Abstract
Meat secondary streams command low value along the meat value chain, with a significant portion of these exiting the food value chain and contributing to global food loss and waste. Valorizing these substantial secondary streams through the efficient extraction of high-biological-value proteins could translate into significant social, economic, and environmental benefits. Protein extraction from meat secondary streams offers a promising approach to enhance their nutritional and commercial value while supporting global food security initiatives. This approach could also help to distant these by-products from their original source, making them more appealing to consumers. The current review evaluates the protein content and valorization potential of meat secondary streams from various animal sources. It further provides a critical assessment of both traditional and emerging protein extraction techniques, highlighting their advantages, limitations, and applications. Existing knowledge gaps are also identified to guide future research. This review aligns the role of protein recovery technologies with the UN’s Sustainable Development Goal target 12.3, which seeks to halve global food waste by 2030.
1. Introduction
The conversation about food loss and food waste is intensifying among policy makers, researchers, and other stakeholders along the food value chain. This is because food loss and waste not only represents a resource problem but also an environmental and economic threat that challenges our moral responsibilities in the 21st century. According to the Food and Agriculture Organization (FAO), [1], food loss and waste (FLW) refers to a decrease in the quantity or quality of food along the food supply chain. This includes the food materials that altogether exit the food supply chain and those that could no longer meet their intended use due to a loss of attributes and overall value. In practice, FLW indices have been further elaborated by the FAO, where food loss involves the actions or decisions of suppliers and occurs from harvest (slaughter/catch) to processing and packaging. FLW indices do not extend to the retail level. On the other hand, food waste, which spans both the retail and consumption level, results from consumers’ behavior, as well as retailers’ and food service providers’ actions or decisions [2,3].
Consequently, reducing food loss could represent a more important focus in the Global South where most FLW occurs at production, handling, and storage stages of the food supply chain, whereas reducing food waste could represent a more strategic focus in the Global North, where these losses mostly occur at the consumer and retail levels. In addition, studies have shown that waste levels are typically high for highly perishable food materials (e.g., animal products) at the consumer level in high-income countries [1,4]. This seems to indicate that strategies employed to reduce FLW may vary across different jurisdictions and will be largely underscored by economic decision-making and the behaviors of actors along the food chain. Regardless of whether this loss occurs at the producer or consumer end of the supply chain, within the context of the circular bio-based economy, it is important that the optimal value within these secondary streams be explored in consideration of health, economy, social inclusivity, food security/nutrition, and environmental benefits.
In addition to the by-products that inevitably exit or find low utilization along the value chain, meat, being a highly perishable food product, is prone to waste at any stage of its product life cycle. The potential of these animal by-products not only lies in their enormous volume but also in their nutrient density. Specifically, these secondary streams are excellent sources of protein (up to about 20%), which could sometimes be higher or comparable to that from other animal sources [5,6]. However, adding value to these by-products requires some degree of innovation, science, and investment. Toldrá et al. [7] highlighted the economic potential of these co-streams in the production of new value-added products and functional ingredients with varying applications within the food industry. The use of protein hydrolysates as flavor enhancers, functional ingredients, or nutritional additives in food of low protein quality has been highlighted in the literature [8]. With increasing concern about chronic hunger and food insecurity, especially in developing economies, the possibility of harvesting the enormous protein resident in this low-value stream will not only contribute to the realization of Sustainable Development Goal target 12.3 but will also be beneficial to the environment and potentially provide an economic incentive to the associated industries.
As part of the effort to ensure that our current food system is as efficient as it possibly can be in the face of increasing population growth and climate change, adequate exploration of protein resources of appropriate quality for sustaining human health and population is a relevant line of research. Regardless of the economic and logistical constraints associated with these valorization processes, redirecting proteins derived from food waste back into the human food chain or into animal feed could enhance the efficiency of our global food system [9]. More importantly, although wastes from the meat and protein industry have been reported to contribute the highest negative environmental impact based on estimated greenhouse gas emissions, they ranked only fourth in terms of total weight of waste produced, after fruit, vegetables, and grains [10,11], highlighting the importance of valorizing this waste stream. Hence, protein can be extracted from these secondary streams, especially given their high biological value and overall content. Several methods have been traditionally used for protein extraction and these may vary depending on the type of protein to be extracted, the intended use of the protein, the food matrix, and the solubility of the protein. These methods include acid/alkali extraction, detergent extraction, enzyme-assisted protein extraction, salt extraction, and denaturation or non-denaturation extraction [12,13,14].
This review includes available protein extraction and isolation methods, as well as their application for animal by-products. Recent advances in protein extraction technology for meat and meat by-products are also discussed. To achieve this, the authors searched CAB Abstracts, Scopus, Agricola, and Medline, using the search terms ‘animal by-products’, ‘meat by-products’, ‘meat secondary streams’ and ‘protein extraction’ to provide a general background and identify reviews. The authors then narrowed the search to specific livestock species. The authors searched for specific types of extraction methods using the same approach. The authors used the same search keywords to provide additional references from the Web of Knowledge (http://www.isiwebofknowledge.com) database. In addition, the authors used the references from the articles obtained following this method to identify additional relevant material. Due to the historical context needed for this review, no date limit was used. The goal of this review is to provide an up-to-date inventory of extraction methods for protein from the meat industry’s secondary streams and to evaluate their overall environmental and economic credentials.
2. Meat Secondary Streams
Meat consumption is rising globally, making meat the most valuable livestock product. Nutrition transition patterns as well as other biological, economic, physical, and social determinants are fueling this trend [15,16]. Consequently, this increase has inadvertently resulted in an increase in the generation of meat secondary streams, also sometimes referred to as meat by-products, fifth quarter meat, or meat co-products (Table 1). While these can represent under-utilized fractions of meat animals anywhere along the meat value chain (i.e., from farm gate to consumer plate), this review will only focus on those secondary streams from slaughterhouse to retail, as these are the portions that can be easily recovered and re-used within the value chain.

Table 1.
Proportion of selected animal by-products from different animal species.
The American Meat Science Association (AMSA) has defined meat as the skeletal muscle and associated tissues derived from mammalian, avian, reptilian, amphibian, and aquatic species commonly harvested for human consumption, including edible offal consisting of organs and non-skeletal muscle tissues [23]; this definition will be employed in this review (Figure 1). The following sections will identify these meat secondary streams from common meat species and highlight some of their current utilizations.
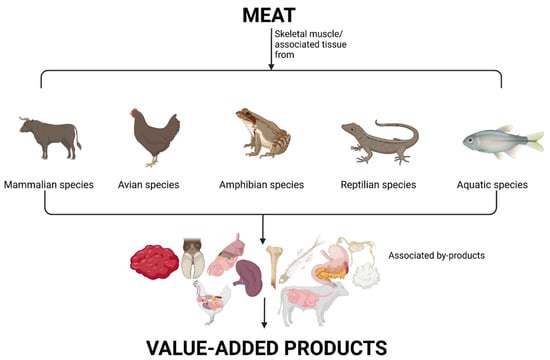
Figure 1.
Definition of meat and their resulting animal by-products (diagram drawn using Biorender).
2.1. Meat Secondary Streams from Mammalian Species
By-products from mammalian animals refer to all parts of live animals that are not part of the dressed carcass [24]. They include all non-skeletal muscle components arising from meat processing and fabrication [17] which, when not channeled for human consumption, are mostly sent for rendering, incineration, or landfill. According to several reports, about 44–56% bovine, 45–51% ovine, and 30–48% porcine animal live weight represents these secondary streams or by-products [25]. Aside from their enormous volume, these low-value streams are highly nutritious, being especially high in high-biological-value proteins, vitamins, and minerals. As an example, Figure 2 shows the proportion of protein, fat, and ash in various porcine meat by-products.
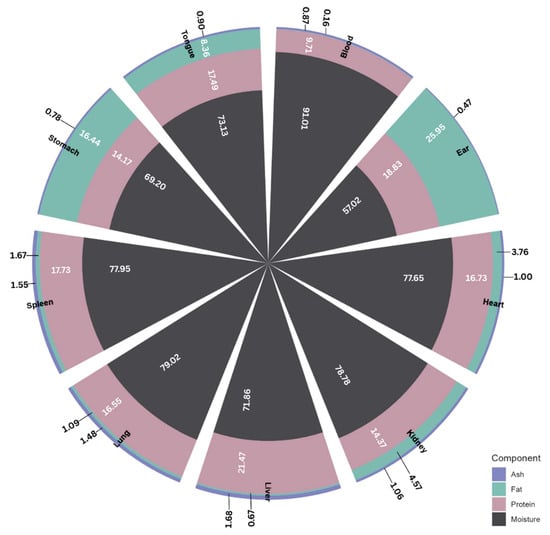
Figure 2.
Proximate analysis of select meat by-products from swine animal.
However, the utilization of meat secondary streams from the mammalian species is especially constrained by food safety and consumer acceptance issues, largely predicated on the advent of bovine spongiform encephalopathy (BSE) in 1982. As a biosecurity and consumer safety measure, the utilization of some of these by-products has been highly regulated in human foods and animal feeds, limiting innovation and economic returns from these secondary streams. Our previous work has highlighted some of the potential utilizations of these by-products within the context of the circular bio-based economy [25]. Moreover, meat secondary streams may also include cut-out trims and bones, meats that do not meet the hygiene standard for human consumption during processing, and expired meat from grocery stores.
2.2. Meat Secondary Streams from Aquatic Species
According to the Food and Agriculture Organization of the United Nations’ report, total fisheries and aquaculture production reached an all-time record of 214 million tonnes in 2020 [22]. About 90.3 million tonnes of these account for global capture fisheries production (including anchoveta, Alaska pollock, skipjack tuna, Atlantic herring, yellowfin tuna, blue whiting, European pilchard, Pacific chub mackerel, Atlantic cod, and largehead hairtail, among others), while about 123 million tonnes represents the production of other aquatic species which include crustaceans, molluscs, other aquatic animals, and algae [22]. However, there are several side streams produced during the processing of these aquatic species that constitute a challenge to global food security and environmental sustainability due to their inefficient utilization and improper disposal.
The non-fillet portion of fish—which includes the head, backbone/frame, tail, blood, skin/scale, fins, belly flap, and visceral parts—accounts for up to 70% of the total fish weight, depending on the size, species, and method of processing, and often has low-value utilization or ends up in landfills [26,27,28,29]. The processing of shrimp generates a large amount of solid waste in the form of head, shell, and viscera, constituting approximately 48–56% of their total weight [30]. According to Ganjeh et al. [29], globally, over 25.4 million tonnes of fish by-products are produced annually, 8–38% being crude protein. Similarly, a large percentage of small pelagic fish species which are usually not used or processed for human consumption purposes finds low utilization along the food value chain. These by-products contain high-quality proteins, long-chain polyunsaturated fatty acids, essential amino acids, vitamins, and minerals, all crucial for human nutrition and with the potential to be further explored in different applications [26,31]. A recent study highlighted the benefits of pre-sorting fish by-products prior to valorization for more optimal value derivatization [32]. Several authors have also explored the prospects of these by-products in protein hydrolysates [33], bioactive peptides [34], and functional additives [35] for food, feed, cosmetic, pharmaceutical, or other industrial applications.
2.3. Meat Secondary Streams from Avian Species
A big representative of the avian species is poultry, which are domesticated birds kept for their meat, eggs, and feathers. These include chickens, turkeys, ducks, geese, and guinea fowl, among others. Of these poultry birds, globally, chickens represent the vast majority at 63%, followed by ducks at 11%, geese at 9%, and turkeys at 5% [36]. With over 70 billion poultry slaughtered or used for meat annually, this animal species is fast becoming the most consumed animal protein source around the world [37,38]. While poultry has been identified as the most efficiently processed among the livestock sector [39], there are still secondary streams being generated that can pose undue burden on the environment and create disposal challenges for the industry. These could include feathers, egg shells, and internal organs, among others.
A major by-product of the commercial egg and hatching egg industries is the spent hens, representing over 6 billion birds globally as estimated in 2019 [40]. In Canada, for example, about 26 million hens produce more than 600 million dozen eggs annually. After about 52–60 weeks of laying eggs, however, these hens reach the end of their production cycle, resulting in significant decline in the rate at which they lay their eggs, making them less profitable for the producers. Producers have limited avenues to utilize or market these secondary streams, leaving a large proportion of them euthanized and inevitably destined for landfills or burial sites or at best composted or rendered. Previous works have shown that these spent hens are compositionally about 68% moisture, 24% protein, and 7% fats [41,42]. Similarly, spent ducks have also been identified as a major underutilized protein source that could find more value along the food value chain [43].
Additionally, chicken dark meat is another major underutilized meat commodity of the poultry processing industry. It usually commands low consumer acceptability due to its dark color, high fat content, and poor shelf-life stability [44]. The pale, soft, exudative (PSE)-like chicken breast meat has also been associated with some quality issues including reduced water-holding capacity and inferior texture, resulting in its low-value proposition and perceived quality downgrade [45]. Previous studies have explored protein extraction strategy as a way to add value to these underutilized commodities and potentially enhance their application in the formulation of further processed food/meat products [44,46,47].
3. Traditional Protein Extraction Methods from Meat and Meat By-Products
3.1. pH Shift Extraction
The pH shift protein extraction method applies the principle whereby in extreme acid (<3.5) or alkaline (>10.5) conditions, further away from the isoelectric point of the protein, proteins partly unfold and positive and negative charges on the myofibrillar, sarcoplasmic, and cytoskeletal proteins experience electrostatic repulsion and are driven further apart, resulting in solubilization of the proteins in water [48,49]. Generally, most muscle proteins are negatively charged at physiological pH. When the pH is lowered by adding acid, the protein side chains become positively charged due to the protonation of the amino group. When a base is added, however, the protein side chains become more negatively charged. As a result of this overall increased protein charge, individual proteins start to repel one another and the interaction between protein and water is enhanced, resulting in increased protein solubilization.
This method—also referred to as acid/alkaline solubilization, or the isoelectric solubilization/precipitation method [42]—has been widely employed in protein extraction from various animal protein sources, particularly by-products from aquatic species, including mussels [50], herring [51], tilapia [52], roach [53], Cape hake [54], harp seal [55], channel catfish, Spanish mackerel, croaker, and mullet [28,56], as well as from mechanically separated turkey meat [57,58], chicken dark meat [59], and the lungs, stomach, and small/large intestine of ox, sheep, and pig [14]. Aside from the benefits associated with the process being carried out under cold conditions, thereby helping the proteins to retain their functionality, Nolsøe and Undeland [48], Momen, Alavi et al. [47], and Kristinsson and Demir [56] have also highlighted many advantages of this method, including (1) the ability to directly mince raw materials without necessarily having to mechanically remove the bone and/or the skin since all contaminating materials with a different density from the proteins can be subsequently removed by gravity, (2) the possibility of efficiently removing neutral and membrane lipids, thereby limiting the risk of lipid oxidation during storage, (3) an increased protein yield due to co-recovery of sarcoplasmic protein in the process, (4) the relatively less labor-intensive nature of this process, (5) the absence of salt in the process of protein solubilization, (6) non-thermal pasteurization due to its antimicrobial potential, and (7) the efficiency in removing toxins such as dioxan, polychlorinated biphenyls, and mercury and arsenic.
The pH shift method of protein extraction started to gain popularity in the early 2000s when scientists at the University of Massachusetts patented the acid/alkaline solubilization process [60,61,62]. Although prior protein extraction methods have utilized similar principles, these scientists modified the experimental conditions to include a low temperature to ensure the functional properties (e.g., gel strength) of the proteins are protected [48]. In general, with this method, protein sources are minced and homogenized in about 5–9 parts of water. The pH of the homogenized mix is then adjusted to either 2.5–3.5 or 10.5–11.5. This condition allows for the solubility of the myofibrillar, cytoskeletal, and sarcoplasmic proteins. Following protein solubility at these pHs, the insoluble components are separated by gravity (e.g., centrifugation) and the supernatant from this process is precipitated by adjusting the pH of the solution to the isoelectric point of the proteins (typically between 5 and 5.5). The precipitated proteins are then recovered under centrifugation and isolated.
Protein recovery is largely determined by how soluble the proteins are at extreme pHs and has ranged between 19% and 96% in different experiments, especially with various aquatic species [47,48]. More importantly, variation in protein recovery from different studies is dependent on the type of aquatic species, the overall chemical composition of the raw material, the period of storage of the fish, whether acid or alkali was used in the extraction process, the type of acid/alkali that was used to achieve the extreme pH, and other variations (e.g., pH, fish-to-water ratio, temperature, time, etc.) inherent to each study. While several studies have reported higher protein recovery/yield from the alkali compared to the acid shift method in aquatic species [52,63,64,65,66,67], others have shown higher protein yield with the acid shift method [51,67,68]. Nolsøe and Undeland [48] concluded that the choice of the method may depend on the application, although the alkali shift method may have some advantages over the acid shift method, including a stronger gel strength, a whiter color, higher lipid removal capability, milder lipid oxidation, and a lower aerobic plate count. The authors further claimed that the functionality of protein generally appeared to be slightly improved following alkali compared to acid extraction processes. It is important to note that the pH shift method has been applied extensively for aquatic species, with only a few studies employing this method for other animal species. Among those are applications in porcine by-products [69], bovine by-products [70], chicken dark meat [44], and spent hens [40,42]. Protein recovery in these species range from 21 and 94%. More studies are still needed to evaluate the efficiency and adaptability of this method to protein extraction from varieties of meat by-products.
