Aquatic Invasive Species in the Protected Areas of the Yucatan Peninsula and Adjacent Marine Zone, Mexico
Abstract
1. Introduction
2. Methods
2.1. Study Area
2.2. Data Collection
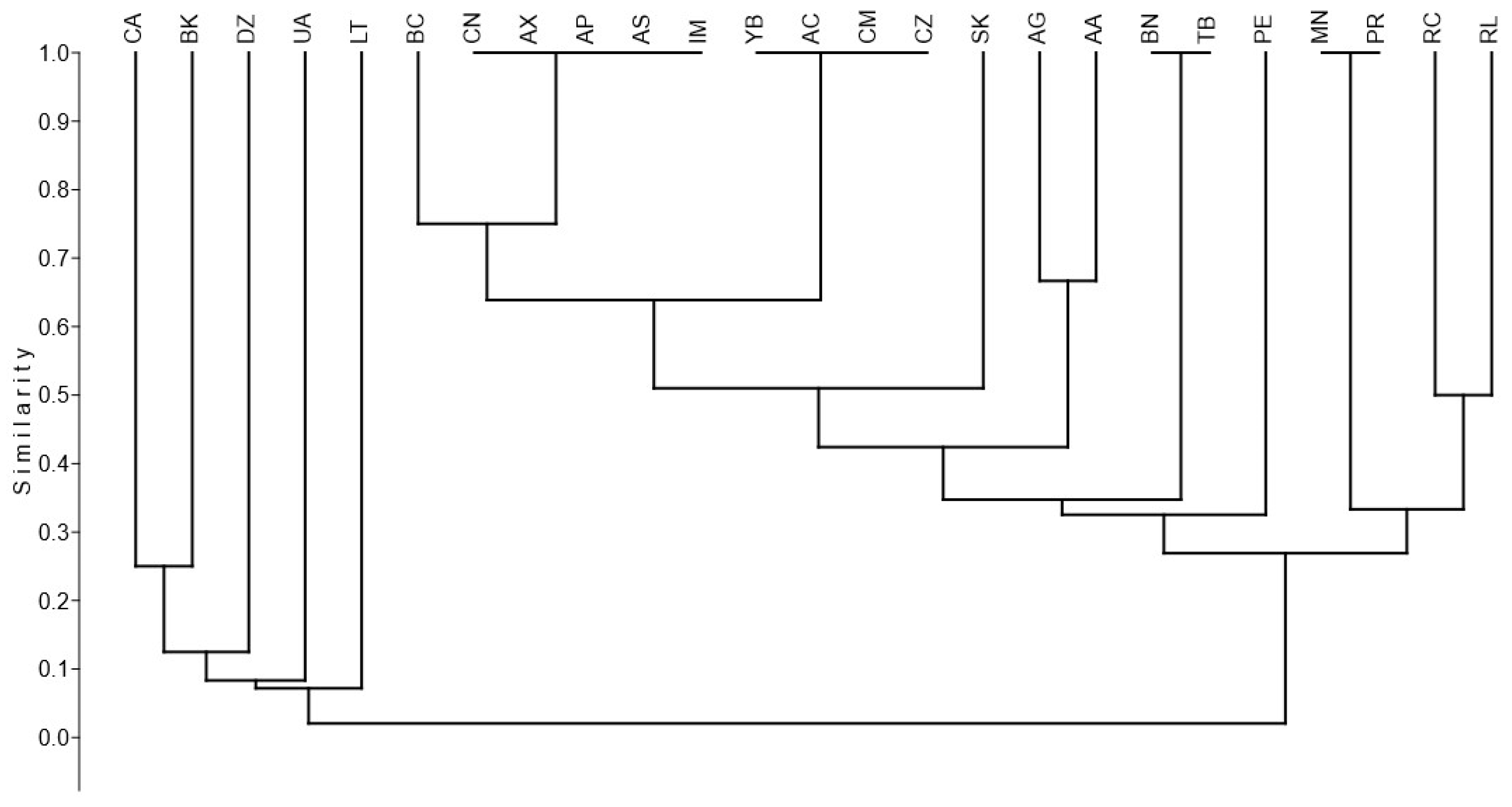
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackburn, T.M.; Bellard, C.; Ricciardi, A. Alien versus native species as drivers of recent extinctions. Front. Ecol. Environ. 2019, 17, 203–207. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- IPBES. Thematic Assessment Report on Invasive Alien Species and Their Control of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2023. [Google Scholar] [CrossRef]
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Change Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef]
- Meira, A.; Carvalho, F.; Castro, P.; Souza, R. Applications of biosensors in non-native freshwater species: A systematic review. NeoBiota 2024, 96, 211–236. [Google Scholar] [CrossRef]
- Cuthbert, R.N.; Pattison, Z.; Taylor, N.G.; Verbrugge, L.; Diagne, C.; Ahmed, D.A.; Leroy, B.; Angulo, E.; Briski, E.; Capinha, C.; et al. Global economic costs of aquatic invasive alien species. Sci. Total Environ. 2021, 775, 145238. [Google Scholar] [CrossRef]
- Rico-Sanchez, A.E.; Haubrock, P.J.; Cuthbert, R.N.; Angulo, E.; Ballesteros-Mejia, L.; Lopez-Lopez, E.; Duboscq-Carra, V.G.; Nunez, M.A.; Diagne, C.; Courchamp, F. Economic costs of invasive alien species in Mexico. In The Economic Costs of Biological Invasions Around the World; Zenni, R.D., McDermott, S., Garcia-Berthou, E., Essl, F., Eds.; Pensoft Publishers: Sofia, Bulgaria, 2021; Volume 67, pp. 459–483. [Google Scholar] [CrossRef]
- Perrin, S.W.; Lundmark, C.; Wenaas, C.P.; Finstad, A.G. Contrasts in perception of the interaction between non-native species and climate change. NeoBiota 2024, 96, 343–361. [Google Scholar] [CrossRef]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; van Kleunen, M.; Kühn, I.; et al. Projecting the continental accumulation of alien species through to 2050. Glob. Change Biol. 2021, 27, 970–982. [Google Scholar] [CrossRef]
- Vitousek, P.M.; D’Antonio, C.M.; Loope, L.L.; Rejmánek, M.; Westbrooks, R. Introduced species: A significant component of human-caused global change. N. Z. J. Ecol. 1997, 21, 1–16. Available online: http://www.jstor.org/stable/24054520 (accessed on 5 January 2025).
