Towards Safe Maritime Decarbonization: Safety Barriers of Methanol Fuel
Abstract
1. Introduction
1.1. Historical Context and Regulatory Motivation
1.2. Alternative Fuel and Methanol’s Role
1.3. Technical Risks and Study Motivation
2. Literature Review
2.1. Properties and Risks
2.2. Human Health Hazards
2.3. Regulatory Framework
3. Methodology
3.1. Review of Existing Knowledge
3.2. Development of a Sub-Hazard-Based Coding System
3.3. Accident Reports Collection
3.4. Hazard Severity and Trends Classification
3.5. Gap Analysis and Safety Barriers
4. Results and Discussion
4.1. Results
4.2. Discussion
5. Validation of Proposed Measures and Impact
6. Conclusions
6.1. Regulatory Implications
6.2. Operational Implications
6.3. Medical Implications
6.4. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deniz, C.; Zincir, B. Environmental and Economical Assessment of Alternative Marine Fuels. J. Clean. Prod. 2016, 113, 438–449. [Google Scholar] [CrossRef]
- IMO. 2023 IMO Strategy on Reduction of GHG Emissions from Ships. 2023. Available online: https://wwwcdn.imo.org/localresources/en/OurWork/Environment/Documents/annex/MEPC%2080/Annex%2015.pdf (accessed on 23 February 2024).
- Alamoush, A.S.; Dalaklis, D.; Ballini, F.; Ölcer, A.I. Consolidating Port Decarbonisation Implementation: Concept, Pathways, Barriers, Solutions, and Opportunities. Sustainability 2023, 15, 14185. [Google Scholar] [CrossRef]
- Alamoush, A.S. Trends in Port Decarbonisation Research: Are We Reinventing the Wheel? Curr. Opin. Environ. Sustain. 2024, 71, 101478. [Google Scholar] [CrossRef]
- Alamoush, A.S.; Ballini, F.; Ölçer, A.I. Management of Stakeholders Engaged in Port Energy Transition. Energy Policy 2024, 188, 114074. [Google Scholar] [CrossRef]
- IMO. Revised GHG Reduction Strategy for Global Shipping Adopted. 2023. Available online: https://www.imo.org/en/MediaCentre/PressBriefings/pages/Revised-GHG-reduction-strategy-for-global-shipping-adopted-.aspx (accessed on 16 September 2024).
- Hansson, J.; Månsson, S.; Brynolf, S.; Grahn, M. Alternative Marine Fuels: Prospects Based on Multi-Criteria Decision Analysis Involving Swedish Stakeholders. Biomass Bioenergy 2019, 126, 159–173. [Google Scholar] [CrossRef]
- Bilgili, L. A systematic review on the acceptance of alternative marine fuels. Renew. Sustain. Energy Rev. 2023, 182, 113367. [Google Scholar] [CrossRef]
- Bilgili, L. Comparative assessment of alternative marine fuels in life cycle perspective. Renew. Sustain. Energy Rev. 2021, 144, 110985. [Google Scholar] [CrossRef]
- Methanol Institute. Comments on Department of Treasury and Internal Revenue Service Notice 2022-58 Request for Comments on Credits for Clean Hydrogen and Clean Fuel Production. 2023. Available online: https://www.methanol.org/wp-content/uploads/2023/05/IRA-Section-45Z_MI_Final.pdf (accessed on 21 May 2025).
- Agarwal, A.K.; Valera, H.; Pexa, M.; Cedík, J. Methanol; Agarwal, A.K., Valera, H., Pexa, M., Cedík, J., Eds.; Springer Singapore: Singapore, 2021; ISBN 978-981-16-1279-4. [Google Scholar]
- DNV. Maritime Forecast to 2050. 2024. Available online: https://brandcentral.dnv.com/original/gallery/10651/files/original/49ba47c0-47c1-4bb1-a8f9-0d728e62b8d6.pdf?utm_campaign=MA_AUTO_DL_24Q3_MartimeForecast_download_incl_SubscriptionBox&utm_medium=email&utm_source=Eloqua (accessed on 12 January 2025).
- Lloyd’s Register. Fuel for Thought: Methanol Report. 2023. Available online: https://www.lr.org/en/knowledge/research-reports/2023/fuel-for-thought-methanol-report (accessed on 22 December 2024).
