Abstract
The ongoing environmental crisis, driven by biodiversity loss and climate change, raises concerns about the impacts on marine systems and human well-being. These environments provide crucial ecosystem services valued at approximately USD 74.5 trillion·year−1 globally. Seagrasses support fisheries, protect coasts, help mitigate climate change, maintain biodiversity, provide food security, and enhance water quality. However, comprehensive assessments of seagrass ecosystem services (SESs) and their impacts are lacking. Focusing on the Brazilian southwest Atlantic, our aim is to bridge this gap and identify key research areas for improved management decisions. Our literature search employed n = 19 paired terms for seagrass in Brazil. We screened 30,351 search returns for 394 relevant documents. Research on SESs has grown over time, and most research has focused on provisioning and supporting ecosystem services: 79.7% of documents mentioned at least one SES, while 24.5% of the documents provided evidence of observed SESs; 31.5% only provided information on expected SESs. Provisioning services were the most observed and expected. Coastal urbanization (54%) and marine food provisioning (17%) were the main drivers impacting SESs. Terrestrial food and material provision (9%) and climate change (8%) were also significant drivers. This study provides key recommendations aimed at fostering further research and management strategies to consider the complete ensemble of ecosystem services for a range of seagrass bioregions, to better understand the provision of and impacts to seagrass services and human well-being at the global scale.
Keywords:
biodiversity; ecological functions; Halodule; Halophila; marine conservation; marine plants; Ruppia 1. Introduction
The ongoing biodiversity and climate change crises have led to concerns about how impacts on marine systems will affect human well-being [1] and biodiversity [2]. Marine ecosystems sustain diverse biogeophysical structures, processes, and functions that deliver direct and indirect benefits to human well-being, also known as ecosystem services (ESs) [3] or the closely related concept of nature’s contributions to people (NCP) [4]. Globally, marine ecosystem services are estimated to be worth USD 74.5 trillion/year, contributing to more than 60% of the total economic value of the biosphere [5], including a wide array of provisioning, regulating, supporting, and cultural services [6]. Therefore, a better comprehension of marine services supply and their responses to environmental stressors is needed to support management actions aimed at the maintenance of human well-being and solutions to emergent environmental challenges (e.g., nature-based solutions [7]).
Seagrass meadows occur in shallow coastal areas along all continents, except Antarctica, covering a total area estimated to be between 160,387 and 2,660,562 km2 across six marine bioregions, ranging from tropical to temperate areas [8]. These ecosystems have recently received significant attention due to their contributions to global climate change mitigation, coastline protection, biodiversity maintenance, food security, water quality, and disease control [9,10]. Seagrass meadows sequester large amounts of organic carbon (termed blue carbon), storing about 140 Mg C ha−1 in the top 1 m of soils, potentially contributing to climate regulation and excess CO2 mitigation through sedimentary trapping and long-term burial of autochthonous and allochthonous organic matter [11,12,13,14]. Wave attenuation and decreased water flow generated by seagrass leaves also offer coastal protection against extreme weather events, contributing to climate adaptation [15]. Seagrass meadows offer habitat, nursery, and feeding grounds for a wide diversity of marine organisms, including endangered (marine turtles, dugong, manatee) and commercial species, such as one fifth of the world’s largest 25 fisheries, including Walleye Pollock [16]. Seagrasses enhance water quality through the uptake and storage of nutrients, contaminants, and microplastics and the removal of pathogens from the water column [10,17]. Seagrass leaves also serve as raw materials for cultural artifacts and fertilizers, providing opportunities for education, recreation, and tourism, and spiritual and religious value for coastal populations [9,18].
Along with macroalgae beds, seagrass meadows ecosystem services (SESs) are estimated to contribute around USD 6.8 USD trillion·year−1 globally [3], although current valuation models and estimates have included only partial functions of seagrass ecosystems, mostly related to commercial fish production (see [19] for a review). Therefore, seagrass protection and restoration are among the recognized nature-based solutions that could help reduce the effects of anthropogenic impact across multiple scales. However, variability in species composition and abundance, genus-specific traits, latitude, and coastal environmental settings may affect seagrass ecological functions (e.g., [9]), indicating that caution is needed when extrapolating the values of SESs across distinct biogeographical areas [8,20,21]. Additionally, people’s relation to and perception of nature and their needs may affect the relative importance and the value of SESs across a region [9,21]. Despite the increasing recognition of SESs, their characterization and evaluation are still lacking in most coastal regions around the world.
In the southwestern Atlantic, seagrass meadows occur intermittently across ~9000 km of the Brazilian coast and a wide latitudinal range from the equator to subtropics (between 4° N and 35° S), associated with several other coastal and marine ecosystems [14,22] (Figure 1). There are six seagrass species currently recognized in Brazil [22] (Figure 2). Along this coast, the distribution, abundance, size, and dynamics of the seagrass meadows depend on hydrological, climatic, oceanographic, and geomorphological conditions which vary along the coastline and offshore islands [22,23,24]. In Brazil, seagrass meadows occur in warm and well-lit waters with low hydrodynamic conditions, coexisting with other aquatic plants, drift and/or rhizophytic macroalgae, and/or rhodoliths (Figure 2). This results in the formation of dense submerged aquatic vegetation (SAV) along the Brazilian tropical and subtropical shores.
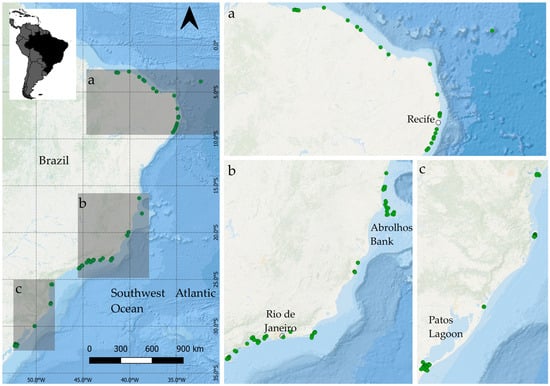
Figure 1.
A map of the known distribution of seagrasses in Brazil (green dots), showing locations mentioned in the text. a, b and c are details of each region.

Figure 2.
Seagrasses in Brazil. (a) Halodule wrightii, (b) Halophila decipiens, (c) Ruppia maritima, (d–f) Halodule wrightii meadows at different scales: (d) population, (e) meadow, (f) seascape scale (drone image). Photos (a–d) J.C.C., (e,f) I.C.
The most widely distributed species is the widgeon grass Ruppia maritima (Ruppiaceae), belonging to a cosmopolitan family [25] and found in both temporarily and permanently flooded coastal lagoons and estuaries. It can thrive in oligohaline and hyperhaline environments (i.e., salinities from 0 to 39) (Figure 2c). This species occurs sporadically along the southwestern Atlantic coast, from Guyana to Argentina. Exceptionally large stands (120 km2) of R. maritima are found in the Patos Lagoon estuary in southern Brazil [14,22] (Figure 1). The other five seagrass species have a more tropical and marine distribution: the shoalgrass Halodule wrightii (Figure 2a), H. emarginata and H. beaudettei (Cymodoceaceae), and the paddle grass Halophila decipiens (Figure 2b) and H. baillonii. These may occur (1) in intertidal-to-shallow subtidal zones in coastal lagoons; (2) as denser populations in smaller rocky-shore-formed embayments or estuaries in the intertidal zone; (3) in larger bays with less turbid waters extending into the subtidal zone; (4) as intertidal-to-shallow subtidal populations, where beach sand is sufficiently stable on the leeward side or amongst the beachrock and coral reefs [22] (Figure 2). Furthermore, H. decipiens may occur in deep populations (>20 m and up to 40 m) in northeast Brazil [26]. SESs are known to vary by genus (=seagrass size, form, and life history) [9].
