Modulating Coffee Fermentation Quality Using Microbial Inoculums from Coffee By-Products for Sustainable Practices in Smallholder Coffee Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Coffee Beans
2.2. Coffee Pulp
2.3. Coffee Wastewater
2.4. Microorganisms
2.5. Analytical Methods
2.5.1. Microorganism Viability
2.5.2. pH
2.5.3. Soluble Solids
2.5.4. Titratable Acidity
2.6. Coffee Cup Quality Evaluation
2.7. Experimental Methods
2.7.1. Bacteria and Yeast Inoculums Optimized Production
2.7.2. Optimization
2.7.3. Experimental Validation
2.8. Coffee Processing with Optimized Inoculums
3. Results
3.1. Dynamic Monitoring of Inoculum Fermentation Parameters
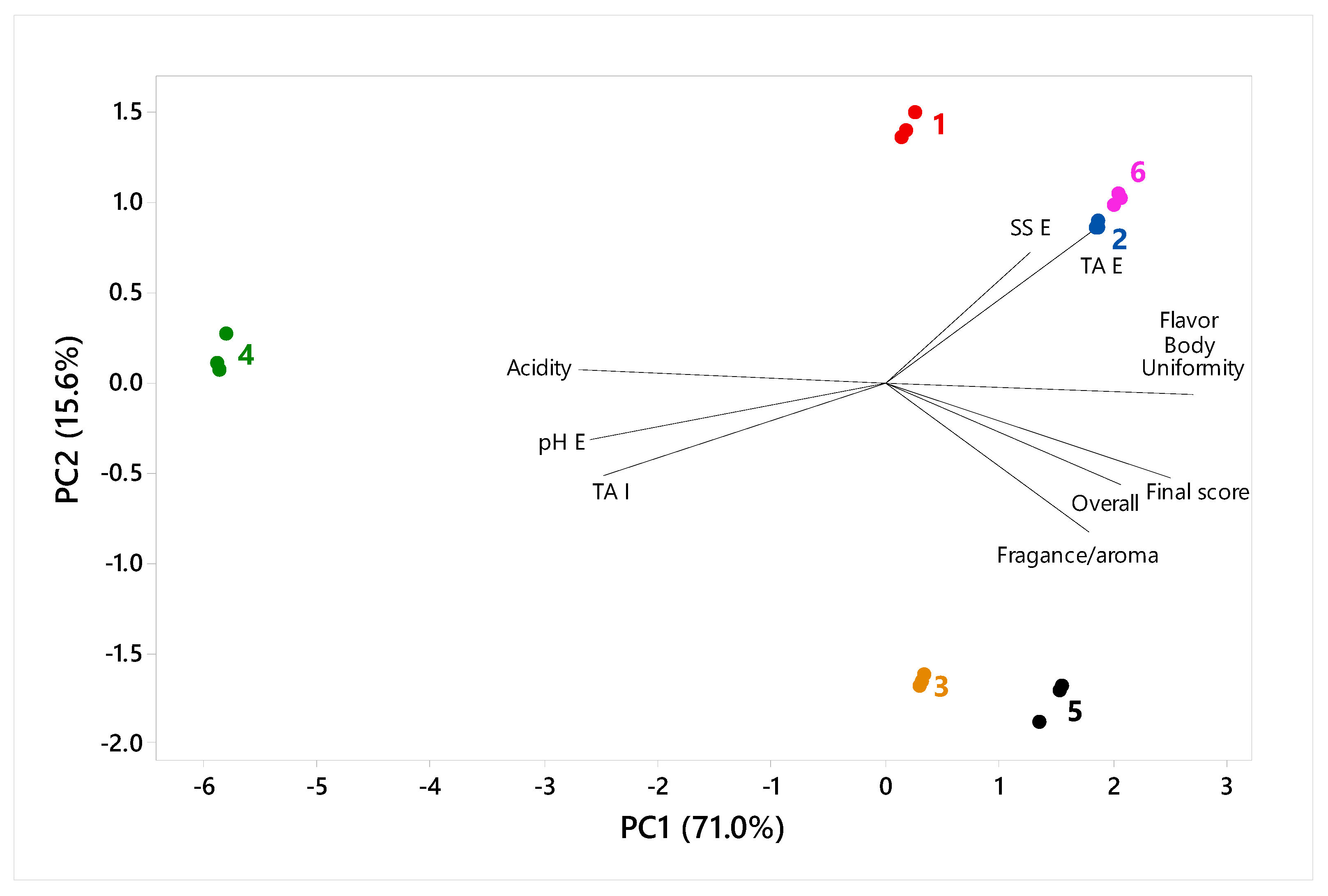
3.2. Statistical Analysis
3.3. Optimization and Validation
3.4. Performance Evaluation of Optimized Inoculum
4. Discussion
4.1. Impact of Coffee By-Products on LAB and Yeast Growth in Different Inoculum Formulations
4.2. Impact of LAB and Yeast Inoculum in Coffee Fermentation and Sensory Profile
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Espíndola Rafael, V. A Business Case to Increase Specialty Coffee Consumption in Producing Countries; Specialty Coffee Association: Santa Ana, CA, USA, 2020. [Google Scholar]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; Vásquez, Z.S.; Medeiros, A.B.P.; Vandenberghe, L.P.S.; Soccol, C.R. Exploring the Impacts of Postharvest Processing on the Aroma Formation of Coffee Beans—A Review. Food Chem. 2019, 272, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Aswathi, K.N.; Shirke, A.; Praveen, A.; Chaudhari, S.R.; Murthy, P.S. Pulped Natural/Honey Robusta Coffee Fermentation Metabolites, Physico-Chemical and Sensory Profiles. Food Chem. 2023, 429, 136897. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Taquez, H.N.; GilPavas, E.; Blatchley, E.R.; Gómez-García, M.Á.; Dobrosz-Gómez, I. Integrated Electrocoagulation-Electrooxidation Process for the Treatment of Soluble Coffee Effluent: Optimization of COD Degradation and Operation Time Analysis. J. Environ. Manag. 2017, 200, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Serna-Jiménez, J.A.; Siles, J.A.; de los Ángeles Martín, M.; Chica, A.F. A Review on the Applications of Coffee Waste Derived from Primary Processing: Strategies for Revalorization. Processes 2022, 10, 2436. [Google Scholar] [CrossRef]
- Zhao, N.; Kokawa, M.; Amini, R.K.; Dong, W.; Kitamura, Y. Isolation of Yeast and LAB from Dry Coffee Pulp and Monitoring of Organic Acids in Inoculated Green Beans. Foods 2023, 12, 2622. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. Antioxidant Activity, Total Polyphenol, Flavonoid and Tannin Contents of Fermented Green Coffee Beans with Selected Yeasts. Fermentation 2019, 5, 29. [Google Scholar] [CrossRef]
