Ecology of Fear: Acclimation and Adaptations to Hunting by Humans
Abstract
1. Introduction
2. The Hunters: Motives and Forms of Hunting and Harvesting
Dynamics of Hunting Cultures and Regulations in Time and Space
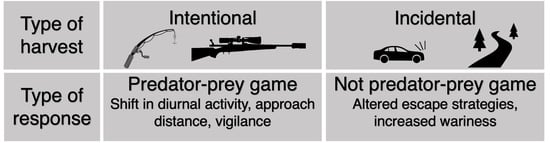
3. The Hunted: Types and Efficacy of Fear Responses
3.1. Responses from Active Hunting (Chase and Capture)
3.2. Responses from Passive Hunting (Sit and Wait)
4. Hunting as a Selective Force: Evolutionary Changes to Populations and Species
5. Unintended Consequences: How Responses of Hunted Prey Change Whole Communities and Ecosystems
6. Conservation and Management
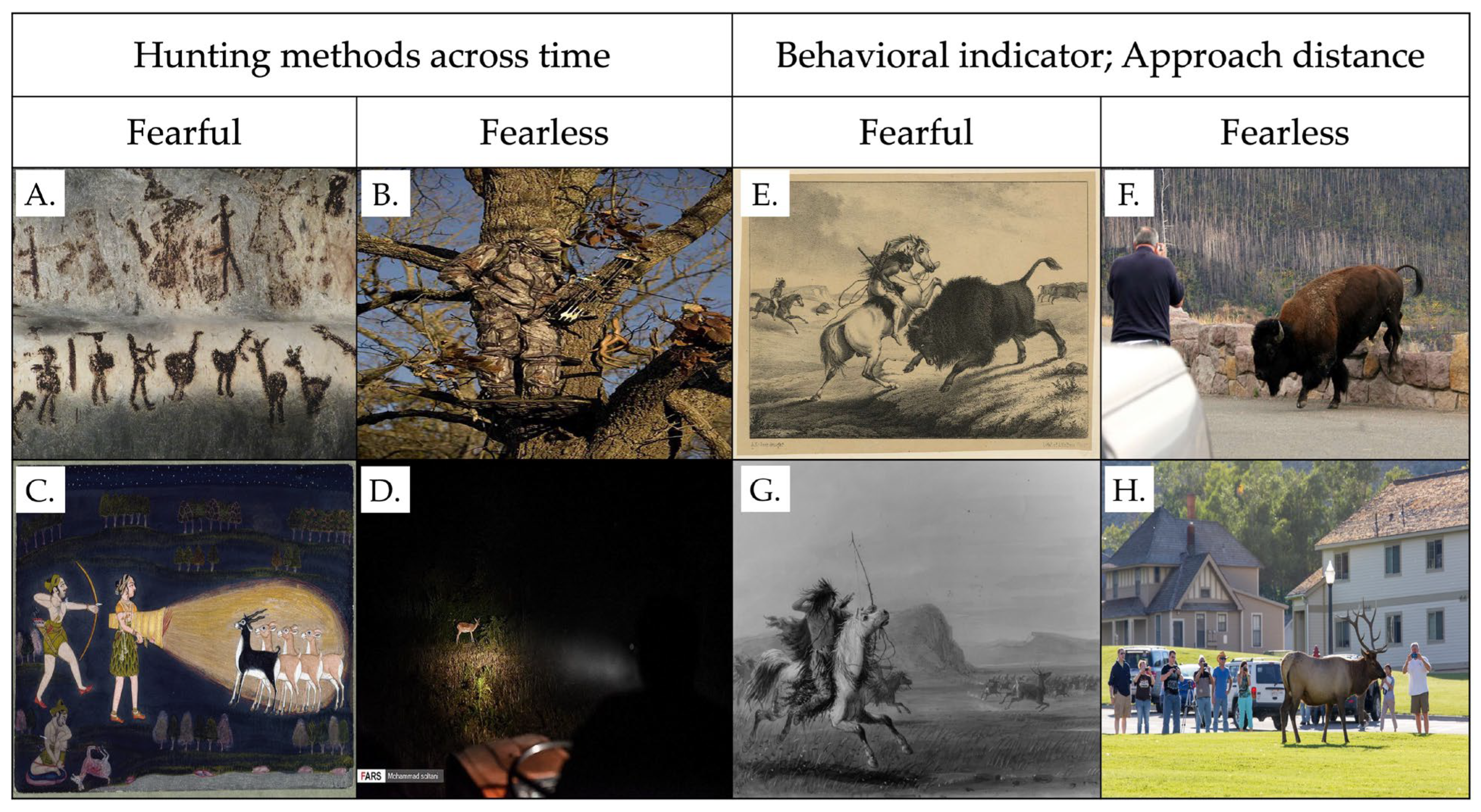
6.1. Fear Responses as Behavioral Indicators
6.2. Hunting for Fear
7. Managing Evolving Prey: Evolutionarily Enlightened Management Strategies
8. Socio-Economics: Fear Responses of Other Species as a Reflection of Ourselves
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bulmer, R. The Strategies of Hunting in New Guinea. Oceania 1968, 38, 302–318. [Google Scholar] [CrossRef]
- Pangau-Adam, M.; Noske, R. Wildlife Hunting and Bird Trade in Northern Papua (Irian Jaya), Indonesia. In Ethno-Ornithology; Routledge: Oxfordshire, UK, 2010; pp. 95–108. [Google Scholar]
- Metcalf, B.M. Adaptation of Behaviour by ’IWo Bird Species as a Result of Habituation to Humans. Aust. Field Ornithol. 2000, 18, 306–312. [Google Scholar]
- Møller, A.P.; Diaz, M.; Flensted-Jensen, E.; Grim, T.; Ibáñez-Álamo, J.D.; Jokimäki, J.; Mänd, R.; Markó, G.; Tryjanowski, P. High Urban Population Density of Birds Reflects Their Timing of Urbanization. Oecologia 2012, 170, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Hendry, A.P.; Farrugia, T.J.; Kinnison, M.T. Human Influences on Rates of Phenotypic Change in Wild Animal Populations. Mol. Ecol. 2008, 17, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Darimont, C.T.; Carlson, S.M.; Kinnison, M.T.; Paquet, P.C.; Reimchen, T.E.; Wilmers, C.C. Human Predators Outpace Other Agents of Trait Change in the Wild. Proc. Natl. Acad. Sci. USA 2009, 106, 952–954. [Google Scholar] [CrossRef]
- Luukkonen, B.Z.; Jones, O.E.; Klaver, R.W. Canada Goose Survival and Recovery Rates in Urban and Rural Areas of Iowa, USA. J. Wildl. Manag. 2021, 85, 283–292. [Google Scholar] [CrossRef]
- Thompson, S.; Vehkaoja, M.; Pellikka, J.; Nummi, P. Ecosystem Services Provided by Beavers Castor spp. Mammal Rev. 2021, 51, 25–39. [Google Scholar] [CrossRef]
- Darimont, C.T.; Fox, C.H.; Bryan, H.M.; Reimchen, T.E. The Unique Ecology of Human Predators. Science 2015, 349, 858–860. [Google Scholar] [CrossRef]
- Smith, J.A.; Suraci, J.P.; Clinchy, M.; Crawford, A.; Roberts, D.; Zanette, L.Y.; Wilmers, C.C. Fear of the Human ‘Super Predator’ Reduces Feeding Time in Large Carnivores. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170433. [Google Scholar] [CrossRef]
- Amory, C. Man Kind? Our Incredible War on Wildlife; Harper and Row: New York, NY, USA, 1974; p. 372. [Google Scholar]
- Wroe, S.; Field, J.; Fullagar, R.; Jermin, L.S. Megafaunal Extinction in the Late Quaternary and the Global Overkill Hypothesis. Alcheringa Australas. J. Palaeontol. 2004, 28, 291–331. [Google Scholar] [CrossRef]
- Surovell, T.; Waguespack, N.; Brantingham, P.J. Global Archaeological Evidence for Proboscidean Overkill. Proc. Natl. Acad. Sci. USA 2005, 102, 6231–6236. [Google Scholar] [CrossRef] [PubMed]
- Aubert, M.; Lebe, R.; Oktaviana, A.A.; Tang, M.; Burhan, B.; Hamrullah; Jusdi, A.; Abdullah; Hakim, B.; Zhao, J.; et al. Earliest Hunting Scene in Prehistoric Art. Nature 2019, 576, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.S. Buffalo Hunt: International Trade and the Virtual Extinction of the North American Bison. Am. Econ. Rev. 2011, 101, 3162–3195. [Google Scholar] [CrossRef]
- Zedeño, M.N.; Ballenger, J.A.M.; Murray, J.R. Landscape Engineering and Organizational Complexity among Late Prehistoric Bison Hunters of the Northwestern Plains. Curr. Anthropol. 2014, 55, 23–58. [Google Scholar] [CrossRef]