- DNV. Methanol as Fuel Heads for the Mainstream in Shipping. 2023. Available online: https://www.dnv.com/expert-story/maritime-impact/Methanol-as-fuel-heads-for-the-mainstream-in-shipping/ (accessed on 1 February 2025).
- DNV. Methanol as Marine Fuel: Environmental Benefits, Technology Readiness, and Economic Feasibility. 2016. Available online: https://www.dnvgl.com (accessed on 11 January 2025).
- Elgohary, M.M.; Seddiek, I.S.; Salem, A.M. Overview of Alternative Fuels with Emphasis on the Potential of Liquefied Natural Gas as Future Marine Fuel. Proc. Inst. Mech. Eng. Part M J. Eng. Marit. Environ. 2015, 229, 365–375. [Google Scholar] [CrossRef]
- Dougal, D. An Introduction to Fire Dynamics; Wiley: Hoboken, NJ, USA, 2013; Available online: https://books.google.com/books/about/An_Introduction_to_Fire_Dynamics.html?id=8Au5oOMAdsoC (accessed on 11 January 2025).
- Methanex Safety Data Sheet. Available online: https://www.methanex.com/wp-content/uploads/UL-METHANOL-EU_v3.3_eSDS_10.17.2023_English.pdf (accessed on 11 October 2024).
- Log, T.; Moi, A.L. Ethanol and Methanol Burn Risks in the Home Environment. Int. J. Environ. Res. Public Health 2018, 15, 2379. [Google Scholar] [CrossRef] [PubMed]
- NJDH. Right to Know Hazardous Substance Fact Sheet. 2016. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/1222.pdf (accessed on 23 January 2025).
- NIOSH Methanol: Systemic Agent|NIOSH|CDC. Available online: https://www.cdc.gov/niosh/ershdb/emergencyresponsecard_29750029.html (accessed on 6 October 2024).
- Ellis, J.; Tanneberger, K. Study on the Use of Ethyl and Methyl Alcohol as Alternative Fuels in Shipping. 2017. Available online: https://www.maritimecyprus.com/wp-content/uploads/2016/06/emsa-study-alternative-fuels-in-shippings-1.pdf (accessed on 21 October 2024).
- Sathish Kumar, T.; Ashok, B. Material Compatibility of SI Engine Components towards Corrosive Effects on Methanol-Gasoline Blends for Flex Fuel Applications. Mater. Chem. Phys. 2023, 296, 127344. [Google Scholar] [CrossRef]
- Methanol Institute. Methanol Safe Handling Manual: 4th Edition Methanol Safe Handling Manual-Health and Safety Module 4th Edition. 2017. Available online: https://www.methanol.org/wp-content/uploads/2017/04/SafeHandlingManual-Health-Safety-Module.pdf (accessed on 12 January 2025).
- ILO. Methanol ICSC: 0057 Methyl Alcohol Carbinol Wood Alcohol Methyl Hydrate. 2018. Available online: https://chemicalsafety.ilo.org/dyn/icsc/showcard.display?p_lang=en&p_card_id=0057&p_version=2 (accessed on 6 October 2024).
- OSHA. Methyl Alcohol (Methanol)|Occupational Safety and Health Administration. Occupational Safety and Health Administration. 2024. Available online: https://www.osha.gov/chemicaldata/474 (accessed on 26 October 2024).
- US EPA Methanol. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/methanol.pdf (accessed on 1 January 2025).
- Mojica, C.V.; Pasol, E.A.; Dizon, M.L.; Kiat, W.A.; Lim, T.R.U.; Dominguez, J.C.; Valencia, V.V.; Tuaño, B.J.P. Chronic Methanol Toxicity through Topical and Inhalational Routes Presenting as Vision Loss and Restricted Diffusion of the Optic Nerves on MRI: A Case Report and Literature Review. eNeurologicalSci 2020, 20, 100258. [Google Scholar] [CrossRef] [PubMed]
- Ashurst, J.V.; Nappe, T.M. Methanol Toxicity. In StatPearls; [Internet]; StatPearls: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482121/ (accessed on 13 October 2024).
- Huang, B.Y. CHAPTER 47 - Toxic and Metabolic Brain Disease. In Imaging of the Brain; Naidich, T.P., Castillo, M., Cha, S., Smirniotopoulos, J.G., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 951–971. [Google Scholar] [CrossRef]
- Liu, D.M.; Zhou, S.; Chen, J.M.; Peng, S.Y.; Xia, W.T. The Intoxication Effects of Methanol and Formic Acid on Rat Retina Function. J. Ophthalmol. 2016, 2016, 4087096. [Google Scholar] [CrossRef] [PubMed]
- IMO. International Code of Safety for Ship Using Gases or Other Low-Flashpoint Fuels (IGF Code). Available online: https://www.imo.org/en/ourwork/safety/pages/igf-code.aspx (accessed on 22 December 2024).
