass); 20% Festulolium (hybrid between Festuca and Lolium sp.); 20% Festuca pratensis (meadow fescue); 10% Phleum pratense (timothy-grass); 13% Festuca rubra (red fescue); 12%—Trifolium repens (white clover). The mixture was sown at a rate of 30 kg ha−1; drilling occurred at the end of October in 2020 and in early November 2021, 2022, and 2023, parallel with the winter wheat drilling operation. The cover crop preceded the spring crops (maize and sunflower) within the rotation and were terminated mechanically in early April 2021, 2022, and 2024, and at the end of April 2023, before the spring crops were sown. Mechanical termination was performed as part of the seedbed preparation for spring crops. Using a soil cultivator, a spring tillage was conducted to incorporate the biomass of the cover crop into the top 10 cm of soil in a non-inversion manner. In the conservative system (CONS), the cover crop served as an additional method for weed suppression, along with the uniform application of herbicides in both systems.
The study area is classified as a temperate continental climate, characterized by an annual precipitation of ~550 mm and an average annual temperature of 10.5 °C. Weather conditions in 2020–2021 were favourable to cover crop establishment and development in terms of rainfall. Moreover, this was the year with the most abundant rainfall (764.3 mm cumulated and 422.2 mm during the cover crop growing season) (Table 1). In 2021–2022 and 2022–2023, the total amount of precipitation during the cover crop growing season (October–April) (322.3 mm) was close to the multiannual mean, while 2023–2024 recorded the lowest cumulative rainfall (206.8 mm) during October–April. In the sowing window (late October–November), average temperatures were above the long-term average, and winter months (December–February) were warmer than the historical average.

Table 1.
Weather data, 2021–2024, ARDDS Moara Domnească, Ilfov.
2.2. Weed and Cover Crop Assessment
Cover crop plant density (m−2), weed species identification, and density (m−2) were determined once annually, in the spring (in early April 2021, 2022, and 2024 and in late April 2023), two days prior to seedbed preparation, on the plots that were to be sown with spring crops, before cover crop termination. Two quadrats (each 50 × 50 cm in size, i.e., 0.25 m2) were randomly positioned along the centre of each plot in both systems (2 samples × 18 plots). To ensure consistency, weed and cover crop density was converted and reported as number of plants m−2.
2.3. Statistical Analyses
To describe the diversity in CONV and CONS plots, the following three indices were calculated: species richness (S: total number of species), Shannon’s diversity (H′), and Pielou’s evenness (E). Shannon’s diversity index (H′) and Pielou’s evenness (E) were calculated using the following equations:
where H′ is Shannon’s diversity, S is total number of species, N is total number of plants per plot, and n is the number of plants per species per plot [40,41]. Evenness (E) values range between 0 and 1.0; a value of 0 corresponds to a community of one species (total dominance), and a value of 1.0 to a community.
H′ = (N ln N − ∑n ln n) N−1,
E = H′/ln(S),
Frequency (F), measuring the occurrence of a particular species across the experimental field, was also analysed following [41,42]:
where F(%) is weed frequency, ∑Zi is number of sampling units containing i species, and n is the total number of sampling units.
F(%) = (∑Zi/n) × 100,
The weed assessment data were subjected to factorial analysis of variance (ANOVA) considering ‘management system’ (i.e., CONV and CONS) and ‘year’ as fixed factors. Treatment means obtained by ANOVA were compared using LSD procedures at a p = 0.05 level of significance.
3. Results
Regardless of season, species richness was lower under CONS compared to CONV (Table 2). Across all experimental plots, the weed flora identified throughout the 4-year period (2021 to 2024) consisted of twelve weed species from ten botanical families (Table 3).

Table 2.
Weed diversity in conventional (CONV) and conservation (CONS) management systems (data are plot mean per year).

Table 3.
Weed species and their frequency as identified across conventional (CONV) and conservation (CONS) system plots.
The total number of species in CONS plots started as low in 2021 (5.5 species compared to 8.5 species under CONV) and fluctuated over the years from 4 (2022) to 6 weed species (2023). Under CONV, species richness started high (S = 8.5, 2021) and ranged throughout the next 3 years from 4 (2024) to 7.5 weed species (2023). In the first two years, the CONS system exhibited lower richness (S) and diversity (H′) compared to CONV; however, in 2023 and 2024, diversity (H′) was higher in CONS at lower or similar richness. Over the four-year period, CONV showed a downward trend in weed diversity, with a consistent drop in 2024 (H′ = 0.35), while in CONS the pattern of change over the years fluctuated. Under both management systems, in the first 3 years, Pielou’s evenness (E) had moderate values from 0.65 (2023, in CONV) to 0.91 (2021, in CONS). Evenness (E) was consistently higher in CONS, suggesting that the management system enabled a better balance of weed community, suppressing the dominance of certain weed species. Moreover, the CONV system had a consistent decline in evenness (E) over the 4-year period, from 0.81 in 2021 to 0.25 in 2024, suggesting that the system favoured the dominance of certain weed species. Overall (average of 4 years), species richness (S) was lower under CONS (S = 5); the weed community was slightly more diverse (H′ = 1.16) and more evenly distributed (E = 0.72) compared to the CONV system, with higher species richness (S = 6.3) as well as lower weed diversity (H′ = 1.14) and evenness (E = 0.62) (Table 2).
Several weed species (Cirsium arvense (L.) Scop., Fumaria officinalis L., Stellaria media (L.) Vill., and Polygonum aviculare L.) declined in spatial distribution (F) under the CONS system, and over the four seasons they became less widespread across these plots. However, these species had a low (<25%) or medium (25–75%) spatial distribution (Table 3). The sharpest drop under CONS was observed for C. arvense (L.) Scop. The species presence frequency (F) in 2022 was 58.3% under CONV and 25% under CONS, while in 2023, it was 16.7% under CONV and completely absent under the CONS system. F. officinalis L. was low-medium widespread in the CONS plots over the first three seasons. Thus, the species spatial distribution (F) ranged from 16.7% (2021 and 2023) to 33.3% (2022). The species was less frequently present across plots under CONS, with a reduced spread (8.3%) recorded only in 2023 (Table 3).
