Ag-Containing Carbon Nanocomposites: Physico-Chemical Properties and Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
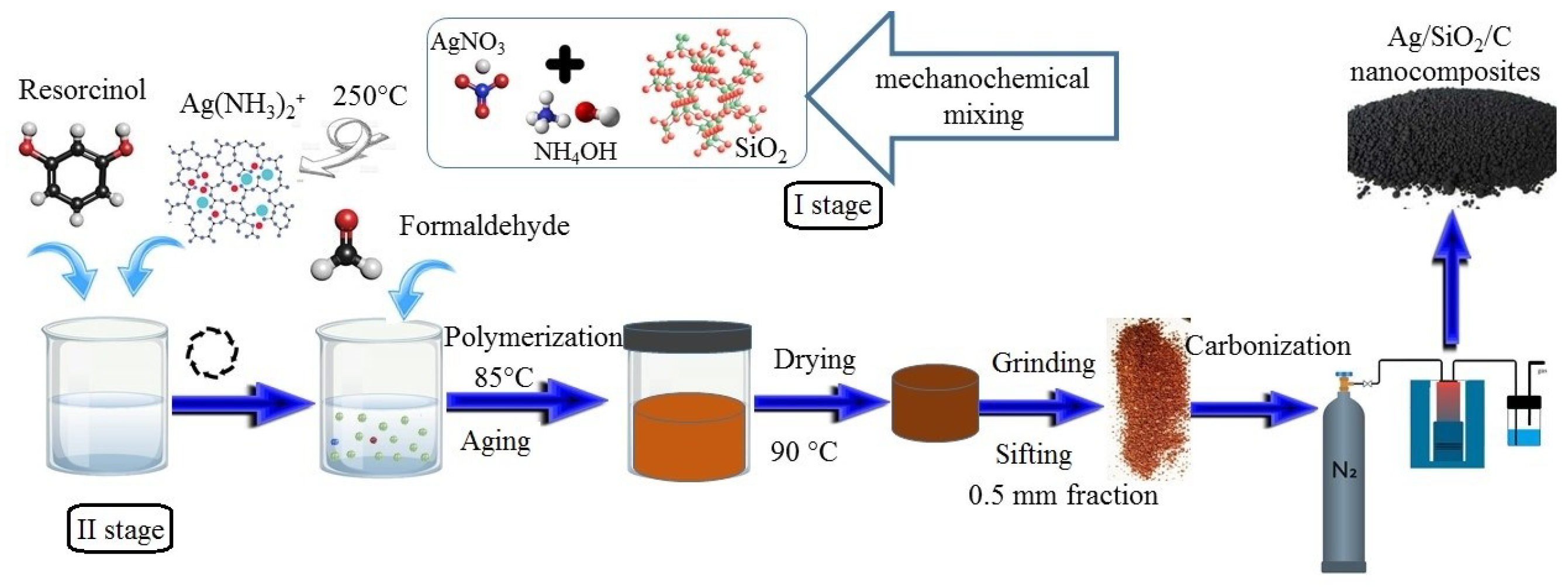
2.2. Sample Synthesis
2.3. Instruments and Measurements
2.4. Adsorption Studies
2.5. Antibacterial Activity Determination
3. Results and Discussion
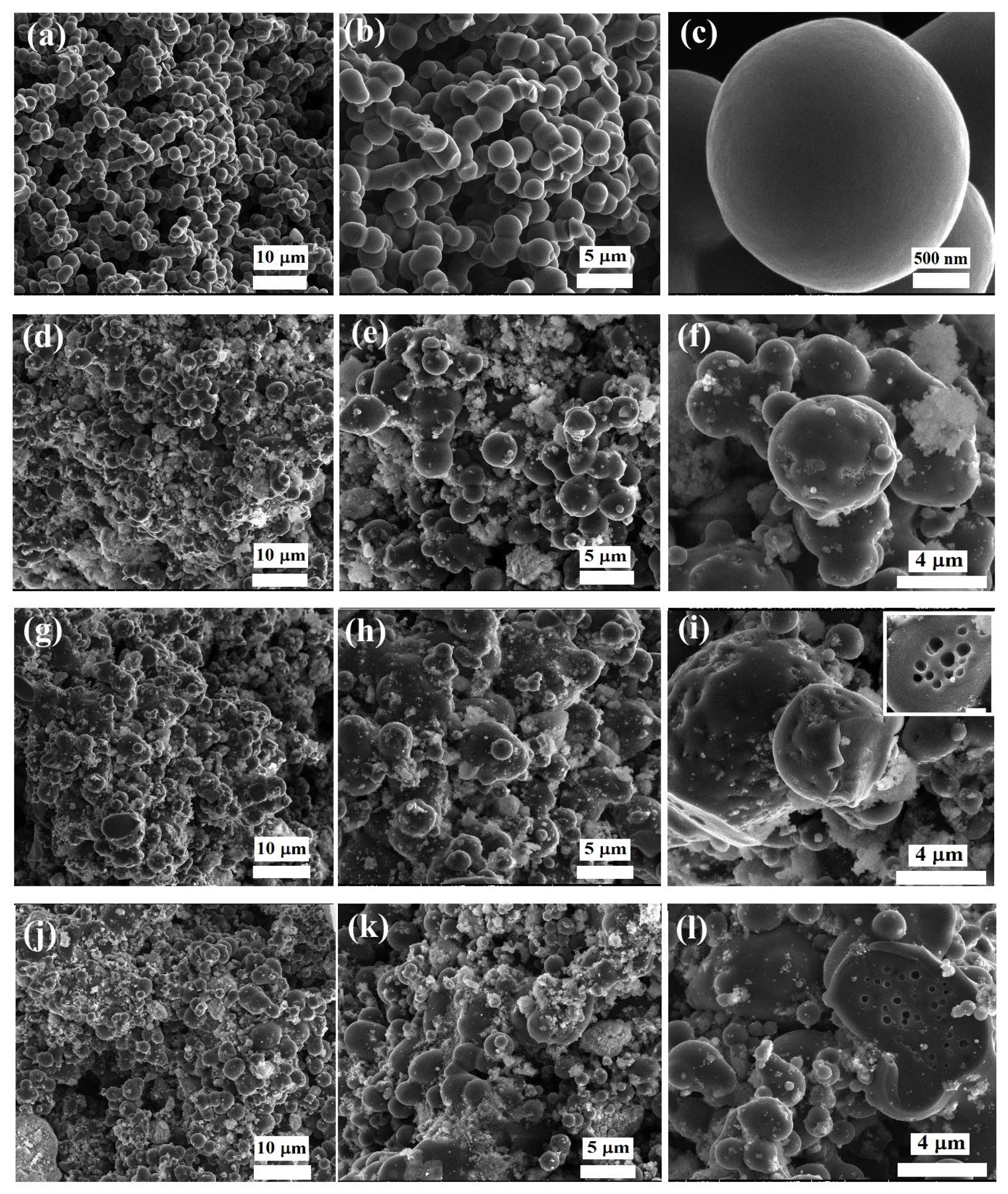
3.1. Characterization of the Adsorbents
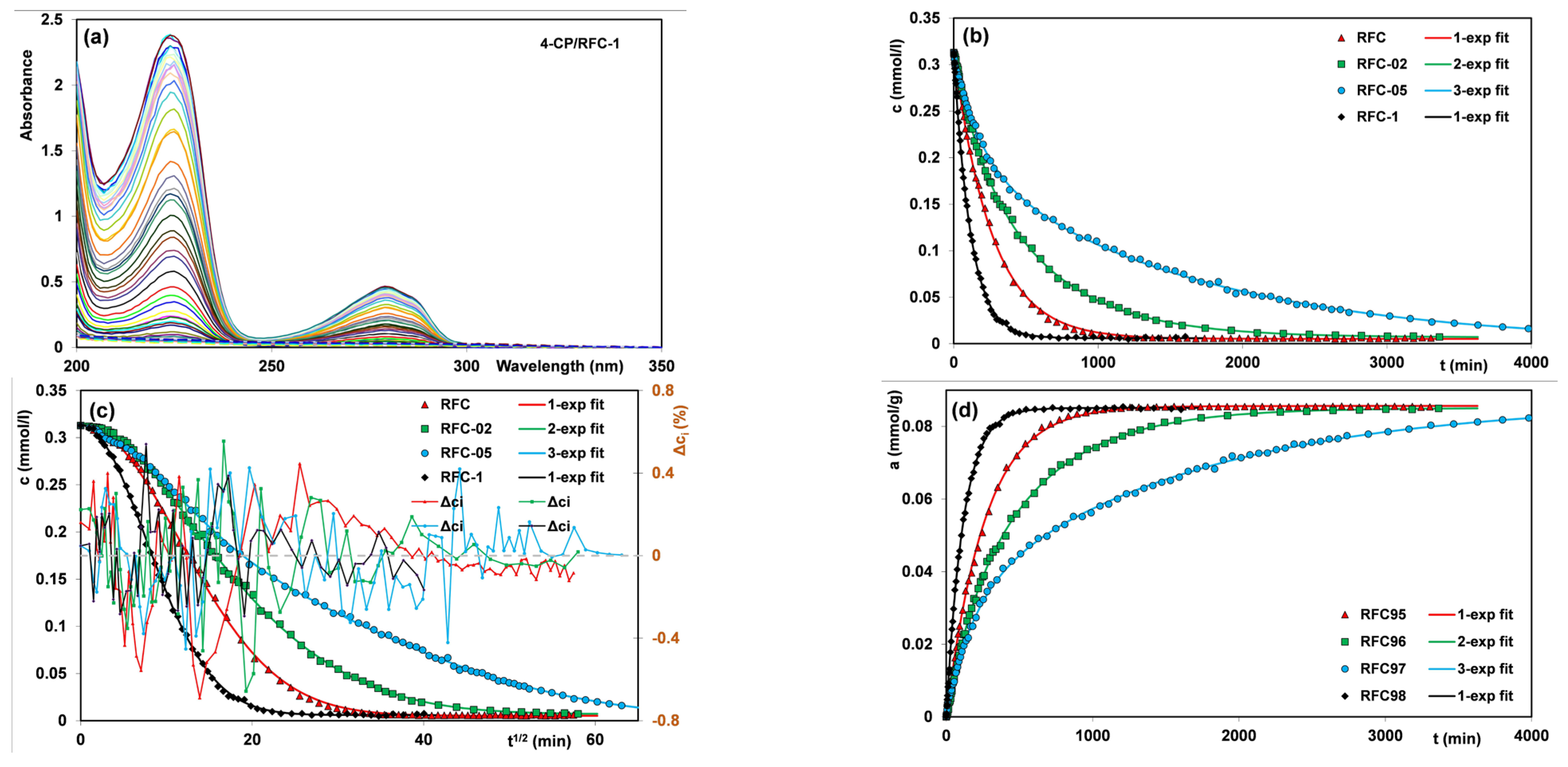
3.2. Adsorption of 4-Chlorophenol
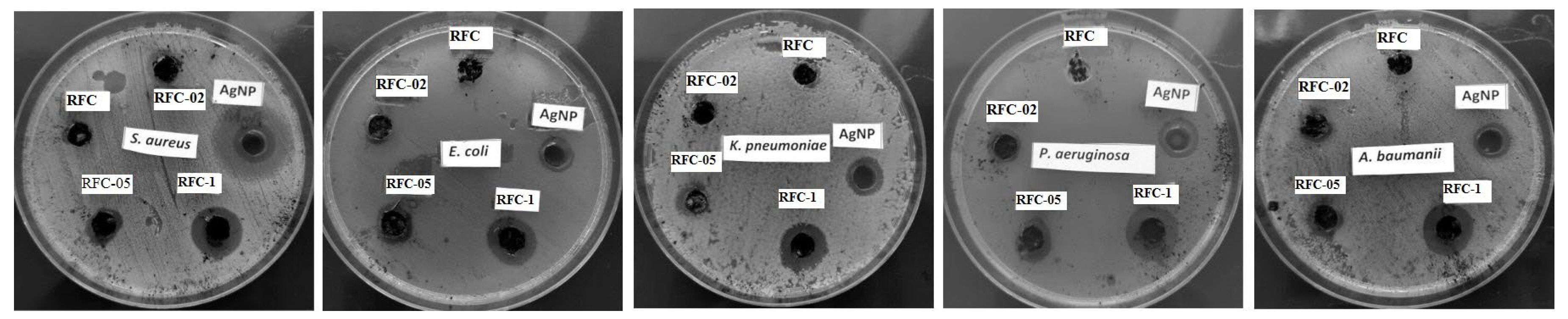
3.3. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Sangamnere, R.; Misra, T.; Bherwani, H.; Kapley, A.; Kumar, R. A Critical Review of Conventional and Emerging Wastewater Treatment Technologies. Sustain. Water Resour. Manag. 2023, 9, 58. [Google Scholar] [CrossRef]
- Pell, M.; Wörman, A. Biological Wastewater Treatment Systems. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 275–290. [Google Scholar]
- Lin, S.H.; Kiang, C.D. Combined Physical, Chemical and Biological Treatments of Wastewater Containing Organics from a Semiconductor Plant. J. Hazard Mater. 2003, 97, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New Perspectives for Advanced Oxidation Processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Grządka, E. Adsorption and Electrokinetic Properties in the System: Beta-Cyclodextrin/Alumina in the Presence of Ionic and Non-Ionic Surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 261–268. [Google Scholar] [CrossRef]
- Raninga, M.; Mudgal, A.; Patel, V.K.; Patel, J.; Kumar Sinha, M. Modification of Activated Carbon-Based Adsorbent for Removal of Industrial Dyes and Heavy Metals: A Review. Mater. Today Proc. 2023, 77, 286–294. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Braga, M.C.B. Adsorption of Organic and Inorganic Pollutants onto Biochars: Challenges, Operating Conditions, and Mechanisms. Bioresour. Technol. Rep. 2021, 15, 100728. [Google Scholar] [CrossRef]