- European Commission. Decarbonising Maritime Transport–FuelEU Maritime-European Commission. Available online: https://transport.ec.europa.eu/transport-modes/maritime/decarbonising-maritime-transport-fueleu-maritime_en (accessed on 22 December 2024).
- European Union. Directive 2014/94/EU on the Deployment of Alternative Fuels Infrastructure. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32014L0094 (accessed on 22 December 2024).
- Parris, D.; Spinthiropoulos, K.; Ragazou, K.; Giovou, A.; Tsanaktsidis, C. Methanol, a Plugin Marine Fuel for Green House Gas Reduction—A Review. Energies 2024, 17, 605. [Google Scholar] [CrossRef]
- IMO Interim Guidelines For The Safety Of Ships Using Methyl/Ethyl Alcohol as Fuel. Available online: https://www.register-iri.com/wp-content/uploads/MSC.1-Circ.1621.pdf (accessed on 22 December 2024).
- CG CVC. USCG Office of Commercial Vessel Compliance (CG-CVC) Mission Management System (MMS) Work Instruction (WI) Category International Convention for the Prevention of Pollution from Ships (MARPOL) Annex VI Title Implementation of Compliance/Enforcement Policy for MARPOL Annex VI Regulation 14, Including IMO 2020 Sulfur Cap Serial. 2020. Available online: https://marinechemistassociation.com/wp-content/uploads/2020/09/CVC-WI-0181.pdf (accessed on 13 January 2025).
- Class NK Safety Requirements for Construction and Equipment of Ships Using Methanol as Fuel. Available online: https://www.classnk.or.jp/hp/pdf/research/rd/2023/08_e03.pdf (accessed on 7 January 2025).
- ABS. Methanol Bunkering: Technical and Operational Advisory. 2024. Available online: https://ww2.eagle.org/content/dam/eagle/advisories-and-debriefs/methanol-bunkering-advisory.pdf (accessed on 16 January 2025).
- BV NR670 Methanol and Ethanol Fuelled Ships|Marine & Offshore. Available online: https://marine-offshore.bureauveritas.com/nr670-methanol-and-ethanol-fuelled-ships (accessed on 22 December 2024).
- DNV. Methanol as an Alternative Fuel for Container Vessels. Available online: https://www.dnv.com/expert-story/maritime-impact/methanol-as-an-alternative-fuel-for-container-vessels/ (accessed on 22 December 2024).
- ISO 20519:2017(en); Ships and Marine Technology—Specification for Bunkering of Liquefied Natural Gas Fuelled Vessels. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/obp/ui/#iso:std:iso:20519:ed-1:v1:en (accessed on 22 December 2024).
- IMO. Resolution MSC.396(95) Amendments to the International Convention on Standards of Training, Certification and WatchKeeping for Seafarers (STCW), 1978, as Amended. 2015. Available online: https://wwwcdn.imo.org/localresources/en/OurWork/HumanElement/Documents/MSC.396(95).pdf (accessed on 18 October 2024).
- IMO. Resolution MSC.397(95) Amendments to Part a of the Seafarers’ Training, Certification and Watchkeeping (STCW) Code. 2015. Available online: https://wwwcdn.imo.org/localresources/en/KnowledgeCentre/IndexofIMOResolutions/MSCResolutions/MSC.397(95)NotyetavailableonIMODOCS.pdf (accessed on 18 October 2024).
- ITF. Considerations of Training Aspects for Seafarers on Ships Powered by Ammonia, Methanol and Hydrogen Photo: IMO With a Special Thanks to. 2024. Available online: https://www.itfglobal.org/sites/default/files/node/resources/files/MJTTF%20Report_Training%20Aspects%20for%20Seafarers_Double%20Spread_20241122.pdf (accessed on 20 October 2024).
- GHS. The Globally Harmonized System of Classification and Labelling of Chemicals (GHS). 2022. Available online: https://www.ilo.org/sites/default/files/wcmsp5/groups/public/@ed_dialogue/@lab_admin/documents/genericdocument/wcms_841722.pdf (accessed on 20 October 2024).