S. media (L.) Vill. had a higher spatial distribution (F) under the CONS system (33.3%) compared to the CONV system (16.7%), but only for the first season (2021). In the following years, the species was completely absent in CONS plots and had a spatial distribution (F) of 25% under the CONV system in 2023 (Table 3). P. aviculare L. had a medium but similar spatial distribution (F) under both systems in 2021 and 2023 (66.7% and 50%, respectively) and experienced a decline across the four seasons (Table 3), with a sharper drop in 2024 under CONS (8.3%).
Over the four years, Veronica hederifolia L., Capsella bursa-pastoris (L.) Medik., Matricaria chamomilla L., and Lamium purpureum L. were the most abundant weed species under both management systems (Table 3). Most of these species remained well-established and frequently observed (F > 75%) across the four years (2021–2024). V. hederifolia was the dominant weed species, the most widely distributed regardless of the management system. The species was highly adaptive under both the CONV and the CONS system, and its presence frequency (F) ranged from 83.3% to 100%. C. bursa-pastoris and M. chamomilla had a wider spatial distribution (F) under the CONS system across the period. Moreover, both species showed a clear increasing trend in spatial distribution (F) under the conservation system over the years. L. purpureum was widespread in 2021 and 2023, was completely absent in 2022, and had a spatial distribution that reduced sharply in 2024 under both systems. Initially, the species was more frequently occurring under CONS (2021); however, by 2024 it reached a lower distribution compared to CONV. In this study, Rumex sp. L. (8.3% in 2021 and 2022), Galium aparine L. (8.3% in 2023), and Setaria viridis (L.) P. Beauv. (33.3% in 2021) had the lowest spatial distribution in the weed community under CONV and were completely absent in the CONS plots (Table 3).
The ANOVA of the weed density indicated a significant ‘management system’ (System), ‘year’, and their interaction effect (Table 4). The effect of CONS on weed density, using cover crops and non-inversion tillage, was distinctly significant (p ≤ 0.01) but dependent on climatic conditions, while the interaction ‘system × year‘ was also distinctly significant (p ≤ 0.01).

Table 4.
Effect of ‘year’, ‘management system’, and their interaction on weed density (plants m−2) as determined by ANOVA, with degrees of freedom (DF), F-values, and statistical significance levels (*** p ≤ 0.001; ** p ≤ 0.01).
Weed density under the CONV system ranged between 55.8 plants m−2 (2021) at its highest level to 13.5 plants m−2 (2022) at its lowest. Under the CONS system, it varied from 17.7 plants m−2 at the lowest again in 2022, up to 44.4 plants m−2 in 2024, which was the second-highest year for the CONV system (Figure 1). Overall (average of the period 2021–2024), weed density was significantly higher under CONV compared to CONS, with 37 plants m−2 and 26 plants m−2, respectively.
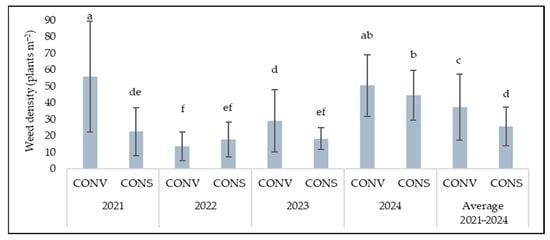
Figure 1.
Weed density (plants m−2) as affected by conventional (CONV) and conservation (CONS) management system for each season (year); columns indicate the mean; values followed by different letters are statistically significant at p ≤ 0.05; bars are standard deviation (σ).
For both cover crops and weed density, ANOVA showed a statistically significant effect with regard to the year of assessment (p ≤ 0.001) (Table 5). Cover crop density reduced significantly in 2022 and maintained a negative trend in the following years (Figure 2a), with non-significant difference between densities. Weed density was low and stable in 2022 and 2023 (Figure 2b), without significant differences between the two years. Weed density was significantly higher in 2024.

Table 5.
Effect of ‘year’ on cover crops and weed density (plants m−2) under conservation system (CONS) as determined by ANOVA, with degrees of freedom (DF), F-values, and statistical significance levels (*** p ≤ 0.001).
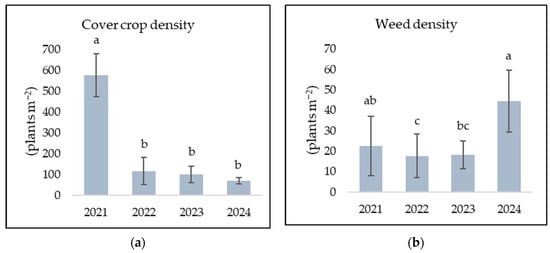
Figure 2.
Conservation (CONS) management system effect on cover crop density (a) and weed density (b) for each season (year); columns indicate the mean; values followed by different letters are statistically significant at p ≤ 0.05; bars are standard deviation (σ).
4. Discussion
This study aimed to evaluate the influence of 4 years (a complete rotation cycle) when applying two management systems, i.e., conservation (minimum tillage and cover crops) and conventional (ploughing without cover crops), on the evolution of the diversity and abundance of weed species before cover crop termination, to determine its effect.
Over the 4-year period, both management systems showed fluctuating patterns in terms of temporal dynamics of species richness (S). CONV exhibited a declining trend in diversity and evenness with a variable richness, while CONS had higher values of evenness and diversity indexes over the 4-year period, at a similar or lower richness than CONV (2023 and 2024) and without a consistent pattern over time. Moreover, the higher evenness under CONS (0.72 versus 0.6 in CONV, average of the four years), indicates a more balanced weed population, especially by the fourth year.
Most weed species assessed were dicotyledonous (92%) and annual (80%). In the final year of the rotation cycle, under both management systems, weed species of the families Plantaginaceae, Lamiaceae, Polygonaceae, Brassicaceae, and Asteraceae held a similar share of the total weed community (20%). Annual weed species and species of the Asteraceae family are the most common in this geographical area [43,44,45]. This study demonstrated within a 4-year time span that changing to a CONS system does not cause a large shift towards a monocotyledonous weed species community within the southeastern region of Romania.
