Geographical Variation in Pasturelands and Their Impact on the Physicochemical Characterization and Fatty Acid Composition of Cheese in Caraș-Severin County, Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Locations of the Study
2.2. Management and Feeding of Animals
2.3. Determination of the Physicochemical Characteristics of Fresh Grass and Grass Hay
2.3.1. Determination of Proximate Composition
2.3.2. Antioxidant Profile of Fresh Grass and Grass Hay
The Preparation of Plant Extracts
Determination of TPC
Determination of Antioxidant Capacity by 1,1-Diphenyl-2-picrylhydrazyl (DPPH)
2.4. Description of the Production Process of the Two Cheeses Samples
2.5. Determination of Physicochemical Characteristics of Cheese
2.5.1. Determining the Cheese’s Proximate Composition
2.5.2. Determination of Fatty Acid Content
2.6. Statistical Analysis
3. Results
3.1. Determination of the Physicochemical Characteristics of Fresh Grass and Grass Hay
3.1.1. Determination of Proximate Composition
3.1.2. Antioxidant Profile of Fresh Grass and Grass Hay
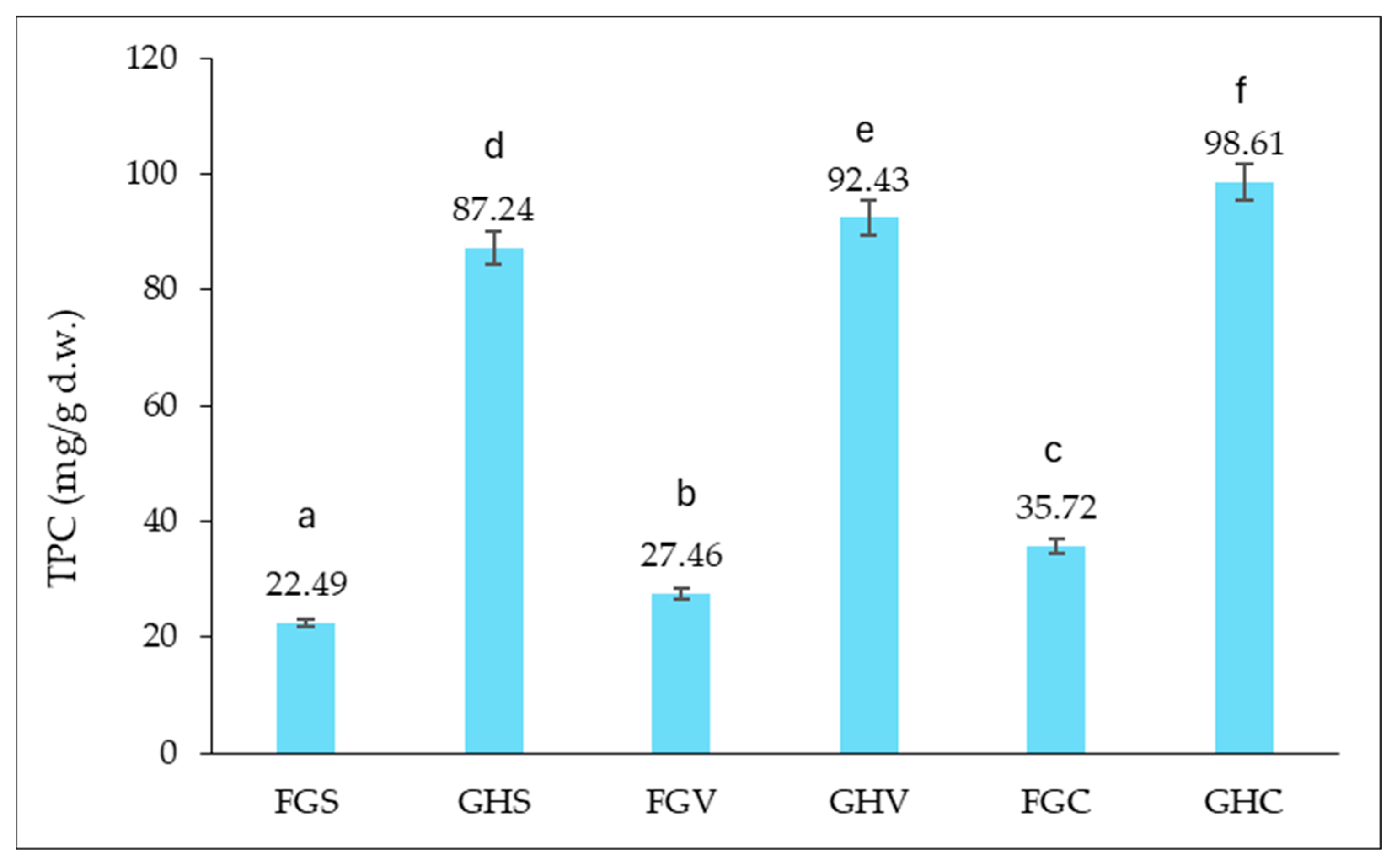
Determination of the Amount of Total Polyphenols Content (TPC)
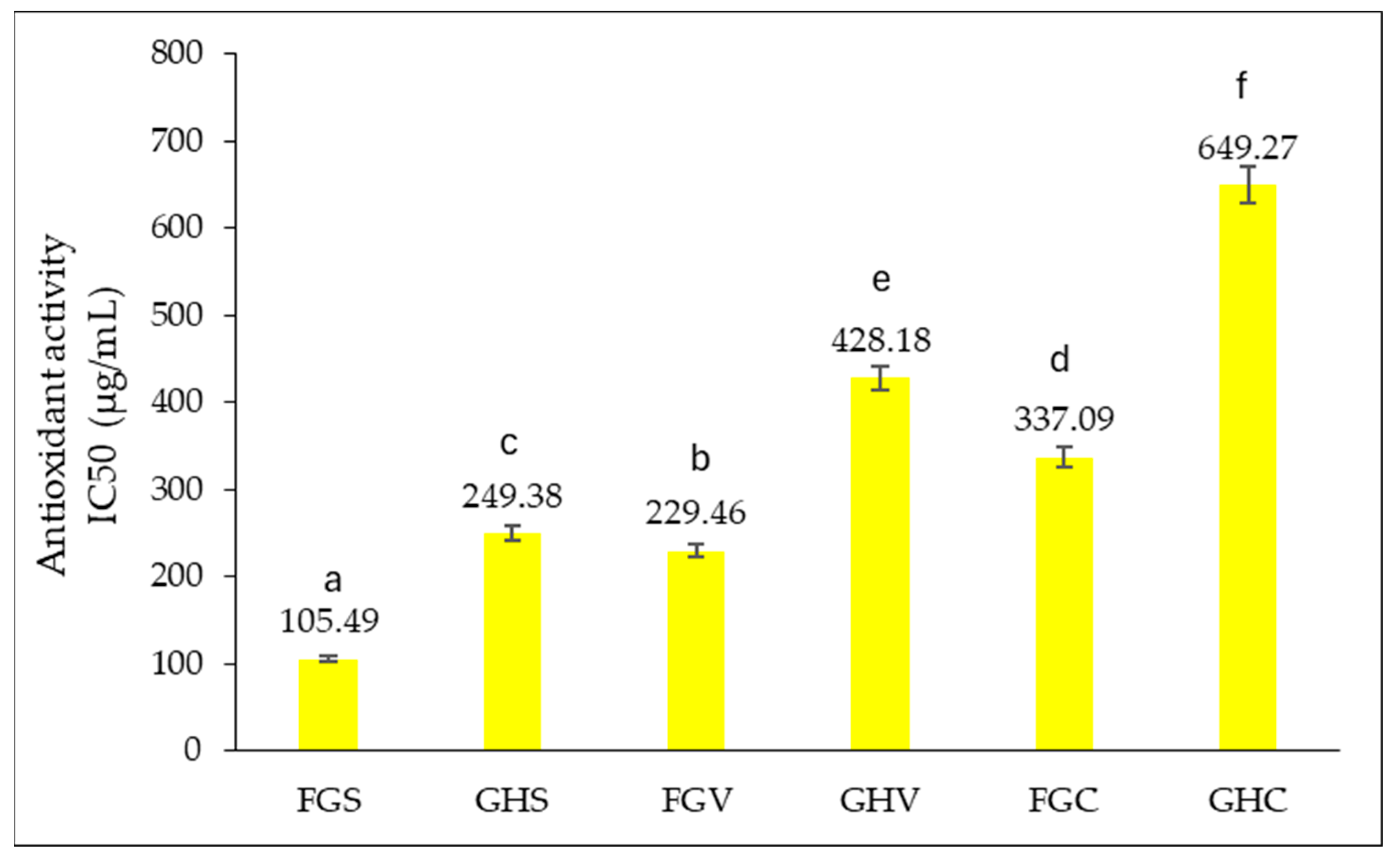
Determination of Antioxidant Capacity by DPPH (1,1-Diphenyl-2-picrylhydrazyl)
3.2. Determination of the Physicochemical Characteristics of Cheese
3.2.1. The Proximate Composition of Cheese Samples
3.2.2. Determination of Fatty Acid Content
3.3. Correlations between Variables
4. Discussions
4.1. Determination of the Physicochemical Characteristics of Fresh Grass and Grass Hay
4.2. Determination of the Physicochemical Characteristics of Cheese
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Ponti, T.; Rijk, B.; Van Ittersum, M.K. The crop yield gap between organic and conventional agriculture. Agric. Syst. 2012, 108, 1–9. [Google Scholar]