- Sofía Torres-Valenzuela, L.; Andrea Serna-Jiménez, J.; Martínez, K. Coffee By-Products: Nowadays and Perspectives. In Coffee-Production and Research; IntechOpen: London, UK, 2020. [Google Scholar]
- Evangelista, S.R.; da Cruz Pedroso Miguel, M.G.; Silva, C.F.; Pinheiro, A.C.M.; Schwan, R.F. Microbiological Diversity Associated with the Spontaneous Wet Method of Coffee Fermentation. Int. J. Food Microbiol. 2015, 210, 102–112. [Google Scholar] [CrossRef]
- Cruz-O’Byrne, R.; Piraneque-Gambasica, N.; Aguirre-Forero, S. Microbial Diversity Associated with Spontaneous Coffee Bean Fermentation Process and Specialty Coffee Production in Northern Colombia. Int. J. Food Microbiol. 2021, 354, 109282. [Google Scholar] [CrossRef]
- Zhang, S.J.; Page-Zoerkler, N.; Genevaz, A.; Roubaty, C.; Pollien, P.; Bordeaux, M.; Mestdagh, F.; Moccand, C. Unlocking the Aromatic Potential of Native Coffee Yeasts: From Isolation to a Biovolatile Platform. J. Agric. Food Chem. 2023, 71, 4665–4674. [Google Scholar] [CrossRef]
- Polanía-Rivera, A.M.; López-Silva, J.; Torres-Valenzuela, L.S.; Plaza-Dorado, J.L. Development of Starter Inoculum for Controlled Arabica Coffee Fermentation Using Coffee By-Products (Pulp and Mucilage Broth), Yeast, and Lactic Acid Bacteria. Fermentation 2024, 10, 516. [Google Scholar] [CrossRef]
- Martins, P.M.M.; Ribeiro, L.S.; da Cruz Pedroso Miguel, M.G.; Evangelista, S.R.; Schwan, R.F. Production of Coffee (Coffea Arabica) Inoculated with Yeasts: Impact on Quality. J. Sci. Food Agric. 2019, 99, 5638–5645. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, S.R.; da Cruz Pedrozo Miguel, M.G.; de Souza Cordeiro, C.; Silva, C.F.; Marques Pinheiro, A.C.; Schwan, R.F. Inoculation of Starter Cultures in a Semi-Dry Coffee (Coffea Arabica) Fermentation Process. Food Microbiol. 2014, 44, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ulhano Braga, A.V.; Miranda, M.A.; Aoyama, H.; Schmidt, F.L. Study on Coffee Quality Improvement by Self-Induced Anaerobic Fermentation: Microbial Diversity and Enzymatic Activity. Food Res. Int. 2023, 165, 112528. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. Coffee Flavour Modification through Controlled Fermentation of Green Coffee Beans by Lactococcus Lactis Subsp. Cremoris. LWT 2020, 120, 108930. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Lu, Y.; Liu, S.Q. In Vitro Bioactivities of Coffee Brews Fermented with the Probiotics Lacticaseibacillus Rhamnosus GG and Saccharomyces Boulardii CNCM-I745. Food Res. Int. 2021, 149, 110693. