Unravelling Complex Interaction among Coastal Management and Marine Biodiversity: A Case Study in Southern Spain
Abstract
1. Introduction
2. Materials and Methods
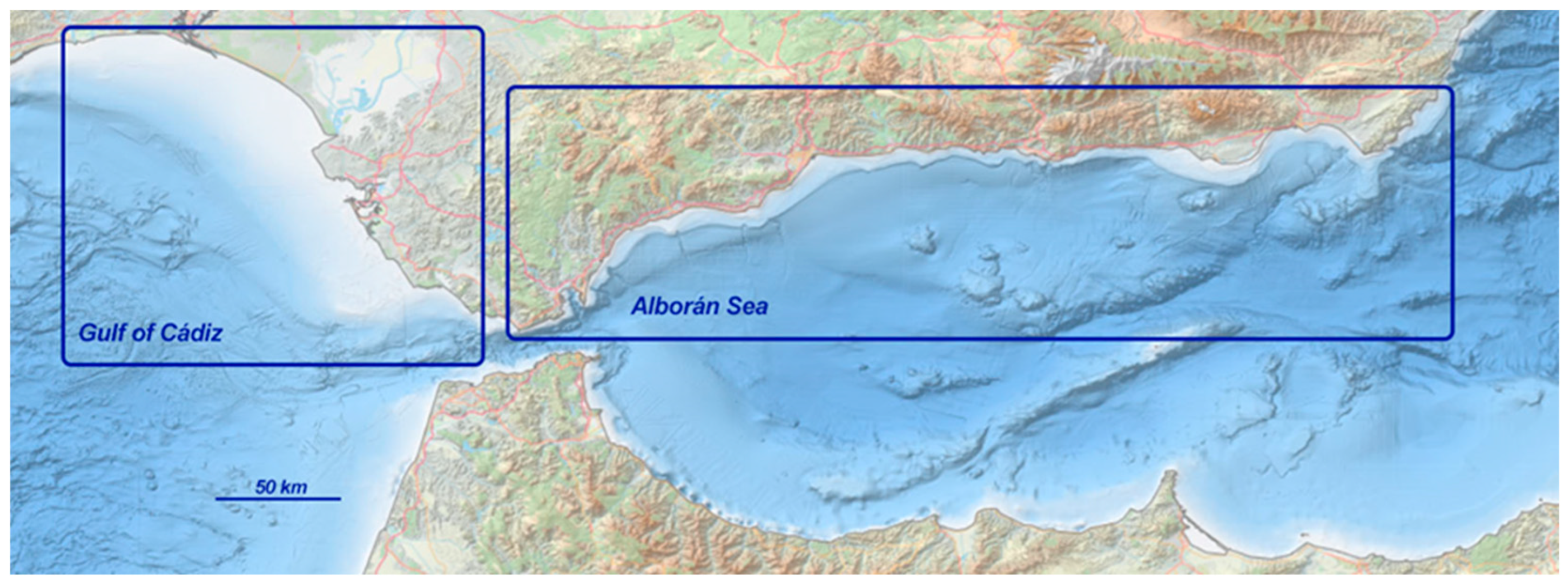
2.1. Study Area
2.2. Indicators Selection and Data Sources
2.3. Temporal Trends of Indicators
2.4. Conceptual Model and Statistical Analysis
2.5. Evaluation of the Path Regression Model
2.6. Final Indicators Selection
3. Results
Model Evaluations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Connor, S.; Ono, R.; Clarkson, C. Pelagic Fishing at 42,000 Years Before the Present and the Maritime Skills of Modern Humans. Science 2011, 334, 1117–1121. [Google Scholar] [CrossRef]
- Stringer, C.B.; Finlayson, J.C.; Barton, R.N.E.; Fernández-Jalvo, Y.; Cáceres, I.; Sabin, R.C.; Rhodes, E.J.; Currant, A.P.; Rodríguez-Vidal, J.; Giles-Pacheco, F.; et al. Neanderthal exploitation of marine mammals in Gibraltar. Proc. Natl. Acad. Sci. USA 2008, 105, 14319–14324. [Google Scholar] [CrossRef]
- Liu, J.; Dietz, T.; Carpenter, S.R.; Folke, C.; Alberti, M.; Redman, C.L.; Schneider, S.H.; Ostrom, E.; Pell, A.N.; Lubchenco, J.; et al. Coupled Human and Natural Systems. AMBIO 2007, 36, 639–649. [Google Scholar] [CrossRef]
- Pope, K.L.; Pegg, M.A.; Cole, N.W.; Siddons, S.F.; Fedele, A.D.; Harmon, B.S.; Ruskamp, R.L.; Turner, D.R.; Uerling, C.C. Fishing for ecosystem services. J. Environ. Manag. 2016, 183, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, E.; Grande, U.; Franzese, P.; Russo, G. Trends and Evolution in the Concept of Marine Ecosystem Services: An Overview. Water 2021, 13, 2060. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020; 244p. [Google Scholar] [CrossRef]
- Myers, R.A.; Worm, B. Rapid worldwide depletion of predatory fish communities. Nature 2003, 423, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Luypaert, T.; Hagan, J.G.; McCarthy, M.L.; Poti, M. Status of Marine Biodiversity in the Anthropocene. In YOUMARES 9—The Oceans: Our Research, Our Future; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer: Cham, Germany, 2020; pp. 57–82. [Google Scholar] [CrossRef]
- Scientific, Technical and Economic Committee for Fisheries (STECF 2021). Monitoring the Performance of the Common Fisheries Policy (STECF-Adhoc-21-01); EUR 28359 EN; Publications Office of the European Union: Luxembourg, 2021; ISBN 978-92-76-36155-8. [CrossRef]
- Agardy, T.S. Marine Protected Areas and Ocean Conservation; R.G. Landes Company and Academic Press, Inc.: Austin, TX, USA, 1997; 244p, ISBN 1-57059-423-6. [Google Scholar]
- McCAY, B.J.; Jones, P.J.S. Marine Protected Areas and the Governance of Marine Ecosystems and Fisheries. Conserv. Biol. 2011, 25, 1130–1133. [Google Scholar] [CrossRef]
- Edgar, G.J.; Russ, G.R.; Babcock, R.C. Marine protected areas. In Marine Ecology; Connell, S.D., Gillanders, B.M., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 534–565. ISBN 0195553020. [Google Scholar]
- Edgar, G.J.; Stuart-Smith, R.D.; Willis, T.J.; Kininmonth, S.; Baker, S.C.; Banks, S.; Barrett, N.S.; Becerro, M.A.; Bernard, A.T.F.; Berkhout, J.; et al. Global conservation outcomes depend on marine protected areas with five key features. Nature 2014, 506, 216–220. [Google Scholar] [CrossRef]
- Convention on Biological Diversity 2004. Decision adopted by the Conference of the Parties to the convention n biological diversity. VII/5. Marine and coastal biodiversity. In Proceedings of the Conference of the Parties to the Convention on Biological Diversity, Seventh Meeting, Kuala Lumpur, Malaysia, 9–20, 27 February 2004. Available online: https://www.cbd.int/doc/decisions/cop-07/cop-07-dec-05-en.pdf (accessed on 1 November 2022).