- González-Ronquillo, M.; Abecia, J.-A.; Gómez, R.; Palacios, C. Effects of weather and other factors on milk production in the Churra dairy sheep breed. J. Anim. Behav. Biometeorol. 2021, 9, 2125. [Google Scholar]
- Mahlehla, M.; Oluremi, O.; Mosebi, P.; Molapo, S.; Ranchobe, M.; Moea, L.; Mochoa, L.; Lefoka, M.; Mantsoe, M. The effect of pasture supplemented diets on the performance of lactating ewes in the foothills of Lesotho. Online J. Anim. Feed. Res. 2021, 11, 189–195. [Google Scholar]
- Koppenberg, M. Markups, organic agriculture and downstream concentration at the example of European dairy farmers. Agric. Econ. 2023, 54, 161–178. [Google Scholar]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary Polyphenol Supplementation in Food Producing Animals: Effects on the Quality of Derived Products. Animals 2021, 11, 401. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: Areview. Meat Sci. 2016, 120, 107–117. [Google Scholar]
- Bou, R.; Codony, R.; Tres, A.; Decker, E.A.; Guardiola, F. Dietary strategies to improve nutritional value, oxidative stability, and sensory properties of poultry products. Crit. Rev. Food Sci. Nutr. 2009, 49, 800–822. [Google Scholar]
- Reynaud, A.; Fraisse, D.; Cornu, A.; Farruggia, A.; Pujos-Guillot, E.; Besle, J.M.; Martin, B.; Lamaison, J.L.; Paquet, D.; Doreau, M.; et al. Variation in content and composition of phenolic compounds in permanent pastures according to botanical variation. J. Agric. Food Chem. 2010, 58, 5485–5494. [Google Scholar]
- Peiretti, P.G.; Tassone, S.; Vahdani, N.; Battelli, G.; Gai, F. Evaluation of the nutritive value and the fatty acid, phenol, tannin and terpenoid contents of nine pastures in an alpine district during the summer season. Agriculture 2020, 10, 42. [Google Scholar] [CrossRef]
- Lucas, A.; Hulin, S.; Michel, V.; Agabriel, C.; Chamba, J.F.; Rock, E.; Coulon, J.B. Relations entre les conditions de production du lait et les teneurs en composés d’intérêt nutritionnel dans le fromage: Étude en conditions réelles de production. INRAE Prod. Anim. 2006, 19, 15–28. [Google Scholar]
