Rainfall Influences the Patterns of Diversity and Species Distribution in Sandy Beaches of the Amazon Coast
Abstract
1. Introduction
2. Materials and Methods
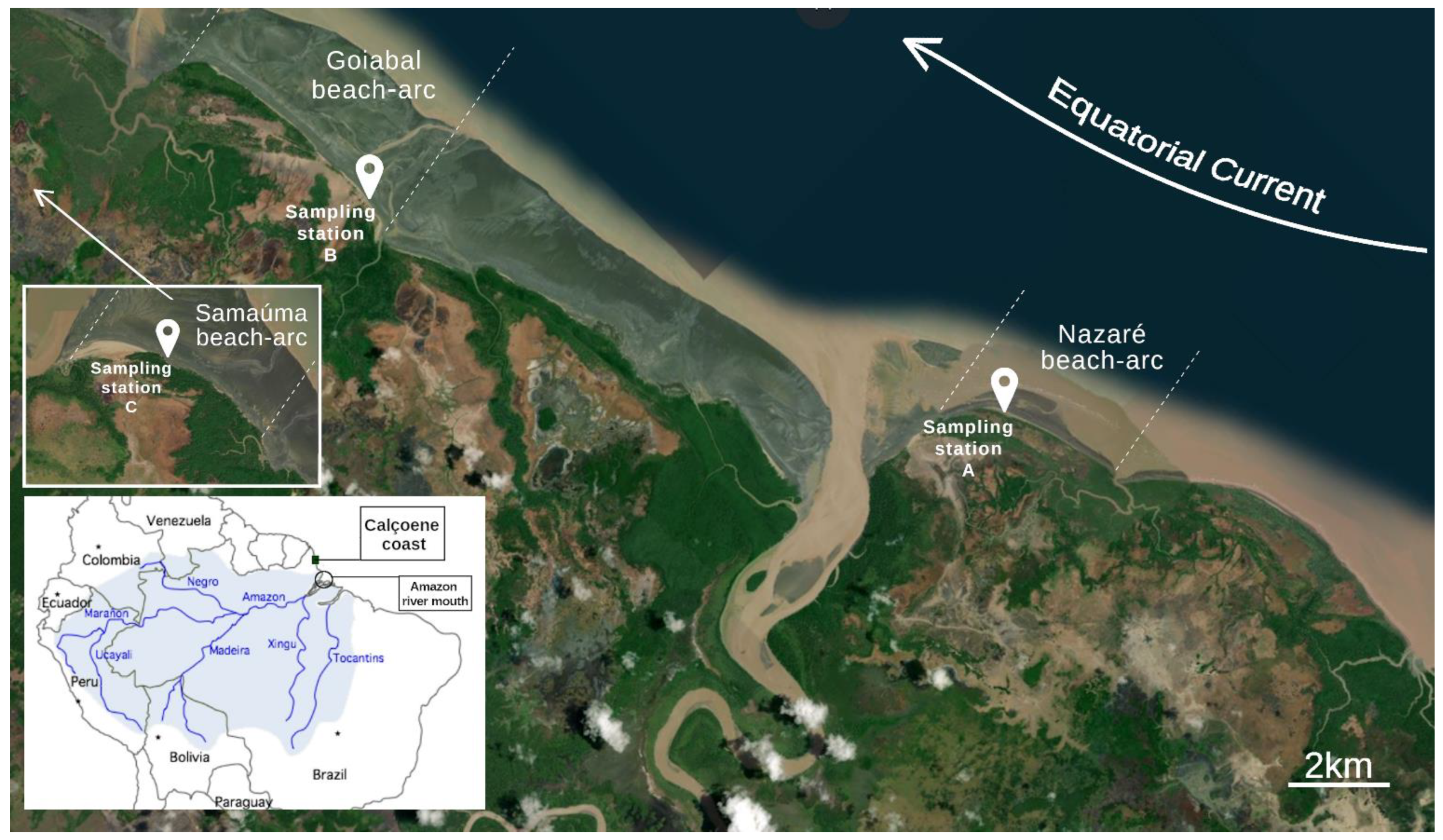
2.1. Study Area and Sampling Procedures
2.2. Data Analysis
3. Results
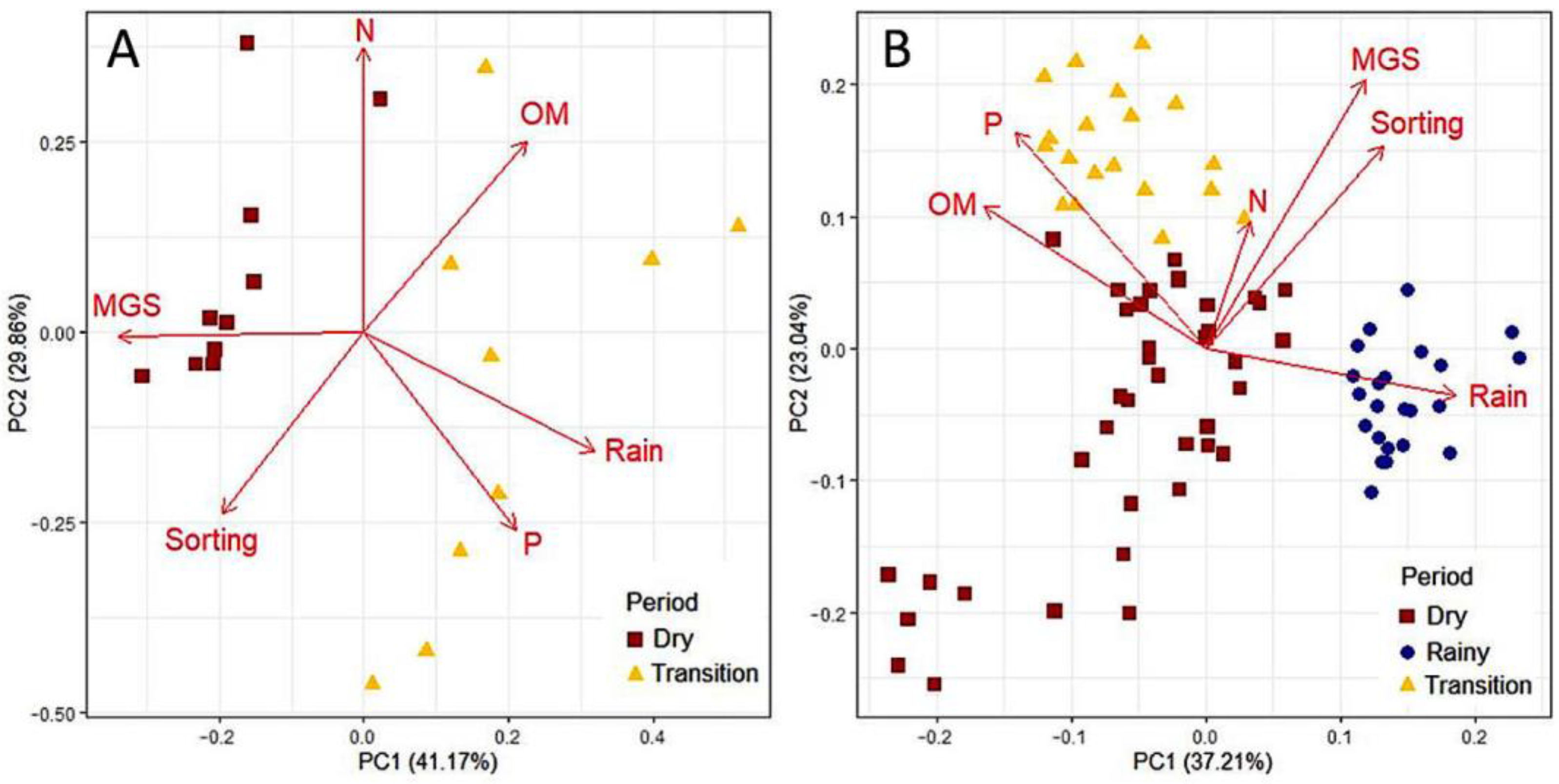
3.1. Environmental Characterization and Habitat Heterogeneity
3.2. Macrobenthic Diversity
3.3. Zonation Patterns
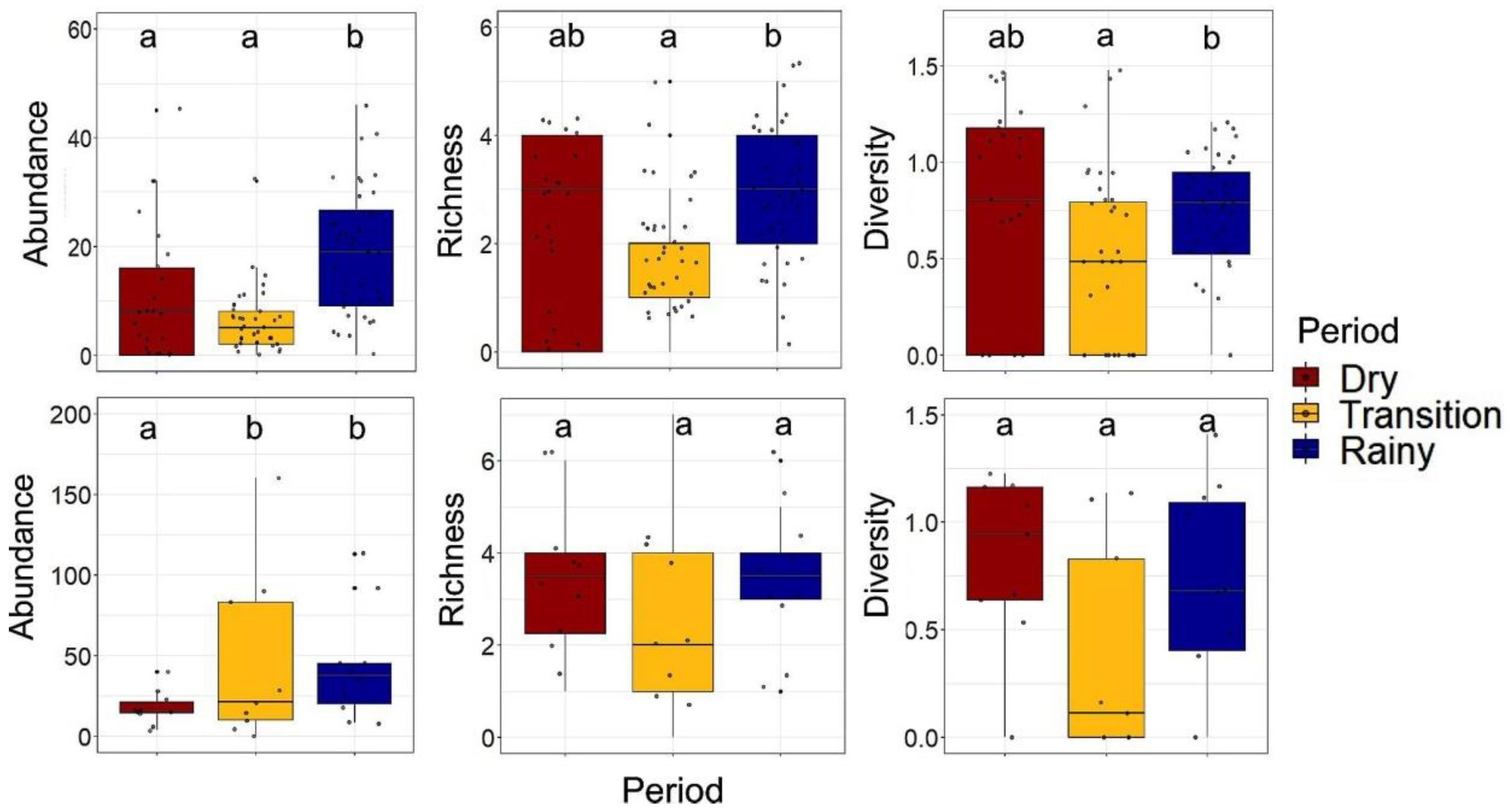
3.4. Temporal Variation in Abundance, Species Richness, and Diversity
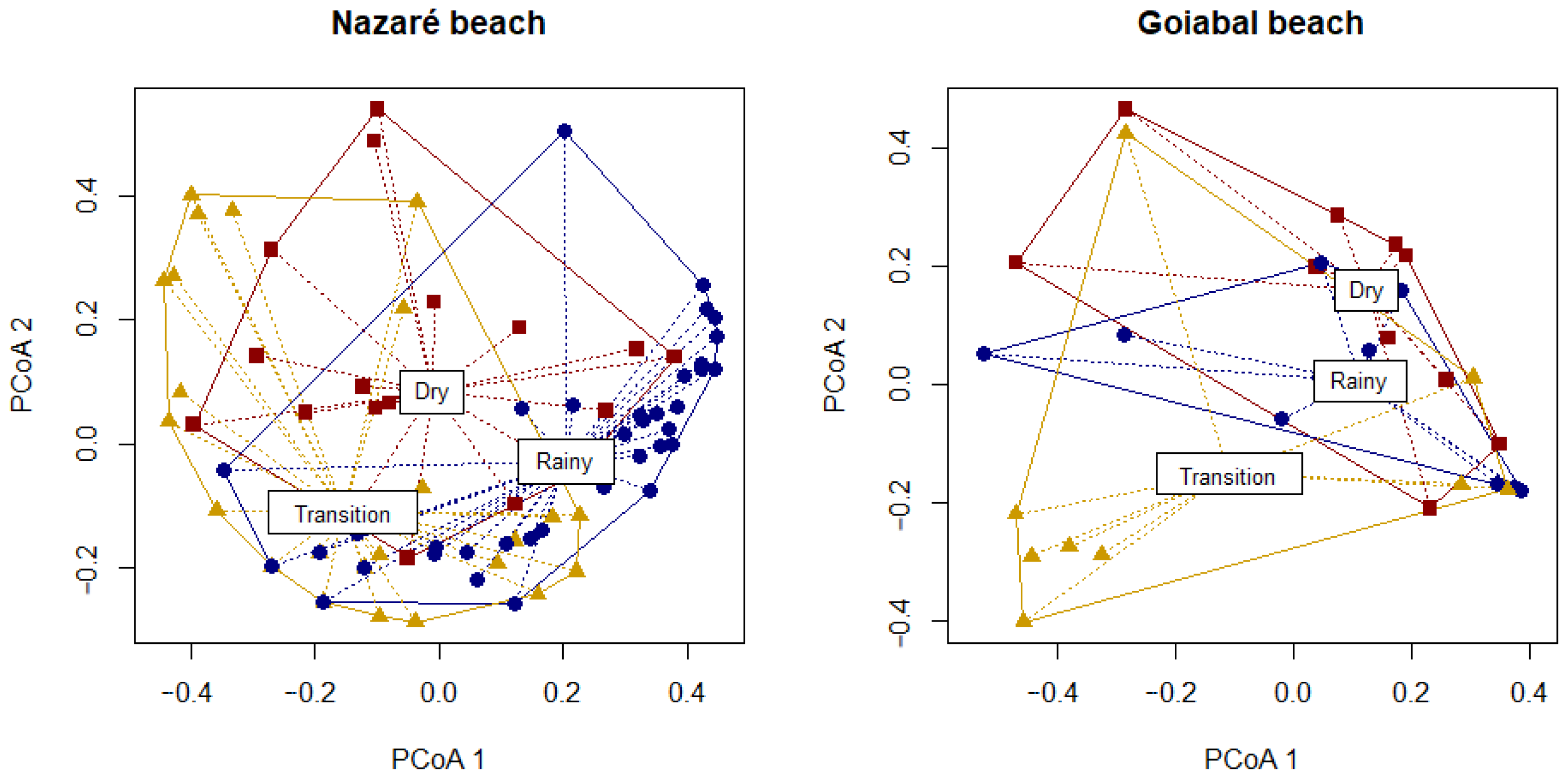
3.5. Beta Diversity
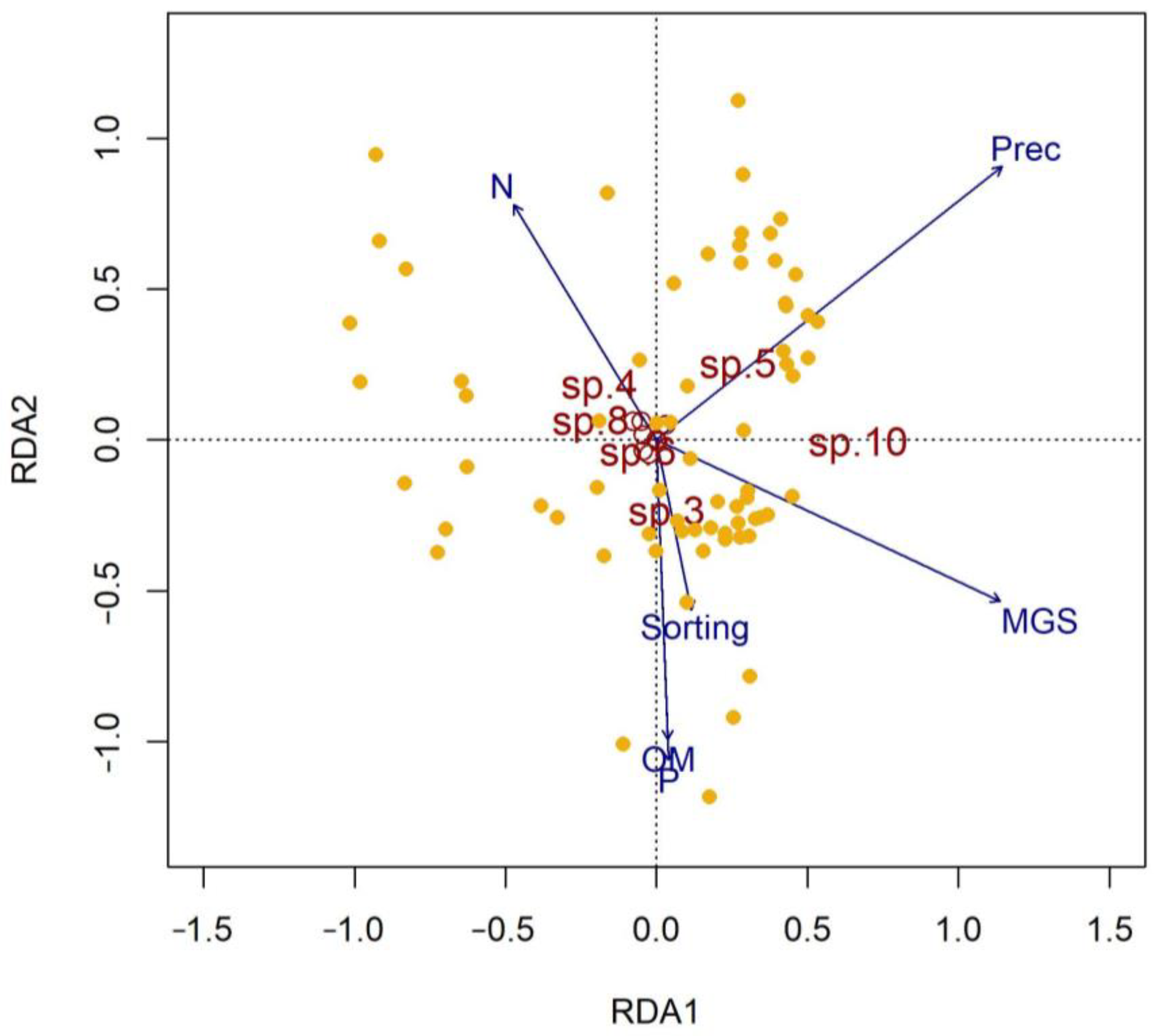
3.6. Influence of Environmental Variables on Macrobenthic Assemblages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nobre, C.A.; Sampaio, G.; Borma, L.S.; Castilla-Rubio, J.C.; Silva, J.S.; Cardoso, M. Land-use and climate change risks in the Amazon and the need of a novel sustainable development program. Proc. Natl. Acad. Sci. USA 2016, 113, 10759–10768. [Google Scholar] [CrossRef]
