Selected Chemical Parameters of Cereal Grain Influencing the Development of Rhyzopertha dominica F.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- -
- Winter wheat, Hondia variety, Triticum aestivum L. subsp. aestivum (DANKO Breeding Plant);
- -
- Winter triticale, Transfer variety, xTriticosecale Wittm. ex A. Camus (Strzelce Breeding Plant);
- -
- Hybrid rye, KWS Dolaro variety, Secale cereale L. (KWS Lochów Polska Breeding Plant);
- -
- Spring barley, Stratus variety, Hordeum vulgare L. (Strzelce Breeding Plant),
- -
- Common oats, Bingo variety, Avena sativa L. (Strzelce Breeding Plant);
- -
- Corn, Rosomak variety, Zea mays L. (Smolice Breeding Plant).
2.2. Chemical Properties of Grain
- -
- Albumins (water-soluble proteins);
- -
- Globulins (proteins soluble in dilute salt solutions);
- -
- Prolamins (proteins soluble in aqueous alcohols);
- -
- Glutelins (proteins soluble in dilute acids or bases) [44].
2.3. Statistical Analysis
3. Results
3.1. Developmental Parameters of R. dominica
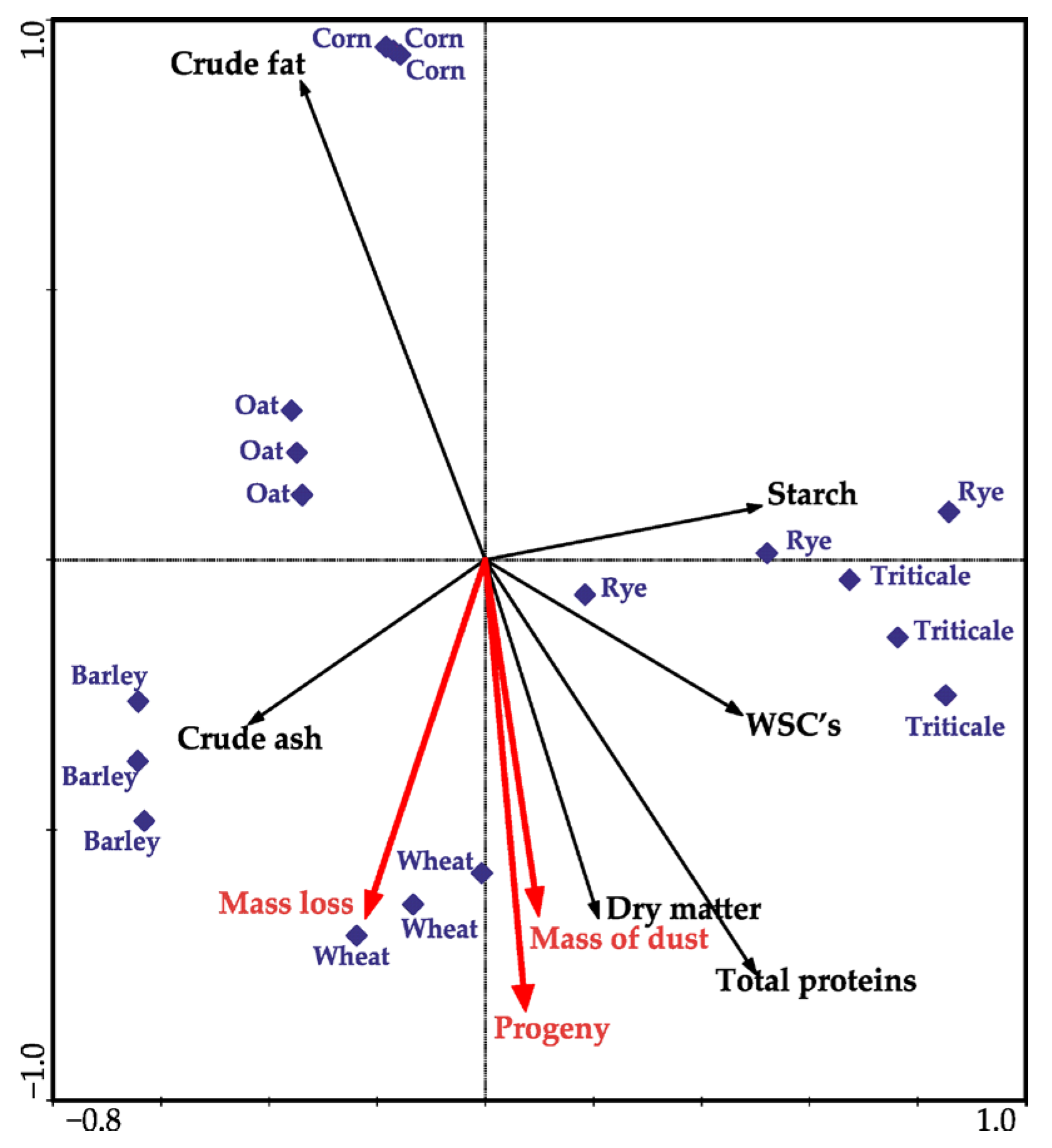
3.2. Chemical Properties of the Tested Cereal Grain
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations World Food Situation. Available online: https://www.fao.org/home/en (accessed on 10 January 2024).