- Falchero, L.; Lombardi, G.; Gorlier, A.; Lonati, M.; Odoardi, M.; Cavallero, A. Variation in fatty acid composition of milk and cheese from cows grazed on two alpine pastures. Dairy Sci. Technol. 2010, 90, 657–672. [Google Scholar]
- Coppa, M.; Ferlay, A.; Monsallier, F.; Verdier-Metz, I.; Pradel, P.; Didienne, R.; Martin, B. Milk fatty acid composition and cheese texture and appearance from cows fed hay or different grazing systems on upland pastures. J. Dairy Sci. 2011, 94, 1132–1145. [Google Scholar] [PubMed]
- Innocente, N.; Praturlon, D.; Corradini, C. Fatty acid profile of cheese produced with milk from cows grazing on mountain pastures. Ital. J. Food Sci./Riv. Ital. Di Sci. Degli Aliment. 2002, 14, 217. [Google Scholar]
- Horwitz, W.; Chichilo, P.; Reynolds, H. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 1970. [Google Scholar]
- Plustea, L.; Negrea, M.; Cocan, I.; Radulov, I.; Tulcan, C.; Berbecea, A.; Popescu, I.; Obistioiu, D.; Hotea, I.; Suster, G. Lupin (Lupinus spp.)-fortified bread: A sustainable, nutritionally, functionally, and technologically valuable solution for bakery. Foods 2022, 11, 2067. [Google Scholar] [CrossRef]
- Floares, D.; Cocan, I.; Alexa, E.; Poiana, M.-A.; Berbecea, A.; Boldea, M.V.; Negrea, M.; Obistioiu, D.; Radulov, I. Influence of Extraction Methods on the Phytochemical Profile of Sambucus nigra L. Agronomy 2023, 13, 3061. [Google Scholar] [CrossRef]
- Banu, C. Tratat de Industrie Alimentara; Alimentare, T., Ed.; ASAB Bucuresti: Bucharest, Romania, 2016; pp. 47–59. [Google Scholar]
- ISO 5534:2004; Cheese and Processed Cheese—Determination of the Total Solids Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO/CD 9877|IDF 258; Milk and Milk Products—Determination of Ash. International Organization for Standardization: Geneva, Switzerland, 2024.