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Toh, M.; Liu, S.Q. Growth, Survival, and Metabolic Activities of Probiotics Lactobacillus Rhamnosus GG and Saccharomyces Cerevisiae Var. Boulardii CNCM-I745 in Fermented Coffee Brews. Int. J. Food Microbiol. 2021, 350, 109229. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Toh, M.; Liu, S.Q. Growth, Survival, and Metabolic Activities of Probiotic Lactobacillus spp. in Fermented Coffee Brews Supplemented with Glucose and Inactivated Yeast Derivatives. Food Res. Int. 2020, 137, 109746. [Google Scholar] [CrossRef]
- Zhang, S.J.; De Bruyn, F.; Pothakos, V.; Contreras, G.F.; Cai, Z.; Moccand, C.; Weckx, S.; De Vuyst, L. Influence of Various Processing Parameters on the Microbial Community Dynamics, Metabolomic Profiles, and Cup Quality During Wet Coffee Processing. Front. Microbiol. 2019, 10, 2621. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; da Silva Vale, A.; de Carvalho Neto, D.P.; Muynarsk, E.S.; Soccol, V.T.; Soccol, C.R. Lactic Acid Bacteria: What Coffee Industry Should Know? Curr. Opin. Food Sci. 2020, 31, 1–8. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Medeiros, A.B.P.; Soccol, V.T.; Neto, E.; Woiciechowski, A.L.; Soccol, C.R. Potential of Lactic Acid Bacteria to Improve the Fermentation and Quality of Coffee during On-Farm Processing. Int. J. Food Sci. Technol. 2016, 51, 1689–1695. [Google Scholar] [CrossRef]
- da Silva Vale, A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Rodrigues, C.; Pagnoncelli, M.G.B.; Soccol, C.R. Effect of Co-Inoculation with Pichia Fermentans and Pediococcus Acidilactici on Metabolite Produced during Fermentation and Volatile Composition of Coffee Beans. Fermentation 2019, 5, 67. [Google Scholar] [CrossRef]
- Bressani, A.P.P.; Martinez, S.J.; Evangelista, S.R.; Dias, D.R.; Schwan, R.F. Characteristics of Fermented Coffee Inoculated with Yeast Starter Cultures Using Different Inoculation Methods. LWT 2018, 92, 212–219. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. Coffee Flavour Modification through Controlled Fermentation of Green Coffee Beans by Saccharomyces Cerevisiae and Pichia Kluyveri: Part II. Mixed Cultures with or without Lactic Acid Bacteria. Food Res. Int. 2020, 136, 109452. [Google Scholar] [CrossRef] [PubMed]
- Vinícius de Melo Pereira, G.; Soccol, V.T.; Brar, S.K.; Neto, E.; Soccol, C.R. Microbial Ecology and Starter Culture Technology in Coffee Processing. Crit. Rev. Food Sci. Nutr. 2017, 57, 2775–2788. [Google Scholar] [CrossRef] [PubMed]