- Miladinov, G. Impacts of Population Growth and Economic Development on Food Security in Low-Income and Middle-Income Countries. Front. Hum. Dyn. 2023, 5, 1121662. [Google Scholar] [CrossRef]
- Galanakis, C.M. The Future of Food. Foods 2024, 13, 506. [Google Scholar] [CrossRef]
- Pretty, J. Intensification for Redesigned and Sustainable Agricultural Systems. Science 2018, 362, eaav0294. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the Intensification of Agriculture for Global Food Security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Pea, A.; Rosa, L.; D’Odorico, P. Global Food Self-Sufficiency in the 21st Century under Sustainable Intensification of Agriculture. Environ. Res. Lett. 2020, 15, 095004. [Google Scholar] [CrossRef]
- Hodges, R.J.; Buzby, J.C.; Bennett, B. Postharvest Losses and Waste in Developed and Less Developed Countries: Opportunities to Improve Resource Use. J. Agric. Sci. 2011, 149, 37–45. [Google Scholar] [CrossRef]
- Wijerathna-Yapa, A.; Pathirana, R. Sustainable Agro-Food Systems for Addressing Climate Change and Food Security. Agriculture 2022, 12, 1554. [Google Scholar] [CrossRef]
- Canali, M.; Amani, P.; Aramyan, L.; Gheoldus, M.; Moates, G.; Östergren, K.; Silvennoinen, K.; Waldron, K.; Vittuari, M. Food Waste Drivers in Europe, from Identification to Possible Interventions. Sustainability 2016, 9, 37. [Google Scholar] [CrossRef]
- Łaba, S.; Cacak-Pietrzak, G.; Łaba, R.; Sułek, A.; Szczepański, K. Food Losses in Consumer Cereal Production in Poland in the Context of Food Security and Environmental Impact. Agriculture 2022, 12, 665. [Google Scholar] [CrossRef]
- Rayees, A.; Shafiya, H.; Showkat, A.; Syed, N.; Yendrambamb, D.; Kounser, J.; Salma, U.; Mohammad Javed, A.; Sait, E.; Barkat, H.; et al. Sustainability-Oriented Innovation in the Agri-Food System: Current Issues and the Road Ahead. Technol. Forecast. Soc. Chang. 2022, 179, 121653. [Google Scholar]
- Doukas, Y.E.; Salvati, L.; Vardopoulos, I. Unraveling the European Agricultural Policy Sustainable Development Trajectory. Land 2023, 12, 1749. [Google Scholar] [CrossRef]
- Cheba, K.; Bąk, I.; Szopik-Depczyńska, K.; Ioppolo, G. Directions of Green Transformation of the European Union Countries. Ecol. Indic. 2022, 136, 108601. [Google Scholar] [CrossRef]
- Cheba, K.; Bąk, I.; Pietrzak, M.B. Conditions of the Green Transformation. The Case of the European Union. Technol. Econ. Dev. Econ. 2023, 29, 438–467. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). The State of Food Security and Nutrition in the World 2023; International Fund for Agricultural Development: Rome, Italy, 2023; ISBN 9789251372265. [Google Scholar]
- FAO. Food Loss and Food Waste: Causes and Solutions; FAO: Rome, Italy, 2011; ISBN 9781788975391. [Google Scholar]
- Flanagan, K.; Robertson, K.; Hanson, C. Reducing Food Loss and Waste: Setting a Global Action Agenda; World Research Institute: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Scherhaufer, S.; Moates, G.; Hartikainen, H.; Waldron, K.; Obersteiner, G. Environmental Impacts of Food Waste in Europe. Waste Manag. 2018, 77, 98–113. [Google Scholar] [CrossRef]
- Campoy-Muñoz, P.; Cardenete, M.A.; Delgado, M.D.C.; Sancho, F. Food Losses and Waste: A Needed Assessment for Future Policies. Int. J. Environ. Res. Public Health 2021, 18, 11586. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development. In Arsenic Research and Global Sustainability—Proceedings of the 6th International Congress on Arsenic in the Environment, AS 2016, Stockholm, Sweden, 19–23 June 2016; UN: New York, NY, USA, 2016; pp. 12–14. [Google Scholar] [CrossRef]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food Security and the Dynamics of Wheat and Maize Value Chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Tufail, T.; Ul Ain, H.B.; Hussain, M.; Farooq, M.A.; Nayik, G.A.; Ansari, M.J. Cereals: An Overview. In Cereal Grains: Composition, Nutritional Attributes, and Potential Applications; CRC Press: Boca Raton, FL, USA, 2023; 336p, ISBN 9781000831849. [Google Scholar]
- Kumar, D.; Kalita, P. Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef]
- Tadesse, M. Post-Harvest Loss of Stored Grain, Its Causes and Reduction Strategies. Food Sci. Qual. Manag. 2020, 96, 26–35. [Google Scholar] [CrossRef]
- Klejdysz, T.; Ignatowicz, S.; Kubasik, W.; Gorzała, G.; Olejarski, P.; Horoszkiewicz, J.; Jajor, E.; Korbas, M.; Pruszyński, G.; Pruszyński, S.; et al. Metodyka Integrowanej Ochrony Magazynów Zbożowych Dla Doradców [Methodology of Integrated Protection of Grain Storage for Advisers]; Instytut Ochrony Roślin—Państwowy Instytut Badawczy: Poznań, Poland, 2017; ISBN 978-83-64655-31-9. [Google Scholar]
- Ahmad, R.; Hassan, S.; Ahmad, S.; Nighat, S.; Devi, Y.K.; Javeed, K.; Usmani, S.; Ansari, M.J.; Erturk, S.; Alkan, M.; et al. Stored Grain Pests and Current Advances for Their Management. In Postharvest Technology—Recent Advances, New Perspectives and Applications; IntechOpen: London, UK, 2021; pp. 1–37. [Google Scholar]
- Boniecki, P.; Koszela, K.; Świerczyński, K.; Skwarcz, J.; Zaborowicz, M.; Przybył, J. Neural Visual Detection of Grain Weevil (Sitophilus granarius L.). Agriculture 2020, 10, 25. [Google Scholar] [CrossRef]
- Kučerová, Z.; Aulický, R.; Stejskal, V. Outdoor Occurrence of Stored-Product Pests (Coleoptera) in the Vicinity of a Grain Store—Short Communication. Plant Prot. Sci. 2005, 41, 86–89. [Google Scholar] [CrossRef]
- Alldrick, A.J. Chemical Safety of Cereal-Based Foods: Risk Management Considerations. Qual. Assur. Saf. Crops Foods 2014, 6, 3–14. [Google Scholar] [CrossRef]
- Hamel, D.; Rozman, V.; Liška, A. Storage of Cereals in Warehouses with or without Pesticides. Insects 2020, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Cooper, J.; Dobson, H. The Benefits of Pesticides to Mankind and the Environment. Crop Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- Kordan, B.; Żuk-Gołaszewska, K.; Nietupski, M.; Słomka, W. Żyto Mieszańcowe Jako Siedlisko Rozwoju Wołka Zbożowego (Sitophilus granarius L.) i Kapturnika Zbożowca (Rhizoperta dominica F.). Prog. Plant Prot./Postępy Ochr. Roślin 2009, 49, 4. [Google Scholar]
- Mebarkia, A.; Guechi, A.; Mekhalif, S.; Makhlouf, M. Biochemical Composition Effect of the Some Cereal Species’ on the Behaviour of Sitophilus granarius L. and Rhyzopertha dominica F. Species in Semi-Arid Zone of Setif, Algeria. J. Agron. 2009, 8, 60–66. [Google Scholar] [CrossRef]
- Nietupski, M.; Bujak, E.; Kordan, B. Development of the Lesser Grain Borer (Rhyzopertha dominica F.) on Malt Barley and Fodder Barley Grain. Prog. Plant Prot. 2013, 53, 59–63. [Google Scholar] [CrossRef]
- Ludwiczak, E.; Nietupski, M.; Laszczak-Dawid, A.; Gabryś, B.; Kordan, B.; Purwin, C. Influence of Chemical Composition and Degree of Fragmentation of Millet Grain on Confused Flour Beetle (Tribolium confusum Duv.) Infestation. Agriculture 2023, 13, 2178. [Google Scholar] [CrossRef]
- Kordan, B.; Nietupski, M.; Ludwiczak, E.; Gabryś, B.; Cabaj, R. Selected Cultivar-Specific Parameters of Wheat Grain as Factors Influencing Intensity of Development of Grain Weevil Sitophilus granarius (L.). Agriculture 2023, 13, 1492. [Google Scholar] [CrossRef]
- Nietupski, M.; Ludwiczak, E.; Cabaj, R.; Purwin, C.; Kordan, B. Fatty Acids Present in Wheat Kernels Influence the Development of the Grain Weevil (Sitophilus granarius L.). Insects 2021, 12, 806. [Google Scholar] [CrossRef]
- Gołębiowska, Z. The Feeding and Fecundity of Sitophilus granarius (L.), Sitophilus oryzae (L.), and Rhyzopertha dominica (F.) in Wheat Grain. J. Stored Prod. Res. 1969, 5, 143–155. [Google Scholar] [CrossRef]
- PN-ISO 5983:2000; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content—Kjeldahl Method. Polski Komitet Normalizacyjny: Warsaw, Poland, 2000.
