Remediation of Polycyclic Aromatic Hydrocarbon-Contaminated Soil Using Microwave-Activated Persulfate Oxidation System
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Soil Samples
2.3. Experimental Procedure
2.4. Analytical Methods
3. Results and Discussion
3.1. MW/PS Performance Under Different Operational Conditions
3.2. MW/PS Performance Associated with Soil Characteristics
3.3. Comparison of MW/PS and TH/PS Systems
3.4. ROS Generated During Activation
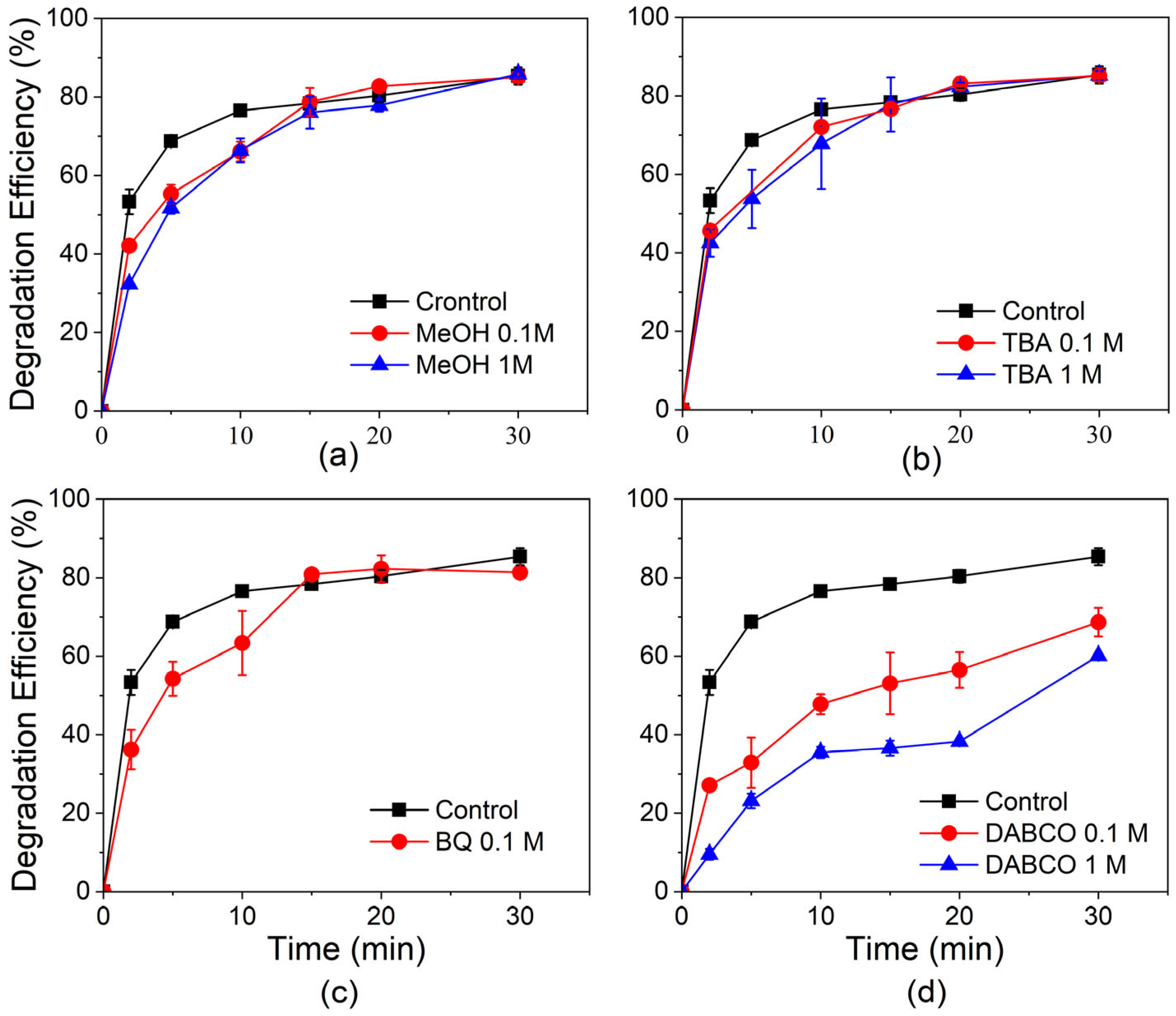
3.5. ROS Validation Using Quenching Experiments
3.6. Oxidation Mechanisms and Degradation Pathways
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Components | Content (%) | Components | Content (%) |
|---|---|---|---|
| Na2O | 0.978 | MnO | 0.233 |
| MgO | 1.122 | Fe2O2 | 14.929 |
| Al2O3 | 11.286 | ZnO | 0.096 |
| SiO2 | 53.300 | Rb2O | 0.068 |
| P2O5 | 0.244 | SrO | 0.089 |
| SO3 | 0.141 | ZrO2 | 0.164 |
| K2O | 2.329 | BaO | 0.127 |
| CaO | 1.811 | PbO | 0.052 |
| TiO2 | 1.459 | L.O.I. | 11.57 |
| Experiment | Conditions | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| qe (μg/g) | k1 (min−1) | R2 | qe (mg/g) | k2 (mg/g·min) | R2 | ||
| Power | 200 W | 73.2138 | 0.3243 | 0.9613 | 82.1934 | 0.0055 | 0.9894 |
| 300 W | 79.2942 | 0.2699 | 0.9219 | 83.3949 | 0.0079 | 0.9908 | |
| 400 W | 79.8311 | 0.4588 | 0.9826 | 86.5131 | 0.0091 | 0.9980 | |
| 600 W | 84.6555 | 0.4004 | 0.9533 | 86.4155 | 0.0106 | 0.9959 | |
| Temperature | 80 °C | 79.6741 | 0.5035 | 0.9844 | 86.5125 | 0.0091 | 0.9980 |
| 60 °C | 65.6981 | 0.1054 | 0.9745 | 89.0150 | 0.0010 | 0.9655 | |
| 40 °C | 62.1284 | 0.0704 | 0.9993 | 89.7173 | 0.0006 | 0.9822 | |
| Persulfate | 11.9 mg/g | 81.1028 | 1.1611 | 0.9913 | 83.3576 | 0.0400 | 0.9960 |
| 23.9 mg/g | 79.6741 | 0.5035 | 0.9844 | 86.5125 | 0.0091 | 0.9980 | |
| 47.6 mg/g | 78.6616 | 0.3516 | 0.9825 | 87.5311 | 0.0058 | 0.9941 | |
| Initial concentration | 50 μg/g | 46.9870 | 0.6853 | 0.9951 | 49.5859 | 0.0134 | 0.9994 |
| 100 μg/g | 76.6741 | 0.5035 | 0.9844 | 86.5125 | 0.0091 | 0.9980 | |
| 200 μg/g | 150.306 | 0.2941 | 0.9451 | 173.351 | 0.0045 | 0.9794 | |
| No. | Intermediates | Chemical Structure |
|---|---|---|
| 1 | Phthalic acid |  |
| 2 | p-Hydroxybenzaldehyde |  |
| 3 | 4-Hydroxy-3-methoxybenzaldehyde |  |
| 4 | 3-Hydroxy-4-methoxybenzoic acid |  |
| 5 | Phenanthrene |  |
| 6 | Dibutyl phthalate |  |
| 7 | 4H-Cyclopenta[def]-phenanthridin-4-one |  |
Appendix B





References