- UN Environment. Enabling Effective and Equitable Marine Protected Areas—Guidance on Combining Governance Approaches; Jones, P.J.S., Murray, R.H., Vestergaard, O., Eds.; Regional Seas Reports and Studies: Nairobi, Kenya, 2019; Volume 203, 52p, Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/27790/MPA.pdf?sequence=1&isAllowed=y (accessed on 2 November 2022).
- European Union. Directive 2008/56/EC of the european parliament and of the council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Union. 2008, 164, 19–40. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0056 (accessed on 1 November 2022).
- BOE-A-2010-20050. Ley 41/2010, de 29 de Diciembre, de Protección Del Medio Marino. «BOE» Núm. 317, de 30 de Diciembre de 2010, pp 108464–108488. Available online: https://www.boe.es/eli/es/l/2010/12/29/41 (accessed on 7 November 2022).
- Mace, P. A new role for MSY in single-species and ecosystem approaches to fisheries stock assessment and management. Fish Fish. 2001, 2, 2–32. [Google Scholar] [CrossRef]
- Arcos, J.M.; Louzao, M.; Oro, D. Fishery Ecosystem Impacts and Management in the Mediterranean: Seabirds Point of View. In Proceedings of the Reconciling Fisheries with Conservation: Proceedings of the Fourth World Fisheries Congress, Vancouver, BC, Canada, 2–6 May 2004; Nielsen, J.L., Dodson, J.J., Friedland, K., Hamon, T.R., Musick, J., Verspoor, E., Eds.; American Fisheries Society, Symposium 49: Bethesda, MD, USA, 2008; pp. 587–595. [Google Scholar]
- Hilborn, R. Future directions in ecosystem based fisheries management: A personal perspective. Fish. Res. 2011, 108, 235–239. [Google Scholar] [CrossRef]
- Berg, T.; Fürhaupter, K.; Teixeira, H.; Uusitalo, L.; Zampoukas, N. The Marine Strategy Framework Directive and the ecosystem-based approach—Pitfalls and solutions. Mar. Pollut. Bull. 2015, 96, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, R.; Amoroso, R.O.; Anderson, C.M.; Baum, J.K.; Branch, T.A.; Costello, C.; de Moor, C.L.; Faraj, A.; Hively, D.; Jensen, O.P.; et al. Effective fisheries management instrumental in improving fish stock status. Proc. Natl. Acad. Sci. USA 2020, 117, 2218–2224. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- UNEP-MAP RAC/SPA. The Mediterranean Sea Biodiversity: State of the Ecosystems, Pressures, Impacts and Future Priorities; Bazairi, H., Ben Ha, S., Boer, F., Cebria, D., De Jua, S., Lima, A., Lleonart, J., Torchi, G., Rais, C., Eds.; RAC/SPA: Tunis, Tunisia, 2010; 100p, Available online: https://www.rac-spa.org/sites/default/files/doc_cop/biodiversity.pdf (accessed on 31 October 2022).
- Abdul-Malak, D.; Livingstone, S.R.; Pollard, D.; Polidoro, B.-A.; Cuttelod, A.; Bariche, M.; Bilecenoglu, M.; Carpenter, K.E.; Collette, B.B.; Francour, P.; et al. Overview of the Conservation Status of the Marine Fishes of the Mediterranean Sea; IUCN: Gland, Switzerland; Malaga, Spain, 2011. [Google Scholar]
- Rodríguez, J. Oceanografía del Mar Mediterráneo; Pirámide: Madrid, Spain, 1982; 174p. [Google Scholar]
- Tintoré, J.; La Violette, P.E.; Blade, I.; Cruzado, A. A Study of an Intense Density Front in the Eastern Alboran Sea: The Almeria–Oran Front. J. Phys. Oceanogr. 1988, 18, 1384–1397. [Google Scholar] [CrossRef]
- Rubin, J.P.; Gil, J.; Ruiz, J.; Cortes, M.D.; Jiménez-Gómez, F.; Parada, M.; Rodríguez, J. La distribución ictioplanctónica y su relación con parámetros físicos, químicos y biológicos en el sector norte del Mar de Alboran. Inf. Tec. Inst. Esp. Oceanogr. 1992, 139, 49. [Google Scholar]
- Gil de Sola, L. Las pesquerías demersales del mar de Alborán, Submediterráneo ibérico: Evolución en los últimos decenios. Inf. Técnico Inst. Español Oceanogr. 1993, 142, 1–179. Available online: http://www.ieo.es/es/informes-tecnicos (accessed on 11 November 2022).
