Abstract
Sustainable management of soils degraded by heavy metals is a major environmental challenge. The aim of this study was to evaluate the acclimatization ability of the hybrid Populus nigra L. × Populus maximowiczii under variable soil moisture conditions. In a greenhouse experiment, it was shown that both soil moisture level and the presence of metals significantly affected plant growth and metabolism. The hybrid showed high nickel (Ni) accumulation at low and medium soil moisture content (LMC, MMC) (BCF 4.56 and 4.99), while copper (Cu) accumulation was highest at MMC (BCF 5.53). Nickel translocation to aerial parts increased after exposure (TF up to 0.63), while Cu translocation was limited (TF below 0.94). Increased humidity promoted the biosynthesis of low molecular weight organic acids (LMWOAs) in roots, with the highest total content recorded in the Cu treatment under high soil moisture content (HMC) (230 μg g−1 FW). In the stems, the highest levels of sum LMWOAs were found under HMC conditions (6764 μg g−1 FW in the control sample), while among the phenolic acids, the highest content of chlorogenic acid (~144 μg g−1 FW) was determined under LMC conditions under Ni stress, which indicates a strong defense response of the plant. The obtained results emphasize the importance of selecting appropriate water conditions in remediation strategies and indicate that the tested poplar hybrid may be a promising tool in improving the quality of degraded soils.
1. Introduction
In Europe, climate change contributes to the increased frequency and intensity of drought and water overload events. Some regions are experiencing increasingly longer periods of drought, which has a negative impact on agriculture, access to drinking water, and ecosystems. Conversely, other areas have to deal with extreme rainfall, causing floods and flooding. In Poland, average annual rainfall is very diverse—the highest is recorded in the Tatra Mountains, and the lowest in Wielkopolska, Mazovia, and especially in Kujawy, Dobrzyń, and Chełmno Land, where in some places it does not exceed 500 mm [1]. The latest studies indicate a decrease in the size of floods and an increase in the occurrence of river droughts throughout the country, except in the mountainous areas of south-eastern Poland, where the size of droughts increases in summer and decreases in winter, while floods intensify in late spring and early autumn [2,3,4]. In the face of climate change, European countries must adapt their water resources, infrastructure, and agriculture management strategies, taking into account, among others, investments in irrigation systems, water resource protection, flood risk management, sustainable agriculture practices, and the rehabilitation of degraded areas, e.g., by using species with high adaptive capacity.
In this context, woody species with high environmental resistance, such as poplars of the Populus genus, are significant. Hybrids of balsam poplars, especially Populus nigra L. × Populus maximowiczii and P. trichocarpa, are widely used in urban greenery and for biomass production due to their rapid growth (up to 2.5 m per year) and low soil requirements [5,6,7]. These poplars show high tolerance to stress conditions and can be cultivated in degraded areas containing toxic heavy metals, metalloids, and organic xenobiotics. Thanks to their extensive root system and intensive transpiration, they are capable of phytostabilizing pollutants in the rhizosphere zone and rhizofiltrating shallow groundwater. Numerous studies have confirmed their effectiveness in remediating soils contaminated with copper (Cu), lead (Pb), zinc (Zn), cadmium (Cd), nickel (Ni), and chromium (Cr) [8,9,10]. Cu and Ni were selected for this study due to their common occurrence in the soil environment, resulting from industrial activity, and their significant physiological effect on plants. Cu is an essential trace element, but in excess it is toxic, disrupting photosynthesis and respiration processes [11]. Conversely, although in trace amounts, Ni participates in nitrogen metabolism and can cause oxidative stress and growth disorders.
Despite tolerance to pollution, variability in water availability, mainly resulting from temporary droughts and heavy rainfall, can limit the effectiveness of remediation using poplar [7]. Dynamic changes in soil moisture make it difficult for plants to adapt and affect the uptake of heavy metals. It is important to use species resistant not only to chemical contamination but also to water fluctuations. Understanding the physiological response of the energy poplar P. nigra L. × P. maximowiczii to water stress is crucial for preparing for changes in soil moisture caused by climate change. It can provide a basis for planning effective remediation strategies in degraded habitats. This knowledge is crucial for its effectiveness in phytoremediation in the ongoing climate change.
Analyses of the content of low molecular weight organic acids (LMWOAs), phenolic compounds, and salicylic acid were carried out. The choice of these molecules results from the fact that they play a key role in plant defense mechanisms, especially in the context of oxidative stress. Oxidative stress, caused by excessive production of reactive oxygen species (ROS), is a common element of plant response [10]. Excess ROS can lead to cellular damage, which is why plants activate defense mechanisms, including the synthesis of compounds with antioxidant activity, including LMWOAs, such as citric, malic, or oxalic acids, which occur in plants as intermediaries of metabolic pathways such as the Krebs cycle. Under stress conditions, including the increasingly common stress of water deficiency, plants can modify the production of these acids to regulate metabolism and osmotic balance. Their role in water stress is related to energy storage and regulation of cell water levels [12]. In turn, phenolic compounds, such as flavonoids and phenolic acids, directly defend against oxidative stress. They are natural antioxidants that neutralize reactive oxygen species, reducing the risk of oxidative damage. Salicylic acid can induce the synthesis of antioxidant enzymes, such as peroxidases or catalases, which neutralize ROS [13]. Therefore, in the face of water stress, heavy metals, and other forms of abiotic stress, plants often increase the production of phenolic compounds to protect cells from oxidative damage.
This study aimed to assess the potential of P. nigra L. × P. maximowiczii as a highly adaptive plant, capable of functioning under conditions of simultaneous metal and water stress. Knowing and understanding the adaptation mechanisms of poplars can contribute to developing effective methods for using them in degraded areas and protecting ecosystems in the face of climate change. So far, the impact of the interaction between heavy metal pollution and soil moisture fluctuations on the physiology of P. nigra L. × P. maximowiczii has been analyzed only to a limited extent, therefore the obtained results make an essential contribution to the development of knowledge on the adaptive strategies of plants in degraded environments.
2. Materials and Methods
2.1. Characteristics of Experimental Materials
The one-year-old cuttings of P. nigra L. × P. maximowiczii used in the experiment were collected in February/March from the plantation of AGRO-WOOD.PL, Dziedzice, Poland (52.22879° N; 17.84689° E). The mean biomass of the cuttings before the experiment was 26.68 ± 4.85 g. Cuttings were prepared according to the European Commission standard [14] (Council Directive 1999/105/EC), i.e., sections of shoots were selected from unbranched specimens, at least 1 cm thick at the thinner end.
2.2. Experiment Design
Poplar seedlings P. nigra L. × P. maximowiczii were rooted for 10 days in Knop’s solution (half strength) and then transferred to 18 × 19 cm pots filled with 2.5 kg of homogeneous soil classified as loamy sand [15]. The plants were divided into 9 experimental variants, including three levels of soil moisture (low, medium, high) and three treatment variants (control, Cu, Ni). Each variant was conducted in 9 replicates, which gave a total of 81 pots. The range of water available to plants was determined as the moisture level between field capacity (FC) and permanent wilting point (PWP) [16]. PWP (% wt.) was calculated based on the maximum hygroscopicity of the soil (MH), according to the formula [17].
PWP = 2MH
MH (% wt.) was determined according to the Mitscherlich method [18]. Twenty ~30 g air-dried soil samples were placed in a vacuum desiccator on porcelain dishes over 10% H2SO4, where relative humidity was settled at 94%. Next, the air was pumped out from the desiccator using a water pump, where the vacuum (negative pressure) reached about 200 hPa. After seven days, the vacuum was removed, and soil samples were weighed, oven-dried at 105 °C for 24 h, and again weighed to determine the gravimetric water content. The FC (% wt.) was determined parallel to maximal hygroscopicity measurements. Twenty air-dried soil samples were collected in 100 cm3 cylinders with perforated bottom. Next, cylinders were placed in containers with water for seven days. In the initial soaking stage (~2 days), the samples were slowly filled with water by gradually raising the water level in the containers. After removing soil samples from the water and completing the gravity draining process (lasting about 1–2 h), soil samples were weighed, oven-dried at 105 °C for 24 h, and again weighed to determine the gravimetric water content.