- Mahingsapun, R.; Tantayotai, P.; Panyachanakul, T.; Samosorn, S.; Dolsophon, K.; Jiamjariyatam, R.; Lorliam, W.; Srisuk, N.; Krajangsang, S. Enhancement of Arabica Coffee Quality with Selected Potential Microbial Starter Culture under Controlled Fermentation in Wet Process. Food Biosci. 2022, 48, 101819. [Google Scholar] [CrossRef]
- Samoggia, A.; Fantini, A. Revealing the Governance Dynamics of the Coffee Chain in Colombia: A State-of-the-Art Review. Sustainability 2023, 15, 13646. [Google Scholar] [CrossRef]
- Wienhold, K.; Goulao, L.F. The Embedded Agroecology of Coffee Agroforestry: A Contextualized Review of Smallholder Farmers’ Adoption and Resistance. Sustainability 2023, 15, 6827. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC INTERNATIONAL; AOAC International: Rockville, MD, USA, 2023. [Google Scholar]
- Mazzafera, P. Chemical Composition of Defective Coffee Beans. Food Chem. 1999, 64, 547–554. [Google Scholar] [CrossRef]
- SCA. Speciality Coffee Asociation Protocols & Best Practices; Specialty Coffee Association: Santa Ana, CA, USA, 2024. [Google Scholar]
- Plaza-Dorado, J.L.; Suárez-Rodríguez, C.; Torres-Valenzuela, L.S. Tuneable Solvents-Based Extraction for the Recovery of Caffeine from Coffee Wastewater. Environ. Prog. Sustain. Energy 2024, 43, e14291. [Google Scholar] [CrossRef]
- Evangelista, S.R.; Silva, C.F.; da Cruz Miguel, M.G.P.; de Souza Cordeiro, C.; Pinheiro, A.C.M.; Duarte, W.F.; Schwan, R.F. Improvement of Coffee Beverage Quality by Using Selected Yeasts Strains during the Fermentation in Dry Process. Food Res. Int. 2014, 61, 183–195. [Google Scholar] [CrossRef]
- Ruta, L.L.; Farcasanu, I.C. Coffee and Yeasts: From Flavor to Biotechnology. Fermentation 2021, 7, 9. [Google Scholar] [CrossRef]
- Osorio, V.; Medina, R.; Acuña, J.R.; Pabón, J.; Álvarez, C.I.; Matallana, L.G.; Fernández-Alduenda, M.R. Transformation of Organic Acids and Sugars in the Mucilage and Coffee Beans during Prolonged Fermentation. J. Food Compos. Anal. 2023, 123, 105551. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Zhao, J. Ecological Diversity, Evolution and Metabolism of Microbial Communities in the Wet Fermentation of Australian Coffee Beans. Int. J. Food Microbiol. 2020, 321, 108544. [Google Scholar] [CrossRef] [PubMed]
- Inés, G.; Mejia, J.; Osorio, G. Microbiología De La Fermentación Del Mucílago De Café Según Su Madurez Y Selección. Cenicafé 2012, 63, 58–78. [Google Scholar]
- Cassimiro, D.M.d.J.; Batista, N.N.; Fonseca, H.C.; Oliveira Naves, J.A.; Coelho, J.M.; Bernardes, P.C.; Dias, D.R.; Schwan, R.F. Wet Fermentation of Coffea Canephora by Lactic Acid Bacteria and Yeasts Using the Self-Induced Anaerobic Fermentation (SIAF) Method Enhances the Coffee Quality. Food Microbiol. 2023, 110, 104161. [Google Scholar] [CrossRef]