- Rodríguez, J. Las Reservas Marinas en el Marco Ecológico y Oceanográfico del Mediterráneo Occidental, in La Gestión de los Espacios Marinos del Mediterráneo Occidental; Guirado, J., Ed.; Instituto de Estudios Almerienses (Diputación de Almería): Almería, Spain, 1995; pp. 13–28. [Google Scholar]
- Cunha, M.R.; Rodrigues, C.F.; Génio, L.; Hilário, A.; Ravara, A.; Pfannkuche, O. Macrofaunal assemblages from mud volcanoes in the Gulf of Cadiz: Abundance, biodiversity and diversity partitioning across spatial scales. Biogeosciences 2013, 10, 2553–2568. [Google Scholar] [CrossRef]
- Díaz del Río, V.; Bruque, G.; Fernández-Salas, L.M.; Rueda, J.L.; González, E.; López, N.; Palomino, D.; López, F.J.; Farias, C.; Sánchez, R.; et al. Volcanes de Fango del Golfo de Cádiz, Proyecto LIFE+ INDEMARES; Fundación Biodiversidad: Madrid, Spain; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2014; pp. 1–128. [Google Scholar]
- Coll, M.; Carreras, M.; Ciércoles, C.; Cornax, M.-J.; Gorelli, G.; Morote, E.; Sáez, R. Assessing Fishing and Marine Biodiversity Changes Using Fishers’ Perceptions: The Spanish Mediterranean and Gulf of Cadiz Case Study. PLoS ONE 2014, 9, e85670. [Google Scholar] [CrossRef]
- Reyes-Martínez, M.J.; Ruiz-Delgado, M.C.; Sánchez-Moyano, J.E.; García-García, F.J. Biodiversity and distribution of macroinfauna assemblages on sandy beaches along the Gulf of Cadiz (SW Spain). Sci. Mar. 2015, 79, 367–377. [Google Scholar] [CrossRef]
- Grinyó, J.; Francescangeli, M.; Santín, A.; Ercilla, G.; Estrada, F.; Mecho, A.; Fanelli, E.; Costa, C.; Danovaro, R.; Company, J.B.; et al. Megafaunal assemblages in deep-sea ecosystems of the Gulf of Cádiz, northeast Atlantic ocean. Deep-Sea Res. 2022, 183 (Pt I), 103738. [Google Scholar] [CrossRef]
- Baillie, J.E.M.; Hilton-Taylor, C.; Stuart, S.N. 2004 IUCN Red List of Threatened Species. A Global Species Assessment; IUCN Press: Cambridge, MA, USA, 2004; Available online: https://www.iucn.org/es/node/23917 (accessed on 23 August 2021).
- Bearzi, G.; Reeves, R.R.; di Sciara, G.N.; Politi, E.; Canadas, A.; Frantzis, A.; Mussi, B. Ecology, status and conservation of short-beaked common dolphins Delphinus delphis in the Mediterranean Sea. Mammal. Rev. 2003, 33, 224–252. [Google Scholar] [CrossRef]
- Báez, J.C.; Real, R.; Camiñas, J.A. Differential distribution within longline transects of loggerhead and swordfish captured by the Spanish Mediterranean surface longline fishery. J. Mar. Biol. Assoc. 2007, 87, 801–803. [Google Scholar] [CrossRef]
- Báez, J.; Real, R.; García-Soto, C.; De La Serna, J.; Macias, D.; Camiñas, J. Loggerhead turtle by-catch depends on distance to the coast, independent of fishing effort: Implications for conservation and fisheries management. Mar. Ecol. Prog. Ser. 2007, 338, 249–256. [Google Scholar] [CrossRef]
- Báez, J.C.; Camiñas, J.A.; Sagarminaga, R.; Torreblanca, D.; Real, R. Capturas no dirigidas de tortuga boba (Caretta caretta Linnaeus, 1758) en aguas de Andalucía y Murcia durante 2004. Munibe 2007, 25, 196–201. [Google Scholar]
- Arcos, J.M.; Bécares, J.; Rodríguez, B.; Ruiz, A. Important Areas for the Conservation of Seabirds in Spain; LIFE04NAT/ES/000049-Sociedad Española de Ornitología (SEO/BirdLife): Madrid, Spain, 2009; pp. 198–224. Available online: https://www.researchgate.net/publication/229067402_Areas_Importantes_para_la_Conservacion_de_las_Aves_Marinas_en_Espana (accessed on 12 November 2022).
- Camiñas, J.A. Estatus y conservación de las Tortugas Marinas en España. In Libro Rojo de los Vertebrados Amenazados; de Andalucía Franco, A., Rodríguez, M., Eds.; Consejería de Medio Ambiente de la Junta de Andalucía: Sevilla, Spain, 2012; pp. 347–380. [Google Scholar]
- Croxall, J.P.; Butchart, S.H.M.; Lascelles, B.; Stattersfield, A.J.; Sullivan, B.; Symes, A.; Taylor, P. Seabird conservation status, threats and priority actions: A global assessment. Bird Conserv. Int. 2012, 22, 1–34. [Google Scholar] [CrossRef]
- de Stephanis, R.; García-Tíscar, S.; Verborgh, P.; Esteban-Pavo, R.; Pérez, S.; Minvielle-Sebastia, L.; Guinet, C. Diet of the social groups of long-finned pilot whales (Globicephala melas) in the Strait of Gibraltar. Mar. Biol. 2008, 154, 603–612. [Google Scholar] [CrossRef]
- WWF. Red Natura 2000 Marina en España. 2014. 151 p. Available online: https://wwfes.awsassets.panda.org/downloads/libro_red_natura_200_marina_en_espana_c.pdf?_ga=2.233157275.483627554.1580121547–1583122700.1572448035 (accessed on 9 November 2022).
- Wold, H. Model construction and evaluation when theoretical knowledge is scarce. In Evaluation of Econometric Models; Kmenta, J., Ramsey, J.B., Eds.; Academic Press: Cambridge, MA, USA, 1980; pp. 47–74. Available online: http://www.nber.org/chapters/c11693 (accessed on 15 February 2023).
- Esposito-Vinzi, V.; Trinchera, L.; Amato, S. PLS Path Modeling: From Foundations to Recent Developments and Open Issues for Model Assessment and Improvement. In Handbook of Partial Least Squares, Concepts, Methods and Applications; Esposito-Vinzi, V., Chin, W.W., Henseler, J., Wang, H., Eds.; Springer Handbooks of Computational Statistics, Springer-Verlag: Berlin/Heidelberg, Germany, 2010; pp. 47–82. [Google Scholar]
- Sánchez, G. PLS Path Modeling with R; Trowchez Editions; University of California: Berkeley, CA, USA, 2013; 235p, Available online: http://www.gastonsanchez.com/PLSPathModelingwithR.pdf (accessed on 16 September 2022).