- Danielson, B.; (Iowa State University, Ames, IA, USA). Personal communication, 2009.
- Seney, E.E. Diet of Kemp’s Ridley Sea Turtles Incidentally Caught on Recreational Fishing Gear in the Northwestern Gulf of Mexico. Chelonian Conserv. Biol. 2016, 15, 132–137. [Google Scholar] [CrossRef]
- Van Waerebeek, K.; Baker, A.N.; Félix, F.; Gedamke, J.; Iñiguez, M.; Sanino, G.P.; Secchi, E.; Sutaria, D.; Van Helden, A.; Wang, Y. Vessel Collisions with Small Cetaceans Worldwide and with Large Whales in the Southern Hemisphere, an Initial Assessment. Lat. Am. J. Aquat. Mamm. 2007, 6, 43–69. [Google Scholar] [CrossRef]
- Wilson, S.M.; Raby, G.D.; Burnett, N.J.; Hinch, S.G.; Cooke, S.J. Looking beyond the Mortality of Bycatch: Sublethal Effects of Incidental Capture on Marine Animals. Biol. Conserv. 2014, 171, 61–72. [Google Scholar] [CrossRef]
- Stokesbury, K.; Carey, J.; Harris, B.; O’Keefe, C. Incidental Fishing Mortality May Be Responsible for the Death of Ten Billion Juvenile Sea Scallops in the Mid-Atlantic. Mar. Ecol. Prog. Ser. 2011, 425, 167–173. [Google Scholar] [CrossRef]
- Andersen, K.H.; Marty, L.; Arlinghaus, R. Evolution of Boldness and Life History in Response to Selective Harvesting. Can. J. Fish. Aquat. Sci. 2018, 75, 271–281. [Google Scholar] [CrossRef]
- Arlinghaus, R.; Laskowski, K.L.; Alós, J.; Klefoth, T.; Monk, C.T.; Nakayama, S.; Schröder, A. Passive Gear-induced Timidity Syndrome in Wild Fish Populations and Its Potential Ecological and Managerial Implications. Fish Fish. 2017, 18, 360–373. [Google Scholar] [CrossRef]
- Biro, P.A.; Post, J.R. Rapid Depletion of Genotypes with Fast Growth and Bold Personality Traits from Harvested Fish Populations. Proc. Natl. Acad. Sci. USA 2008, 105, 2919–2922. [Google Scholar] [CrossRef]
- Brown, C.R.; Brown, M.B. Where Has All the Road Kill Gone? Curr. Biol. 2013, 23, R233–R234. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; Brown, C.R. Intense Natural Selection on Morphology of Cliff Swallows (Petrochelidon pyrrhonota) a Decade Later: Did the Population Move between Adaptive Peaks? Auk 2011, 128, 69–77. [Google Scholar] [CrossRef]
- Swaddle, J.P.; Lockwood, R. Wingtip Shape and FLight Performance in the European Starling Sturnus Vulgaris. Ibis 2003, 145, 457–464. [Google Scholar] [CrossRef]
- Sih, A.; Ferrari, M.C.; Harris, D.J. Evolution and Behavioural Responses to Human-induced Rapid Environmental Change. Evol. Appl. 2011, 4, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Catullo, R.A.; Llewelyn, J.; Phillips, B.L.; Moritz, C.C. The Potential for Rapid Evolution under Anthropogenic Climate Change. Curr. Biol. 2019, 29, R996–R1007. [Google Scholar] [CrossRef]
- Alberti, M. Cities of the Anthropocene: Urban Sustainability in an Eco-Evolutionary Perspective. Philos. Trans. R. Soc. B Biol. Sci. 2024, 379, 20220264. [Google Scholar] [CrossRef]
- Fischer, A.; Sandström, C.; Delibes-Mateos, M.; Arroyo, B.; Tadie, D.; Randall, D.; Hailu, F.; Lowassa, A.; Msuha, M.; Kereži, V. On the Multifunctionality of Hunting—An Institutional Analysis of Eight Cases from Europe and Africa. J. Environ. Plan. Manag. 2013, 56, 531–552. [Google Scholar] [CrossRef]
- von Essen, E.; van Heijgen, E.; Gieser, T. Hunting Communities of Practice: Factors behind the Social Differentiation of Hunters in Modernity. J. Rural Stud. 2019, 68, 13–21. [Google Scholar] [CrossRef]
- Bichel, N.; Hart, A. A History of Hunting and Hunting Perceptions. In Trophy Hunting; Springer Nature: Singapore, 2023; pp. 19–93. [Google Scholar]
- Festa-Bianchet, M.; Mysterud, A. Hunting and Evolution: Theory, Evidence, and Unknowns. J. Mammal. 2018, 99, 1281–1292. [Google Scholar] [CrossRef]
- Coulson, T.; Schindler, S.; Traill, L.; Kendall, B.E. Predicting the Evolutionary Consequences of Trophy Hunting on a Quantitative Trait. J. Wildl. Manag. 2018, 82, 46–56. [Google Scholar] [CrossRef]
- Chen, R.S.; Soulsbury, C.D.; Lebigre, C.; Ludwig, G.; Van Oers, K.; Hoffman, J.I. Effects of Hunting on Genetic Diversity, Inbreeding and Dispersal in Finnish Black Grouse (Lyrurus tetrix). Evol. Appl. 2023, 16, 625–637. [Google Scholar] [CrossRef]
