Sustainable Biomass Valorization by Solid-State Fermentation with the Mutant Strain Trichoderma viride M5-2 of Forage Legumes to Improve Their Nutritional Composition as Animal Feed
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Species
2.2. Whole Forage Flour Meals of Legumes Substrate Preparation
2.3. Observation of the Cultural Characteristics of the Fungus T. viride M5-2 in Legume Flours
2.4. Solid-State Fermentation Process
2.5. Cellulolytic Capacity of T. viride M5-2 in Solid-State Fermentation Process
2.6. Enzyme Activities
2.7. CMCase Enzyme Activity
2.8. PFase Enzyme Activity
2.9. Chemical Analysis of Solid-State Fermentation Process
2.10. Physical Analysis of Solid-State Fermentation Process
2.11. Determination of Packing Volume
2.12. Determination of Solubility
2.13. The Water Adsorption Capacity (WAC)
2.14. Determination of T. viride M5-2 Structural Changes of the Whole Forage Flour Meals from the Legumes L. purpureus and M. pruriens, by Fourier Transform Infrared Spectroscopy (ATR-FT-IR) Analysis
2.15. Statistical Analysis
3. Results
3.1. Growth of the Lignocellulolytic Fungus T. viride M5-2 on Whole Forage Flour Meals of L. purpureus and M. pruriens
3.2. Cellulolytic Capacity of T. viride M5-2 in the Degradation of Legumes in the Solid-State Fermentation Process (SSF)
3.3. Chemical Analysis of Solid-State Fermentation Process by T. viride M5-2
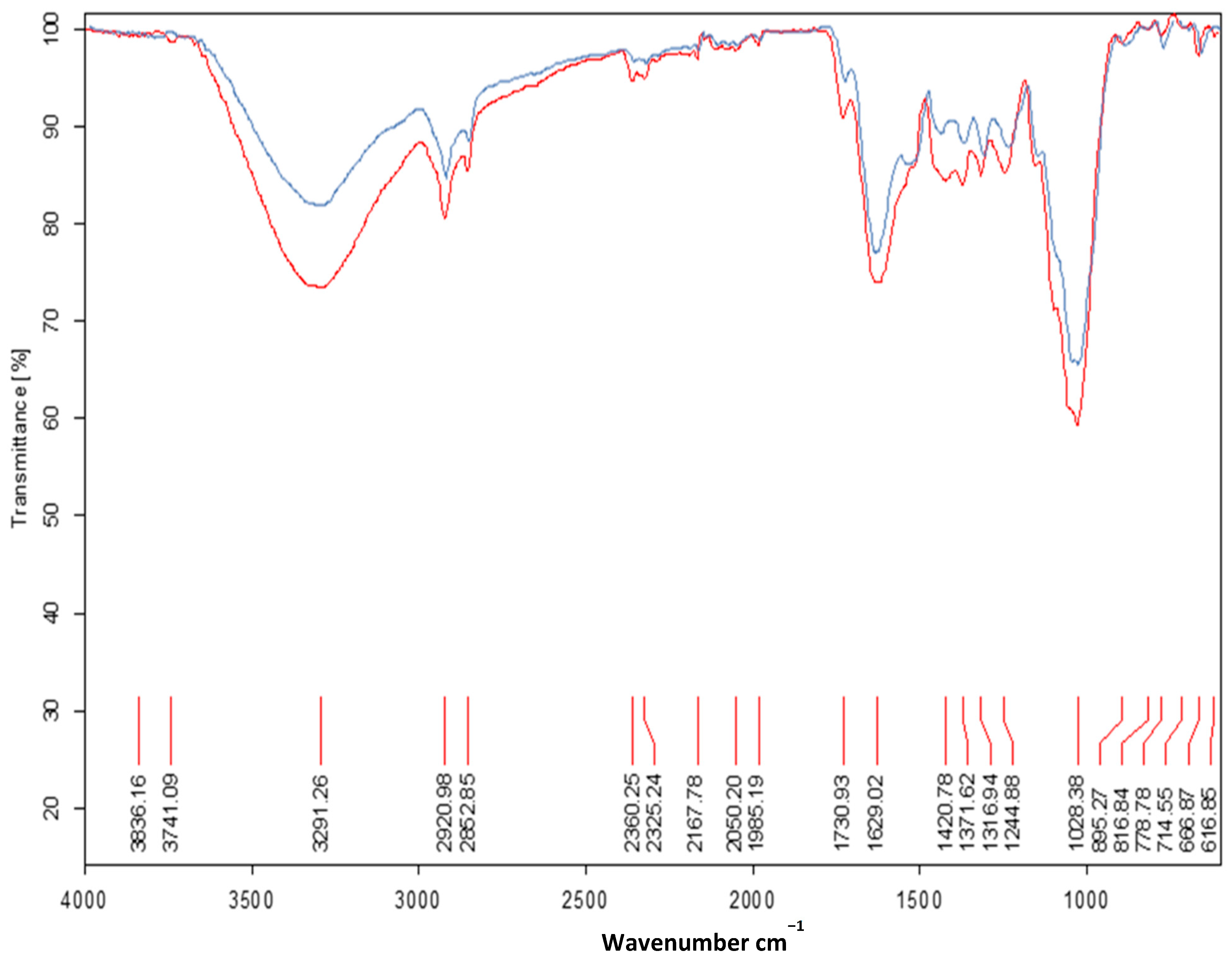
3.4. Determination of T. viride M5-2 Molecular Changes in Whole Forage Flour Meals from the Legumes L. purpureus and M. pruriens, by Fourier Transform Infrared Spectroscopy (ATR-FT-IR) Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikusika, O.O.; Akinmoladun, O.F.; Mpendulo, C.T. Enhancement of the Nutritional Composition and Antioxidant Activities of FruitPomaces and Agro-Industrial Byproducts through Solid-State Fermentation for Livestock Nutrition: A Review. Fermentation 2024, 10, 227. [Google Scholar] [CrossRef]
- Savón, L.; Scull, I.; Dihigo, L.E.; Martínez, M.; Albert, A.; Leiva, L. Tropical forage meals: An alternative for sustainable monogastric species production. Multifunct. Grassl. A Chang. World 2008, 2, 487–497. [Google Scholar]
- Ezegbe, C.C.; Nwosu, J.N.; Owuamanam, C.I.; Victor-Aduloju, T.A.; Nkhata, S.G. Proximate composition and anti-nutritional factors in Mucuna pruriens (velvet bean) seed flour as affected by several processing methods. Heliyon 2023, 9, e18728. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yoo, J.; Zhang, T.; Yang, K.; Guo, J.; Pan, S. Mixed fermentationof navel orange peel by Trichoderma viride and Aspergillus niger: Effects on the structural and functional properties of soluble dietary fiber. Food Biosci. 2024, 57, 103545. [Google Scholar] [CrossRef]