- Le-Minh, N.; Sivret, E.C.; Shammay, A.; Stuetz, R.M. Factors Affecting the Adsorption of Gaseous Environmental Odors by Activated Carbon: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 341–375. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Detoxification of Pesticide Waste via Activated Carbon Adsorption Process. J. Hazard Mater. 2010, 175, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Kondracki, B.; Szewczuk-Karpisz, K. Chemical Modification of Biochars as a Method to Improve Its Surface Properties and Efficiency in Removing Xenobiotics from Aqueous Media. Chemosphere 2023, 312, 137238. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An Overview of the Modification Methods of Activated Carbon for Its Water Treatment Applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, X.; Qian, Y. Synthetic Methodologies for Carbon Nanomaterials. Adv. Mater. 2010, 22, 1963–1966. [Google Scholar] [CrossRef]
- Han, Z.; Adeleye, A.S.; Keller, A.A. Engineered Nanomaterials for Water Treatment. In Encyclopedia of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 418–455. [Google Scholar]
- Zahid, M.U.; Pervaiz, E.; Hussain, A.; Shahzad, M.I.; Niazi, M.B.K. Synthesis of Carbon Nanomaterials from Different Pyrolysis Techniques: A Review. Mater. Res. Express 2018, 5, 052002. [Google Scholar] [CrossRef]
- Galaburda, M.V.; Bogatyrov, V.M.; Skubiszewska-ZiĿba, J.; Oranska, O.I.; Sternik, D.; Gunko, V.M. Synthesis and Structural Features of Resorcinol Formaldehyde Resin Chars Containing Nickel Nanoparticles. Appl. Surf. Sci. 2016, 360, 722–730. [Google Scholar] [CrossRef]
- Foong, S.Y.; Liew, R.K.; Lee, C.L.; Tan, W.P.; Peng, W.; Sonne, C.; Tsang, Y.F.; Lam, S.S. Strategic Hazard Mitigation of Waste Furniture Boards via Pyrolysis: Pyrolysis Behavior, Mechanisms, and Value-Added Products. J. Hazard Mater. 2022, 421, 126774. [Google Scholar] [CrossRef] [PubMed]
- Galaburda, M.; Bogatyrov, V.; Oranska, O.; Gun’ko, V.; Skubiszewska-Zięba, J.; Urubkov, I. Synthesis and Characterization of Carbon Composites Containing Fe, Co, Ni Nanoparticles. J. Therm. Anal. Calorim. 2015, 122, 553–561. [Google Scholar] [CrossRef]
- Monge, F.A.; Jagadesan, P.; Bondu, V.; Donabedian, P.L.; Ista, L.; Chi, E.Y.; Schanze, K.S.; Whitten, D.G.; Kell, A.M. Highly Effective Inactivation of SARS-CoV-2 by Conjugated Polymers and Oligomers. ACS Appl. Mater. Interfaces 2020, 12, 55688–55695. [Google Scholar] [CrossRef]