While several studies have pointed out the promising nature of this protein extraction method, one of the downsides is the amount of acid or alkali needed to achieve those extreme pHs and the cost and environmental implications of using these reagents. Also, the large volume of water that is needed to execute this process may pose a resource management challenge through overexploitation of water resources. A variation of this method includes salt in the extraction processes [71,72], because it is believed that salt potentially provides a stabilizing effect against protein denaturation. Other methods, mostly used in the laboratory environment to extract myofibrillar proteins from a variety of raw materials, have been described in the literature using phosphate buffer at a pH between 7 and 8 [12,13,73,74,75]. Zubair [42] described a salt-aided extraction method to separate and isolate sarcoplasmic, myofibrillar, and stromal protein using phosphate buffer in a step-by-step approach at different pHs. It remains to be assessed, however, how this method could be adopted in a commercial protein processing environment.
3.2. Salt Extraction
In the laboratory setting, salt soluble proteins such as myofibrillar proteins can typically be extracted using high-ionic-strength solutions, mostly sodium chloride (NaCl). The process involves homogenizing minced meat in buffered salt solution for a specified period with subsequent centrifugation to separate salt soluble proteins (supernatant) from the insoluble components [76]. Aside from the impact of salt type and concentration, other factors such as pH, dilution ratio, mixing conditions, time, temperature, muscle fiber types, and post-mortem storage have been recognized to impact protein extractability and yield during the salt extraction process [76,77,78,79]. In general, studies have shown that a pH above 6 maximizes protein extraction, and protein solubility increases with NaCl concentration [76,80,81]. A salt concentration between 9 and 12% has been identified as most effective for protein extraction from ground meat [78]. Munasinghe and Sakai [82] compared the protein extractability of NaCl to that of potassium chloride (KCl) and lithium chloride (LiCl) at a particular range of molarity within specified physiological pHs. They concluded that NaCl (1.2 M) had the highest protein extractability followed by LiCl and KCl (1.1 M). Furthermore, Gordon and Barbut [83] have shown that monovalent chloride salts (e.g., NaCl, KCl, and LiCl) extracted six native proteins (including actomyosin, 520 kDa; myosin, 425 kDa; Z-nin, 300 kDa; myomesin, 157 kDa; synemin, 240 kDa; and an unidentified 260 kDa protein) from lean chicken breast meat, and that the amounts and ratios of proteins extracted from the lean meat could vary with these monovalent salts. At this time, studies specifically employing salt extraction in meat by-products are very scarce. In the early 1970s, Young and Lawrie [84] evaluated the effect of salt concentration on protein extractability from sheep lung and stomach tissues over a range of pH values. They observed decreased protein extraction with increasing salt concentration, even at extreme pH values. In fact, 1 M NaCl solution significantly inhibited protein extraction from these by-products, undermining the suitability of this method for these tissues. Dewitt et al. [85] even found that there is a significant reduction in recovered protein from beef heart when sodium chloride is added. Other studies also reported more water-soluble proteins (21–79%) compared to salt soluble proteins (5–23%) in pork liver [86], turkey liver [87], buffalo liver [88], and other meat by-products [89,90,91], with the former largely dominated by low-molecular-weight proteins whereas the later mostly comprised high-molecular-weight proteins. These studies also demonstrated the significant impact of salt in the extraction process on the functional properties (emulsification, gelling and foaming) of the extracted proteins. While it seems evident that salt extraction may not be the most ideal for these by-products, more studies are still needed to further verify this claim and evaluate the practicality, feasibility, environmental implications, and economy of employing this method of protein extraction for animal by-products derived from different animal species, especially in commercial settings.
3.3. Chemical Hydrolysis
Characterized by its ease and suitability for protein production at the industrial scale, chemical hydrolysis is a cost-effective protein extraction process that utilizes acids or bases. For example, collagen and hydrolyzed collagen (gelatin) are predominant proteins from fish processing by-products, and acid extraction has been widely employed for the isolation of acid-soluble collagen [92]. Previous studies have employed different acid species including lactic acid, formic acid, citric acid, and acetic acid for the extraction of collagen from different fish processing by-products, with recovery yield ranging between 12% and 70% depending on the tissue [93,94,95,96]. These chemicals disrupt the crosslinks of the collagen helix, thereby enhancing the extraction process. Both sulfuric and hydrochloric acids at high temperature and pressure, as well as sodium, calcium, and potassium hydroxides, are also commonly used in this chemical hydrolysis process [92,97]. Although this method may be cost-effective and convenient, it is less environmentally friendly due to the use of these chemical species. Also, it is limited due to its severity and the non-specific nature of its peptide production.
3.4. Denaturing Solutions and Detergent Extraction
In the past, anionic detergent (e.g., sodium dodecyl sulphate, SDS) has also been utilized in laboratory settings for protein extraction from meat industry by-products with recovery rates between 68 and 82% [14,98]. The study by Malva et al. [12] comparing denaturing (solutions containing urea, thiourea, and dithiothreitol) and non-denaturing protein extraction methods for meat and fish found that the former resulted in good extractability of proteins and fragments with a low molecular weight such as actin, tropomyosin, and myosin light chain 1 and 2, whereas the latter led to major extraction of high-molecular-weight proteins, such as myosin heavy chain, α-actinin, and desmin. Some reports have identified this method of protein extraction using SDS as a potential solution for the toxic lysinoalanine that could be formed when alkaline extraction is executed at elevated temperature (>60 °C), with the intention of improving protein yield [99]. The recovered proteins from this process have also been reported to be bland and possess improved functionality [100]. More recently, Khumalo et al. [101] optimized the extraction of keratin from waste chicken feathers using a mixture of SDS, sodium bisulphite, and urea. However, the use of a detergent like SDS has declined significantly in food research settings, especially due to its affinity to bind with extracted proteins and the inherent difficulty of eliminating it from the SDS–protein complex [99]. This inevitably leads to the utilization of more solvents (e.g., 40% methanol or 60% acetone, with 10% KCl) to ensure complete removal with no residue of this detergent in the extracted protein [100]. This comes with increasing safety, cost, and environmental concerns, limiting their wide utilization for protein extraction in large-scale food applications.
3.5. Surimi Extraction/Processing
A traditional protein extraction method worth mentioning due to its success in the recovery of fish protein is the surimi processing technology. This process that originated in Japan more than 900 years ago involves repeated washing of minced raw fish materials in cold water (5–10 °C) or a slightly alkaline solution with the intent of concentrating functional myofibrillar proteins and removing undesirable flavor components, pigments, blood, and fat [102]. The surimi process provides an opportunity to add value by extracting proteins from underutilized fish species with little to no commercial value possibly due to their small size or some inherent technological issues [103]. The complete surimi processing steps involve fish harvesting, offloading/sorting, heading/gutting, deboning/mincing, washing/dewatering, refining, screw pressing, stabilization using a cryoprotectant, and freezing [104]. The protein yield from surimi processing has progressively improved from the early 1980s due to advancements in the field. The fish species and the processing parameters employed also impact the yield from this process [104,105]. Several studies have reported between 50 and 70% protein yield from different species [56], but when compared to pH shift methods, a lower protein yield has always been reported for surimi processing [67]. Surimi comes in block form, is stored frozen, and is primarily used for gelling foods such as kamaboko, fish balls, crabsticks, crab legs/meat, and others [102].
While this method has undergone several developments over the years, it has mostly been applied to both cold water and tropical fish species, with limited application to other meat species or meat by-products. However, some studies have applied similar processes to mechanically recovered poultry meat [106,107], chicken breast [108], spent hens [109], mechanically separated pork [110], chopped beef and pork [111], pork spleen [112], and beef heart [113]. Tina et al. [114] highlighted the potential and drawbacks of this technology in the valorization of meat secondary streams, especially in the poultry sector. Although this process could generally be beneficial for protein functionality, the surimi process requires large amounts of fresh water and discharges copious amount of wastewater with high organic load, potentially posing resource management and environmental issues [103,115]. Furthermore, aside from the lower protein yield compared to the pH shift method, the surimi process still leaves significant wastes as the parts of fishes typically used in the process are fillets, leaving the other by-products as potential waste streams.
4. Novel Methods for Protein Extraction
Over the years, researchers have explored new ideas to mitigate the inadequacies of traditional protein extraction methods. Some of these recent advances are discussed in this section.
4.1. Enzyme-Assisted Extraction
Enzymes are catalysts that are effective in small quantities under favorable reaction conditions. Proteases derived from plants, animals, and microorganisms have been used to aid the extraction of proteins and bioactive peptides from animal by-products. Proteases have been broadly categorized into aspartic, cysteine, glutamic, metallo-, asparagine, serine, and threonine proteases based on their principal catalytic residues [116]. For protease-assisted hydrolysis of animal proteins, alcalase and neutrase [117], pepsin from porcine gastric mucosa [118,119], and keratinase [120] are among the enzymes commonly utilized. Being the most used method in the production of hydrolysates and bioactive peptides [121], enzyme hydrolysis primarily helps to release more reactive R-groups, which are normally inactive in the complex structure of parent proteins through the unfolding of the protein chain structure, thereby promoting hydrophobicity [122] and in turn encouraging their precipitation. The characteristics of proteases, in terms of their cleavage site specificity, optimal temperature, optimal pH of activity, enzyme-to-substrate ratio, and time of exposure to the peptide bonds of proteins, all determine the size, composition, and molecular weights of the proteins or peptides that result from their hydrolytic actions [121]. Therefore, the sequences of bioactive peptides from a protein reserve depend on the individual proteases or their combinations used for hydrolysis. Pretreatment methods such as hydrothermal [123,124] and ultrasonic [125] treatments have been applied to processed proteins from animal by-products and collagen to break complex protein bonds and improve enzyme accessibility and hydrolysis.
Meat (mixture of pork and beef residues; chicken) and fish (salmon viscera) by-products were subjected to endogenous and exogenous (alcalase 2.4 L and papain from Carica papaya) enzymatic hydrolysis to produce protein-rich hydrolysates [126]. While endogenous enzymes were found to achieve a level of hydrolysis of proteins, the addition of alcalase and papain improved protein solubilization, yield, and protein recovery at an enzyme/substrate ratio as low as 0.05% in the beef/pork mixture and 0.02% in chicken by-products and salmon viscera over an extended time of hydrolysis (up to 2 h). The same authors also reported a reduction in peptide chain length and molecular weights in pork/beef and chicken by-products with the addition of exogenous enzymes. However, this was not the case with salmon viscera, which showed no significant difference in the sizes of peptides for exogenous and endogenous enzyme activities. Plant proteases from Bromelia karatas and Bromelia pinguin had comparable effects on chicken and fish by-products, as with the use of a commercial exogenous enzyme (bromelain), as they produced protein hydrolysates with over 50% bioactive peptides (<1.35 kDa).
In most cases, low-molecular-weight proteins/protein hydrolysates result when enzymes are employed in protein extraction. Consequently, the enzyme type, the amount used, and the processing conditions will have significant effects on the degree of hydrolysis, protein recovery, and the physicochemical properties of the resulting protein hydrolysates [92]. While this method has been widely applied to fish and aquaculture by-products, enzyme-assisted extraction has also been used to extract protein from chicken skin, a major by-product of the chicken processing industry [127,128]. These authors employed commercial proteases, including alcalase, protamex, pancreatic trypsin, neutrase, and flavourzyme, at an enzyme-to-substrate ratio of 2:100 (w/w) for enzymatic recovery of protein hydrolysates from chicken skin. Alcalase yielded the highest nitrogen content and hence protein recovery (66%) compared to the other enzymes. All enzymes generated protein purity between 87 and 92%, with alcalase resulting in the highest purity overall. Although their main objective was not protein extraction, other authors also applied similar techniques to recover protein hydrolysates from goat viscera [129], sheep viscera [8], pig bone [130], bovine blood [131], sardine by-products [132,133], raw herring [134], eggshell membrane [135], blue shark skin [136], by-products of Atlantic salmon and yellowtail kingfish [137], and a combination of meat waste [138]. Other studies also employed papain or bromelin [139,140], pepsin [141,142], and alcalase [143] in the optimization of collagen extraction from chicken feet. This method has been reported with enhanced functional properties compared to other traditional protein extraction methods.
Although the cost of the enzymes, the environmental dependence of the process (e.g., pH, incubation temperature), and the specificity of the enzymes may be the limiting factors in the commercial application of the enzyme-assisted protein extraction method, it has the benefit of being a fast, safe, and easily controlled technique with low energy consumption. Furthermore, enzymatic hydrolysis of protein could enhance some important biological activities and functional properties of protein, thereby expanding their technological, functional, and sensorial applications. Protein hydrolysates of filefish by-products obtained from the action of different proteases have been found to have antimicrobial properties. In the study by Hatab et al. [144], pH shift was used to extract proteins from filefish by-products before subjecting the extracted proteins to the activities of different proteases, including neutrase, papain, pepsin, and trypsin at a 1.5 w/w enzyme/substrate ratio and their optimum conditions of activity. They reported that protein hydrolysates obtained after 120 min hydrolysis of filefish by-products with trypsin and papain and their mixture inhibited the growth of Staphylococcus aureus, Bacillus cereus, Salmonella enteridis, and Escherichia coli. Several authors have also elaborated on several biological activities of protein hydrolysates extracted from livers of different animal species [145,146]. Enzymes, both endogenous and exogenous, in their optimum conditions and aided by pretreatment methods can reduce high-molecular-weight complex storage proteins in meat and fish by-products to lower-molecular-weight bioactive hydrolysates and peptides, with various value-added applications along the food value chain.
4.2. Ultrasound/Cavitation-Assisted Extraction
Ultrasonication is considered a green technology and an improvement on the traditional extraction processes [147]. The ultrasonication principle of protein extraction efficiency is the cavitation effects of ultrasound, which could cause pressure fluctuation. This cavitation induces the formation, growth, and collapse of microbubbles at the center of an aqueous solution, allowing for cell disruption and mass transfer to the medium [148,149]. Acoustics, hydrodynamics, optics, and particle cavitation are the main types of cavitation. These cavitation types may be produced by varying cavitation production methods [150]. However, only acoustic and hydrodynamic cavitation generate the desired and suitable intensity for chemical or physical processes [149].
Ultrasound-assisted extraction (UAE) could enhance yield and protein recovery compared with those from typical processes for protein extraction [151]. The ultrasound-assisted process is a promising technique for the food technology sector, presenting low environmental impact and lower energy and solvent consumption (mostly using water as solvent), and it is in accordance with green chemistry technology and sustainable concepts [149]. Although UAE improves protein extraction, there are some procedure variables that still impair its effective application, especially at the industrial level, reinforcing the importance of further studies given its potential benefits [152].
Ran and Wang [125] compared the use of ultrasound pretreatment of pepsin extraction with an acetic acid extraction method for the extraction of collagen from cattle tendons. In this study, the ultrasonic and pepsin treatments in the presence of 0.5 molL−1 acetic acid led to a higher extraction efficiency of collagen from cattle tendon than that observed with pepsin treatment without ultrasonic treatment. A peak yield of 6.2% was reported, compared with the 2.4% yield achieved without ultrasonic treatment. The results show that the ultrasonic–pepsin tandem method can effectively improve the efficiency of pepsin extraction of natural collagen without compromising collagen quality. With respect to the available literature, UAE does not only improve the efficiency of protein extraction and recovery but also improves the functional properties of the extracted protein hydrolysates. Analysis of UAE protein hydrolysates by differential scanning calorimetry, circular dichroism, and fluorescence spectroscopy predicted that ultrasound treatment could cause partial protein hydrolysis and unfolding, leading to increased surface hydrophobicity, surface net charge, and gelling properties. Ultrasound also promoted the storage modulus and solubility of duck liver protein isolate [153]. In a similar study on the effect of ultrasound treatment on the functional properties of protein hydrolysates from chicken eggshell membrane, Jain and Anal [135] reported that the cavitation effect of the ultrasonication process denatured protein hydrolysates generated from the UAE pretreatment, which contributed to improved functional properties such as solubility, foaming and emulsifying properties, and water-holding capacity as compared to the untreated hydrolysates. During the ultrasonic process, the production of bubbles and the cavitation effect led to the shearing and heating of the material, which resulted in a high mass transfer rate and improved diffusion [135,154]. Furthermore, the cavitation effect of ultrasonication treatment was responsible for the high protein extraction yield and degree of protein modification (DPM) in the study by Duppeti et al. aiming to optimize UAE of flavor compounds from shrimp by-products [155]. The optimum DPM value indicated that sonication markedly influenced protein denaturation, as evidenced by the higher trichloroacetic acid-soluble protein content [155].
However, a serious drawback of ultrasonic irradiation for a long time is the cavitation effect that can give rise to high temperatures, high shear force, and high pressures inside the medium. It could also break the hydrogen bonds and van der Waals interactions in the polypeptide chains, leading to the denaturation of proteins/enzymes. Several authors have indicated that ultrasound waves can destroy the hydrogen bonds and hydrophobic interactions and thus unfold the proteins by changing their conformation (Table 2). Some amino acids in ultrasound pork liver protein isolates, such as serine, threonine, cysteine, and tyrosine, were reported to be significantly reduced compared to conventional pork liver protein isolate in a study by Zou et al. [156]. This trend was attributed to the heat generated by the ultrasonic cavitation and alkali treatment of pork protein isolate [156,157].