- Boulton, C.A.; Lenton, T.M.; Boers, N. Pronounced loss of Amazon rainforest resilience since the early 2000s. Nat. Clim. Chang. 2022, 12, 271–278. [Google Scholar] [CrossRef]
- Castello, L.; McGrath, D.G.; Hess, L.L.; Coe, M.T.; Lefebvre, P.A.; Petry, P.; Macedo, M.N.; Renó, V.F.; Arantes, C.C. The vulnerability of Amazon freshwater ecosystems. Conserv. Lett. 2013, 6, 217–229. [Google Scholar] [CrossRef]
- Heino, J.; Gronroos, M. Exploring species and site contributions to beta diversity in stream insect assemblages. Oecologia 2017, 183, 151–160. [Google Scholar] [CrossRef]
- Legendre, P.; De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef]
- Muehe, D. Brazilian Coast vulnerability to climate change. Pan-Am. J. Aquat. Sci. 2010, 5, 173–183. [Google Scholar]
- Santos, V.F.; Short, A.D.; Mendes, A.C. Beaches of the Amazon Coast: Amapá and West Pará. In Brazilian Beach Systems; Short, A.D., Klein, A.H.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 67–93. [Google Scholar]
- Marinelli-Filho, J.E.; Monteiro, R.C.P. Widespread microplastics distribution at an Amazon macrotidal sandy beach. Mar. Pollut. Bull. 2019, 145, 219–223. [Google Scholar] [CrossRef]
- Myneni, R.B.; Yang, W.; Nemani, R.R.; Huete, A.R.; Dickinson, R.E.; Knyazikhin, Y.; Didan, K.; Fu, R.; Juárez, R.I.N.; Saatchi, S.S.; et al. Large seasonal swings in leaf area of Amazon rainforests. Proc. Natl. Acad. Sci. USA 2007, 104, 4820–4823. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.C.; Pereira, L.C.C.; Costa, R.M.; Jiménez, J.A. Management of estuarine beaches on the Amazon coast through the application of recreational carrying capacity indices. Tour. Manag. 2017, 59, 216–225. [Google Scholar] [CrossRef]
- Baia, R.; Rollnic, M.; Venekey, V. Seasonality of pluviosity and saline intrusion drive meiofauna and nematodes on an Amazon freshwater-oligohaline beach. J. Sea Res. 2021, 170, 102022. [Google Scholar] [CrossRef]
- Nunes, F.L.D.; Norris, R.D.; Knowlton, N. Long distance dispersal and connectivity in amphi-Atlantic corals at regional and basin scales. PLoS ONE 2011, 6, e22289. [Google Scholar] [CrossRef]