- Liu, H.; Huang, J.; Mao, J.; Chen, Z.; Chen, G.; Lai, Y. Transparent Antibacterial Nanofiber Air Filters with Highly Efficient Moisture Resistance for Sustainable Particulate Matter Capture. iScience 2019, 19, 214–223. [Google Scholar] [CrossRef]
- Gough, C.R.; Callaway, K.; Spencer, E.; Leisy, K.; Jiang, G.; Yang, S.; Hu, X. Biopolymer-Based Filtration Materials. ACS Omega 2021, 6, 11804–11812. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Wan, K.; Kong, H.; Guo, L.; Wang, Y.; Liu, X.; Wei, G. Recent Advance in Biomass Membranes: Fabrication, Functional Regulation, and Antimicrobial Applications. Carbohydr. Polym. 2023, 305, 120537. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver Nanoparticles as an Effective Disinfectant: A Review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef]
- Malachová, K.; Praus, P.; Rybková, Z.; Kozák, O. Antibacterial and Antifungal Activities of Silver, Copper and Zinc Montmorillonites. Appl. Clay Sci. 2011, 53, 642–645. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial Resistance to Silver Nanoparticles and How to Overcome It. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zienkiewicz-Strzałka, M.; Deryło-Marczewska, A.; Skorik, Y.A.; Petrova, V.A.; Choma, A.; Komaniecka, I. Silver Nanoparticles on Chitosan/Silica Nanofibers: Characterization and Antibacterial Activity. Int. J. Mol. Sci. 2019, 21, 166. [Google Scholar] [CrossRef]
- Prasad, R.; Karchiyappan, T. Advanced Research in Nanosciences for Water Technology; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-02380-5. [Google Scholar]
- Egger, S.; Lehmann, R.P.; Height, M.J.; Loessner, M.J.; Schuppler, M. Antimicrobial Properties of a Novel Silver-Silica Nanocomposite Material. Appl. Environ. Microbiol. 2009, 75, 2973–2976. [Google Scholar] [CrossRef] [PubMed]
- Garba, Z.N.; Zhou, W.; Lawan, I.; Xiao, W.; Zhang, M.; Wang, L.; Chen, L.; Yuan, Z. An Overview of Chlorophenols as Contaminants and Their Removal from Wastewater by Adsorption: A Review. J. Environ. Manag. 2019, 241, 59–75. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization, International Agency for Research on Cancer: Lyon, France, 2014; Volume 83, Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/08/14-002.pdf (accessed on 23 November 2023).