- McConnell, L.L.; Osorio, C.; Hofmann, T. The future of agriculture and food: Sustainable approaches to achieve zero hunger. J. Agric. Food Chem. 2023, 71, 13165–13167. [Google Scholar] [CrossRef]
- Pandey, D.K.; Singh, S.; Kumar, S.D.; Sing, T.M.; Dixit, S.; Sawargaonkar, G. Nutrient profiling of lablab bean (Lablab purpureus) from northeastern India: A potential legume for plant-based meat alternatives. J. Food Compos. Anal. 2023, 119, 105252. [Google Scholar] [CrossRef]
- Yoon, L.W.; Ang, T.N.; Ngoh, G.C.; Seak, A.M.C. Fungal solid-state fermentationand various methods of enhancement in cellulase production. Biomass Bioenergy 2014, 67, 319–338. [Google Scholar] [CrossRef]
- Malgas, S.; Thorensen, M.; van Dyk, J.S.; Pletschke, B.I. Time dependence of enzyme synergism during the degradation of model and natural lignocellulosic substrates. Enzym. Microb. Technol. 2017, 103, 1–11. [Google Scholar] [CrossRef]
- Cebrián, M.; Ibarruri, J. Filamentous fungi processing by solid-state fermentation. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 251–292. [Google Scholar]
- Hernández, D.J.M.; Ferrera, C.R.; Alarcón, A. Trichoderma: Importancia agrícola, biotecnológica, y sistemas de fermentación para producir biomasa y enzimas de interés industrial. Chil. J. Agric. Anim. Sci. 2019, 35, 98–112. [Google Scholar] [CrossRef]
- Plouhinec, L.; Neugnot, V.; Lafond, M.; Berrin, J.G. Carbohydrate-active enzymes in animal feed. Biotechnol. Adv. 2023, 65, 108145. [Google Scholar] [CrossRef]
- Bulgari, D.; Alias, C.; Peron, G.; Ribaudo, G.; Gianoncelli, A.; Savino, S.; Gobbi, E. Solid-state fermentation of Trichoderma spp.: A new way to valorize the agricultural digestate and produce value-added bioproducts. J. Agric. Food Chem. 2023, 71, 3994–4004. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Pandey, K.R.; Joshi, Y.R.; Lamichhane, S.K. An overview of multifaceted role of Trichoderma spp. for sustainable agriculture. Arch. Agric. Environ. Sci. 2021, 6, 72–79. [Google Scholar] [CrossRef]
- Hamdan, N.T.; Jasim, H.M. Cellulase from Trichoderma longibrachiatum Fungus: A Review. World Bull. Public Health 2021, 4, 52–68. [Google Scholar]
- Ma, X.; Li, S.; Tong, X.; Liu, K. An overview on the status and future prospects in Aspergillus cellulase production. Review article. Environ. Res. 2024, 244, 117866. [Google Scholar] [CrossRef] [PubMed]
- de França Passos, D.; Pereira, N., Jr.; de Castro, A.M. A comparative review of recent advances in cellulases production by Aspergillus, Penicillium and Trichoderma strains and their use for lignocellulose deconstruction. Curr. Opin. Green Sustain. Chem. 2018, 14, 60–66. [Google Scholar] [CrossRef]