- Summary of 2012–2013 Illinois Deer Seasons; Forest Wildlife Program, Illinois Department of Natural Resources: Springfield, IL, USA, 2013; p. 10.
- Spoon, J.; Norbu Sherpa, L. Beyul Khumbu: The Sherpa and Sagarmatha (Mount Everest) National Park and Buffer Zone, Nepal. In Protected Landscapes and Cultural and Spiritual Values; Values of Protected Landscapes and Seascapes; IUCN, GTZ and Obra Social de Caixa Catalunya: Heidelberg, Germany, 2008; Volume 2, p. 68. [Google Scholar]
- Lama, N. Role of “Beyul” System in Biodiversity Conservation: A Case Study of Kyimalung in Gorkha, Nepal. Ph.D. Thesis, Tribhuvan University, Kathmandu, Nepal, 2016. [Google Scholar]
- Abraham, J.O.; Mumma, M.A. Elevated Wildlife-Vehicle Collision Rates during the COVID-19 Pandemic. Sci. Rep. 2021, 11, 20391. [Google Scholar] [CrossRef]
- Gordo, O.; Brotons, L.; Herrando, S.; Gargallo, G. Rapid Behavioural Response of Urban Birds to COVID-19 Lockdown. Proc. R. Soc. B 2021, 288, 20202513. [Google Scholar] [CrossRef]
- Behera, A.K.; Kumar, P.R.; Priya, M.M.; Ramesh, T.; Kalle, R. The Impacts of COVID-19 Lockdown on Wildlife in Deccan Plateau, India. Sci. Total Environ. 2022, 822, 153268. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, K.M.; Brown, J.S.; Middleton, A.D.; Power, M.E.; Brashares, J.S. Landscapes of Fear: Spatial Patterns of Risk Perception and Response. Trends Ecol. Evol. 2019, 34, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Laundré, J.W.; Gurung, M. The Ecology of Fear: Optimal Foraging, Game Theory, and Trophic Interactions. J. Mammal. 1999, 80, 385–399. [Google Scholar] [CrossRef]
- Lima, S.L.; Dill, L.M. Behavioral Decisions Made Under the Risk of Predation: A Review and Prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Suttle, K.B. Effects of Top Predator Species on Direct and Indirect Interactions in a Food Web. Ecology 2001, 82, 2072–2081. [Google Scholar] [CrossRef]
- Cromsigt, J.P.G.M.; Kuijper, D.P.J.; Adam, M.; Beschta, R.L.; Churski, M.; Eycott, A.; Kerley, G.I.H.; Mysterud, A.; Schmidt, K.; West, K. Hunting for Fear: Innovating Management of Human-Wildlife Conflicts. J. Appl. Ecol. 2013, 50, 544–549. [Google Scholar] [CrossRef]
- Thaker, M.; Vanak, A.T.; Owen, C.R.; Ogden, M.B.; Niemann, S.M.; Slotow, R. Minimizing Predation Risk in a Landscape of Multiple Predators: Effects on the Spatial Distribution of African Ungulates. Ecology 2011, 92, 398–407. [Google Scholar] [CrossRef]
- Marantz, S.A.; Long, J.A.; Webb, S.L.; Gee, K.L.; Little, A.R.; Demarais, S. Impacts of Human Hunting on Spatial Behavior of White-Tailed Deer (Odocoileus virginianus). Can. J. Zool. 2016, 94, 853–861. [Google Scholar] [CrossRef]
- Stankowich, T. Ungulate Flight Responses to Human Disturbance: A Review and Meta-Analysis. Biol. Conserv. 2008, 141, 2159–2173. [Google Scholar] [CrossRef]
- Ciuti, S.; Northrup, J.M.; Muhly, T.B.; Simi, S.; Musiani, M.; Pitt, J.A.; Boyce, M.S. Effects of Humans on Behaviour of Wildlife Exceed Those of Natural Predators in a Landscape of Fear. PLoS ONE 2012, 7, e50611. [Google Scholar] [CrossRef] [PubMed]
- Parsons, A.W.; Wikelski, M.; Keeves von Wolff, B.; Dodel, J.; Kays, R. Intensive Hunting Changes Human-Wildlife Relationships. PeerJ 2022, 10, e14159. [Google Scholar] [CrossRef]
- Lone, K.; Loe, L.E.; Meisingset, E.L.; Stamnes, I.; Mysterud, A. An Adaptive Behavioural Response to Hunting: Surviving Male Red Deer Shift Habitat at the Onset of the Hunting Season. Anim. Behav. 2015, 102, 127–138. [Google Scholar] [CrossRef]
- Kilgo, J.C.; Labisky, R.F.; Fritzen, D.E. Influences of Hunting on the Behavior of White-Tailed Deer: Implications for Conservation of the Florida Panther. Conserv. Biol. 1998, 12, 1359–1364. [Google Scholar]
- Sasaki, K.; Fox, S.F.; Duvall, D. Rapid Evolution in the Wild: Changes in Body Size, Life-history Traits, and Behavior in Hunted Populations of the Japanese Mamushi Snake. Conserv. Biol. 2009, 23, 93–102. [Google Scholar] [CrossRef]