Table 2.
Potential methods for extracting protein from meat by-products.
4.3. Microwave-Assisted Extraction
Microwaves are non-ionizing electromagnetic radiations with a frequency range of 0.3–300 GHz, which corresponds to wavelengths of 1 mm and 1 m, respectively [182]. They are effective and selective non-contact heat sources that facilitate the transfer of energy and reduce thermal gradient. Microwaves can pass through mediums and barriers into biological samples to cause the desired absorption of microwave energy by the matrices of these biological samples, leading to the collision of molecules and pressure build-up, the generation of heat, temperature rise, a loss of moisture, the rupture of cells of biological molecules, and leaching or the release of high-value bioactive components [183]. Microwave-assisted extraction (MAE) generally involves the enhancement in the release of bioactive compounds from biological cells into extraction solvents, leading to improved leaching of these compounds into the solvents at reduced times and improved efficiencies compared to traditional solvent extraction methods [182,184]. The moisture content of the biological matrix is the main target of MAE, and the heat and mass gradients work in the same direction towards the openings of the ruptured cells, leading to increased yields at reduced times, while for conventional extraction processes, heat and mass transfer are in opposite directions in the cells, as heat flows from the heating medium into the cells while leached mass flows out [185].
The extraction of gelatin from the skin of different animals has been enhanced using MAE. Liu et al. [186] investigated the exposure (5–90 min) of rabbit skin to MAE during the extraction of gelatin (using water as solvent) and compared the yield, structure, and properties with those obtained from water bath extraction. Microwave treatment at 5–30 min yielded gelatin with higher strength compared to water bath extraction with more powerful triple-helix-like structures after 16–18 h of maturation. Pigskin gelatin was extracted using MAE (using water as solvent), as reported by Feng et al. [187] who reported an improvement in the solubility properties of pigskin gelatin, resulting from the breakdown of its polymer subunits at 25 °C. Also, MAE improved the hydrophobicity of gelatin as treatment time increased from 5 to 30 min, resulting in the reduction in surface tension, improved foaming properties, and increased interface properties (adsorption) at the oil–water interface. Microwave-assisted alkali extraction of proteins from chicken feathers was studied by Lee et al. [188]. Different sodium hydroxide concentrations (0.1–2 M) and microwave power levels (100–800 W) were combined to achieve morphological changes in chicken feathers towards the production of protein hydrolysates. This study reported that microwave–alkali-assisted extraction led to increased denaturation of keratin in feathers, thereby increasing the production of proteins (26.74 mg/mL) and amino acids (69.4 mg/g) in the hydrolysates, compared to autoclave–alkali and conventional heating–alkali extraction methods. This underscores the importance of microwave treatment in the extraction of proteins from animal by-products into smaller moieties and their enhanced release into the extraction solvents.
While this method is considered “green” due to its eco-friendliness, overall cost-effectiveness, reduced extraction time, decreased solvent volume, prevention of thermal degradation, and increased energy efficiency, all these benefits are dependent on a number of factors including the solvent system, solvent-to-feed ratio, microwave power and temperature, extraction time and cycle, and the nature of the feedstock matrix [9,167]. If these factors are not adequately optimized for specific process, it is possible that all potential benefits of this process may not be realized. The hot and cold regions formed within the sample matrix system may also allow for irregular and uncontrolled degradation that may impact the effectiveness and efficiency of this process [9] (Table 2).
4.4. Pulse Electric Field Extraction
Conventional extraction systems comprise cell damage practices and involve the use of substantial mechanical force or thermal energy and the use of hazardous chemicals for a longer processing time [189,190]. Emerging non-thermal technologies that use high voltage to generate an electric field and perform extraction are pulsed electric field (PEF) technology and high-voltage electrical discharge (HVED). As a substitute for the conventional method, the development of green extraction techniques such as PEF is seen as a significant step in recovering by-products from food wastes. PEF is reported as a novel technique that can decrease solvent usage, heating steps, and extraction time to recover by-products [190].
PEF technology involves placing a material between two electrodes, through which direct-current high-voltage pulses (kV) are applied in specific short time periods ranging between microseconds and milliseconds. The high-voltage pulses are responsible for the generation of an electric field, and with a high level of generated electric field, a phenomenon known as electroporation could occur [189,191]. Electroporation is a phenomenon that occurs when high-voltage PEF is applied on a biological cell, which can lead to the increase in membrane permeability of usually non-permeable molecules [192]. The electric field strength and the processing time are the main processing parameters that characterize PEF treatment. This treatment can efficiently optimize the extraction of valuable compounds within a shorter time while minimizing solvent (mostly water) and energy consumption [193].
Currently, PEF-assisted extraction has found several applications in the recovery process of valuable molecules from the food waste stream, with meat by-products not being an exception. It is an emerging technique that holds promise for the utilization of waste materials. As part of the effort to divert from conventional extraction techniques, Arshad et al. [190] suggested that PEF treatment of food wastes needs urgent models for optimum processing operation at the industrial level, particularly economic viability under practical working conditions as a response to the call for new approaches for meat waste conversion to value-added products. In a study that utilized PEF in the extraction of protein from mussel, Zhou et al. [169] observed higher protein extraction yield with PEF (77.1%) compared to other traditional protein extraction methods. Ghosh et al. [194] used the combination of PEF and mechanical pressing to successfully extract functional protein molecules from deteriorated chicken breast meat. Although these authors did not compare the result of their PEF study with traditional protein extraction methods, they concluded that this method can be utilized for optimized extraction of functional molecules from the waste meat biomass using this non-thermal, chemical-free process.
It is important to note that the efficiency of PEF-assisted extraction is influenced by the characteristics of the tissues and cells under extraction [193,195,196]. Other important factors include the electric field strength, the number of pulses, time of exposure, and the ratio of material to solvent, among other extraction conditions [169,170]. A study that applied PEF for protein extraction from abalone visceral mass concluded that the optimal conditions for its protein extraction were a ratio of material to solvent of 4:1, PEF intensity strength of 20 kV/cm, and treatment time of 600 μs. Wang et al. [197] utilized PEF for protein extraction from the head, skin, and viscera of rainbow trout and sole fish by applying electric field strength ranging from 1 to 3 kV/cm, with energy levels between 123 and 300 kJ/kg, for 15 to 24 h. Protein extraction efficiency of about 80% was reported. Also, a significant enhancement in the antioxidant capacity (Oxygen Radical Absorbance Capacity, ORAC) in extracts obtained from the skin and head of rainbow trout and sole fish was observed, which was attributed to the PEF treatment [193]. In another study by La Fuente et al. [198], the influence of pulse electric field-assisted extraction on the extraction of protein from sea bass by-products indicated an increase in the protein content (1.2–4.5 times) of sea bass side stream extracts under optimal PEF conditions when compared to conventional extraction methods. The PEF technique has been found to be useful in improving protein extraction rates and yields and maintaining the extracted protein quality [29].
The primary advantage of PEF as a pretreatment for enhancing extraction processes is its non-thermal nature compared to pretreatments based on heating [191]. No significant increase in the temperature of the matrix results during electroporation due to the low energy requirements for electroporating cells, preventing the negative effects of heating on the quality and purity of the extracts. Though PEF has been recognized as a non-thermal technology, ohmic heating can be generated under high treatment intensity, which can have negative effects on the quality and appearance of solid food materials, such as cooking effects on fresh meat [199,200]. Moreover, the equipment for PEF requires a high economic investment, as it must be specifically designed and adapted for each type of sample matrix. Among other limitations, the electrochemical reactions that take place between the electrodes and the surface of the material when a current is applied, which include fouling and corrosion of the electrodes, electrolysis of water, migration of electrode components, and chemical changes in the by-product extract stream (protein extract in this case) during treatment, are some of the identified challenges attributed to the use of the PEF-assisted extraction process [201,202] (Table 2).
4.5. Supercritical Fluid Extraction
Supercritical fluids (SFs) refer to solvents in a critical state where both their pressure and temperature are above the critical point such that they are conferred with unique intermediate characteristics of transportability like gases (e.g., high permeability and low viscosity) and the solubility power of liquids [29,203]. In this state, the density of an SF (which can be modified by changing its pressure and/or temperature) is similar to a liquid, its viscosity similar to a gas, and its diffusivity intermediate between the two states [204]. These properties enable SFs to more efficiently penetrate solid matrices and enhance the extraction of the desired compounds. Although various solvents (e.g., methanol, water, carbon dioxide, n-pentane, etc.) can be utilized as SFs, the critical properties of pressure and temperature, toxicity, price, and solvation power of the solvents need to be considered when selecting the most suitable solvent for any particular application [172,205]. Generally, supercritical fluid extraction (SFE) is said to be an alternative to conventional protein extraction methods, with advantages such as a cheaper cost, ease of accessibility, high solute diffusivity, ease of recycling, lower viscosity, lower solvent consumption, high reproducibility, high quality of final product, and the possibility of controlling the solvating power through the adjustment of temperature and pressure [29,206]. For most supercritical extraction methods, carbon dioxide (CO2) has been widely preferred due to its safe, non-toxic, non-flammable, non-corrosive, cost-effective, easily available, and easily recoverable nature. Its low-value critical parameters (temperature of 31.2 °C and pressure of 73.8 bar), as well as its favorable physicochemical properties (including its inert, nonpolar, non-flammable, odorless, and tasteless nature), make it especially desirable as an effective SF for a range of applications [172,173,204]. However, due to its low polarity, supercritical CO2 (SCO2) is less effective in extracting more polar compounds and as such requires some polar modifiers (or co-solvents, e.g., ethanol, methanol, water, etc.) to improve its extraction efficiency.
Proteins are not very soluble in supercritical SCO2. This could explain the limited application of SFE for protein extraction in the literature [207]. This method has been mostly applied to the extraction of some other valuable compounds from plant and animal-based matrices (especially waste from aquatic species) including antioxidants, polyunsaturated fatty acids, vitamins, essential oils, and natural pigments, among others [208,209,210]. Furthermore, while SFE of proteins from biological materials can employ water as an SF [9,211], its practical application for protein extraction has been limited because the high temperature (above 374 °C) and pressure (above 220 bars) needed to achieve this critical point for water may result in significant challenge for equipment design and overall personnel safety. Furthermore, this condition may also pose issues with energy consumption, corrosion of construction materials, and potential degradation of some heat-sensitive compounds [212]. In the literature, the SFE method has generally not been widely employed in the valorization of meat by-products. Hence, more research is needed to explore and optimize this technology using different green fluids for the efficient extraction of proteins from meat by-products for higher-value utilizations.
Some studies have attempted to extract proteins from seafood by-products using SFE. Park et al. [213] performed supercritical CO2 (SCO2) extraction from mackerel visceral under a constant pressure of 25 MPa and a temperature range of 35 to 45 °C. They observed an increase in the extracted total amino acids from SCO2-treated samples (48.8 mg/100 g) compared with untreated samples (33.7 mg/100 g). The authors further concluded that SCO2 treatment minimized the denaturation of proteins and could be a better replacement for conventional solvent extraction. Rahman et al. [214] treated bovine heart, an important meat by-product rich in myofibrillar proteins, amino acids, and other bioactive substances, with SCO2 at 40 °C and three pressure regimes (20, 30, and 40 MPa). They also reported significantly higher protein (80.27 g/100 g at 30 MPa and 82.67 g/100 g at 40 MPa) and total amino acids (80.39 g/100 g at 30 MPa and 79.05 g/100 g at 40 MPa) in defatted bovine heart compared with the control sample extracted with organic solvents under atmospheric conditions (70.62 g/100 g proteins and 68.993 g/100 g total amino acids). In other approaches, SCO2 extraction has been used as an important preliminary step for the extraction of pure protein hydrolysates from fish by-products. In separate attempts, Haque et al. [215] and Melgosa et al. [207] used SCO2 to initially defat by-products of yellow corvina and sardines, respectively, and then applied subcritical water extraction to obtain fish protein hydrolysates. For supercritical defatted sardine wastes, protein contents were higher in their fish protein hydrolysates (91.3 g/100 g) at 140 °C of SCO2 extraction and hydrolysis than those of the control samples (70.0 g/100 g) at the same temperature [207]. For SCO2 defatted yellow corvina head and viscera wastes, amino acid contents were improved by initial extraction and were found to be dependent on the subcritical water extraction method applied. The application of SFE has potential for increased protein extraction from meat/fish by-products, whether used as the direct extraction method, with a solvent modifier, or as a precursor to other extraction methods.
4.6. Liquid Biphasic Floatation
Liquid biphasic flotation (LBF) is a well-known technique for separating, concentrating, and purifying biological materials like protein and DNA [216]. This method has been extensively used in extracting and separating proteins from microalgae [217,218,219,220,221]. Relatively fewer studies have reported on LBF protein extraction from plants [222] and animal sources [216]. The technique is based on the principle of utilizing two immiscible liquid phases (they do not mix but can co-exist in a system) to selectively extract and purify proteins from complex mixtures.
A typical example of the two liquid phases is a polymer (e.g., polyethylene glycol) and a salt solution (e.g., ammonium sulfate) [216]. Proteins distribute these phases based on their solubility, size, charge, and hydrophobicity. Air bubbles are introduced into the system in order to concentrate the protein molecules in the upper liquid phase for easier recovery. The bubbles attach to the surface of the liquid phase, hence the term ‘floatation’.
The LBF procedure operates under relatively mild conditions due to the low interfacial tension between the two phases [216], which helps to retain protein structure and function. The specific partitioning of proteins also ensures high purity levels and recovery yields. The LBF method scores high on simplicity and scalability, making it suitable for both laboratory and industrial applications [219]. Chia et al. [223] went on to use a tri-phase flotation system, an enhanced version of the LBF, to extract and purify protein with better results. The optimized conditions of 70% v/v of t-butanol, 40% w/v of salt solution, 0.5% w/v of biomass, pH 5.54, a 1:1 ratio of salt to t-butanol solution, and 10 min of air flotation achieved up to 87.2% protein recovery and 56.7% separation efficiency [223].
4.7. Deep Eutectic Solvent Extraction
A deep eutectic solvent (DES) is an environmentally friendly solvent that is obtained by mixing the components capable of forming a eutectic mixture. It is also known as an ionic liquid analogue [224]. DESs, along with their bio-analogues, natural deep eutectic solvents (NaDESs), are presently evolving as a novel category of favorable liquid media to eliminate harmful organic solvents while moving towards the ecological extraction methods [225]. NaDESs are green solvents used in several applications as they present promising advantageous characteristics due to their low cost, low to non-toxicity, and biodegradability for environmentally friendly processes [226,227,228,229]. DESs are characterized by biodegradability, sustainability, low volatility, low vapor pressure, low toxicity, non-flammability, and chemical tunability [230].
In a study by Abbott et al. [231], DESs were obtained by mixing choline chloride (melting point, 302 °C) and urea (melting point, 133 °C) in a molar ratio of 1:2. This eutectic mixture had a melting point of 12 °C, which was much lower than that of each component in the solvent [174]. The melting point of a DES is an indicator that directly determines the lowest limit of its application temperature, which can be used as an indicator of its applicable extraction temperature range. Combining DESs with other novel extraction methods for protein and bioactive compound extraction from meat by-products has been reported to produce greater yield recovery. The use of UAE along with DESs is a powerful method which can break the structure of cell wall and release intracellular bioactive compounds [225].
NaDESs were used to extract bioactive compounds from marine by-products: codfish bones, mussel meat, and tuna vitreous humor [226]. The DES was prepared using lactic acid, fructose, and urea. Extracts obtained from mussels were found to be mainly composed of proteins and lipids, although this high protein content was not only attributed to the use of the DES but also due to the structural composition of the material. In a similar study by Rodrigues et al. [232], the application of betaine/polyol-based deep eutectic systems was explored for the recovery of bioactive protein derivative-rich extracts contained in sardine processing waste streams. Extractions using DESs as alternative solvents were compared to conventional solid/liquid extraction with water. In that study, the impact of different process parameters (operating temperature [25, 45, and 80 °C] and extraction time [6 and 18 h]) on the extraction performance of DESs was evaluated for a fixed solid/liquid ratio (1:80 gdry residue/gsolvent). The study showed that protein yields slightly increased with a decreasing solid/liquid ratio. The extracts obtained with DESs also presented some significant differences in amino acid composition when compared to the water extract.
The protein extraction efficiency of DESs in the studies stated earlier are in corroboration with the review by Zhou et al. [174], with the extraction efficiency of protein by DESs being over 90% in most studies. This high extraction efficiency is obtained following the optimization of several DES systems prior to the main extraction. Despite the high extraction efficiency of DES-assisted protein extraction, it is necessary to overcome the challenges of recovery and recycling of DESs to improve the feasibility and efficiency of such extraction processes, as there are factors that affect the recovery and efficiency of extraction processes using DESs, which include but are not limited to the effect of the solid-to-solvent ratio, temperature, time, pH, and viscosity, as reported by Saini et al. [225].