- De Dianous, V.; Fiévez, C. ARAMIS Project: A More Explicit Demonstration of Risk Control through the Use of Bow–Tie Diagrams and the Evaluation of Safety Barrier Performance. J. Hazard. Mater. 2006, 130, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Methanol Institute. Methanol Railcar and Tanker Truck Accident Response. 2024. Available online: https://www.methanol.org/wp-content/uploads/2018/03/railcarandtankertruckresponse.pdf (accessed on 12 December 2024).
- NFPA. Buy NFPA 69, Standard (NFPA, Ed.). 2024. Available online: https://www.nfpa.org/product/nfpa-69-standard/p0069code#about-this-product (accessed on 23 October 2024).
- IMO. FSS Code International Code for Fire Safety Systems. 2015. Available online: www.imo.org (accessed on 21 December 2024).
- NIOSH. 1988 Osha Pel Project—Methyl Alcohol|NIOSH|CDC. In National Institue for Occupational Safety and Health.; 2011. Available online: https://archive.cdc.gov/www_cdc_gov/niosh/pel88/67-56.html (accessed on 21 January 2025).
- MCA. The Ship Captain’s Medical Guide, 23rd ed.; Maritime Coastguard Agency (MCA): Southampton, UK, 2019. [Google Scholar]
- WHO. International Medical Guide for Ships, 3rd ed.; WHO: Avenue, Switzerland, 2007. [Google Scholar]
| GHS Classification | Main Hazards | Coding | Sub. HAZID/Exposure Method | Notes |
|---|---|---|---|---|
| Health Hazards | Toxicity (T) | T1 | Dermal | Contact |
| T2 | Ocular | Splash/contact | ||
| T3 | Inhalation | Vapor inhalation | ||
| T4 | Ingestion | Accidently as alcohol | ||
| Physical Hazards | Flammability (F) | F1 | Highly flammable liquid | Low flashpoint (9–12 °C) |
| F2 | Invisible flames | Colorless during Daylight | ||
| F3 | No smoke/no soot | Methanol burns efficiently | ||
| F4 | Flammable Methanol Solution | Methanol + Water | ||
| Explosivity (E) | E1 | Tank explosion/explosion | Confinement led to an explosion (explosive range between 6.7% and 36%) |
| Severity | Criteria |
|---|---|
| Fatality |
|
| Severe |
|
| Mild |
|
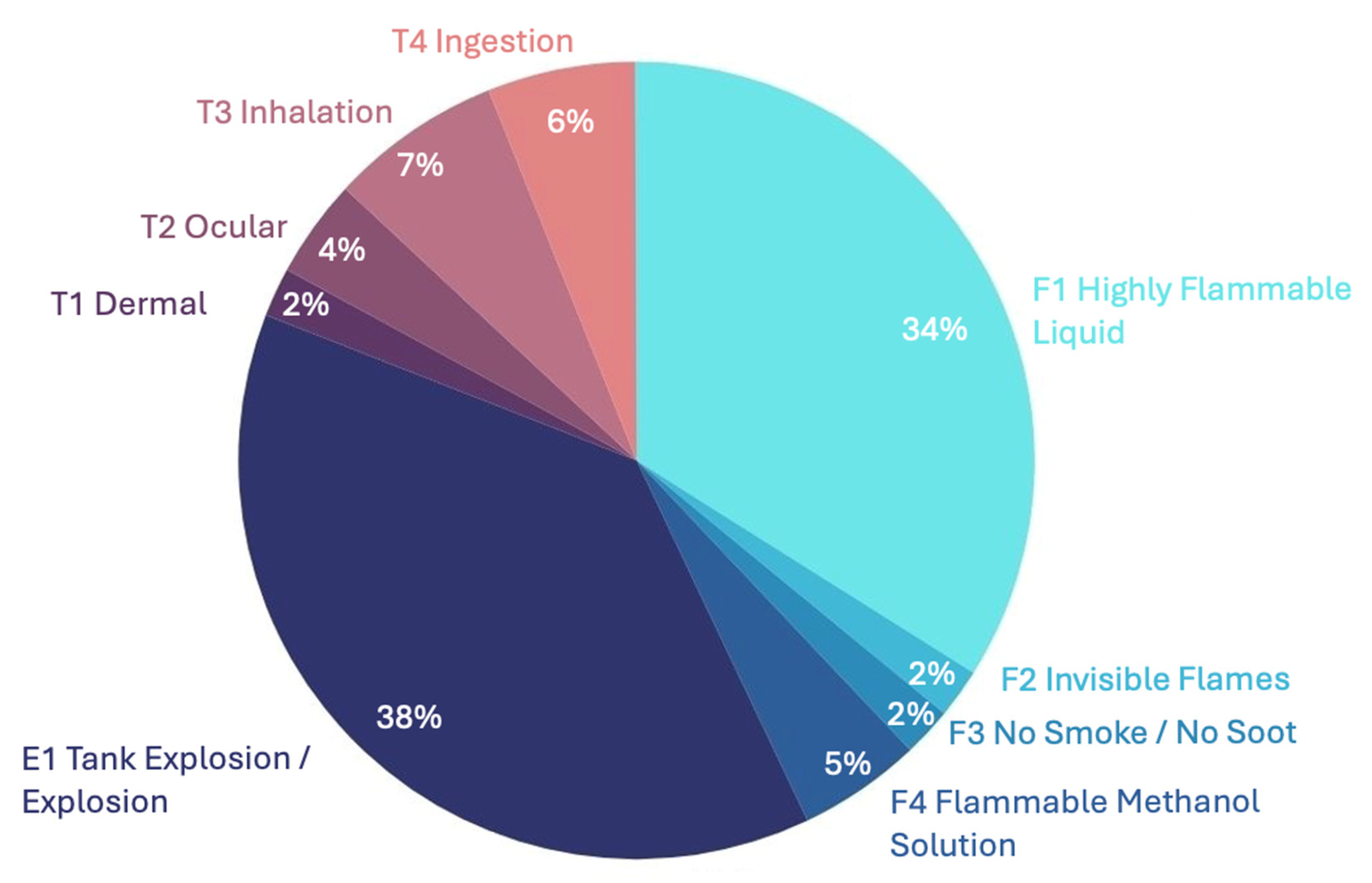
| Color Code | GHS Classification | Main Hazards | Sub-Hazard Coding System | Code Segments for Accident Reports | Frequency |
|---|---|---|---|---|---|
| ● | Physical hazards | Flammability (F) | F1 Highly Flammable Liquid | 18 | 3.40 × 10−1 |
| ● | F2 Invisible Flames | 1 | 1.89 × 10−2 | ||
| ● | F3 No smoke/No soot | 1 | 1.89 × 10−2 | ||
| ● | F4 Flammable Methanol Solution (methanol–water) | 3 | 5.66 × 10−2 | ||
| ● | Explosivity (E) | E1 Tank explosion/explosion | 20 | 3.77 × 10−1 | |
| ● | Health hazards | Toxicity (T) | T1 Dermal | 1 | 1.89 × 10−2 |
| ● | T2 Ocular | 2 | 3.77 × 10−2 | ||