- Barroso, C.X.; Lotufo, T.M.; Matthews-Cascon, H. Biogeography of Brazilian prosobranch gastropods and their Atlantic relationships. J. Biogeogr. 2016, 43, 2477–2488. [Google Scholar] [CrossRef]
- Tosetto, E.G.; Bertrand, A.; Neumann-Leitão, S.; Nogueira Júnior, M. The Amazon river plume, a barrier to animal dispersal in the Western Tropical Atlantic. Sci. Rep. 2022, 12, 523. [Google Scholar]
- Amaral, A.C.Z.; Jablonski, S. Conservation of marine and coastal biodiversity in Brazil. Conserv. Biol. 2005, 19, 625–631. [Google Scholar] [CrossRef]
- Amaral, A.C.Z.; Corte, G.N.; Rosa Filho, J.S.; Denadai, M.R.; Colling, L.A.; Borzone, C.A.; Omena, E.P.; Zalmon, I.R.; Rocha-Barreira, C.A.; Rosa, L.C.; et al. Brazilian sandy beaches: Characteristics, ecosystem services, impacts, knowledge and priorities. Braz. J. Oceanogr. 2016, 64, 5–16. [Google Scholar] [CrossRef]
- Rosa-Filho, J.S.; Almeida, M.F.; Aviz, D.E. Spatial and temporal changes in the benthic fauna of a macrotidal Amazon sandy beach, Ajuruteua, Brazil. J. Coast. Res. 2009, 56, 1796. [Google Scholar]