- Valiño, E.C.; Dustet, J.C.; Pérez, H.; Brandão, L.R.; Rosa, A.C.; Scull, I. Transformation of Mucuna pruriens with cellulolytics fungi strains as functional food. Acad. J. Microbiol. Res. 2016, 4, 62–71. [Google Scholar]
- Valiño, E.; Elías, A.; Rodríguez, M.; Albelo, N. Evaluation using Fourier Transformed-infrared spectroscopy (FT-IR) of fermentation by the strain Trichoderma viride M5-2 from the cell walls of sugarcane (Saccharum officinarum Lin) bagasse pretreated. Int. J. Chem. Biomol. Sci. 2015, 1, 134–140. [Google Scholar]
- Valiño, E.; Alberto, M.; Dustet, J.C.; Albelo, N. Production of lignocellulases enzymes from Trichoderma viride M5-2 in wheat bran (Triticum aestivum) and purification of their laccases. Cuba. J. Agric. Sci. 2020, 54, 55–56. [Google Scholar]
- Valiño, E.; Savón, L.; Elías, A.; Rodríguez, M.; Albelo, N. Nutritive value improvement of seasonal legumes Vigna unguiculata, Canavalia ensiformis, Stizolobium niveum, Lablab purpureus, through processing their grains with Trichoderma viride M5-2. Cuba. J. Agric. Sci. 2015, 49, 81–89. [Google Scholar]
- Pérez-Soler, H.; Dustet-Mendoza, J.C.; Valiño-Cabrera, E. Incremento de la calidad nutritiva potencial de la harina de follaje de Stizolobium niveum (Mucuna) mediante fermentación en estado sólido con el hongo Trichoderma viride M5-2. Rev. CENIC. Cienc. Químicas 2016, 47, 30–33. [Google Scholar]
- Sánchez, M.F.D.; Martín-Cabrejas, M.Á.; Pérez, M.M.; Savón, L.L.; Valdés, Y.A.; Benítez, V.; Torres Cárdenas, V.; Coto Valdés, G.; González, A.M.; Sarmiento, M.; et al. A temporary legume sprouts: An alternative for animal feeding1 Germinados de leguminosas temporales: Una alternativa para la alimentación animal. Cuba. J. Agric. Sci. 2017, 51, 381. [Google Scholar]
- Sosa, A.; González, N.; García, Y.; Marrero, Y.; Valiño, E.; Galindo, J.; Sosa, D.; Alberto, M.; Roque, D.; Albelo, N.; et al. Collection of microorganisms with potential as additives for animal nutrition at the Institute of Animal Science. Cuba. J. Agric. Sci. 2018, 51, 311–319. [Google Scholar]
- Eveleigh, D.E.; Mandels, M.; Andreotti, R.; Roche, C. Measurement of saccharifying cellulase. Biotechnol. Biofuels 2009, 2, 381–390. [Google Scholar] [CrossRef]
- Mandels, M.; Medeiros, J.E.; Andreotti, R.E.; Bissett, F.H. Enzymatic hydrolysis of cellulose: Evaluation of cellulase culture filtrates under use conditions. Biotechnol. Bioeng. 1981, 23, 2009–2026. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; Chapter 4. Animal Feed; Latimer, G., Jr., Ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Scull, I.R.; Savón, L.V.; Spengler, I.S.; Herrera, M.V.; González, V.L. Potentiality of the forage meal of Stizolobium niveum and Stizolobium aterrimum as a nutraceutical for animal feeding. Cuba. J. Agric. Sci. 2018, 52, 223–234. [Google Scholar]