- ISO/TS 17837:2008; Processed Cheese Products—Determination of Nitrogen Content and Crude Protein Calculation—Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO 3433:2008; Determination of Fat Content Cheese. International Organization for Standardization: Geneva, Switzerland, 2008.
- Poșta, D.S.; Radulov, I.; Cocan, I.; Berbecea, A.A.; Alexa, E.; Hotea, I.; Iordănescu, O.A.; Băla, M.; Cântar, I.C.; Rózsa, S. Hazelnuts (Corylus avellana L.) from spontaneous flora of the west part of Romania: A source of nutrients for locals. Agronomy 2022, 12, 214. [Google Scholar] [CrossRef]
- Paszczyk, B.; Polak-Śliwińska, M.; Zielak-Steciwko, A.E. Chemical Composition, Fatty Acid Profile, and Lipid Quality Indices in Commercial Ripening of Cow Cheeses from Different Seasons. Animals 2022, 12, 198. [Google Scholar] [CrossRef]
- Taboada, N.; Van Nieuwenhove, C.; Alzogaray, S.L.; Medina, R. Influence of autochthonous cultures on fatty acid composition, esterase activity and sensory profile of Argentinean goat cheeses. J. Food Compos. Anal. 2015, 40, 86–94. [Google Scholar]
- Younge, B.; Murphy, J.; Rath, M. Nutrient metabolism in the rumen and milk production in cows fed on grass-silage and fresh grass based diets. Livest. Prod. Sci. 2004, 88, 43–54. [Google Scholar]
- Muhakka, M.; Wijaya, A.; Ammar, M. Nutritional dried matter, crude protein and crude fiber on lowland tidal grass fermented by probiotic microorganisms for use Bali cattle feed. Anim. Prod. 2015, 17, 24–29. [Google Scholar]
- Rochana, A.; Indriani, N.P.; Ayuningsih, B.; Hernaman, I.; Dhalika, T.; Rahmat, D.; Suryanah, S. Feed Forage and Nutrition Value at Altitudes During the Dry Season in West Java. Anim. Prod. 2016, 18, 85–93. [Google Scholar]
- Mir, P.; Bittman, S.; Hunt, D.; Entz, T.; Yip, B. Lipid content and fatty acid composition of grasses sampled on different dates through the early part of the growing season. Can. J. Anim. Sci. 2006, 86, 279–290. [Google Scholar]
- Stergiadis, S.; Allen, M.; Chen, X.; Wills, D.; Yan, T. Prediction of nutrient digestibility and energy concentrations in fresh grass using nutrient composition. J. Dairy Sci. 2015, 98, 3257–3273. [Google Scholar]
- Sheppard, S.C.; Cattani, D.J.; Ominski, K.H.; Biligetu, B.; Bittman, S.; McGeough, E.J. Sainfoin production in western Canada: A review of agronomic potential and environmental benefits. Grass Forage Sci. 2019, 74, 6–18. [Google Scholar]
- Petrović, M.P.; Stanković, M.S.; Anđelković, B.S.; Babić, S.; Zornić, V.G.; Vasiljević, S.; Dajić-Stevanović, Z.P. Quality parameters and antioxidant activity of three clover species in relation to the livestock diet. Not. Bot. Horti Agrobot. 2016, 44, 201–208. [Google Scholar]
- Rapisarda, S.; Abu-Ghannam, N. Polyphenol characterization and antioxidant capacity of multi-species swards grown in Ireland—Environmental sustainability and nutraceutical potential. Sustainability 2022, 15, 634. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar]