- Selim, S.A.; Blanchard, J.L.; Bedford, J.; Webb, T.J. Direct and indirect effects of climate and fishing on changes in coastal ecosystem services: A historical perspective from the North Sea. Reg. Environ. Chang. 2014, 16, 341–351. [Google Scholar] [CrossRef]
- Fu, C.; Large, S.; Knight, B.; Richardson, A.J.; Bundy, A.; Reygondeau, G.; Boldt, J.; van der Meeren, G.I.; Torres, M.A.; Sobrino, I.; et al. Relationships among fisheries exploitation, environmental conditions, and ecological indicators across a series of marine ecosystems. J. Mar. Syst. 2015, 148, 101–111. [Google Scholar] [CrossRef]
- Coll, M.; Shannon, L.; Kleisner, K.; Juan-Jordá, M.; Bundy, A.; Akoglu, A.; Banaru, D.; Boldt, J.; Borges, M.; Cook, A.; et al. Ecological indicators to capture the effects of fishing on biodiversity and conservation status of marine ecosystems. Ecol. Indic. 2016, 60, 947–962. [Google Scholar] [CrossRef]
- Jouffray, J.B.; Blasiak, R.; Norström, A.V.; Österblom, H.; Nyström, M. The Blue Acceleration: The Trajectory of Human Expansion into the Ocean. One Earth 2020, 2, 43–54. [Google Scholar] [CrossRef]
- Abdulla, A.; Gomei, M.; Maison, E.; Piante, C. Status of Marine Protected Areas in the Mediterranean Sea; IUCN: Málaga, Spain; WWF: Gland, Switzerland, 2008; 152p. [Google Scholar]
- Borja, A.; Elliott, M.; Andersen, J.H.; Berg, T.; Carstensen, J.; Halpern, B.S.; Heiskanen, A.-S.; Korpinen, S.; Lowndes, J.S.S.; Martin, G.; et al. Overview of Integrative Assessment of Marine Systems: The Ecosystem Approach in Practice. Front. Mar. Sci. 2016, 3, 20. [Google Scholar] [CrossRef]
- Cochrane, S.K.J.; Andersen, J.H.; Berg, T.; Blanchet, H.; Borja, A.; Carstensen, J.; Elliott, M.; Hummel, H.; Niquil, N.; Renaud, P.E. What Is Marine Biodiversity? Towards Common Concepts and Their Implications for Assessing Biodiversity Status. Front. Mar. Sci. 2016, 3, 248. [Google Scholar] [CrossRef]
- Uusitalo, L.; Blanchet, H.; Andersen, J.H.; Beauchard, O.; Berg, T.; Bianchelli, S.; Cantafaro, A.; Carstensen, J.; Carugati, L.; Cochrane, S.; et al. Indicator-Based Assessment of Marine Biological Diversity–Lessons from 10 Case Studies across the European Seas. Front. Mar. Sci. 2016, 3, 159. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Coll, M.; Fraschetti, S.; Giakoumi, S.; Goldsborough, D.; Mačić, V.; Mackelworth, P.; Rilov, G.; Stelzenmüller, V.; Albano, P.G.; et al. Twelve Recommendations for Advancing Marine Conservation in European and Contiguous Seas. Front. Mar. Sci. 2020, 7, 565968. [Google Scholar] [CrossRef]
- Vasilakopoulos, P.; Maravelias, C.D.; Tserpes, G. The Alarming Decline of Mediterranean Fish Stocks. Curr. Biol. 2014, 24, 1643–1648. [Google Scholar] [CrossRef]
- Piroddi, C.; Coll, M.; Steenbeek, J.; Moy, D.M.; Christensen, V. Modelling the Mediterranean marine ecosystem as a whole: Addressing the challenge of complexity. Mar. Ecol. Prog. Ser. 2015, 533, 47–65. [Google Scholar] [CrossRef]
- Piroddi, C.; Coll, M.; Liquete, C.; Macias, D.; Greer, K.; Buszowski, J.; Steenbeek, J.; Danovaro, R.; Christensen, V. Historical changes of the Mediterranean Sea ecosystem: Modelling the role and impact of primary productivity and fisheries changes over time. Sci. Rep. 2017, 7, srep44491. [Google Scholar] [CrossRef]
- Guidetti, P.; Di Franco, A.; Calò, A.; Belharet, M.; Claudet, J.; Carbonara, P.; Coll, M.; Corrales, X.; Font, T.; Ligas, A.; et al. Marine Protected Areas: Network(s) for Enhancement of Sustainable Fisheries in EU Mediterranean Waters Safe Net: Sustainable Fisheries in EU Mediterranean Waters through Network of MPAs; Publications Office of the EU: Luxembourg, 2019. Available online: https://op.europa.eu/fr/publication-detail/-/publication/d6a7167b-f17a-11ea-991b-01aa75ed71a1/language-en/format-PDF/source-153083541 (accessed on 16 November 2022).
- Bevilacqua, S.; Katsanevakis, S.; Micheli, F.; Sala, E.; Rilov, G.; Sarà, G.; Malak, D.A.; Abdulla, A.; Gerovasileiou, V.; Gissi, E.; et al. The Status of Coastal Benthic Ecosystems in the Mediterranean Sea: Evidence from Ecological Indicators. Front. Mar. Sci. 2020, 7, 475. [Google Scholar] [CrossRef]
- Claudet, J.; Loiseau, C.; Sostres, M.; Zupan, M. Underprotected Marine Protected Areas in a Global Biodiversity Hotspot. One Earth 2020, 2, 380–384. [Google Scholar] [CrossRef]
- Torres, M.; Coll, M.; Heymans, J.J.; Christensen, V.; Sobrino, I. Food-web structure of and fishing impacts on the Gulf of Cadiz ecosystem (South-western Spain). Ecol. Model. 2013, 265, 26–44. [Google Scholar] [CrossRef]
- Tudela, S.; Kai, A.K.; Maynou, F.; El Andalossi, M.; Guglielmi, P. Driftnet fishing and biodiversity conservation: The case study of the large-scale Moroccan driftnet fleet operating in the Alboran Sea (SW Mediterranean). Biol. Conserv. 2005, 121, 65–78. [Google Scholar] [CrossRef]
- Commission Decision (EU). 2017/848 of 17 May 2017 laying down criteria and methodological standards on good environmental status of marine waters and specifications and standardised methods for monitoring and assessment, and repealing Decision 2010/477/EU (Text with EEA relevance). C/2017/2901. Off. J. Eur. Union L 2017, 125, 43–74. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017D0848 (accessed on 3 November 2022).
- Convention on Biological Diversity 2010. Decision Adopted by The Conference Of The Parties To The Convention On Biological Diversity at its Tenth Meeting X/2. In Proceedings of the Strategic Plan for Biodiversity 2011–2020 and the Aichi Biodiversity Targets, Nagoya, Japan, 18–29 October 2010; Agenda Item 4.4. Available online: https://www.cbd.int/doc/decisions/cop-10/cop-10-dec-02-en.pdf (accessed on 3 November 2022).
