1. Introduction
In the pursuit of sustainable and greener development in the polyurethane (PU) manufacturing industries, there have been various attempts to replace fossil-based polyols with bio-derived feedstock such as biomasses and vegetable oils [
1,
2,
3,
4]. One of the most abundant bio-resources that attracts global interest as a sustainable alternative are lignocellulosic biomasses [
3,
5]. These can be obtained from various sources, including forest residues, agricultural wastes, and bagasse [
6]. The main structure of lignocellulosic biomass mainly consists of cellulose, hemicelluloses, and lignin, all of which contain functional hydroxyl groups [
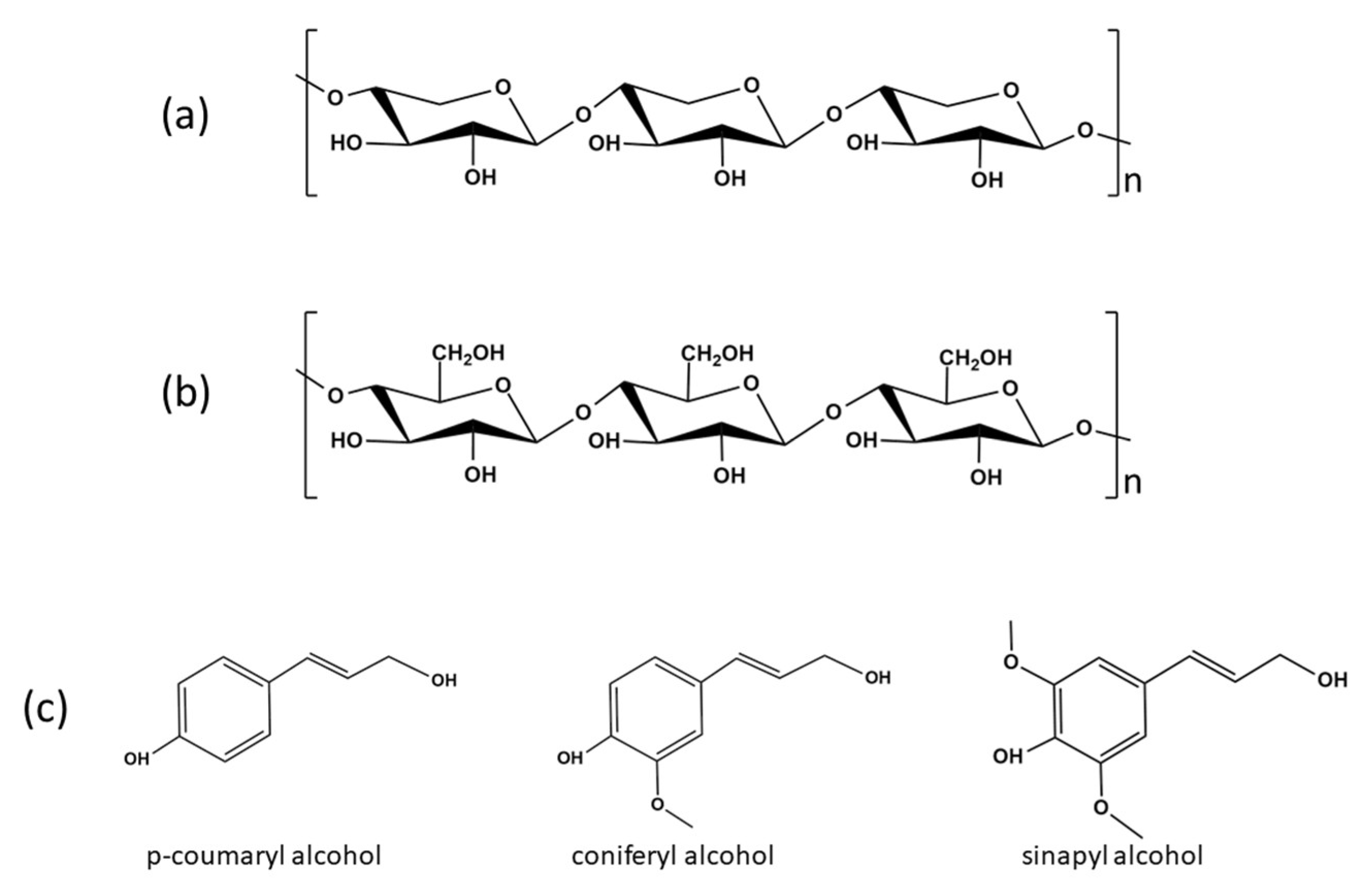
7]. Cellulose is a polymer made of repeating β-glucose connected via glycosidic linkages as shown in
Figure 1a [
8]. Hemicellulose is a branched heteropolymer of pentose and hexose sugars [
7,
9]. One of the most predominant types of hemicelluloses is xylan which consists of repeating D-xylopyranose units as shown in
Figure 1b [
10]. On the other hand, lignin is a complex aromatic polymer that is mainly made from coniferyl hydroxyl, p-coumaryl hydroxyl, and sinapyl hydroxyl, whose structures are shown in
Figure 1c [
11]. Lignocellulosic biomass is generally converted into liquid polyols via oxypropylation or liquefaction process for various PU product applications, including flexible and rigid PU foams [
12,
13].