The main disadvantage of DESs in extraction is high viscosity, which hinders the dissolution of proteins and affects the solvent mass transfer phenomena, heat transfer rate, and conductivity [174]. This advanced approach has various advantages which include a higher extraction efficiency, reduced processing time, and low solvent requirement compared to conventional extraction methods with organic solvents [225,233,234] (Table 2).
4.8. Electroactivation Methods
Electroactivation methods use electrical energy to enhance or modify the extraction proteins. When electrical fields are applied, they alter the properties of the solvent or the biological material itself, facilitating protein release and limiting or completely eliminating the need for chemicals in extracting proteins [235,236]. Hence, it is referred to as a “green technology”. When an electrical current is passed through water, resulting in its electrolysis, it generates two reactive solutions, the anolyte and catholyte. The anolyte (acidic water) contains active oxygen species like ozone or free radicals, which can break down cell walls or membranes, making proteins more accessible. The catholyte (alkaline water) has an increased hydrogen ion concentration, which facilitates the solubilization of proteins. Electric activation methods were successfully applied in plants [235], comparable even to conventional methods that use chemicals [236]. Ghosh et al. [194] extracted proteins that showed antioxidant properties from waste chicken breast muscle. A related study by Robin et al. [237] also retrieved bioactive dipeptides from chicken meat. In the aforementioned chicken studies, electroactivation was coupled with mechanical pressing. Electroactivation was also used to improve the solubility and emulsifying properties of whey protein-only samples and also whey–canola composite protein samples [238]. Electroactivation alters the ionic environment (pH and oxidation–reduction potential), which in turn affects protein charge. This alters protein–water interactions and the tendency to aggregate, hence improving their solubility and extractability.
In electric activation, the electrical fields can also directly disrupt biological cell membranes. This results in pores in the membranes, and hence the process is often called electroporation. Because of the pores formed, intracellular proteins are more easily released into the medium. Electroporation has been successfully demonstrated in microorganisms [239] and microalgae [240].
4.9. Thermal Hydrolysis
Thermal hydrolysis, also known as superheated water extraction [241], uses high temperature and pressure to break down proteins into smaller molecular peptides and volatile fatty acids [242]. The high temperatures (usually above 100 °C) disrupt bonds that stabilize the complex structure of proteins—hydrogen bonds, disulfide bridges, and hydrophobic interactions. The protein then unfolds, facilitating its hydrolysis. Thermal energy also cleavages the peptide bonds between amino acids resulting, in shorter peptides and free amino acids. When protein is broken down into smaller fractions, it generally becomes more soluble.
The pressure aids in raising the boiling point of water, allowing higher temperatures to be used without evaporating the liquid. This increases the efficiency of bond breaking in protein structures. Pressure also allows for deeper heat penetration into the sample, improving the efficiency of hydrolysis. In addition, depending on the selected parameters, this process can also help with completing the inactivation of any biological agents, including prions, leaving behind the primary beneficial components, such as amino acids, fatty acids, and minerals, which can be reformed into safe, valuable nutrient products for different industries [243,244]. This point makes this method ideal for meat by-products from ruminant animals.
This method has been widely applied at varying pH ranges with great success in the treatment of sludge from wastewater treatment plants [245,246] and value recovery from specified risk materials [247]. In addition to the recovery of proteins and other biological molecules, this method, when applied to sludge, confers several advantages; improving biogas production, destroying pathogens, and reducing sludge volume and odor. Wang et al. [248] recovered close to 50% of hydrolysates on a dry matter basis from tilapia skin using thermal hydrolysis. However, in the same study, enzymatic proteolysis fared better based on the reaction time, the use of chemicals, and better control of the size of resulting peptides. With some modifications to improve efficiency, Tasaki [241] designed a two-stage thermal hydrolysis process and successfully extracted keratin (70%) from hog hair, a significant achievement compared to no or poor keratin yield from keratinous animal body parts in previous attempts [249,250]. This novel approach had the added advantage of not requiring chemicals, making it environmentally friendly, but it could be cost-intensive in terms of equipment acquisition and process scale-up.
4.10. Pressurized Liquid Extraction
Pressurized liquid extraction (PLE), also referred to as accelerated solvent extraction (or sometimes as pressurized fluid extraction, pressurized hot solvent extraction, subcritical solvent extraction, or high-pressure solvent extraction), is a high-throughput, clean, and green extraction technology that employs solvents below their critical point to extract a wide range of organic compounds from biological, environmental, and food matrices within a short period of time [29]. The concept of PLE is based on the application of high pressure to maintain the solvents in liquid state at temperatures higher than their atmospheric boiling points [251,252]. Among other changes in the physicochemical properties of solvents that occur during PLE are (1) an enhanced mass transfer rate and (2) decreased solvent surface tension and viscosity. All these result in increased solubility of analytes, deeper and easier penetration of solvents into the sample matrix, and an overall faster extraction process and higher extraction yield compared to conventional extraction methods [251,253]. Ultimately, PLE results in reduced extraction time and a reduced amount of solvent used.
Among solvents that have been utilized with this advanced technique to extract various organic components, the authors have reported using ethanol, hexane, ethyl acetate, ethyl lactate, propane, n-butane, or D-limonene, highlighting the versatility of PLE [203,252]. The possibility of using non-toxic solvents such as water as an extraction solvent makes this method particularly attractive within the context of environmental sustainability and cost-effectiveness. When water is used as extraction solvent, this method is usually called subcritical water extraction [254,255]. Subcritical water extraction uses the unique properties of subcritical water held at sufficient pressure (1–22.1 MPa) and temperature between its boiling point (100 °C) and its critical point (374 °C). At this state, the viscosity, dielectric constant, and surface tension of water will gradually reduce as temperature and pressure increase but its diffusivity properties get better due to the disruption of hydrogen bonds.
As it relates to animal-based tissue matrices, PLE has been mostly applied to the extraction of high-value-added components from fish and aquaculture side streams. In a study aiming to optimize green PLE techniques to extract protein from sea bass processing side streams, de la Fuente et al. [198] observed that PLE-assisted extraction improved protein extraction by about 1.2 to 4.5 times compared to the conventional extraction process and even in a shorter extraction time period. A protein recovery rate of up to 61% was observed for the visceral tissues. Furthermore, the authors observed that proteins extracted using the optimal PLE extraction method contained a larger amount of lower-molecular-weight protein fragments compared to those extracted with the traditional method, which subsequently improved the total antioxidant capacity of the PLE-extracted protein compared to that of the control, as reported in a previous study. Similar results were reported when PLE was applied to extract protein from salmon [256] and gilthead sea bream [257] processing side streams, with protein recovery of up to 92 and 78%, respectively, from the visceral tissues and overall enhanced total antioxidant capacity.
Other studies have also applied the optimized subcritical water extraction method to extract chitin from shrimp cephalothorax by-products [258], amino acids from waste fish (white croaker) entrails [259], protein hydrolysates from shell fish waste streams [260], and bioactive protein extracts from cod frames [261]. All these studies concluded that subcritical water extraction could be a viable alternative for the valorization of waste streams from the meat industry. Surprisingly, however, no studies have applied this method to by-products from other animal sources. While this process has limitations in not being a selective extraction process and not being suitable for thermosensitive compounds, potential opportunities could be explored using this process to extract protein from other animal by-products. However, although PLE is considered a fast, simple, effective, and easily automated extraction process (e.g., Dionex ASE 200/300 and Buchi and Fluid Management Systems), its application at the industrial level is still in its early stage and more work is still required to make it applicable for commercial operations.
4.11. Steam Explosion-Assisted Extraction
Although first invented by Mason in 1928 and widely used for the pretreatment of lignocellulosic biomasses, especially cellulose, hemicellulose, and lignin, steam explosion technology is fast becoming an innovative thermophysico-chemical process used on a wide range of biomasses including meat by-products due to its lower environmental impact, lower energy consumption, and overall treatment efficiency and cost effectiveness [262,263]. Steam explosion (SE), also referred to as instant catapult SE, steam flash explosion, and high-density steam flash explosion technology in the scientific literature, involves subjecting specific biomass to high temperature (between 110 and 260 °C) and pressure (0.04–5 MPa) for a predetermined short period of time. This period is followed by sudden explosive decompression (≤0.00875 s), converting thermal energy into mechanical energy, which results in cell wall rupturing, permitting easier release of small-molecular-weight components from the matrix and increasing the sample contact surface area [262,264]. The kinetic energy generated during the steam explosion disrupts the protein microstructure and initiates the release of a large number of fragmented cells, increasing the protein extraction yield. Moreover, this process may alter the structure of protein, its groups, and intermediates, impacting its functional properties. Overall, SE which provides mechanical destructuration through a combination of vapocracking and explosive decompression [265], may facilitate biomass’ solvent and enzyme accessibility, as well as improving digestibility and fermentation.
Dong et al. [180] applied SE to fresh bighead carp backbones using saturated steam at 159 ± 0.5 °C for 2 min. This procedure led to about 54% protein recovery without decalcification steps and without the use of toxic reagents. Although these authors did not demonstrate the impact of SE on the technological/functional attributes of the extracted protein, it seems evident that this procedure exposed more enzyme sites for subsequent hydrolysis reaction, resulting in accelerated peptide release and hydrolysates with predominantly <3000 Da molecular weight fragments following three enzymatic hydrolysis cycles. This could have also contributed to the higher free radical scavenging activities for the SE-treated protein compared to an alternative extraction method. Similarly, Zhang et al. [181] used SE (1.5–2.5 MPa, 200–245 °C for 10–30 min) to extract collagen peptides from cattle bone. Up to 62.5% protein recovery was recorded. These authors also found that SE facilitated the release of peptides from the collagen by destroying both peptide and disulphide bonds as well as enzymatic crosslinks with a significant reduction in molecular weight.
Other studies have applied this method for the extraction and/or bio-utilization of keratin from wool [266,267], feather [268,269], and hoof shell [270], as well as to valorize bone [271]. Furthermore, Scopel et al. [272] applied SE for the extraction of gelatin from chromium tanned leather wastes, resulting in up to three times the extraction yield compared to the control. It is important to note that optimizing processing parameters (e.g., time, temperature, pressure, and explosion power density, among others) for SE extraction will be crucial to ensure that protein functionality is not sacrificed for extraction yield.
While this method has been widely acclaimed for its economic, environmental, and efficiency prospects, it seems to lack in the aspect of protein recovery, with fairly low recovery rates generally reported in the literature (Table 2).
4.12. Others Emerging Methods and Combinations of Technologies
Aside from all the methods described in the previous sections (Figure 3), other emerging novel techniques have also been reported in the literature. A recent study has extracted type 1 collagen from tilapia scales by bubbling ultrafine bubbles of oxygen, carbon dioxide, and ozone into a diluted acetic acid solution [273]. This author concluded that using ultrafine CO2 bubbles resulted in the highest collagen yield, and that this procedure can promote resource recycling in a simple, mild, cost-effective, and environmentally safe manner. Castro-Muñoz [274] provided an overview of some membrane technologies, especially those based on pressure-driven membrane operations (e.g., microfiltration, ultrafiltration, nanofiltration, and reverse osmosis) and electro-membrane processes (e.g., electrodialysis, electrodialysis with bipolar membrane, electro-membrane filtration, and electrodialysis with ultrafiltration membrane), and their potential in the valorization of meat by-products. They concluded that the use of these technologies will allow for efficient utilization of meat by-products, promoting the principles of the sustainable circular economy.
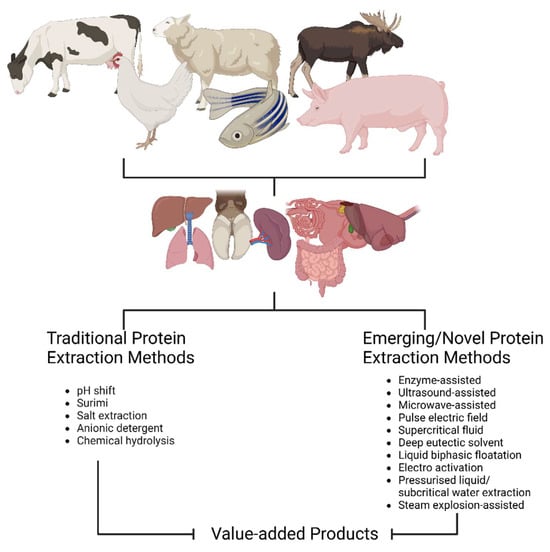
Figure 3.
Traditional and emerging protein extraction methods for meat by-products (diagram drawn using Biorender).
Substantial prospects exist for a combination of several of these green extraction methods or traditional/green extraction methods to improve the yield and efficiency of the process. Melgosa et al. [207] utilized two different green extraction techniques, whereby SFE was used to defat waste streams of sardine (S. pilchardus) before subcritical water extraction/hydrolysis of the protein was performed. These authors observed higher protein purity and yield when these two methods were used in sequence, and they proposed that coupling these methods could provide significant practical advantages. Similarly, Li et al. [170] reported an improved hydrolysis rate, nitrogen recovery, total amino nitrogen content, and degree of hydrolysis for abalone visceral protein when a pulse electric field was coupled with enzymatic protein extraction. In fact, the integrated process further improved some functional properties of the protein including solubility, emulsifying capacity, and emulsifying stability. The same was true when Asaduzzaman et al. [275] combined enzymatic hydrolysis (with pepsin) and subcritical water extraction in the isolation of type 1 collagen from bone and skin of mackerel. The authors observed enhanced antioxidant activities when subcritical water hydrolysis was applied to pepsin-solubilized collagen. Kim et al. [276] also found that treatment of porcine myocardium with salt extraction (sodium pyrophosphate) and ultrasonication had synergistic effects on protein extraction, and Zou et al. [156] reported up to a 55.4% increase in protein yield for chicken liver when ultrasound was combined with alkali protein extraction. Furthermore, aside from its proven benefit of microbial destruction and enzyme inactivation, studies have shown the capability of cold plasma technology to induce structural changes in proteins, thereby enhancing some functional properties of proteins [277,278]. As such, some studies have coupled this technology with pH shift [279] and ultrasound- [280] assisted protein extraction, showing great promise. Combining cold plasma with other protein extraction technologies could have great prospects for meat by-product valorization; however, no work has explored this possibility in the literature. Future works should explore the prospects of combining these methods for meat by-product valorization.
5. Conclusion and Future Perspectives
The efficient utilization of agricultural food processing by-products is pivotal in our efforts to reduce FLW as well as to eliminate its negative impacts on the climate, humanity, and the environment. This is particularly important for animal-based by-products, especially ruminant animals, which have been reported to have much greater contribution to global greenhouse gas emissions (Figure 4). It is however ironic that more recently, less focus has been given to valorizing the waste emanating from the production of these land animals despite their significant impact on the environment. Consequently, these enormous by-products have only found utilization in low-value outlets (e.g., composting, energy/heat generation, or outright disposal in the landfills). Efficiently diverting these secondary streams back into the food value chain will command more value propositions and provide more benefits to the industry, the environment, and consumers alike. In the face of the ever-growing human population, it is increasingly becoming imperative to explore alternative protein sources. Isolating these high-biological-value proteins inherent in these always available and enormous animal by-products with the intention of using them in other food formulations could be part of our global strategies to combat world hunger and meet the ever-increasing protein demands of our ever-growing world population.
Figure 4.
Contribution of different food items (per kilogram) to kilograms of greenhouse gas emissions (adapted from United Nations: Climate Action. Accessed on 28 April 2025 from https://www.un.org/en/climatechange/science/climate-issues/food#:~:text=Animal%2Dbased%20foods%2C%20especially%20red,carbon%20dioxide%20stored%20in%20forests).
It seems evident from the current work that while there are many protein extraction techniques, research works that have explored emerging or novel protein extraction methods for meat by-products have mostly focused on those from aquatic species and less on by-products from other land animals. This could be due to some ethical, religious, and legislative restrictions in the utilization of by-products from, for example, ruminant animals. These restrictions, either self-imposed from the consumer end of the chain or as a result of legislative requirements from policy makers, play a significant role in limiting innovation in the valorization of these by-products and re-integrating them back into the food value chain. Acknowledging that these animals are an integral part of our livestock production system and that their enormous by-products are constantly posing a significant threat to our environment, alongside the associated economic and social ramifications, should be an awakening call for policy makers, researchers, and other stakeholders to focus on devising safe, viable, and efficient innovations to divert these secondary streams into a more value-added space. To achieve this, it is important to explore protein extraction methods that guarantee nutritional quality applicable to marketable products, satisfactory yield, and a reduction in cost and process time, with none or minimal environmental impact.
Given the inadequate environmental credential of most traditional protein extraction methods, investment in these novel/emerging technologies for protein extraction from these animal by-products could bring significant returns, not only economically but also socially and environmentally. It will be imperative that research on these novel technologies such as SFE, subcritical water extraction, cold plasma, SE, and PEF focuses on by-products, especially from land animals, in order to optimize their conditions for significant value recovery. This is crucial as most of these methods require process development, optimization, and control for specific applications, and most research efforts in the literature have largely applied these novel green methods to alternative matrices (e.g., plant and aquatic species).
Moreover, exploration of combinations of these technologies for more efficient value recovery will be a viable area of future research. It is important to note however that although potential exists for most of these novel protein extraction methods in the valorization of meat by-products, some of these technologies are cost-prohibitive, especially for scale-up and commercial production. Intentional investment and collaboration from both the government and the industry may be necessary to make this upscaling realistic in the real world.