- Acosta, J.J.; Ámez, M.A.; Araujo, H.; Castro, B.; Castro, J.A.; Cebrián, J.L.; Gancedo, R.; García, J.M.; Marín, M.; Morlán, R.; et al. Análisis de la Actividad Pesquera de la Flota Española de Aguas Ibéricas Atlánticas y su Uso en la Gestión de Stocks (2017-2019). Proyecto SAP: Seguimiento y Análisis de la Actividad Pesquera en el Área ICES. SAP: Seguimiento y Análisis de la Actividad Pesquera en el Área ICES; 2019; 84p. Available online: http://www.ieo.es/documents/2796493/5496504/SAP-Analisis_actividad_pesquera_espa%C3%B1ola_aguas_atlanticas_2017-19/36310d6f-0487-4208-9393-8a8436b7416b (accessed on 5 September 2022). [CrossRef]
- Mora, C.; Sale, P. Ongoing global biodiversity loss and the need to move beyond protected areas: A review of the technical and practical shortcomings of protected areas on land and sea. Mar. Ecol. Prog. Ser. 2011, 434, 251–266. [Google Scholar] [CrossRef]
- Shanks, A.L.; Grantham, B.A.; Carr, M.H. Propagule dispersal distance and the size and spacing of marine reserves. Ecol. Appl. 2003, 13, 159–169. [Google Scholar] [CrossRef]
- Palumbi, S.R. Marine reserves and ocean neighborhoods: The Spatial Scale of Marine Populations and Their Management. Annu. Rev. Environ. Resour. 2004, 29, 31–68. [Google Scholar] [CrossRef]
- García-Charton, J.A.; Pérez-Ruzafa, A.; Sánchez-Jerez, P.; Bayle-Sempere, J.T.; Reñones, O.; Moreno, D. Multi-scale spatial heterogeneity, habitat structure, and the effect of marine reserves on Western Mediterranean rocky reef fish assemblages. Mar. Biol. 2004, 144, 161–182. [Google Scholar] [CrossRef]
- García-Charton, J.; Pérez-Ruzafa, A.; Marcos, C.; Claudet, J.; Badalamenti, F.; Benedetti-Cecchi, L.; Falcón, J.; Milazzo, M.; Schembri, P.J.; Stobart, B.; et al. Effectiveness of European Atlanto-Mediterranean MPAs: Do they accomplish the expected effects on populations, communities and ecosystems? J. Nat. Conserv. 2008, 16, 193–221. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; González-Wangüemert, M.; Lenfant, P.; Marcos, C.; García-Charton, J.A. Effects of fishing protection on the genetic structure of fish populations. Biol. Conserv. 2006, 129, 244–255. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; García-Charton, J.A.; Marcos, C. North East Atlantic vs. Mediterranean Marine Protected Areas as Fisheries Management Tool. Front. Mar. Sci. 2017, 4, 245. [Google Scholar] [CrossRef]
- Hogg, K.; Noguera-Méndez, P.; Semitiel-García, M.; Gray, T.; Young, S. Controversies over stakeholder participation in marine protected area (MPA) management: A case study of the Cabo de Palos-Islas Hormigas MPA. Ocean Coast. Manag. 2017, 144, 120–128. [Google Scholar] [CrossRef]
- Hogg, K.; Semitiel-García, M.; Noguera-Méndez, P.; García-Charton, J.A. A governance analysis of Cabo de Palos-Islas Hormigas and Cabo de Gata-Níjar Marine Protected Areas, Spain. Mar. Policy 2021, 127, 102944. [Google Scholar] [CrossRef]
- Fox, H.E.; Mascia, M.B.; Basurto, X.; Costa, A.; Glew, L.; Heinemann, D.; Karrer, L.B.; Lester, S.E.; Lombana, A.V.; Pomeroy, R.S.; et al. Reexamining the science of marine protected areas: Linking knowledge to action. Conserv. Lett. 2011, 5, 1–10. [Google Scholar] [CrossRef]
- Rojo, I.; Anadón, J.D.; García-Charton, J.A. Exceptionally high but still growing predatory reef fish biomass after 23 years of protection in a Marine Protected Area. PLoS ONE 2021, 16, e0246335. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.; García-Bellido, E.; Prados, S. La Red Natura 2000 marina. Ambienta 2017, 119, 82–95. Available online: https://www.miteco.gob.es/ministerio/pags/Biblioteca/Revistas/pdf_AM%2FPDF_AM_Ambienta_2017_119_82_95.pdf (accessed on 4 October 2021).
- Cabral, R.B.; Bradley, D.; Mayorga, J.; Goodell, W.; Friedlander, A.M.; Sala, E.; Costello, C.; Gaines, S.D. A global network of marine protected areas for food. Proc. Natl. Acad. Sci. USA 2020, 117, 28134–28139. [Google Scholar] [CrossRef]
- Vilas, D.; Coll, M.; Corrales, X.; Steenbeek, J.; Piroddi, C.; Calò, A.; Di Franco, A.; Font, T.; Guidetti, P.; Ligas, A.; et al. The effects of marine protected areas on ecosystem recovery and fisheries using a comparative modelling approach. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1885–1901. [Google Scholar] [CrossRef]
- Ban, N.C.; Davies, T.E.; Aguilera, S.E.; Brooks, C.; Cox, M.; Epstein, G.; Evans, L.S.; Maxwell, S.M.; Nenadovic, M. Social and ecological effectiveness of large marine protected areas. Glob. Environ. Chang. 2017, 43, 82–91. [Google Scholar] [CrossRef]
- EC (1995) 95/540/EC: Council Decision of 7 December 1995 on the Conclusion of an Agreement in the Form of An Exchange of Letters Concerning the Provisional Application of the Agreement on Cooperation in the Sea Fisheries Sector between the European Community and the Kingdom of Morocco Initialled in Brussels on 13 November 1995. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31995D0540 (accessed on 3 November 2022).