- Van Soest, P.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Savón, L.; Scull, I.; Orta, M.; Martínez, M. Whole-grain foliage flours from three tropical legumes for poultry feed. Chemical composition, physical properties, and phytochemical screening. Cuba. J. Agric. Sci. 2007, 41, 359–361. [Google Scholar]
- Savón, L.; Gutiérrez, O.; González, T.; Orta, M. Manual de Caracterización Fisicoquímica de Alimentos, 1st ed.; EDICA, Ed.; EDICA: La Habana, Cuba, 1999. [Google Scholar]
- Di Rienzo, J.; Casanoves, F.; Balzarini, M.; González, L.; Tablada, M.; Robledo, Y.C. InfoStat Versión 2017. In Grupo InfoStat, FCA; Universidad Nacional de Córdoba: Córdoba, Argentina, 2017. [Google Scholar]
- Duncan, B. Multiple ranges and multiple F test. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Matas Baca, M.Á.; Flores-Córdova, M.A.; Pérez Álvarez, S.; Rodríguez Roque, M.J.; Salas Salazar, N.A.; Soto Caballero, M.C.; Sánchez Chávez, E. Trichoderma fungi as an agricultural biological control in México. Rev. Chapingo. Ser. Hortic. 2023, 29, 79–114. [Google Scholar]
- Bézier, S.; Stiegler, M.; Hitzenhammer, E.; Schmoll, M. Screening for genes involved in cellulase regulation by expression under the control of a novel constitutive promoter in Trichoderma reesei. Curr. Res. Biotechnol. 2022, 4, 238–246. [Google Scholar]
- Bamidele, M.O.; Bamikale, M.B.; Cárdenas-Hernández, E.; Bamidele, M.A.; Castillo-Olvera, G.; Sandoval-Cortes, J.; Aguilar, C.N. Bioengineering in Solid-State Fermentation for next sustainable food bioprocessing. Next Sustain. 2025, 6, 100105. [Google Scholar] [CrossRef]
- Alberto Vazquez, M.; Saa, L.R.; Valiño, E.; Torta, L.; Laudicina, V.A. Microbiological Aspects and Enzymatic Characterization of Curvularia kusanoi L7: Ascomycete with Great Biomass Degradation Potentialities. J. Fungi 2024, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.A.; Valiño, E.C.; Torta, L.; Laudicina, A.; Sardina, M.T.; Mirabile, G. Potencialidades del consorcio microbiano Curvularia kusanoi-Trichoderma pleuroticola como pretratamiento biológico para la degradación de fuentes fibrosas. Rev. MVZ Córdoba 2022, 27, 12. [Google Scholar]
- Singh, A.; Bajarb, S.; Devia, A.; Pantc, D. An overview on the recent developments in fungal cellulase production and their industrial applications. Bioresour. Technol. Rep. 2021, 14, 100652. [Google Scholar] [CrossRef]
- Valdivia, A.L.; Matos, M.M.; Rodríguez, Z.; Pérez, Y.; Rubio, Y.; Vega, J. Enzymatic additives and their use on animal rearing. Cuba. J. Agric. Sci. 2019, 53, 341–352. [Google Scholar]
- Zhao, C.; Deng, L.; Fang, H. Mixed culture of recombinant Trichoderma reesei and Aspergillus niger for cellulase production to increase the cellulose degrading capability. Biomass Bioenergy 2018, 112, 93–98. [Google Scholar] [CrossRef]