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic invasive species: Challenges for the future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef]
- Saunders, D.L.; Meeuwig, J.J.; Vincent, A.C. Freshwater protected areas: Strategies for conservation. Conserv. Biol. 2002, 16, 30–41. [Google Scholar] [CrossRef]
- Kiruba-Sankar, R.; Praveen, J.; Saravanan, K.; Lohith, K.; Raymond, J.; Velmurugan, A.; Dam, S. Invasive species in freshwater ecosystems—Threats to ecosystem services. In Biodiversity and Climate Change Adaptation in Tropical Islands; Sivaperuman, C., Velmurugan, A., Kumar, A., Jaisankar, I., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 257–296. [Google Scholar] [CrossRef]
- Baxter, C.V.; Fausch, K.D.; Murakami, M.; Chapman, P.L. Fish invasion restructures stream and forest food webs by interrupting reciprocal prey subsidies. Ecology 2004, 85, 2656–2663. [Google Scholar] [CrossRef]
- Dimitriadis, C.; Fournari-Konstantinidou, I.; Sourbès, L.; Koutsoubas, D.; Katsanevakis, S. Long term interactions of native and invasive species in a marine protected area suggest complex cascading effects challenging conservation outcomes. Diversity 2021, 13, 71. [Google Scholar] [CrossRef]
- Bax, N.; Williamson, A.; Agüero, M.; Gonzalez, E.; Geeves, W. Marine invasive alien species: A threat to global biodiversity. Mar. Policy 2003, 27, 313–323. [Google Scholar] [CrossRef]
- Foxcroft, L.C.; Richardson, D.M.; Pyšek, P.; Genovesi, P. Invasive alien plants in protected areas: Threats, opportunities, and the way forward. In Plant Invasions in Protected Areas. Invading Nature—Springer Series in Invasion Ecology; Foxcroft, L.C., Richardson, D.M., Pyšek, P., Genovesi, P., Eds.; Springer: Dordrecht, The Netherlands, 2013; Volume 7, pp. 621–639. [Google Scholar] [CrossRef]
- Braun, M.; Schindler, S.; Essl, F. Distribution and management of invasive alien plant species in protected areas in Central Europe. J. Nat. Conserv. 2016, 33, 48–57. [Google Scholar] [CrossRef]
- Holenstein, K.; Simonson, W.D.; Smith, K.G.; Blackburn, T.M.; Charpentier, A. Non-native species surrounding protected areas influence the community of non-native species within them. Front. Ecol. Evol. 2021, 8, 625137. [Google Scholar] [CrossRef]
- CONABIO. Provincias Biogeográficas de México, Escala 1: 4,000,000. Available online: http://geoportal.conabio.gob.mx/descargas/mapas/imagen/96/rbiog4mgw (accessed on 7 January 2025).