The utilization of bio-derived materials in the manufacturing processes of PU products has led to the establishment of new formulations and protocols. One crucial aspect that profoundly affects the properties of these products is the reaction kinetics during the PU manufacturing process [
14,
15,
16,
17,
18]. Alfeche et al. (2023) successfully modeled the physicomechanical properties of rigid PU foam based on coconut oil, focusing on the gel reaction kinetics and gel time [
19]. It was found that PU reaction kinetics are largely influenced by the chemical properties of the polyol, such as heat capacities, functionalities, and hydroxyl values.
The hydroxyl groups of the polyol can be classified as primary, secondary, and hindered-secondary, with each category playing a crucial role in determining the distinct characteristics of the resulting PU networks [
20,
21]. The presence of primary hydroxyl groups leads to the formation of more compact PU networks. Conversely, the predominance of secondary hydroxyl groups introduces detrimental dangling chains that act as plasticizers within the PU matrix. Moreover, the presence of hindered-secondary hydroxyl groups in the middle of the carbon chain introduces high steric hindrance, which restricts urethane crosslinking [
22]. Hence, a comprehensive understanding of the hydroxyl moieties in the polyol provides invaluable insights that can contribute to the enhancement of PU product properties through improved and optimized design and formulation.
The hydroxyl functional moieties are generally characterized via titration and nuclear magnetic resonance spectroscopic analysis [
1,
23]. These experimental techniques, however, may be unavailable or implausible to certain laboratories and can be costly, time-intensive, space-consuming, imprecise, tedious, produce wastes, and involve health and safety risks. Hence, computational methods are explored as an alternative approach to address sustainability and practicality in such polyol characterizations.
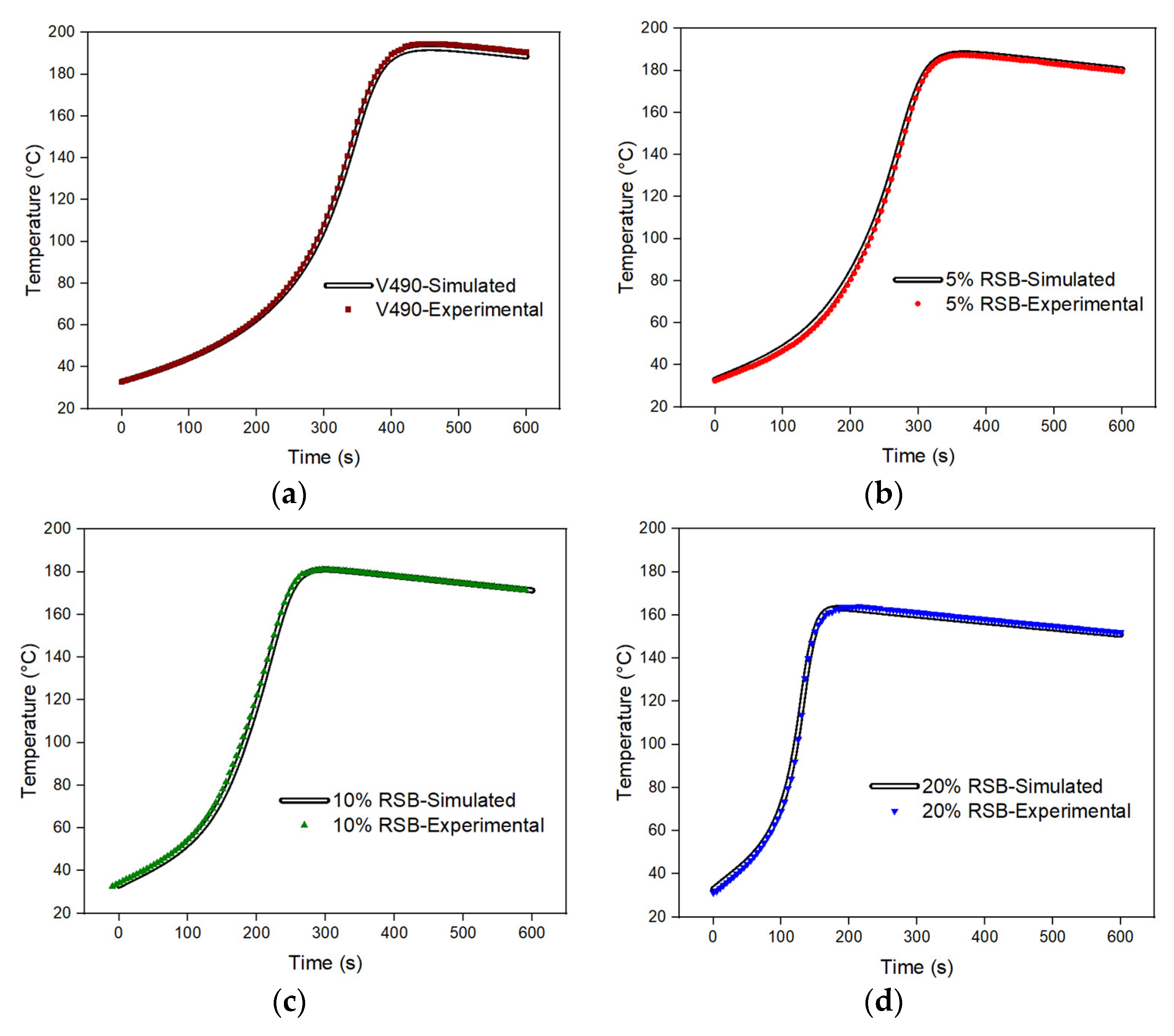
Ghoreishi et al. (2014) have developed a method that predicts the hydroxyl moieties of petroleum-based polyols by employing curve-fitting techniques on PU gel reaction temperature profiles [
20]. They further examined and modeled the influence of hydroxyl moieties on the reaction kinetics and temperature profiles during gel formation. However, it should be noted that the existing model still necessitates the utilization of alcohol compounds as references for accurate predictions [
20].