- Rosa-Filho, J.S.; Gomes, T.P.; Almeida, M.F.; Silva, R.F. Benthic fauna of macrotidal sandy beaches along a small-scale morphodynamic gradient on the Amazon coast (Algodoal Island, Brazil). J. Coast. Res. 2012, 64, 435–439. [Google Scholar]
- Santos, T.M.T.; Almeida, M.F.; Aviz, D.; Rosa-Filho, J.S. Patterns of spatial and temporal distribution of the macrobenthic fauna on an estuarine macrotidal sandy beach on the Amazon coast (Brazil). Mar. Ecol. 2021, 42, e12675. [Google Scholar] [CrossRef]
- Muller-Karger, F.E.; McClain, C.R.; Richardson, P.L. The dispersal of the Amazon River. Nature 1988, 333, 56–59. [Google Scholar] [CrossRef]
- Bernardino, A.; Mazzuco, A.C.; Souza, F.M.; Santos, T.M.T.; Sanders, C.J.; Massone, C.G.; Costa, R.F.; Silva, A.E.B.; Ferreira, T.O.; Nóbrega, G.N.; et al. The novel mangrove environment and composition of Amazon Delta. Curr. Biol. 2022, 32, 3636–3640. [Google Scholar] [CrossRef]
- BRASIL. Panorama da Erosão Costeira no Brasil; Ministério do Meio Ambiente: Brasília, Brazil, 2018.