The water properties of soil used in the pot experiment in terms of MH, PWP, and FC are shown in Table 1.

Table 1.
Water properties of soil used in a pot experiment, where MH is the maximal hygroscopicity, PWP is the permanent wilting point, and FC is the field capacity.
The mean amount of plant water available ranged between 5.4 and 38.8%. This range has been used to determine three water regimes, which were applied in the pot experiment: (1) low soil moisture content (LMC) between 5.4 and 16.0% (on average ~11.0%), (2) medium soil moisture content (MMC) between 16.1 and 27.0% (on average ~22.0%), and (3) high soil moisture content (HMC) between 27.1 and 38.8% (on average ~33%). The given water regime was maintained in the pots by daily measurements of each pot mass and by adding an appropriate amount of water to maintain the assumed soil moisture content.
The soil used in the experiment was collected from the natural environment from an area not contaminated with heavy metals. It was then appropriately sieved and prepared for experimental purposes. To model the conditions of heavy metal contamination in a controlled manner, Cu and Ni salts were introduced into the soil immediately after transplanting the plants. For this purpose, copper (II) nitrate trihydrate (Cu(NO3)2 × 3H2O) and nickel (II) nitrate hexahydrate (Ni(NO3)2 × 6H2O) were used at a concentration of 100 mg·kg−1 of dry soil mass (DW). This procedure allowed the creation of three experimental variants within each soil moisture level: control (without metal addition), with the addition of Cu, and with the addition of Ni. According to geochemical data for European soils, the adopted concentration corresponds to a moderate level of contamination.
The plants were grown for 4 weeks in greenhouse conditions. After this period, biometric parameters were assessed, and analyses of metal content and the level of selected secondary metabolites, including LMWOAs and phenolic compounds, were performed.
2.3. Sample Preparation and Determination of LMWOAs and Phenolic Compounds
After the experiment, the plant material was divided into three parts: the rhizosphere, the roots, and the stems (with shoots referring to green shoots and leaves). Rhizosphere samples were divided into two equal parts (~1–5 g) and analyzed. One part of the sample was extracted with water (20 mL at pH 2 acidified with concentrated HCl) by shaking on an orbital shaker at room temperature (for 12 h) [19]. The aqueous extracts were filtered through Whatman #42 filters (Munktell, Grycksbo, Sweden) and extracted three times with ethyl acetate (5 mL, 5 min) (LC-MS LiChrosolv, Merch, Darmstadt, Germany) [20]. The volume of ethyl acetate was reduced to ~3 mL on a rotary evaporator at 40 °C (Rotavapor R-300, Buchi, Flawil, Switzerland), transferred to an amber glass vial, and evaporated at room temperature to dryness. The second part was mixed with 5 mL 80% methanol, sonicated, and shaken for 5 h. The extracted methanolic samples were also evaporated to dryness. The residue was dissolved in 80% methanol, then both extracts were combined for analysis [21].
Since the poplar roots were very delicate and thin after the experiment, it was decided not to remove the remaining rhizosphere from the roots to avoid damaging the roots and losing valuable plant material. As a result, the remaining rhizosphere on the roots was treated as primarily root material rather than soil material, ensuring the representativeness of the samples in the context of studies related to plant activity. About 1–5 g of P. nigra L. × P. maximowiczii root and stems (stems including green shoots and leaves) were powdered in liquid nitrogen using a chilled mortar, transferred to 50 mL centrifuge tubes, and kept frozen at −80 °C until analysis [19]. For LMWOAs analysis of tissue samples, 5 mL of H2O was added, and the mixture was heated in a water bath (60 min, 40 °C) to denature the degradative enzymes. For phenolic compound analysis, ground tissue samples were mixed with 80% methanol and HCl (99:1), sonicated, and shaken for 5 h [21].
Aqueous and methanolic solutions (rhizosphere, roots, and stems samples) were centrifuged (at 3600 rpm min−1 for 15 min at 25 °C) (Universal 320R Hettich Zentrifugen, Tuttlingen, Germany) before analysis and filtered through 0.2 μm nylon filters.
The determination of LMWOAs and phenolics was performed using a Waters Acquity H-class Ultra Performance Liquid Chromatography (UPLC) system, with separation on an Acquity UPLC BEH C18 column (2.1 mm × 150 mm, 1.7 μm, Waters, Milford, MA, USA) thermostated at 35 °C according to the method described by [21]. Compounds were identified by comparing the retention times of the analyzed peaks with those of reference standards or by adding a known quantity of the standard to the samples, followed by reanalysis [19]. The results were expressed in micrograms per gram of fresh weight (FW) of sample [μg g−1 FW].
2.4. Determination of Salicylic Acid
Salicylic acid (free form) was analyzed in the obtained methanolic extracts of poplar stems using a Waters Alliance 2695 Chromatograph coupled with a Waters 2475 Multi-λ Fluorescence Detector (Waters Corporation, Milford, MA, USA) according to the method by [22]. The isocratic elution was performed in a Waters Spherisorb ODS2 column (100 × 4.6 mm, 3 μm) at a 1.5 mL min−1 flowrate using a potassium acetate buffer (0.2 M, pH 5.0). The fluorometric detection was carried out at 295 nm for excitation and 405 nm for emission. The metabolite was identified according to the retention time in a given chromatographic setup and quantified using an appropriate calibration curve.
2.5. Methodology of Metals Analysis
2.5.1. Gases and Reagents
High–purity argon (purity 99.999%, N–5.) was purchased from Linde Gaz (Kraków, Poland). All calibration solutions were prepared from ICP analytical standards (PrimAg® Reference Materials) from ROMIL (Cambridge, UK). Water purification system (Milli–Q, Merck Millipore, Darmstadt, Germany) ensured high–pure deionized water (≥18 MΩ cm resistivity).
2.5.2. Sample Preparation
0.300 ± 0.001 g of samples (soil, roots, and stems) were mineralized with 7.0 mL of 65% nitric acid (Merck, Darmstadt, Germany) using the microwave digestion system, Mars 6 Xpress (CEM, Matthews, NC, USA). The digestion process was conducted in three stages of 20 min each: (1) ramping the temperature, (2) holding at 180 °C, and (3) cooling. Afterward, samples were filtered using filter papers (previously rinsed with 200 mL of deionized water) and filled up to 15 mL.
2.5.3. Instrumentation
The spectrometer, Agilent 5110 ICP–OES SVDV (Santa Clara, CA, USA) was used with standard operating parameters, i.e., RF generator power 1.2 kW, gas flows: 12 L min−1 (plasma), 0.7 L min−1 (nebulizer), 1.0 L min−1 (auxiliary), and synchronous vertical dual view (SVDV) with 8 mm viewing height for radial view. A single measurement was 3 replicates (5 s each). The following emission lines were used: Ni 231.604 nm, Cu 327.395 nm, Co 238.892 nm, Si 288.158 nm, Fe 238.204 nm, Al 396.152 nm, Zn 213.857 nm, Na 588.995 nm, Mg 279.553 nm, K 766.491 nm, and Ca 422.673 nm. Detection limits (DLs), established using 3−sigma criteria, were Ni 0.002, Cu 0.009, Co 0.009, Si 0.011, Fe 0.039, Al 0.17, Zn 0.20, Na 0.33, Mg 0.51, K 2.1, and Ca 2.7 mg kg−1 dry weight (DW). The uncertainty (k = 2) was <20% for the complete analytical process (including sample preparation). Quality control was ensured with several certified reference materials (CRMs), i.e., leaves (INCT−TL−1), wood (NIST SRM 2790), and soil (NIST SRM 2709a), and acceptable recovery (80–120%) was obtained for most of the determined elements.