- Gan, S.; Lau, E.V.; Ng, H.K. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.; Rocha, B.A.; Souza, M.C.O.; Bocato, M.Z.; Azevedo, L.F.; Adeyemi, J.A.; Santana, A.; Campiglia, A.D. Polycyclic aromatic hydrocarbons (PAHs): Updated aspects of their determination, kinetics in the human body, and toxicity. J. Toxicol. Environ. Health Part B Crit. Rev. 2023, 26, 28–65. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Li, T.J.; Liu, Y.; Ma, L.M. Spatial distribution of polycyclic aromatic hydrocarbon contamination in urban soil of China. Chemosphere 2019, 230, 498–509. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Yan, K.; Yuan, Z.; Feng, D.; Wang, H.; Wu, L.; Xu, J. Polycyclic aromatic hydrocarbons (PAHs) pollution and risk assessment of soils at contaminated sites in China over the past two decades. J. Clean. Prod. 2024, 450, 141876. [Google Scholar] [CrossRef]
- Kan, H.; Wu, D.; Wang, T.; Qu, G.; Zhang, P.; Jia, H.; Sun, H. Crystallographic manganese oxides enhanced pyrene contaminated soil remediation in microwave activated persulfate system. Chem. Eng. J. 2021, 417, 127916. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R.; Megharaj, M. Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef]
- Li, S.; Tang, J.C.; Yu, C.; Liu, Q.L.; Wang, L. Efficient degradation of anthracene in soil by carbon-coated nZVI activated persulfate. J. Hazard. Mater. 2022, 431, 128581. [Google Scholar] [CrossRef]
- Gou, Y.L.; Zhao, Q.Y.; Yang, S.C.; Qiao, P.W.; Cheng, Y.J.; Song, Y.; Sun, Z.P.; Zhang, T.F.; Wang, L.; Liu, Z.H. Enhanced degradation of polycyclic aromatic hydrocarbons in aged subsurface soil using integrated persulfate oxidation and anoxic biodegradation. Chem. Eng. J. 2020, 394, 125040. [Google Scholar] [CrossRef]
- Guo, J.Y.; Wen, X.Y.; Yang, J.W.; Fan, T. Removal of benzo(a)pyrene in polluted aqueous solution and soil using persulfate activated by corn straw biochar. J. Environ. Manag. 2020, 272, 111058. [Google Scholar] [CrossRef]
- Fagan, W.P.; Zhao, J.; Villamena, F.A.; Zweier, J.L.; Weavers, L.K. Synergistic, aqueous PAH degradation by ultrasonically-activated persulfate depends on bulk temperature and physicochemical parameters. Ultrason. Sonochem. 2020, 67, 105172. [Google Scholar] [CrossRef]
- García-Cervilla, R.; Santos, A.; Romero, A.; Lorenzo, D. Remediation of soil contaminated by lindane wastes using alkaline activated persulfate: Kinetic model. Chem. Eng. J. 2020, 393, 124646. [Google Scholar] [CrossRef]
- Jiang, C.L.; Yang, Y.; Zhang, L.; Lu, D.; Lu, L.L.; Yang, X.X.; Cai, T.M. Degradation of Atrazine, Simazine and Ametryn in an arable soil using thermal-activated persulfate oxidation process: Optimization, kinetics, and degradation pathway. J. Hazard. Mater. 2020, 400, 123201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Tian, H.F.; Wang, Z.X.; Zhu, L.F.; Liu, X.S.; Wang, Y.; Sun, Y.F. Degradation of PAHs in soil by activated persulfate system with activated carbon supported iron-based bimetal. Sci. Total Environ. 2023, 866, 161323. [Google Scholar] [CrossRef]