- Vinícius de Melo Pereira, G.; Neto, E.; Soccol, V.T.; Medeiros, A.B.P.; Woiciechowski, A.L.; Soccol, C.R. Conducting Starter Culture-Controlled Fermentations of Coffee Beans during on-Farm Wet Processing: Growth, Metabolic Analyses and Sensorial Effects. Food Res. Int. 2015, 75, 348–356. [Google Scholar] [CrossRef]
- Smit, B.A.; Vlieg, J.E.T.v.H.; Engels, W.J.M.; Meijer, L.; Wouters, J.T.M.; Smit, G. Identification, Cloning, and Characterization of a Lactococcus Lactis Branched-Chain α-Keto Acid Decarboxylase Involved in Flavor Formation. Appl. Environ. Microbiol. 2005, 71, 303–311. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Lassabliere, B.; Yu, B.; Zhao, F.F.; Zhao, F.F.; Chen, Y.; Liu, S.Q. Potential of Lactic Acid Bacteria to Modulate Coffee Volatiles and Effect of Glucose Supplementation: Fermentation of Green Coffee Beans and Impact of Coffee Roasting. J. Sci. Food Agric. 2019, 99, 409–420. [Google Scholar] [CrossRef]
- Martinez, S.J.; Bressani, A.P.P.; Dias, D.R.; Simão, J.B.P.; Schwan, R.F. Effect of Bacterial and Yeast Starters on the Formation of Volatile and Organic Acid Compounds in Coffee Beans and Selection of Flavors Markers Precursors during Wet Fermentation. Front. Microbiol. 2019, 10, 1287. [Google Scholar] [CrossRef]
- Sampaio, V.d.M.; Wiele, N.; Vale, A.d.S.; Woiciechowski, A.L.; Karp, S.G.; Soccol, C.R.; Pereira, G.V.d.M. Modulation of Aroma and Chemical Composition of Coffee Beans through Simultaneous and Sequential Inoculation of Pichia fermentans and Pediococcus pentosaceus during Wet Fermentation. Syst. Microbiol. Biomanuf. 2024. [Google Scholar] [CrossRef]
- Moreira, R.F.A.; Trugo, L.C.; De Maria, C.A.B. Componentes Voláteis Do Café Torrado. Parte II. Compostos Alifáticos, Alicíclicos e Aromáticos. Quim. Nova 2000, 23, 195–203. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. The Crucial Role of Yeasts in the Wet Fermentation of Coffee Beans and Quality. Int. J. Food Microbiol. 2020, 333, 108796. [Google Scholar] [CrossRef] [PubMed]
- Bravim, D.G.; de Oliveira, T.M.; Rosário, D.K.A.D.; Batista, N.N.; Schwan, R.F.; Coelho, J.M.; Bernardes, P.C. Inoculation of Yeast and Bacterium in Wet-Processed Coffea Canephora. Food Chem. 2023, 400, 134107. [Google Scholar] [CrossRef] [PubMed]
- Galarza, G.; Figueroa, J.G. Volatile Compound Characterization of Coffee (Coffea Arabica) Processed at Different Fermentation Times Using SPME–GC–MS. Molecules 2022, 27, 2004. [Google Scholar] [CrossRef]