Recovery (%) = Detected value (mg kg−1)/Certified value (mg kg−1) × 100%
The bioconcentration factor (BCF) was calculated as a ratio of metal content in roots to its concentration in soil at the experiment endpoint. Translocation factor (TF) was calculated as a ratio of metal content in shoots to roots [23,24].
2.6. Statistical Analysis and Calculations
Statistical analyses were performed using Statistica ver. 13.3 by TIBCO Software Inc. (Polo Alto, CA, USA). The differences in growth and biochemical parameters and element concentration were assessed by two-dimensional analysis of variance (ANOVA) with ‘soil moisture’ and ‘treatment’ as experimental factors at α = 95%. Analyses were followed by the multiple comparisons Tukey’s HSD as a post hoc analysis for significant effects. Test assumptions were checked with Levene’s test assessed homogeneity of variance [25]. A simple correlation analysis was performed for the analyzed poplar roots and shoots parameters, and only significant Pearson correlation coefficients were presented.
For Principal Component Analysis (PCA), the data were normalized using the clusterSim library to prevent any single variable from disproportionately influencing the results. Additionally, all variables were zero-centered to ensure comparability. The PCA was conducted in a two-step approach. First, separate PCA analyses were performed for Cu and Ni accumulation in shoots, roots, and woody stems (on plot Cu and Ni, respectively). Another PCA was conducted for metal accumulation (Ca, Fe, K, Mg, Na, Si, Zn) in shoots (Me_S) and roots (Me_R), as well as for metabolite groups. Metabolites were categorized as follows: LMWOAs_R: lactic, citric, succinic, and fumaric acids in roots. LMWOAs_S: quinic, oxalic, malonic, lactic, citric, acetic, malic, succinic, and fumaric acids in shoots. Vanillic, syringic, chlorogenic, and salicylic acids were classified as C6C1 structural acids, whereas caffeic, p-coumaric, ferulic, and sinapic acids were classified as C6C3.
Variables included in each PCA were selected based on the correlation matrix, ensuring the removal of multicollinearity and improving the explanatory power of the first principal component (PC1). Afterward, PC1 scores from these preliminary analyses were extracted and used in the final PCA to reduce data dimensionality while preserving the variance of the entire dataset. The data were visualized using the ggpubr library [26]. All statistical analyses were conducted using R software (ver. 4.2; R Development Core Team, Vienna, Austria).
3. Results and Discussion
3.1. Plant Growth and Biomass Production
In the control system (without Cu or Ni), where three levels of soil moisture content were used, the tested poplar plants did not show toxic symptoms. They exhibited 100% survival under all soil moisture systems. However, biomass production differed significantly between water regimes (Figure 1).
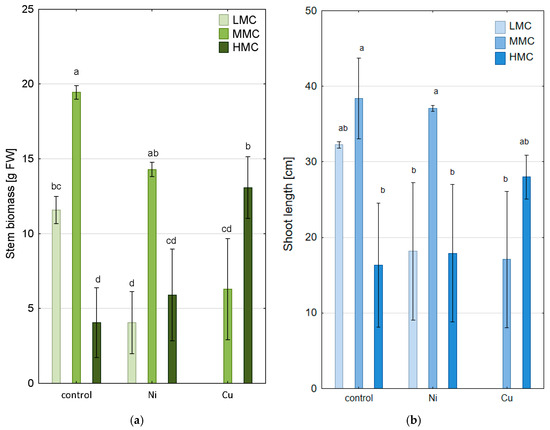
Figure 1.
Stem biomass (a) and length (b) of P. nigra L. × P. maximowiczii in experimental variants (mean ±SE; significant differences between means were indicated with different lettering according to a post hoc Tukey’s test following the two-way ANOVA for fixed ‘soil moisture × treatment’ analysis at α = 95% (LMC—low moisture content, MMC—medium moisture content, HMC—high moisture content).
Lower biomass was observed in the LMC system compared to the MMC system (Figure 1a). This finding is also supported by other studies on the response of poplars to low water content (drought) [27,28], which indicated a limitation of biomass growth. At the same time, the lowest biomass was recorded in the HMC system. This observation is consistent with similar studies on willow varieties (Salix spp.), which indicated that Salix grew well in well-drained conditions while flooding significantly inhibited the growth of shoots of Salix clones to varying degrees [29]. Our studies clearly showed that P. nigra L. × P. maximowiczii coped better in the LMC system than in the HMC system, which was particularly evident in the case of shoot weight (Figure 1b).
Regardless of the given soil moisture content and the stress related to the presence of Ni in the system, P. nigra L. × P. maximowiczii did not show necrotic changes or leaf fall. The trends in biomass growth were identical to those in the control system, but the length and weight of shoots after the experiment were significantly lower (Figure 1a,b). This is because stress reactions resulting from the presence of Ni in the environment significantly impact plant development by disturbing the water balance, thus limiting the growth of plants [30].
Similarly to Ni, excess Cu in the soil can interfere with photosynthetic processes, nitrogen metabolism, and oxidation pathways [31]. The addition of Cu at a concentration corresponding to average pollution resulted in poplar death in the LMC system. In the MMC system, in the presence of Cu, the biomass of P. nigra L. × P. maximowiczii plants was significantly lower compared to the control system and the system containing Ni. Meanwhile, the HMC system in the presence of Cu became the most favorable system in terms of poplar biomass for given water conditions.
3.2. Copper and Nickel Accumulation
The investigated poplar hybrid exhibited distinct patterns of metal (Cu, Ni) uptake, which were differently influenced by soil moisture (Figure 2).
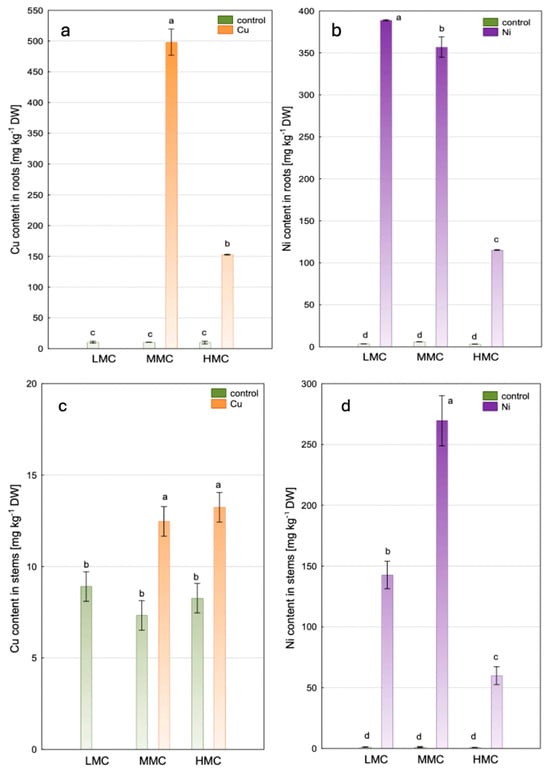
Figure 2.
The content of Cu (a,c) and Ni (b,d) in roots and stems of P. nigra L. × P. maximowiczii in experimental variants (mean ± SE; significant differences between means were indicated with different lettering according to a post hoc Tukey’s test following the two-way ANOVA for fixed ‘soil moisture × treatment’ analysis at α = 95% (LMC—low moisture content, MMC—medium moisture content, HMC—high moisture content).