- PN-ISO 6492:2005; Animal Feeding Stuffs—Determination of Fat Content. Polski Komitet Normalizacyjny: Warsaw, Poland, 2005.
- PN-R-64785:1994; Pasze—Oznaczanie Zawartości Skrobi Metodą Polarymetryczną. Polski Komitet Normalizacyjny: Warsaw, Poland, 1994.
- PN-R-64784:1994; Feeds—Determination of Sugar Content. Polski Komitet Normalizacyjny: Warsaw, Poland, 1994.
- Nucia, A. Białka Gluteninowe—Charakterystyka i Ich Wpływ Na Właściwości Reologiczne Pszenicy. Praca Przeglądowa. Agron. Sci. 2018, 73, 5–16. [Google Scholar] [CrossRef]
- Wieser, H.; Kieffer, R. Correlations of the Amount of Gluten Protein Types to the Technological Properties of Wheat Flours Determined on a Micro-Scale. J. Cereal Sci. 2001, 34, 19–27. [Google Scholar] [CrossRef]
- Osborne, T.B. The Vegetable Proteins, by Thomas B. Osborne; Longmans: London, UK, 1924; Volume 44. [Google Scholar] [CrossRef]
- Ter Braak, C.J.R.; Smilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination (Version 4); Microcomputer Power: Ithaca, NY, USA, 1998. [Google Scholar]
- Chandrashekar, A.; Satyanarayana, K.V. Disease and Pest Resistance in Grains of Sorghum and Millets. J. Cereal Sci. 2006, 44, 287–304. [Google Scholar] [CrossRef]
- Germinara, G.S.; De Cristofaro, A.; Rotundo, G. Bioactivity of Short-Chain Aliphatic Ketones against Adults of the Granary Weevil, Sitophilus granarius (L.). Pest Manag. Sci. 2012, 68, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Sheeba, T.; Bai, M.R. Feeding Preference and Reproductive Fitness of Rhyzopertha dominica (Fabricius, 1792) in a Choice-Based Feed Paradigm. Indian J. Sci. Technol. 2021, 14, 850–857. [Google Scholar] [CrossRef]
- Edde, P.A. Corrigendum to “A Review of the Biology and Control of Rhyzopertha dominica (F.) the Lesser Grain Borer” [Journal of Stored Products Research 48 (2012) 1-18]. J. Stored Prod. Res. 2012, 48, 105. [Google Scholar] [CrossRef]
- Perišić, V.; Perišić, V.; Vukajlović, F.; Pešić, S.; Predojević, D.; Đekić, V.; Luković, K. Feeding Preferences and Progeny Production of Rhyzopertha dominica (Fabricius 1792) (Coleoptera: Bostrichidae) in Small Grains. Biol. Nyssana 2018, 9, 55–61. [Google Scholar] [CrossRef]