- Arriaga, L.; Aguilar, V.; Alcocer, J. Aguas Continentales y Diversidad Biológica de México; Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2002; Available online: https://www.biodiversidad.gob.mx/pais/regiones-hidrologicas-prioritarias-de-Mexico (accessed on 7 January 2025).
- Arriaga, L.; Vázquez, E.; González, J.; Jiménez, R.; Muñoz, E.; Aguilar, V. Regiones Marinas Prioritarias de México; Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 1998; Available online: https://www.biodiversidad.gob.mx/pais/regiones-marinas-prioritarias-de-mexico (accessed on 8 January 2025).
- Arriaga, L.; Espinoza-Rodríguez, J.M.; Aguilar-Zúñiga, C.; Martínez-Romero, E.; Gómez-Mendoza, L.; Loa, E. Regiones Terrestres Prioritarias de México; Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2000; Available online: https://bioteca.biodiversidad.gob.mx/janium-bin/detalle.pl?Id=20250103185318 (accessed on 8 January 2025).
- CONANP. Listado de las Áreas Naturales Protegidas de México. Available online: https://sig.conanp.gob.mx/General (accessed on 7 January 2025).
- CONABIO. Sistema Nacional de Información Sobre Biodiversidad. Available online: https://www.snib.mx/ (accessed on 6 January 2025).
- GBIF Global Biodiversity Information Facility (2025) GBIF México. Available online: https://www.gbif.org/ (accessed on 8 January 2025).
- CONANP. Programa de Manejo Reserva de la Biosfera Ría Celestún; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2000; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/celestun.pdf (accessed on 10 January 2025).
- CONANP. Programa de Manejo Parque Nacional Arrecifes de Xcalak; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2004; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/Xcalak_ok.pdf (accessed on 11 January 2025).
- CONANP. Programa de Conservación y Manejo Parque Nacional Arrecife Alacranes; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2006; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/alacranes_ok.pdf (accessed on 11 January 2025).
- CONANP. Programa de Conservación y Manejo Reserva de la Biosfera Los Petenes; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2006; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/petenes_final.pdf (accessed on 13 January 2025).
- CONANP. Programa de Conservación y Manejo Área de Protección de Flora y Fauna Bala’an K’aax; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2007; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/Final_BallanKaxx.pdf (accessed on 17 January 2025).
- CONANP. Programa de Conservación y Manejo Reserva de la Biosfera Ría Lagartos; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2007; Available online: https://www.conanp.gob.mx/anp/consulta/PCM_RiaLagartos.pdf (accessed on 14 January 2025).
- CONANP. Programa de Manejo Área de Protección de Flora y Fauna Manglares de Nichupté; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2014; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/2015/Manglares_de_Nichupt%C3%A9.pdf (accessed on 15 January 2025).
- CONANP. Programa de Manejo Complejo Sian Ka’an: Reserva de la Biosfera Sian Ka’an, Área de Protección de Flora y Fauna Uaymil y Reserva de la Biosfera Arrecifes de Sian Ka’an; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2014; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/2015/Complejo_Sian_Ka_an.pdf (accessed on 20 January 2025).
- CONANP. Programa de Manejo Parque Nacional Isla Contoy; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2015; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/2015/Isla_Contoy_completo.pdf (accessed on 25 January 2025).
- CONANP. Programa de Manejo Reserva de la Biosfera Tiburón Ballena; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2015; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/2015/Tiburon_Ballena_Libro.pdf (accessed on 21 January 2025).