The accumulation of Cu in roots reached 500 mg kg−1 following Cu addition to the soil and plant cultivation in the MMC system and decreased significantly in the HMC system (153 mg kg−1) (Figure 2a). The uptake of Ni showed a similar pattern to that of Cu, with the highest level for the LMC system, followed by the MMC system, and significantly lower values in the HMC system (389, 357, and 115 mg kg−1, respectively) (Figure 2b). In the roots of control plants, the mean content amounted to 10.2 mg kg−1 (Cu) and 4.3 mg kg−1 (Ni). Metal accumulation in shoots was lower compared to roots, and Ni content reached significantly higher values than Cu. In the shoots of Cu-treated plants, its content reached approximately 13 mg kg−1 regardless of soil moisture, compared to the control (8.2 mg kg−1 on average) (Figure 2c). The accumulation of Ni amounted to 270 mg kg−1 in the MMC system and significantly decreased in the LMC and HMC systems (143 and 60 mg kg−1, respectively), while the shoots of control plants accumulated as low as 1.2 mg kg−1 on average (Figure 2d).
The BCF values were elevated following metal treatment in the MMC system, and for Ni also in the LMC system, ranging from 4.6 to 5.5. A significantly lower metal uptake in roots was noted in the HMC system for both metals. The addition of Cu lowered its translocation ratio to shoots (TF < 0.1) compared to the control, regardless of soil moisture. In contrast, Ni addition elevated its translocation from roots to shoots in the MMC and HMC systems; however, the TF did not exceed 1 (Table 2).

Table 2.
Bioconcentration factor (BCF) and translocation factor (TF) values calculated for Cu and Ni accumulation by P. nigra L. × P. maximowiczii (differences between means were indicated with different lettering according to a post hoc Tukey’s test following the two-way ANOVA for fixed ‘soil moisture × treatment’ analysis for Cu and Ni separately at α = 95% (LMC—low moisture content, MMC—medium moisture content, HMC—high moisture content).
Opposite to our results, Kacálková et al. [32] reported lower accumulation of Cu in roots and relatively higher uptake of Cu in shoots than in roots of P. nigra L. × P. maximowiczii in a field study, with mean values of 9.61, 7.43, and 5.03 mg kg−1 DW for twigs, leaves, and roots, respectively. Consequently, the obtained BCF values did not exceed 1 and were significantly lower than in the present pot experiment despite comparable metal levels in the soil. In another study, Kacálková et al. [33], Ni uptake was also much lower, with values ranging from 0.58–1.78 mg kg−1 DW, and mainly non-significant differences were noted between plant organs. Migeon et al. [34] revealed the second-highest tolerance of P. nigra L. × P. maximowiczii out of 21 poplar hybrids in a screening hydroponic study using 10 µM solutions of metal salts (Cd, Zn, Cu, or Ni). Stems of the investigated hybrid accumulated ~8 mg Cu kg−1 DW, which was comparable to our results; however, much lower content was found for Ni (~31 mg kg−1). Simultaneously, much higher leaf accumulation was found for Zn (~1380 mg kg−1 DW) and Cd (~60 mg kg−1 DW). However, metals were added as chlorides, which could influence the uptake and tolerance.
Differences in the uptake of particular mineral nutrients were observed between experimental variants (Tables S1 and S2). In plants growing in the HMC system, the content of Ca was the lowest in both roots and shoots, and the treatment did not significantly affect its uptake. A similar pattern was observed for Mg. The highest Mg content was noted in the MMC system, while it was lower and comparable in the LMC and HMC systems. A similar effect of soil moisture was found for Fe and Al content in roots. In the case of monovalent ions, non-significant differences were noted for K, while a significant effect of soil moisture was observed for Na, with the highest level in roots and the lowest in shoots observed in the MMC system. The content of Zn was the highest in the MMC system (on average) and in the roots of Cu- or shoots of Ni-treated plants.
The study by D’Oria et al. [35] revealed changes in the net uptake of 20 nutrients in Brassica napus and Triticum aestivum under drought conditions, along with an analysis of 183 differentially expressed genes related to the ionome in leaves in B. napus. The study identified three patterns of gene expression due to drought: upregulation (transport of Co and Cl), downregulation (transport of N, P, Mo, B, and Ni), and mixed levels (transport of S, K, Mg, Zn, Cu, Fe, and Mn). The patterns of gene regulation were discussed in the context of the crosstalk established between mineral nutrients. This suggested that a decrease in Fe uptake occurred as a specific response to drought before a reduction in growth, leading to a decrease in Zn (and Mn) uptake, which was also observed in the present study at LMC, though in the roots. In contrast, Li et al. [27] reported that drought decreased the uptake of Mg and Mn in fine roots and increased the uptake of Fe in fine and moderate roots, but decreased it in large roots in the tree species Cunninghamia lanceolata. Additionally, severe drought increased the accumulation of K, Na, Ca, P, Fe, and Al in leaves after 45 days and Mn and Mg after 15 days [36]. Considering differences in ionomics under water deficit, plants develop different strategies to counteract drought stress via the regulation of mineral compositions in phloem and xylem depending on severity and duration.
3.3. Physiological Processes Related to Soil Moisture and Cu and Ni Tolerance
To investigate the differences in the biochemical response of poplar resulting from different soil moisture levels and their correlation with the phytoextraction capacity of Cu and Ni, we analyzed the changes in the content and profile of LMWOAs, phenolic compounds in roots and stems, and salicylic acid in poplar stems.
3.3.1. LMWOAs Content in Rhizosphere and Roots
The study showed that the content of LMWOAs in the rhizosphere was below the detection limit, and the LMWOA content in the roots of the tested poplar hybrid (P. nigra L. × P. maximowiczii) was low (Table 3).

Table 3.
The content of LMWOAs [μg g−1 FW] in the roots of P. nigra × P. maximowiczii in experimental variants (LMC—low moisture content, MMC—medium moisture content, HMC—high moisture content).
An increase in soil moisture in the control system resulted in elevated biosynthesis of succinic acid in the roots, from 24.1 µg g−1 FW in the LMC system to 70.2 and 145 µg g−1 FW at higher moisture values, and citric acid from 9.62 µg g−1 FW in the LMC system to 25.5 and 73.8 µg g−1 FW at higher moisture values (average for all systems). These observations were confirmed by correlation analysis, which showed a significant positive correlation between the citric acid level in roots and soil moisture (R = 0.61). In contrast, the addition of Ni caused the opposite effect for succinic acid, decreasing from 149 µg g−1 FW in the LMC system to 116 and 28.8 µg g−1 FW at higher moisture values. The highest content of 104 µg g−1 FW for citric acid was found in the MMC system (Table 3). In plants exposed to Cu, the highest content of succinic and citric acids in roots was determined for the HMC systems, amounting to 178 and 50.5 µg g−1 FW, respectively. The presented results indicate variability in the content of acids depending on the metal and soil moisture. This is also confirmed by studies by Shi et al. [37,38]; these studies showed increased levels of oxalic, malic, and citric acids in the roots of P. × canescens and P. nigra in response to Pb exposure. Furthermore, the amounts of acids differed between the two poplar species, which was related to species-specific plant tolerance to changes in soil moisture [39].
Additionally, literature data indicate that citric and malic acids are present in high concentrations in xylem sap, suggesting that both are important for the xylem transport of Cu in Salix integra Thunb. [40], Zn in Sedum alfredii [41], and Cd in Oryza sativa and Brassica juncea [42]. It is worth noting that differences in plant species, type of metal, and dose can lead to conflicting results, which are further influenced by soil moisture.
3.3.2. LMWOAs and Phenolic Compounds Content in Stems
Short-chain LMWOAs, such as oxalic, succinic, malic, malonic, and citric acids, contribute to plant tolerance by reducing toxicity through chelation and maintaining low concentrations of free ions in the cytoplasm [12,43,44]. Elevated levels of LMWOAs have been observed in several plants exposed to Cu, Pb, or Zn [38,45,46]. This is because LMWOAs form negatively charged ions and, therefore, can balance the increased concentration of metal ions in cells and regulate cell pH and osmotic potential [47,48]. Their content is also related to environmental conditions, such as soil water content [7,46]. In the shoots of P. nigra L. × P. maximowiczii, the presence of nine LMWOAs was demonstrated (Table 4).