Despite the widespread occurrence of seagrass meadows along the Brazilian coast, the perception and value of SESs along the southwestern Atlantic is considered very low [8]. To our knowledge, there is no comprehensive assessment of SESs along the Brazilian coast. This knowledge gap could potentially hamper the adoption of ecosystem-based management practices in the region. Our goal is, therefore, to assess and synthesize the existing knowledge of ecosystem services provided by seagrass meadows along the Brazilian coast in terms of their temporal variation, types of services, relative frequency, variation between seagrasses genera and the drivers, activities, pressures, and state environmental changes that are associated with and impact SESs. By comparing the latent and evident knowledge of SESs in Brazil, as well as relating them to known patterns of SESs in other regions, we aim to identify critical knowledge gaps to be further considered in future investigations for improved management decisions.
2. Materials and Methods
2.1. Literature Review and Document Selection
We used a literature review to obtain data regarding SESs. Based on our preliminary investigation, the Google Scholar search engine yielded far more results than Scopus and Web of Science, encompassing all the references listed by them as well as significant amounts of national gray papers, which were desirable but which Scopus and Web of Science missed. Therefore, we opted to use Google Scholar as our primary source. We did not limit the literature search to a specific starting date, and searches were also conducted in local languages, including citations. Since Google Scholar does not support Boolean operators, we conducted multiple specific searches using paired terms. The pairs consisted of the country (or its local language equivalent name) and a seagrass-related term, which included “seagrass”, “submerged aquatic vegetation SAV”, or a relevant genera for Brazil, such as “Halodule”, “Diplanthera”, “Ruppia”, “Halophila”, or their local language equivalents (e.g., in Brazilian Portuguese: “Grama marinha”, “Erva marinha”, “Angiosperma marinha”, “Fanerógama marinha”, “Vegetação Aquática Submersa”, “capim agulha”, or “lixo-capim”). This search approach resulted in the use of 19 paired search terms for seagrasses in Brazil (see Supplementary Material Table S1). As Google Scholar gives far more returns than Scopus or Web of Science and multiple searches generate far more repeated documents, the quantity of returns was a challenge. To manage the search results, we established a cutoff criterion for screening the first 2500 results for each pair of terms. However, if the total number of results (n) was inferior to 2500, we screened all the records. The initial screening yielded a total of 30,351 records, including duplicates.
The selection criteria used to further examine the results from Google Scholar was a document that met the following conditions: (1) the document was written in English, Spanish, or Portuguese; (2) the document had full text available, either as peer-reviewed item or being documented in the gray literature (i.e., citations and conference abstracts were not included). Subsequently, we included the retained documents (see below) in a bibliographic database. We obtained full copies of these documents and screened them to identify those containing relevant information about seagrasses in Brazil. We used the ROSES (Reporting standards for Systematic Evidence Syntheses in environmental research) protocol and report card, and details of the synthesis process can be found in the ROSES flowchart (Supplementary Material Figure S1).
2.2. Data Extraction and Typologies
The initial Excel database was standardized with a unique reference number assigned to each source, along with relevant bibliographic information (Author(s), Date, Title, etc.), and a copy of the publication. Using the retained documents, we proceeded to examine, categorize, and tabulate each document based on the following criteria: (1) SESs which were referred to by the author(s) in the text, typically in the introduction or in the discussion, but not explicitly demonstrated or proven in the study (termed “Expected”); (2) SESs which were actually demonstrated or proven in the study/document (termed “Observed”); (3) the seagrass genera to which expected or observed services were associated; and (4) impacts on SESs. The category “Observed” encompassed both the SESs that were explicitly mentioned by the author(s) and those indirectly supported by evidence presented within the results of the study (e.g., if an author investigated the occurrence of a certain species of mollusk collected from a seagrass bed and did not explicitly mention the provision of habitat, this was implicitly demonstrated in the study). We utilized an initial list of 28 seagrass ecosystem services compiled by Nordlund et al. [9], to which we added pathogen removal [27], buffering of ocean acidification [28], as well as a category termed “others” for those cases that could not be assigned to the specific categories (Supplementary Material Table S2).
We carried out a title analysis by conducting a word count on the bibliographic references of all 314 retained documents that mentioned SESs. To achieve this, we extracted the titles from the reference database software, creating a single document that was then imported into a word cloud generator [29], resulting in an initial word list of 2425 words (Supplementary Material Table S3). Through a stepwise process, which included editing (if necessary, including translation into English), removing nonspecific words, combining very similar words, and applying a cut-off of five or less repeated words, we refined the list to 77 words, representing key words in the document titles. Subsequently, we prepared a word cloud, and specifically selected those associated with SESs as another method of assessing the most important SESs.
To best categorize human impacts on SESs, we also retrieved information on impacts mentioned in the documents. As this information was often presented as a mixture of drivers, activities, pressures, or even state environmental changes (DAPSIR), we used categories defined in Table A3 from Griffiths et al. [30] (previously adapted from Smith et al. [31] and Elliot et al. [32]) to quantify the drivers, activities, and pressures affecting SESs (Supplementary Material Table S4). Since some pressures could fit into multiple drivers or activities, we exercised our best judgment and considered the context, as described by the authors, when feasible. When drivers were nonspecific, we categorized them into the most relevant activity (or driver) category. It was assumed here that the frequencies of mention of key words in titles, SESs, drivers, activities, and pressures in the documents was a faithful quantitative indicator of their relative importance.
3. Results
3.1. General Patterns in Expected and Observed Seagrass Ecosystem Services
We obtained a total of 474 documents, of which 394 considered some aspect or mention of seagrasses in Brazil (Supplementary Material Table S5). Out of these, 314 documents (79.7%) mentioned (either expected or observed) at least one SES. Specifically, 77 documents (24.5%) provided evidence of observing at least one SES in Brazil that was not initially expected. On the other hand, 99 documents (31.5%) only provided information on expected SESs that were not observed. The average number of expected SESs was almost two times higher than the number of observed SESs (mean (SE) = 2.69 (0.16) and 1.45 (0.09), respectively), with a maximum number of 17 expected SESs listed in a single document and a maximum of 8 observed (Figure 3). The proportion of expected and observed SESs was significantly different (Chi2 = 53, df = 17, p < 0.001) (Figure 3). The number of expected SESs was a nonlinear predictor of the mean number of observed SESs, as observed SESs increased up to about four expected services, but then decreased beyond that (Supplementary Material Figure S2).
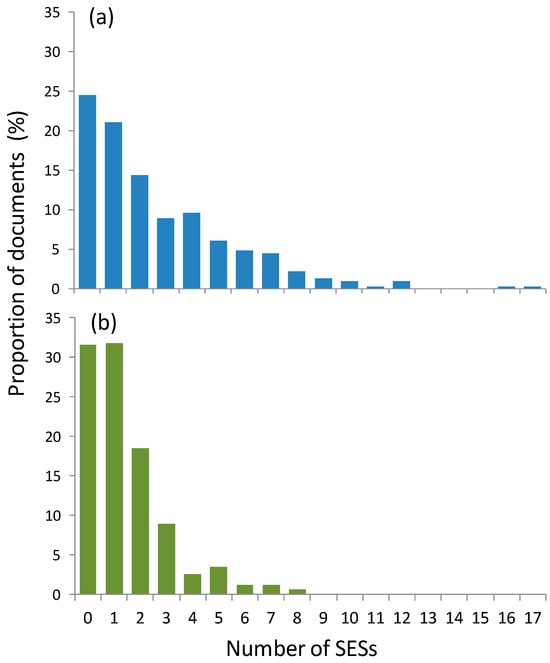
Figure 3.