- CONANP. Programa de Manejo Área de Protección de Flora y Fauna La Porción Norte y la Franja Costera Oriental, Terrestres y Marinas de la Isla de Cozumel; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2016; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/2017/Isla%20Cozumel%20(completo).pdf (accessed on 28 January 2025).
- CONANP. Programa de Manejo Parque Nacional Costa Occidental de Isla Mujeres, Punta Cancún y Punta Nizuc; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2016; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/2017/Isla%20Mujeres%20Punta%20Canc%C3%BAn.pdf (accessed on 26 January 2025).
- CONANP. Programa de Manejo Parque Nacional Dzibilchantún; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2016; Available online: https://www.conanp.gob.mx/acciones/pdf/PMDZIBILCHANTUNCompleto.pdf (accessed on 29 January 2025).
- CONANP. Programa de Manejo Área de Protección de Flora y Fauna Yum Balam; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2019; Available online: https://www.conanp.gob.mx/programademanejo/PMYumBalam.pdf (accessed on 24 January 2025).
- CONANP. Programa de Manejo Reserva de la Biosfera Caribe Mexicano; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2019; Available online: https://www.conanp.gob.mx/programademanejo/PMCaribeMexicano.pdf (accessed on 27 January 2025).
- INE. Programa de Manejo del Área de Protección de Flora y Fauna Laguna de Términos; Instituto Nacional de Ecología: Mexico City, Mexico, 1997; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/APFFTerminos.pdf (accessed on 17 January 2025).
- INE. Programa de Manejo Parque Marino Nacional Arrecifes de Cozumel; Instituto Nacional de Ecología: Mexico City, Mexico, 1998; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/cozumel.pdf (accessed on 19 January 2025).
- INE. Programa de Manejo Parque Nacional Arrecife de Puerto Morelos; Instituto Nacional de Ecología: Mexico City, Mexico, 2000; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/puerto_morelos.pdf (accessed on 18 January 2025).
- INE. Programa de Manejo Reserva de la Biosfera Banco Chinchorro; Instituto Nacional de Ecología: Mexico City, Mexico, 2000; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/chinchorro.pdf (accessed on 24 January 2025).
- INE. Programa de Manejo Reserva de la Biosfera Calakmul; Instituto Nacional de Ecología: Mexico City, Mexico, 2000; Available online: https://www.conanp.gob.mx/que_hacemos/pdf/programas_manejo/calakmul.pdf (accessed on 20 January 2025).
- CONANP. Estudio Previo Justificativo Para el Establecimiento del Área Natural Protegida Parque Nacional Bajos del Norte, Golfo de México; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2023; Available online: https://www.conanp.gob.mx/pdf/separata/EPJ-PN-BajosDelNorte.pdf (accessed on 30 January 2025).
- CONANP. Estudio Previo Justificativo Para el Establecimiento del Área Natural Protegida Arrecifes del Golfo de México-Sur; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2024; Available online: https://www.conanp.gob.mx/pdf/separata/EPJ-PN-ArrecifesDelGolfoDeMexico-Sur.pdf (accessed on 30 January 2025).
- CONABIO. Método de Evaluación Rápida de Invasividad (MERI) Para Especies exóticas en México; Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2015; Available online: https://www.biodiversidad.gob.mx/media/1/especies/Invasoras/files/Instrutivo_MERI_2020.pdf (accessed on 8 January 2025).
- PAST Core Team. PAST: A Software for Scientific Data Analysis. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 10 January 2025).