- Popescu, R.; Voiculescu, A.; Cojocaru, D. Polyphenol content in alpine meadows of the Carpathians. J. Mt. Sci. 2016, 13, 281–290. [Google Scholar]
- Ionescu, A.; Vasilescu, M. Comparative analysis of polyphenol content in meadow plants from different altitudes. Rom. Agric. Res. 2018, 35, 77–85. [Google Scholar]
- Rey, A.I.; Hopia, A.; Kivikari, R.; Kahkönen, M. Use of natural food/plant extracts: Cloudberry (Rubus chamaemorus), beetroot (Beta vulgaris “Vulgaris”) or willow herb (Epilobium angustifolium) to reduce lipid oxidation of cooked pork patties. LWT Food Sci. Technol. 2005, 38, 363–370. [Google Scholar]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, E. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, F.L.; Shankar, C.G.; Girija, S. Chemical composition and antioxidant property of holy basil (Ocimum sanctum L.) leaves, stems, and inflorescence and their in vitro callus cultures. J. Agric. Food Chem. 2007, 55, 9109–9117. [Google Scholar] [PubMed]
- Wang, S.Y.; Chen, C.T.; Wang, C.Y. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem. 2006, 101, 1160–1168. [Google Scholar]
- Vasiljević, S.; Radinović, I.; Branković, G.; Krstić, S.; Prodanović, S.; Živanović, T.; Katanski, S. Evaluation of a diverse collection of red clover for forage quality and antioxidant activity. Biotechnol. Agron. Soc. Environ. 2022, 26, 210–223. [Google Scholar]
- Haenlein, G.F.W. Goat milk in human nutrition. Small Rumin. Res. 2004, 51, 155–163. [Google Scholar]
- Raynal-Ljutovac, K.; Lagriffoul, G.; Paccard, P.; Guillet, I.; Chilliard, Y. Composition of goat and sheep milk products: An update. Small Rumin. Res. 2008, 79, 57–72. [Google Scholar]
- Bertoni, G.; Varisco, G. Influence of environmental factors on milk composition and yield. In Proceedings of the 15th ICAR, New Delhi, India, 15–19 December 2001; Volume 4, pp. 11–15. [Google Scholar]
- Bonanno, A.; Di Grigoli, A.; Mazza, F.; Vitale, F. Effect of grazing system on milk yield and quality of dairy sheep. Animal 2013, 7, 1240–1246. [Google Scholar]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science; Springer: Boston, MA, USA, 2000. [Google Scholar]
- Ojedapo, L.; Tona, G.; Amao, S.; Adeneye, J. Yield, composition and coagulation time of unsalted and salted soft cheese prepared from the milk of White Fulani cows. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 378–383. [Google Scholar]
- Alexa, E.; Danciu, C.; Cocan, I.; Negrea, M.; Morar, A.; Obistioiu, D.; Dogaru, D.; Berbecea, A.; Radulov, I. Chemical composition and antimicrobial potential of Satureja hortensis L. in fresh cow cheese. J. Food Qual. 2018, 2018, 8424035. [Google Scholar]