- McGrath, D.J.; Terhune, T.M.; Martin, J.A. Northern Bobwhite Foraging Response to Hunting: Hunting Influences Bobwhite Foraging Behavior. J. Wildl. Manag. 2018, 82, 966–976. [Google Scholar] [CrossRef]
- Verdade, L.M. The Influence of Hunting Pressure on the Social Behavior of Vertebrates. Rev. Bras. Biol. 1996, 56, 1–13. [Google Scholar]
- Crawshaw, P.G. Effects of Hunting on the Reproduction of the Paraguayan Caiman (Caiman yacare) in the Pantanal of Mato Grosso, Brazil. In Neotropical Wildlife Use and Conservation; Chicago University Press, Chicago: Chicago, IL, USA, 1991; pp. 145–153. [Google Scholar]
- Halliday, T.R. The Extinction of the Passenger Pigeon Ectopistes Migratorius and Its Relevance to Contemporary Conservation. Biol. Conserv. 1980, 17, 157–162. [Google Scholar] [CrossRef]
- Sacks, B.N.; Blejwas, K.M.; Jaeger, M.M. Relative Vulnerability of Coyotes to Removal Methods on a Northern California Ranch. J. Wildl. Manag. 1999, 63, 939. [Google Scholar] [CrossRef]
- Séquin, E.S.; Jaeger, M.M.; Brussard, P.F.; Barrett, R.H. Wariness of Coyotes to Camera Traps Relative to Social Status and Territory Boundaries. Can. J. Zool. 2003, 81, 2015–2025. [Google Scholar] [CrossRef]
- Heinlein, B.W.; Urbanek, R.E.; Olfenbuttel, C.; Dukes, C.G. Effects of Different Attractants and Human Scent on Mesocarnivore Detection at Camera Traps. Wildl. Res. 2020, 47, 338. [Google Scholar] [CrossRef]
- Little, A.R.; Demarais, S.; Gee, K.L.; Webb, S.L.; Riffell, S.K.; Gaskamp, J.A.; Belant, J.L. Does Human Predation Risk Affect Harvest Susceptibility of White-Tailed Deer during Hunting Season?: Predation Risk and Harvest Susceptibility. Wildl. Soc. Bull. 2014, 38, 797–805. [Google Scholar] [CrossRef]
- Lima, S.L.; Bednekoff, P.A. Temporal Variation in Danger Drives Antipredator Behavior: The Predation Risk Allocation Hypothesis. Am. Nat. 1999, 153, 649–659. [Google Scholar] [CrossRef]
- Coltman, D.W. Evolutionary Rebound from Selective Harvesting. Trends Ecol. Evol. 2008, 23, 117–118. [Google Scholar] [CrossRef]
- Kuparinen, A.; Merilä, J. Detecting and Managing Fisheries-Induced Evolution. Trends Ecol. Evol. 2007, 22, 652–659. [Google Scholar] [CrossRef]
- Allendorf, F.W.; Hard, J.J. Human-Induced Evolution Caused by Unnatural Selection through Harvest of Wild Animals. Proc. Natl. Acad. Sci. USA 2009, 106, 9987–9994. [Google Scholar] [CrossRef]
- Nash, J. Naming Places-on and around Kangaroo Island. S. Aust. Geogr. J. 2014, 112, 69–73. [Google Scholar] [CrossRef]
- Seersholm, F.V.; Grealy, A.; McDowell, M.C.; Cole, T.L.; Arnold, L.J.; Prideaux, G.J.; Bunce, M. Ancient DNA from Bulk Bone Reveals Past Genetic Diversity of Vertebrate Fauna on Kangaroo Island, Australia. Quat. Sci. Rev. 2021, 262, 106962. [Google Scholar] [CrossRef]
- Boyce, M.S.; Krausman, P.R. Special Section: Controversies in Mountain Sheep Management. J. Wildl. Manag. 2018, 82, 5–7. [Google Scholar] [CrossRef]
- Coltman, D.W.; O’Donoghue, P.; Jorgenson, J.T.; Hogg, J.T.; Strobeck, C.; Festa-Bianchet, M. Undesirable Evolutionary Consequences of Trophy Hunting. Nature 2003, 426, 655–658. [Google Scholar] [CrossRef]
- Pigeon, G.; Festa-Bianchet, M.; Coltman, D.W.; Pelletier, F. Intense Selective Hunting Leads to Artificial Evolution in Horn Size. Evol. Appl. 2016, 9, 521–530. [Google Scholar] [CrossRef]
- Garel, M.; Cugnasse, J.-M.; Maillard, D.; Gaillard, J.-M.; Hewison, A.M.; Dubray, D. Selective Harvesting and Habitat Loss Produce Long-term Life History Changes in a Mouflon Population. Ecol. Appl. 2007, 17, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Jachmann, H.; Berry, P.S.M.; Imae, H. Tusklessness in African Elephants: A Future Trend. Afr. J. Ecol. 1995, 33, 230–235. [Google Scholar] [CrossRef]
- Haldane, J.B.S. The Selective Elimination of Silver Foxes in Eastern Canada. J. Genet. 1942, 44, 296–304. [Google Scholar] [CrossRef]
- Rauw, W.M.; Kanis, E.; Noordhuizen-Stassen, E.N.; Grommers, F.J. Undesirable Side Effects of Selection for High Production Efficiency in Farm Animals: A Review. Livest. Prod. Sci. 1998, 56, 15–33. [Google Scholar] [CrossRef]