CONS demonstrated an earlier impact on the weed community, reducing the spatial presence of species such as C. arvense (L.) Scop., F. officinalis L., S. media (L.) Vill., and P. aviculare L., starting with the second year. Over the 4-year period, S. viridis (L.) P. Beauv., Rumex sp. L., and G. aparine L. had a low prevelence under CONV and were absent under the CONS system. Rumex sp. and G. aparine need access to sunlight for effective establishment; therefore, the cover crop, which had the highest density in 2021, might have hindered these species’ emergence. McKenzie-Gopsill and Farooque (2023) [46] linked weed suppression, including Rumex sp., to allelopathy and/or nutrient availability preventing weed seed germination. S. viridis also thrives in open soil, and the canopy of cover crop species might have reduced the space and light resources needed for the species emergence [47,48]. Reduced soil disturbance may help regulate population build-up of C. arvese and P. aviculare, which thrive in disturbed soil, whereas F. officinalis is small-seeded and has poor seedling vigour. Long-term research (seven years) under a Mediterranean climate showed that the F. officinalis seed bank increases under conservation tillage [49] because light soil disturbance favours germination. However, Weber et al. (2014) [50] found that F. officinalis was persistent under both ploughing and reduced tillage. Hence, the species is well-adapted to a broader range of soil disturbance systems. Therefore, cover crops should have been crucial in suppressing F. officinalis density under CONS. In a five-year study, Restuccia et al. (2020) [42] demonstrated that cover crops can reduce the weed seed bank, including F. officinalis, by 40–57%, and the growth of perennial dicotyledons may be suppressed by competition or allelopathy. Phan et al. (2022) [51] noted that the extent of C. arvense development was constrained by the ability of winter cover crops to compete with it for nitrogen. Bicksler and Masiunas (2009) [52] found that cover crops with high biomass affect thistle shoot emergence. Weed suppression via competition with dense cover crops seems to be effective for species like P. aviculare or L. purpureum by monopolizing light and resources, filling the bare soil that some species depend on for establishment. Dorn et al. (2015) [53] reported that cover crops with rapid development and high soil coverage have a high suppressive behaviour, but their effect depends on the season (year). L. purpureum is a facultative winter annual and germinates almost solely during autumn when once-ripe seeds are subjected to cool (5–20 °C), moist, and good-light conditions [54]. In the current study, while present during most years, L. purpureum was found to be absent in 2022, most likely due to its sensitivity to climatic conditions during autumn, as the poor weather in 2022 did not favor emergence and may have triggered secondary dormancy [55].
In this study, the weed community was reduced to a similar range of species by 2024, under both management systems. However, in the final year of the rotation cycle, weed diversity (H′) and evenness (E) strongly differed by management system (H′ = 0.65 under CONS and 0.35 under CONV, and E = 0.42 under CONS and 0.25 under CONV, respectively, because of the dominance of V. hederifolia. A higher weed diversity and evenness under conservation practices align with the result of Adeux et al. (2022) [56]. Their findings illustrate that using cover crops for cropping system diversification increases species richness and results in weed communities being more evenly distributed. Similarly, Hofmeijer et al. (2021) [57] found that, across Europe, undertaking practices that increase crop diversity (intercropping, undersowing, and winter cover crops) increases long-term weed diversity without increasing weed densities. Weed diversity is generally higher in conservation systems [22], although it is often accompanied by a shift in species composition [20,21,22]. According to Adeux et al. (2023) [58], using cover crops over the long term can control weeds, changing weed flora over time. The same authors note that, in the long term, under intensive weed management systems, including tillage and herbicides, cover crops have reduced contributions to weed management [58].
In this study, the same herbicide regime was applied to both management systems (CONV and CONS) by standard agricultural practices for each cash crop within the sunflower–winter wheat–maize–sunflower rotation. Some of the herbicides used in this study contain soil-persistent compounds (i.e., imazamox) that can alter the composition and density of the weed community. The imidazolinone group, including imazamox, has a reported persistence of up to 300 days [59]. However, imazamox persistence generally ranges up to 90 days under anaerobic field conditions [60,61,62] and shows even faster rates of degradation when exposed to light [61,63]. Soil acidity, moisture, clay content, and organic matter can also accelerate its degradation depending on microbial activity and residue properties [64]. Nevertheless, in no-till, low-pH soils with high organic matter, the persistence remains high under dry conditions [65]. Applying imazamox under minimum or zero tillage significantly reduced weed density and improved soil moisture compared to conventional tillage, particularly when residues were retained [66,67]. In the current study, the interval of more than one year between herbicide application and weed data collection significantly reduces the likelihood that the residual effects of the active substance significantly influenced the structure of the weed community in the two systems (CONV and CONS).