- Serrapica, F.; Masucci, F.; Di Francia, A.; Napolitano, F.; Braghieri, A.; Esposito, G.; Romano, R. Seasonal variation of chemical composition, fatty acid profile, and sensory properties of a mountain pecorino cheese. Foods 2020, 9, 1091. [Google Scholar] [CrossRef] [PubMed]

| Sample | Abbreviation |
|---|---|
| Fresh grass from Sacu | FGS |
| Grass hay from Sacu | GHS |
| Fresh grass from Văliug | FGV |
| Grass hay from Văliug | GHV |
| Fresh grass from Cozia | FGC |
| Grass hay from Cozia | GHC |
| Soft cow cheese from Sacu | SCCS |
| Mature cow cheese from Sacu | MCCS |
| Soft cow cheese from Văliug | SCCV |
| Mature cow cheese from Văliug | MCCV |
| Soft cow cheese from Cozia | SCCC |
| Mature cow cheese from Cozia | MCCC |
| Sample | Chemical Parameters | |||||
|---|---|---|---|---|---|---|
| Moisture (g/100 g) | Protein (g/100 g) | Lipids (g/100 g) | Ash (g/100 g) | Carbohydrates (g/100 g) | Energy Value (kcal/100 g) | |
| FGS | 83.60 ± 2.75 a | 2.60 ± 0.09 f | 0.37 ± 0.01 e | 3.09 ± 0.10 f | 10.34 ± 0.34 | 55.09 ± 1.89 |
| GHS | 7.82 ± 0.24 d | 9.95 ± 0.33 c | 2.46 ± 0.08 b | 7.26 ± 0.22 c | 72.51 ± 2.51 | 351.98 ± 12.22 |
| FGV | 86.70 ± 3.05 b | 4.08 ± 0.12 e | 0.46 ± 0.01 d | 3.76 ± 0.12 e | 5.00 ± 0.16 | 40.46 ± 1.37 |
| GHV | 8.25 ± 0.27 e | 11.41 ± 0.36 b | 2.77 ± 0.09 b | 7.89 ± 0.26 b | 69.68 ± 2.39 | 349.29 ± 12.14 |
| FGC | 89.30 ± 3.08 c | 5.57 ± 0.18 d | 0.55 ± 0.01 c | 4.17 ± 0.14 d | 0.71 ± 0.02 | 29.27 ± 1.01 |
| GHC | 9.10 ± 0.30 f | 13.05 ± 0.43 a | 3.43 ± 0.11 a | 8.54 ± 0.27 a | 65.88 ± 2.27 | 346.59 ± 12.08 |
| Samples | Chemical Parameters | |||||
|---|---|---|---|---|---|---|
| Moisture (g/100 g) | Protein (g/100 g) | Lipids (g/100 g) | Ash (g/100 g) | Carbohydrates (g/100 g) | Energy Value (kcal/100 g) | |
| SCCS | 57.71 ± 2.05 B | 16.25 ± 0.54 A | 23.11 ± 0.79 A | 1.88 ± 0.05 A | 1.05 ± 0.03 B | 277.19 ± 9.65 A |
| MCCS | 53.28 ± 1.84 b | 16.87 ± 0.57 a | 26.37 ± 0.90 a | 2.04 ± 0.06 a | 1.44 ± 0.04 c | 310.57 ± 10.71 a |
| SCCV | 57.17 ± 2.02 B | 16.59 ± 0.56 AB | 23.72 ± 0.82 A,B | 1.97 ± 0.05 A | 0.55 ± 0.01 A | 282.04 ± 9.79 B |
| MCCV | 52.69 ± 1.79 a,b | 16.98 ± 0.60 a | 27.01 ± 0.92 a,b | 2.19 ± 0.08 b | 1.13 ± 0.03 b | 315.53 ± 10.94 a,b |
| SCCC | 56.24 ± 1.84 A | 16.82 ± 0.57 B | 24.18 ± 0.81 B | 2.23 ± 0.08 B | 0.53 ± 0.01 A | 287.02 ± 9.89 C |
| MCCC | 51.32 ± 1.73 a | 17.50 ± 0.61 b | 27.93 ± 0.95 b | 2.47 ± 0.09 c | 0.78 ± 0.02 a | 324.49 ± 10.91 b |
| Fatty Acids, (g/100 g Fatty Acids) | SCCS | MCCS | SCCV | MCCV | SCCC | MCCC |
|---|---|---|---|---|---|---|
| C8:0 | 0.34 ± 0.02 a | 2.38 ± 0.11 e | 1.24 ± 0.06 c | 1.67 ± 0.07 d | 1.09 ± 0.05 b | 1.17 ± 0.05 c |
| C10:0 | 1.14 ± 0.05 c | 3.30 ± 0.15 e | 0.89 ± 0.04 b | 1.56 ± 0.07 d | 0.84 ± 0.04 a,b | 0.71 ± 0.04 a |
| C12:0 | 2.78 ± 0.13 c | 2.88 ± 1.13 c | 2.018 ± 0.10 b | 3.21 ± 0.15 d | 1.58 ± 0.08 a | 1.62 ± 0.07 a |
| C14:1 | 0.58 ± 0.03 b | 0.36 ± 0.02 a | 0.66 ± 0.03 b | 0.58 ± 0.03 b | 0.60 ± 0.03 b | 0.56 ± 0.03 b |