- Vázquez, M.A.; Cabrera, E.C.V.; Aceves, M.A.; Mallol, J.L.F. Cellulolytic and ligninolytic potential of new strains of fungi for the conversion of fibrous substrates. Biotechnol. Res. Innov. 2019, 3, 177–186. [Google Scholar] [CrossRef]
- Liu, H.; Sun, J.; Leu, S.Y.; Chen, S. Toward a fundamental understanding of cellulase-lignin interactions in the whole slurry enzymatic saccharification process. Biofuels Bioprod. Biorefining 2016, 10, 648–663. [Google Scholar] [CrossRef]
- Dustet, J.C.; Izquierdo, E. Application of mass and energy balances to the solid-state fermentation process of sugarcane bagasse with Aspergillus niger. Appl. Biotechnol. 2004, 21, 85–91. [Google Scholar]
- Ghose, T.K. Measurement of Cellulase Activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Yi, J.; Chen, X.; Wen, Z.; Fan, Y. Improving the functionality of pea protein with laccase-catalyzed crosslinking mediated by chlorogenic acid. Food Chem. 2024, 433, 137344. [Google Scholar] [CrossRef]
- Martínez, M.; Sarmiento, L.; Santos, R.H.; Villafranca, M.; Londres, S. Digestive and carcass indicators of Rhode Island Red chickens, which intake rocessed Mucuna pruriens, in two rearing systems. Technical note. Cuba. J. Agric. Sci. 2022, 56, 121–126. [Google Scholar]
- Jung, N.; Meyer, A.S. Solid-State Fungal Fermentation for Better Plant Foods. Food Sci. Nutr. Cases 2025, fsncases20250001. [Google Scholar] [CrossRef]
- Shubha, K.; Choudhary, A.K.; Mukherjee, A.; Kumar, S.; Saurabh, K.; Kumar, R.; Das, A.A. Chemometric study comparing nutritional profiles and functional attributes of two botanical forms of Lablab Bean (Lablab purpureus (L.) Sweet). S. Afr. J. Bot. 2024, 173, 320–329. [Google Scholar] [CrossRef]
- Sowdhanyaa, D.; Singha, J.; Rasanea, P.; Kaura, S.; Kaura, J.; Ercislib, S.; Vermac, H. Nutritional significance of velvet bean (Mucuna pruriens) and opportunities for its processing into value-added products. J. Agric. Food Res. 2024, 15, 100921. [Google Scholar] [CrossRef]
- Díaz, M.; Martín-Cabrejas, M.Á.; González, A.; Torres, V.; Noda, A. Biotransformation of Vigna unguiculata during the germination process. Cuba. J. Agric. Sci. 2007, 41, 161. [Google Scholar]
- Williams, B.A.; Mikkelsen, D.; Flanagan, B.M. “Dietary fibre”: Moving beyond the “soluble/insoluble” classification for monogastric nutrition, with an emphasis on humans and pigs. J. Anim. Sci. Biotechnol. 2019, 10, 45. [Google Scholar] [CrossRef]
- Savón, L.; Scull, I.; Orta, M.; Torres, V. Physicochemical characterization of the fibrous fraction of five tropical foliage meals for monogastric species. Cuba. J. Agric. Sci. 2004, 38, 281–286. [Google Scholar]