- de Carvalho-Souza, G.F.; Torres, M.; Farias, C.; Acosta, J.J.; Tornero, J.; Sobrino, I.; Ramos, F.; Llope, M. International politics must be considered together with climate and fisheries regulation as a driver of marine ecosystems. Glob. Environ. Chang. 2021, 69, 102288. [Google Scholar] [CrossRef]
- Airoldi, L.; Beck, M.W. Loss, Status and Trends for Coastal Marine Habitats of Europe. In Oceanography and Marine Biology: An Annual Review 45; Gibson, R.N., Atkinson, R.J.A., Gordon, J.D.M., Eds.; CRC Press: Boca Raton, FL, USA, 2007; Chapter 7; pp. 345–405. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F., Jr. Fishing Down Marine Food Webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Albouy, C.; Ben Rais Lasram, F.; Cheung, W.W.L.; Christensen, V.; Karpouzi, V.S.; Guilhaumon, F.; Mouillot, D.; Paleczny, M.; et al. The Mediterranean Sea under siege: Spatial overlap between marine biodiversity, cumulative threats and marine reserves. Glob. Ecol. Biogeogr. 2012, 21, 465–480. [Google Scholar] [CrossRef]
- Carbonell, A.; Martín, P.; De Ranieri, S.; WEDIS team. Discards of the Western Mediterranean Trawl Fleets. Rapports et Procès-Verbaux des Réunions. Commission Internationale pour L’Exploration Scientifique de la Mer Méditerranée, 35. 1998, pp. 392–393. Available online: http://hdl.handle.net/10508/6895 (accessed on 22 June 2022).
- Pauly, D.; Ulman, A.; Piroddi, C.; Bultel, E.; Coll, M. ‘Reported’ versus ‘likely’ fisheries catches of four Mediterranean countries. Sci. Mar. 2014, 78, 11–17. [Google Scholar] [CrossRef]
- Lloret, J.; Biton-Porsmoguer, S.; Carreño, A.; Di Franco, A.; Sahyoun, R.; Melià, P.; Claudet, J.; Sève, C.; Ligas, A.; Belharet, M.; et al. Recreational and small-scale fisheries may pose a threat to vulnerable species in coastal and offshore waters of the western Mediterranean. ICES J. Mar. Sci. 2019, 77, 2255–2264. [Google Scholar] [CrossRef]
- Casalduero, M.G. La pesca marítima recreativa: En el marco de la política pesquera común. Rev. Catalan-Dret Ambient. 2021, 12, 1–36. [Google Scholar] [CrossRef]
- Francour, P.; Ganteaume, A.; Poulain, M. Effects of boat anchoring in Posidonia oceanica seagrass beds in the Port-Cros National Park (NW Mediterranean Sea). Aquat. Conserv. 1999, 9, 391–400. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V. Primary production required to sustain global fisheries. Nature 1995, 374, 255–257. [Google Scholar] [CrossRef]
- Pauly, D.; Watson, R. Background and interpretation of the ‘Marine Trophic Index’ as a measure of biodiversity. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 415–423. [Google Scholar] [CrossRef]
- Baeta, F.; Costa, M.J.; Cabral, H. Changes in the trophic level of Portuguese landings and fish market price variation in the last decades. Fish. Res. 2009, 97, 216–222. [Google Scholar] [CrossRef]
- Muñoz, J.L.; Cárdenas, S. La pesca artesanal en el caladero de Conil (Golfo de Cádiz). In Acuicultura, Pesca y Marisqueo en el Golfo de Cádiz; Morales, J.L., Mata, A.J., Rodríguez, A., Revilla, C.J., Eds.; Dirección General de Pesca y Acuicultura de la Junta de Andalucía: Sevilla, Spain, 2005; pp. 204–223. Available online: https://www.researchgate.net/publication/258158121_Acuicultura_Pesca_y_Marisqueo_en_el_Golfo_de_Cadiz (accessed on 23 September 2022).
- Camiñas, J.A. Pesquerías Artesanales Mediterráneas. El caso Andaluz. Revista de Estudios Agrosociales. MAPA 1990, 155 (Enero-Marzo) pp. 83–117. Available online: https://www.miteco.gob.es/ministerio/pags/Biblioteca/Revistas/pdf%5Freas%2Fr151%5F04%2Epdf (accessed on 23 September 2022).
- Castilla-Espino, D.; García del Hoyo, J.J.; Domínguez, J.M.; Suárez, A. Un modelo de gestión para las pesquerías de boquerón y voraz basado en la dinámica de sistemas que toma en consideración las condiciones medioambientales. In Cultura, Mercados y Gestión de la Pesca Artesanal en el Golfo de Cádiz García; del Hoyo, J.J., Ed.; Universidad de Huelva, Servicio de Publicaciones: Huelva, Spain, 2014; pp. 45–70. [Google Scholar]
- García del Hoyo, J.J. (Ed.) Cultura, Mercados y Gestión de la Pesca Artesanal en el Golfo de Cádiz; Universidad de Huelva, Servicio de Publicaciones: Huelva, Spain, 2014; 133p. [Google Scholar]
- García del Hoyo, J.J.; Toribio, R.J.; Ordaz, F.G. Análisis de las interrelaciones entre la evolución de la flota atunera española y el sector conservero. Stud. Appl. Econ. 2019, 37, 81–100. [Google Scholar] [CrossRef]
- de Madariaga, C.J.; del Hoyo, J.J.G. Enhancing of the cultural fishing heritage and the development of tourism: A case study in Isla Cristina (Spain). Ocean Coast. Manag. 2018, 168, 1–11. [Google Scholar] [CrossRef]
- Vila, Y.; Silva, L.; Millán, M.; Ramos, F.; Gil, J.; Jiménez, M.P. Los Recursos Pesqueros del Golfo de Cádiz: Estado Actual de Explotación; Informe Técnico; Instituto Español de Oceanografía; 2004; 200p. Available online: http://www.repositorio.ieo.es (accessed on 15 February 2023).