- Lercari, D.; Defeo, O.; Celentano, E. Consequences of a freshwater canal discharge on the benthic community and its habitat on an exposed sandy beach. Mar. Pollut. Bull. 2002, 44, 1397–1404. [Google Scholar] [CrossRef]
- Laurino, I.A.; Turra, A. The threat of freshwater input on sandy beaches: A small-scale approach to assess macrofaunal changes related to salinity reduction. Mar. Environ. Res. 2021, 171, 105459. [Google Scholar] [CrossRef]
- Thomaz, S.M.; Bini, L.M.; Bozelli, R.L. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 2006, 579, 1–13. [Google Scholar] [CrossRef]
- Corte, G.N.; Schlacher, T.A.; Checon, H.H.; Barboza, C.A.M.; Siegle, E.; Coleman, R.A.; Amaral, A.C.Z. Storm effects on intertidal invertebrates: Increased beta diversity of few individuals and species. PeerJ 2017, 5, e3360. [Google Scholar] [CrossRef]
- Lansac-Tôha, F.M.; Heino, J.; Bini, L.M.; Peláez, O.; Baumgartner, M.T.; Quirino, B.A.; Pineda, A.; Meira, B.R.; Florêncio, F.M.; Oliveira, F.R.; et al. Cross-Taxon Congruence of Taxonomic and Functional Beta-Diversity Facets Across Spatial and Temporal Scales. Front. Environ. Sci. 2022, 10, 903074. [Google Scholar] [CrossRef]
- Vilhena, J.C.E.; Amorim, A.; Ribeiro, L.; Duarte, B.; Pombo, M. Baseline study of trace metal contaminations in the sediment of the intertidal zone of Amazonian Oceanic Beaches. Front. Mar. Sci. 2021, 8, 671390. [Google Scholar] [CrossRef]
- Corte, G.N.; Gonçalves-Souza, T.; Checon, H.H.; Siegle, E.; Coleman, R.A.; Amaral, A.C.Z. When time affects space: Dispersal ability and extreme weather events determine metacommunity organization in marine sediments. Mar. Environ. Res. 2018, 136, 139–152. [Google Scholar] [CrossRef]
- Folk, R.L.; Ward, W.C. Brazos River Bar: A study in the significance of grain size parameters. J. Sediment. Petrol. 1957, 27, 3–26. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis—Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy e Soil Science Society of America, cap. 29: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Kjeldahl, J. A new method for the determination of nitrogen in organic matter. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Mehlich, A. Determination of P, Ca, Mg, K, Na and NH4; Soil Test Division, Department of Agriculture: Raleigh, NC, USA, 1953.
- O’Hara, R.B.; Kotze, D.J. Do not log-transform data count. Methods Ecol. Evol. 2010, 1, 118–122. [Google Scholar] [CrossRef]
- Morisita, M. Iσ-Index, a measure of dispersion of individuals. Pop. Ecol. 1962, 4, 1–7. [Google Scholar] [CrossRef]
- Smith-Gill, S.J. Cytophysiological basis of disruptive pigmentary patterns in the leopard frog Rana pipiens. J. Morphol. 1975, 146, 35–54. [Google Scholar] [CrossRef]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2016, 9, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Baselga, A. Partitioning abundance-based multiple-site dissimilarity into components: Balanced variation in abundance and abundance gradients. Methods Ecol. Evol. 2017, 8, 799–808. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R—A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 4 March 2022).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2022. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 4 March 2022).
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer Verlag: New York, NY, USA, 2016. [Google Scholar]
- Defeo, O.; McLachlan, A. Patterns, processes and regulatory mechanisms in sandy beach macrofauna: A multi-scale analysis. Mar. Ecol. Prog. Ser. 2005, 295, 1–20. [Google Scholar] [CrossRef]
- Barboza, F.R.; Defeo, O. Global diversity patterns in sandy beach macrofauna: A biogeographical analysis. Sci. Rep. 2015, 5, 14515. [Google Scholar] [CrossRef]
- Defeo, O.; McLachlan, A.; Schoeman, D.S.; Schlacher, T.A.; Dugan, J.; Jones, A.; Lastra, M.; Scapini, F. Threats to sandy beach ecosystems: A review. Estuar. Coast. Shelf Sci. 2009, 81, 1–12. [Google Scholar] [CrossRef]
- Kowalewski, M.; Domènech, R.; Martinelli, J. Vanishing Clams on an Iberian Beach: Local Consequences and Global Implications of Accelerating Loss of Shells to Tourism. PLoS ONE 2014, 9, e83615. [Google Scholar] [CrossRef]
- Orlando, L.; Ortega, L.; Defeo, O. Urbanization effects on sandy beach macrofauna along an estuarine gradient. Ecol. Indic. 2020, 111, 106036. [Google Scholar] [CrossRef]
- Corte, G.N.; Checon, H.H.; Shah Esmaeili, Y.; Defeo, O.; Turra, A. Evaluation of the effects of urbanization and environmental features on sandy beach macrobenthos highlights the importance of submerged zones. Mar. Pollut. Bull. 2022, 182, 113962. [Google Scholar] [CrossRef]