- Reid, I.D. Biodegradation of lignin. Can. J. Bot. 1995, 73 (Suppl. S1), 1011–1018. [Google Scholar] [CrossRef]
- Hindrichsen, I.K.; Kreuzer Madsen, M.J.; Bach, K.E. Fiber and Lignin Analysis in Concentrate, Forage, and Feces: Detergent versus Enzymatic-Chemical Method. J. Dairy Sci. 2006, 89, 2168–2176. [Google Scholar] [CrossRef]
- Engels, F.M.; Jung, H.G. Alfalfa stem tissues: Cell-wall development and lignification. Ann. Bot. 1998, 82, 561–568. [Google Scholar] [CrossRef]
- García, Y.; Ibarra, A.; Valiño, E.C.; Dustet, J.; Oramas, A.; Albelo, N. Study of a solid fermentationsystem with agitation in the Biotransformation of sugarcane bagasse by the Trichoderma viride strain M5-2. Cuba. J. Agric. Sci. 2002, 36, 265–270. [Google Scholar]
- Sadh, P.K.; Saharan, P.; Duhan, J.S. Bio-augmentation of antioxidants and phenolic content of Lablab purpureus by solid state fermentation with GRAS filamentous fungi. Resour.-Effic. Technol. 2017, 3, 285–292. [Google Scholar] [CrossRef]
- Murphy, A.M.; Colucci, P.E. A tropical forage solution to poor quality diets: A review of Lablab purpureus. Livest. Res. Rural. Dev. 1999, 11, 1999. [Google Scholar]
- Guo, Q.; Luo, J.; Zhang, X.; Zhi, J.; Yin, Z.; Zhang, J.; Zhang, B.; Chen, L. A Comprehensive Review of the Chemical Constituents and Functional Properties of Adzuki Beans (Vigna angulariz). J. Agric. Food Chem. 2025, 73, 6361–6384. [Google Scholar] [CrossRef]
- Hettiarachchi, H.-O.; Gunathilake, K.-P. Physicochemical and functional properties of seed flours obtained from germinated and non-germinated Canavalia gladiata and Mucuna pruriens. Heliyon 2023, 9, 19653. [Google Scholar] [CrossRef]
- Alcívar, J.L.; Martínez, M.P.; Lezcano, P.; Scull, I.; Valverde, A. Technical note on physical-chemical composition of Sacha inchi (Plukenetia volubilis) cake. Cuba. J. Agric. Sci. 2020, 54, 19–23. [Google Scholar]
- Martínez, M.; Vives, Y.; Rodríguez, B.; Pérez, O.G.; Herrera, M. Nutritional value of palm kernel meal, fruit of the royal palm tree (Roystonea regia), for feeding broilers. Cuba. J. Agric. Sci. 2021, 55, 303–313. [Google Scholar]
- Scull, I.; Savón, L.; Valiño, E.; Ramos, Y. Composición fitoquímica de la harina de forraje de mucuna (Styzolobium aterrimum) fermentada con el hongo Trichoderma viride. Multiciencias 2015, 15, 265–270. [Google Scholar]
- Rodríguez, Z.; Martínez, M.; Sarmiento, L.; Pérez, M.; Dihigo, L.E.; Núñez, O.; Herrera, F.R.; Hernández, Y. Harina de forraje de Mucuna deeringiana en algunos grupos fisiológicos microbianos e indicadores fermentativos del ciego de pollos de ceba. Cuba. J. Agric. Sci. 2012, 46, 193–198. [Google Scholar]