Author Contributions
Conceptualization, O.P.S.; resources, O.P.S., J.M., and J.W.; writing—original draft preparation, O.P.S., I.E.B., D.T.M., and A.B.O.; writing—review and editing, O.P.S., I.E.B., D.T.M., Y.F., J.M., T.T., J.W., and A.B.O.; visualization, O.P.S., I.E.B., D.T.M., Y.F., J.M., T.T., J.W., and A.B.O.; supervision, O.P.S., J.M., and J.W.; project administration, O.P.S., J.M., and J.W.; funding acquisition, O.P.S., J.M., and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by A-base funding from the Agriculture and Agri-Food Canada, Project ID: J-003168 under the project title “Evaluating the Canadian meat industry by-products and the valorisation options for value recovery”.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to Arun Kommadath for their help with putting together some of the figures in this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO. The State of Food and Agriculture: Moving Forward on Food Loss and Waste Reduction; FAO Licence: CC BY-NC-SA 3.0 IGO, Global; FAO: Rome, Italy, 2019; p. 182. [Google Scholar]
- Cattaneo, A.; Sánchez, M.V.; Torero, M.; Vos, R. Reducing food loss and waste: Five challenges for policy and research. Food Policy 2021, 98, 101974. [Google Scholar] [CrossRef] [PubMed]
- Ominski, K.; McAllister, T.; Stanford, K.; Mengistu, G.; Kebebe, E.G.; Omonijo, F.; Cordeiro, M.; Legesse, G.; Wittenberg, K. Utilization of by-products and food waste in livestock production systems: A Canadian perspective. Anim. Front. 2021, 11, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B.; Hanson, C.; Waite, R.; Searchinger, T.; Lomax, J. Reducing Food Loss and Waste. Working Paper, Installment 2 of Creating a Sustainable Food Future; World Resources Institute: Washington, DC, USA, 2013; Available online: https://www.wri.org/research/reducing-food-loss-and-waste?page (accessed on 2 March 2025).
- Mora, L.; Toldrá-Reig, F.; Reig, M.; Toldrá, F. Chapter 8-Possible Uses of Processed Slaughter Byproducts. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press: New York, NY, USA, 2019; pp. 145–160. [Google Scholar]
- Mullen, A.M.; Álvarez, C. Offal: Types and Composition. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: New York, NY, USA, 2016; pp. 152–157. [Google Scholar]
- Toldrá, F.; Aristoy, M.C.; Mora, L.; Reig, M. Innovations in value-addition of edible meat by-products. Meat Sci. 2012, 92, 290–296. [Google Scholar] [CrossRef]
- Bhaskar, N.; Modi, V.K.; Govindaraju, K.; Radha, C.; Lalitha, R.G. Utilization of meat industry by products: Protein hydrolysate from sheep visceral mass. Bioresour. Technol. 2007, 98, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Kamal, H.; Le, C.F.; Salter, A.M.; Ali, A. Extraction of protein from food waste: An overview of current status and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2455–2475. [Google Scholar] [CrossRef]
- Costello, C.; Birisci, E.; McGarvey, R.G. Food waste in campus dining operations: Inventory of pre- and post-consumer mass by food category, and estimation of embodied greenhouse gas emissions. Renew. Agric. Food Syst. 2016, 31, 191–201. [Google Scholar] [CrossRef]
- Chen, C.; Chaudhary, A.; Mathys, A. Nutritional and environmental losses embedded in global food waste. Resour. Conserv. Recycl. 2020, 160, 104912. [Google Scholar] [CrossRef]
- Malva, A.d.; Albenzio, M.; Santillo, A.; Russo, D.; Figliola, L.; Caroprese, M.; Marino, R. Methods for Extraction of Muscle Proteins from Meat and Fish Using Denaturing and Nondenaturing Solutions. J. Food Qual. 2018, 2018, 8478471. [Google Scholar] [CrossRef]
- Dara, P.K.; Geetha, A.; Mohanty, U.; Raghavankutty, M.; Mathew, S.; Chandragiri Nagarajarao, R.; Rangasamy, A. Extraction and Characterization of Myofibrillar Proteins from Different Meat Sources: A Comparative Study. J. Bioresour. Bioprod. 2021, 6, 367–378. [Google Scholar] [CrossRef]
- Gault, N.F.S.; Lawrie, R.A. Efficiency of protein extraction and recovery from meat industry by-products. Meat Sci. 1980, 4, 167–190. [Google Scholar] [CrossRef]
- Pinto, J.; Boavida-Dias, R.; Matos, H.A.; Azevedo, J. Analysis of the Food Loss and Waste Valorisation of Animal By-Products from the Retail Sector. Sustainability 2022, 14, 2830. [Google Scholar] [CrossRef]
- Mathijs, E. Exploring future patterns of meat consumption. Meat Sci. 2015, 109, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.A.; Mullen, A.M.; O’Neill, E.; Drummond, L.; Álvarez, C. Opportunities and perspectives for utilisation of co-products in the meat industry. Meat Sci. 2018, 144, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. Journal of Food Science and Technology 2012, 49, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Plavnik, I.; Hurwitz, S. Organ weights and body composition in chickens as related to the energy and amino acid requirements: Effects of strain, sex, and age. Poult. Sci. 1983, 62, 152–163. [Google Scholar] [CrossRef]
- Okareh, O.T.; Awe, A.O.; Sridhar, M.K.C. Effect of processed feather waste as mulch on crop growth and soil fertilization. J Agric. Eco Res. Int. 2015, 4, 25–35. [Google Scholar] [CrossRef]
- Iwujia, T.; Iheanachoa, G.; Ogambaa, M.; Odunfab, O. Relationship between live weight, internal organs, and body part weights of broiler chickens. Malay. Anim. Husband. J. 2022, 2, 64–66. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture (SOFIA); Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; Available online: https://www.fao.org/documents/card/en/c/cc0461en (accessed on 15 January 2025).
- Boler, D.D.; Woerner, D.R. What is meat? A perspective from the American Meat Science Association. Anim. Front. 2017, 7, 8–11. [Google Scholar] [CrossRef]
- AMSA. What are Meat Byproducts? American Meat Science Association: Savoy, IL, USA, 2015; Available online: https://meatscience.org/students/meat-judging-program/meat-judging-news/article/2015/11/20/what-are-animal-byproducts (accessed on 16 March 2021).
- Soladoye, P.O.; Juárez, M.; Estévez, M.; Fu, Y.; Álvarez, C. Exploring the prospects of the fifth quarter in the 21st century. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1439–1461. [Google Scholar] [CrossRef]
- Abdollahi, M.; Wu, H.; Undeland, I. Impact of processing technology on macro-and micronutrient profile of protein-enriched products from fish backbones. Foods 2021, 10, 950. [Google Scholar] [CrossRef]
- Tan, Y.; Gao, H.; Chang, S.K.C.; Bechtel, P.J.; Mahmoud, B.S.M. Comparative studies on the yield and characteristics of myofibrillar proteins from catfish heads and frames extracted by two methods for making surimi-like protein gel products. Food Chem. 2019, 272, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, S.K.C. Protein extraction from catfish byproducts and physicochemical properties of the protein isolates. J. Food Sci. 2021, 86, 3061–3074. [Google Scholar] [CrossRef] [PubMed]
- Ganjeh, A.M.; Saraiva, J.A.; Pinto, C.A.; Casal, S.; Silva, A.M.S. Emergent technologies to improve protein extraction from fish and seafood by-products: An overview. Appl. Food Res. 2023, 3, 100339. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Bhaskar, N.; Mahendrakar, N.S. Carotenoids in different body components of Indian shrimps. J. Sci. Food Agric. 2005, 85, 167–172. [Google Scholar] [CrossRef]
- Wu, H.; Forghani, B.; Abdollahi, M.; Undeland, I. Lipid oxidation in sorted herring (Clupea harengus) filleting co-products from two seasons and its relationship to composition. Food Chem. 2022, 373, 131523. [Google Scholar] [CrossRef] [PubMed]
- van Berlo, E.; Undeland, I.; Abdollahi, M. Physicochemical and functional properties of protein isolated from herring co-products; effects of catching season, pre-sorting, and co-product combination. Food Chem. 2023, 398, 133947. [Google Scholar] [CrossRef]
- Wisuthiphaet, N.; Kongruang, S.; Chamcheun, C. Production of fish protein hydrolysates by acid and enzymatic hydrolysis. J. Med. Bioeng. 2015, 4, 466–470. [Google Scholar] [CrossRef]
- Borrajo, P.; Pateiro, M.; Barba, F.J.; Mora, L.; Franco, D.; Toldrá, F.; Lorenzo, J.M. Antioxidant and Antimicrobial Activity of Peptides Extracted from Meat By-products: A Review. Food Anal. Methods 2019, 12, 2401–2415. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Jaczynski, J. Gelation of protein recovered from whole Antarctic krill (Euphausia superba) by isoelectric solubilization/precipitation as affected by functional additives. J. Agric. Food Chem. 2007, 55, 1814–1822. [Google Scholar] [CrossRef]
- Pym, R. Poultry genetics and breeding in developing countries: Genetic diversity of genetic resources. In FAO Poultry Development Review; FAO: Rome, Italy, 2013; pp. 1–3. [Google Scholar]
- GCDL. Our World in Data: Yearly Number of Animals Slaughtered for MEAT, World, 1961–2018. 2020. Available online: https://ourworldindata.org/grapher/animals-slaughtered-for-meat?tab=table (accessed on 27 July 2022).
- Das, P.K.; Samanta, I. Role of backyard poultry in South-East Asian countries: Post COVID-19 perspective. World’s Poult. Sci. J. 2021, 77, 415–426. [Google Scholar] [CrossRef]
- Mottet, A.; Tempio, G. Global poultry production: Current state and future outlook and challenges. World’s Poult. Sci. J. 2017, 73, 245–256. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J. Conventional use and sustainable valorization of spent egg-laying hens as functional foods and biomaterials: A review. Bioresour. Bioprocess. 2022, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Composition, color, and texture of Thai indigenous and broiler chicken muscles. Poult. Sci. 2004, 83, 123–128. [Google Scholar] [CrossRef]
- Zubair, M. Proteins Derived Bionanocomposites from Poultry By-Product for Food Packaging Applications. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2017. [Google Scholar]
- Nurkhoeriyati, T.; Huda, N.; Ahmad, R. Gelation properties of spent duck meat surimi-like material produced using acid–alkaline solubilization methods. J. Food Sci. 2011, 76, S48–S55. [Google Scholar] [CrossRef]
- Moayedi, V.; Omana, D.A.; Chan, J.; Xu, Y.; Betti, M. Alkali-aided protein extraction of chicken dark meat: Composition and stability to lipid oxidation of the recovered proteins. Poult. Sci. 2010, 89, 766–775. [Google Scholar] [CrossRef]
- Barbut, S. Pale, soft, and exudative poultry meat—Reviewing ways to manage at the processing plant. Poult. Sci. 2009, 88, 1506–1512. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Han, M.-Y.; Qian, C.; Xu, X.-L.; Zhou, G.-H. Application of isoelectric solubilization/precipitation processing to improve gelation properties of protein isolated from pale, soft, exudative (PSE)-like chicken breast meat. LWT-Food Sci. Technol. 2016, 72, 141–148. [Google Scholar] [CrossRef]
- Momen, S.; Alavi, F.; Aider, M. Alkali-mediated treatments for extraction and functional modification of proteins: Critical and application review. Trends Food Sci. Technol. 2021, 110, 778–797. [Google Scholar] [CrossRef]
- Nolsøe, H.; Undeland, I. The acid and alkaline solubilization process for the isolation of muscle proteins: State of the art. Food Bioprocess. Technol. 2009, 2, 1–27. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Hultin, H.O. Changes in conformation and subunit assembly of cod myosin at low and high pH and after subsequent refolding. J. Agric. Food Chem. 2003, 51, 7187–7196. [Google Scholar] [CrossRef]
- Vareltzis, P.K.; Undeland, I. Protein isolation from blue mussels (Mytilus edulis) using an acid and alkaline solubilisation technique—Process characteristics and functionality of the isolates. J. Sci. Food Agric. 2012, 92, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Undeland, I.; Kelleher, S.D.; Hultin, H.O. Recovery of functional proteins from herring (Clupea harengus) light muscle by an acid or alkaline solubilization process. J. Agric. Food Chem. 2002, 50, 7371–7379. [Google Scholar] [CrossRef]
- Chomnawang, C.; Yongsawatdigul, J. Protein Recovery of Tilapia Frame By-Products by pH-Shift Method. J. Aquat. Food Prod. Technol. 2013, 22, 112–120. [Google Scholar] [CrossRef]
- Nisov, A.; Kakko, T.; Alakomi, H.-L.; Lantto, R.; Honkapää, K. Comparison of enzymatic and pH shift methods to extract protein from whole Baltic herring (Clupea harengus membras) and roach (Rutilus rutilus). Food Chem. 2022, 373, 131524. [Google Scholar] [CrossRef] [PubMed]
- Batista, I.; Pires, C.; Nelhas, R.; Godinho, V. Acid and alkaline-aided protein recovery from Cape hake by-products. In Seafood Research from Fish to Dish. Quality, Safety and Processing of Wild and Farmed Fish; Academic Publishers: Wageningen, The Netherlands, 2006; pp. 427–438. [Google Scholar]
- Shahidi, F.; Synowiecki, J. Alkali-assisted extraction of proteins from meat and bone residues of harp seal (Phoca groenlandica). Food Chem. 1996, 57, 317–321. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Demir, N. Functional fish protein ingredients from fish species of warm and temperate waters: Comparison of acid-and alkali-aided processing vs. conventional surimi processing. In Alaska Sea Grant College Program; University of Alaska Fairbanks: Fairbanks, AK, USA, 2003; pp. 277–295. [Google Scholar]
- Hrynets, Y.; Omana, D.A.; Xu, Y.; Betti, M. Effect of acid-and alkaline-aided extractions on functional and rheological properties of proteins recovered from mechanically separated turkey meat (MSTM). J. Food Sci. 2010, 75, E477–E486. [Google Scholar] [CrossRef]
- Hrynets, Y.; Omana, D.A.; Xu, Y.; Betti, M. Comparative study on the effect of acid- and alkaline-aided extractions on mechanically separated turkey meat (MSTM): Chemical characteristics of recovered proteins. Process Biochem. 2011, 46, 335–343. [Google Scholar] [CrossRef]
- Omana, D.A.; Moayedi, V.; Xu, Y.; Betti, M. Alkali-aided protein extraction from chicken dark meat: Textural properties and color characteristics of recovered proteins. Poult. Sci. 2010, 89, 1056–1064. [Google Scholar] [CrossRef]
- Hultin, H.O.; Kelleher, S.D. Process for Isolating a Protein Composition from a Muscle Source and Protein Composition. U.S. Patent US6288216B1, 11 September 2001. [Google Scholar]
- Hultin, H.O.; Kelleher, S.D. Protein Composition and Process for Isolating a Protein Composition from a Muscle Source. U.S. Patent US6451975B1, 17 September 2002. [Google Scholar]
- Hultin, H.O.; Kelleher, S.D. Process for Isolating a Protein Composition from a Muscle Source and Protein Composition. Google Patents MXPA97008100A, 12 November 1998. [Google Scholar]
- Abdollahi, M.; Undeland, I. A novel cold biorefinery approach for isolation of high quality fish oil in parallel with gel-forming proteins. Food Chem. 2020, 332, 127294. [Google Scholar] [CrossRef]
- Álvarez, C.; Lélu, P.; Lynch, S.A.; Tiwari, B.K. Optimised protein recovery from mackerel whole fish by using sequential acid/alkaline isoelectric solubilization precipitation (ISP) extraction assisted by ultrasound. LWT 2018, 88, 210–216. [Google Scholar] [CrossRef]
- Hinchcliffe, J.; Carlsson, N.G.; Jönsson, E.; Sundell, K.; Undeland, I. Aquafeed ingredient production from herring (Clupea harengus) by-products using pH-shift processing: Effect from by-product combinations, protein solubilization-pH and centrifugation force. Anim. Feed. Sci. Technol. 2019, 247, 273–284. [Google Scholar] [CrossRef]