- Jourde, J.; Dupuy, C.; Nguyen, H.T.; Mizrahi, D.; de Pracontal, N.; Bocher, P. Low benthic macrofauna diversity in dynamic, tropical tidal mudflats: Migrating banks on Guiana’s Coast, South America. Estuaries Coast. 2017, 40, 1159–1170. [Google Scholar] [CrossRef]
- Jorge-Romero, G.; Lercari, D.; Ortega, L.; Defeo, O. Long-term ecological footprints of a man-made freshwater discharge onto a sandy beach ecosystem. Ecol. Indic. 2019, 96, 412–420. [Google Scholar] [CrossRef]
- Medeiros, E.L.; Fernandes, G.V.; Henry-Silva, G.G. Distribution and density of the mollusk Donax striatus (Linnaeus, 1767) in a tropical estuarine region in the Brazilian semi-arid. Braz. J. Biol. 2015, 75, 914–922. [Google Scholar] [CrossRef]
- Checon, H.H.; Vieira, D.C.; Corte, G.N.; Sousa, E.C.P.M.; Fonseca, G.; Amaral, A.C.Z. Defining soft bottom habitats and potential indicator species as tools for monitoring coastal systems: A case study in a subtropical bay. Ocean Coast. Manag. 2018, 164, 68–78. [Google Scholar] [CrossRef]
- Mucciolo, S.; Desiderato, A.; Leal, S.M.; Mastrodonato, M.; Lana, P.C.; Freire, C.A. Variability in the degree of euryhalinity of neotropical estuarine annelids. J. Exp. Mar. Biol. Ecol. 2021, 544, 151617. [Google Scholar] [CrossRef]
- Rosa-Filho, J.S.; Aviz, D. Macrobenthic communities of an Amazonian estuary (Guajará Bay, Brazil): Temporal and spatial changes. J. Coast. Res. 2013, 65, 123–128. [Google Scholar] [CrossRef]
- Holdrich, D.M.; Harrison, K. The sphaeromatid isopod genus Sphaeromopsis Holdrich & Jones in African, Australian and South American waters. Crustaceana 1981, 41, 286–300. [Google Scholar]
- Flynn, M.N.; Wakabara, Y.; Tararam, A.S. Macrobenthic associations of the lower and upper marshes of a tidal flat colonized by Spartina alterniflora in Cananeia Lagoon Estuarine Region (Southeastern Brazil). Bull. Mar. Sci. 1998, 63, 427–442. [Google Scholar]
- Lana, P.C.; Christofoletti, R.; Gusmão, J.B., Jr.; Barros, T.L.; Spier, D.; Costa, T.M.; Soares-Gomes, A.; Santos, C.S.G. Benthic Estuarine Assemblages of the Southeastern Brazil Marine Ecoregion (SBME). In Brazilian Estuaries; Lana, P.C., Bernardino, A., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Thrush, S.F.; Hewitt, J.E.; Cummings, V.J.; Norkko, A.; Chiantore, M. β-Diversity and Species Accumulation in Antarctic Coastal Benthos: Influence of Habitat, Distance and Productivity on Ecological Connectivity. PLoS ONE 2010, 5, e11899. [Google Scholar] [CrossRef] [PubMed]
- Astorga, A.; Death, R.; Death, F.; Paavola, R.; Chakraborty, M.; Muotka, T. Habitat heterogeneity drives the geographical distribution of beta diversity: The case of New Zealand stream invertebrates. Ecol. Evol. 2014, 4, 2693–2702. [Google Scholar] [CrossRef]
- Moritz, C.; Meynard, C.N.; Devictor, V.; Guizien, K.; Labrune, C.; Guarini, J.-M.; Mouquet, N. Disentangling the role of connectivity, environmental filtering, and spatial structure on metacommunity dynamics. Oikos 2013, 122, 1401–1410. [Google Scholar] [CrossRef]
- Heino, J.; Melo, A.S.; Siqueira, T.; Soininen, J.; Valanko, S.; Bini, L.M. Metacommunity organisation, spatial extent and dispersal in aquatic systems: Patterns, processes and prospects. Freshw. Biol. 2015, 60, 845–869. [Google Scholar] [CrossRef]
- McLachlan, A.; Defeo, O. The Ecology of Sandy Beaches; Academic Press: Waltham, MA, USA; Elsevier: Waltham, MA, USA, 2017. [Google Scholar]
- Gandara-Martins, A.L.; Borzone, C.A.; Rosa, L.C.; Caron, E. Ocorrência de três espécies do gênero Bledius (Coleoptera, Staphylinidae, Oxytelinae) nas praias arenosas expostas do Paraná, Brasil. Braz. J. Aquat. Sci. Technol. 2010, 14, 23–30. [Google Scholar] [CrossRef]
- Vieira, J.V.; Borzone, C.A.; Lorenzi, L.; Carvalho, F.G. Human impact on the benthic macrofauna of two beach environments with different morphodynamic characteristics in Southern Brazil. Braz. J. Oceanogr. 2012, 60, 135–148. [Google Scholar] [CrossRef]
- Linley, J.R.; Evans, H.T.; Evans, F.D.S. A qualitative study of autogeny in a naturally occurring population of Culicoides furens (Diptera: Ceratopogonnidae). J. Anim. Ecol. 1970, 39, 169–183. [Google Scholar] [CrossRef]
- Hagan, C.E.; Kettle, D.S. Habitats of Culicoides spp. in an intertidal zone of southeast Queensland, Australia. Med. Vet. Entomol. 1990, 4, 105–115. [Google Scholar] [CrossRef]