- Kordan, B.; Gabryś, B.; Załuski, D.; Zdunowski, K. Podatność Wybranych Gatunków Zbóż Na Żerowanie Trojszyka Ulca (Tribolium confusum DUV.). Prog. Plant Prot./Postępy Ochr. Roślin 2011, 51, 1559–1562. [Google Scholar]
- Ajayi, E.O.; Oladipupo, S.O.; Ajisafe, O. Influence of Processing and Substrate Variety on Survival and Development of Tribolium confusum (Coleoptera: Tenebrionidae). Arch. Phytopathol. Plant Prot. 2019, 52, 356–370. [Google Scholar] [CrossRef]
- Wong, N.; Lee, C.-Y. Relationship between Population Growth of the Red Flour Beetle Tribolium Castaneum and Protein and Carbohydrate Content in Flour and Starch. J. Econ. Entomol. 2011, 104, 2087–2094. [Google Scholar] [CrossRef]
- Nemati-Kalkhoran, M.; Razmjou, J.; Borzoui, E.; Naseri, B. Comparison of Life Table Parameters and Digestive Physiology of Rhyzopertha dominica (Coleoptera: Bostrichidae) Fed on Various Barley Cultivars. J. Insect Sci. 2018, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Hendrival, H.; Afriani, D.; Aryani, D.S. Susceptibility and Damage Cereals to Infestation Rhyzopertha Dominica (F.) (Coleoptera: Bostrichidae) in Storage. J. Agro 2019, 6, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Mebarkia, A.; Rahbé, Y.; Guechi, A.; Bouras, A.; Makhlouf, M.; Sciences, F.; Insa, I.; Biology, F.; Pasteur, B.L.; Mebarkia, C.A. Susceptibility of Twelve Soft Wheat Varieties (Triticum aestivum) to Sitophilus granarius (L.) (Coleoptera: Curculionidae) Agricultural Experimental Station of the Field Crop Institute of Setif-Algeria. Agric. Biol. J. N. Am. 2010, 1, 571–578. [Google Scholar]
- Piasecka-Kwiatkowska, D.; Gawlak, M.; Niewiada, A.; Nawrot, J.; Warchlewski, J.R.; Fornal, J.; Grundas, S. Wpływ Chemicznych Właściwości Ziarna Trzech Odmian Pszenicy Na Intensywnosc Zerowania i Tempo Rozwoju Populacji Wołka Zbożowego (Sitophilus granarius L.). Prog. Plant Prot. 2005, 45, 1688255. [Google Scholar]
- Rajnincová, D.; Špaleková, A.; Gálová, Z.; Romanová, K. The Protein Profile of Cereals, Pseudocereals and Legumes. J. Food Sci. Technol. Mysore 2019, 7, 49–53. [Google Scholar]