Proportion of documents that expected or observed at least one seagrass ecosystem service (n = 314) as a function of number of (a) expected or (b) observed seagrass ecosystem services.
The title analysis returned 2425 words of which 77 were retained (Figure 4). The most common words in titles of documents that mentioned SESs in Brazil was, not surprisingly, “Brazil(ian)” (n = 192 times), followed by “Halodule wrightii” (n = 97), “marine” (n = 83), “coast(al)”, “seagrass(es)” (n = 57), and “estuary” (n = 40) (Figure 4; Table S2). Many retained words mentioned regions, states, or places where seagrasses are particularly abundant or well studied. Eighteen (23%) words could be related to one or more SESs categories, but almost all of these referred to provisioning services, the most commonly used being “habitat(s)” (n = 34), “fish(es)” (n = 32), “T. manatus” (n = 26), and “Callinectes sapidus” (n = 9), which related to invertebrate habitat, fish habitat, nursery habitat, and vertebrate habitat including birds (other than fish) (Table S2).

Figure 4.
Word cloud formed from the 77 most used words in the titles of documents which mentioned seagrass ecosystem services in Brazil. The size of the word is proportional to the number of times used.
SESs were less often mentioned (either as expected or observed) in the past than in the current decade. While only about a third of studies mentioned SESs in the 1930s–1950s, 95% of studies mention SESs in this decade (Figure 5). Clearly, there has also been a large increase in the research output regarding seagrasses and seagrass habitats in Brazil (Figure 5). However, when we consider the evolution of the number of SESs being mentioned in the literature over time, we found a dichotomy between the number of SESs expected and observed over time. On average, more SESs were observed per study in the 1970s and 1980s than were expected (Figure 6). This seems to have changed in the 1990s, as the mean number of SESs observed per study declines somewhat over time.
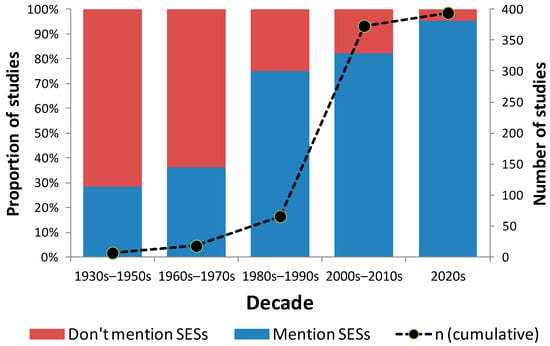
Figure 5.
Number of studies on seagrass or components of the seagrass habitat in Brazil over time and the proportion of studies which do or do not mention seagrass ecosystems services over the different decades.
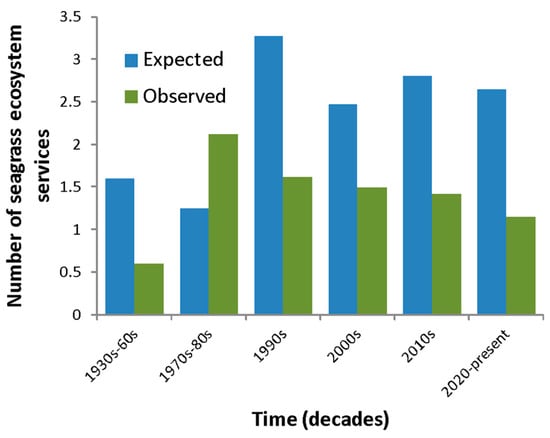
Figure 6.
Mean number of seagrass ecosystem services (SESs) expected and observed in studies on seagrass, or components of the seagrass habitat, in Brazil over time.
Most of the studies on seagrasses in Brazil addressed provisioning services for both documents assessing expected and observed SESs (Figure 7). Supporting services were followed by regulating services, which in turn were followed by cultural services for both types of SESs; observed SESs were always less frequent than expected ones (a reduction of 17% for supporting SESs, 26% for regulating SESs, and 9% for cultural SESs). Regarding specific SESs, habitat provision for invertebrate and vertebrate species including birds, as well as carbon sequestration and source of information, had proportionally more observed than expected cases. On the other hand, sediment stabilization, coastal protection, and water purification were proportionally less observed than expected (Figure 8 and Figure S3). Six SESs that were mentioned as expected were never observed in the documents studied: education, pathogen control, compost fertilizer, spiritual and religious value, recreation, and animal food from seagrass-associated species (Figure 8).
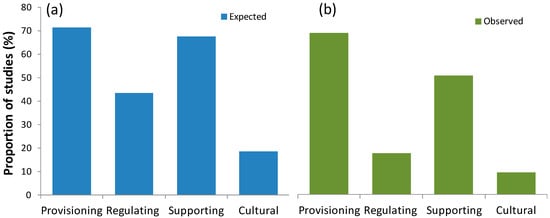
Figure 7.
Proportion of studies which addressed seagrasses or seagrass habitats in Brazil as (a) expected or (b) observed different types of seagrass ecosystem services.
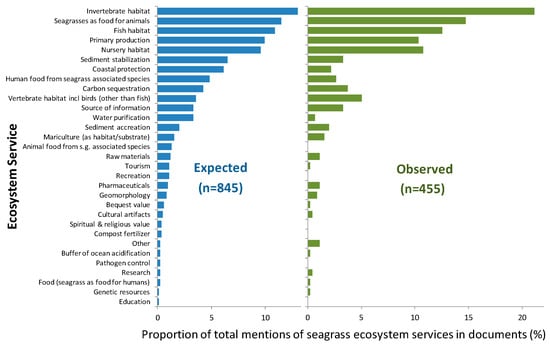
Figure 8.
Relative proportion of studies on seagrasses or seagrass habitats in Brazil: expected or observed, for 23 seagrass ecosystem services.
3.2. Seagrass Genera and Species
Of those documents that mentioned SESs in Brazil, almost half (49%) referred to shoalgrass (Halodule wrightii), one third (29%) to widgeon grass (Ruppia maritima), and one tenth (10.9%) to paddlegrass (Halophila decipiens) (Figure 9). In Halodule spp., the most expected and observed SESs were provisioning and supporting services (Figure 10). In contrast, provisioning and regulating services each corresponded to about one third of the expected SESs for Ruppia spp., while over half of the observed services were actually regulating and one quarter cultural. Halophila spp. also presented a very different pattern with half of the SESs expected to be provisioning (the others divided equally). However, regulating and supporting services were the most observed, and no provisioning services were observed (Figure 10). Finally, for those documents that contemplated multiple genera, the expected proportions of the four different service types were more equally divided, while in the observed services, a greater proportion of provisioning and lower proportion of supporting services were found.
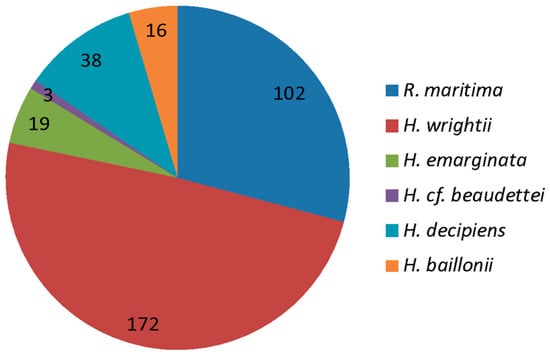
Figure 9.
Relative frequency and number of documents which mentioned seagrass ecosystem services in Brazil as a function of seagrass species. Numbers are absolute values.