Table 4.
The content of LMWOAs [μg g−1 FW] in stems of P. nigra × P. maximowiczii in experimental variants (LMC—low moisture content, MMC—medium moisture content, HMC—high moisture content).
Similar to the roots, the highest concentrations were found for citric and succinic acids, followed by malonic, lactic, and acetic acids. The remaining acids were detected at significantly lower levels. The lowest contents in the control MMC system for citric, malonic, and lactic acids were 1040, 38.5, and 4.94 µg g−1 FW, respectively. The highest content of citric acid, 1893 µg g−1 FW, was found in the LMC system, while the highest contents of malonic and lactic acids were found in the HMC system, amounting to 225 and 14.8 µg g−1 FW, respectively. A similar situation was observed for malonic acid in the system containing the addition of Ni. In the control system, it was also shown that an increase in soil moisture simultaneously caused an increase in the content of succinic acid in the shoots of the studied hybrid, amounting to 242, 1667, and 4663 µg g−1 FW for the LMC, MMC, and HMC systems, respectively. In the systems containing the addition of Ni ions, depending on soil moisture, there was a decrease in the content of citric acid (2145, 1862, and 675 µg g−1 FW for the LMC, MMC, and HMC systems, respectively), succinic acid (3037, 2089, and 1471 µg g−1 FW for the LMC, MMC, and HMC systems, respectively), and malonic acid (99.9, 40.0, and 71.4 µg g−1 FW for the LMC, MMC, and HMC systems, respectively). Thus, the active synthesis of these LMWOAs could significantly improve ROS detoxification capacity, osmotic regulation, membrane stability, and drought tolerance [49]. The comparative content of organic substances significantly depended on specific metabolites under given conditions. Among all identified LMWOAs, succinic, citric, and malic acids showed the most significant increases in response to changes in soil moisture. A similar relationship was observed in the system containing Cu. A significant decrease in the content of these LMWOAs in the shoots was demonstrated with an increase in soil moisture. Quinic acid was identified in the control system only for the MMC and HMC systems, and in the presence of Ni or Cu only for the HMC system. The observations were confirmed by correlation analysis, which showed a significant negative correlation between the citric acid content in shoots and soil moisture (R = −0.53) and a positive correlation between the quinic acid content in shoots and soil moisture (R = 0.64). At the same time, it was confirmed that the succinic acid content varied depending on soil moisture and the added metal (Table 4). Studies by Sazanova et al. [50], confirmed an increase in the succinic acid content in P. sylvestris shoots compared to the control, under the influence of stress resulting from increased Cd and Cu concentrations.
The same studies also showed that the presence of Ni did not significantly affect the accumulation of succinate in plants. Our experiment clearly demonstrated that changes in the content of succinic acid depended on soil moisture, and we also showed that the presence of Ni significantly increased its levels in poplar shoots. A common phenomenon characteristic of plants is the higher accumulation of short-chain LMWOAs in response to heavy metals [44,51]. Acids such as citrate, malate, oxalate, malonate, aconitate, and tartrate closely bind and thus detoxify heavy metal ions [43]. In the leaves of Amaranthus cruentus and A. caudatus, a Cd-induced increase in malate, citrate, and oxalate content was observed. The content of succinate increased only in A. cruentus and decreased in A. caudatus [44]. The results of the presented experiment show changes in the content of LMWOAs not only due to the presence of metals but also due to variations in soil moisture, which cause significant differences in plant acclimatization to the given conditions. The changes observed in the concentration of identified acids in P. nigra exposed to Cu and Ni, as well as the amount of water available to the plants, indicate that both factors significantly impact carbon metabolism in P. nigra L. × P. maximowiczii.
In the analyzed samples, phenolic acids with the C6C1 structure (vanillic and syringic acid) and with the C6C3 structure (chlorogenic, caffeic, p-coumaric, ferulic, and sinapic acid) were detected (Table 5).

Table 5.
The content of phenolic acids [μg g−1 FW] in stems of P. nigra × P. maximowiczii in experimental variants (LMC—low moisture content, MMC—medium moisture content, HMC—high moisture content).
The content of syringic, chlorogenic, caffeic, ferulic, and sinapic acids significantly increased in the control group in the HMC system. Ni addition in LMC significantly increased the content of all acids compared to almost all control levels, reaching the highest values for nearly all acids. In the case of the MMC system, the Ni-treatment increased only the syringic acid content compared to the control at the same moisture level. In the HMC system treated with Ni, a decrease in the content of all acids was observed except vanillic acid (Table 5). The content of chlorogenic, syringic, caffeic, p-coumaric, ferulic, and sinapic acids significantly increased in the MMC system under Cu addition. Whereas, under Cu and HMC treatment conditions, the content of p-coumaric acid increased, while the content of chlorogenic, caffeic, ferulic, and sinapic acids decreased. Phenolic compounds are considered resistance markers to various abiotic stresses [52,53]. Many plants respond to drought stress by increasing their phenolic compound content. The poplar hybrid reduced the content of most analyzed acids under soil moisture limitation, while Ni stress at LMC increased the content of most acids, and Cu stress increased the content of some acids in the MMC system.
The findings suggest that P. nigra L. × P. maximoviczii accumulates certain phenolic acids under Ni and Cu stress across varying soil moisture content, highlighting that soil moisture levels influence the response of the poplar hybrid to metal stress. The activation of defense mechanisms against metal stress varies accordingly. The enhancement of synthesis is attributed to the modification of the phenylpropanoid biosynthetic pathway, involving the regulation of key genes encoding the main enzymes [54,55,56,57].
For P. nigra L. × P. maximowiczii, significant correlations between Cu content and p-coumaric acid and Ni content and syringic acid were indicated. Negative correlations were also confirmed between some phenolic acids (chlorogenic, caffeic, vanillic, ferulic, and sinapic acids) and biomass, as well as shoot length under the influence of stress factors. The results indicate that P. nigra L. × P. maximowiczii increased the production of phenolic acids in stress response, which was one of its defense mechanisms, but at the same time, limited its growth and development, resulting in reduced shoot biomass and length.
Salicylic acid was determined in the stems of the tested hybrid as a measure of the plants’ response to the given stress factors (Cu or Ni) under different soil moisture systems during cultivation. The decrease in soil moisture induced increased salicylic acid biosynthesis, from 1.7 µg g−1 FW in the HMC system to 7.7 and 6.8 µg g−1 FW at lower moisture content (average for all systems). In contrast, the addition of Cu or Ni caused a significant decrease in the content of salicylic acid in stems compared to the control plants at each level of soil moisture (on average, from 7.3 µg g−1 FW for the control to 4.4 and 3.1 µg g−1 FW for Ni and Cu, respectively). As a result, the lowest content of this metabolite was observed with the addition of metals in the HMC system, while the largest decrease compared to the control was noted under MMC system conditions for Cu (Table 5). These observations were confirmed by correlation analysis, which showed a significant negative relationship between the level of salicylic acid in stems and soil moisture (R = −0.61). Furthermore, the increase in salicylic acid content correlated positively with the biomass of aboveground organs obtained under variable substrate moisture systems (R = 0.70 and 0.55, respectively, for the shoots’ biomass and length).