- Comino, I.; De Lourdes Moreno, M.; Real, A.; Rodríguez-Herrera, A.; Barro, F.; Sousa, C. The Gluten-Free Diet: Testing Alternative Cereals Tolerated by Celiac Patients. Nutrients 2013, 5, 4250–4268. [Google Scholar] [CrossRef]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) Guideline for Coeliac Disease and Other Gluten-Related Disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Rosell, C.M.; Barro, F.; Sousa, C.; Mena, M.C. Cereals for Developing Gluten-Free Products and Analytical Tools for Gluten Detection. J. Cereal Sci. 2014, 59, 354–364. [Google Scholar] [CrossRef]
- Bouchard, J.; Malalgoda, M.; Storsley, J.; Malunga, L.; Netticadan, T.; Thandapilly, S.J. Health Benefits of Cereal Grain-and Pulse-Derived Proteins. Molecules 2022, 27, 3746. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, W.; Górska-Warsewicz, H.; Rejman, K.; Czeczotko, M.; Zwolińska, J. How Important Are Cereals and Cereal Products in the Average Polish Diet? Nutrients 2019, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Urošević, D.; Knežević, D.; Branković, G.; Novoselskaya-Dragovich, A.Y.; Kudryavtsev, A.M.; Matković Stojšin, M.; Mićanović, D.; Zečević, V. Protein Content and Amino Acid Composition in Seed of Bread. Orig. Sci. Artic. Protein 2023, 55, 301–318. [Google Scholar]
- Geirsdóttir, Ó.G.; Pajari, A.M. Protein—A Scoping Review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67, 1–15. [Google Scholar] [CrossRef]
- Cenni, S.; Sesenna, V.; Boiardi, G.; Casertano, M.; Russo, G.; Reginelli, A.; Esposito, S.; Strisciuglio, C. The Role of Gluten in Gastrointestinal Disorders: A Review. Nutrients 2023, 15, 1615. [Google Scholar] [CrossRef] [PubMed]
- Union Comission Implementing Regulation (EU) No 828/2014 of 30 July 2014 on the Requirements for the Provision of Information to Consumers on the Absence or Reduced Presence of Gluten in Food Text with EEA Relevance. 2014. Available online: https://eur-lex.europa.eu/eli/reg_impl/2014/828/oj (accessed on 17 January 2024).
- Astuti, L.P.; Rizali, A.; Firnanda, R.; Widjayanti, T. Physical and Chemical Properties of Flour Products Affect the Development of Tribolium castaneum. J. Stored Prod. Res. 2020, 86, 101555. [Google Scholar] [CrossRef]