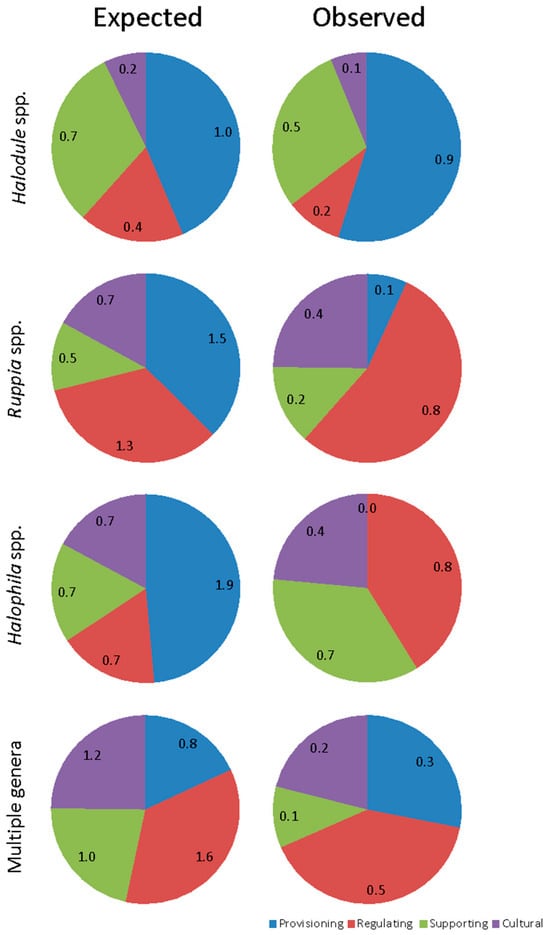
Figure 10.
Relative frequency of documents which mentioned seagrass ecosystems services in Brazil as a function of seagrass taxons and expected and observed categories of services. Numbers are absolute (mean) values.
3.3. Drivers, Activities, Pressures, and State Environmental Changes
Coastal urbanization was responsible for over half (54%) of the perceived impacts on SESs along the Brazilian coast (Figure 11a), mostly due to the impacts of coastal infrastructure causing smothering of seagrass, nutrient enrichment, contaminant exposure, changes in salinity, substratum loss, and increased turbidity (Figure 11b,c). Coastal urbanization was also responsible for impacts caused by transport and shipping, especially pressures such as abrasion (=direct interaction with seagrass from infrastructure causing physical damage to plants).
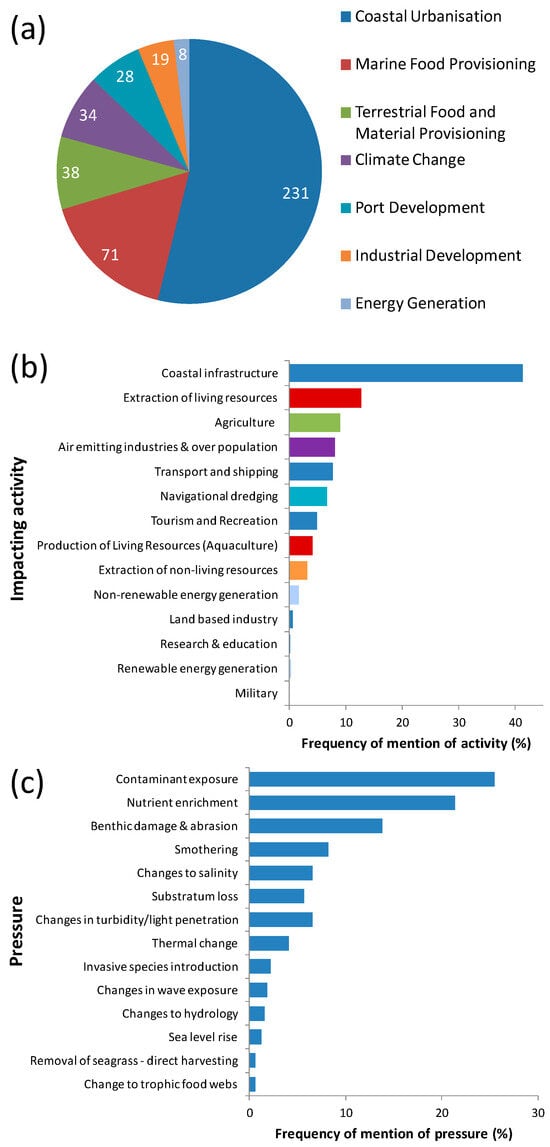
Figure 11.
Relative frequency of documented (a) drivers, (b) activities, and (c) pressures which impact seagrass ecosystems services in Brazil. Colors of bars in (b) relate to driver colors in (a); numbers in (a) are absolute number of documents.
Marine food provisioning was considered the second most important driver of impact (17%) (Figure 11a), but this driver and its two associated activities (production of living resources—aquaculture, and extraction of living resources) were the least well specified in terms of stressors that could be categorized. For example, “shrimp farming” or “overfishing” were often mentioned as impactful, but without mentioning the specific stressors involved. Of the stressors mentioned, benthic damage and abrasion, change to trophic food webs, and removal of seagrass—direct harvesting were associated with extraction of living resources, while abrasion was associated with aquaculture.
The third most important driver impacting SESs (9%) was terrestrial food and material provision, mainly due to nutrient enrichment, increased turbidity, and contaminant exposure associated with agricultural runoff, pesticides, and herbicides. The fourth most important driver impacting SESs (8%) was climate change (activity of air emitting industries and over-population) in all its stressors (thermal change, salinity change, sea level rise, and changes in wave exposure). Tourism and recreation was another activity which was not well specified in terms of stressors that could be categorized (“tourism”), although abrasion was considered an important pressure. Other important pressures were contaminant exposure due to nonrenewable energy generation (oil), extraction of nonliving resources (heavy metal suspension), and port development and substratum loss due to port development (Figure 11).
4. Discussion
This study demonstrates that there is a mismatch between what was perceived and what was detected as SESs along the Brazilian coast. Based on the available documents, it was expected that provisioning SESs (such as invertebrate habitat, fish habitat, nursery [habitat for juveniles], and vertebrate habitat including birds [other than fish]) would be as common and important as supporting services (such as seagrasses as food for animals [e.g., dugong eats seagrass] and primary production). However, the observations revealed that supporting, regulating, and cultural services were less often observed than expected, which suggests that our knowledge of these services (such as sediment stabilization, coastal protection, and water purification) is less complete.
In contrast to our findings, Nordlund et al. [9] requested opinions about SESs from seagrass experts based upon their perspective, which was equivalent to our “expected” results. The authors found that the provision of research, vertebrate and invertebrate habitats, nursery, water purification, education, and recreation were the most frequently globally expected SESs. However, the provision of education, research, and recreation were very little expected in the present study, and education and recreation were not observed at all. Our results corroborated the expected global importance of seagrasses as providing vertebrate, invertebrate, and nursery habitat, as well as demonstrating that these SESs were amongst the most frequently observed in the southwest Atlantic (Figure 12). As an expected seagrass ecosystem service, the provision of water purification only ranked twelfth and stood out as a seagrass ecosystem service less observed than expected in Brazil.

Figure 12.
Illustration of some seagrass ecosystem services in Brazil: (a) invertebrate habitat, (b) vertebrate habitat (green turtle), (c) primary production, (d) human food from seagrass-associated species, (e) carbon sequestration (in soil core). Photos (a,d,e) JCC, photos (b,c) IC.