In the final year of the rotation cycle (2024), V. hederifolia was the dominant species across CONV plots. V. hederifolia is an annual weed species predominant in winter wheat fields, capable of vegetatively overwintering and completing its lifecycle in the spring [68]. The ecology of the species is defined by its interaction with different crop management practices. V. hederifolia seeds require burial for germination, as they undergo the process of after-ripening, which reduces their dormancy [69]. The germination process is favoured by low temperatures of 4–8 °C [70]. Conventional tillage ensures the uniform distribution of the species’ seeds to depths that favour their germination [69], as V. hederifolia seeds within the total weed seed bank increase [71] and are more numerous with increasing soil depth [49]. Moreover, the species can produce between 426 (in normal-density wheat crops) and 980 seeds per plant (in the absence of competition), which gives it a high invasive potential in systems where soil disturbance is frequent [72]. Milder winter conditions during the study period, in addition to the physiological traits of V. hederifolia, impacted the species’ establishment in both management systems. Climatic data (Table 1) suggest that the 2021–2024 period was marked by suitable rainfall and positive winter temperatures, promoting germination and establishment of seeds. In the CONV system, ploughing ensured the necessary thermal conditions in the soil profile level for seed germination, and seed burial ensured the physiological process of after-ripening. Winkler et al. (2015) [73] found conventional tillage to be more favourable to the weed species compared to minimum tillage, and V. hederifolia was the most frequent weed species in this system. Conversely, Kolářová et al. (2014) [70] stated that reduced tillage (no-till) tends to favour species that germinate in autumn, such as Veronica species, because the seeds remain on the surface and are not buried deeply, which facilitates germination at low temperatures. Adeux et al. (2023) [58] note that a decrease in Veronica species seedbank is facilitated by the use of cover crops. Other studies [74] support the hypothesis that V. hederifolia populations are lower in conservative tillage systems compared to conventional systems, because the seeds require burial or tillage for germination, even though the seed bank is larger in the latter. In this study, under the conservation system, the dominance of V. hederifolia was balanced by C. bursa-pastoris (L.) Medik. and M. chamomilla L., creating a slightly more stable weed community (Table 3). V. hederifolia is an annual weed that can coexist with winter crops and withstand moderate shading [72], as well as low nutrient levels [75]. In addition, the species biological traits make it resilient to weed suppression using mulch, since it can cycle through seasonal dormancy [75]. Reduced tillage favours shallow-germinating Veronica species [76]. Schmidt et al. (2019) [77] noted similar results in the Veronica species population, linked to seed persistence (more than five years) and its ability germinate in cold conditions, both in autumn and spring seasons, and to develop rapidly. Therefore, the cover crop was unable to break the species life cycle [77,78]. Biennial species such as C. bursa-pastoris are favoured by conservation tillage because it increases the seedbank [79]; thus, when the cover crop establishment is poor, it favours the species emergence and development. M. chamomilla is an opportunistic weed in long-term reduced tillage systems; refs. [80,81] reported that the species was more tolerant than other weeds to cover crop mulches.
Under southeastern Romanian conditions, the results for 2021–2024 demonstrated that CONS, with cover crops and non-inversion tillage, was effective in controlling weed density. Overall (average of the period 2021–2024), under CONS weed density was reduced by 31%, from 37.2 plants/m−2 under CONV to 25.7 plants/m−2, and the difference was statistically significant (p ≤ 0.01). This agrees with similar studies that reported minimum tillage and soil cover to reduce weed density compared to conventional tillage, while species diversity remained comparable [82]. ANOVA on weed density highlighted the effect of the management system, although significant in reducing weed density (p ≤ 0.01), is dependent on season (year), with the latter having a highly significant effect (p ≤ 0.001) on both weed and cover crop density (Figure 1 and Figure 2). In this study, it is evident that the high cover crop stands in 2021 resulted in lower weed density under CONS (Figure 2). The significant difference in cover crop density between the first year (2021) and subsequent years is likely due to weather conditions, particularly autumn and winter precipitation, as well as temperatures. The cumulative rainfall of 422.2 mm during the cover crop vegetative growth from October 2020 to April 2021 was significantly higher than the multiannual mean of 367.3 mm and compared to subsequent years, providing adequate soil moisture for seed germination and the establishment of the cover crop. Additionally, the favourable temperature in October 2020 (14.3 °C) most likely favoured an early emergence and fast canopy establishment. In 2022, 2023, and 2024, sowing the cover crop in early November was less favourable for its establishment due to temperatures. Subsequently, during the development of the cover crop, lower cumulative precipitation affected its density. Although cover crop density was significantly lower in these years (2022–2024) compared to 2021, weed density under the CONS system remained relatively constant. Therefore, weed density in 2022 was significantly lower compared to 2021 but similar to the weed density in 2023 (Figure 2b), suggesting a possible residual effect of the cover crop competition in 2021, which reduced the contribution to the weed seedbank in 2022. Different studies observed that cover crop use can reduce weed seed deposits [83,84] and the seed set of some short-cycled weeds [58]. Hence, a considerably lower cover crop density in the following years (2022–2024) reduced the weed suppresion effect, resulting in a higher weed density in 2024. This weed density increase might also have been potentially due to weather conditions in late spring before cover crop termination. April 2024 recorded the highest average temperature (14.7 °C) of the 4-year period and sufficient precipitation (43 mm) (Table 1) to favour weed germination and growth, to outcompete a low-density cover crop. Weather conditions also favoured the emergence of the weeds in both systems. In the final year of the rotation cycle, the difference in weed density between CONS and CONV was not statistically significant (Figure 1). However, weed density was more stable under CONS and remained lower compared to CONV (Figure 1). The conservation management system provided consistency in controlling weed density, with an average standard deviation of 11.8 (σ between 6.7 and 15.1). In contrast, the absence of a cover crop in CONV allowed weeds to fully exploit the favourable weather conditions, resulting in higher weed densities and a lower stability (average σ = 20.0, ranging between 8.8 and 33.8). Osipitan et al. (2019) [85] correlated cover crops’ weed suppression capacity to the seeding rate. Even though CONS maintained the weed suppression efficiency over CONV, cover crop density needs to be effectively managed to sufficiently control weeds in the warming weather conditions associated with climate change.
5. Conclusions
The weed community composition was specific to the southeastern Romania weed flora. Species richness was reduced under the conservation agricultural practices of non-inversion tillage and cover crop. Cover crops combined with non-inversion tillage demonstrated effectiveness in controlling weed density; however, the effect was also dependent on climate conditions. The conservation management system proved a stable weed control strategy for southeastern Romania crop conditions. In the last year of the rotation cycle, under both cropping systems, the weed flora was composed of the same species (V. hederifolia, C. bursa-pastoris, L. purpureum, M. chamomilla, and P. aviculare); however, year-over-year, the conservation system ensured a more stable weed suppression capacity. Crop management (i.e., diverse and longer rotations and herbicide application) and conservation agricultural practices (i.e., optimization of cover crop species or mixtures; cover crop seeding rate and densities) should be further explored for their potential to control these persistent weed species.