- Brei, B.; Cribb, B.W.; Merritt, D.J. Effects of seawater components on immature Culicoides molestus (Skuse) (Diptera: Ceratopogonidae). Aust. J. Entomol. 2003, 42, 119–123. [Google Scholar] [CrossRef]
- Cardoso, R.S.; Mattos, G.; Caetano, C.H.S.; Cabrini, T.M.B.; Galhardo, L.B.; Meireis, F. Effects of environmental gradients on sandy beach macrofauna of a semi-enclosed bay. Mar. Ecol. 2012, 33, 106–116. [Google Scholar] [CrossRef]
- Ray, S.; Choudhury, A. Population Ecology of Culicoides peliliouensis Tok. in the Hooghly Estuary, Sagar Island, India. Int. J. Trop. Insect Sci. 1988, 9, 17–25. [Google Scholar] [CrossRef]
- Pagliosa, P.R.; Barboza, F.A.R. Assessing the environment-benthic fauna coupling in protected and urban areas of southern Brazil. Biol. Conserv. 2006, 129, 408–417. [Google Scholar] [CrossRef]
- Pearson, T.H.; Rosenberg, R. Macrobenthic succession in relation to organic enrichment and pollution in the benthic environment. Oceanogr. Mar. Biol. Ann. Rev. 1976, 16, 229–311. [Google Scholar]
- Schooler, N.K.; Dugan, J.E.; Hubbard, D.M.; Straughan, D. Local scale processes drive long-term change in biodiversity of sandy beach ecosystems. Ecol. Evol. 2017, 7, 4822–4834. [Google Scholar] [CrossRef] [PubMed]
- Luijendijk, A.; Hagenaars, G.; Ranasinghe, R.; Baart, F.; Donchtys, G.; Aarninkhof, S. The state of the world’s beaches. Sci. Rep. 2018, 8, 6641. [Google Scholar] [CrossRef] [PubMed]

| Variables | Goiabal | Nazaré | ||||
|---|---|---|---|---|---|---|
| Dry | Transition | Rainy | Dry | Transition | Rainy | |
| Precipitation (mm) | 20 | 70 | 200 | 50 | 120 | 400 |
| Mean grain size (phi) | 3.19 ± 0.1 | 2.72 ± 0.3 | - | 2.9 ± 0.4 | 3.29 ± 0.1 | 3.23 ± 0.2 |
| Sorting (phi) | 1.3 ± 0.1 | 1.29 ± 0.1 | - | 1.26 ± 0.1 | 1.31 ± 0.1 | 1.31 ± 0.1 |
| Organic matter (%) | 5.35 ± 0.7 | 5.08 ± 0.6 | - | 9.75 ± 2.3 | 7.15 ± 0.4 | 5.13 ± 0.7 |
| Nitrogen (%) | 0.13 ± 0.1 | 0.11 ± 0.1 | - | 0.07 ± 0.1 | 0.1 ± 0.1 | 0.09 ± 0.1 |
| Phosphorus (mg/cm³) | 38.3 ± 5.2 | 48.2 ± 9.0 | - | 52.3 ± 8.0 | 70.3 ± 10.2 | 40.5 ± 13.5 |
| Taxa | Goiabal | Nazaré | Samaúma | ||||
|---|---|---|---|---|---|---|---|
| Dry | Transition | Rainy | Dry | Transition | Rainy | ||
| Bledius sp. | - | 1.000 * | - | - | - | - | 1.000 * |
| Culicoides sp. | 0.826 * | 0.884 * | 1.000 * | - | - | - | - |
| Cumacea | −0.079 | - | 0.656 * | 0.569 * | −0.032 | 0.512 * | 0.835 * |
| Laeonereis acuta | −0.238 | 0.712 * | 0.708 * | 0.899 * | 1.000 * | - | 1.000 * |
| Nephtys fluviatis | 0.511 * | 0.618 * | 0.509 * | 0.524 * | 0.504 | 0.505 * | 0.508 * |
| Sphaeromopsis mourei | 0.549 * | 0.534 * | 0.674 * | 0.530 * | 0.414 | 0.527 * | 0.513 * |
| Goiabal | Nazaré | |||||
|---|---|---|---|---|---|---|
| d.f. | Deviance | p-Value | d.f. | Deviance | p-Value | |
| Abundance | ||||||
| Period | 2 | 9.767 | 0.008 | 2 | 26.991 | <0.001 |
| Residual | 27 | 44.213 | 103 | 122.82 | ||
| Richness | ||||||
| Period | 2 | 1.587 | 0.452 | 2 | 10.627 | 0.005 |
| Residual | 27 | 103 | 142.18 | |||
| Diversity (H’) | ||||||
| Period | 2 | 1.618 | 0.217 | 2 | 4.691 | 0.011 |
| Residual | 27 | 103 | ||||
| β Bray | β Bal | β Gra | |
|---|---|---|---|
| Nazaré | |||
| Dry vs. Trans | 0.41 | 98% | 2% |
| Dry vs. Rainy | 0.63 | 41% | 59% |
| Trans vs. Rainy | 0.57 | 25% | 75% |
| Goiabal | |||
| Dry vs. Trans | 0.82 | 67% | 33% |
| Dry vs. Rainy | 0.46 | 15% | 85% |
| Trans vs. Rainy | 0.84 | 93% | 7% |
| Nazaré vs. Goiabal | |||
| Dry | 0.4 | 63% | 37% |
| Transition | 0.82 | 80% | 20% |
| Rainy | 0.49 | 57% | 43% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Checon, H.H.; Costa, H.H.R.; Corte, G.N.; Souza, F.M.; Pombo, M. Rainfall Influences the Patterns of Diversity and Species Distribution in Sandy Beaches of the Amazon Coast. Sustainability 2023, 15, 5417. https://doi.org/10.3390/su15065417
Checon HH, Costa HHR, Corte GN, Souza FM, Pombo M. Rainfall Influences the Patterns of Diversity and Species Distribution in Sandy Beaches of the Amazon Coast. Sustainability. 2023; 15(6):5417. https://doi.org/10.3390/su15065417
Chicago/Turabian StyleChecon, Helio H., Hugo H. R. Costa, Guilherme N. Corte, Fernanda M. Souza, and Maíra Pombo. 2023. "Rainfall Influences the Patterns of Diversity and Species Distribution in Sandy Beaches of the Amazon Coast" Sustainability 15, no. 6: 5417. https://doi.org/10.3390/su15065417
APA StyleChecon, H. H., Costa, H. H. R., Corte, G. N., Souza, F. M., & Pombo, M. (2023). Rainfall Influences the Patterns of Diversity and Species Distribution in Sandy Beaches of the Amazon Coast. Sustainability, 15(6), 5417. https://doi.org/10.3390/su15065417