- Bogatyrov, V.M.; Gun’ko, V.M.; Galaburda, M.V.; Oranska, O.I.; Petryk, I.S.; Tsyganenko, K.S.; Savchuk, Y.I.; Chobotarov, A.Y.; Rudenchyk, T.V.; Rozhnova, R.A.; et al. The Effect of Photoactivated Transformations of Ag+ and Ag0 in Silica Fillers on Their Biocidal Activity. Res. Chem. Intermed. 2019, 45, 3985–4001. [Google Scholar] [CrossRef]
- Gun’ko, V.M. Composite Materials: Textural Characteristics. Appl. Surf. Sci. 2014, 307, 444–454. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Mikhalovsky, S.V. Evaluation of Slitlike Porosity of Carbon Adsorbents. Carbon N. Y. 2004, 42, 843–849. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic Models of Sorption: A Theoretical Analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Blachnio, M.; Zienkiewicz-Strzalka, M.; Derylo-Marczewska, A.; Nosach, L.V.; Voronin, E.F. Chitosan–Silica Composites for Adsorption Application in the Treatment of Water and Wastewater from Anionic Dyes. Int. J. Mol. Sci. 2023, 24, 11818. [Google Scholar] [CrossRef] [PubMed]
- Haerifar, M.; Azizian, S. Fractal-Like Kinetics for Adsorption on Heterogeneous Solid Surfaces. J. Phys. Chem. C 2014, 118, 1129–1134. [Google Scholar] [CrossRef]
- Zienkiewicz-Strzalka, M.; Blachnio, M. Nitrogenous Bases in Relation to the Colloidal Silver Phase: Adsorption Kinetic, and Morphology Investigation. Appl. Sci. 2023, 13, 3696. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C. In Vitro Antibacterial Activity and Mechanism of Silver Nanoparticles against Foodborne Pathogens. Bioinorg. Chem. Appl. 2014, 2014, 581890. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiao, S.Z.; Liu, H.; Chen, J.; Orpe, A.; Zhao, D.; Lu, G.Q.M. Extension of The Stöber Method to the Preparation of Monodisperse Resorcinol-Formaldehyde Resin Polymer and Carbon Spheres. Angew. Chem. Int. Ed. 2011, 50, 5947–5951. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yang, H.; Zhang, Y.; Hu, Q.; Li, S.; He, W. Synthesis of Size-Controlled Carbon Microspheres from Resorcinol/Formaldehyde for High Electrochemical Performance. New Carbon Mater. 2021, 36, 616–624. [Google Scholar] [CrossRef]