Author Contributions
M.R., C.B., C.M., A.M., M.J.N. and V.P.V. contributed to the discussion and conceptualization of the manuscript; writing, original draft—C.B. and M.R.; writing, reviewing and editing—V.P.V.; supervision and project administration—V.P.V., C.M., A.M. and M.J.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Syngenta Crop Protection AG and the University of Agronomic Science and Veterinary Medicine of Bucharest through Moara Domnească Agricultural Research and Development Didactic Station, contract number 25986/20.10.2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Andrei Măruțescu, Max John Newbert, and Vasileios P. Vasileiadis were employed by the company Syngenta Crop Protection AG. The authors declare that this study received funding from Syngenta Crop Protection AG. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Abbreviations
The following abbreviations are used in this manuscript:
| CONV | Conventional crop management system based on ploughing tillage |
| CONS | Conservative crop management system using cover crops and non-inversion tillage (chiselling) |
References
- Friedrich, T. Degradation of natural resources and measures for mitigation. In Handbook of the International Seminar on Enhancing Extension of Conservation, Proceedings of the Agriculture Techniques in Asia and the Pacific, Zhengzhou, China, 24–26 October 2007; Asian and Pacific Centre for Agricultural Engineering and Machinery (APCAEM): Beijing, China; Ministry of Agriculture of the People’s Republic: Beijing, China, 2007; pp. 24–26. [Google Scholar]
- Kassam, A.; Friedrich, T.; Derpsch, R.; Kienzle, J. Overview of the worldwide spread of conservation agriculture. Field Actions Sci. Rep. 2015, 8, 1–11. [Google Scholar]
- Prestele, R.; Hirsch, A.L.; Davin, E.L.; Seneviratne, S.I.; Verburg, P.H. A spatially explicit representation of conservation agriculture for application in global change studies. Glob. Chang. Biol. 2018, 24, 4038–4053. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, N.; Govaerts, B.; Verachtert, E.; Castellanos-Navarrete, A.; Mezzalama, M.; Wall, P.C.; Chocobar, A.; Deckers, J.; Sayre, K.D. Conservation agriculture, improving soil quality for sustainable production systems? In Advances in Soil Science: Food Security and Soil Quality; CRC Press eBooks: Boca Raton, FL, USA, 2010; pp. 137–208. [Google Scholar]
- Rhoton, F.E. Influence of time on soil response to No-Till practices. Soil Sci. Soc. Am. J. 2000, 64, 700–709. [Google Scholar] [CrossRef]
- McVay, K.A.; Budde, J.A.; Fabrizzi, K.; Mikha, M.M.; Rice, C.W.; Schlegel, A.J.; Peterson, D.E.; Sweeney, D.W.; Thompson, C. Management Effects on Soil Physical Properties in Long-Term Tillage Studies in Kansas. Soil Sci. Soc. Am. J. 2006, 70, 434–438. [Google Scholar] [CrossRef]
- Reicosky, D.C. Conservation tillage is not conservation agriculture. J. Soil Water Conserv. 2015, 70, 103A–108A. [Google Scholar] [CrossRef]
- Rusu, T.; Bogdan, I.; Moraru, P.I.; Pop, A.I.; Duda, B.M.; Coste, C. Research results on conservative tillage systems in the last 50 years at USAMV Cluj-Napoca. ProEnviron. Promediu 2015, 8, 105–111. [Google Scholar]
- Mubvumba, P.; DeLaune, P.B.; Hons, F.M. Enhancing long-term no-till wheat systems with cover crops and flash grazing. Soil Secur. 2022, 8, 100067. [Google Scholar] [CrossRef]
- Subbulakshmi, S.; Chandrasekaran, H.; Saravanan, N.; Subbian, P. Conservation tillage—An ecofriendly management practices for agriculture. Res. J. Agric. Biol. Sci. 2009, 5, 1098–1110. [Google Scholar]
- Pittelkow, C.M.; Liang, X.; Linquist, B.A.; Van Groenigen, K.J.; Lee, J.; Lundy, M.E.; Van Gestel, N.; Six, J.; Venterea, R.T.; Van Kessel, C. Productivity limits and potentials of the principles of conservation agriculture. Nature 2015, 517, 365–368. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops—A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Lal, R. Restoring soil quality to mitigate soil degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Nichols, V.; Verhulst, N.; Cox, R.; Govaerts, B. Weed dynamics and conservation agriculture principles: A review. Field Crops Res. 2015, 183, 56–68. [Google Scholar] [CrossRef]
- Marin, D.I.; Teodor, R.U.S.U.; Mihalache, M.; Ilie, L.; Nistor, E.; Bolohan, C. Influence of soil tillage upon production and energy efficiency in wheat and maize crops. AgroLife Sci. J. 2015, 4, 43–47. [Google Scholar]
- Rusu, T.; Bogdan, I.; Marin, D.I.; Moraru, P.I.; Pop, A.I.; Duda, B.M. Effect of conservation agriculture on yield and protecting environmental resources. AgroLife Sci. J. 2015, 4, 141–145. [Google Scholar]
- Weber, J.F.; Kunz, C.; Peteinatos, G.; Zikeli, S.; Gerhards, R. Weed control using conventional tillage, reduced tillage, No-Tillage, and cover crops in organic soybean. Agriculture 2017, 7, 43. [Google Scholar] [CrossRef]
- Sims, B.; Corsi, S.; Gbehounou, G.; Kienzle, J.; Taguchi, M.; Friedrich, T. Sustainable Weed Management for Conservation Agriculture: Options for Smallholder Farmers. Agriculture 2018, 8, 118. [Google Scholar] [CrossRef]