| ● | T3 Inhalation | 4 | 7.55 × 10−2 | ||
| ● | T4 Ingestion | 3 | 5.66 × 10−2 | ||
| Total occurrences | 53 | - | |||
| Sub-Hazard Coding System | Severity | Recorded Severity | Frequencies in the Same Severity Level | Frequencies in all Severity Level |
|---|---|---|---|---|
| E1 Tank explosion/explosion | Fatality | 15 | 7.89 × 10−1 | 3.41 × 10−1 |
| F4 Flammable methanol solution | Fatality | 1 | 5.26 × 10−2 | 2.27 × 10−2 |
| T3 Inhalation | Fatality | 2 | 1.05 × 10−1 | 4.55 × 10−2 |
| T4 Ingestion | Fatality | 1 | 5.26 × 10−2 | 2.27 × 10−2 |
| E1 Tank explosion/explosion | Severe Injury | 13 | 6.50 × 10−1 | 4.55 × 10−2 |
| F1 Highly flammable liquid | Severe Injury | 6 | 3.00 × 10−1 | 2.27 × 10−2 |
| T3 Inhalation | Severe Injury | 1 | 5.00 × 10−2 | 2.27 × 10−2 |
| E1 Tank explosion/explosion | Mild Injury | 2 | 4.00 × 10−1 | 2.27 × 10−2 |
| F1 Highly flammable liquid | Mild Injury | 1 | 2.00 × 10−1 | 2.95 × 10−1 |
| T1 Dermal | Mild Injury | 1 | 2.00 × 10−1 | 1.36 × 10−1 |
| T3 Inhalation | Mild Injury | 1 | 2.00 × 10−1 | 2.27 × 10−2 |
| TOTAL | 44 | |||
| Barrier Provided Through IMO Tools | The Barrier Should Be in Place (From Incident Review, Severity Level) | ||||
|---|---|---|---|---|---|
| Code | IGF Code, MSC.1/Circ.1621 and SOLAS | System, Equipment and PPE | Human Error Elimination | Legal Framework | First Aid |
| F1 | -Inerting the methanol tank using nitrogen or other inert gases. -Usage of Foam (AR-AFFF). -Material safety data sheet MSDS Ch.VI/5.1. | -MOC value validation for Inert gas system provides more durability. -The amount of AR-AFFF application rate needs to be standardized. | Methanol risk perception needs to be elevated. | Stipulated requirements and validation for enhancing the durability of the Inert gas system. | Amendments to the IMO Medical Guide 3rd edition address the trended hazards with reactive measures to contain the medical consequences and support the measures detailed in the methanol product material safety data sheet (MSDS). |
| F2 | No mandatory requirements for colorless flame detection during firefighting, only fixed gas detectors are stipulated. | Thermal imaging camera (TIC); as part of firefighting teams’ standard equipment. | Methanol Safety awareness—emphasize the TIC usage. | Stipulated requirements for thermal imaging camera (TIC) application. Emergency Standard Operating Procedure (SOP). | |
| F3 | -No mandatory requirements for vapor detections except for gas release during a spill. | Thermal imaging camera (TIC); as part of firefighting teams’ standard equipment. | Methanol Safety Awareness. | Stipulated requirements for TIC application. | |