- Collado-Vides, L.; González-González, J.; Ezcurra, E. Patrones de distribución ficofloristica en el sistema lagunar de Nichupté, Quintana Roo, México. Acta Botánica Mex. 1995, 31, 19–32. [Google Scholar] [CrossRef]
- Espinoza-Avalos, J.; Aguilar-Rosas, L.E.; Aguilar-Rosas, R.; Gómez-Poot, J.M.; Raigoza-Figueras, R. Presencia de caulerpaceae (Chlorophyta) en la Península de Yucatán, México. Bot. Sci. 2015, 93, 845–854. [Google Scholar] [CrossRef]
- Rendón-Hernández, E.; Ayala-Pérez, L.A.; Golubov, J.; Torres-Lara, R. Especies exóticas invasoras y sus implicaciones sobre los bosques de manglar en las Reservas de la Biosfera Ría Celestún y Ría Lagartos. Madera Bosques 2024, 30, 3042627. [Google Scholar] [CrossRef]
- Endañú-Huerta, E.; López-Contreras, J.E.; Amador-Del Ángel, L.E.; Guevara-Carrió, E.; Alderete-Chávez, A.; Brito-Pérez, R. Flora exótica naturalizada e invasora del Área de Protección de Flora y Fauna Laguna de Términos, Campeche: Estado actual, impactos, necesidades y perspectivas. In Problemas Contemporáneos Regionales del Sureste Mexicano El Caso del Estado de Campeche; Amador-Del Ángel, L.E., Frutos-Cortés, M., Eds.; Universidad Autónoma del Carmen: Ciudad del Carmen, Mexico, 2015; pp. 280–307. [Google Scholar]
- Amador-Del Ángel, L.E.; Endañú-Huerta, E.; López-Contreras, J.E.; Wakida-Kusunoki, A.T.; Guevara-Carrió, E.; Brito-Pérez, R. Fauna exótica establecida e invasora en el Área de Protección de Flora y Fauna Laguna de Términos, Campeche: Estado actual, impactos, necesidades y perspectivas. In Problemas Contemporáneos Regionales del Sureste Mexicano El Caso del Estado de Campeche; Amador-Del Ángel, L.E., Frutos-Cortés, M., Eds.; Universidad Autónoma del Carmen: Ciudad del Carmen, Mexico, 2015; pp. 128–154. [Google Scholar]
- Trinidad-Ocaña, C.; Juárez-Flores, J.; Sánchez, A.J.; Barba-Macías, E. Diversidad de moluscos y crustáceos acuáticos en tres zonas en la cuenca del Río Usumacinta, México. Rev. Mex. Biodivers. 2018, 89, S65–S78. [Google Scholar] [CrossRef]
- Gómez-Ponce, M.A.; Bolaños-Martínez, N.; Díaz-Jaimes, P.; Bortolini-Rosales, J.L.; Castellanos, P. A new record of a tiger shrimp Penaeus monodon Fabricius, 1798 breeding female in the coast of Campeche, Mexico. Lat. Am. J. Aquat. Res. 2020, 48, 150–155. [Google Scholar] [CrossRef]
- Wakida-Kusunoki, A.T.; Rojas-González, R.I.; González-Cruz, A.; Del Ángel, A.; Sánchez-Cruz, J.L.; López, N.A. Presence of giant tiger shrimp Penaeus monodon Fabricius, 1798 on the Mexican coast of the Gulf of Mexico. BioInvasions Rec. 2013, 2, 325–328. [Google Scholar] [CrossRef]
- Wakida-Kusunoki, A.T.; De Anda-Fuentes, D.; López-Téllez, N.A. Presence of giant tiger shrimp Penaeus monodon (Fabricius, 1798) in eastern Peninsula of Yucatan coast, Mexico. Lat. Am. J. Aquat. Res. 2016, 44, 155–158. [Google Scholar] [CrossRef]
- Wakida-Kusunoki, A.T.; Cruz-Sánchez, J.L.; López-Téllez, N.A. A review of recent sightings and reports of the giant tiger shrimp Penaeus monodon (Decapoda: Penaeidae) on the Mexican coast of the Gulf of Mexico (2012–2019). Rev. De Biol. Mar. Oceanografía 2021, 56, 83–88. [Google Scholar] [CrossRef]
- Simoes, N.; Wakida-Kusunoki, A.T.; Cruz-Sánchez, J.L.; Alvarez, F.; Villalobos-Hiriart, J.L. On the presence of Charybdis (Charybdis) hellerii (A. Milne-Edwards, 1867) on the Mexican coast of the Gulf of Mexico. BioInvasions Rec. 2019, 8, 670–674. [Google Scholar] [CrossRef]
- Torres-Castro, I.L.; Vega-Cendejas, M.A.; Schmitter-Soto, J.J.; Palacio-Aponte, G.; Rodiles-Hernández, R. Ictiofauna de Sistemas Cárstico-Palustres Con Impacto Antrópico: Los Petenes de Campeche, México. Rev. Biol. Tropical 2009, 57, 141–157. Available online: https://www.scielo.sa.cr/scielo.php?pid=S0034-77442009000100014&script=sci_arttext (accessed on 5 January 2025). [CrossRef]
- Vega-Cendejas, M.A. Estudio de Caso: Los Peces de la Reserva de Calakmul. In La Biodiversidad en Campeche: Estudio de Estado; Villalobos-Zapata, G.J., Mendoza, J., Eds.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico; Gobierno del Estado de Campeche: Campeche, Mexico; Universidad Autónoma de Campeche: Campeche, Mexico; El Colegio de la Frontera Sur: Campeche, Mexico, 2010; Available online: https://bioteca.biodiversidad.gob.mx/janium/Documentos/7371.pdf (accessed on 3 January 2025).