- Chen, R.; Nuhfer, N.T.; Moussa, L.; Morris, H.R.; Whitmore, P.M. Silver Sulfide Nanoparticle Assembly Obtained by Reacting an Assembled Silver Nanoparticle Template with Hydrogen Sulfide Gas. Nanotechnology 2008, 19, 455604. [Google Scholar] [CrossRef]
- Bakardjieva, S.; Mares, J.; Koci, E.; Tolasz, J.; Fajgar, R.; Ryukhtin, V.; Klementova, M.; Michna, Š.; Bibova, H.; Holmestad, R.; et al. Effect of Multiply Twinned Ag(0) Nanoparticles on Photocatalytic Properties of TiO2 Nanosheets and TiO2 Nanostructured Thin Films. Nanomaterials 2022, 12, 750. [Google Scholar] [CrossRef]
- Van der Veeken, P.L.R.; Chakraborty, P.; van Leeuwen, H.P. Accumulation of Humic Acid in DET/DGT Gels. Env. Sci. Technol. 2010, 44, 4253–4257. [Google Scholar] [CrossRef] [PubMed]
- Perino, A.; Klymchenko, A.; Morere, A.; Contal, E.; Rameau, A.; Guenet, J.-M.; Mély, Y.; Wagner, A. Structure and Behavior of Polydiacetylene-Based Micelles. Macromol. Chem. Phys. 2011, 212, 111–117. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Bailey, E.; Beetsch, N.; Wait, D.; Oza, H.; Ronnie, N.; Sobsey, M. Methods, Protocols, Guidance and Standards for Performance Evaluation for Point-of-Use Water Treatment Technologies: History, Current Status, Future Needs and Directions. Water 2021, 13, 1094. [Google Scholar] [CrossRef]
- Nasir, A.M.; Adam, M.R.; Mohamad Kamal, S.N.E.A.; Jaafar, J.; Othman, M.H.D.; Ismail, A.F.; Aziz, F.; Yusof, N.; Bilad, M.R.; Mohamud, R.; et al. A Review of the Potential of Conventional and Advanced Membrane Technology in the Removal of Pathogens from Wastewater. Sep. Purif. Technol. 2022, 286, 120454. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, A.; Tehrani, M.S.; Husain, S.W.; Anbia, M.; Azar, P.A. Adsorption of Chlorophenols from Aqueous Solution over Amino-Modified Ordered Nanoporous Silica Materials. J. Nanostruct. Chem. 2014, 4, 114. [Google Scholar] [CrossRef]
- Deryło-Marczewska, A.; Zienkiewicz-Strzałka, M.; Skrzypczyńska, K.; Świątkowski, A.; Kuśmierek, K. Evaluation of the SBA-15 Materials Ability to Accumulation of 4-Chlorophenol on Carbon Paste Electrode. Adsorption 2016, 22, 801–812. [Google Scholar] [CrossRef]