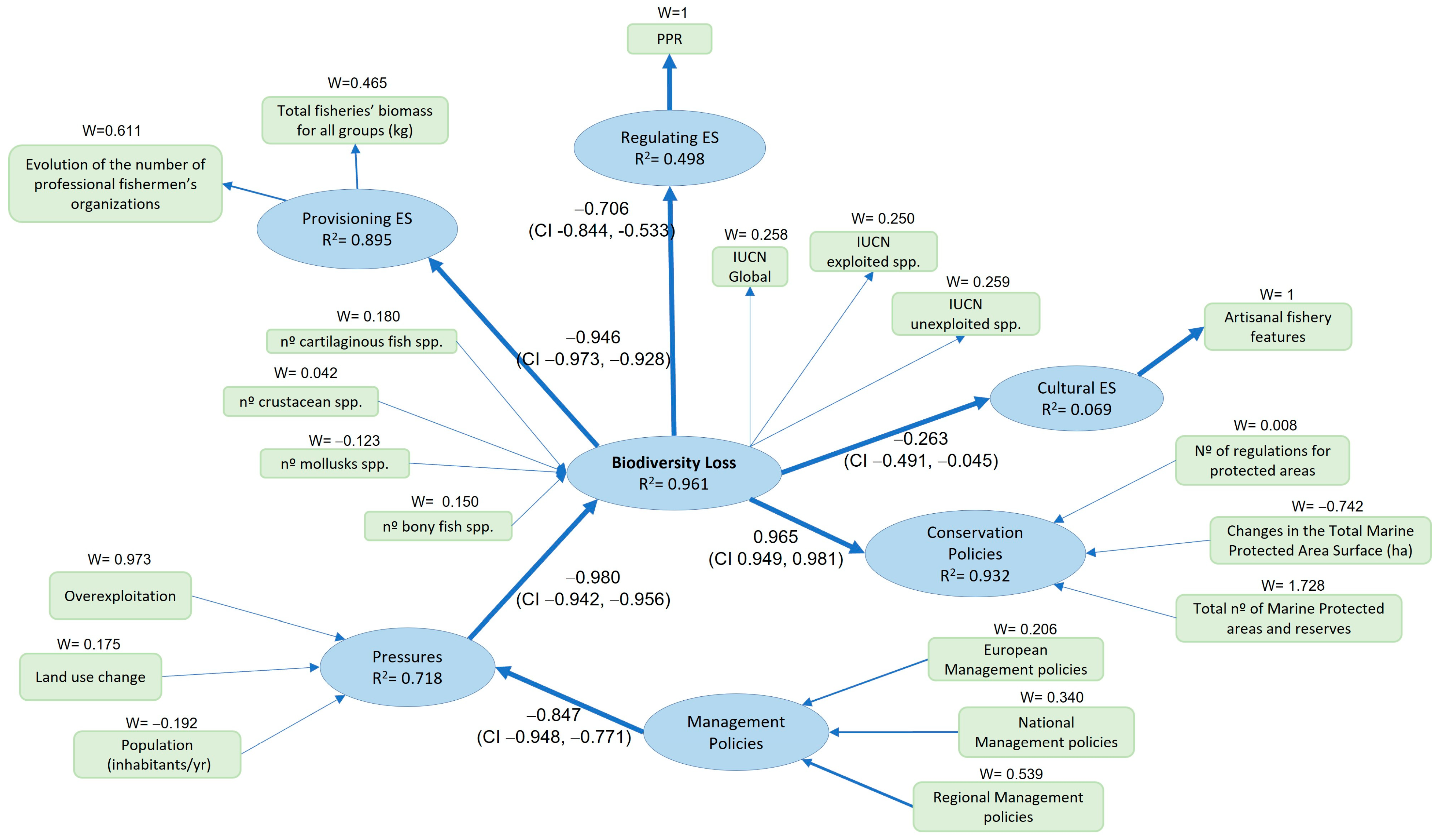
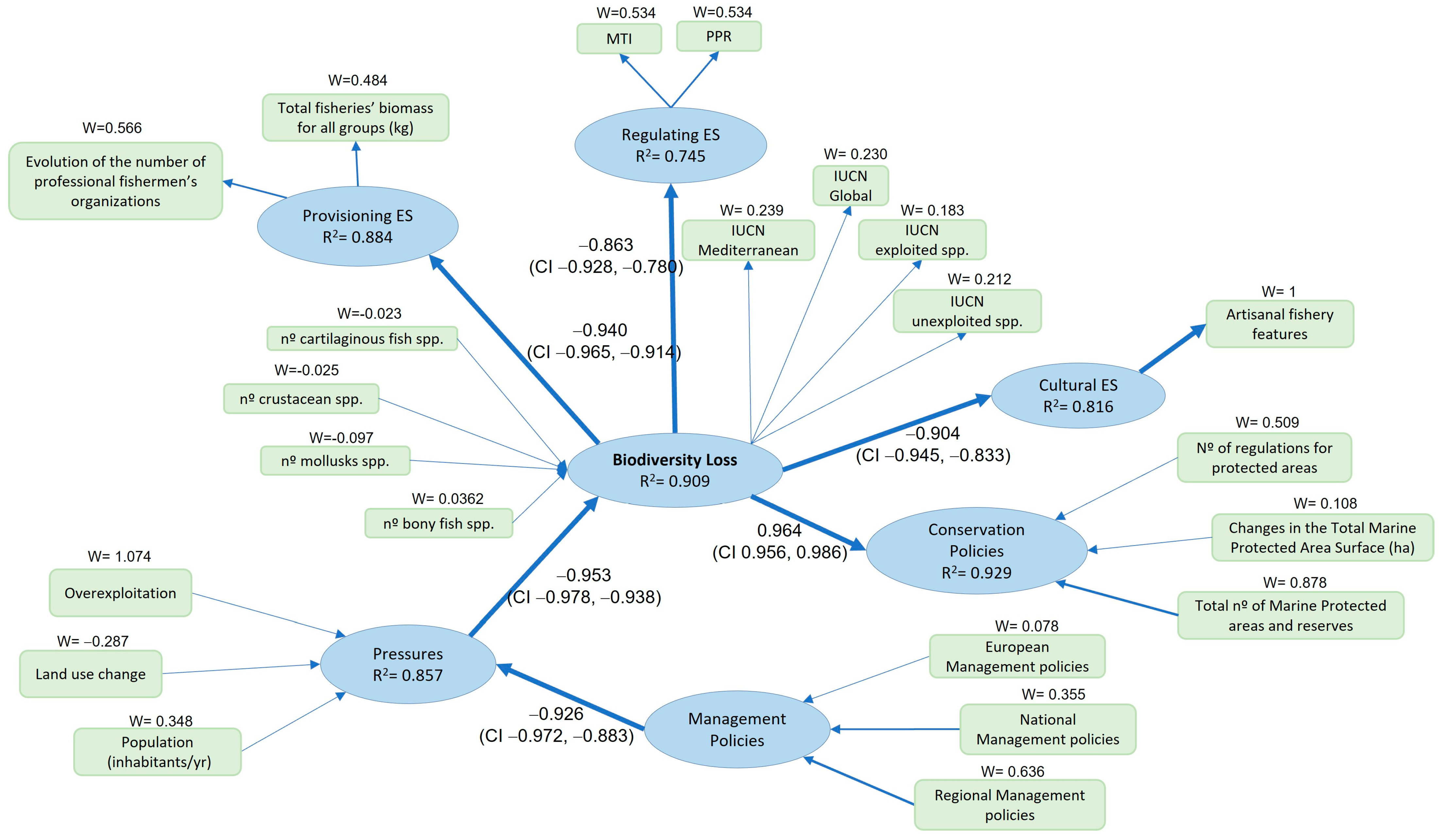
| Marine Area | R2 | F | R2 (Bootstrap) | S.E. | Lower Confidence Limit (95%) | Upper Confidence Limit (95%) |
|---|---|---|---|---|---|---|
| Gulf of Cádiz | 0.961 | 804.409 | 0.957 | 0.014 | 0.914 | 0.984 |
| Alborán | 0.909 | 330.068 | 0.922 | 0.019 | 0.879 | 0.959 |
| Model | GoF | GoF (Bootstrap) | S.E. | Lower Confidence Limit (95%) | Upper Confidence Limit (95%) |
|---|---|---|---|---|---|
| Gulf of Cádiz | |||||
| Overall model | 0.729 | 0.719 | 0.051 | 0.602 | 0.836 |
| Outer model | 0.966 | 0.941 | 0.057 | 0.804 | 1.000 |
| Inner model | 0.876 | 0.872 | 0.014 | 0.829 | 0.898 |
| Alborán | |||||
| Overall model | 0.804 | 0.797 | 0.069 | 0.654 | 0.921 |
| Outer model | 0.966 | 0.942 | 0.069 | 0.803 | 1.000 |
| Inner model | 0.956 | 0.952 | 0.013 | 0.915 | 0.972 |
| Cronbach’s α | Dillon–Goldstein ρ | |||
|---|---|---|---|---|
| Latent Variable | GC | A | GC | A |
| Biodiversity loss | 0.971 | 0.945 | 0.977 | 0.956 |
| Regulating ES | 0.859 | 0.934 | ||
| Provisioning ES | 0.839 | 0.918 | 0.925 | 0.961 |
| Latent Variables | Manifest Variables | Loadings | Communalities | Loadings | Communalities |
|---|---|---|---|---|---|
| Gulf of Cádiz | Alborán | ||||
| Management policies (formative) | European Management policies | 0.635 | 0.403 | 0.655 | 0.428 |
| National Management policies | 0.915 | 0.837 | 0.924 | 0.853 | |
| Regional Management policies | 0.946 | 0.896 | 0.977 | 0.954 | |
| Pressures (formative) | Population (inhabitants/yr) | −0.911 | 0.829 | −0.901 | 0.813 |
| Overexploitation | 0.997 | 0.995 | 0.994 | 0.988 | |
| Land use change | −0.836 | 0.699 | −0.858 | 0.736 | |
| Biodiversity Loss (Mixed) | nº bony fish spp. (formative) | 0.962 | 0.925 | 0.913 | 0.834 |
| nº cartilaginous fish spp. (formative) | 0.915 | 0.838 | 0.847 | 0.717 | |
| nº molluscs spp. (formative) | 0.642 | 0.412 | 0.436 | 0.190 | |
| nº crustacean spp. (formative) | 0.928 | 0.861 | 0.803 | 0.645 | |
| UICN (fisheries) (reflective) | 0.963 | 0.928 | 0.785 | 0.616 | |
| IUCN (unexploited) (reflective) | 0.919 | 0.845 | 0.866 | 0.750 | |
| IUCN (Global) (reflective) | 0.980 | 0.960 | 0.956 | 0.913 | |
| IUCN (Mediterranean) (reflective) | 0.856 | 0.733 | |||