- Zhao, D.; Liao, X.Y.; Yan, X.L.; Huling, S.G.; Chai, T.Y.; Tao, H. Effect and mechanism of persulfate activated by different methods for PAHs removal in soil. J. Hazard. Mater. 2013, 254, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, C.W.; Li, Q.B. Treatment of refractory organics in strongly alkaline dinitrodiazophenol wastewater with microwave irradiation-activated persulfate. Chemosphere 2020, 254, 126773. [Google Scholar] [CrossRef]
- Kan, H.S.; Wang, T.C.; Yu, J.X.; Qu, G.Z.; Zhang, P.; Jia, H.Z.; Sun, H.W. Remediation of organophosphorus pesticide polluted soil using persulfate oxidation activated by microwave. J. Hazard. Mater. 2021, 401, 123361. [Google Scholar] [CrossRef]
- Feng, Y.; Tao, Y.; Meng, Q.Q.; Qu, J.H.; Ma, S.Y.; Han, S.Y.; Zhang, Y. Microwave-combined advanced oxidation for organic pollutants in the environmental remediation: An overview of influence, mechanism, and prospective. Chem. Eng. J. 2022, 441, 135924. [Google Scholar] [CrossRef]
- Kostas, E.T.; Beneroso, D.; Robinson, J.P. The application of microwave heating in bioenergy: A review on the microwave pre-treatment and upgrading technologies for biomass. Renew. Sustain. Energy Rev. 2017, 77, 12–27. [Google Scholar] [CrossRef]
- Peng, L.B.; Deng, D.Y.; Ye, F.T. Efficient oxidation of high levels of soil-sorbed phenanthrene by microwave-activated persulfate: Implication for in situ subsurface remediation engineering. J. Soils Sediments 2016, 16, 28–37. [Google Scholar] [CrossRef]
- Qu, J.H.; Liu, R.X.; Bi, X.W.; Li, Z.R.; Li, K.G.; Hu, Q.; Zhang, X.R.; Zhang, G.S.; Ma, S.Y.; Zhang, Y. Remediation of atrazine contaminated soil by microwave activated persulfate system: Performance, mechanism and DFT calculation. J. Clean. Prod. 2023, 399, 136546. [Google Scholar] [CrossRef]
- Wu, D.; Kan, H.S.; Zhang, Y.; Wang, T.C.; Qu, G.Z.; Zhang, P.; Jia, H.Z.; Sun, H.W. Pyrene contaminated soil remediation using microwave/magnetite activated persulfate oxidation. Chemosphere 2022, 286, 131787. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.F.; Li, C.Q.; Wang, Z.; Zhao, S.; Xu, Y.X.; Wang, S.G. Polycyclic aromatic hydrocarbons degradation mechanisms in methods using activated persulfate: Radical and non-radical pathways. Chem. Eng. J. 2023, 473, 145319. [Google Scholar] [CrossRef]
- Miao, D.; Zhao, S.; Zhu, K.C.; Zhang, P.; Wang, T.C.; Jia, H.Z.; Sun, H.W. Activation of persulfate and removal of ethyl-parathion from soil: Effect of microwave irradiation. Chemosphere 2020, 253, 126679. [Google Scholar] [CrossRef]