- Moritz, M. Use of Modified SBA-15 and MCF Mesoporous Silicas as Adsorbents for Chlorogenic Acid. A Comparative Study. Przemysł. Chem. 2015, 1, 112–115. [Google Scholar] [CrossRef]
- Liu, C.; Shaw, L. Nanoparticulate Materials and Core/Shell Structures Derived from Wet Chemistry Methods. In Encyclopedia of Nanotechnology; Springer Netherlands: Dordrecht, The Netherlands, 2016; pp. 2579–2597. [Google Scholar]
- El-Toni, A.M.; Habila, M.A.; Labis, J.P.; ALOthman, Z.A.; Alhoshan, M.; Elzatahry, A.A.; Zhang, F. Design, Synthesis and Applications of Core–Shell, Hollow Core, and Nanorattle Multifunctional Nanostructures. Nanoscale 2016, 8, 2510–2531. [Google Scholar] [CrossRef]
- Okolo, B.; Park, C.; Keane, M.A. Interaction of Phenol and Chlorophenols with Activated Carbon and Synthetic Zeolites in Aqueous Media. J. Colloid Interface Sci. 2000, 226, 308–317. [Google Scholar] [CrossRef]
- Berro, Y.; Gueddida, S.; Lebègue, S.; Pasc, A.; Canilho, N.; Kassir, M.; Hassan, F.E.H.; Badawi, M. Atomistic Description of Phenol, CO and H2O Adsorption over Crystalline and Amorphous Silica Surfaces for Hydrodeoxygenation Applications. Appl. Surf. Sci. 2019, 494, 721–730. [Google Scholar] [CrossRef]
- Blachnio, M.; Derylo-Marczewska, A.; Winter, S.; Zienkiewicz-Strzalka, M. Mesoporous Carbons of Well-Organized Structure in the Removal of Dyes from Aqueous Solutions. Molecules 2021, 26, 2159. [Google Scholar] [CrossRef] [PubMed]
- Kohsari, I.; Mohammad-Zadeh, M.; Minaeian, S.; Rezaee, M.; Barzegari, A.; Shariatinia, Z.; Koudehi, M.F.; Mirsadeghi, S.; Pourmortazavi, S.M. In Vitro Antibacterial Property Assessment of Silver Nanoparticles Synthesized by Falcaria Vulgaris Aqueous Extract against MDR Bacteria. J. Solgel Sci. Technol. 2019, 90, 380–389. [Google Scholar] [CrossRef]
- Taha, A.; Ben Aissa, M.; Da’na, E. Green Synthesis of an Activated Carbon-Supported Ag and ZnO Nanocomposite for Photocatalytic Degradation and Its Antibacterial Activities. Molecules 2020, 25, 1586. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Emam, H.E.; El-Rafie, M.H.; Ahmed, H.B.; Zahran, M.K. Room Temperature Synthesis of Metallic Nanosilver Using Acacia to Impart Durable Biocidal Effect on Cotton Fabrics. Fibers Polym. 2015, 16, 1676–1687. [Google Scholar] [CrossRef]