| Cultural ES (reflective) | Artisanal fishery features | 1.000 | 1.000 | ||
| Regulating ES (reflective) | PPR | 1.000 | 0.936 | 0.876 | |
| ITM | 0.936 | 0.877 | |||
| Provisioning ES (reflective) | Total fisheries’ biomass for all groups (kg) | 0.906 | 0.822 | 0.956 | 0.913 |
| Fishermen | 0.947 | 0.896 | 0.966 | 0.934 | |
| Conservation Policies (formative) | Number of regulations for protected areas | 0.186 | 0.035 | 0.331 | 0.110 |
| Total Number of Marine Protected areas and reserves | 0.995 | 0.990 | 0.998 | 0.996 | |
| Changes in the Total Marine Protected Area Surface (ha) | 0.971 | 0.943 | 0.981 | 0.963 | |
| Marine Area | Centre | Opened | Closed |
|---|---|---|---|
| Gulf Cádiz | Marina El Terrón | 1996 | 2008 |
| Museo Marítimo Matalascañas | 2002 | 2011 | |
| Centro de Interpretación del Atún de Almadraba | 2008 | 2011 | |
| Alborán Sea | Aula del mar de Benalmádena | 1989 | 2011 |
| Aula del Mar de Málaga | 1989 | - | |
| Aula del Mar “El Corralete” Cabo de Gata | 2000 | 2007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcorlo, P.; García-Tiscar, S.; Vidal-Abarca, M.R.; Suárez-Alonso, M.L.; Santos-Martín, F. Unravelling Complex Interaction among Coastal Management and Marine Biodiversity: A Case Study in Southern Spain. Sustainability 2023, 15, 6544. https://doi.org/10.3390/su15086544
Alcorlo P, García-Tiscar S, Vidal-Abarca MR, Suárez-Alonso ML, Santos-Martín F. Unravelling Complex Interaction among Coastal Management and Marine Biodiversity: A Case Study in Southern Spain. Sustainability. 2023; 15(8):6544. https://doi.org/10.3390/su15086544
Chicago/Turabian StyleAlcorlo, Paloma, Susana García-Tiscar, María Rosario Vidal-Abarca, María Luisa Suárez-Alonso, and Fernando Santos-Martín. 2023. "Unravelling Complex Interaction among Coastal Management and Marine Biodiversity: A Case Study in Southern Spain" Sustainability 15, no. 8: 6544. https://doi.org/10.3390/su15086544
APA StyleAlcorlo, P., García-Tiscar, S., Vidal-Abarca, M. R., Suárez-Alonso, M. L., & Santos-Martín, F. (2023). Unravelling Complex Interaction among Coastal Management and Marine Biodiversity: A Case Study in Southern Spain. Sustainability, 15(8), 6544. https://doi.org/10.3390/su15086544