- Shang, X.; Liu, X.T.; Ma, X.Y.; Zhang, Z.G.; Lin, C.Y.; He, M.C.; Ouyang, W. Efficient degradation of chlorpyrifos and intermediate in soil by a novel microwave induced advanced oxidation process: A two-stage reaction. J. Hazard. Mater. 2024, 464, 133001. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Palanisami, T.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. In-Situ Remediation Approaches for the Management of Contaminated Sites: A Comprehensive Overview. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 236, pp. 1–115. [Google Scholar]
- Chen, L.W.; Hu, X.X.; Cai, T.M.; Yang, Y.; Zhao, R.D.; Liu, C.; Li, A.Y.; Jiang, C.L. Degradation of Triclosan in soils by thermally activated persulfate under conditions representative of in situ chemical oxidation (ISCO). Chem. Eng. J. 2019, 369, 344–352. [Google Scholar] [CrossRef]
- Zhu, S.S.; Li, X.J.; Kang, J.; Duan, X.G.; Wang, S.B. Persulfate activation on crystallographic manganese oxides: Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants. Environ. Sci. Technol. 2019, 53, 307–315. [Google Scholar] [CrossRef]
- Wu, L.; Sun, R.; Li, Y.X.; Sun, C.J. Sample preparation and analytical methods for polycyclic aromatic hydrocarbons in sediment. Trends Environ. Anal. Chem. 2019, 24, e00074. [Google Scholar] [CrossRef]
- Tang, M.D.; Zhang, Y.G. Enhancing the activation of persulfate using nitrogen-doped carbon materials in the electric field for the effective removal of p-nitrophenol. RSC Adv. 2021, 11, 38003–38015. [Google Scholar] [CrossRef]
- Kang, Y.G.; Yoon, H.; Lee, C.S.; Kim, E.J.; Chang, Y.S. Advanced oxidation and adsorptive bubble separation of dyes using MnO2-coated Fe3O4 nanocomposite. Water Res. 2019, 151, 413–422. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Scandura, P.; Vagliasindi, F.G.A. Modelling of in situ microwave heating of hydrocarbon-polluted soils: Influence of soil properties and operating conditions on electric field variation and temperature profiles. J. Geochem. Explor. 2017, 174, 91–99. [Google Scholar] [CrossRef]
- Falciglia, P.P.; De Guidi, G.; Catalfo, A.; Vagliasindi, F.G.A. Remediation of soils contaminated with PAHs and nitro-PAHs using microwave irradiation. Chem. Eng. J. 2016, 296, 162–172. [Google Scholar] [CrossRef]