- Gjedrem, T. Selection and Breeding Programs in Aquaculture; Springer: Dordrecht, The Netherlands, 2005; p. 360. [Google Scholar]
- De Roos, A.M.; Boukal, D.S.; Persson, L. Evolutionary Regime Shifts in Age and Size at Maturation of Exploited Fish Stocks. Proc. R. Soc. B Biol. Sci. 2006, 273, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Hard, J.J.; Gross, M.R.; Heino, M.; Hilborn, R.; Kope, R.G.; Law, R.; Reynolds, J.D. Evolutionary Consequences of Fishing and Their Implications for Salmon. Evol. Appl. 2008, 1, 388–408. [Google Scholar] [CrossRef]
- Edeline, E.; Carlson, S.M.; Stige, L.C.; Winfield, I.J.; Fletcher, J.M.; James, J.B.; Haugen, T.O.; Vøllestad, L.A.; Stenseth, N.C. Trait Changes in a Harvested Population Are Driven by a Dynamic Tug-of-War between Natural and Harvest Selection. Proc. Natl. Acad. Sci. USA 2007, 104, 15799–15804. [Google Scholar] [CrossRef] [PubMed]
- Hard, J. Evolution of Chinook Salmon Life History under Size-Selective Harvest. In Evolution Illuminated: Salmon and Their Relatives; Oxford University Press: New York, NY, USA, 2004; pp. 315–337. [Google Scholar]
- Hyer, K.; Schleusner, C.J. Chinook Salmon Age, Sex, and Length Analysis from Selected Escapement Projects on the Yukon River; US Fish and Wildlife Service Report; Office of Subsistence Management, Fisheries Information Services Division: Washington, DC, USA, 2005. [Google Scholar]
- Olsen, E.M.; Heino, M.; Lilly, G.R.; Morgan, M.J.; Brattey, J.; Ernande, B.; Dieckmann, U. Maturation Trends Indicative of Rapid Evolution Preceded the Collapse of Northern Cod. Nature 2004, 428, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Clutton-Brock, T.; Lonergan, M. Culling Regimes and Sex Ratio Biases in Highland Red Deer. J. Appl. Ecol. 1994, 31, 521–527. [Google Scholar] [CrossRef]
- Cooley, H.S.; Wielgus, R.B.; Koehler, G.M.; Robinson, H.S.; Maletzke, B.T. Does Hunting Regulate Cougar Populations? A Test of the Compensatory Mortality Hypothesis. Ecology 2009, 90, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Kvalnes, T.; Sæther, B.; Haanes, H.; Røed, K.H.; Engen, S.; Solberg, E.J. Harvest-induced Phenotypic Selection in an Island Population of Moose, Alces Alces. Evolution 2016, 70, 1486–1500. [Google Scholar] [CrossRef]
- Mumme, R.L.; Schoech, S.J.; Woolfenden, G.E.; Fitzpatrick, J.W. Life and Death in the Fast Lane: Demographic Consequences of Road Mortality in the Florida Scrub-Jay. Conserv. Biol. 2000, 14, 501–512. [Google Scholar] [CrossRef]
- Miller, R.B. Have the Genetic Patterns of Fishes Been Altered by Introductions or by Selective Fishing? J. Fish. Board Can. 1957, 14, 797–806. [Google Scholar] [CrossRef]
- Leclerc, M.; Zedrosser, A.; Swenson, J.E.; Pelletier, F. Hunters Select for Behavioral Traits in a Large Carnivore. Sci. Rep. 2019, 9, 12371. [Google Scholar] [CrossRef]
- Ordiz, A.; Støen, O.-G.; Sæbø, S.; Kindberg, J.; Delibes, M.; Swenson, J.E. Do Bears Know They Are Being Hunted? Biol. Conserv. 2012, 152, 21–28. [Google Scholar] [CrossRef]
- Harris, R.B.; Wall, W.A.; Allendorf, F.W. Genetic Consequences of Hunting: What Do We Know and What Should We Do? Wildl. Soc. Bull. 2002, 30, 634–643. [Google Scholar]
- Werner, E.E.; Peacor, S.D. A Review of Trait-mediated Indirect Interactions in Ecological Communities. Ecology 2003, 84, 1083–1100. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Krivan, V.; Ovadia, O. Trophic Cascades: The Primacy of Trait-mediated Indirect Interactions. Ecol. Lett. 2004, 7, 153–163. [Google Scholar] [CrossRef]
- Audzijonyte, A.; Kuparinen, A.; Gorton, R.; Fulton, E.A. Ecological Consequences of Body Size Decline in Harvested Fish Species: Positive Feedback Loops in Trophic Interactions Amplify Human Impact. Biol. Lett. 2013, 9, 20121103. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.G.; Paetkau, D.; Lewis, D.; McLellan, B.N.; Proctor, M.; Strobeck, C. Genetic Tagging of Free-Ranging Black and Brown Bears. Wildl. Soc. Bull. 1999, 27, 616–627. [Google Scholar]