One clear difference compared with the global expectations in Nordlund et al. [9] was the importance of seagrass as food for animals which was highly (second) ranked as both an expected and observed SES in Brazil (Table 1). This is because seagrasses are primary producers, thus providing food for associated primary consumers (herbivores and detritivores in general). In Brazil, conservation efforts have been focused on studies on two important seagrass herbivores—the Antillean manatee (Trichechus manatus manatus), which is critically endangered [33], and the green turtle (Chelonia mydas) (Figure 12b) [34,35]. Due to their preference of sheltered shallow habitats with favorable conditions for foraging and resting, these species co-occur along with seagrass meadows [36]. Both species also have iconic conservation projects associated with them, the Projeto Peixe-Boi and Projeto TAMAR, which have focused substantial efforts on captive breeding and habitat protection, as well as combating illegal hunting of the manatee and the preservation of nesting sites and combat of illegal hunting and egg collecting for marine turtles. These projects have raised the conservation profile of these megaherbivores, indirectly focusing attention on the seagrasses which support them, with substantial resources being invested in mapping of their distribution and habitats [37] (Table 1). Another difference we found regarded the SES provision for research, which was ubiquitous to all bioregions globally, but scored very low in our study. This was because we used a different criterion for inclusion, as we only scored research when seagrass habitat or elements of the biota within was used as a research subject to test a specific hypothesis. This contrasts to Nordlund et al. [9], who scored research if it was carried out on seagrasses or seagrass habitats—clearly, the group of seagrass experts in that study scored research in all cases.

Table 1.
Eight key recommendations for future research and management strategies for seagrasses in the southwest Atlantic.
In contrast to our observations, a recent literature review of SESs by Lima et al. [38], who used the Millennium Ecosystem Assessment Board classification scheme [4], reported far more studies focused on regulating services (42%) than on cultural services (21%), supporting services (20%), and provisioning services (17%). They identified carbon sequestration, food provision, maintenance of habitat and biodiversity/nursery of habitats, storm protection/ extreme events, and opportunities for recreation and tourism as the focus of most studies. Although our study also identified maintenance of habitat and biodiversity/nursery habitats and food provision (=human food from seagrass-associated species) as very important SESs (Figure 12), there were also some clear differences from their study. For example, carbon sequestration was ranked lower in our study, as were tourism and recreation, which may indicate their lesser importance or that we have a regional knowledge gap, so further study is needed.
The increasing number of studies focused on SESs from the 2000s supports global increases in studies on SESs, ranging from ecological assessments to stakeholders’ perceptions and monetary valuation [38]. Similar trends have been observed for other marine ecosystems worldwide, with discrepancies among distinct geographical regions indicating an over-representation of ES assessments in Europe and North America [39]. Such global trends resulted from the publication of seminal works such as Constanza et al. [3] and Daily [40] on the value of the world’s ecosystem services, which triggered research, policies, and application of the concept.
The idea of nature’s service as ecosystem services first appeared in the ecological literature in the 1970s [41]. Similarly, our findings indicated that SESs have been reported in Brazil since the 1930s (but not in the context of benefits of nature to humans). These results suggest a long-term awareness of the importance of seagrass ecosystem functioning to human well-being, which has recently been strengthened by the recognition of seagrass declines and the greater focus on their positive role in tackling emerging environmental challenges, such as climate change through carbon offsetting (Table 1). Importantly, historical SES assessments, along with studies not appropriately framed into the ES concept, indicate that a significant amount of information is available for integrated management approaches along the Brazilian coast. We actually found a decrease in observed SESs over time from the 1980s on. This suggests a worldwide overestimation of SESs, without a proper consideration of specific environmental conditions, species traits, and spatio-temporal dynamics [9,21], or even the use of inadequate ES indicators [42].
Although R. maritima presents the widest latitudinal distribution along the Brazilian coast, H. wrightii is the most common seagrass and provides dense meadows in Brazil (Figure 2), mainly concentrated on the northeast coast [14,22], which is reflected in its citation (almost 50%) in studies with SESs. The concentration of services in the results observed as provision may be a direct reflection not only of the seagrass distribution, but the dependence on artisanal fisheries for food security in the northeastern region, especially invertebrate gleaning (Figure 12d), as already demonstrated for other tropical regions [21,43]. In fact, quite a lot of information on SESs was available in the literature focusing on traditional knowledge in communities which use seagrasses, but this was mostly in Portuguese and often in gray literature (theses and dissertations) and focused on areas with R. maritima in the south of Brazil or H. wrightii on the northeast coast. Despite the growing number of studies on seagrass in Brazil, especially regarding carbon sequestration, there are still large concentrations of studies in a few areas, as was demonstrated in the word cloud (Figure 4)—SESs were spatially concentrated in the “Northeast(ern)”, “Ceará” (state), “Pernambuco” (state), and “Itamaracá” (an island), “Bahia” (state), “Abrolhos”, “Rio de Janeiro” (state), “south(ern)” and Lagoa dos Patos (Patos Lagoon) (Figure 1), which are consistently where most studies on seagrasses have also been concentrated, as previously observed [22].
This is the first study that has systematically reviewed and provided qualitative information on human impacts to SESs as they are perceived (threats) by authors in publications of SESs in the southwest Atlantic. Quantitative data on the relative importance of human impacts on seagrasses on a regional basis is fundamental for the management of seagrasses, as it can direct the strategic deployment of limited resources [44], feed into frameworks for assessing threats to ecosystem service integrity [45], or contribute to regional mapping of cumulative human impacts on marine ecosystems [46] (Table 1). We found that the top driver of human pressure to seagrasses, coastal urbanization caused by impacts of coastal infrastructure and transport and shipping, was compatible with global expert opinions, which ranked urban/industrial runoff and urban/port infrastructure development as the two anthropogenic activities most impacting seagrasses [44]. This is particularly relevant as seagrass meadows are most usually found in estuaries and bays where ports and cities are also found. Globally, agricultural runoff, dredging, and trawling were ranked the third to fifth most impacting anthropogenic activities to seagrasses, respectively [44]. Our results highlighted marine food provisioning as the second most frequent driver of change, mainly due to the impacts of the extraction of living resources (which includes trawling). In our study, the third most frequent driver was terrestrial food and material provisioning due to the impact of agriculture (which encompasses agricultural runoff), the fourth was climate change, and the fifth was port development, which included impacts from navigational dredging. Major pressures to seagrass habitats and SESs in the southwest Atlantic were contaminant exposure, nutrient enrichment, and benthic damage and abrasion. As can be seen from the above comparisons, and as was apparent in the documents we analyzed, human impacts on seagrasses are often presented or mentioned as a mixture of drivers, activities, pressures, or even state environmental changes, so it was very useful to be able to frame human impacts into Griffiths et al.’s drivers, activities, pressures, or state environmental changes (DAPSIR) framework [30], and we would recommend the use of compatible frameworks in other studies of seagrasses (Table 1).
Due to a lack of detailed mapping for most of the regions, the total extension of the Brazilian seagrass meadows is still uncertain [14,47], making it difficult to quantify SES provision, loss, or erosion due to human impact or even perform a monetary valuation. Based on the known distribution of Halodule spp. and R. maritima and average meadow size, recent reviews have estimated between 730 km2 and 800 km2 [14,47]. Those numbers are certainly underestimated, since deeper meadows and Halophila spp. banks are very little known (H. decipiens has been recorded up to a 62 m depth off Recife, Pernambuco [48]). Due to many regional gaps, there is still no consensus about the global extension of seagrass meadows, with recent estimates in the literature varying between 200,000 km2 and 600,000 km2 [8,49]. A dearth of mapping efforts or funding, technical difficulties of mapping deep seagrass beds, and spatial variability hamper the estimation in most areas, particularly at greater depths [50,51]. We also need to advance the appraisal of the rapid decline and loss of seagrass meadows and the subsequent change in the delivery of ecosystem services. The high global rate of decline in seagrasses (7% per year in two decades from 1990) [52] makes them amongst the most threatened and vulnerable ecosystems on earth [28]. To the use of satellite images, remote underwater vehicles [53], and scientific surveys, the inclusion of seagrasses in citizen science is an additional tool which can be used for finding and mapping seagrass meadows [54] (Table 1).