| C14:0 | 9.97 ± 0.50 a,b | 10.93 ± 0.53 d | 10.08 ± 0.49 b | 9.61 ± 0.46 a | 11.09 ± 0.55 d | 10.45 ± 0.49 c |
| C15:0 | 2.76 ± 0.13 c | 2.84 ± 0.13 c | 2.24 ± 0.11 b | 1.46 ± 0.07 a | 2.13 ± 0.11 b | 2.25 ± 0.09 b |
| C16:1 | 1.17 ± 0.05 b | 0.82 ± 0.04 a | 2.07 ± 0.10 c | 1.11 ± 0.05 b | 1.04 ± 0.05 a,b | 0.96 ± 0.04 a |
| C16:0 | 35.99 ± 1.76 f | 33.42 ± 1.65 e | 32.49 ± 1.61 d | 31.53 ± 1.58 c | 27.37 ± 1.35 a | 28.08 ± 1.36 b |
| C17:0 | 1.35 ± 0.07 c | 1.22 ± 0.05 b,c | 1.06 ± 0.05 b | 1.47 ± 0.06 d | 0.72 ± 0.03 a | 0.82 ± 0.03 a |
| C18:0 | 12.27 ± 0.59 a,b | 13.25 ± 0.64 c | 11.94 ± 0.59 a | 12.66 ± 0.62 b | 14.38 ± 0.71 d | 15.65 ± 0.77 e |
| C18:1 | 25.57 ± 1.28 b | 22.76 ± 1.12 a | 28.43 ± 1.40 c | 28.85 ± 1.42 c | 31.22 ± 1.55 d | 30.63 ± 1.51 d |
| C18:1, n9 trans | 0.01 ± 0.001 a | 0.34 ± 0.02 c | 0.60 ± 0.03 d | 0.03 ± 0.001 a | 0.54 ± 0.03 d | 0.10 ± 0.01 b |
| C18:1, trans-11 | 0.97 ± 0.05 b | nd * | 0.74 ± 0.04 a | 0.96 ± 0.04 b | 0.83 ± 0.04 a | 1.00 ± 0.05 b |
| C18:1, trans-12 | 1.36 ± 0.07 b | 2.22 ± 0.11 d | 0.99 ± 0.05 a | 2.09 ± 0.09 c,d | 1.05 ± 0.05 a | 1.94 ± 0.10 c |
| C18:2 | 1.32 ± 0.07 a | 1.39 ± 0.07 a | 2.42 ± 0.10 b | 1.41 ± 0.07 a | 3.99 ± 0.18 c | 3.85 ± 0.17 c |
| C18:2, n8, 11 | 0.43 ± 0.02 a | 0.68 ± 0.03 b | 0.95 ± 0.04 f | 0.76 ± 0.04 d | 0.88 ± 0.04 e | 0.64 ± 0.03 b |
| C19:1 | 0.25 ± 0.01 b,c | 0.20 ± 0.01 a,b | 0.57 ± 0.03 e | 0.31 ± 0.02 d | 0.28 ± 0.01 c,d | 0.14 ± 0.01 a |
| C20:0 | 0.23 ± 0.01 a,b | 0.24 ± 0.01 b | 0.21 ± 0.01 a,b | 0.20 ± 0.01 a,b | 0.18 ± 0.01 a | 0.21 ± 0.01 a,b |
| Other | 1.17 ± 0.05 a,b | 0.77 ± 0.04 d | 0.40 ± 0.02 c | 0.53 ± 0.03 d | 0.19 ± 0.01 b | 0.13 ± 0.01 a |
| MUFA | 29.90 ± 0.88 b | 26.70 ± 0.75 a | 34.06 ± 0.99 c | 33.93 ± 0.95 c | 35.56 ± 1.04 d | 35.32 ± 1.02 d |
| PUFA | 1.74 ± 0.04 a | 2.07 ± 0.05 b | 3.37 ± 0.09 c | 2.17 ± 0.06 b | 4.87 ± 0.14 d | 4.49 ± 0.12 d |
| SFA | 66.82 ± 1.84 e | 70.46 ± 1.97 f | 62.17 ± 1.73 c | 63.37 ± 1.78 d | 59.38 ± 1.61 a | 60.96 ± 1.65 b |
| TPC | IC50 | C8:0 | C10:0 | C12:0 | C14:1 | C14:0 | C15:0 | C16:1 | C16:0 | C17:0 | C18:0 | C18:1 | C18:1 trans | C18:1, trans-11 | C18:1, trans-12 | C 18:2 | C 18:2, n8, 11 | C19:1 | C20:0 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | 1.00 | |||||||||||||||||||
| IC50 | 0.74 | 1.00 | ||||||||||||||||||
| C8:0 | −0.24 | −0.59 | 1.00 | |||||||||||||||||
| C10:0 | −0.40 | −0.61 | 0.98 | 1.00 | ||||||||||||||||
| C12:0 | 0.17 | −0.42 | 0.76 | 0.63 | 1.00 | |||||||||||||||
| C14:1 | 0.24 | 0.37 | −0.87 | −0.91 | −0.41 | 1.00 | ||||||||||||||
| C14:0 | −0.60 | −0.20 | 0.07 | 0.26 | −0.52 | −0.46 | 1.00 | |||||||||||||
| C15:0 | −0.35 | −0.53 | 0.38 | 0.38 | −0.03 | −0.50 | 0.45 | 1.00 | ||||||||||||
| C16:1 | −0.29 | −0.20 | −0.40 | −0.42 | −0.18 | 0.68 | −0.41 | −0.14 | 1.00 | |||||||||||
| C16:0 | −0.02 | −0.65 | 0.57 | 0.42 | 0.73 | −0.22 | −0.45 | 0.48 | 0.20 | 1.00 | ||||||||||