- Gaynor, K.M.; Hojnowski, C.E.; Carter, N.H.; Brashares, J.S. The Influence of Human Disturbance on Wildlife Nocturnality. Science 2018, 360, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Clinchy, M.; Zanette, L.Y.; Roberts, D.; Suraci, J.P.; Buesching, C.D.; Newman, C.; Macdonald, D.W. Fear of the Human “Super Predator” Far Exceeds the Fear of Large Carnivores in a Model Mesocarnivore. Behav. Ecol. 2016, 27, 1826–1832. [Google Scholar] [CrossRef]
- Ordiz, A.; Saebø, S.; Kindberg, J.; Swenson, J.E.; Støen, O.-G. Seasonality and Human Disturbance Alter Brown Bear Activity Patterns: Implications for Circumpolar Carnivore Conservation? Anim. Conserv. 2017, 20, 51–60. [Google Scholar] [CrossRef]
- Berger, J. Fear, Human Shields and the Redistribution of Prey and Predators in Protected Areas. Biol. Lett. 2007, 3, 620–623. [Google Scholar] [CrossRef]
- Muhly, T.B.; Semeniuk, C.; Massolo, A.; Hickman, L.; Musiani, M. Human Activity Helps Prey Win the Predator-Prey Space Race. PLoS ONE 2011, 6, e17050. [Google Scholar] [CrossRef]
- Atickem, A.; Loe, L.E.; Stenseth, N.C. Individual Heterogeneity in Use of Human Shields by Mountain Nyala. Ethology 2014, 120, 715–725. [Google Scholar] [CrossRef]
- Ale, S.B. Ecology of the Snow Leopard and the Himalayan Tahr in Sagarmatha (Mt. Everest) National Park, Nepal. Ph.D. Thesis, University of Illinois at Chicago, Chicago, IL, USA, 2007. [Google Scholar]
- Gurung, M. Ecology of Fear: A Case Study of Blue Sheep and Snow Leopards in the Nepal Himalayas. Ph.D. Thesis, University of Illinois at Chicago, Chicago, IL, USA, 2002. [Google Scholar]
- Morris, D.W.; Kotler, B.P.; Brown, J.S.; Sundararaj, V.; Ale, S.B. Behavioral Indicators for Conserving Mammal Diversity. Ann. N. Y. Acad. Sci. 2009, 1162, 334–356. [Google Scholar] [CrossRef]
- Ale, S.B.; Brown, J.S. Prey Behavior Leads to Predator: A Case Study of the Himalayan Tahr and the Snow Leopard in Sagarmatha (Mt. Everest) National Park, Nepal. Isr. J. Ecol. Evol. 2009, 55, 315–327. [Google Scholar] [CrossRef]
- Bamforth, D.B. Historical Documents and Bison Ecology on the Great Plains. Plains Anthropol. 1987, 32, 1–16. [Google Scholar] [CrossRef]
- Driver, J.C.; Maxwell, D. Bison Death Assemblages and the Interpretation of Human Hunting Behaviour. Quat. Int. 2013, 297, 100–109. [Google Scholar] [CrossRef]
- Flores, D. Bison Ecology and Bison Diplomacy: The Southern Plains from 1800–1850. In Agriculture, Resource Exploitation, and Environmental Change; Routledge: London, UK, 2021; pp. 47–67. [Google Scholar]
- Laundré, J.W.; Hernández, L.; Ripple, W.J. The Landscape of Fear: Ecological Implications of Being Afraid. Open Ecol. J. 2010, 3, 1–7. [Google Scholar] [CrossRef]
- Speziale, K.L.; Lambertucci, S.A.; Olsson, O. Disturbance from Roads Negatively Affects Andean Condor Habitat Use. Biol. Conserv. 2008, 141, 1765–1772. [Google Scholar] [CrossRef]
- Brown, J.S.; Kotler, B.P. Hazardous Duty Pay and the Foraging Cost of Predation: Foraging Cost of Predation. Ecol. Lett. 2004, 7, 999–1014. [Google Scholar] [CrossRef]
- Nowak, K.; Hill, R.A.; Wimberger, K.; le Roux, A. Risk-Taking in Samango Monkeys in Relation to Humans at Two Sites in South Africa. In Ethnoprimatology. Developments in Primatology: Progress and Prospects; Springer: New York, NY, USA, 2016; pp. 301–314. [Google Scholar]
- Leighton, P.A.; Horrocks, J.A.; Kramer, D.L. Conservation and the Scarecrow Effect: Can Human Activity Benefit Threatened Species by Displacing Predators? Biol. Conserv. 2010, 143, 2156–2163. [Google Scholar] [CrossRef]
- Proffitt, K.M.; Grigg, J.L.; Hamlin, K.L.; Garrott, R.A. Contrasting Effects of Wolves and Human Hunters on Elk Behavioral Responses to Predation Risk. J. Wildl. Manag. 2009, 73, 345–356. [Google Scholar] [CrossRef]
- Cleveland, S.M.; Hebblewhite, M.; Thompson, M.; Henderson, R. Linking Elk Movement and Resource Selection to Hunting Pressure in a Heterogeneous Landscape. Wildl. Soc. Bull. 2012, 36, 658–668. [Google Scholar] [CrossRef]