- Amador-Del Ángel, L.E.; Wakida-Kusunoki, A.T.; Guevara-Carrió, E.C.; Brito-Pérez, R.; Cabrera-Rodríguez, P. Peces Invasores de Agua Dulce en la Región de la Laguna de Términos, Campeche. UNACAR Tecnociencia 2009, 3, 11–28. Available online: https://biblat.unam.mx/hevila/UNACARtecnociencia/2009/no2/2.pdf (accessed on 4 January 2025).
- Ayala-Pérez, L.A.; Ramos-Miranda, J.; Flores-Hernández, D. La Comunidad de Peces de la Laguna de Términos: Estructura Actual Comparada. Biol. Trop. 2003, 50, 783–793. Available online: https://www.redalyc.org/articulo.oa?id=44911882020 (accessed on 18 January 2025).
- Amador-Del Ángel, L.E.; Guevara, E.; Brito, R.; Wakida-Kusunoki, A.T.; Cabrera-Rodríguez, P. Aportaciones recientes al estudio de la ictiofauna del Área de Protección de Flora y Fauna Laguna de Términos, Campeche. In Aspectos Hidrológicos y Ambientales en la Laguna de Términos; Ruíz, A., Canedo, Y., Zavala, J.C., Campos, S.C., Sabido, M.Y., Ayala, L.A., Amador-Del Ángel, L.E., Eds.; Universidad Autónoma del Carmen: Ciudad del Carmen, Mexico, 2012; pp. 128–154. [Google Scholar]
- Álvarez-Pliego, N.; Sánchez, A.J.; Florido, R.; Salcedo, M.A. First record of South American suckermouth armored catfishes (Loricariidae, Pterygoplichthys spp.) in the Chumpan River system, southeast Mexico. BioInvasions Records 2015, 4, 309–314. [Google Scholar] [CrossRef]
- Ayala-Pérez, L.A.; Pineda-Peralta, A.D.; Álvarez-Guillen, H.; Amador-Del Ángel, L.E. El pez diablo (Pterygoplichthys spp.) en las cabeceras estuarinas de la Laguna de Términos, Campeche. In Especies Invasoras Acuáticas: Casos de Estudio en Ecosistemas de México; Low, A., Quijón, P., Peters, E., Eds.; Secretaría de Medio Ambiente y Recursos Naturales: Mexico City, Mexico; Instituto Nacional de Ecología y Cambio Climático: Mexico City, Mexico; University of Prince Edward Island: Charlottetown, PE, Canada, 2014; pp. 313–336. Available online: https://agua.org.mx/biblioteca/especies-invasoras-acuaticas-casos-de-estudio-en-ecosistemas-de-mexico-2/ (accessed on 16 January 2025).
- Rendón, E.; Bernal, J.F.; Hernández, B.; Müller, E. Estrategias de atención a especies exóticas invasoras en áreas naturales protegidas de competencia federal en México. In Principales Retos que Enfrenta México Ante las Especies Exóticas Invasoras; Born-Schmidt, G., de Alba, F., Parpal, J., Koleff, P., Eds.; Centro de Estudios Sociales y de Opinión Pública: Mexico City, Mexico; Cámara de Diputados: Mexico City, Mexico, 2017; pp. 209–224. [Google Scholar]
- Wakida-Kusunoki, A.T.; Amador-Del Ángel, L.E. Nuevos Registros de los Plecos Pterygoplichthys pardalis (Castelnau 1855) y P. disjunctivus (Weber 1991) (Siluriformes: Loricariidae) en el Sureste de México. Hidrobiológica 2008, 18, 251–256. Available online: https://hidrobiologica.izt.uam.mx/index.php/revHidro/article/view/916 (accessed on 19 January 2025).