- Abdollahi, M.; Undeland, I. Physicochemical and gel-forming properties of protein isolated from salmon, cod and herring by-products using the pH-shift method. LWT 2019, 101, 678–684. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Liang, Y. Effect of pH-shift processing and surimi processing on Atlantic croaker (Micropogonias undulates) muscle proteins. J. Food Sci. 2006, 71, C304–C312. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Theodore, A.E.; Demir, N.; Ingadottir, B. A comparative study between acid-and alkali-aided processing and surimi processing for the recovery of proteins from channel catfish muscle. J. Food Sci. 2005, 70, C298–C306. [Google Scholar] [CrossRef]
- Costa, C.G.C.D.; Paula, M.M.d.O.; Massingue, A.A.; Torres Filho, R.d.A.; Ramos, E.M.; Carneiro, J.d.D.d.S. Protein concentrates obtained from pig by-products using pH-shifting technique: A preliminary study. Ciência Rural 2019, 49, e20181048. [Google Scholar] [CrossRef]
- Lynch, S.A.; Álvarez, C.; O’Neill, E.E.; Keenan, D.F.; Mullen, A.M. Optimization of protein recovery from bovine lung by pH shift process using response surface methodology. J. Sci. Food Agric. 2018, 98, 1951–1960. [Google Scholar] [CrossRef]
- Park, J.D.; Hung, C.H.; Kim, J.S.; Choi, Y.J.; Cho, D.M.; Cho, M.S. Surimi processing using acid and alkali solubilization of fish muscle protein. J. Korean Soc. Food Sci. Nutr. 2003, 32, 400–405. [Google Scholar]
- Batista, I. Recovery of proteins from fish waste products by alkaline extraction. Eur. Food Res. Technol. 1999, 210, 84–89. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Wang, X.-X.; Ma, F.; Xu, B.-C.; Li, P.-J.; Chen, C.-G. Origin of high-pressure induced changes in the properties of reduced-sodium chicken myofibrillar protein gels containing CaCl2: Physicochemical and molecular modification perspectives. Food Chem. 2020, 319, 126535. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Li, P.-J.; Wang, X.-X.; Cai, K.-Z.; Chen, C.-G. Combined effect of CaCl2 and high pressure processing on the solubility of chicken breast myofibrillar proteins under sodium-reduced conditions. Food Chem. 2018, 269, 236–243. [Google Scholar] [CrossRef]
- Hashimoto, K.; Watabe, S.; KoNo, M.; Shiro, K. Muscle protein composition of sardine and mackerel. Bull. Jpn. Soc. Sci. Fish. 1979, 45, 1435–1441. [Google Scholar] [CrossRef]
- Lan, Y.H.; Novakofski, J.; Carr, T.R.; McKeith, F.K. Assay and Storage Conditions Affect Yield of Salt Soluble Protein from Muscle. J. Food Sci. 1993, 58, 963–967. [Google Scholar] [CrossRef]
- Foegeding, E.A. Functional Properties of Turkey Salt-Soluble Proteins. J. Food Sci. 1987, 52, 1495–1499. [Google Scholar] [CrossRef]
- Gillett, T.A.; Meiburg, D.E.; Brown, C.L.; Simon, S. Parameters affecting meat protein extraction and interpretation of model system data for meat emulsion formation. J. Food Sci. 1977, 42, 1606–1610. [Google Scholar] [CrossRef]
- Vega-Warner, V.; Merkel, R.A.; Smith, D.M. Composition, solubility and gel properties of salt soluble proteins from two bovine muscle types. Meat Sci. 1999, 51, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.L.; Lou, X.; Wang, C.; Moody, W.G.; Harmon, R.J. Protein Extraction From Chicken Myofibrils Irrigated with Various Polyphosphate and NaCl Solutions. J. Food Sci. 2000, 65, 96–100. [Google Scholar] [CrossRef]
- Richardson, R.I.; Jones, J.M. The effects of salt concentration and pH upon water-binding, water-holding and protein extractability of turkey meat. Int. J. Food Sci. Technol. 1987, 22, 683–692. [Google Scholar] [CrossRef]
- Munasinghe, D.M.S.; Sakai, T. Sodium chloride as a preferred protein extractant for pork lean meat. Meat Sci. 2004, 67, 697–703. [Google Scholar] [CrossRef]
- Gordon, A.; Barbut, S. Effect of chloride salts on protein extraction and interfacial protein film formation in meat batters. J. Sci. Food Agric. 1992, 58, 227–238. [Google Scholar] [CrossRef]
- Young, R.H.; Lawrie, R.A. Utilization of edible protein from meat industry by-products and waste: I. Factors influencing the extractability of protein from bovine and ovine stomach and lungs. Int. J. Food Sci. Technol. 1974, 9, 69–78. [Google Scholar] [CrossRef]
- DeWitt, C.A.M.; Gomez, G.; James, J.M. Protein extraction from beef heart using acid solubilization. J. Food Sci. 2002, 67, 3335–3341. [Google Scholar] [CrossRef]
- Steen, L.; Glorieux, S.; Goemaere, O.; Brijs, K.; Paelinck, H.; Foubert, I.; Fraeye, I. Functional Properties of Pork Liver Protein Fractions. Food Bioprocess. Technol. 2016, 9, 970–980. [Google Scholar] [CrossRef]
- Zouari, N.; Fakhfakh, N.; Amara-Dali, W.B.; Sellami, M.; Msaddak, L.; Ayadi, M.A. Turkey liver: Physicochemical characteristics and functional properties of protein fractions. Food Bioprod. Process. 2011, 89, 142–148. [Google Scholar] [CrossRef]
- Devatkal, S.; Mendiratta, S.K.; Kondaiah, N.; Sharma, M.C.; Anjaneyulu, A.S.R. Physicochemical, functional and microbiological quality of buffalo liver. Meat Sci. 2004, 68, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nuckles, R.O.; Smith, D.M.; Merkel, R.A. Meat by-product protein composition and functional properties in model systems. J. Food Sci. 1990, 55, 640–643. [Google Scholar] [CrossRef]
- Krasnowska, G.; Górska, I.; Gergont, J. Evaluation of functional properties of offal proteins. Food/Nahrung 1995, 39, 149–155. [Google Scholar] [CrossRef]
- Rivera, J.A.; Sebranek, J.G.; Rust, R.E.; Tabatabai, L.B. Composition and protein fractions of different meat by-products used for petfood compared with mechanically separated chicken (MSC). Meat Sci. 2000, 55, 53–59. [Google Scholar] [CrossRef]
- Yuan, Z.; Ye, X.; Hou, Z.; Chen, S. Sustainable utilization of proteins from fish processing by-products: Extraction, biological activities and applications. Trends Food Sci. Technol. 2024, 143, 104276. [Google Scholar] [CrossRef]
- Carpio, K.C.R.; Bezerra, R.S.; Cahú, T.B.; Monte, F.T.D.D.; Neri, R.C.A.; Silva, J.F.D.; Santos, P.R.D.; Carvalho, R.P.; Galeno, D.M.L.; Inhamuns, A.J. Extraction and characterization of collagen from the skin of Amazonian freshwater fish pirarucu. Braz. J. Med. Biol. Res. 2023, 56, e12564. [Google Scholar] [CrossRef]
- Truong, T.M.; Nguyen, V.M.; Tran, T.T.; Le, T.M. Characterization of Acid-Soluble Collagen from Food Processing By-Products of Snakehead Fish (Channa striata). Processes 2021, 9, 1188. [Google Scholar] [CrossRef]
- Guan, Y.; He, J.; Chen, J.; Li, Y.; Zhang, X.; Zheng, Y.; Jia, L. Valorization of Fish Processing By-Products: Microstructural, Rheological, Functional, and Properties of Silver Carp Skin Type I Collagen. Foods 2022, 11, 2985. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.A.; Shakir, K.A.; Walsh, M.K. Functional Properties of Collagen Extracted from Catfish (Silurus triostegus) Waste. Foods 2022, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, R.S.; Volpato, J.A.; Silva, I.C. Bioactive peptides extracted from hydrolyzed animal byproducts for dogs and cats. Anim. Front. 2024, 14, 38–45. [Google Scholar] [CrossRef]
- Areas, J.A.G. Lipid protein interactions in offal protein isolates: Effect of several solvents on lipid extraction. J. Food Sci. 1985, 50, 1392–1395. [Google Scholar] [CrossRef]
- Rao, B.J.; Kumar, P. Methods of Proteins Recovery from Abattoir Offals—An Overview. J. Meat Sci. Technol. 2016, 4, 76–83. [Google Scholar]
- Ellison, N.S.; Gault, N.F.; Lawrie, R.A. Removal of sodium dodecyl sulphate from complex with recovered offal proteins. Meat Sci. 1980, 4, 77–78. [Google Scholar] [CrossRef]
- Khumalo, M.; Sithole, B.; Tesfaye, T. Valorisation of waste chicken feathers: Optimisation of keratin extraction from waste chicken feathers by sodium bisulphite, sodium dodecyl sulphate and urea. J. Environ. Manag. 2020, 262, 110329. [Google Scholar] [CrossRef]
- Santana, P.; Huda, N.; Yang, T.A. Technology for production of surimi powder and potential of applications. Int. Food Res. J. 2012, 19, 1313–1323. [Google Scholar]
- Martín-Sánchez, A.M.; Navarro, C.; Pérez-Álvarez, J.A.; Kuri, V. Alternatives for efficient and sustainable production of surimi: A review. Compr. Rev. Food Sci. Food Saf. 2009, 8, 359–374. [Google Scholar] [CrossRef]
- Leadbitter, D.; Guenneugues, P.; Park, J. The Production of Surimi and Surimi Seafood from Tropical Fish—A Landscape View of the Industry. 2020. Available online: https://certificationandratings.org/wp-content/uploads/2023/04/Surimi-Landscape-Report-Final-2.pdf (accessed on 28 December 2024).
- Park, J.W.; Nozaki, H.; Suzuki, T.; Beliveau, J.-L. Historical review of surimi technology and market developments. Surimi Surimi Seaf. 2013, 1, 1. [Google Scholar]
- Stangierski, J.; Baranowska, H.M.; Rezler, R.; Kijowski, J. Enzymatic modification of protein preparation obtained from water-washed mechanically recovered poultry meat. Food Hydrocoll. 2008, 22, 1629–1636. [Google Scholar] [CrossRef]
- Cortez-Vega, W.R.; Fonseca, G.G.; Prentice, C. Optimization of parameters for obtaining surimi-like material from mechanically separated chicken meat using response surface methodology. J. Food Sci. Technol. 2015, 52, 763–772. [Google Scholar] [CrossRef]
- Jin, S.-K.; Kim, I.-S.; Choi, Y.-J.; Park, G.-B.; Yang, H.-S. Quality Characteristics of Chicken Breast Surimi as Affected by Water Washing Time and pH Adjustment. Asian-Australas. J. Anim. Sci. 2008, 21, 449–455. [Google Scholar] [CrossRef]
- Jin, S.K.; Kim, I.S.; Jung, H.J.; Kim, D.H.; Choi, Y.J.; Hur, S.J. The Development of Sausage Including Meat from Spent Laying Hen Surimi. Poult. Sci. 2007, 86, 2676–2684. [Google Scholar] [CrossRef]
- Wimmer, M.P.; Sebranek, J.G.; McKelTh, F.K. Washed mechanically separated pork as a surimi-like meat-product ingredient. J. Food Sci. 1993, 58, 254–258. [Google Scholar] [CrossRef]
- Park, S.; Brewer, M.S.; Novakofski, J.; Bechtel, P.J.; McKeith, F.K. Process and characteristics for a surimi-like material made from beef or pork. J. Food Sci. 1996, 61, 422–427. [Google Scholar] [CrossRef]
- Toldrà, M.; Taberner, P.; Parés, D.; Carretero, C. Surimi-like protein ingredient from porcine spleen as lean meat replacer in emulsion-type sausages. Meat Sci. 2021, 182, 108640. [Google Scholar] [CrossRef]
- Desmond, E.M.; Kenny, T.A. Preparation of surimi-like extract from beef hearts and its utilisation in frankfurters. Meat Sci. 1998, 50, 81–89. [Google Scholar] [CrossRef]
- Tina, N.; Nurul, H.; Ruzita, A. Surimi-like material: Challenges and prospects. Int. Food Res. J. 2010, 17, 509–517. [Google Scholar]
- Venugopal, V. Valorization of seafood processing discards: Bioconversion and bio-refinery approaches. Front. Sustain. Food Syst. 2021, 5, 611835. [Google Scholar] [CrossRef]
- Agbowuro, A.A.; Huston, W.M.; Gamble, A.B.; Tyndall, J.D.A. Proteases and protease inhibitors in infectious diseases. Med. Res. Rev. 2018, 38, 1295–1331. [Google Scholar] [CrossRef] [PubMed]
- Matulessy, D.N.; Erwanto, Y.; Nurliyani, N.; Suryanto, E.; Abidin, M.Z.; Hakim, T.R. Characterization and functional properties of gelatin from goat bone through alcalase and neutrase enzymatic extraction. Vet. World 2021, 14, 2397. [Google Scholar] [CrossRef] [PubMed]
- Abedinia, A.; Ariffin, F.; Huda, N.; Nafchi, A.M. Extraction and characterization of gelatin from the feet of Pekin duck (Anas platyrhynchos domestica) as affected by acid, alkaline, and enzyme pretreatment. Int. J. Biol. Macromol. 2017, 98, 586–594. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stefan, L.M.; Anton, E.D.; Berger, D.; Matei, C.; Negreanu-Pirjol, T.; Moldovan, L. Physicochemical and biological properties of gelatin extracted from marine snail Rapana venosa. Mar. Drugs 2019, 17, 589. [Google Scholar] [CrossRef]
- Laba, W.; Rodziewicz, A. Biodegradation of hard keratins by two Bacillus strains. Jundishapur J. Microbiol. 2014, 7, e8896. [Google Scholar] [CrossRef]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.-h.; Zhang, W.-g. A review of antioxidant peptides derived from meat muscle and by-products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef]
- Morales, D.; Miguel, M.; Garcés-Rimón, M. Pseudocereals: A novel source of biologically active peptides. Crit. Rev. Food Sci. Nutr. 2021, 61, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tu, D.; Shen, Q.; Dai, Z. Fish scale valorization by hydrothermal pretreatment followed by enzymatic hydrolysis for gelatin hydrolysate production. Molecules 2019, 24, 2998. [Google Scholar] [CrossRef]
- Pérez-Aguilar, H.; Lacruz-Asaro, M.; Arán-Ais, F. Towards a circular bioeconomy: High added value protein recovery and recycling from animal processing by-products. Sustain. Chem. Pharm. 2022, 28, 100667. [Google Scholar] [CrossRef]
- Ran, X.G.; Wang, L.Y. Use of ultrasonic and pepsin treatment in tandem for collagen extraction from meat industry by-products. J. Sci. Food Agric. 2014, 94, 585–590. [Google Scholar] [CrossRef]
- Lapenña, D.; Vuoristo, K.S.; Kosa, G.; Horn, S.J.; Eijsink, V.G.H. Comparative assessment of enzymatic hydrolysis for valorization of different protein-rich industrial byproducts. J. Agric. Food Chem. 2018, 66, 9738–9749. [Google Scholar] [CrossRef] [PubMed]
- Safar Razavizadeh, R.; Farmani, J.; Motamedzadegan, A. Enzyme-assisted extraction of chicken skin protein hydrolysates and fat: Degree of hydrolysis affects the physicochemical and functional properties. J. Am. Oil Chem. Soc. 2022, 99, 621–632. [Google Scholar] [CrossRef]
- Fallah-Delavar, M.; Farmani, J. Recovery and characterization of enzymatic protein hydrolyzates and fat from chicken skin. J. Am. Oil Chem. Soc. 2018, 95, 1151–1161. [Google Scholar] [CrossRef]
- de Queiroz, A.L.M.; Bezerra, T.; Kênia, A.; de Freitas Pereira, S.; da Silva, M.E.C.; de Almeida Gadelha, C.A.; Gadelha, T.S.; Pacheco, M.; Teresa, B.; Madruga, M.S. Functional protein hydrolysate from goat by-products: Optimization and characterization studies. Food Biosci. 2017, 20, 19–27. [Google Scholar] [CrossRef]
- Pagán, J.; Ibarz, A.; Falguera, V.; Benítez, R. Enzymatic hydrolysis kinetics and nitrogen recovery in the protein hydrolysate production from pig bones. J. Food Eng. 2013, 119, 655–659. [Google Scholar] [CrossRef]
- Bah, C.S.F.; Bekhit, A.E.-D.A.; McConnell, M.A.; Carne, A. Generation of bioactive peptide hydrolysates from cattle plasma using plant and fungal proteases. Food Chem. 2016, 213, 98–107. [Google Scholar] [CrossRef]
- Batista, I.; Ramos, C.; Mendonça, R.; Nunes, M.L. Enzymatic Hydrolysis of Sardine (Sardina pilchardus) By-products and Lipid Recovery. J. Aquat. Food Prod. Technol. 2009, 18, 120–134. [Google Scholar] [CrossRef]
- Chiodza, K.; Goosen, N.J. Emulsion formation during enzymatic protein hydrolysis and its effect on protein recovery and molecular weight distribution of protein hydrolysates from sardine (Sardina pilchardus) by-products. Biomass Convers. Biorefinery 2023, 14, 24069–24080. [Google Scholar] [CrossRef]
- Liceaga-Gesualdo, A.M.; Li-Chan, E.C.Y. Functional properties of fish protein hydrolysate from herring (Clupea harengus). J. Food Sci. 1999, 64, 1000–1004. [Google Scholar] [CrossRef]
- Jain, S.; Anal, A.K. Optimization of extraction of functional protein hydrolysates from chicken egg shell membrane (ESM) by ultrasonic assisted extraction (UAE) and enzymatic hydrolysis. LWT-Food Sci. Technol. 2016, 69, 295–302. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, J.C.; Kurozawa, L.E.; Netto, F.M.; Hubinger, M.D. Optimization of the enzymatic hydrolysis of Blue shark skin. J. Food Sci. 2011, 76, C938–C949. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Franco, C.; Zhang, W. Process optimisation and physicochemical characterisation of enzymatic hydrolysates of proteins from co-products of Atlantic Salmon (Salmo salar) and Yellowtail Kingfish (Seriola lalandi). Int. J. Food Sci. Technol. 2012, 47, 2397–2404. [Google Scholar] [CrossRef]