- Altendorf, K.B.; Laundré, J.W.; González, C.A.L.; Brown, J.S. Assessing Effects of Predation Risk on Foraging Behavior of Mule Deer. J. Mammal. 2001, 82, 430–439. [Google Scholar] [CrossRef]
- Blossey, B.; Hare, D. Myths, Wishful Thinking, and Accountability in Predator Conservation and Management in the United States. Front. Conserv. Sci. 2022, 3, 881483. [Google Scholar] [CrossRef]
- MacKenzie, J.M. Hunting and African Societies. In The Empire of Nature; Manchester University Press: Manchester, UK, 2017; pp. 54–84. ISBN 1-5261-1958-7. [Google Scholar]
- Hinde, S.L.; Hinde, H.B.G. The Last of the Masai, 1st ed.; Heinemann: London, UK, 1901. [Google Scholar]
- Herfindal, I.; Linnell, J.D.; Moa, P.F.; Odden, J.; Austmo, L.B.; Andersen, R. Does Recreational Hunting of Lynx Reduce Depredation Losses of Domestic Sheep? J. Wildl. Manag. 2005, 69, 1034–1042. [Google Scholar] [CrossRef]
- Núñez-Regueiro, M.M.; Branch, L.C.; Derlindati, E.; Gasparri, I.; Marinaro, S.; Nanni, S.; Núñez Godoy, C.; Piquer-Rodríguez, M.; Soto, J.R.; Tálamo, A. Open Standards for Conservation as a Tool for Linking Research and Conservation Agendas in Complex Socio-Ecological Systems. Curr. Opin. Environ. Sustain. 2020, 44, 6–15. [Google Scholar] [CrossRef]
- Ruid, D.B.; Paul, W.J.; Roell, B.J.; Wydeven, A.P.; Willging, R.C.; Jurewicz, R.L.; Lonsway, D.H. Wolf–Human Conflicts and Management in Minnesota, Wisconsin, and Michigan. In Recovery of Gray Wolves in the Great Lakes Region of the United States: An Endangered Species Success Story; Springer: New York, NY, USA, 2009; pp. 279–295. [Google Scholar]
- Gaynor, K.M.; McInturff, A.; Brashares, J.S. Contrasting Patterns of Risk from Human and Non-human Predators Shape Temporal Activity of Prey. J. Anim. Ecol. 2022, 91, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Invasive Wild Pigs in Ontario. Available online: https://www.ontario.ca/page/invasive-wild-pigs-ontario#:~:text=When%20wild%20pigs%20are%20exposed,way%20of%20controlling%20their%20spread (accessed on 22 November 2023).
- Karnowski, S. A Population of Hard-to-Eradicate ‘Super Pigs’ in Canada Is Threatening to Invade the US; Assoicated Press: New York, NY, USA, 2023. [Google Scholar]
- Palumbi, S.R. Evolution Explosion: How Humans Cause Rapid Evolutionary Change; WW Norton & Company: New York, NY, USA, 2002; ISBN 0-393-32338-2. [Google Scholar]
- Ashley, M.V.; Willson, M.F.; Pergams, O.R.W.; O’Dowd, D.J.; Gende, S.M.; Brown, J.S. Evolutionarily Enlightened Management. Biol. Conserv. 2003, 111, 115–123. [Google Scholar] [CrossRef]
- Hendry, A.P.; Kinnison, M.T. Perspective: The Pace of Modern Life: Measuring Rates of Contemporary Microevolution. Evolution 1999, 53, 1637–1653. [Google Scholar] [CrossRef]
- Jørgensen, C.; Ernande, B.; Fiksen, Ø. Size-selective Fishing Gear and Life History Evolution in the Northeast Arctic Cod. Evol. Appl. 2009, 2, 356–370. [Google Scholar] [CrossRef]
- Czorlich, Y.; Aykanat, T.; Erkinaro, J.; Orell, P.; Primmer, C. Rapid Evolution in Salmon Life History Induced by Direct and Indirect Effects of Fishing. Science 2022, 376, 420–423. [Google Scholar] [CrossRef]
- Diamond, S.E.; Martin, R.A. Evolution in Cities. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 519–540. [Google Scholar] [CrossRef]
- Pergams, O.R.; Lawler, J.J. Recent and Widespread Rapid Morphological Change in Rodents. PLoS ONE 2009, 4, e6452. [Google Scholar] [CrossRef][Green Version]
- Galbreath, R.; Brown, D. The Tale of the Lighthouse Keeper’s Cat: Discovery and Extinction of the Stephens Island Wren (Traversia lyalli). Notornis 2004, 51, 193–200. [Google Scholar]
- Chevin, L.-M.; Gallet, R.; Gomulkiewicz, R.; Holt, R.D.; Fellous, S. Phenotypic Plasticity in Evolutionary Rescue Experiments. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120089. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, E. High Importance of Indirect Evolutionary Rescue in a Small Food Web. Ecol. Lett. 2023, 26, 2110–2121. [Google Scholar] [CrossRef]