| C17:0 | 0.26 | −0.36 | 0.62 | 0.45 | 0.97 | −0.20 | −0.70 | −0.07 | 0.01 | 0.80 | 1.00 | |||||||||
| C18:0 | 0.24 | 0.69 | −0.32 | −0.21 | −0.63 | −0.16 | 0.55 | −0.05 | −0.58 | −0.80 | −0.73 | 1.00 | ||||||||
| C18:1 | 0.29 | 0.75 | −0.89 | −0.83 | −0.68 | 0.72 | −0.02 | −0.67 | 0.17 | −0.82 | −0.61 | 0.50 | 1.00 | |||||||
| C18:1 trans | −0.89 | −0.43 | −0.21 | −0.03 | −0.52 | 0.15 | 0.54 | 0.10 | 0.49 | −0.29 | −0.55 | −0.08 | 0.15 | 1.00 | ||||||
| C18:1, trans-11 | 0.72 | 0.70 | −0.80 | −0.89 | −0.27 | 0.82 | −0.54 | −0.53 | 0.21 | −0.26 | −0.10 | 0.13 | 0.73 | −0.37 | 1.00 | |||||
| C18:1, trans-12 | 0.34 | 0.18 | 0.68 | 0.64 | 0.54 | −0.73 | −0.08 | −0.06 | −0.66 | 0.07 | 0.41 | 0.24 | −0.39 | −0.59 | −0.36 | 1.00 | ||||
| C18:2 | −0.03 | 0.58 | −0.75 | −0.61 | −0.95 | 0.36 | 0.52 | −0.18 | −0.03 | −0.90 | −0.96 | 0.78 | 0.80 | 0.39 | 0.33 | −0.37 | 1.00 | |||
| C18:2, n8, 11 | −0.60 | −0.07 | −0.31 | −0.16 | −0.39 | 0.34 | 0.20 | −0.48 | 0.52 | −0.50 | −0.40 | −0.04 | 0.45 | 0.81 | −0.11 | −0.38 | 0.39 | 1.00 | ||
| C19:1 | −0.46 | −0.36 | −0.27 | −0.25 | −0.06 | 0.58 | −0.33 | −0.25 | 0.94 | 0.17 | 0.08 | −0.66 | 0.12 | 0.61 | 0.06 | −0.62 | −0.11 | 0.68 | 1.00 | |
| C20:0 | −0.06 | −0.45 | 0.75 | 0.66 | 0.51 | −0.66 | −0.06 | 0.71 | −0.14 | 0.73 | 0.49 | −0.28 | −0.89 | −0.30 | −0.58 | 0.48 | −0.65 | −0.58 | −0.23 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibric, A.-I.; Cocan, I.; Alexa, E.; Jianu, C.; Negrea, M.; Dragoescu, A.A.; Jurcuț, R.-C.; Iancu, T. Geographical Variation in Pasturelands and Their Impact on the Physicochemical Characterization and Fatty Acid Composition of Cheese in Caraș-Severin County, Romania. Sustainability 2024, 16, 7179. https://doi.org/10.3390/su16167179
Ibric A-I, Cocan I, Alexa E, Jianu C, Negrea M, Dragoescu AA, Jurcuț R-C, Iancu T. Geographical Variation in Pasturelands and Their Impact on the Physicochemical Characterization and Fatty Acid Composition of Cheese in Caraș-Severin County, Romania. Sustainability. 2024; 16(16):7179. https://doi.org/10.3390/su16167179
Chicago/Turabian StyleIbric, Alexandra-Ioana, Ileana Cocan, Ersilia Alexa, Călin Jianu, Monica Negrea, Alina Andreea Dragoescu, Raul-Cristian Jurcuț, and Tiberiu Iancu. 2024. "Geographical Variation in Pasturelands and Their Impact on the Physicochemical Characterization and Fatty Acid Composition of Cheese in Caraș-Severin County, Romania" Sustainability 16, no. 16: 7179. https://doi.org/10.3390/su16167179
APA StyleIbric, A.-I., Cocan, I., Alexa, E., Jianu, C., Negrea, M., Dragoescu, A. A., Jurcuț, R.-C., & Iancu, T. (2024). Geographical Variation in Pasturelands and Their Impact on the Physicochemical Characterization and Fatty Acid Composition of Cheese in Caraș-Severin County, Romania. Sustainability, 16(16), 7179. https://doi.org/10.3390/su16167179