Here, we have provided a systematic and quantitative panorama of the SESs and the threats posed to them by humans in the southwest Atlantic in Brazil. The role of Brazilian seagrass meadows has been demonstrated and quantified with respect to the maintenance of biodiversity [55,56]; habitat for fish [57,58,59,60], including important commercial species [61,62]; soil genesis; carbon sequestration; and climate mitigation [14]. Focusing on the Brazilian southwest Atlantic we do not know for certain how much seagrass we have, so it is difficult to fix a baseline in order to know how much seagrass we have probably lost. However, we do know that seagrass protection and restoration are potential strategies within nature-based solutions (NBSs), which is an integrated approach that addresses the twin crises of climate change and biodiversity [63], at the same time as supporting several sustainable development goals. According to a recent report [64], Brazil already has the legal framework and policies aligned with NBS, which can contribute to Brazil meeting its climate commitments, so Brazil is well positioned to advance this ecological and social agenda in blue carbon ecosystems as we expand our knowledge of their services and threats.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su152014722/s1, Figure S1: ROSES (Reporting standards for Systematic Evidence Syntheses in environmental research) systematic review flowchart; Figure S2: Mean number of observed seagrass ecosystems services as a function of expected. Bars = ±SE; Figure S3: Relationship between the proportions of 23 expected and observed seagrass ecosystem services mentioned in studies on seagrasses or seagrass habitats in Brazil, detailing the ten most commonly expected/observed services; Table S1: Searches of seagrasses in Brazil: combinations of search terms and the number of screened records from the Google Scholar search engine (with total returns in parentheses when n > 2500 for the search term); Table S2: List of seagrass ecosystem services used in this study and the type of service; Supplementary Table S3: List of words (with count ≥ 6) used in the title analysis and word cloud and seagrass ecosystem services and type implied by the words, where relevant; Supplementary Table S4: Griffiths et al. (2020)’s [30] DAPSIR for seagrass used for categorization; Table S5: A list of all documents used in the study.
Author Contributions
Conceptualization, J.C.C., M.L., K.M.M., R.A.M., P.A.H., P.R., F.A.C., X.O. and M.C.; methodology, J.C.C., L.S.A., J.G.d.S., C.B.B.d.B. and M.C.; validation, J.C.C.; formal analysis, J.C.C.; investigation, J.C.C., L.S.A., J.G.d.S., C.B.B.d.B. and B.S.V.M.D.; resources, J.C.C., L.S.A., J.G.d.S., C.B.B.d.B., B.S.V.M.D., M.L., V.E.d.S., K.M.M., R.A.M., M.V.-B., I.C., P.A.H., P.R., F.A.C., X.O. and M.C.; data curation, J.C.C., L.S.A., J.G.d.S., C.B.B.d.B. and B.S.V.M.D.; writing—original draft preparation, J.C.C., M.L., V.E.d.S., K.M.M. and M.C.; writing—review and editing, J.C.C., L.S.A., J.G.d.S., C.B.B.d.B., B.S.V.M.D., M.L., V.E.d.S., K.M.M., R.A.M., M.V.-B., I.C., P.A.H., P.R., F.A.C., X.O. and M.C.; visualization, R.A.M. and X.O.; supervision, J.C.C.; project administration, J.C.C.; funding acquisition, J.C.C., M.L., V.E.d.S., K.M.M., R.A.M., P.A.H., P.R., F.A.C., X.O. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico, grant number 407770/2021-6.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available in supplementary materials or from the corresponding author.
Acknowledgments
We thank Vesselina Mihneva and Violin Raykov for inviting us to contribute to the Special Issue “Understanding and Conserving Marine Biodiversity and Ecosystem Services” and the four reviewers for their positive contributions to our manuscript. PAH thanks Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (Fapesc), Biodiversa, CAPES and CNPq for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barbier, E.B. Marine ecosystem services. Curr. Biol. 2017, 27, R507–R510. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Marcoval, M.A.; Bazzini, S.M.; Vallina, M.V.; Marco, S. Coastal marine biodiversity: Challenges and threats. In Marine Ecology in a Changing World; Arias, A.H., Menendez, M.C., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 43–67. [Google Scholar]
- Costanza, R.; D’Arge, R.; Groot, R.d.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Kadykalo, A.N.; López-Rodriguez, M.D.; Ainscough, J.; Droste, N.; Ryu, H.; Ávila-Flores, G.; Le Clec’h, S.; Muñoz, M.C.; Nilsson, L.; Rana, S. Disentangling ‘ecosystem services’ and ‘nature’s contributions to people’. Ecosyst. People 2019, 15, 269–287. [Google Scholar] [CrossRef]
- Costanza, R.; de Groot, R.; Sutton, P.; van der Ploeg, S.; Anderson, S.J.; Kubiszewski, I.; Farber, S.; Turner, R.K. Changes in the global value of ecosystem services. Glob. Environ. Chang. 2014, 26, 152–158. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Current State and Trends; Island Press: Washington, DC, USA, 2005; p. 966. [Google Scholar]
- Manes, S.; Vale, M.M.; Malecha, A.; Pires, A.P. Nature-based solutions promote climate change adaptation safeguarding ecosystem services. Ecosyst. Serv. 2022, 55, 101439. [Google Scholar] [CrossRef]
- McKenzie, L.J.; Nordlund, L.M.; Jones, B.L.; Cullen-Unsworth, L.C.; Roelfsema, C.; Unsworth, R.K.F. The global distribution of seagrass meadows. Environ. Res. Lett. 2020, 15, 074041. [Google Scholar] [CrossRef]
- Nordlund, L.M.; Koch, E.W.; Barbier, E.B.; Creed, J.C. Seagrass ecosystem services and their variability across genera and geographical regions. PLoS ONE 2016, 11, e0163091. [Google Scholar] [CrossRef]
- de los Santos, C.B.; Scott, A.; Arias-Ortiz, A.; Jones, B.; Kennedy, H.; Mazarrasa, I.; McKenzie, L.; Nordlund, L.M.; de la Torre-Castro, M.; Unsworth, R.K. Seagrass ecosystem services: Assessment and scale of benefits. In Out of the Blue: The Value of Seagrasses to the Environment and to People; Potouroglou, M., Grimsditch, G., Weatherdon, L., Lutz, S., Eds.; United Nations Environment Programme: Nairobi, Kenya, 2020; pp. 19–21. [Google Scholar]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012, 5, 505. [Google Scholar] [CrossRef]