| F4 | -Usage of alcohol resistance AFFF (AR-AFFF). -Checklist for bunker safety. -Electrical bonding. | -Formal Safety Assessment (FSA) for the usage of Compressed Air foam system (CAFS). -Calculation for coverage time needs to be standardized. | Elevate the risk perception of using water only for fighting methanol fires. | Stipulated requirements for crew training to elevate methanol risk perception. -Adopt criteria for AR-AFFF concentrate quantity calculation, application rate and coverage time. | |
| E1 | -Hazards zone classification to zero, one, and two. -Use explosion-proof electrical apparatus. -eliminate any sources of ignition. | - | Methanol Safety Awareness. | Stipulated requirements for visualization of hazardous zones at safety plans in conspicuous places. | |
| T1 | -Tank monitoring for overflow risk. -Generic requirements for PPE without specific technical details. | PPE: Acid gloves Chemical suits to contain the spill without exposure. | Methanol Safety Awareness. | Stipulated requirements for specific technical details. | |
| T2 | Generic requirements for PPE without specific technical details. | PPE: face mask or Hood suitable for methanol exposure. | Methanol Safety Awareness. | Stipulated requirements for specific technical details. | |
| T3 | -Portable gas detectors are available during bunkering. -Electrical bonding. | Single gas detector (methanol) for 100% of operators with toxicity indicator not only flammability. PPE: Hood suitable for methanol exposure. | Training in methanol TLV and STEL awareness. | Stipulated requirements for personal single gas detectors for each crew member involved in methanol operation. | |
| T4 | -Checklist for bunker safety. | PPE: Hood suitable for methanol exposure | Methanol Safety Awareness Course. | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, A.M.; Metwalli, M.M.A.; Alamoush, A.S. Towards Safe Maritime Decarbonization: Safety Barriers of Methanol Fuel. Sustainability 2025, 17, 4896. https://doi.org/10.3390/su17114896
Ismail AM, Metwalli MMA, Alamoush AS. Towards Safe Maritime Decarbonization: Safety Barriers of Methanol Fuel. Sustainability. 2025; 17(11):4896. https://doi.org/10.3390/su17114896
Chicago/Turabian StyleIsmail, Ahmed M., Mahmoud M. Attia Metwalli, and Anas S. Alamoush. 2025. "Towards Safe Maritime Decarbonization: Safety Barriers of Methanol Fuel" Sustainability 17, no. 11: 4896. https://doi.org/10.3390/su17114896
APA StyleIsmail, A. M., Metwalli, M. M. A., & Alamoush, A. S. (2025). Towards Safe Maritime Decarbonization: Safety Barriers of Methanol Fuel. Sustainability, 17(11), 4896. https://doi.org/10.3390/su17114896