- Syed, M.I.S.; Chaudhary, F.K.; Prithiv, R.V.; Prabakaran, V. Role of Physico-Chemical Parameters in Stored Wheat for Resistance to Rhyzopertha dominica (Coleoptera: Bostrychidae) Role of Physico-Chemical Parameters in Stored Wheat for Resistance to Rhyzopertha dominica. Environ. Ecol. 2023, 41, 1235–1240. [Google Scholar]
- Đukić, N.; Radonjić, A.; Popović, B.; Kljajić, P.; Pražič-Golić, M.; Andrić, G. The Impact of the Protein-Carbohydrate Ratio in Animal Feed and the Initial Insect Population Density on the Development of the Red Flour Beetle, Tribolium castaneum. J. Stored Prod. Res. 2022, 97, 101983. [Google Scholar] [CrossRef]
- Nietupski, M.; Ciepielewska, D.; Fornal, Ł. Wpływ Żróżnicowania Chemicznego Białek w Ziarnie Wybranych Odmian Pszenicy Na Rozwój Szkodników Magazynowych. Prog. Plant Prot./Postępy Ochr. Roślin 2006, 46, 420–423. [Google Scholar]
- Kosewska, O.; Przemieniecki, S.W.; Koronkiewicz, S.; Nietupski, M. Effect of Different Chemical Properties of Cereal Grains on the Foraging and Microbiome of the Rice Weevil (Sitophilus oryzae L.). Int. Agrophys. 2024, 38, 165–176. [Google Scholar] [CrossRef]

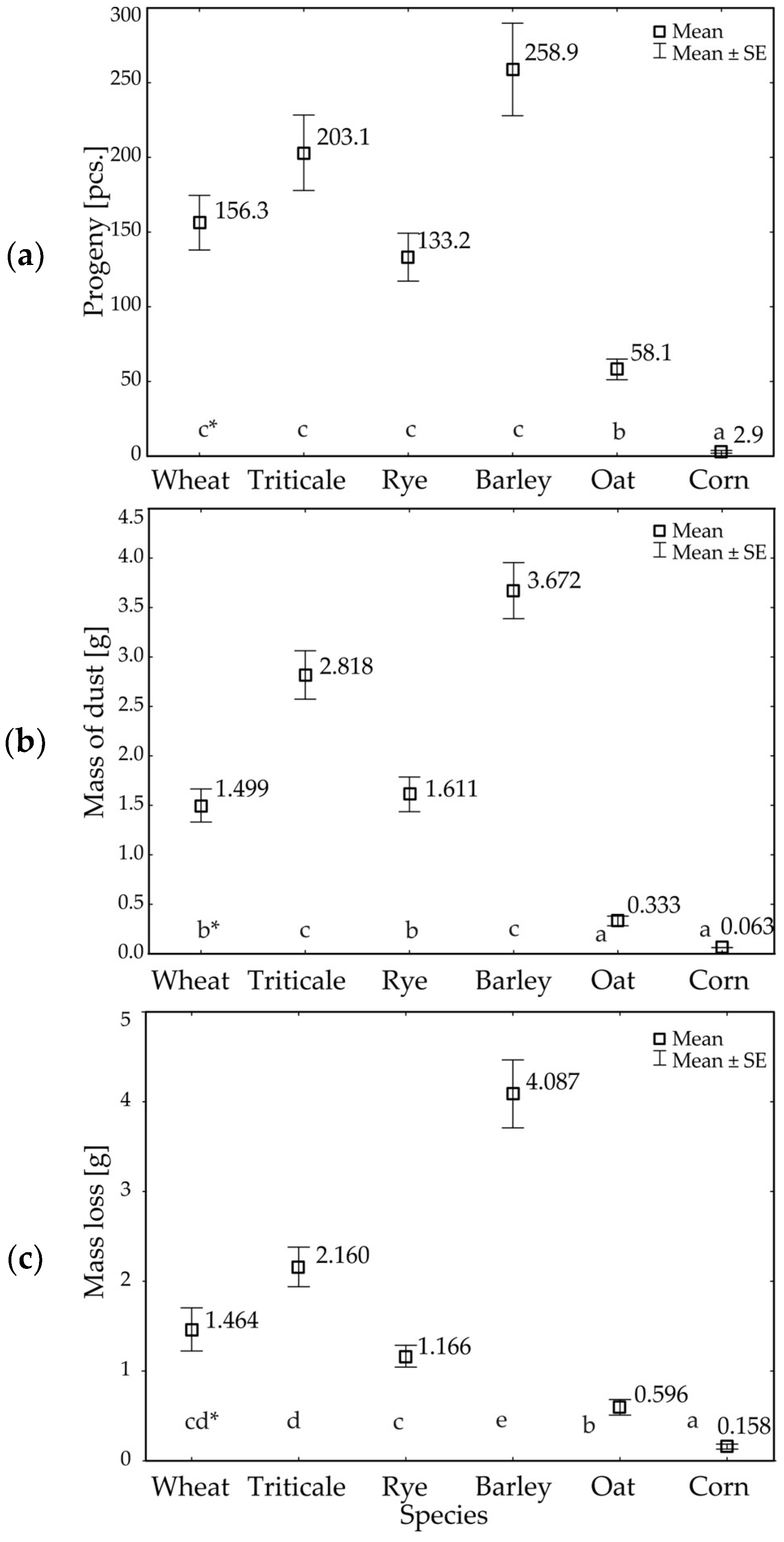
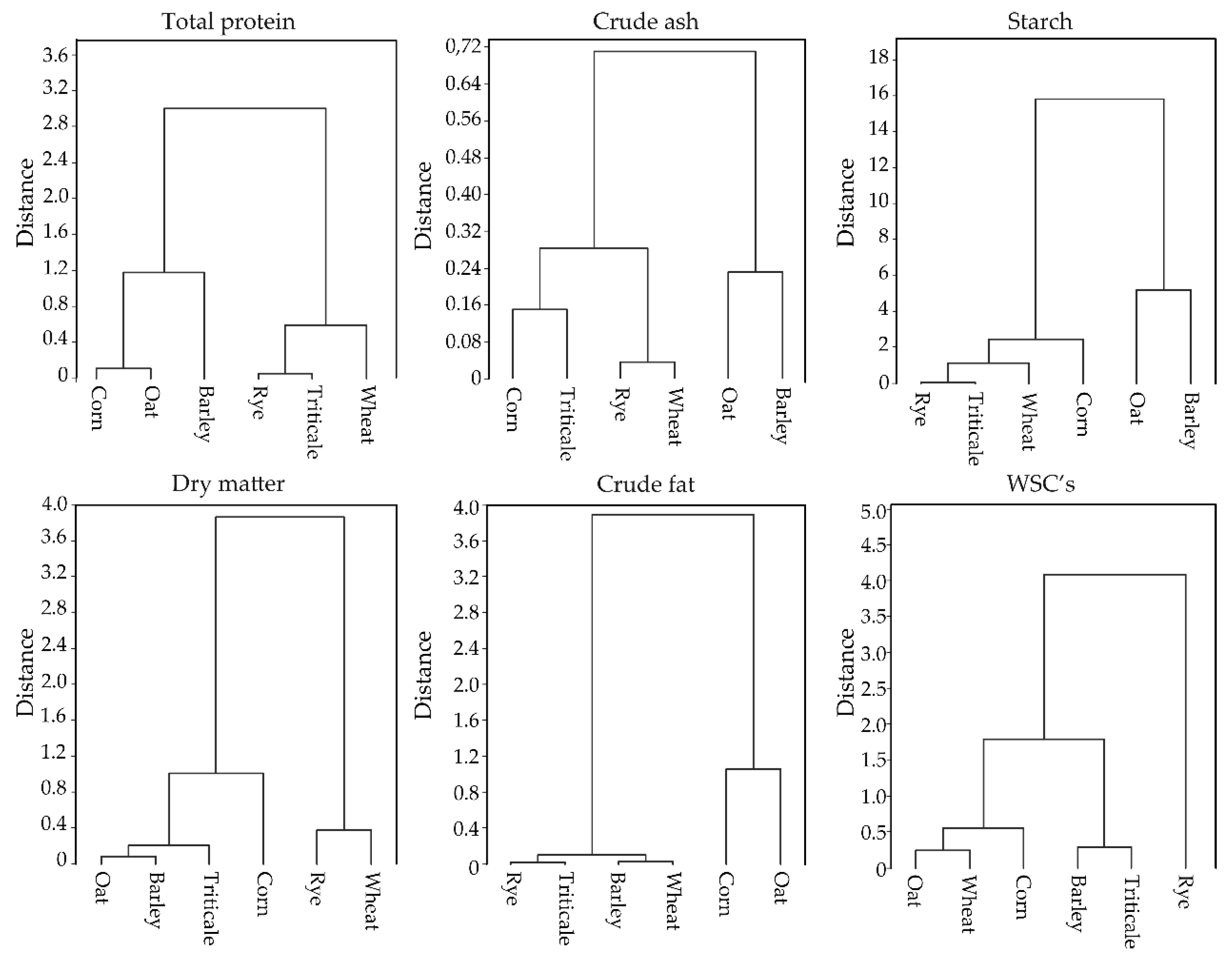
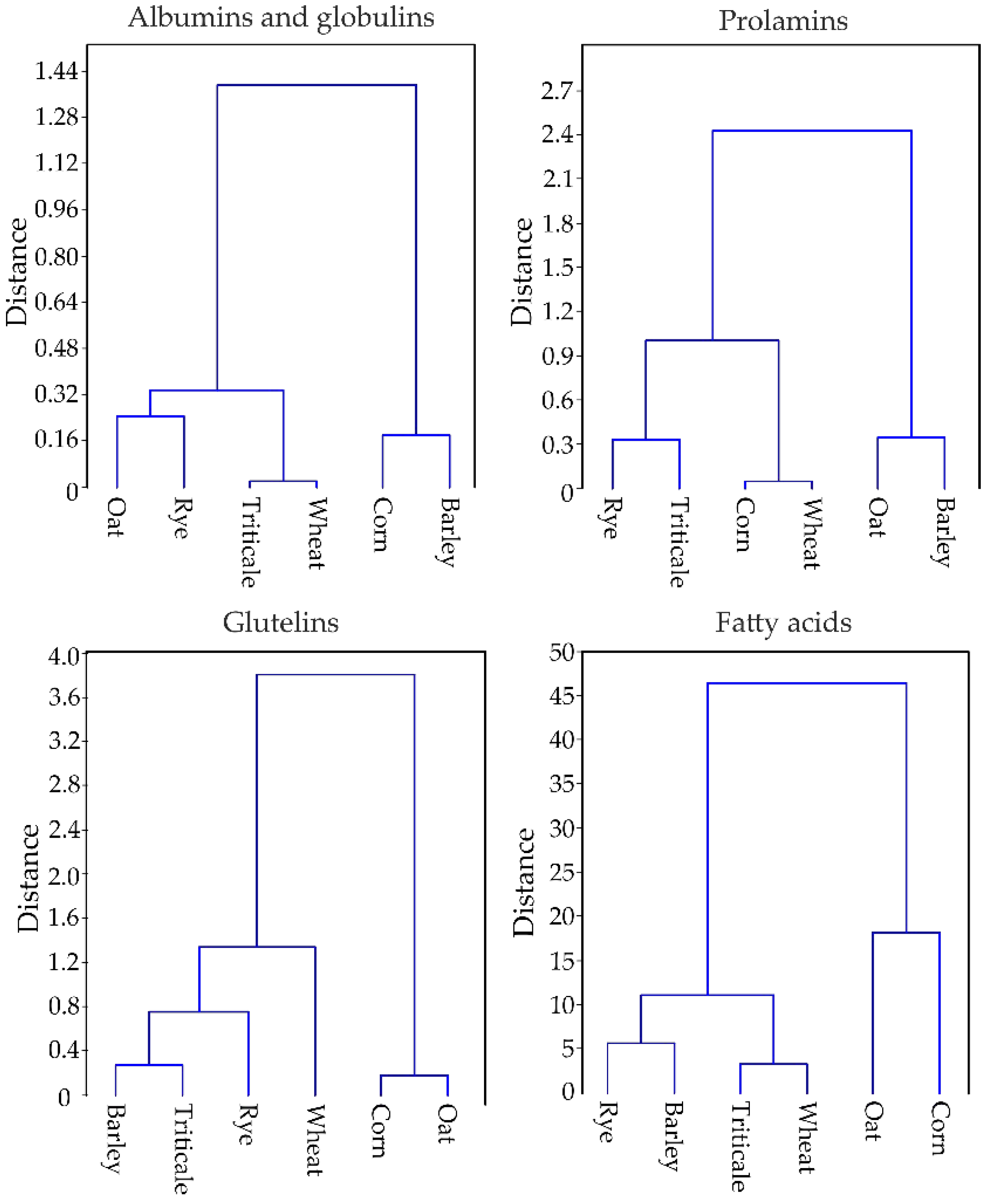
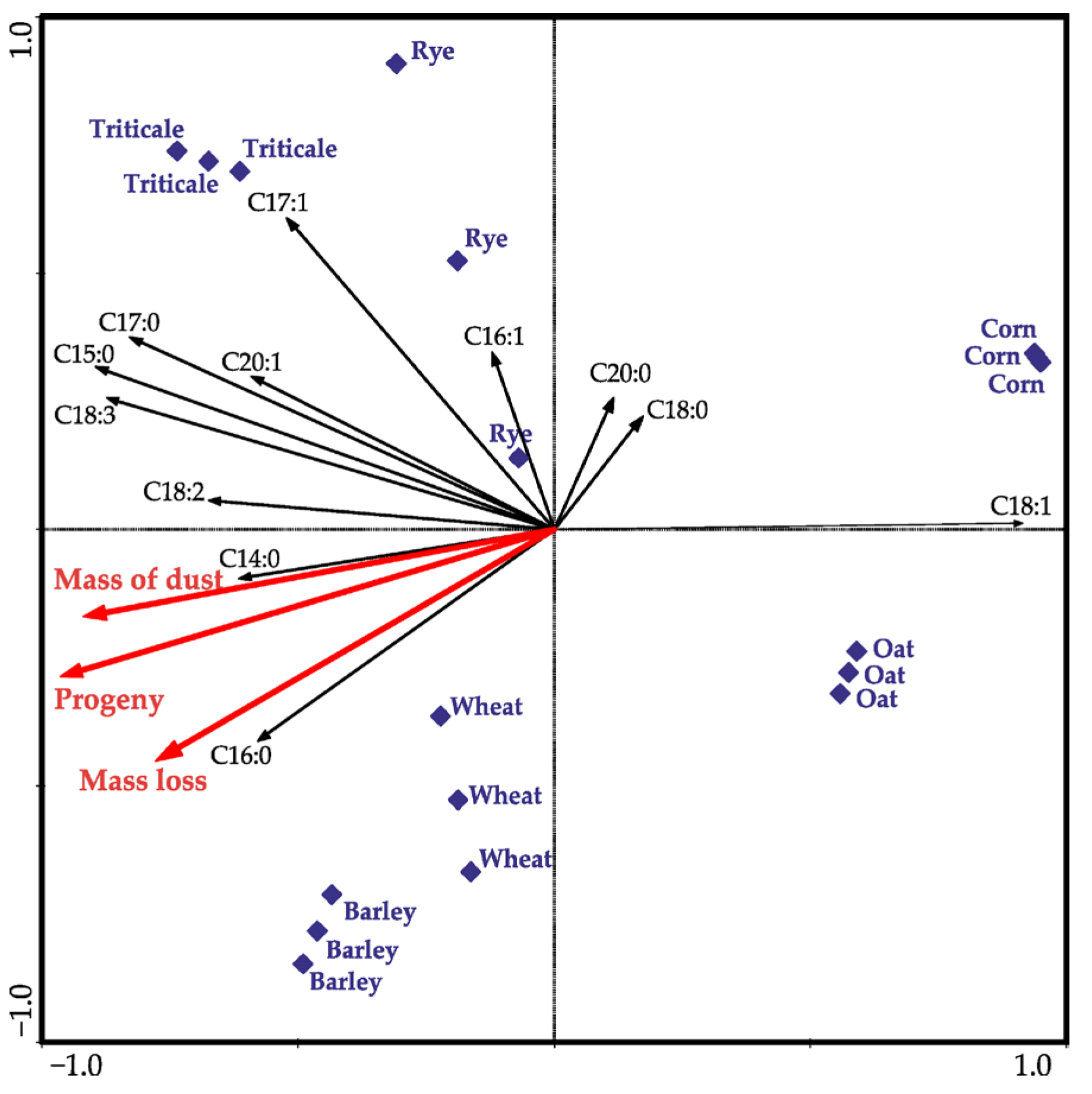
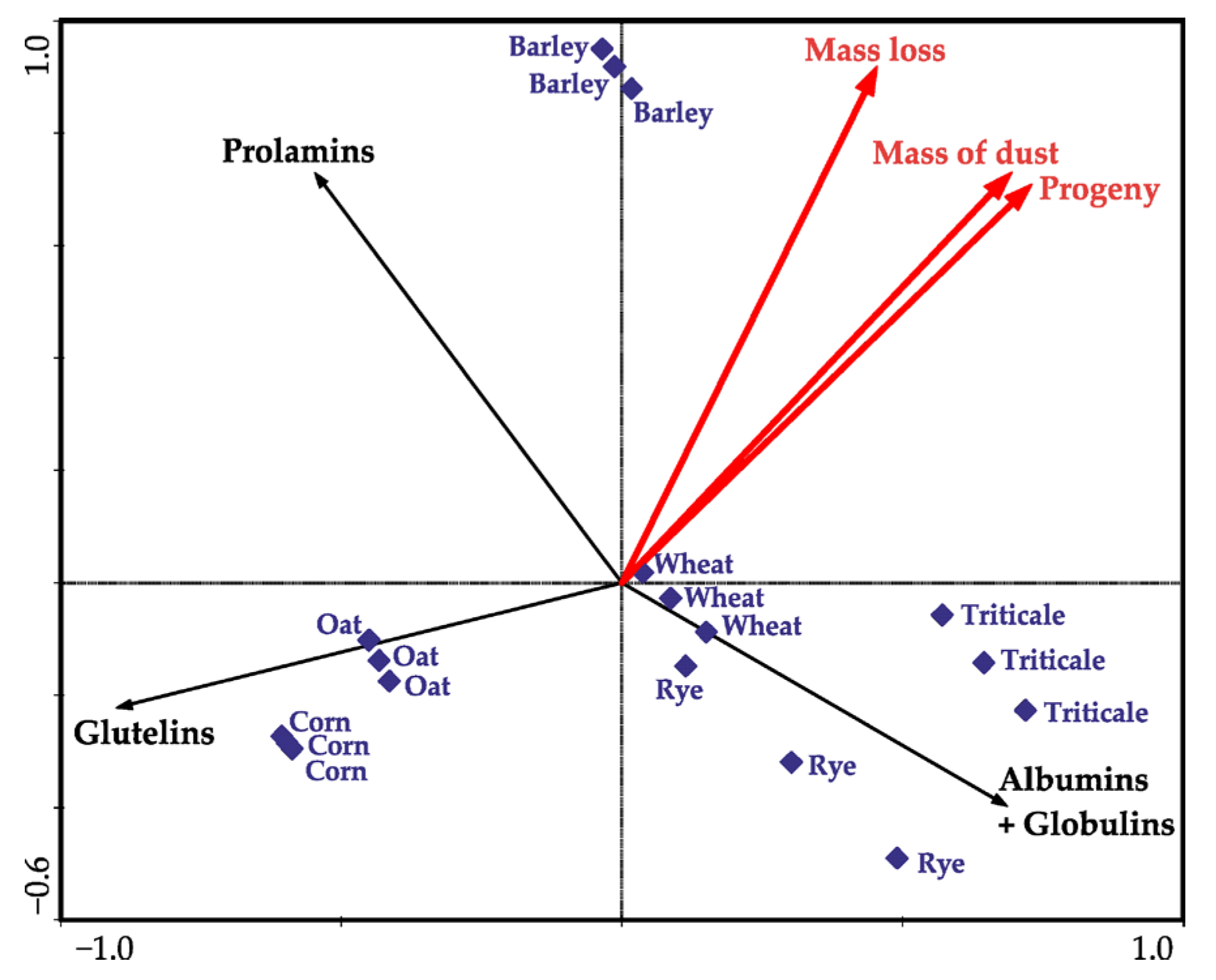
| df * | F Values | p ** | |
|---|---|---|---|
| Progeny of beetles | 5 | 81.47 | 0.00 |
| Mass of dust | 5 | 92.54 | 0.00 |
| Loss of grain mass | 5 | 55.65 | 0.00 |
| df * | F Values | p ** | |
|---|---|---|---|
| Dry matter | 5 | 757.42 | 0.00 |
| Crude ash | 5 | 254.79 | 0.00 |
| Total protein | 5 | 421.00 | 0.00 |
| Crude fat | 5 | 660.25 | 0.00 |
| Starch | 5 | 14,190.98 | 0.00 |
| WSCs | 5 | 6834.16 | 0.00 |
| Wheat | Triticale | Rye | Barley | Oat | Corn | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry matter | 89.59 ± 0.09 | e * | 86.22 ± 0.08 | b | 89.05 ± 0.07 | d | 86.52 ± 0.06 | c | 86.40 ± 0.04 | bc | 85.24 ± 0.01 | a |
| Crude ash | 1.6 ± 0.00 | c | 1.49 ± 0.02 | b | 1.65 ± 0.04 | c | 1.87 ± 0.02 | d | 2.2 ± 0.02 | e | 1.28 ± 0.01 | a |