To date, comprehensive understanding has been scarce regarding the hydroxyl moieties found in lignocellulosic biomasses. This study addresses this scarcity by accurately predicting the primary, secondary, and hindered-secondary hydroxyl moieties present in a mixture of rice straw-based (RSB) and petroleum-based polyols via iterative computational method using MATLAB computer software without the need for reference compounds. For this purpose, a script was developed that can solve multiple simultaneous differential equations. These equations describe the kinetics and thermodynamics of the PU gelling reaction, based on the experimental inputs, assumptions, and heuristics described and derived from previous studies [
14,
20,
24]. Utilizing the provided inputs, the script conducts an energy balance analysis to generate an expression for the temperature profile. Subsequently, the script employs temperature profile curve fitting by comparing the experimental and simulated data. This procedure serves as a criterion for predicting the fraction of each type of hydroxyl moiety. The corresponding fractions of hydroxyl moieties from the best-fit temperature profiles are then recorded and saved.
One notable aspect of the script developed in this study is that it is designed to operate with minimal human intervention. The script is a one-time user input, where the necessary parameters are provided, and it takes responsibility for conducting the iteration processes automatically. This reduces the potential for human error and enhances the efficiency of the analysis. Furthermore, the script code is designed and optimized to facilitate the encapsulation of foaming simulations, thereby enabling seamless and effortless modifications to accommodate future investigations involving foaming reactions. This sophisticated implementation ensures enhanced scientific rigor, allowing researchers to delve into the intricacies of foaming phenomena with greater ease and flexibility. By employing the script code, researchers can efficiently manipulate and refine the parameters and variables associated with foaming reactions, fostering a deeper understanding of their underlying mechanisms and paving the way for further advancements in this field of study.
Hence, this study presents an important step towards a more comprehensive understanding of the hydroxyl moieties in lignocellulosic biomasses. By employing an iterative computational method and developing a script that automates the simulation process, the present study provides a scientific approach to quantitatively characterize the hydroxyl moieties in polyol mixtures. The script’s validity was demonstrated through its successful application to the gel temperature profile, reinforcing its utility as a valuable tool in future research and industrial applications.