- Toro-Ramírez, A.; Wakida-Kusunoki, A.T.; Amador-Del Ángel, L.E.; Cruz-Sánchez, J.L. Common snook Centropomus undecimalis (Bloch, 1792) preys on the invasive Amazon sailfin catfish Pterygoplichthys pardalis (Castelnau, 1855) in the Palizada River, Campeche, southeastern Mexico. J. Appl. Ichthyol. 2014, 30, 532–534. [Google Scholar] [CrossRef]
- Guzmán-Méndez, I.A.; Rivera-Madrid, R.; Díaz-Jaimes, P.; García-Rivas, M.C.; Aguilar-Espinosa, M.; Arias-González, J.E. First genetically confirmed record of the invasive devil firefish Pterois miles (Bennett, 1828) in the Mexican Caribbean. BioInvasions Rec. 2017, 6, 99–103. [Google Scholar] [CrossRef]
- Aguilar-Perera, A.; Tuz-Sulub, A. Non-native, invasive red lionfish (Pterois volitans Linnaeus, 1758: Scorpaenidae), is first recorded in the southern Gulf of Mexico, off the northern Yucatan Peninsula, Mexico. Aquat. Invasions 2010, 5, S9–S12. [Google Scholar] [CrossRef]
- Aguilar-Perera, A.; Quijano-Puerto, L.; Hernández-Landa, R.C. Lionfish invaded the mesophotic coral ecosystem of the Parque Nacional Arrecife Alacranes, Southern Gulf of Mexico. Mar. Biodivers. 2017, 47, 15–16. [Google Scholar] [CrossRef]
- Arias-González, J.E.; González-Gándara, C.; Cabrera, J.L.; Christensen, V. Predicted impact of the invasive lionfish Pterois volitans on the food web of a Caribbean coral reef. Environ. Res. 2011, 111, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Arredondo-Chávez, A.T.; Sánchez-Jiménez, J.A.; Ávila-Morales, O.G.; Torres-Chávez, P.; Herrerias-Diego, Y.; Medina-Nava, M.; Madrigal-Gurdi, X.; Campos-Mendoza, A.; Domínguez-Domínguez, O.; Caballero-Vázquez, J.A. Spatio-temporal variation in the diet composition of red lionfish, Pterois volitans (Actinopterygii: Scorpaeniformes: Scorpaenidae), in the Mexican Caribbean: Insights into the ecological effect of the alien invasion. Acta Ichthyol. Piscat. 2016, 46, 185–200. [Google Scholar] [CrossRef]
- Cobián-Rojas, D.; Schmitter-Soto, J.J.; Aguilar, C.M.; Aguilar-Perera, A.; Ruiz-Zárate, M.A.; González-Sansón, G.; Chevalier, P.P.; Herrera, R.; García, A.; Corrada, R.I.; et al. The community diversity of two Caribbean MPAs invaded by lionfish does not support the biotic resistance hypothesis. J. Sea Res. 2018, 134, 26–33. [Google Scholar] [CrossRef]
- Loreto-Viruel, R.M.; Rendón-Hernández, E.; Espindola, S. Plan de Acción Nacional Para el Manejo y Control del pez León en México; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico; Amigos de Sian Ka’an, A.C.: Cancún, Mexico; Mesoamerican Reef Fund: Mexico City, Mexico, 2023; Available online: https://marfund.org/es/wp-content/uploads/2023/03/Mexico-Plan-Accion_Pez-leon_feb23_opt.pdf (accessed on 1 February 2025).