- Zhu, C.; Zhu, F.; Liu, C.; Chen, N.; Zhou, D.; Fang, G.; Gao, J. Reductive Hexachloroethane Degradation by S2O8•– with Thermal Activation of Persulfate under Anaerobic Conditions. Environ. Sci. Technol. 2018, 52, 8548–8557. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-Y.; Hou, Y.-Z.; Lin, Y.-L.; Deng, Y.-G.; Hua, S.-J.; Du, Y.-F.; Chen, C.-W.; Wu, C.-H. Investigation of iohexol degradation kinetics by using heat-activated persulfate. Chem. Eng. J. 2020, 379, 122403. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Wu, Y.; Chen, H.; Shi, Y.; Liu, M.; Dong, W. Degradation of ibuprofen by thermally activated persulfate in soil systems. Chem. Eng. J. 2019, 356, 799–810. [Google Scholar] [CrossRef]
- Sahoo, B.M.; Panda, J.; Banik, B.K. Thermal and non-thermal effects of microwaves in synthesis. J. Indian Chem. Soc. 2018, 95, 1311–1319. [Google Scholar] [CrossRef]
- Hu, Y.N.; He, Y.Z.; Cheng, H.F. Microwave-induced degradation of N-nitrosodimethylamine (NDMA) sorbed in zeolites: Effect of mineral surface chemistry and non-thermal effect of microwave. J. Clean. Prod. 2018, 174, 1224–1233. [Google Scholar] [CrossRef]
- Remya, N.; Lin, J.G. Current status of microwave application in wastewater treatment-A review. Chem. Eng. J. 2011, 166, 797–813. [Google Scholar] [CrossRef]
- Qi, C.D.; Liu, X.T.; Zhao, W.; Lin, C.Y.; Ma, J.; Shi, W.X.; Sun, Q.; Xiao, H. Degradation and dechlorination of pentachlorophenol by microwave-activated persulfate. Environ. Sci. Pollut. Res. 2015, 22, 4670–4679. [Google Scholar] [CrossRef]
- Zhao, L.J.; Yang, S.Y.; Wang, L.L.; Shi, C.; Huo, M.Q.; Li, Y. Rapid and simple spectrophotometric determination of persulfate in water by microwave assisted decolorization of Methylene Blue. J. Environ. Sci. 2015, 31, 235–239. [Google Scholar] [CrossRef]
- Chen, L.; Duan, J.; Du, P.H.; Sun, W.L.; Lai, B.; Liu, W. Accurate identification of radicals by in-situ electron paramagnetic resonance in ultraviolet-based homogenous advanced oxidation processes. Water Res. 2022, 221, 118747. [Google Scholar] [CrossRef]
- Liang, C.J.; Su, H.W. Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar] [CrossRef]
- Yu, J.F.; Tang, L.; Pang, Y.; Zeng, G.M.; Feng, H.P.; Zou, J.J.; Wang, J.J.; Feng, C.Y.; Zhu, X.; Ouyang, X.L.; et al. Hierarchical porous biochar from shrimp shell for persulfate activation: A two-electron transfer path and key impact factors. Appl. Catal. B 2020, 260, 118160. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; He, S.Y.; Wu, S.H.; Yang, C.P. Singlet oxygen: Properties, generation, detection, and environmental applications. J. Hazard. Mater. 2024, 461, 132538. [Google Scholar] [CrossRef]
- Aubry, J.-M.; Bouttemy, S. Preparative oxidation of organic compounds in microemulsions with singlet oxygen generated chemically by the sodium molybdate/hydrogen peroxide system1. J. Am. Chem. Soc. 1997, 119, 5286–5294. [Google Scholar] [CrossRef]