- Szucs, M.; Angulo, M.; Costa, C.; Márquez, M. Meat waste valorization through protein hydrolysis using different types of proteases. Recent. Prog. Mater. 2021, 3, 1–17. [Google Scholar] [CrossRef]
- Dhakal, D.; Koomsap, P.; Lamichhane, A.; Sadiq, M.B.; Anal, A.K. Optimization of collagen extraction from chicken feet by papain hydrolysis and synthesis of chicken feet collagen based biopolymeric fibres. Food Biosci. 2018, 23, 23–30. [Google Scholar] [CrossRef]
- Mokhtar, N.D.; Wahab, W.A.; Hamdan, N.A.; Hadi, H.A.; Abu Hassan, M.S.; Bunnori, N.M. Extraction, optimization and characterization of collagen from chicken (Gallus gallus domesticus) feet. In Proceedings of the 5th International Conference on Chemical, Agricultural, Biological and Environmental Sciences, Kyoto, Japan, 18–19 April 2017; pp. 18–19. [Google Scholar]
- Zhou, C.; Li, Y.; Yu, X.; Yang, H.; Ma, H.; Yagoub, A.E.A.; Cheng, Y.; Hu, J.; Otu, P.N.Y. Extraction and characterization of chicken feet soluble collagen. LWT 2016, 74, 145–153. [Google Scholar] [CrossRef]
- AraÚJo, Í.B.d.S.; Bezerra, T.K.A.; Nascimento, E.S.d.; Gadelha, C.A.d.A.; Santi-Gadelha, T.; Madruga, M.S. Optimal conditions for obtaining collagen from chicken feet and its characterization. Food Sci. Technol. 2018, 38, 167–173. [Google Scholar] [CrossRef]
- Woo, H.; Jeong, G.A.; Choi, H.; Lee, C.J. Characterization of Low-Molecular-Weight Collagen from Korean Native Chicken Feet Hydrolyzed Using Alcalase. J. Microbiol. Biotechnol. 2023, 33, 656–661. [Google Scholar] [CrossRef]
- Hatab, S.; Chen, M.-L.; Miao, W.; Lin, J.; Wu, D.; Wang, C.; Yuan, P.; Deng, S. Protease hydrolysates of filefish (Thamnaconus modestus) byproducts effectively inhibit foodborne pathogens. Foodborne Pathog. Dis. 2017, 14, 656–666. [Google Scholar] [CrossRef]
- Zou, Y.; Shahidi, F.; Shi, H.; Wang, J.; Huang, Y.; Xu, W.; Wang, D. Values-added utilization of protein and hydrolysates from animal processing by-product livers: A review. Trends Food Sci. Technol. 2021, 110, 432–442. [Google Scholar] [CrossRef]
- Arslan, B.; Xiong, Y.L.; Soyer, A. Antioxidant properties of bovine liver protein hydrolysates and their practical application in biphasic systems. J. Sci. Food Agric. 2023, 104, 2980–2989. [Google Scholar] [CrossRef]
- Mintah, B.K.; He, R.; Dabbour, M.; Xiang, J.; Jiang, H.; Agyekum, A.A.; Ma, H. Characterization of edible soldier fly protein and hydrolysate altered by multiple-frequency ultrasound: Structural, physical, and functional attributes. Process Biochem. 2020, 95, 157–165. [Google Scholar] [CrossRef]
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- Bernardi, S.; Lupatini-Menegotto, A.L.; Kalschne, D.L.; Moraes Flores, É.L.; Bittencourt, P.R.S.; Colla, E.; Canan, C. Ultrasound: A suitable technology to improve the extraction and techno-functional properties of vegetable food proteins. Plant Foods Hum. Nutr. 2021, 76, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.; Tayal, R.K.; Pandit, A. Cavitation: A technology on the horizon. Curr. Sci. 2006, 91, 35–46. [Google Scholar]
- Kingwascharapong, P.; Chaijan, M.; Karnjanapratum, S. Ultrasound-assisted extraction of protein from Bombay locusts and its impact on functional and antioxidative properties. Sci. Rep. 2021, 11, 17320. [Google Scholar] [CrossRef]
- Ojha, K.S.; Aznar, R.; O’Donnell, C.; Tiwari, B.K. Ultrasound technology for the extraction of biologically active molecules from plant, animal and marine sources. TrAC Trends Anal. Chem. 2020, 122, 115663. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, L.; Li, P.; Cai, P.; Zhang, M.; Sun, Z.; Sun, C.; Geng, Z.; Xu, W.; Xu, X. Effects of ultrasound assisted extraction on the physiochemical, structural and functional characteristics of duck liver protein isolate. Process Biochem. 2017, 52, 174–182. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Cavitation Technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Appl. Sci. 2019, 9, 766. [Google Scholar] [CrossRef]
- Duppeti, H.; Nakkarike Manjabhatta, S.; Bheemanakere Kempaiah, B. Optimization of ultrasonic-assisted extraction of flavor compounds from shrimp by-products and characterization of flavor profile of the extract. Ultrason. SonoChem. 2023, 101, 106651. [Google Scholar] [CrossRef]
- Zou, Y.; Bian, H.; Li, P.; Sun, Z.; Sun, C.; Zhang, M.; Geng, Z.; Xu, W.; Wang, D. Optimization and physicochemical properties of nutritional protein isolate from pork liver with ultrasound-assisted alkaline extraction. Anim. Sci. J. 2018, 89, 456–466. [Google Scholar] [CrossRef]
- Friedman, M. Lysinoalanine in Food and in Antimicrobial Proteins. In Impact of Processing on Food Safety; Jackson, L.S., Knize, M.G., Morgan, J.N., Eds.; Springer: Greer, SC, USA, 1999; pp. 145–159. [Google Scholar]
- Surasani, V.K.R. Acid and alkaline solubilization (pH shift) process: A better approach for the utilization of fish processing waste and by-products. Environ. Sci. Pollut. Res. 2018, 25, 18345–18363. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Ying, R.-F.; Shi, L.-E. Physicochemical and functional characteristics of proteins treated by a pH-shift process: A review. Int. J. Food Sci. Technol. 2021, 56, 515–529. [Google Scholar] [CrossRef]
- Lee, C.H. A Simple Outline of Methods for Protein Isolation and Purification. Endocrinol. Metab. 2017, 32, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef] [PubMed]
- Sert, D.; Rohm, H.; Struck, S. Ultrasound-Assisted Extraction of Protein from Pumpkin Seed Press Cake: Impact on Protein Yield and Techno-Functionality. Foods 2022, 11, 4029. [Google Scholar] [CrossRef]
- Suchintita Das, R.; Tiwari, B.K.; Chemat, F.; Garcia-Vaquero, M. Impact of ultrasound processing on alternative protein systems: Protein extraction, nutritional effects and associated challenges. Ultrason. Sonochemistry 2022, 91, 106234. [Google Scholar] [CrossRef]
- Ampofo, J.; Ngadi, M. Ultrasound-assisted processing: Science, technology and challenges for the plant-based protein industry. Ultrason. Sonochem. 2022, 84, 105955. [Google Scholar] [CrossRef]
- Barrios, C.; Fernández-Delgado, M.; López-Linares, J.C.; García-Cubero, M.T.; Coca, M.; Lucas, S. A techno-economic perspective on a microwave extraction process for efficient protein recovery from agri-food wastes. Ind. Crops Prod. 2022, 186, 115166. [Google Scholar] [CrossRef]
- Peñas, E.; Hernandez-Ledesma, B.; Martinez-Villaluenga, C. Microwave-assisted extraction of plant proteins. In Green Protein Processing Technologies from Plants: Novel Extraction and Purification Methods for Product Development; Springer: Belin, Germany, 2023; pp. 211–236. [Google Scholar]
- Zhou, Y.; He, Q.; Zhou, D. Optimization extraction of protein from mussel by high-Intensity pulsed electric fields. J. Food Process. Preserv. 2017, 41, e12962. [Google Scholar] [CrossRef]
- Li, M.; Lin, J.I.E.; Chen, J.; Fang, T. Pulsed electric field-assisted enzymatic extraction of protein from abalone (Haliotis discus hannai Ino) viscera. J. Food Process Eng. 2016, 39, 702–710. [Google Scholar] [CrossRef]
- Rocha, C.M.R.; Genisheva, Z.; Ferreira-Santos, P.; Rodrigues, R.; Vicente, A.A.; Teixeira, J.A.; Pereira, R.N. Electric field-based technologies for valorization of bioresources. Bioresour. Technol. 2018, 254, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gullón, B.; Wang, M.; Gullón, P.; Lorenzo, J.M.; Barba, F.J. The Application of Supercritical Fluids Technology to Recover Healthy Valuable Compounds from Marine and Agricultural Food Processing By-Products: A Review. Processes 2021, 9, 357. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Supercritical Fluid Extraction of Bioactive Compounds from Plant Materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, W.; Zhang, N.; Soladoye, O.P.; Zhang, Y.; Fu, Y. Deep eutectic solvents as new media for green extraction of food proteins: Opportunity and challenges. Food Res. Int. 2022, 161, 111842. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Bowen, H.; Durrani, R.; Delavault, A.; Durand, E.; Chenyu, J.; Yiyang, L.; Lili, S.; Jian, S.; Weiwei, H.; Fei, G. Application of deep eutectic solvents in protein extraction and purification. Front. Chem. 2022, 10, 912411. [Google Scholar] [CrossRef]
- Suthar, P.; Kaushal, M.; Vaidya, D.; Thakur, M.; Chauhan, P.; Angmo, D.; Kashyap, S.; Negi, N. Deep eutectic solvents (DES): An update on the applications in food sectors. J. Agric. Food Res. 2023, 14, 100678. [Google Scholar] [CrossRef]
- Karabulut, G.; Köroğlu, D.G.; Feng, H.; Karabulut, Z. Sustainable fungi-based protein extraction from agro-waste mushroom stem using deep eutectic solvents. Food Chemistry X 2024, 24, 101931. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, W.; Zhang, X.-D.; Dai, Z.-Y.; Zhang, Y.-Q. Steam explosion-assisted extraction of protein from fish backbones and effect of enzymatic hydrolysis on the extracts. Foods 2021, 10, 1942. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Qi, L.; Xv, X.; Li, X.; Guo, Y.; Jia, W.; Zhang, C.; Richel, A. Application of steam explosion treatment on the collagen peptides extraction from cattle bone. Innov. Food Sci. Emerg. Technol. 2023, 85, 103336. [Google Scholar] [CrossRef]
- Sharma, P.; Zalpouri, R. Microwave-assisted extraction of proteins and carbohydrates from marine resources. In Innovative and Emerging Technologies in the Bio-Marine Food Sector; Academic Press: New York, NY, USA, 2022; pp. 361–374. [Google Scholar]
- Sosa-Hernández, J.E.; Escobedo-Avellaneda, Z.; Iqbal, H.M.N.; Welti-Chanes, J. State-of-the-art extraction methodologies for bioactive compounds from algal biome to meet bio-economy challenges and opportunities. Molecules 2018, 23, 2953. [Google Scholar] [CrossRef] [PubMed]
- Leonelli, C.; Veronesi, P.; Cravotto, G. Microwave-assisted extraction: An introduction to dielectric heating. In Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–14. [Google Scholar]
- Gomez, L.; Tiwari, B.; Garcia-Vaquero, M. Emerging extraction techniques: Microwave-assisted extraction. In Sustainable Seaweed Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–224. [Google Scholar]
- Liu, T.; Dai, H.; Ma, L.; Yu, Y.; Tang, M.; Li, Y.; Hu, W.; Feng, X.; Zhang, Y. Structure of Hyla rabbit skin gelatin as affected by microwave-assisted extraction. Int. J. Food Prop. 2019, 22, 1594–1607. [Google Scholar] [CrossRef]
- Feng, X.; Dai, H.; Zhu, J.; Ma, L.; Yu, Y.; Zhu, H.; Wang, H.; Sun, Y.; Tan, H.; Zhang, Y. Improved solubility and interface properties of pigskin gelatin by microwave irradiation. Int. J. Biol. Macromol. 2021, 171, 1–9. [Google Scholar] [CrossRef]
- Lee, Y.S.; Phang, L.-Y.; Ahmad, S.A.; Ooi, P.T. Microwave-alkali treatment of chicken feathers for protein hydrolysate production. Waste Biomass Valorization 2016, 7, 1147–1157. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green. Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Qureshi, M.I.; Khan, N.; Ahmad, M.H.; Liu, Z.W.; Aadil, R.M. Effective valorization of food wastes and by-products through pulsed electric field: A systematic review. J. Food Process Eng. 2021, 44, e13629. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Maza, M.; Álvarez, I.; Raso, J. Utilising pulsed electric field processing to enhance extraction processes. In Innovative Food Processing Technologies: A Comprehensive Review; Elsevier: Amsterdam, The Netherlands, 2020; pp. 281–287. [Google Scholar]
- Golberg, A.; Sack, M.; Teissie, J.; Pataro, G.; Pliquett, U.; Saulis, G.; Stefan, T.; Miklavcic, D.; Vorobiev, E.; Frey, W. Energy-efficient biomass processing with pulsed electric fields for bioeconomy and sustainable development. Biotechnol. Biofuels 2016, 9, 94. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kalompatsios, D.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Pulsed electric field applications for the extraction of bioactive compounds from food waste and by-products: A critical review. Biomass 2023, 3, 367–401. [Google Scholar] [CrossRef]
- Ghosh, S.; Gillis, A.; Sheviryov, J.; Levkov, K.; Golberg, A. Towards waste meat biorefinery: Extraction of proteins from waste chicken meat with non-thermal pulsed electric fields and mechanical pressing. J. Clean. Prod. 2019, 208, 220–231. [Google Scholar] [CrossRef]
- Bocker, R.; Silva, E.K. Pulsed electric field assisted extraction of natural food pigments and colorings from plant matrices. Food Chem. X 2022, 15, 100398. [Google Scholar] [CrossRef] [PubMed]
- Puértolas, E.; Koubaa, M.; Barba, F.J. An overview of the impact of electrotechnologies for the recovery of oil and high-value compounds from vegetable oil industry: Energy and economic cost implications. Food Res. Int. 2016, 80, 19–26. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Pallarés, N.; Bäuerl, C.; Collado, M.C.; Dar, B.N.; Barba, F.J. Role of Extracts Obtained from Rainbow Trout and Sole Side Streams by Accelerated Solvent Extraction and Pulsed Electric Fields on Modulating Bacterial and Anti-Inflammatory Activities. Separations 2021, 8, 187. [Google Scholar] [CrossRef]
- de la Fuente, B.; Pallarés, N.; Barba, F.J.; Berrada, H. An Integrated Approach for the Valorization of Sea Bass (Dicentrarchus labrax) Side Streams: Evaluation of Contaminants and Development of Antioxidant Protein Extracts by Pressurized Liquid Extraction. Foods 2021, 10, 546. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; van de Ven, R.; Suwandy, V.; Fahri, F.; Hopkins, D.L. Effect of pulsed electric field treatment on cold-boned muscles of different potential tenderness. Food Bioprocess. Technol. 2014, 7, 3136–3146. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.-D.A. Current and future prospects for the use of pulsed electric field in the meat industry. Crit. Rev. Food Sci. Nutr. 2019, 59, 1660–1674. [Google Scholar] [CrossRef]
- Calleja-Gomez, M.; Pallares, N.; Salgado-Ramos, M.; Barba, F.J.; Berrada, H.; Castagnini, J.M. Sustainable processing of food side streams and underutilized leftovers into high-added-value chemicals assisted by pulsed electric fields-and high-pressure processing-based technologies. TrAC Trends Anal. Chem. 2024, 171, 117506. [Google Scholar] [CrossRef]
- Pataro, G.; Ferrari, G. Limitations of pulsed electric field utilization in food industry. In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–310. [Google Scholar]
- Zhou, J.; Wang, M.; Saraiva, J.A.; Martins, A.P.; Pinto, C.A.; Prieto, M.A.; Simal-Gandara, J.; Cao, H.; Xiao, J.; Barba, F.J. Extraction of lipids from microalgae using classical and innovative approaches. Food Chem. 2022, 384, 132236. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub-and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Herrero, M.; Castro-Puyana, M.; Ibáñez, E. Supercritical fluid extraction. In Natural Product Extraction; The Royal Society of Chemistry: Cambridge, UK, 2013; p. 196. [Google Scholar]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [PubMed]
- Melgosa, R.; Trigueros, E.; Sanz, M.T.; Cardeira, M.; Rodrigues, L.; Fernández, N.; Matias, A.A.; Bronze, M.R.; Marques, M.; Paiva, A. Supercritical CO2 and subcritical water technologies for the production of bioactive extracts from sardine (Sardina pilchardus) waste. J. Supercrit. Fluids 2020, 164, 104943. [Google Scholar] [CrossRef]
- Ling Wen Xia, F.; Supri, S.; Djamaludin, H.; Nurdiani, R.; Leong Seng, L.; Wee Yin, K.; Rovina, K. Turning waste into value: Extraction and effective valorization strategies of seafood by-products. Waste Manag. Bull. 2024, 2, 84–100. [Google Scholar] [CrossRef]