Salicylic acid belongs to the secondary metabolites of the hydroxybenzoic group and regulates the plant’s reaction to stressors [58,59]. Its concentration in plant tissue can be used as a biochemical marker of oxidative stress triggered by environmental abiotic stimuli [60,61,62]. Despite its well-recognized function among numerous plant species, the salicylic acid content in the stems of P. nigra L. × P. maximowiczii decreased following metal addition to the soil for each soil moisture system applied, and a reverse response was observed for the hybrid (biomass decrease did not trigger the induction of salicylic acid biosynthesis). This phenomenon can be attributed to the hybrid-specific response pattern aimed at activating other avoidance or detoxification mechanisms towards Cu and Ni.
3.4. Principal Component Analysis of Investigated Trails
Principal component analysis (PCA) revealed that the first two principal components together explained 65.3% of the total variance (Figure 3).
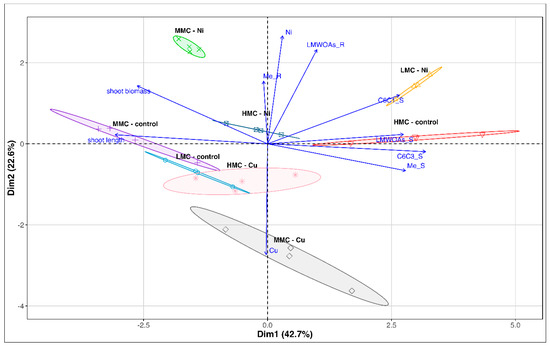
Figure 3.
Principal component analysis (PCA) of investigated parameters of roots (R) and stems (S) of P. nigra × P. maximowiczii in experimental variants (LMC—low moisture content, MMC—medium moisture content, HMC—high moisture content, Me—essential metals (Ca, Mg, K, Fe, Zn, Si), LMWOAs—low-molecular-weight organic acids, C6C1—benzoic acids, C6C3—simple phenylpropanoids).
The first dimension (Dim1, 42.7% of the variance) primarily differentiated samples based on biomass and shoot length, which negatively correlated with metabolite content and metal accumulation in shoots (C6C3_S, LMWOAs_S, Me_S). The second dimension (Dim2, 22.6% of the variance) reflected the influence of toxic metals (Ni, Cu) and separated plants based on metabolite presence and metal accumulation in roots (LMWOAs_R, Me_R).
The positioning of the LMC—control and MMC—control groups indicated higher biomass and shoot length, accompanied by lower overall metabolite levels. In contrast, the HMC—control group exhibited elevated metabolite levels and metal accumulation in shoots even without additional toxic metal exposure. This pattern suggested a potential effect of elevated soil moisture on plant metabolism (Figure 3). These observations were confirmed with correlation analysis for categorized trails (Figure 4).
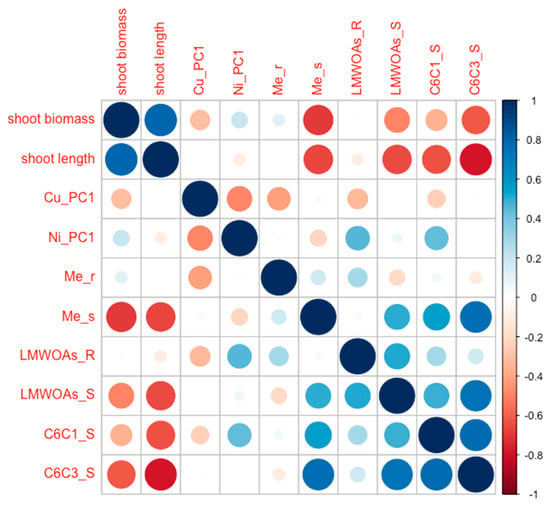
Figure 4.
Correlation coefficient values for PCA analysis (R—roots, S—stems of P. nigra × P. maximowiczii in experimental variants; Me—essential metals (Ca, Mg, K, Fe, Zn, Si), LMWOAs—low-molecular-weight organic acids, C6C1—benzoic acids, C6C3—simple phenylpropanoids).
The clustering of Ni-exposed plants (LMC—Ni, MMC—Ni, HMC—Ni) reflected increased metabolite synthesis and metal accumulation in roots as a response to Ni-induced stress. These plants exhibited enhanced Ni accumulation, confirming their ability to metal uptake. Moreover, HMC—Ni and MMC—Ni plants displayed a stronger increase in metabolite production and metal accumulation in roots compared to the LMC—Ni variant. The elevated phenols accumulation, particularly of the C6C1 group, was observed in the poplar shoots (Figure 3 and Figure 4).
The Cu-exposed plants (in both MMC and HMC) showed distinct metabolic responses compared to plants exposed to Ni, with a concurrent increase in Cu accumulation. This may result from a different mechanism of Cu toxicity, triggering a diverse profile of defense-related metabolites, compared to Ni. Unlike Ni, Cu appeared to exert its toxic effects without prompting secondary metabolite production (Figure 3 and Figure 4).
4. Conclusions
The conducted studies have shown that the adaptive capacity of the P. nigra L. × P. maximowiczii hybrid to the presence of heavy metals, such as Cu and Ni, depends to a significant extent on the level of soil moisture. The moisture content of the substrate affected both the content of organic and phenolic acids in the roots and shoots of the plants, and metal supplementation modulated their levels in different ways, depending on the compound and organ. For example, medium soil moisture promoted salicylic acid accumulation in the stems, while increased moisture promoted the biosynthesis of succinic and citric acids in the roots. In turn, the presence of Cu and Ni reduced the content of these acids in the shoots. The obtained results indicate a significant role of the interaction of stress factors—soil moisture and the presence of heavy metals—in shaping the metabolic response of plants.
However, it should be emphasized that the experiment was short-term and was conducted in greenhouse conditions in pots. Therefore, the observed reactions cannot directly relate to long-term environmental conditions. Further studies, covering extended periods and field conditions, are necessary to fully understand the mechanisms of physiological response and the potential use of poplar in phytoremediation activities of soils contaminated with heavy metals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17114989/s1, Table S1: The content of mineral nutrients in roots of P. nigra × P. maximowiczii in experimental variants; Table S2: The content of mineral nutrients in stems of P. nigra × P. maximowiczii in experimental variants.
Author Contributions
Conceptualization, investigation, formal analysis, methodology, visualization, supervision, writing—original draft, writing—review and editing, funding acquisition, Z.M.; investigation, formal analysis, methodology, writing—original draft, writing—review and editing, M.G.; statistical analysis, visualization, formal analysis, writing—original draft, writing—review and editing, K.D.; methodology, writing—review and editing, A.I.; statistical analysis, visualization, M.R.; methodology, P.N. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Science Center, Poland: decision no. DEC-2021/05/X/NZ9/00299.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors express their sincere appreciation to Włodzimierz Krzesiński for providing access to the greenhouse at the Poznań University of Life Sciences, Department of Vegetable Crops, Poznań University of Life Sciences, Dąbrowskiego 159, 60-594 Poznan, Poland.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kożuchowski, K.M. Stowarzyszenie Klimatologów Polskich. Opady Atmosferyczne w Polsce. 2015. Available online: https://klimatolodzy.pl/images/attachements/Kozuchowski_opady.pdf (accessed on 31 March 2025).