- Jimenez, E.J.M.; Martins, P.M.M.; de Oliveira Vilela, A.L.; Batista, N.N.; da Rosa, S.D.V.F.; Dias, D.R.; Schwan, R.F. Influence of Anaerobic Fermentation and Yeast Inoculation on the Viability, Chemical Composition, and Quality of Coffee. Food Biosci. 2023, 51, 102218. [Google Scholar] [CrossRef]
- Lyon, S.W. A Cross-Disciplinary Approach Needs to Be at the Core of Sustainability. Sustainability 2023, 15, 15954. [Google Scholar] [CrossRef]
- Calderon, N.; Jiang, G.Z.; Gibney, P.A.; Dando, R. A Consumer Assessment of Fermented Green Coffee Beans with Common Beer/Wine Yeast Strains for Novel Flavor Properties. Fermentation 2023, 9, 865. [Google Scholar] [CrossRef]
- Kwak, H.S.; Jeong, Y.; Kim, M. Effect of Yeast Fermentation of Green Coffee Beans on Antioxidant Activity and Consumer Acceptability. J. Food Qual. 2018, 2018, 5967130. [Google Scholar] [CrossRef]
- Rosário, D.K.A.D.; da Silva Mutz, Y.; Vieira, K.M.; Schwan, R.F.; Bernardes, P.C. Effect of Self-Induced Anaerobiosis Fermentation (SIAF) in the Volatile Compounds and Sensory Quality of Coffee. Eur. Food Res. Technol. 2024, 250, 667–675. [Google Scholar] [CrossRef]
- Martins, P.M.M.; Batista, N.N.; Naves, J.A.O.; Dias, D.R.; Schwan, R.F. Use of Microencapsulated Starter Cultures by Spray Drying in Coffee under Self-Induced Anaerobiosis Fermentation (SIAF). Food Res. Int. 2023, 172, 113189. [Google Scholar] [CrossRef] [PubMed]

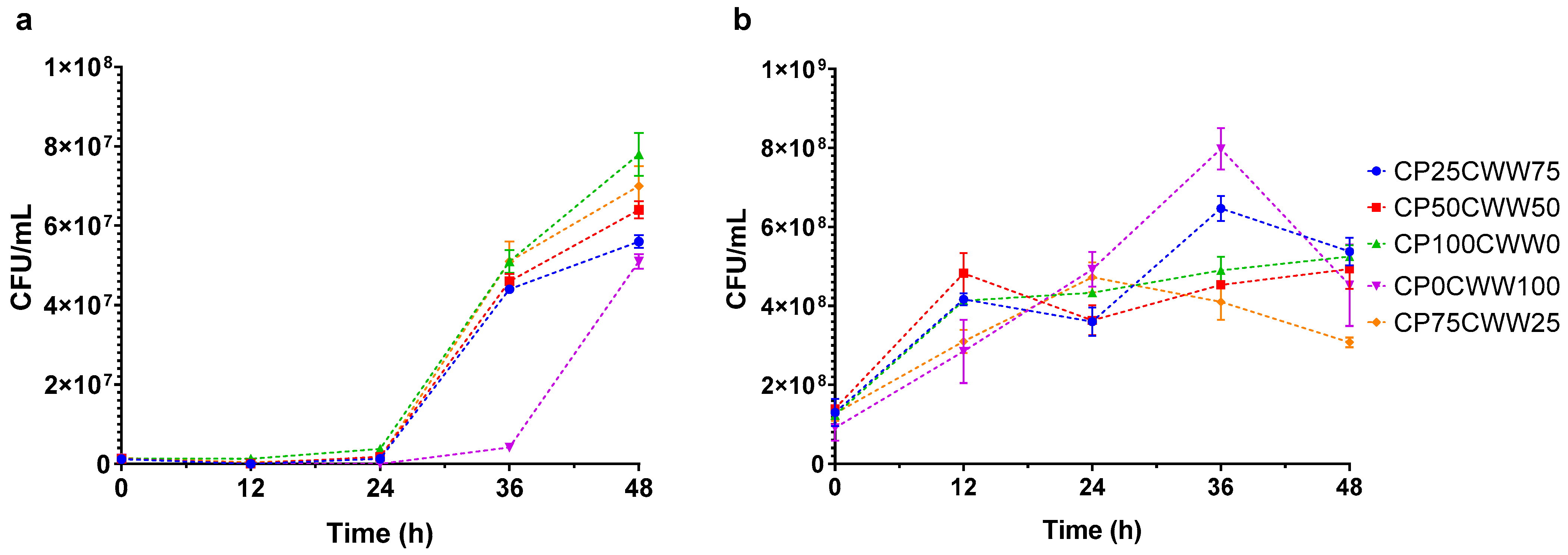
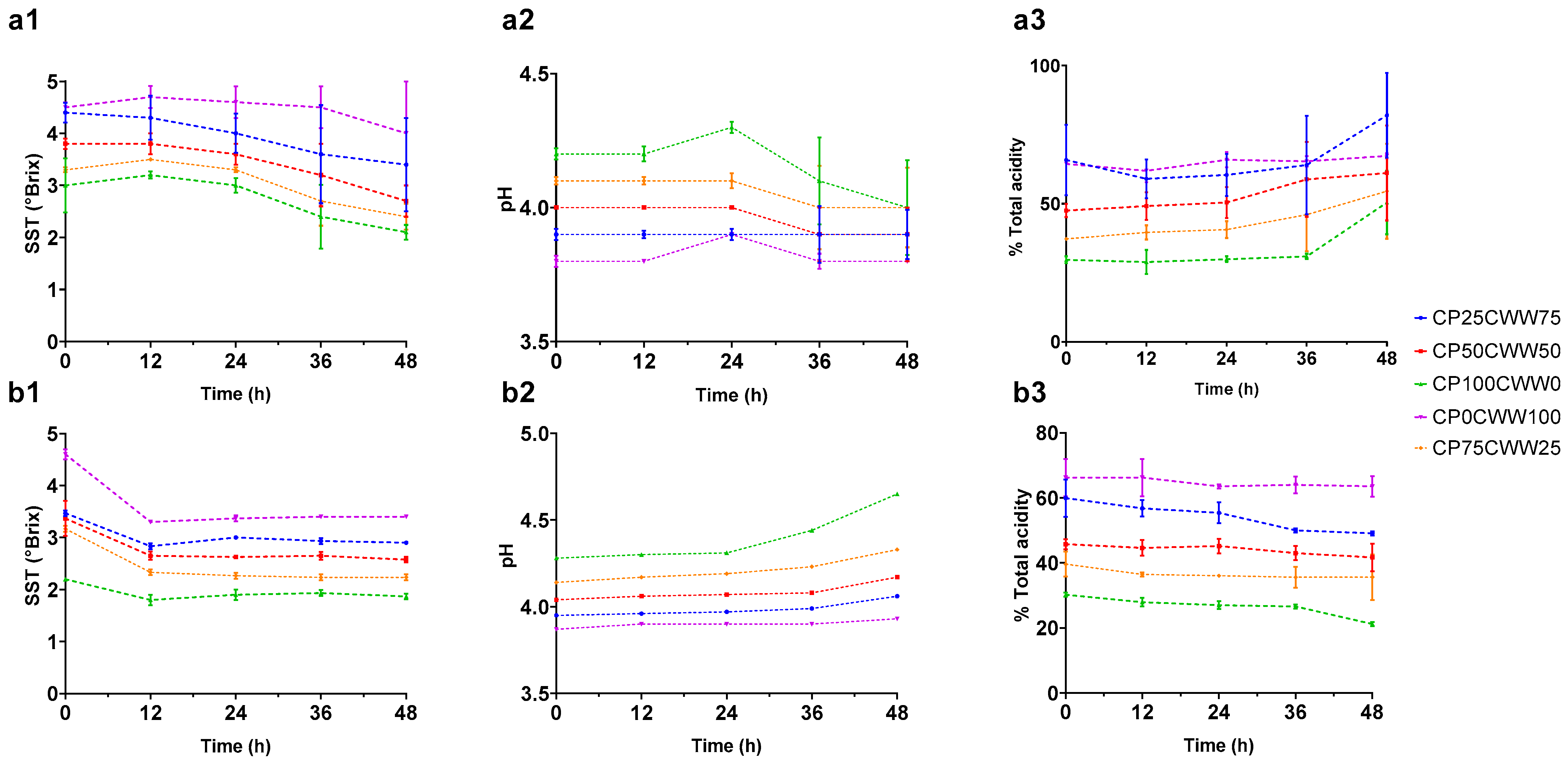
| Run | Components (%) | Process Variable Time (h) | Response Variable | ||
|---|---|---|---|---|---|
| CP | CWW | LAB | Yeast | ||
| 1 | 25 | 75 | 36 | 12 | Relative Microbial Growth (RG) |
| 2 | 25 | 75 | 48 | 36 | |
| 3 | 50 | 50 | 36 | 12 | |
| 4 | 50 | 50 | 48 | 36 | |
| 5 | 75 | 25 | 48 | 36 | |
| 6 | 50 | 50 | 36 | 12 | |
| 7 | 100 | 0 | 48 | 36 | |
| 8 | 50 | 50 | 48 | 36 | |
| 9 | 50 | 50 | 48 | 36 | |
| 10 | 50 | 50 | 36 | 12 | |
| 11 | 0 | 100 | 48 | 36 | |
| 12 | 0 | 100 | 36 | 12 | |
| 13 | 75 | 25 | 36 | 12 | |
| 14 | 100 | 0 | 36 | 12 | |
| 15 | 50 | 50 | 48 | 36 | |
| 16 | 50 | 50 | 36 | 12 | |
| 17 | 50 | 50 | 48 | 36 | |
| 18 | 50 | 50 | 36 | 12 | |
| Exp. Number (n) | Components | Response Variable Relative Growth (RG) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Process Variable | LAB | Process Variable | Yeast | |||||||
| X1 | X2 | X3 | X3 | |||||||
| CP (%) | CWW (%) | Time (h) | Observed | Linear Model | Quadratic Model | Time (h) | Observed | Linear Model | Quadratic Model | |
| 1 | 25 | 75 | 36 | 34.33 | 24.23 | 24.68 | 12 | 5.32 | 5.46 | 5.50 |