Model Description
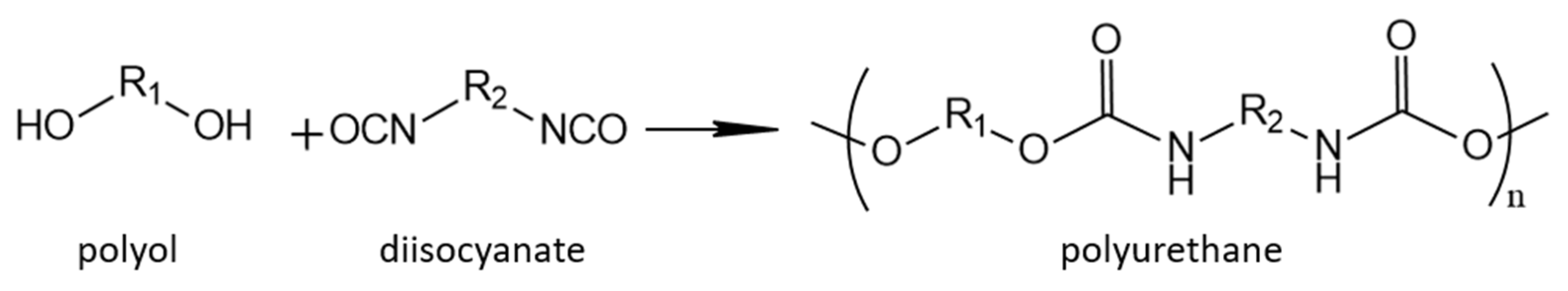
The PU gelling reaction occurs when the polyol and polyisocyanate are mixed, resulting in macromolecules with urethane structures, thus forming PUs as shown in
Figure 2 [
25]. The relationships between the concentrations of the reactants in
Figure 2 are employed to develop a gel reaction kinetic expression as presented in Equation (1) [
24]. In Equation (1),
is the summation of gel reaction rates of polyol mixtures,
is the reaction rate constant of gel
,
is the concentration of the gelling catalyst,
is the concentration of the isocyanate groups,
is the concentration of hydroxyl groups of polyol
i, and
is the gel reaction rate of polyol
i.
Moreover, the rate equations are considered to be elementary as in Equation (2). In Equation (2),
ri represents the rate of any reaction
i in
Table 1,
k represents the rate constant for any reaction, and
X1 and
X2 represent the concentration of the reacting moieties involved in the reaction. Additionally, a study by Zhao et. al. (2013) on catalyst impact on PU foam polymerization verified that Equation (3) provides a good estimate of catalyzed reaction rate constants, where
c represents any particular catalyst/s used [
24]. In Equation (3),
ki represents the overall rate constant for reaction
i,
kuncat represents the uncatalyzed reaction rate constant, and
kcat j represents the reaction rate constant in the presence of catalyst
c (
cat c).
The computational counterpart of the characterization of the gelling temperature of the reacting system (
T) as a function of time (
t) can be found as the solution of the thermodynamic differential equation (
) reflected in Equation (4) [
24]. In Equation (4), ∑
i∆
Hi ×
ri represents the instantaneous heat released from the reactions happening in the duration of the gelling process,
UA∆
T represents the instantaneous heat transfer from the system to the surroundings, and ∑ (
n ×
Cp) represents the instantaneous heat capacity of the mixture. With a database or guess of pertinent physical, thermodynamic, and kinetic parameters and information on initial conditions, the solution to Equation (2) can be found although the process may be lengthy and challenging as it involves more than one differential equation.
In the lens of the foam temperature profiling, several exothermic reactions take place. These reactions can be both gelling and blowing reactions [
13,
26]. Among these two types of reactions, only the gelling reactions are influenced by the polyol’s hydroxyl functionalities [
27]. Thus, this study focuses only on the investigation of the PU gelling reactions identified by the previous studies as presented in
Table 1 [
20,
24,
28]. Aside from the polyol-isocyanate reactions (reactions 1 to 12), reactions 13 and 14 can also take place depending on the other ingredients present.
Moreover, the kinetic parameters such as k0 (reaction rate constant at 25 °C), h (heat or reaction), E (activation energy), and U (heat transfer coefficient) used in this study are based on the values reported by Ghoreishi et al. (2014) for each type of hydroxyl moiety as summarized in
Table 2 [
20].