- Chasi-Brito, N.V.; Navarrete-Zambrano, M.M.; Campozano-Marcillo, G.A. Bromatological value of Mucuna pruriens Georgina velvet and Canavalia ensiformis. Effect on productive parameters. Rev. Cienc. Agropecu. ALLPA 2025, 8, 42–62. [Google Scholar]
- Catagua, D.; Dustet Mendoza, J.C.; Valiño Cabrera, E.C.; De la Cruz, K. Influencia de los parámetros físicos sobre los factores antinutricionales de la harina de L. mutabilis Sweet sometida a fermentación sólida con A. niger y T. viride. Rev. Univ. Y Soc. 2022, 14, 20–28. [Google Scholar]
- Catagua, D.; Dustet Mendosa, J.; Valiño Cabrera, E. Mejora del valor nutritivo de harina de follaje de Lupinus mutabilis Sweet mediante fermentación en estado sólido con las cepas Aspergillus niger J1 y Trichoderma viride M5-2. La Granja Rev. Cienc. La Vida 2025, 41, 140–150. [Google Scholar] [CrossRef]
- Lilliefors, H. On the Kolmogorov-Smirnov Test for Normality with Mean and Variance Unknown. J. Am. Stat. Assoc. 1967, 62, 399–402. [Google Scholar] [CrossRef]
- Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Olkin, I., Hotelling, H., Eds.; Stanford University Press: Redwood City, CA, USA, 1960; pp. 278–292. [Google Scholar]


| Cellulolytic Activity | Legumes | Fermentation Time (h) | SE and p | |||
|---|---|---|---|---|---|---|
| 24 | 48 | 72 | 96 | |||
| CMCase (IU/mL) | L. purpureus | 2.09 c | 1.18 b | 1.22 b | 1.00 a | ±0.03 p < 0.0001 |
| M. pruriens | 3.15 e | 3.29 f | 2.57 d | 2.00 c | ||
| Cellulolytic Activity | Legumes | Fermentation Time (h) | SE and p | |||
|---|---|---|---|---|---|---|
| 24 | 48 | 72 | 96 | |||
| PFase (IU/mL) | M. pruriens | 0.74 d | 0.46 b | 0.39 a | 0.38 a | ±0.02 p < 0.0001 |
| L. purpureus | 0.49 b | 0.60 c | 0.64 c | 0.63 c | ||
| Indicator | Legumes | Fermentation Time (h) | SE and p | |||
|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | |||
| pH | L. purpureus | 6.13 b | 6.83 d | 7.46 g | 7.37 f | ±0.004 p < 0.0001 |
| M. pruriens | 6.03 a | 6.71 c | 6.72 c | 7.12 e | ||
| Indicators (% of DM) | Legumes | Fermentation Time (h) | SE and p | |||
|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | |||
| DM * | L. purpureus | 28.17 d | 28.34 d | 27.82 c | 27.86 c | ±0.10 p < 0.0001 |
| M. pruriens | 28.76 e | 26.38 a | 27.14 b | 27.14 b | ||
| CP | L. purpureus | 13.63 a | 17.15 c | 15.36 b | 17.03 c | ±0.13 p < 0.0001 |
| M. pruriens | 19.04 d | 21.34 e | 22.05 f | 21.27 e | ||
| TP | L. purpureus | 11.30 a | 13.24 b | 13.21 b | 14.40 c | ±0.13 p < 0.0001 |
| M. pruriens | 17.58 d | 19.53 e | 21.74 g | 20.61 f | ||
| NDF | L. purpureus | 67.83 b | 67.83 b | 66.74 a | 67.90 b | ±0.29 p < 0.0001 |
| M. pruriens | 73.06 d | 68.48 b | 69.56 c | 69.83 c | ||
| ADF | L. purpureus | 51.44 a | 51.18 a | 52.97 c | 54.31 d | ±0.18 p < 0.01 |
| M. pruriens | 52.27 b | 52.54 b,c | 52.64 b,c | 54.78 d | ||