| 2 | 25 | 75 | 48 | 44.30 | 42.22 | 42.33 | 36 | 11.52 | 10.19 | 10.12 |
| 3 | 50 | 50 | 36 | 34.06 | 30.65 | 35.16 | 12 | 6.88 | 5.89 | 6.31 |
| 4 | 50 | 50 | 48 | 51.12 | 46.13 | 47.15 | 36 | 9.18 | 8.49 | 7.80 |
| 5 | 75 | 25 | 48 | 45.87 | 50.03 | 50.14 | 36 | 6.87 | 6.80 | 6.73 |
| 6 | 50 | 50 | 36 | 28.40 | 30.65 | 35.16 | 12 | 6.38 | 5.89 | 6.31 |
| 7 | 100 | 0 | 48 | 53.14 | 53.94 | 51.29 | 36 | 7.03 | 5.10 | 6.91 |
| 8 | 50 | 50 | 48 | 38.06 | 46.13 | 47.15 | 36 | 7.17 | 8.49 | 7.80 |
| 9 | 50 | 50 | 48 | 42.71 | 46.13 | 47.15 | 36 | 8.30 | 8.49 | 7.80 |
| 10 | 50 | 50 | 36 | 35.38 | 30.65 | 35.16 | 12 | 6.97 | 5.89 | 6.31 |
| 11 | 0 | 100 | 48 | 34.40 | 38.32 | 35.67 | 36 | 13.19 | 11.89 | 13.70 |
| 12 | 0 | 100 | 36 | 1.92 | 17.80 | 6.06 | 12 | 3.85 | 5.03 | 3.93 |
| 13 | 75 | 25 | 36 | 33.34 | 37.07 | 37.52 | 12 | 5.16 | 6.32 | 6.36 |
| 14 | 100 | 0 | 36 | 34.53 | 43.49 | 31.76 | 12 | 6.07 | 6.74 | 5.64 |
| 15 | 50 | 50 | 48 | 53.09 | 46.13 | 47.15 | 36 | 6.44 | 8.49 | 7.80 |
| 16 | 50 | 50 | 36 | 37.80 | 30.65 | 35.16 | 12 | 6.71 | 5.89 | 6.31 |
| 17 | 50 | 50 | 48 | 52.48 | 46.13 | 47.15 | 36 | 6.75 | 8.49 | 7.80 |
| 18 | 50 | 50 | 36 | 36.07 | 30.65 | 35.16 | 12 | 6.05 | 5.89 | 6.31 |
| R2 | 0.6736 | 0.8364 | 0.7321 | 0.8797 | ||||||
| Adj-R2 | 0.6036 | 0.7682 | 0.6747 | 0.8295 | ||||||
| RMSE | 6.6516 | 4.7093 | 1.1311 | 0.7598 | ||||||
| Parameter | Experimental Value | Predicted Value | RE (%) |
|---|---|---|---|
| RGlab | 45.98 ± 4.30 | 51.3 | 10.35 |
| RGyeast | 12.14 ± 1.16 | 13.70 | 11.33 |
| Fermentation | SS (°Brix) | pH | TA (%) | |||
|---|---|---|---|---|---|---|
| Initial | End | Initial | End | Initial | End | |
| 1 | 5.3 | 5.9 | 5.04 | 3.84 | 13.21 | 65.76 |
| 2 | 5.1 | 3.1 | 4.88 | 3.78 | 12.31 | 54.95 |
| 3 | 2.3 | 3.3 | 4.58 | 4.77 | 31.83 | 33.03 |
| 4 | 4.3 | 3.1 | 4.48 | 4.20 | 20.72 | 33.03 |
| 5 | 4.4 | 3.0 | 4.61 | 3.90 | 16.81 | 46.24 |
| 6 | 5.0 | 6.1 | 5.38 | 3.86 | 7.51 | 62.43 |
| Fermentation | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Characteristic | Score | |||||
| Fragrance/aroma | 7.75 | 8 | 8 | 7.75 | 8.25 | 8 |
| Flavor | 8 | 8 | 8 | 7.75 | 8 | 8 |
| Aftertaste | 7.75 | 7.75 | 7.75 | 7.75 | 7.75 | 7.75 |
| Acidity | 7.75 | 7.75 | 7.75 | 8 | 7.75 | 7.75 |
| Body | 7.75 | 7.75 | 7.75 | 7.5 | 7.75 | 7.75 |
| Uniformity | 10 | 10 | 10 | 10 | 10 | 10 |
| Sweetness | 10 | 10 | 10 | 10 | 10 | 10 |
| Clean cup | 10 | 10 | 10 | 10 | 10 | 10 |
| Balance | 7.75 | 7.75 | 7.75 | 7.5 | 7.75 | 7.75 |
| Overall | 7.75 | 8 | 8 | 7.75 | 8 | 8 |
| Final score | 84.5 | 85 | 85 | 84 | 85.25 | 85 |