- Wan, D.; Li, W.B.; Wang, G.H.; Lu, L.L.; Wei, X.B. Degradation of p-Nitrophenol using magnetic Fe0<Fe3O4<Coke composite as a heterogeneous Fenton-like catalyst. Sci. Total Environ. 2017, 574, 1326–1334. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, K.; Zhao, H. The debatable role of singlet oxygen in persulfate-based advanced oxidation processes. Water Res. 2023, 235, 119925. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, K.; Chen, T.; Xiong, Y.; Li, Y.; Addisu, A.; Pillai, S.C.; Dionysiou, D.D.; Wang, D. Regulating the generation of singlet oxygen (1O2) in Advanced oxidation processes by catalyst design for water treatment. Chem. Eng. J. 2024, 500, 156532. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.G.; Zhang, Y.L.; Wu, X.; Liu, Y. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Qiu, R.; Zhang, P.; Feng, G.; Ni, X.; Miao, Z.; Wei, L.; Sun, H. Enhanced thermal activation of persulfate by coupling hydrogen peroxide for efficient degradation of pyrene. Chemosphere 2022, 303, 135057. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, F.; Jian, H.; Zhen, K.; Zhang, P.; Tang, X.; Fu, Z.; Xu, W.; Wang, C.; Sun, H. Pyrene degradation in an aqueous system using ferrous citrate complex activated persulfate over a wide pH range. J. Environ. Chem. Eng. 2021, 9, 106733. [Google Scholar] [CrossRef]
- Song, S. Study on Microwave Dielectric Property of Coastal Soil. Master’s Dissertation, Zhejiang University, Hangzhou, China, 2012. Available online: https://www.dissertationtopic.net/doc/1674622 (accessed on 15 May 2025).







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wang, Z.; Hou, C.; Li, H.; Chen, W.; Li, H.; Chen, H.; Shi, L. Remediation of Polycyclic Aromatic Hydrocarbon-Contaminated Soil Using Microwave-Activated Persulfate Oxidation System. Sustainability 2025, 17, 4897. https://doi.org/10.3390/su17114897
Guo Y, Wang Z, Hou C, Li H, Chen W, Li H, Chen H, Shi L. Remediation of Polycyclic Aromatic Hydrocarbon-Contaminated Soil Using Microwave-Activated Persulfate Oxidation System. Sustainability. 2025; 17(11):4897. https://doi.org/10.3390/su17114897
Chicago/Turabian StyleGuo, Yuanming, Zhen Wang, Chenglin Hou, Hongrui Li, Wenhao Chen, Hongchao Li, Haoming Chen, and Lin Shi. 2025. "Remediation of Polycyclic Aromatic Hydrocarbon-Contaminated Soil Using Microwave-Activated Persulfate Oxidation System" Sustainability 17, no. 11: 4897. https://doi.org/10.3390/su17114897
APA StyleGuo, Y., Wang, Z., Hou, C., Li, H., Chen, W., Li, H., Chen, H., & Shi, L. (2025). Remediation of Polycyclic Aromatic Hydrocarbon-Contaminated Soil Using Microwave-Activated Persulfate Oxidation System. Sustainability, 17(11), 4897. https://doi.org/10.3390/su17114897