- Singh, V.P.; Barman, K.K.; Singh, R.; Sharma, A.R. Weed Management in Conservation Agriculture Systems. In Conservation Agriculture; Farooq, M., Siddique, K.H.M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 39–77. [Google Scholar]
- Bo, M.; Nicolas, M.-J.; Raphaël, C.; Judith, W.; Jürgen, S.; van der Weide, R.; Ludovic, B.; Peter, K.J.; Per, K. European Perspectives on the Adoption of Nonchemical Weed Management in Reduced-Tillage Systems for Arable Crops. Weed Technol. 2013, 27, 231–240. [Google Scholar] [CrossRef]
- Aušra, S.; Vaclovas, B.; Alfredas, S.; Vaida, S.; Lenkis, A.; Rasa, K. Weed Abundance, Seed Bank in Different Soil Tillage Systems, and Straw Retention. Agronomy 2025, 15, 1105. [Google Scholar] [CrossRef]
- Derrouch, D.; Chauvel, B.; Cordeau, S.; Dessaint, F. Functional shifts in weed community composition following adoption of conservation agriculture. Weed Res. 2021, 62, 103–112. [Google Scholar] [CrossRef]
- Trichard, A.; Alignier, A.; Chauvel, B.; Petit, S. Identification of weed community traits response to conservation agriculture. Agric. Ecosyst. Environ. 2013, 179, 179–186. [Google Scholar] [CrossRef]
- Baraibar, B.; Hunter, M.C.; Schipanski, M.E.; Hamilton, A.; Mortensen, D.A. Weed suppression in cover crop monocultures and mixtures. Weed Sci. 2018, 66, 121–133. [Google Scholar] [CrossRef]
- Sharma, T.; Das, T.K.; Maity, P.P.; Biswas, S.; Sudhishri, S.; Govindasamy, P.; Raj, R.; Sen, S.; Singh, T.; Paul, A.K.; et al. Long-Term Conservation Agriculture Influences Weed Diversity, Water Productivity, Grain Yield, and Energy Budgeting of Wheat in North-Western Indo-Gangetic Plains. Sustainability 2023, 15, 7290. [Google Scholar] [CrossRef]
- Aliste, M.; Ros, C.; Garrido, I.; Martínez, C.M.; Vera, A.; Siles, J.; Contreras, F.; Flores, P.; Hellín, P.; Fenoll, J.; et al. Green manure from cover crops enhances pesticide degradation and soil biological health. J. Hazard. Mater. 2025, 495, 138984. [Google Scholar] [CrossRef]
- Kocira, A.; Staniak, M.; Tomaszewska, M.; Kornas, R.; Cymerman, J.; Panasiewicz, K.; Lipińska, H. Legume cover crops as one of the elements of strategic weed management and soil quality improvement. A review. Agriculture 2020, 10, 394. [Google Scholar] [CrossRef]
- Wittwer, R.A.; van der Heijden, M.G. Cover crops as a tool to reduce reliance on intensive tillage and nitrogen fertilization in conventional arable cropping systems. Field Crops Res. 2020, 249, 107736. [Google Scholar] [CrossRef]
- Kruidhof, H.M.; Bastiaans, L.; Kropff, M.J. Ecological weed management by cover cropping: Effects on weed growth in autumn and weed establishment in spring. Weed Res. 2008, 48, 492–502. [Google Scholar] [CrossRef]
- Kunz, C.; Strum, D.J.; Varnholt, D.; Walker, F.; Gerhards, R. Allelopathic effects and weed suppressive ability of cover crops. Plant Soil Environ. 2016, 62, 60–66. [Google Scholar] [CrossRef]
- Osipitan, O.A.; Dille, J.A.; Assefa, Y.; Knezevic, S.Z. Cover Crop for early Season Weed Suppression in Crops: Systematic Review and Meta-Analysis. Agron. J. 2018, 110, 2211–2221. [Google Scholar] [CrossRef]
- Leskovšek, R.; Eler, K.; Zamljen, S.A. Weed suppression and maize yield influenced by cover crop mixture diversity and tillage. Agric. Ecosyst. Environ. 2025, 383, 109530. [Google Scholar] [CrossRef]
- Gerhards, R.; Schappert, A. Advancing cover cropping in temperate integrated weed management. Pest Manag. Sci. 2020, 76, 42–46. [Google Scholar] [CrossRef]
- Zannopoulos, S.; Gazoulis, I.; Kokkini, M.; Antonopoulos, N.; Kanatas, P.; Kanetsi, M.; Travlos, I. The potential of three summer legume cover crops to suppress weeds and provide ecosystem Services—A review. Agronomy 2024, 14, 1192. [Google Scholar] [CrossRef]
- Schappert, A.; Schumacher, M.; Gerhards, R. Weed control ability of single sown cover crops compared to species mixtures. Agronomy 2019, 9, 294. [Google Scholar] [CrossRef]
- Wittwer, R.A.; Dorn, B.; Jossi, W.; Van Der Heijden, M.G.A. Cover crops support ecological intensification of arable cropping systems. Sci. Rep. 2017, 7, 41911. [Google Scholar] [CrossRef]
- Büchi, L.; Wendling, M.; Amossé, C.; Jeangros, B.; Charles, R. Cover crops to secure weed control strategies in a maize crop with reduced tillage. Field Crops Res. 2020, 247, 107583. [Google Scholar] [CrossRef]
- Vincent-Caboud, L.; Peigné, J.; Casagrande, M.; Silva, E.M. Overview of Organic Cover Crop-Based No-Tillage technique in Europe: Farmers’ practices and research challenges. Agriculture 2017, 7, 42. [Google Scholar] [CrossRef]
- Mihalașcu, C.; Bolohan, C.; Tudor, V.; Mihalache, M.; Teodorescu, R.I. Research on the growth and development of some varieties of Lavandula angustifolia (Mill.) in the south-east of Romania. Rom. Biotechnol. Lett. 2020, 25, 2180–2187. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Vasileiadis, V.P.; van Dijk, W.; Verschwele, A.; Holb, I.J.; Vámos, A.; Urek, G.; Leskovsek, R.; Furlan, L.; Sattin, M. Farm-scale evaluation of herbicide band application integrated with inter-row mechanical weeding for maize production in four European regions. Weed Res. 2016, 56, 313–322. [Google Scholar] [CrossRef]