- Kundzewicz, Z.W.; Piniewski, M.; Mezghani, A.; Okruszko, T.; Pińskwar, I.; Kardel, I.; Hov, Ø.; Szcześniak, M.; Szwed, M.; Benestad, R.E.; et al. Assessment of Climate Change and Associated Impact on Selected Sectors in Poland. Acta Geophys. 2018, 66, 1509–1523. [Google Scholar] [CrossRef]
- Pińskwar, I.; Choryński, A.; Graczyk, D.; Kundzewicz, Z.W. Observed Changes in Extreme Precipitation in Po-land: 1991–2015 versus 1961–1990. Theor. Appl. Clim. 2019, 135, 773–787. [Google Scholar] [CrossRef]
- Raczyński, K.; Dyer, J. Changes in Streamflow Drought and Flood Distribution over Poland Using Trend De-composition. Acta Geophys. 2024, 72, 2773–2794. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, B.; Li, H.; Huang, J.; Jiang, L.; Zhang, X.; Tan, Z.; Wu, Z.; Qin, X.; Feng, C.; et al. Soil Heavy Metals and Phytoremediation by Populus Deltoides Alter the Structure and Function of Bacterial Community in Mine Ecosystems. Appl. Soil. Ecol. 2022, 172, 104359. [Google Scholar] [CrossRef]
- Tuskan, G.A.; Walsh, M.E. Short-Rotation Woody Crop Systems, Atmospheric Carbon Dioxide and Carbon Management: A U.S. Case Study. For. Chron. 2011, 77, 259–264. [Google Scholar] [CrossRef]
- Tschaplinski, T.J.; Abraham, P.E.; Jawdy, S.S.; Gunter, L.E.; Martin, M.Z.; Engle, N.L.; Yang, X.; Tuskan, G.A. The Nature of the Progression of Drought Stress Drives Differential Metabolomic Responses in Populus Deltoides. Ann. Bot. 2019, 124, 617–626. [Google Scholar] [CrossRef]
- Zalesny, R.S.; Wiese, A.H.; Bauer, E.O.; Riemenschneider, D.E. Sapflow of Hybrid Poplar (Populus Nigra L.×P. Maximowiczii A. Henry ‘NM6’) during Phytoremediation of Landfill Leachate. Biomass Bioenergy 2006, 30, 784–793. [Google Scholar] [CrossRef]
- Guerra, F.; Duplessis, S.; Kohler, A.; Martin, F.; Tapia, J.; Lebed, P.; Zamudio, F.; González, E. Gene Expression Analysis of Populus Deltoides Roots Subjected to Copper Stress. Environ. Exp. Bot. 2009, 67, 335–344. [Google Scholar] [CrossRef]
- Solti, Á.; Sárvári, É.; Szöllosi, E.; Tóth, B.; Mészáros, I.; Fodor, F.; Szigeti, Z. Stress Hardening under Long-Term Cadmium Treatment Is Correlated with the Activation of Antioxidative Defence and Iron Acquisition of Chloro-plasts in Populus. Z. Naturforsch. Sect. C J. Biosci. 2016, 71, 323–334. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; de Jonge, M.D.; Mckenna, B.A.; Donner, E.; Webb, R.I.; Paterson, D.J.; Howard, D.L.; Ryan, C.G.; Glover, C.J.; et al. In Situ Distribution and Speciation of Toxic Copper, Nickel, and Zinc in Hydrated Roots of Cowpea. Plant Physiol. 2011, 156, 663–673. [Google Scholar] [CrossRef]
- Panchal, P.; Miller, A.J.; Giri, J. Organic Acids: Versatile Stress-Response Roles in Plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Cichoński, J.; Michalik, P.; Chrzanowski, G. Effect of Heavy Metal Stress on Phenolic Compounds Accumulation in Winter Wheat Plants. Molecules 2022, 28, 241. [Google Scholar] [CrossRef]
- European Council. Council Directive 1999/105/EC of 22 December 1999 on the Marketing of Forest Reproductive Material. Off. J. Eur. Commun. 2000, L11, 17–40. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; pp. 1–236. [Google Scholar]
- Novák, V.; Havrila, J. Method to Estimate the Critical Soil Water Content of Limited Availability for Plants. Biologia 2006, 61, S289–S293. [Google Scholar] [CrossRef]
- Trzecki, S. Możliwość wyznaczania wilgotności trwałego więdnięcia roślin na podstawie maksymalnej higroskopijności i zawartości części spławialnych w glebach mineralnych. Rocz. Glebozn. 1976, 27, 11–18. [Google Scholar]
- Rzasa, S.; Owczarzak, W.; Spychalski, W. Methodological Advances Used to Analyse Maximal Hygroscopic Water in Soils of Different Structure. Int. Agrophys. 1993, 7, 213–220. [Google Scholar]
- Magdziak, Z.; Gąsecka, M.; Budka, A.; Goliński, P.; Mleczek, M. Profile and Concentration of the Low Molecular Weight Organic Acids and Phenolic Compounds Created by Two-Year-Old Acer Platanoides Seedlings Growing under Different As Forms. J. Hazard. Mater. 2020, 392, 122280. [Google Scholar] [CrossRef]
- Baziramakenga, R.; Simard, R.R.; Leroux, G.D. Determination of Organic Acids in Soil Extracts by Ion Chroma-tography. Soil. Biol. Biochem. 1995, 27, 349–356. [Google Scholar] [CrossRef]
- Gąsecka, M.; Krzymińska-Bródka, A.; Magdziak, Z.; Czuchaj, P.; Bykowska, J. Phenolic Compounds and Organic Acid Composition of Syringa Vulgaris L. Flowers Infusions. Molecules 2023, 28, 5159. [Google Scholar] [CrossRef]
- Raskin, I.; Turner, I.M.; Melander, W.R. Regulation of Heat Production in the Inflorescences of an Arum Lily by Endogenous Salicylic Acid. Proc. Natl. Acad. Sci. USA 1989, 86, 2214–2218. [Google Scholar] [CrossRef]
- Baker, A.J.M. Accumulators and Excluders-strategies in the Response of Plants to Heavy Metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A Fern That Hyperaccumulates Arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecka, K.; Gawrysiak, P.; Woźniak, M.; Rybak, M. Metal Accumulation and Tolerance of Energy Willow to Copper and Nickel under Simulated Drought Conditions. Sustainability 2023, 15, 13084. [Google Scholar] [CrossRef]
- Kassambara, A. Ggpubr “Ggplot2” Based Publication Ready Plots. R Package Version 0.4.0. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 31 March 2025).