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative Green Technologies of Intensification for Valorization of Seafood and Their By-Products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef]
- Gondo, T.F.; Jönsson, M.; Karlsson, E.N.; Sandahl, M.; Turner, C. Extractability, selectivity, and comprehensiveness in supercritical fluid extraction of seaweed using ternary mixtures of carbon dioxide, ethanol, and water. J. Chromatogr. A 2023, 1706, 464267. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Yang, Y.; Miller, D.J. Extraction of organic pollutants from environmental solids with sub-and supercritical water. Anal. Chem. 1994, 66, 2912–2920. [Google Scholar] [CrossRef]
- Park, J.Y.; Back, S.S.; Chun, B.S. Protein properties of mackerel viscera extracted by supercritical carbon dioxide. J. Environ. Biol. 2008, 29, 443–448. [Google Scholar]
- Rahman, M.S.; Seo, J.-K.; Choi, S.-G.; Gul, K.; Yang, H.-S. Physicochemical characteristics and microbial safety of defatted bovine heart and its lipid extracted with supercritical-CO2 and solvent extraction. LWT 2018, 97, 355–361. [Google Scholar] [CrossRef]
- Haque, A.R.; Park, J.-S.; Ho, T.C.; Roy, V.C.; Ali, M.S.; Kiddane, A.T.; Kim, G.-D.; Chun, B.-S. Characterization of oil and amino acids obtained from yellow corvina by-products using subcritical and supercritical fluids. J. Supercrit. Fluids 2023, 199, 105970. [Google Scholar] [CrossRef]
- Tham, P.E.; Ng, Y.J.; Sankaran, R.; Khoo, K.S.; Chew, K.W.; Yap, Y.J.; Malahubban, M.; Aziz Zakry, F.A.; Show, P.L. Recovery of protein from dairy milk waste product using alcohol-salt liquid biphasic flotation. Processes 2019, 7, 875. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Krishnamoorthy, R.; Tao, Y.; Chu, D.-T.; Show, P.L. Liquid biphasic flotation for the purification of C-phycocyanin from Spirulina platensis microalga. Bioresour. Technol. 2019, 288, 121519. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Lim, J.W.; Lee, S.Y.; Lam, M.K.; Show, P.L. Optimization of protein extraction from Chlorella Vulgaris via novel sugaring-out assisted liquid biphasic electric flotation system. Eng. Life Sci. 2019, 19, 968–977. [Google Scholar] [CrossRef]
- Phong, W.N.; Show, P.L.; Teh, W.H.; Teh, T.X.; Lim, H.M.Y.; binti Nazri, N.S.; Tan, C.H.; Chang, J.-S.; Ling, T.C. Proteins recovery from wet microalgae using liquid biphasic flotation (LBF). Bioresour. Technol. 2017, 244, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, R.; Manickam, S.; Yap, Y.J.; Ling, T.C.; Chang, J.-S.; Show, P.L. Extraction of proteins from microalgae using integrated method of sugaring-out assisted liquid biphasic flotation (LBF) and ultrasound. Ultrason. Sonochem. 2018, 48, 231–239. [Google Scholar] [CrossRef]
- Sankaran, R.; Show, P.L.; Cheng, Y.-S.; Tao, Y.; Ao, X.; Nguyen, T.D.P.; Van Quyen, D. Integration process for protein extraction from microalgae using liquid biphasic electric flotation (LBEF) system. Mol. Biotechnol. 2018, 60, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Saw, H.S.; Sankaran, R.; Khoo, K.S.; Chew, K.W.; Phong, W.N.; Tang, M.S.Y.; Lim, S.S.; Mohd Zaid, H.F.; Naushad, M.; Show, P.L. Application of a liquid biphasic flotation (LBF) system for protein extraction from Persiscaria tenulla leaf. Processes 2020, 8, 247. [Google Scholar] [CrossRef]
- Chia, S.R.; Mak, K.Y.; Khaw, Y.J.; Suhaidi, N.; Chew, K.W.; Show, P.L. An efficient and rapid method to extract and purify protein–Liquid Triphasic Flotation system. Bioresour. Technol. 2019, 294, 122158. [Google Scholar] [CrossRef]
- Mainberger, S.; Kindlein, M.; Bezold, F.; Elts, E.; Minceva, M.; Briesen, H. Deep eutectic solvent formation: A structural view using molecular dynamics simulations with classical force fields. Mol. Phys. 2017, 115, 1309–1321. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, A.; Panesar, P.S.; Thakur, A. Potential of deep eutectic solvents in the extraction of value-added compounds from agro-industrial by-products. Appl. Food Res. 2022, 2, 100211. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Cardeira, M.; Matias, A.A.; Bronze, M.R.; Fernández, N. Lactic acid-based natural deep eutectic solvents to extract bioactives from marine by-products. Molecules 2022, 27, 4356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Kalhor, P.; Ghandi, K. Deep eutectic solvents for pretreatment, extraction, and catalysis of biomass and food waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Leonardo, I.C.; Gaspar, F.B.; Roseiro, L.C.; Duarte, A.R.C.; Matias, A.A.; Paiva, A. Unveiling the potential of betaine/polyol-based deep eutectic systems for the recovery of bioactive protein derivative-rich extracts from sardine processing residues. Sep. Purif. Technol. 2021, 276, 119267. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Gerliani, N.; Hammami, R.; Aïder, M. Extraction of protein and carbohydrates from soybean meal using acidic and alkaline solutions produced by electro-activation. Food Sci. Nutr. 2020, 8, 1125–1138. [Google Scholar] [CrossRef]
- Gerzhova, A.; Mondor, M.; Benali, M.; Aider, M. A comparative study between the electro-activation technique and conventional extraction method on the extractability, composition and physicochemical properties of canola protein concentrates and isolates. Food Biosci. 2015, 11, 56–71. [Google Scholar] [CrossRef]
- Robin, A.; Ghosh, S.; Gabay, B.; Levkov, K.; Golberg, A. Identifying critical parameters for extraction of carnosine and anserine from chicken meat with high voltage pulsed electric fields and water. Innov. Food Sci. Emerg. Technol. 2022, 76, 102937. [Google Scholar] [CrossRef]
- Momen, S.; Aider, M. Production of highly soluble and functional whey/canola proteins through complexation using alkaline electro-activation. Food Hydrocoll. 2023, 137, 108395. [Google Scholar] [CrossRef]
- Ohshima, T.; Tanino, T.; Guionet, A.; Takahashi, K.; Takaki, K. Mechanism of pulsed electric field enzyme activity change and pulsed discharge permeabilization of agricultural products. Jpn. J. Appl. Phys. 2021, 60, 060501. [Google Scholar] [CrossRef]
- Eleršek, T.; Flisar, K.; Likozar, B.; Klemenčič, M.; Golob, J.; Kotnik, T.; Miklavčič, D. Electroporation as a solvent-free green technique for non-destructive extraction of proteins and lipids from Chlorella vulgaris. Front. Bioeng. Biotechnol. 2020, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, K. A novel thermal hydrolysis process for extraction of keratin from hog hair for commercial applications. Waste Manag. 2020, 104, 33–41. [Google Scholar] [CrossRef]
- Wilson, C.A.; Novak, J.T. Hydrolysis of macromolecular components of primary and secondary wastewater sludge by thermal hydrolytic pretreatment. Water Res. 2009, 43, 4489–4498. [Google Scholar] [CrossRef]
- Schmidt, E. Method for Producing Non-Infectious Products from Infectious Organic Waste Material. United State Patents US8278081B2, 2 October 2012. [Google Scholar]
- Somerville, R.A.; Fernie, K.; Smith, A.; Andrews, R.; Schmidt, E.; Taylor, D.M. Inactivation of a TSE agent by a novel biorefinement system. Process Biochem. 2009, 44, 1060–1062. [Google Scholar] [CrossRef]
- Lu, H.-W.; Xiao, S.; Le, T.; Al-Omari, A.; Higgins, M.; Boardman, G.; Novak, J.; Murthy, S. Evaluation of Solubilization Characteristics of Thermal Hydrolysis Process; Water Environment Federation: Alexandria, VA, USA, 2014. [Google Scholar]
- Yan, W.; Song, M.; Zhou, Y. Redistribution of perfluorooctanoic acid in sludge after thermal hydrolysis: Location of protein plays a major role. Water Res. 2023, 241, 120135. [Google Scholar] [CrossRef]
- Mekonnen, T.H.; Mussone, P.G.; Stashko, N.; Choi, P.Y.; Bressler, D.C. Recovery and characterization of proteinacious material recovered from thermal and alkaline hydrolyzed specified risk materials. Process Biochem. 2013, 48, 885–892. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Liu, J.; Wang, Y.; Liu, S.; Sun, M. Comparison between thermal hydrolysis and enzymatic proteolysis processes for the preparation of tilapia skin collagen hydrolysates. Czech J. Food Sci. 2013, 31, 1–4. [Google Scholar] [CrossRef]
- Bhavsar, P.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Rovero, G.; Tonin, C. Comparative study on the effects of superheated water and high temperature alkaline hydrolysis on wool keratin. Text. Res. J. 2017, 87, 1696–1705. [Google Scholar] [CrossRef]
- Esteban, M.B.; García, A.J.; Ramos, P.; Márquez, M.d.C. Sub-critical water hydrolysis of hog hair for amino acid production. Bioresour. Technol. 2010, 101, 2472–2476. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Chapter 13—Pressurized Liquid Extraction. In Liquid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. [Google Scholar]
- Bruno, S.F.; Ekorong, F.J.A.A.; Karkal, S.S.; Cathrine, M.S.B.; Kudre, T.G. Green and innovative techniques for recovery of valuable compounds from seafood by-products and discards: A review. Trends Food Sci. Technol. 2019, 85, 10–22. [Google Scholar] [CrossRef]
- Ramos, L.; Kristenson, E.M.; Brinkman, U.A.T. Current use of pressurised liquid extraction and subcritical water extraction in environmental analysis. J. Chromatogr. A 2002, 975, 3–29. [Google Scholar] [CrossRef]
- Wani, T.A.; Masoodi, F.A.; Dar, M.M.; Akhter, R.; Sharma, O.C. Subcritical treatment of olive oil: Minor phenolic composition and antioxidant properties of the solvent extracts. LWT 2021, 147, 111584. [Google Scholar] [CrossRef]
- de la Fuente, B.; Pallarés, N.; Berrada, H.; Barba, F.J. Salmon (Salmo salar) Side Streams as a Bioresource to Obtain Potential Antioxidant Peptides after Applying Pressurized Liquid Extraction (PLE). Mar. Drugs 2021, 19, 323. [Google Scholar] [CrossRef]
- de la Fuente, B.; Pallarés, N.; Berrada, H.; Barba, F.J. Development of Antioxidant Protein Extracts from Gilthead Sea Bream (Sparus aurata) Side Streams Assisted by Pressurized Liquid Extraction (PLE). Mar. Drugs 2021, 19, 199. [Google Scholar] [CrossRef]
- Espíndola-Cortés, A.; Moreno-Tovar, R.; Bucio, L.; Gimeno, M.; Ruvalcaba-Sil, J.L.; Shirai, K. Hydroxyapatite crystallization in shrimp cephalothorax wastes during subcritical water treatment for chitin extraction. Carbohydr. Polym. 2017, 172, 332–341. [Google Scholar] [CrossRef]
- Kang, K.; Quitain, A.T.; Daimon, H.; Noda, R.; Goto, N.; Hu, H.Y.; Fujie, K. Optimization of amino acids production from waste fish entrails by hydrolysis in sub and supercritical water. Can. J. Chem. Eng. 2001, 79, 65–70. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Matias, A.A.; Paiva, A. Recovery of antioxidant protein hydrolysates from shellfish waste streams using subcritical water extraction. Food Bioprod. Process. 2021, 130, 154–163. [Google Scholar] [CrossRef]
- Melgosa, R.; Marques, M.; Paiva, A.; Bernardo, A.; Fernández, N.; Sá-Nogueira, I.; Simões, P. Subcritical Water Extraction and Hydrolysis of Cod (Gadus morhua) Frames to Produce Bioactive Protein Extracts. Foods 2021, 10, 1222. [Google Scholar] [CrossRef]
- Wang, C.; Lin, M.; Yang, Q.; Fu, C.; Guo, Z. The Principle of Steam Explosion Technology and Its Application in Food Processing By-Products. Foods 2023, 12, 3307. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Ma, Y.; Qin, X.; Guo, Y.; Zhang, C. Steam explosion as a green method to treat animal waste: A mini-review. Process Saf. Environ. Prot. 2024, 181, 43–52. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Rosseto, M.; Alessandretti, I.; Krein, D.D.C.; Emer, C.D.; Loss, R.A.; Dettmer, A.; Pizzutti, I.R. Extraction and improvement of protein functionality using steam explosion pretreatment: Advances, challenges, and perspectives. J. Food Sci. Technol. 2024, 61, 1215–1237. [Google Scholar] [CrossRef]
- Maniet, G.; Schmetz, Q.; Jacquet, N.; Temmerman, M.; Gofflot, S.; Richel, A. Effect of steam explosion treatment on chemical composition and characteristic of organosolv fescue lignin. Ind. Crops Prod. 2017, 99, 79–85. [Google Scholar] [CrossRef]
- Tonin, C.; Zoccola, M.; Aluigi, A.; Varesano, A.; Montarsolo, A.; Vineis, C.; Zimbardi, F. Study on the Conversion of Wool Keratin by Steam Explosion. Biomacromolecules 2006, 7, 3499–3504. [Google Scholar] [CrossRef]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E.-D.A. Keratin: Dissolution, extraction and biomedical application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, R.; Zhao, W. Improving digestibility of feather meal by steam flash explosion. J. Agric. Food Chem. 2014, 62, 2745–2751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, W.; Yang, R. Steam flash explosion assisted dissolution of keratin from feathers. ACS Sustain. Chem. Eng. 2015, 3, 2036–2042. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, H.; Zhang, C.; Qin, X.; Jia, W.; Xu, X.; Richel, A.; Zheng, Q. Liquefaction of porcine hoof shell to prepare peptone substitute by instant catapult steam explosion. J. Biosci. Bioeng. 2020, 129, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Shen, Q.; Guo, Y.; Liu, J.; Zhang, H.; Jia, W.; Xu, X.; Zhang, C. An advanced strategy for efficient recycling of bovine bone: Preparing high-valued bone powder via instant catapult steam-explosion. Food Chem. 2022, 374, 131614. [Google Scholar] [CrossRef]
- Scopel, B.S.; Restelatto, D.; Baldasso, C.; Dettmer, A.; Santana, R.M.C. Steam explosion as pretreatment to increase gelatin extraction yield from chromium tanned leather wastes. Environ. Prog. Sustain. Energy 2019, 38, 367–373. [Google Scholar] [CrossRef]
- Kuwahara, J. Extraction of type I collagen from tilapia scales using acetic acid and ultrafine bubbles. Processes 2021, 9, 288. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; García-Depraect, O.; León-Becerril, E.; Cassano, A.; Conidi, C.; Fíla, V. Recovery of protein-based compounds from meat by-products by membrane-assisted separations: A review. J. Chem. Technol. Biotechnol. 2021, 96, 3025–3042. [Google Scholar] [CrossRef]
- Asaduzzaman, A.K.M.; Getachew, A.T.; Cho, Y.-J.; Park, J.-S.; Haq, M.; Chun, B.-S. Characterization of pepsin-solubilised collagen recovered from mackerel (Scomber japonicus) bone and skin using subcritical water hydrolysis. Int. J. Biol. Macromol. 2020, 148, 1290–1297. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, Y.H.; Kim, Y.E.; Jung, S.K.; Lee, N.H.; Song, K.-M. Effects of salts on ultrasonic extraction of protein from porcine myocardium. Food Bioprod. Process. 2018, 108, 12–17. [Google Scholar] [CrossRef]
- Sharath Kumar, N.; Dar, A.H.; Dash, K.K.; Kaur, B.; Pandey, V.K.; Singh, A.; Fayaz, U.; Shams, R.; Mukarram, S.A.; Kovács, B. Recent advances in cold plasma technology for modifications of proteins: A comprehensive review. J. Agric. Food Res. 2024, 16, 101177. [Google Scholar] [CrossRef]
- Koddy, J.K.; Miao, W.; Hatab, S.; Tang, L.; Xu, H.; Nyaisaba, B.M.; Chen, M.; Deng, S. Understanding the role of atmospheric cold plasma (ACP) in maintaining the quality of hairtail (Trichiurus Lepturus). Food Chem. 2021, 343, 128418. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, X.; Li, J.; Pan, D.; Du, L. Enhancing the functionalities of chickpea protein isolate through a combined strategy with pH-shifting and cold plasma treatment. Innov. Food Sci. Emerg. Technol. 2024, 93, 103607. [Google Scholar] [CrossRef]
- Zheng, X.; Zou, B.; Zhang, J.; Cai, W.; Na, X.; Du, M.; Zhu, B.; Wu, C. Recent advances of ultrasound-assisted technology on aquatic protein processing: Extraction, modification, and freezing/thawing-induced oxidation. Trends Food Sci. Technol. 2024, 144, 104309. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).