| Total protein | 12.4 ± 0.04 | d | 11.63 ± 0.06 | c | 11.72 ± 0.04 | c | 10.67 ± 0.06 | b | 9.15 ± 0.11 | a | 9.32 ± 0.03 | a |
| Crude fat | 0.60 ± 0.03 | a | 0.5 ± 0.03 | a | 0.48 ± 0.02 | a | 0.56 ± 0.02 | a | 3.03 ± 0.18 | b | 4.53 ± 0.07 | c |
| Starch | 61.17 ± 0.06 | d | 59.83 ± 0.05 | c | 59.78 ± 0.09 | c | 51.82 ± 0.04 | b | 44.44 ± 0.06 | a | 62.8 ± 0.06 | e |
| WSCs | 2.65 ± 0.01 | c | 3.61 ± 0.01 | d | 6.92 ± 0.01 | f | 4.02 ± 0.02 | e | 2.3 ± 0.03 | b | 1.87 ± 0.01 | a |
| df * | F Values | p ** | |
|---|---|---|---|
| Fatty acids/Name of Acid | |||
| C 14:0/myristic acid | 5 | 199.98 | 0.00 |
| C 15:0/pentadecanoic acid | 5 | 83.87 | 0.00 |
| C 16:0/palmitic acid | 5 | 945.12 | 0.00 |
| C 16:1/palmitoleic acid | 5 | 109.26 | 0.00 |
| C 17:0/margaric acid | 5 | 92.1 | 0.00 |
| C 17:1/margaroleic acid | 5 | 217.87 | 0.00 |
| C 18:0/stearic acid | 5 | 114.44 | 0.00 |
| C 18:1/oleic acid | 5 | 4061.95 | 0.00 |
| C 18:2/inoleic acid | 5 | 614.30 | 0.00 |
| C 18:3/α-linoleic acid | 5 | 12,384.3 | 0.00 |
| C 20:0/araquinic acid | 5 | 89.89 | 0.00 |
| C 21:0/henicosanoic acid | 5 | 1756.2 | 0.00 |
| Proteins | |||
| Albumins + globulins | 5 | 101.48 | 0.00 |
| Prolamins | 5 | 557.23 | 0.00 |
| Glutelin | 5 | 645.22 | 0.00 |
| Wheat | Triticale | Rye | Barley | Oat | Corn | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C 14:0 * | 0.07 ± 0.01 | b | 0.18 ± 0.01 | c | 0.24 ± 0.01 | d | 0.26 ± 0.00 | d | 0.18 ± 0.02 | c | 0.01 ± 0.00 | a |
| C 15:0 | 0.05 ± 0.01 | b | 0.14 ± 0.01 | c | 0.13 ± 0.01 | c | 0.11 ± 0.01 | c | 0.01 ± 0.01 | a | 0.01 ± 0.01 | a |
| C 16:0 | 16.59 ± 0.06 | b | 18.39 ± 0.03 | c | 18.16 ± 0.04 | c | 22.12 ± 0.03 | d | 21.45 ± 0.02 | d | 8.91 ± 0.18 | a |
| C 16:1 | 0.08 ± 0.01 | b | 0.10 ± 0.01 | c | 0.18 ± 0.01 | e | 0.07 ± 0.01 | ab | 0.15 ± 0.01 | d | 0.05 ± 0.00 | a |
| C 17:0 | 0.07 ± 0.01 | b | 0.11 ± 0.01 | c | 0.12 ± 0.00 | c | 0.07 ± 0.01 | b | 0.02 ± 0.01 | a | 0.02 ± 0.01 | a |
| C 17:1 | 0.02 ± 0.00 | b | 0.20 ± 0.01 | c | 0.32 ± 0.01 | d | 0.07 ± 0.01 | bc | 0.05 ± 0.01 | b | 0.01 ± 0.01 | a |