- Restuccia, A.; Scavo, A.; Lombardo, S.; Pandino, G.; Fontanazza, S.; Anastasi, U.; Abbate, C.; Mauromicale, G. Long-Term effect of cover crops on species abundance and diversity of weed flora. Plants 2020, 9, 1506. [Google Scholar] [CrossRef]
- Neblea, M.A.; Marian, M.C. Study concerning alien flora from Dâmbovița county (Romania). Curr. Trends Nat. Sci. 2022, 11, 178–194. [Google Scholar] [CrossRef]
- Camen-Comănescu, P.; Mihai, D.C.; Raicu, M.; Sîrbu, C.; Oprea, A.; Anastasiu, P. Alien flora from Buzău county—Romania. Acta Horti Bot. Bucurestiensis 2023, 49, 49–76. [Google Scholar] [CrossRef]
- Răduțoiu, D.; Simion, I.; Boruz, V. Alien flora from Dolj county, Romania/Flora alohtonă din județul Dolj, România. Ann. Univ. Craiova Ser. Geogr. 2024, 25, 48–75. [Google Scholar] [CrossRef]
- McKenzie-Gopsill, A.; Farooque, A. Incorporated cover crop residue suppresses weed seed germination. Weed Biol. Manag. 2023, 23, 48–57. [Google Scholar] [CrossRef]
- Rueda-Ayala, V.; Jaeck, O.; Gerhards, R. Investigation of biochemical and competitive effects of cover crops on crops and weeds. Crop Prot. 2015, 71, 79–87. [Google Scholar] [CrossRef]
- Sebastian, J.; Dinneny, J.R. Setaria viridis: A Model for Understanding Panicoid Grass Root Systems. In Genetics and Genomics of Setaria; Doust, A., Diao, X., Eds.; Springer: Cham, Switzerland, 2017; Volume 19, pp. 177–193. [Google Scholar]
- Fracchiolla, M.; Stellacci, A.M.; Cazzato, E.; Tedone, L.; Ali, S.A.; De Mastro, G. Effects of Conservative Tillage and Nitrogen Management on Weed Seed Bank after a Seven-Year Durum Wheat—Faba Bean Rotation. Plants 2018, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Sekutowski, T.; Owsiak, Z. Variability of weed infestation of winter wheat cultivars in relation to tillage systems. Prog. Plant Prot. 2014, 54, 178–184. [Google Scholar] [CrossRef][Green Version]
- Phan, H.T.; Wacker, T.S.; Thorup-Kristensen, K. Winter cover crops favor cereal crop in N competition against creeping thistle Cirsium arvense (L.) Scop. Soil Tillage Res. 2022, 216, 105261. [Google Scholar] [CrossRef]
- Bicksler, A.J.; Masiunas, J.B. Canada Thistle (Cirsium arvense) Suppression with Buckwheat or Sudangrass Cover Crops and Mowing. Weed Technol. 2009, 23, 556–563. [Google Scholar] [CrossRef]
- Dorn, B.; Jossi, W.; Van Der Heijden, M.G.A. Weed suppression by cover crops: Comparative on-farm experiments under integrated and organic conservation tillage. Weed Res. 2015, 55, 586–597. [Google Scholar] [CrossRef]
- Baskin, J.; Baskin, C.C.; Parr, J. Field emergence of Lamium amplexicaule L. and L. purpureum L. in relation to the annual seed dormancy cycle. Weed Res. 1986, 26, 185–190. [Google Scholar] [CrossRef]
- Karlsson, L.M.; Milberg, P. Variation within species and inter-species comparison of seed dormancy and germination of four annual Lamium species. Flora Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 409–420. [Google Scholar] [CrossRef]
- Adeux, G.; Yvoz, S.; Biju-Duval, L.; Cadet, E.; Farcy, P.; Fried, G.; Guillemin, J.; Meunier, D.; Munier-Jolain, N.; Petit, S.; et al. Cropping system diversification does not always beget weed diversity. Eur. J. Agron. 2022, 133, 126438. [Google Scholar] [CrossRef]
- Hofmeijer, M.A.; Melander, B.; Salonen, J.; Lundkvist, A.; Zarina, L.; Gerowitt, B. Crop diversification affects weed communities and densities in organic spring cereal fields in northern Europe. Agric. Ecosyst. Environ. 2021, 308, 107251. [Google Scholar] [CrossRef]
- Adeux, G.; Rodriguez, A.; Penato, C.; Antichi, D.; Carlesi, S.; Sbrana, M.; Bàrberi, P.; Cordeau, S. Long-term cover cropping in tillage-based systems filters weed community phenology: A seedbank analysis. Field Crops Res. 2023, 291, 108769. [Google Scholar] [CrossRef]
- Gehrke, V.R.; Fipke, M.V.; Avila, L.A.d.; Camargo, E.R. Understanding the Opportunities to Mitigate Carryover of Imidazolinone Herbicides in Lowland Rice. Agriculture 2021, 11, 299. [Google Scholar] [CrossRef]
- Vischetti, C.; Casucci, C.; Perucci, P. Relationship between changes of soil microbial biomass content and imazamox and benfluralin degradation. Biol. Fertil. Soils 2002, 35, 13–17. [Google Scholar] [CrossRef]
- Buerge, I.J.; Kasteel, R.; Bächli, A.; Poiger, T. Behavior of the Chiral Herbicide Imazamox in Soils: Enantiomer Composition Differentiates between Biodegradation and Photodegradation. Environ. Sci. Technol. 2019, 53, 5733–5740. [Google Scholar] [CrossRef]
- Rani, D.; Duhan, A.; Punia, S.S.; Yadav, D.B.; Duhan, S. Behavior of pre-mix formulation of imazethapyr and imazamox herbicides in two different soils. Environ. Monit. Assess. 2018, 191, 33. [Google Scholar] [CrossRef]
- Quivet, E.; Faure, R.; Georges, J.; Païssé, J.-O.; Herbreteau, B.; Lantéri, P. Photochemical Degradation of Imazamox in Aqueous Solution: Influence of Metal Ions and Anionic Species on the Ultraviolet Photolysis. J. Agric. Food Chem. 2006, 54, 3641–3645. [Google Scholar] [CrossRef]