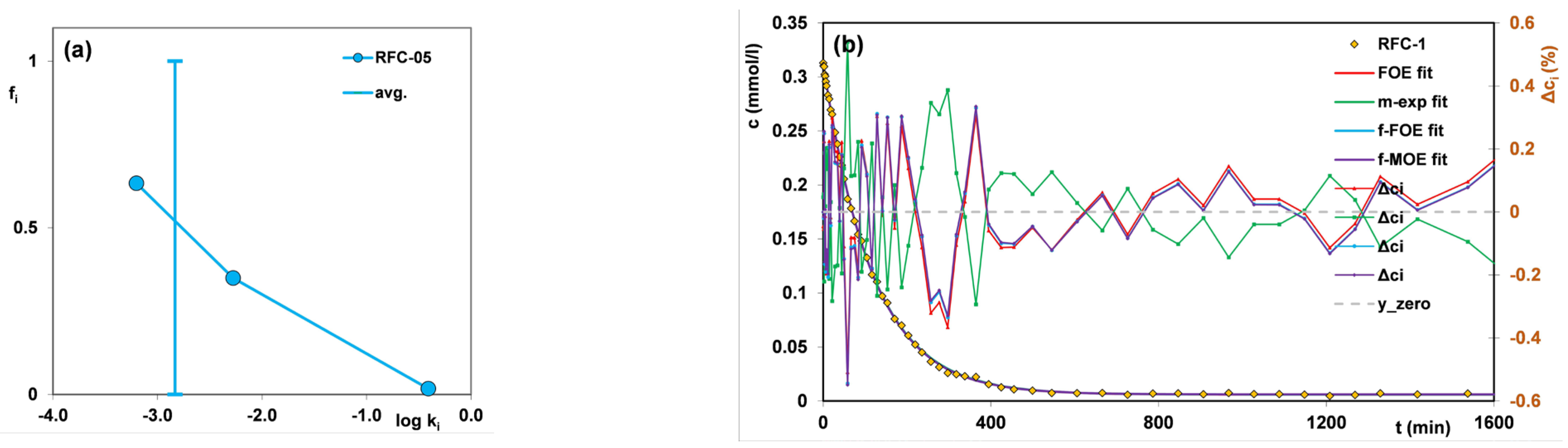
| Kinetic Model | General Equation | Half-Time Expression |
|---|---|---|
| First-order equation (FOE) [35] | t0.5~1/k1 | |
| Multi-exponential equation (m-exp) [36] | t0.5~(ln 2)/ki | |
| Fractal-like MOE equation (f-MOE) [37,38] Fractal-like FOE equation (f-FOE) [37,38] | t0.5~[ln(2 − f2)]1/p/k1 t0.5~[(ln2)]1/p/k1 |
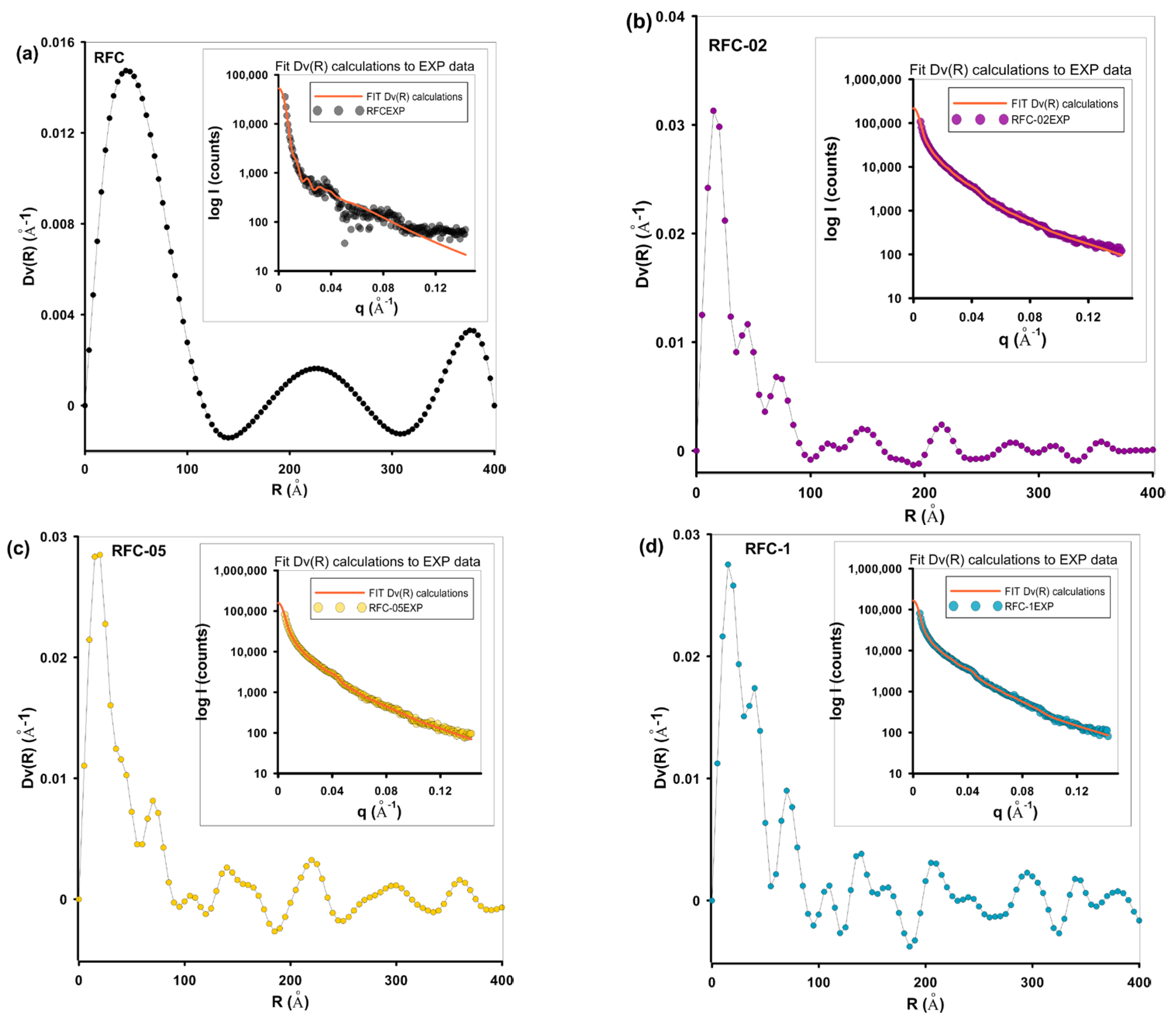
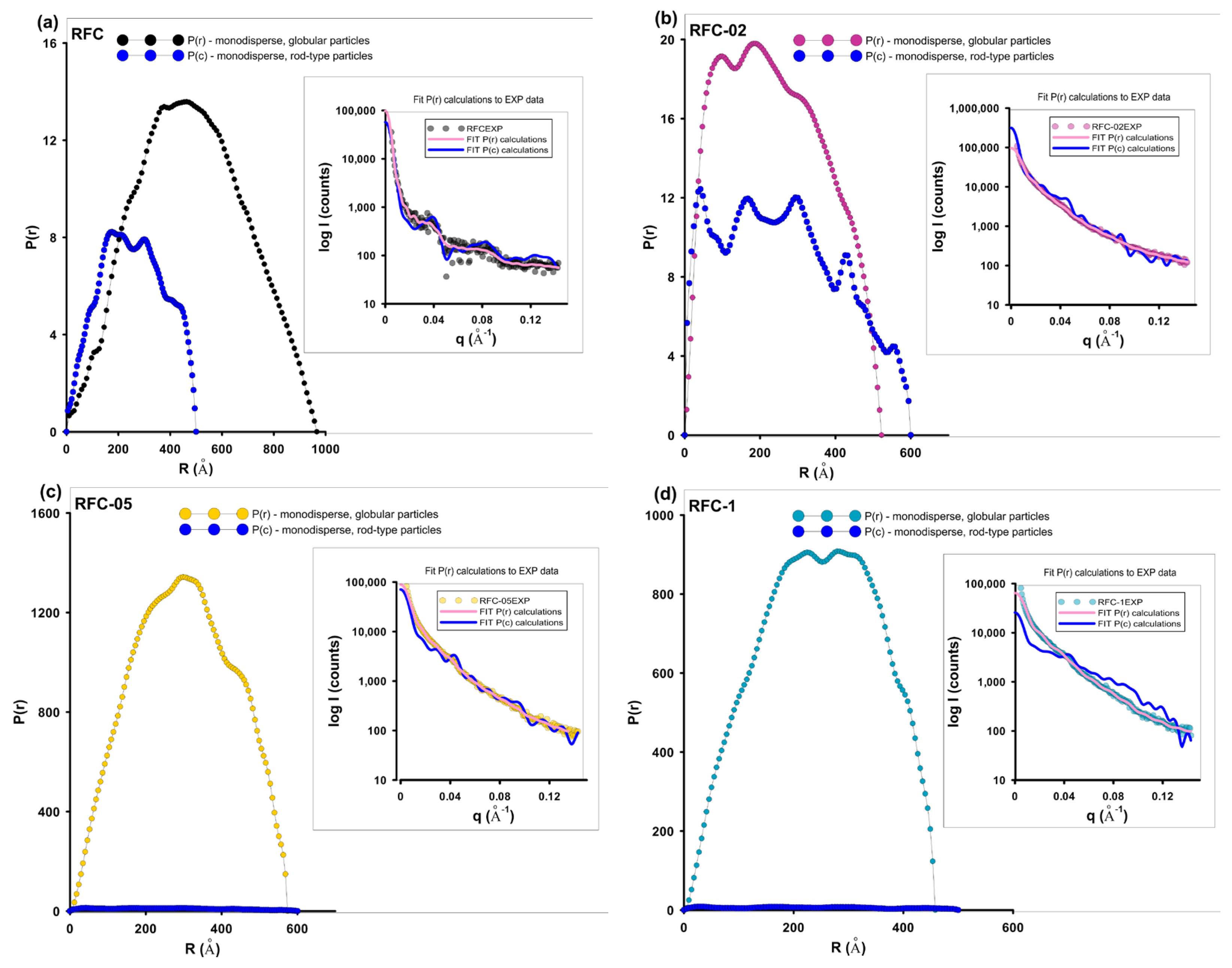
| Sample | Dv(R)a (nm) | PDDF b (nm) | Dmax (PDDF) c (nm) | Rgd (nm) |
|---|---|---|---|---|
| PDDF e | ||||
| RFC | 5.2 | 48 | 96 (50) | 37/17 f |
| RFC-02 | 1.8 | 20 | 52 (60) | 19/21.3 f |
| RFC-05 | 1.9 | 31 | 57 | 23 |
| RFC-1 | 1.7/4.0 | 26 | 46 | 19 |
| Sample | SBET (m2/g) | Smicro (m2/g) | Smeso (m2/g) | Vp (cm3/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Vmacro (cm3/g) | Vmicro/Vp | Vmeso/Vp | Raverage |
|---|---|---|---|---|---|---|---|---|---|---|
| RFC | 529 | 507 | 21 | 0.226 | 0.217 | 0.009 | - | 0.958 | 0.042 | 0.7 |
| RFC-02 | 450 | 392 | 56 | 0.378 | 0.172 | 0.188 | 0.018 | 0.456 | 0.496 | 6.5 |
| RFC-05 | 481 | 434 | 46 | 0.312 | 0.187 | 0.118 | 0.007 | 0.601 | 0.377 | 5.4 |
| RFC-1 | 466 | 421 | 44 | 0.309 | 0.183 | 0.118 | 0.008 | 0.593 | 0.382 | 4 |
| Adsorbent | fit | f2 | p | log k * | t0.5 (min) | ueq | 1-R2 |
|---|---|---|---|---|---|---|---|
| RFC | FOE | 0 | -- | −2.42 | 183 | 0.995 | 3.17·10−4 |
| m-exp | -- | -- | −2.42 | 181 | 0.995 | 1.78·10−4 | |
| f-FOE | 0 | 1.05 | −2.42 | 187 | 0.982 | 2.16·10−4 | |
| f-MOE | −0.13 | 1.02 | −2.39 | 187 | 0.982 | 2.14·10−4 | |
| RFC-02 | FOE | 0 | -- | −2.63 | 294 | 0.987 | 1.48·10−3 |
| m-exp | -- | -- | −2.62 | 289 | 0.993 | 3.68·10−4 | |
| f-FOE | 0 | 0.89 | −2.64 | 288 | 0.977 | 4.94·10−4 | |
| f-MOE | 0.44 | 1.01 | −2.81 | 291 | 0.982 | 3.92·10−4 | |
| RFC-05 | FOE | 0 | -- | −2.84 | 480 | 0.965 | 1.23·10−2 |
| m-exp | -- | -- | −2.83 | 470 | 1.000 | 3.70·10−4 | |
| f-FOE | 0 | 0.66 | −2.93 | 487 | 1.000 | 1.54·10−3 | |
| f-MOE | 0.39 | 0.74 | −3.12 | 489 | 1.000 | 1.43·10−3 | |