- Côté, I.M.; Green, S.J.; Hixon, M.A. Predatory fish invaders: Insights from Indo-Pacific lionfish in the western Atlantic and Caribbean. Biol. Conserv. 2013, 164, 50–61. [Google Scholar] [CrossRef]


| Taxonomic Group | Family | Taxon | Protected Areas | Reference Number |
|---|---|---|---|---|
| Macroalgae | Caulerpaceae | Caulerpa taxifolia ▲ (M.Vahl) C.Agardh 1817 | AA, AG | [25,29,48] |
| Caulerpa verticillata ■ J. Agardh, 1847 | AA, AP, AC, AS, AX, AG, BC, CM, IM, CN, CZ, PE, MN, PR, RC, RL, SK, YB | [25,28,29,34,35,37,38,40,41,44,45,48,51,52,53] | ||
| Ulvaceae | Ulva Lactuca ● (Linnaeus, 1753) | AA, AP, AS, AX, BC, IM, CN, RL, SK | [25,26,28,29,32,34,35,44,45,53] | |
| Plants | Hydrocharitaceae | Egeria densa ▲ Planch. | CA, DZ | [25,39,46] |
| Pontederiaceae | Eichhornia crassipes ▲ (Mart.) Solms | CA, LT, PE, SK, UA | [25,34,46,54] | |
| Hydrozoans | Cordylophoridae | Cordylophora caspia ▲ (Pallas, 1771) | AG | [25,26,48] |
| Plumulariidae | Plumularia setacea ■ (Linnaeus, 1758) | AA, AG | [25,26,48] | |
| Mollusks | Thiaridae | Melanoides tuberculata ▲ (O. F. Müller, 1774) | LT, SK | [25,34,55] |
| Tarebia granifera ▲ (Lamarck, 1816) | LT | [25,55,56] | ||
| Crustaceans | Penaeidae | Penaeus monodon ▲ (Fabricius, 1798) | LT, RC, RL | [25,26,53,55,57,58,59,60] |
| Portunidae | Charybdis (Charybdis) helleri ■ (A. Milne-Edwards, 1867) | LT | [25,61] | |
| Fish | Cichlidae | Oreochromis aureus ▲ (Steindachner, 1864) | LT, PE | [25,26,62] |
| Oreochromis mossambicus ▲ (Peters, 1852) | CA | [25,63] | ||
| Oreochromis niloticus ▲ (Linnaeus, 1758) | BK, CA, LT, PE, RC | [25,31,53,62,63,64,65] | ||
| Parachromis managuensis ■ (Günther, 1867) | LT | [25,55,64,66] | ||
| Parachromis motaguensis ■ (Günther, 1867) | LT | [25,55] | ||
| Cyprinidae | Ctenopharyngodon idella ▲ (Valenciennes, 1844) | LT | [25,55,64,66] | |
| Cyprinus carpio ▲ (Linnaeus, 1758) | LT | [25,55] | ||
| Loricariidae | Pterygoplichthys disjunctivus ▲ (Weber, 1991) | LT | [25,55,64,66,67,68,69,70] | |
| Pterygoplichthys pardalis ■ (Castelnau, 1855) | LT | [25,55,64,66,67,68,69,70,71] | ||
| Scorpaenidae | Pterois miles ▲ (Bennett, 1828) | BC | [72] | |
| Pterois volitans ▲ (Linnaeus, 1758) | AA, AP, AC, AS, AX, AG, BN, BC, CM, IM, CN, CZ, PE, SK, TB, YB | [25,35,36,37,38,40,41,47,48,69,73,74,75,76,77,78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rendón-Hernández, E.; Ayala-Pérez, L.A.; Golubov, J.; Torres-Lara, R.; Vega-Rodríguez, B.I. Aquatic Invasive Species in the Protected Areas of the Yucatan Peninsula and Adjacent Marine Zone, Mexico. Sustainability 2025, 17, 5017. https://doi.org/10.3390/su17115017
Rendón-Hernández E, Ayala-Pérez LA, Golubov J, Torres-Lara R, Vega-Rodríguez BI. Aquatic Invasive Species in the Protected Areas of the Yucatan Peninsula and Adjacent Marine Zone, Mexico. Sustainability. 2025; 17(11):5017. https://doi.org/10.3390/su17115017
Chicago/Turabian StyleRendón-Hernández, Eduardo, Luis Amado Ayala-Pérez, Jordan Golubov, Ricardo Torres-Lara, and Brenda Iliana Vega-Rodríguez. 2025. "Aquatic Invasive Species in the Protected Areas of the Yucatan Peninsula and Adjacent Marine Zone, Mexico" Sustainability 17, no. 11: 5017. https://doi.org/10.3390/su17115017
APA StyleRendón-Hernández, E., Ayala-Pérez, L. A., Golubov, J., Torres-Lara, R., & Vega-Rodríguez, B. I. (2025). Aquatic Invasive Species in the Protected Areas of the Yucatan Peninsula and Adjacent Marine Zone, Mexico. Sustainability, 17(11), 5017. https://doi.org/10.3390/su17115017