- Serrano, O.; Lovelock, C.E.; Atwood, T.B.; Macreadie, P.I.; Canto, R.; Phinn, S.; Arias-Ortiz, A.; Bai, L.; Baldock, J.; Bedulli, C.; et al. Australian vegetated coastal ecosystems as global hotspots for climate change mitigation. Nat. Commun. 2019, 10, 4313. [Google Scholar] [CrossRef]
- Mazarrasa, I.; Lavery, P.; Duarte, C.M.; Lafratta, A.; Lovelock, C.E.; Macreadie, P.I.; Samper-Villarreal, J.; Salinas, C.; Sanders, C.J.; Trevathan-Tackett, S.; et al. Factors Determining Seagrass Blue Carbon Across Bioregions and Geomorphologies. Glob. Biogeochem. Cycles 2021, 35, e2021GB006935. [Google Scholar] [CrossRef]
- Hatje, V.; Copertino, M.; Patire, V.F.; Ovando, X.; Ogbuka, J.; Johnson, B.J.; Kennedy, H.; Masque, P.; Creed, J.C. Vegetated coastal ecosystems in the Southwestern Atlantic Ocean are an unexploited opportunity for climate change mitigation. Commun. Earth Environ. 2023, 4, 160. [Google Scholar] [CrossRef]
- Ondiviela, B.; Losada, I.J.; Lara, J.L.; Maza, M.; Galván, C.; Bouma, T.J.; van Belzen, J. The role of seagrasses in coastal protection in a changing climate. Coast. Eng. 2014, 87, 158–168. [Google Scholar] [CrossRef]
- Unsworth, R.K.F.; Nordlund, L.M.; Cullen-Unsworth, L.C. Seagrass meadows support global fisheries production. Conserv. Lett. 2019, 12, e12566. [Google Scholar] [CrossRef]
- Sanchez-Vidal, A.; Canals, M.; de Haan, W.P.; Romero, J.; Veny, M. Seagrasses provide a novel ecosystem service by trapping marine plastics. Sci. Rep. 2021, 11, 254. [Google Scholar] [CrossRef]
- Cunha-Lignon, M.; Mendonça, J.T.; Conti, L.A.; Souza Barros, K.V.; Magalhães, K.M. Mangroves and Seagrasses. In Blue Economy: An Ocean Science Perspective; Urban, E.R., Jr., Ittekkot, V., Eds.; Springer Nature: Singapore, 2022; pp. 55–85. [Google Scholar] [CrossRef]
- Dewsbury, B.M.; Bhat, M.; Fourqurean, J.W. A review of seagrass economic valuations: Gaps and progress in valuation approaches. Ecosyst. Serv. 2016, 18, 68–77. [Google Scholar] [CrossRef]
- Koch, E.W.; Barbier, E.B.; Silliman, B.R.; Reed, D.J.; Perillo, G.M.; Hacker, S.D.; Granek, E.F.; Primavera, J.H.; Muthiga, N.; Polasky, S.; et al. Non-linearity in ecosystem services: Temporal and spatial variability in coastal protection. Front. Ecol. Environ. 2009, 7, 29–37. [Google Scholar] [CrossRef]
- Nordlund, L.M.; Jackson, E.L.; Nakaoka, M.; Samper-Villarreal, J.; Beca-Carretero, P.; Creed, J.C. Seagrass ecosystem services—What’s next? Mar. Pollut. Bull. 2018, 134, 145–151. [Google Scholar] [CrossRef]
- Copertino, M.S.; Creed, J.C.; Lanari, M.O.; Magalhães, K.; Barros, K.; Lana, P.C.; Sordo, L.; Horta, P.A. Seagrass and Submerged Aquatic Vegetation (VAS) Habitats off the Coast of Brazil: State of knowledge, conservation and main threats. Braz. J. Oceanogr. 2016, 64, 53–80. [Google Scholar] [CrossRef]
- Dominguez, J.M.L. The Coastal Zone of Brazil: An Overview. J. Coast. Res. 2006, I, 16–20. [Google Scholar]
- Dillenburg, S.R.; Hesp, P.A. Geology and Geomorphology of Holocene Coastal Barriers of Brazil; Springer Science & Business Media: Berlin, Germany, 2008; Volume 107. [Google Scholar]
- Kantrud, H.A. Wigeongrass (Ruppia maritima L.): A Literature Review; U.S. Fish and Wildlife Service, Fish and Wildlife Research 10: Washington, DC, USA, 1991; Volume 10, p. 58.
- Laborel-Deguen, F. Nota preliminar sobre a ecologia das pradarias de faner’ogamas marinhas nas costas dos estados de Pernambuco e da Paraiba. Trab. Inst. Oceanog. Univ. Recife 1963, 3, 39–51. [Google Scholar]
- Lamb, J.B.; Van De Water, J.A.; Bourne, D.G.; Altier, C.; Hein, M.Y.; Fiorenza, E.A.; Abu, N.; Jompa, J.; Harvell, C.D. Seagrass ecosystems reduce exposure to bacterial pathogens of humans, fishes, and invertebrates. Science 2017, 355, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Ricart, A.M.; Ward, M.; Hill, T.M.; Sanford, E.; Kroeker, K.J.; Takeshita, Y.; Merolla, S.; Shukla, P.; Ninokawa, A.T.; Elsmore, K.; et al. Coast-wide evidence of low pH amelioration by seagrass ecosystems. Glob. Chang. Biol. 2021, 27, 2580–2591. [Google Scholar] [CrossRef] [PubMed]
- Wordclouds.com. Available online: https://www.wordclouds.com/ (accessed on 29 May 2023).
- Griffiths, L.L.; Connolly, R.M.; Brown, C.J. Critical gaps in seagrass protection reveal the need to address multiple pressures and cumulative impacts. Ocean Coast. Manag. 2020, 183, 104946. [Google Scholar] [CrossRef]
- Smith, C.J.; Papadopoulou, K.-N.; Barnard, S.; Mazik, K.; Elliott, M.; Patrício, J.; Solaun, O.; Little, S.; Bhatia, N.; Borja, A. Managing the Marine Environment, Conceptual Models and Assessment Considerations for the European Marine Strategy Framework Directive. Front. Mar. Sci. 2016, 3, 144. [Google Scholar] [CrossRef]
- Elliott, M.; Burdon, D.; Atkins, J.P.; Borja, A.; Cormier, R.; de Jonge, V.N.; Turner, R.K. “And DPSIR begat DAPSI(W)R(M)!”—A unifying framework for marine environmental management. Mar. Pollut. Bull. 2017, 118, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, A.C.O.d.; Lima, D.d.S.; Alves, M.D.d.O.; Borges, J.C.G.; Marmontel, M.; Carvalho, V.L.; Santos, F.R.d. Don’t let me down: West Indian manatee, Trichechus manatus, is still critically endangered in Brazil. J. Nat. Conserv. 2022, 67, 126169. [Google Scholar] [CrossRef]
- Gama, L.R.; Fuentes, M.; Trevizani, T.H.; Pellizzari, F.; Lemons, G.E.; Seminoff, J.A.; Domit, C. Trophic ecology of juvenile green turtles in the Southwestern Atlantic Ocean: Insights from stable isotope analysis and niche modelling. Mar. Ecol. Prog. Ser. 2021, 678, 139–152. [Google Scholar] [CrossRef]
- Fernandes, A.; Bondioli, A.C.V.; Solé, M.; Schiavetti, A. Seasonal Variation in the Behavior of Sea Turtles at a Brazilian Foraging Area. Chelonian Conserv. Biol. 2017, 16, 93–102. [Google Scholar] [CrossRef]
- Alves, M.D.O.; Schwamborn, R.; Borges, J.C.G.; Marmontel, M.; Costa, A.F.; Schettini, C.A.F.; Araújo, M.E. Aerial survey of manatees, dolphins and sea turtles off northeastern Brazil: Correlations with coastal features and human activities. Biol. Conserv. 2013, 161, 91–100. [Google Scholar] [CrossRef]
- Alves, M.D.; Kinas, P.G.; Marmontel, M.; Borges, J.C.G.; Costa, A.F.; Schiel, N.; Araújo, M.E. First abundance estimate of the Antillean manatee (Trichechus manatus manatus) in Brazil by aerial survey. J. Mar. Biol. Assoc. U.K. 2016, 96, 955–966. [Google Scholar] [CrossRef]