| RFC-1 | FOE | 0 | -- | −2.06 | 80 | 0.994 | 2.37·10−4 |
| m-exp | -- | -- | −2.07 | 81 | 0.994 | 1.83·10−4 | |
| f-FOE | 0 | 1 | −2.06 | 81 | 0.980 | 2.31·10−4 | |
| f-MOE | −0.02 | 1.01 | −2.06 | 81 | 0.980 | 2.30·10−4 |
| Adsorbent | FOE (%) | m-exp (%) | f-FOE (%) | f-MOE (%) |
|---|---|---|---|---|
| RFC | 0.71 | 0.54 | 0.59 | 0.59 |
| RFC-02 | 1.40 | 0.72 | 0.82 | 0.74 |
| RFC-05 | 3.63 | 0.65 | 1.30 | 1.26 |
| RFC-1 | 0.57 | 0.51 | 0.56 | 0.57 |
| Bacterial Strain | Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|---|
| RFC | RFC-02 | RFC-05 | RFC-1 | AgNPs | Cefepime | |
| Staphylococcus aureus ATCC 25923 | 0 | 12 | 13 | 15 | 12 | 28 |
| Escherichia coli ATCC 25922 | 0 | 10 | 12 | 15 | 10 | 33 |
| Klebsiella pneumoniae ATCC 700603 | 0 | 10 | 12 | 15 | 11 | 24 |
| Pseudomonas aeruginosa ATCC 27853 | 0 | 11 | 13 | 16 | 12 | 26 |
| Acinetobacter baumannii ATCC 19606 | 0 | 11 | 12 | 15 | 11 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galaburda, M.; Zienkiewicz-Strzalka, M.; Blachnio, M.; Bogatyrov, V.; Kutkowska, J.; Choma, A.; Derylo-Marczewska, A. Ag-Containing Carbon Nanocomposites: Physico-Chemical Properties and Antimicrobial Activity. Sustainability 2023, 15, 16817. https://doi.org/10.3390/su152416817
Galaburda M, Zienkiewicz-Strzalka M, Blachnio M, Bogatyrov V, Kutkowska J, Choma A, Derylo-Marczewska A. Ag-Containing Carbon Nanocomposites: Physico-Chemical Properties and Antimicrobial Activity. Sustainability. 2023; 15(24):16817. https://doi.org/10.3390/su152416817
Chicago/Turabian StyleGalaburda, Mariia, Malgorzata Zienkiewicz-Strzalka, Magdalena Blachnio, Viktor Bogatyrov, Jolanta Kutkowska, Adam Choma, and Anna Derylo-Marczewska. 2023. "Ag-Containing Carbon Nanocomposites: Physico-Chemical Properties and Antimicrobial Activity" Sustainability 15, no. 24: 16817. https://doi.org/10.3390/su152416817
APA StyleGalaburda, M., Zienkiewicz-Strzalka, M., Blachnio, M., Bogatyrov, V., Kutkowska, J., Choma, A., & Derylo-Marczewska, A. (2023). Ag-Containing Carbon Nanocomposites: Physico-Chemical Properties and Antimicrobial Activity. Sustainability, 15(24), 16817. https://doi.org/10.3390/su152416817