- Lyons, S.K.; Smith, F.A.; Brown, J.H. Of Mice, Mastodons and Men: Human-Mediated Extinctions on Four Continents. Evol. Ecol. Res. 2004, 6, 339–358. [Google Scholar]
- Araujo, B.B.A.; Oliveira-Santos, L.G.R.; Lima-Ribeiro, M.S.; Diniz-Filho, J.A.F.; Fernandez, F.A.S. Bigger Kill than Chill: The Uneven Roles of Humans and Climate on Late Quaternary Megafaunal Extinctions. Quat. Int. 2017, 431, 216–222. [Google Scholar] [CrossRef]
- Andermann, T.; Faurby, S.; Turvey, S.T.; Antonelli, A.; Silvestro, D. The Past and Future Human Impact on Mammalian Diversity. Sci. Adv. 2020, 6, eabb2313. [Google Scholar] [CrossRef]
- Bibi, F.; Kiessling, W. Continuous Evolutionary Change in Plio-Pleistocene Mammals of Eastern Africa. Proc. Natl. Acad. Sci. USA 2015, 112, 10623–10628. [Google Scholar] [CrossRef]
- Becerra-Valdivia, L.; Higham, T. The Timing and Effect of the Earliest Human Arrivals in North America. Nature 2020, 584, 93–97. [Google Scholar] [CrossRef]
- Prates, L.; Rivero, D.; Perez, S.I. Changes in Projectile Design and Size of Prey Reveal the Central Role of Fishtail Points in Megafauna Hunting in South America. Sci. Rep. 2022, 12, 16964. [Google Scholar] [CrossRef] [PubMed]
- Benson, E. The Urbanization of the Eastern Gray Squirrel in the United States. J. Am. Hist. 2013, 100, 691–710. [Google Scholar] [CrossRef]
- Mccleery, R.A. Changes in Fox Squirrel Anti-Predator Behaviors across the Urban–Rural Gradient. Landsc. Ecol. 2009, 24, 483–493. [Google Scholar] [CrossRef]
- Van Horn, G.; Aftandilian, D. City Creatures: Animal Encounters in the Chicago Wilderness; University of Chicago Press: Chicago, IL, USA, 2020; ISBN 0-226-28929-X. [Google Scholar]
- Merwe, M.V.D.; Brown, J.S.; Jackson, W.M. The Coexistence of Fox (Sciurus niger) and Gray (S. caroliniensis) Squirrels in the Chicago Metropolitan Area. Urban Ecosyst. 2005, 8, 335–347. [Google Scholar] [CrossRef]
- Waller, D.M.; Reo, N.J. First Stewards: Ecological Outcomes of Forest and Wildlife Stewardship by Indigenous Peoples of Wisconsin, USA. Ecol. Soc. 2018, 23, art45. [Google Scholar] [CrossRef]
- Crête, M. The Distribution of Deer Biomass in North America Supports the Hypothesis of Exploitation Ecosystems. Ecol. Lett. 1999, 2, 223–227. [Google Scholar] [CrossRef]
- Arcese, P.; Schuster, R.; Campbell, L.; Barber, A.; Martin, T.G. Deer Density and Plant Palatability Predict Shrub Cover, Richness, Diversity and Aboriginal Food Value in a North American Archipelago. Divers. Distrib. 2014, 20, 1368–1378. [Google Scholar] [CrossRef]
- Reo, N.J.; Whyte, K.P. Hunting and Morality as Elements of Traditional Ecological Knowledge. Hum. Ecol. 2012, 40, 15–27. [Google Scholar] [CrossRef]
- McCorquodale, S.M. Cultural Contexts of Recreational Hunting and Native Subsistence and Ceremonial Hunting: Their Significance for Wildlife Management. Wildl. Soc. Bull. 1997, 25, 568–573. [Google Scholar]
- Eltringham, S.K. Wildlife Resources and Economic Development; John Wiley and Sons: New York, NY, USA, 1984. [Google Scholar]
- Kitchell, M. New Survey Shows Americans Spent $394 Billion Participating in Hunting, Fishing, and Wildlife-Associated Activities in 2022. Available online: https://www.fws.gov/press-release/2023-10/americans-spent-394b-hunting-fishing-and-wildlife-associated-activities-22 (accessed on 10 November 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potratz, E.J.; Holt, R.D.; Brown, J.S. Ecology of Fear: Acclimation and Adaptations to Hunting by Humans. Sustainability 2024, 16, 1216. https://doi.org/10.3390/su16031216
Potratz EJ, Holt RD, Brown JS. Ecology of Fear: Acclimation and Adaptations to Hunting by Humans. Sustainability. 2024; 16(3):1216. https://doi.org/10.3390/su16031216
Chicago/Turabian StylePotratz, Emily J., Robert D. Holt, and Joel S. Brown. 2024. "Ecology of Fear: Acclimation and Adaptations to Hunting by Humans" Sustainability 16, no. 3: 1216. https://doi.org/10.3390/su16031216
APA StylePotratz, E. J., Holt, R. D., & Brown, J. S. (2024). Ecology of Fear: Acclimation and Adaptations to Hunting by Humans. Sustainability, 16(3), 1216. https://doi.org/10.3390/su16031216