- Li, Z.; Wang, X.; Liu, Y.; Zhou, Y.; Qian, Z.; Yu, Z.; Wu, N.; Bian, Z. Water Uptake and Hormone Modulation Responses to Nitrogen Supply in Populus Simonii under PEG-Induced Drought Stress. Forests 2022, 13, 907. [Google Scholar] [CrossRef]
- Kulczyk-Skrzeszewska, M.; Kieliszewska-Rokicka, B. Influence of Drought and Salt Stress on the Growth of Young Populus nigra ‘Italica’ Plants and Associated Mycorrhizal Fungi and Non-Mycorrhizal Fungal Endo-phytes. New For. 2022, 53, 679–694. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, F.; Wang, Y.; Ding, Z.; Yang, X.; Zhu, Z. Differences in Uptake and Accumulation of Copper and Zinc by Salix Clones under Flooded versus Non-Flooded Conditions. Chemosphere 2020, 241, 125059. [Google Scholar] [CrossRef]
- Clemens, S. Toxic Metal Accumulation, Responses to Exposure and Mechanisms of Tolerance in Plants. Bio Chim. 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Trudić, B.; Kebert, M.; Popović, B.M.; Štajner, D.; Orlović, S.; Galović, V.; Pilipović, A. The Effect of Heavy Metal Pollution in Soil on Serbian Poplar Clones. Sumar. List 2013, 137, 287–295. [Google Scholar]
- Kacálková, L.; Tlustoš, P.; Száková, J. Phytoextraction of Risk Elements by Willow and Poplar Trees. Int. J. Phytore Mediat. 2015, 17, 414–421. [Google Scholar] [CrossRef]
- Kacálková, L.; Tlustos, P.; Szakova, J. Chromium, Nickel, Cadmium, and Lead Accumulation in Maize, Sunflower, Willow, and Poplar. Pol. J. Environ. Stud. 2014, 23, 753–761. [Google Scholar]
- Migeon, A.; Richaud, P.; Guinet, F.; Blaudez, D.; Chalo, M. Hydroponic Screening of Poplar for Trace Element Tolerance and Accumulation. Int. J. Phytoremediat. 2012, 14, 350–361. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, A.; Courbet, G.; Billiot, B.; Jing, L.; Pluchon, S.; Arkoun, M.; Maillard, A.; Roux, C.P.L.; Trouverie, J.; Etienne, P.; et al. Drought Specifically Downregulates Mineral Nutrition: Plant Ionomic Content and Associated Gene Expression. Plant Direct 2022, 6, e402. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, L.; Huang, X.; Zou, Z.; Zhang, M.; Guo, W.; Addo-Danso, S.D.; Zhou, L. Mineral Nutrient Uptake, Accumulation, and Distribution in Cunninghamia Lanceolata in Response to Drought Stress. Plants 2023, 12, 2140. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhou, J.; Li, J.; Ma, C.; Zhang, Y.; Deng, S.; Yu, W.; Luo, Z. Bin Lead Exposure-Induced Defense Responses Result in Low Lead Translocation from the Roots to Aerial Tissues of Two Contrasting Poplar Species. Environ. Ment. Pollut. 2021, 271, 116346. [Google Scholar] [CrossRef]
- Shi, W.; Li, J.; Kan, D.; Yu, W.; Chen, X.; Zhang, Y.; Ma, C.; Deng, S.; Zhou, J.; Fayyaz, P.; et al. Sulfur Metabolism, Organic Acid Accumulation and Phytohormone Regulation Are Crucial Physiological Processes Modulating the Different Tolerance to Pb Stress of Two Contrasting Poplars. Tree Physiol. 2022, 42, 1799–1811. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail; Shahid, M.A.; Babar, A. Role of Sugars, Amino Acids and Organic Acids in Improving Plant Abiotic Stress Tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, C.; Chen, H.; Zhang, J.; White, J.C.; Chen, G.; Xing, B. Xylem-Based Long-Distance Transport and Phloem Remobilization of Copper in Salix Integra Thunb. J. Hazard. Mater. 2020, 392, 122428. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Tian, S.K.; Yang, X.E.; Wang, X.C.; Brown, P.; Li, T.Q.; He, Z.L. Enhanced Root-to-Shoot Translocation of Cadmium in the Hyperaccumulating Ecotype of Sedum Alfredii. J. Exp. Bot. 2008, 59, 3203–3213. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yu, H.; Li, T.; Wu, Y. Effect of Cadmium Stress on Inorganic and Organic Components in Xylem Sap of High Cadmium Accumulating Rice Line (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2019, 168, 330–337. [Google Scholar] [CrossRef]
- Anjum, N.A.; Hasanuzzaman, M.; Hossain, M.A.; Thangavel, P.; Roychoudhury, A.; Gill, S.S.; Merlos Rodrigo, M.A.; Adam, V.; Fujita, M.; Kizek, R.; et al. Jacks of Metal/Metalloid Chelation Trade in Plants—An Overview. Front. Plant Sci. 2015, 6, 192. [Google Scholar] [CrossRef]
- Osmolovskaya, N.; Dung, V.V.; Kuchaeva, L. The Role of Organic Acids in Heavy Metal Tolerance in Plants. Biol. Commun. 2018, 63, 9–16. [Google Scholar] [CrossRef]
- Hou, X.; Han, H.; Cai, L.; Liu, A.; Ma, X.; Zhou, C.; Wang, G.; Meng, F. Pb Stress Effects on Leaf Chlorophyll Fluo-rescence, Antioxidative Enzyme Activities, and Organic Acid Contents of Pogonatherum Crinitum Seedlings. Flora 2018, 240, 82–88. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Gąsecka, M.; Magdziak, Z.; Rybak, M.; Budzyńska, S.; Rutkowski, P.; Niedzielski, P.; Mleczek, M. Drought Differently Modifies Tolerance and Metal Uptake in Zn- or Cu-Treated Male and Female Salix × Fragilis L. Forests 2024, 15, 562. [Google Scholar] [CrossRef]
- López-Bucio, J.; Nieto-Jacobo, M.F.; Ramírez-Rodríguez, V.; Herrera-Estrella, L. Organic Acid Metabolism in Plants: From Adaptive Physiology to Transgenic Varieties for Cultivation in Extreme Soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Ma, W.; Gao, S.; Jin, Z.; Yue, Q.; Yao, Y. Transcriptomic and Phosphoproteomic Profiling and Metabo-lite Analyses Reveal the Mechanism of NaHCO3-Induced Organic Acid Secretion in Grapevine Roots. BMC Plant Biol. 2019, 19, 389. [Google Scholar] [CrossRef]
- Guo, X.; Xin, Z.; Yang, T.; Ma, X.; Zhang, Y.; Wang, Z.; Ren, Y.; Lin, T. Metabolomics Response for Drought Stress Tolerance in Chinese Wheat Genotypes (Triticum Aestivum). Plants 2020, 9, 520. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Alekseeva-Popova, N.V.; Drozdova, I.V.; Belyaeva, A.I.; Kalimova, I.B.; Pavlova, N.I.; Shavarda, A.L. Effects of Heavy Metals on the Metabolome of Pinus sylvestris (Pinaceae). Dokl. Biol. Sci. 2022, 507, 364–372. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, L.; Du, Z.; Sun, X.; Amombo, E.; Fan, J.; Fu, J. Effects of Cadmium Exposure on Growth and Metabolic Profile of Bermudagrass [Cynodon dactylon (L.) Pers.]. PloS ONE 2014, 29, e115279. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Effects of Drought Stress on Phenolic Accumulation in Greenhouse-Grown Olive Trees (Olea europaea). Biochem. Syst. Ecol. 2020, 92, 104112. [Google Scholar] [CrossRef]
- Varela, M.C.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic Compounds as Indicators of Drought Resistance in Shrubs from Patagonian Shrublands (Argentina). Plant Physiol. Biochem. 2016, 104, 81–91. [Google Scholar] [CrossRef]
- Mao, X.F.; Xu, X.B.; Chen, L.L. Effects of Heavy Metal PB and CD Stress on Physiological Characteristics of Japa-nese Honeysuckle. Appl. Ecol. Environ. Res. 2019, 17, 6415–6427. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Mishra, B.; Sangwan, R.S.; Mishra, S.; Jadaun, J.S.; Sabir, F.; Sangwan, N.S. Effect of Cadmium Stress on Inductive Enzymatic and Nonenzymatic Responses of ROS and Sugar Metabolism in Multiple Shoot Cultures of Ashwa-gandha (Withania Somnifera Dunal). Protoplasma 2014, 251, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Q.; Lu, H.; Li, J.; Yang, D.; Liu, J.; Yan, C. Phenolic Metabolism and Related Heavy Metal Toler-ance Mechanism in Kandelia Obovata under Cd and Zn Stress. Ecotoxicol. Environ. Saf. 2019, 169, 134–143. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic Acid to Decrease Plant Stress. Environ. Chem. Lett. 2016, 15, 101–123. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Borowiak, K.; Bandurska, H.; Golinski, P. Salicylic Acid—A Potential Biomarker of Tobacco Bel-W3 Cell Death Developed as a Response to Ground Level Ozone under Ambient Conditions. Acta Biol. Hung. 2012, 63, 231–249. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Mleczek, M.; Gąsecka, M.; Magdziak, Z.; Budka, A.; Chadzinikolau, T.; Kaczmarek, Z.; Goliński, P. Copper and Nickel Co-Treatment Alters Metal Uptake and Stress Parameters of Salix Purpurea × Viminalis. J. Plant Physiol. 2017, 216, 125–134. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Mleczek, M. Salicylic Acid Accumulation as a Result of Cu, Zn, Cd and Pb Interactions in Com-mon Reed (Phragmites australis) Growing in Natural Ecosystems. Acta Physiol. Plant 2017, 39, 182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).