| Cel | L. purpureus | 42.07 f | 39.28 b | 41.67 e | 43.88 g | ±0.12 p < 0.0001 |
| M. pruriens | 39.66 c | 39.97 c,d | 38.41 a | 40.14 d | ||
| Lig | L. purpureus | 9.17 a | 10.87 c | 10.93 c | 10.20 b | ±0.12 p < 0.0001 |
| M. pruriens | 12.33 e | 11.38 d | 13.35 f | 13.98 g | ||
| Hem | L. purpureus | 16.39 c,d | 16.65 c,d | 13.77 a | 13.59 a | ±0.29 p < 0.0001 |
| M. pruriens | 20.79 e | 15.94 c | 16.92 d | 15.05 b | ||
| Physical Indicators | Legumes | Fermentation Time (h) | ||||
|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | SE and p | ||
| V (g/mL) | L. purpureus | 4.05 e | 3.60 d | 3.35 b | 4.05 e | ±0.04 p < 0.0001 |
| M. pruriens | 3.48 c | 3.22 a | 3.32 a,b | 3.25 a,b | ||
| WAC (g/g) | L. purpureus | 3.71 b | 3.76 b | 2.85 a | 4.36 c | ±0.13 p = 0.0005 |
| M. pruriens | 4.66 c,d | 5.14 e | 4.68 c,d | 4.78 d,e | ||
| ABC (meq) | L. purpureus | 0.51 c | 0.42 a | 0.59 g | 0.47 b | ±0.0018 p < 0.0001 |
| M. pruriens | 0.63 h | 0.58 f | 0.53 d | 0.57 e | ||
| BBC (meq) | L. purpureus | 0.36 e | 0.31 a | 0.45 g | 0.36 e | ±0.0013 p < 0.0001 |
| M. pruriens | 0.32 b | 0.41 f | 0.35 d | 0.33 c | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saa, L.R.; Valiño Cabrera, E.C.; Savón Valdés, L.L.; García Hernández, Y.; Dustet Mendoza, J.C.; Alberto Vazquez, M. Sustainable Biomass Valorization by Solid-State Fermentation with the Mutant Strain Trichoderma viride M5-2 of Forage Legumes to Improve Their Nutritional Composition as Animal Feed. Sustainability 2025, 17, 4990. https://doi.org/10.3390/su17114990
Saa LR, Valiño Cabrera EC, Savón Valdés LL, García Hernández Y, Dustet Mendoza JC, Alberto Vazquez M. Sustainable Biomass Valorization by Solid-State Fermentation with the Mutant Strain Trichoderma viride M5-2 of Forage Legumes to Improve Their Nutritional Composition as Animal Feed. Sustainability. 2025; 17(11):4990. https://doi.org/10.3390/su17114990
Chicago/Turabian StyleSaa, Luis Rodrigo, Elaine Cristina Valiño Cabrera, Lourdes Lucila Savón Valdés, Yaneisy García Hernández, Julio César Dustet Mendoza, and Maryen Alberto Vazquez. 2025. "Sustainable Biomass Valorization by Solid-State Fermentation with the Mutant Strain Trichoderma viride M5-2 of Forage Legumes to Improve Their Nutritional Composition as Animal Feed" Sustainability 17, no. 11: 4990. https://doi.org/10.3390/su17114990
APA StyleSaa, L. R., Valiño Cabrera, E. C., Savón Valdés, L. L., García Hernández, Y., Dustet Mendoza, J. C., & Alberto Vazquez, M. (2025). Sustainable Biomass Valorization by Solid-State Fermentation with the Mutant Strain Trichoderma viride M5-2 of Forage Legumes to Improve Their Nutritional Composition as Animal Feed. Sustainability, 17(11), 4990. https://doi.org/10.3390/su17114990