- Lima, M.A.C.; Bergamo, T.F.; Ward, R.D.; Joyce, C.B. A review of seagrass ecosystem services: Providing nature-based solutions for a changing world. Hydrobiologia 2023, 850, 2655–2670. [Google Scholar] [CrossRef]
- Liquete, C.; Piroddi, C.; Drakou, E.G.; Gurney, L.; Katsanevakis, S.; Charef, A.; Egoh, B. Current Status and Future Prospects for the Assessment of Marine and Coastal Ecosystem Services: A Systematic Review. PLoS ONE 2013, 8, e67737. [Google Scholar] [CrossRef] [PubMed]
- Daily, G.C. Nature’s Services: Societal Dependence on Natural Ecosystems; Island Press: Washington, DC, USA, 1997. [Google Scholar]
- Costanza, R.; De Groot, R.; Braat, L.; Kubiszewski, I.; Fioramonti, L.; Sutton, P.; Farber, S.; Grasso, M. Twenty years of ecosystem services: How far have we come and how far do we still need to go? Ecosyst. Serv. 2017, 28, 1–16. [Google Scholar] [CrossRef]
- Boerema, A.; Rebelo, A.J.; Bodi, M.B.; Esler, K.J.; Meire, P. Are ecosystem services adequately quantified? J. Appl. Ecol. 2017, 54, 358–370. [Google Scholar] [CrossRef]
- Furkon; Nessa, N.; Ambo-Rappe, R.; Cullen-Unsworth, L.C.; Unsworth, R.K.F. Social-ecological drivers and dynamics of seagrass gleaning fisheries. Ambio 2020, 49, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Grech, A.; Chartrand-Miller, K.; Erftemeijer, P.; Fonseca, M.; McKenzie, L.; Rasheed, M.; Taylor, H.; Coles, R. A comparison of threats, vulnerabilities and management approaches in global seagrass bioregions. Environ. Res. Lett. 2012, 7, 024006. [Google Scholar] [CrossRef]
- Maron, M.; Mitchell, M.G.E.; Runting, R.K.; Rhodes, J.R.; Mace, G.M.; Keith, D.A.; Watson, J.E.M. Towards a Threat Assessment Framework for Ecosystem Services. Trends Ecol. Evol. 2017, 32, 240–248. [Google Scholar] [CrossRef]
- Micheli, F.; Halpern, B.S.; Walbridge, S.; Ciriaco, S.; Ferretti, F.; Fraschetti, S.; Lewison, R.; Nykjaer, L.; Rosenberg, A.A. Cumulative Human Impacts on Mediterranean and Black Sea Marine Ecosystems: Assessing Current Pressures and Opportunities. PLoS ONE 2013, 8, e79889. [Google Scholar] [CrossRef]
- Soares, M.O.; Bezerra LE, A.; Copertino, M.; Lopes, B.D.; Barros KV, S.; Rocha-Barreira, C.A.; Maia, R.C.; Beloto, N.; Cotovicz, L.C. Blue Carbon Ecosystems in Brazil: Overview and an Urgent Call for Conservation and Restoration. Front. Mar. Sci. 2022, 9, 797411. [Google Scholar] [CrossRef]
- Oliveira, E.C.; Pirani, J.R.; Giulietti, A.M. The Brazilian seagrasses. Aquat. Bot. 1983, 16, 251–267. [Google Scholar] [CrossRef]
- Short, F.; Carruthers, T.; Dennison, W.; Waycott, M. Global seagrass distribution and diversity: A bioregional model. J. Exp. Mar. Biol. Ecol. 2007, 350, 3–20. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, R.K.; McKenzie, L.J.; Collier, C.J.; Cullen-Unsworth, L.C.; Duarte, C.M.; Eklöf, J.S.; Jarvis, J.C.; Jones, B.L.; Nordlund, L.M. Global challenges for seagrass conservation. Ambio 2019, 48, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [PubMed]
- Coles, R.; McKenzie, L.; De’ath, G.; Roelofs, A.; Lee Long, W. Spatial distribution of deepwater seagrass in the inter-reef lagoon of the Great Barrier Reef World Heritage Area. Mar. Ecol. Prog. Ser. 2009, 392, 57–68. [Google Scholar] [CrossRef]
- Jones, B.L.; Unsworth, R.K.; McKenzie, L.J.; Yoshida, R.L.; Cullen-Unsworth, L.C. Crowdsourcing conservation: The role of citizen science in securing a future for seagrass. Mar. Pollut. Bull. 2018, 134, 210–215. [Google Scholar] [CrossRef]
- Magris, R.A.; Costa, M.D.P.; Ferreira, C.E.L.; Vilar, C.C.; Joyeux, J.-C.; Creed, J.C.; Copertino, M.S.; Horta, P.A.; Sumida, P.Y.G.; Francini-Filho, R.B.; et al. A blueprint for securing Brazil’s marine biodiversity and supporting the achievement of global conservation goals. Divers. Distrib. 2021, 27, 198–215. [Google Scholar] [CrossRef]
- Misturini, D.; Colling, L.A. Can short-term meteorological events alter subtropical estuarine macrobenthic assemblages in seagrass meadows (Patos Lagoon Estuary—Southern Brazil)? Estuar. Coast. Shelf Sci. 2021, 261, 107532. [Google Scholar] [CrossRef]
- Lemos, V.M.; Lanari, M.; Copertino, M.; Secchi, E.R.; de Abreu, P.C.O.; Muelbert, J.H.; Garcia, A.M.; Dumont, F.C.; Muxagata, E.; Vieira, J.P. Patos Lagoon estuary and adjacent marine coastal biodiversity long-term data. Earth Syst. Sci. Data 2022, 14, 1015–1041. [Google Scholar] [CrossRef]
- Pereira, P.H.C.; Ferreira, B.P.; Rezende, S.M. Community structure of the ichthyofauna associated with seagrass beds (Halodule wrightii) in Formoso River estuary—Pernambuco, Brazil. An. Da Acad. Bras. De Cienc. 2010, 82, 617–628. [Google Scholar] [CrossRef]
- Costa, M.D.P.; Possingham, H.P.; Muelbert, J.H. Incorporating early life stages of fishes into estuarine spatial conservation planning. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 1013–1030. [Google Scholar] [CrossRef]
- Costa, A.C.P.; Garcia, T.M.; Paiva, B.P.; Ximenes Neto, A.R.; Soares, M.O. Seagrass and rhodolith beds are important seascapes for the development of fish eggs and larvae in tropical coastal areas. Mar. Environ. Res. 2020, 161, 105064. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.E.L.; Assis, I.O.; Campos-Silva, J.V.; Paulino, G.V.B.; Fabre, N.N. Relative importance of habitat mosaics for fish guilds in the northeastern coast of Brazil. Reg. Stud. Mar. Sci. 2022, 50, 102145. [Google Scholar] [CrossRef]
- Costa, M.D.P.; Muelbert, J.H.; Vieira, J.; Castello, J.P. Dealing with temporal variation and different life stages of whitemouth croaker Micropogonias furnieri (Actinopterygii, Sciaenidae) in species distribution modeling to improve essential estuarine fish habitat identification. Hydrobiologia 2015, 762, 195–208. [Google Scholar] [CrossRef]
- Seddon, N.; Smith, A.; Smith, P.; Key, I.; Chausson, A.; Girardin, C.; House, J.; Srivastava, S.; Turner, B. Getting the message right on nature-based solutions to climate change. Glob. Chang. Biol. 2021, 27, 1518–1546. [Google Scholar] [CrossRef] [PubMed]
- Nature-Based Solutions in Brazil: An Overview of Policies and Experiences CDP Worldwide. 2022. Available online: https://cdn.cdp.net/cdp-production/cms/reports/documents/000/006/563/original/UK_PACT_Leaflet_%2810%29.pdf?1664526067 (accessed on 2 August 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).