| C 18:0 | 0.91 ± 0.02 | a | 1.36 ± 0.01 | b | 2.03 ± 0.01 | d | 1.62 ± 0.01 | c | 1.87 ± 0.08 | d | 1.44 ± 0.04 | b |
| C 18:1 | 13.66 ± 0.09 | c | 11.60 ± 0.04 | a | 17.87 ± 0.05 | d | 12.93 ± 0.09 | b | 46.84 ± 0.96 | f | 39.19 ± 0.07 | e |
| C 18:2 | 64.39 ± 0.12 | e | 61.273 ± 0.03 | e | 52.54 ± 0.09 | c | 57.07 ± 0.08 | d | 28.36 ± 0.82 | a | 49.48 ± 0.27 | b |
| C 18:3 | 3.63 ± 0.04 | c | 5.66 ± 0.01 | e | 6.85 ± 0.01 | f | 4.74 ± 0.08 | d | 0.31 ± 0.01 | a | 0.51 ± 0.01 | b |
| C 20:0 | 0.10 ± 0.01 | a | 0.17 ± 0.01 | b | 0.26 ± 0.01 | c | 0.25 ± 0.01 | c | 0.15 ± 0.01 | b | 0.26 ± 0.01 | c |
| C 21:0 | 0.44 ± 0.00 | b | 0.81 ± 0.01 | e | 1.3 ± 0.01 | f | 0.68 ± 0.02 | d | 0.63 ± 0.01 | c | 0.11 ± 0.01 | a |
| Albumins + globulins | 4.03 ± 0.02 | d | 4.00 ± 0.05 | d | 3.96 ± 0.11 | d | 2.87 ± 0.01 | b | 3.61 ± 0.07 | c | 2.61 ± 0.07 | a |
| Prolamins | 2.17 ± 0.01 | c | 1.49 ± 0.08 | b | 1.02 ± 0.05 | a | 3.87 ± 0.02 | e | 3.38 ± 0.02 | d | 2.24 ± 0.06 | c |
| Glutelin | 4.25 ± 0.07 | d | 3.05 ± 0.05 | b | 2.39 ± 0.06 | a | 3.45 ± 0.05 | c | 6.24 ± 0.01 | e | 6.49 ± 0.09 | e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ludwiczak, E.; Nietupski, M.; Gabryś, B.; Purwin, C.; Kordan, B. Selected Chemical Parameters of Cereal Grain Influencing the Development of Rhyzopertha dominica F. Sustainability 2024, 16, 7178. https://doi.org/10.3390/su16167178
Ludwiczak E, Nietupski M, Gabryś B, Purwin C, Kordan B. Selected Chemical Parameters of Cereal Grain Influencing the Development of Rhyzopertha dominica F. Sustainability. 2024; 16(16):7178. https://doi.org/10.3390/su16167178
Chicago/Turabian StyleLudwiczak, Emilia, Mariusz Nietupski, Beata Gabryś, Cezary Purwin, and Bożena Kordan. 2024. "Selected Chemical Parameters of Cereal Grain Influencing the Development of Rhyzopertha dominica F." Sustainability 16, no. 16: 7178. https://doi.org/10.3390/su16167178
APA StyleLudwiczak, E., Nietupski, M., Gabryś, B., Purwin, C., & Kordan, B. (2024). Selected Chemical Parameters of Cereal Grain Influencing the Development of Rhyzopertha dominica F. Sustainability, 16(16), 7178. https://doi.org/10.3390/su16167178