| Sensory notes | Fragrance of tangerine with walnut, aroma of almond and grapefruit, flavor of tangerine, hazelnut, and molasses, lingering honey-like aftertaste of sugarcane and walnut, creamy body, citrus honey-like acidity. | Fragrance of vanilla and maple syrup, aroma of red apple with honey, flavor of vanilla, butter, and orange, molasses aftertaste with tangerine, silky body, tangerine citrus acidity. | Fragrance of honey with hazelnut, aroma of orange and maple syrup, creamy flavor of grapefruit, tangerine, and butter, honey aftertaste with vanilla, creamy body, citrus honey-like acidity. | Fragrance of molasses and hazelnut, aroma of maple syrup with molasses, flavor of coriander seed, celery, and molasses, herbal and molasses aftertaste, citrus honey-like acidity, light and silky body. | Fragrance of delicate mango with toasted hazelnut, aroma of orange with honey, delicate flavor of vanilla, molasses, and tropical fruits, caramel aftertaste with a subtle hint of honey, silky body, bright citrus acidity. | Fragrance of honey and molasses, aroma of maple syrup with anise, flavor of lime, tangerine, and maple syrup, honey-like aftertaste, syrupy body, juicy citrus acidity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duque-Buitrago, L.-F.; Calderón-Gaviria, K.-D.; Torres-Valenzuela, L.-S.; Sánchez-Tamayo, M.-I.; Plaza-Dorado, J.-L. Modulating Coffee Fermentation Quality Using Microbial Inoculums from Coffee By-Products for Sustainable Practices in Smallholder Coffee Production. Sustainability 2025, 17, 1781. https://doi.org/10.3390/su17051781
Duque-Buitrago L-F, Calderón-Gaviria K-D, Torres-Valenzuela L-S, Sánchez-Tamayo M-I, Plaza-Dorado J-L. Modulating Coffee Fermentation Quality Using Microbial Inoculums from Coffee By-Products for Sustainable Practices in Smallholder Coffee Production. Sustainability. 2025; 17(5):1781. https://doi.org/10.3390/su17051781
Chicago/Turabian StyleDuque-Buitrago, Luisa-Fernanda, Karen-Dayana Calderón-Gaviria, Laura-Sofia Torres-Valenzuela, Martha-Isabel Sánchez-Tamayo, and José-Luis Plaza-Dorado. 2025. "Modulating Coffee Fermentation Quality Using Microbial Inoculums from Coffee By-Products for Sustainable Practices in Smallholder Coffee Production" Sustainability 17, no. 5: 1781. https://doi.org/10.3390/su17051781
APA StyleDuque-Buitrago, L.-F., Calderón-Gaviria, K.-D., Torres-Valenzuela, L.-S., Sánchez-Tamayo, M.-I., & Plaza-Dorado, J.-L. (2025). Modulating Coffee Fermentation Quality Using Microbial Inoculums from Coffee By-Products for Sustainable Practices in Smallholder Coffee Production. Sustainability, 17(5), 1781. https://doi.org/10.3390/su17051781