- Locke, M.A.; Zablotowicz, R.M.; Bauer, P.J.; Steinriede, R.W.; Gaston, L.A. Conservation cotton production in the southern United States: Herbicide dissipation in soil and cover crops. Weed Sci. 2005, 53, 717–727. [Google Scholar] [CrossRef]
- Moyer, J.R.; Coen, G.; Dunn, R.; Smith, A.M. Effects of Landscape Position, Rainfall, and Tillage on Residual Herbicides. Weed Technol. 2010, 24, 361–368. [Google Scholar] [CrossRef]
- Blackshaw, R.E.; Molnar, L.J. Integration of Conservation Tillage and Herbicides for Sustainable Dry Bean Production. Weed Technol. 2008, 22, 168–176. [Google Scholar] [CrossRef]
- Shrirao, T.; Kanase, N.; Goud, V.V.; Jadhao, S.; Konde, N.; Bhoyar, S.; Ravali, E. Effect of tillage and weed management on soil properties and yield of soybean in vertisols. Int. J. Adv. Biochem. Res. 2024, 8, 844–851. [Google Scholar] [CrossRef]
- Tóth, E.; Dorner, Z.; Nagy, J.G.; Zalai, M. How Weed Flora Evolves in Cereal Fields in Relation to the Agricultural Environment and Farming Practices in Different Sub-Regions of Eastern Hungary. Agronomy 2025, 15, 1033. [Google Scholar] [CrossRef]
- Roberts, H.; Lockett, P.M. Seed dormancy and periodicity of seedling emergence in Veronica hederifolia L. Weed Res. 1978, 18, 41–48. [Google Scholar] [CrossRef]
- Kolářová, M.; Tyšer, L.; Soukup, J. Weed vegetation of arable land in the Czech Republic: Environmental a management factors determining weed species composition. Biologia 2014, 69, 443–448. [Google Scholar] [CrossRef]
- Auškalnienė, O.; Auškalnis, A. The influence of tillage system on diversities of soil weed seed bank. Agron. Res. 2009, 7, 156–161. [Google Scholar]
- Lutman, P.J.W.; Wright, K.J.; Berry, K.; Freeman, S.E.; Tatnell, L. Estimation of seed production by Myosotis arvensis, Veronica hederifolia, Veronica persica and Viola arvensis under different competitive conditions. Weed Res. 2011, 51, 499–507. [Google Scholar] [CrossRef]
- Winkler, J.; Trojan, V.; Hrubešová, V. Effects of the tillage technology and the forecrop on weeds in stands of winter wheat. Acta Univ. Agric. Silvic. Mendel. Brun. 2015, 63, 477–483. [Google Scholar] [CrossRef]
- Pardo, G.; Cirujeda, A.; Perea, F.; Verdú, A.M.C.; Mas, M.T.; Urbano, J.M. Effects of reduced and conventional tillage on weed communities: Results of a long-term experiment in southwestern Spain. Planta Daninha 2019, 37, e019201336. [Google Scholar] [CrossRef]
- Mennan, H.; Zandstra, B.H. The Effects of Depth and Duration of Seed Burial on Viability, Dormancy, Germination, and Emergence of Ivyleaf Speedwell (Veronica hederifolia). Weed Technol. 2006, 20, 438–444. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Smagacz, J.; Kwiatkowski, C.A.; Harasim, E.; Woźniak, A. Weed flora and soil seed bank composition as affected by tillage system in Three-Year Crop rotation. Agriculture 2020, 10, 186. [Google Scholar] [CrossRef]
- Schmidt, J.H.; Junge, S.; Finckh, M.R. Cover crops and compost prevent weed seed bank buildup in herbicide-free wheat-potato rotations under conservation tillage. Ecol. Evol. 2019, 9, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, H.; Wang, C.; Cheng, J.; Qiang, S. A comparative study reveals the key biological traits causing bioinvasion differences among four alien species of genus Veronica in China. J. Plant Ecol. 2023, 16, rtac068. [Google Scholar] [CrossRef]
- Ahmed, H.T.; Francis, A.; Clements, D.R.; Dyck, E.; Ross, N.; Upadhyaya, M.K.; Hall, L.M.; Martin, S.L. The Biology of Canadian Weeds. 159. Capsella bursa-pastoris (L.) Medik. Can. J. Plant Sci. 2021, 102, 529–552. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Gill, G.S.; Preston, C. Tillage system effects on weed ecology, herbicide activity and persistence: A review. Aust. J. Exp. Agric. 2006, 46, 1557–1570. [Google Scholar] [CrossRef]
- Sturm, D.J.; Kunz, C.; Gerhards, R. Inhibitory effects of cover crop mulch on germination and growth of Stellaria media (L.) Vill. , Chenopodium album L. and Matricaria chamomilla L. Crop Prot. 2016, 90, 125–131. [Google Scholar] [CrossRef]
- Anyoni, O.G.; Ekwangu, J.; Tumwebaze, S.; Obia, A. Minimum Tillage and Soil Surface Cover Reduced Weed Density but Not Diversity Over Four Growing Cycles. East. Afr. J. Agric. Biotechnol. 2024, 7, 39–58. [Google Scholar] [CrossRef]
- Hossain, M.M.; Begum, M.; Hashem, A.; Rahman, M.M.; Haque, M.E.; Bell, R.W. Continuous Practice of Conservation Agriculture for 3–5 Years in Intensive Rice-Based Cropping Patterns Reduces Soil Weed Seedbank. Agriculture 2021, 11, 895. [Google Scholar] [CrossRef]
- Nichols, V.; English, L.; Carlson, S.; Gailans, S.; Liebman, M. Effects of long-term cover cropping on weed seedbanks. Front. Agron. 2020, 2, 591091. [Google Scholar] [CrossRef]
- Osipitan, O.A.; Dille, J.A.; Assefa, Y.; Radicetti, E.; Ayeni, A.; Knezevic, S.Z. Impact of cover crop management on level of weed Suppression: A Meta-Analysis. Crop Sci. 2019, 59, 833–842. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).