Evaluation of Antioxidant, Antimicrobial, and Anticancer Properties of Onion Skin Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Onion Skin (OS) Waste
2.3. Extraction of OS
2.4. Phytochemical Characterization
2.5. Antioxidant Activity
2.6. HPLC Analysis
2.7. Evaluation of In Vitro Anti-Inflammatory Activity
2.8. Antibacterial Activity of the OS Crude Extracts
2.9. Cytotoxic Properties of the OS Crude Extracts
2.9.1. Cell Culture
2.9.2. Cell Proliferation Assay (MTT Assay)
2.9.3. Spheroid Formation and Treatment
2.9.4. Cell Viability in Spheroids
2.9.5. Quantitative Real-Time PCR
2.9.6. Scratch Wound-Healing Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of TPC, TFC, and Individual Polyphenols of the Extracts
3.2. HPLC Analysis of the Extracts
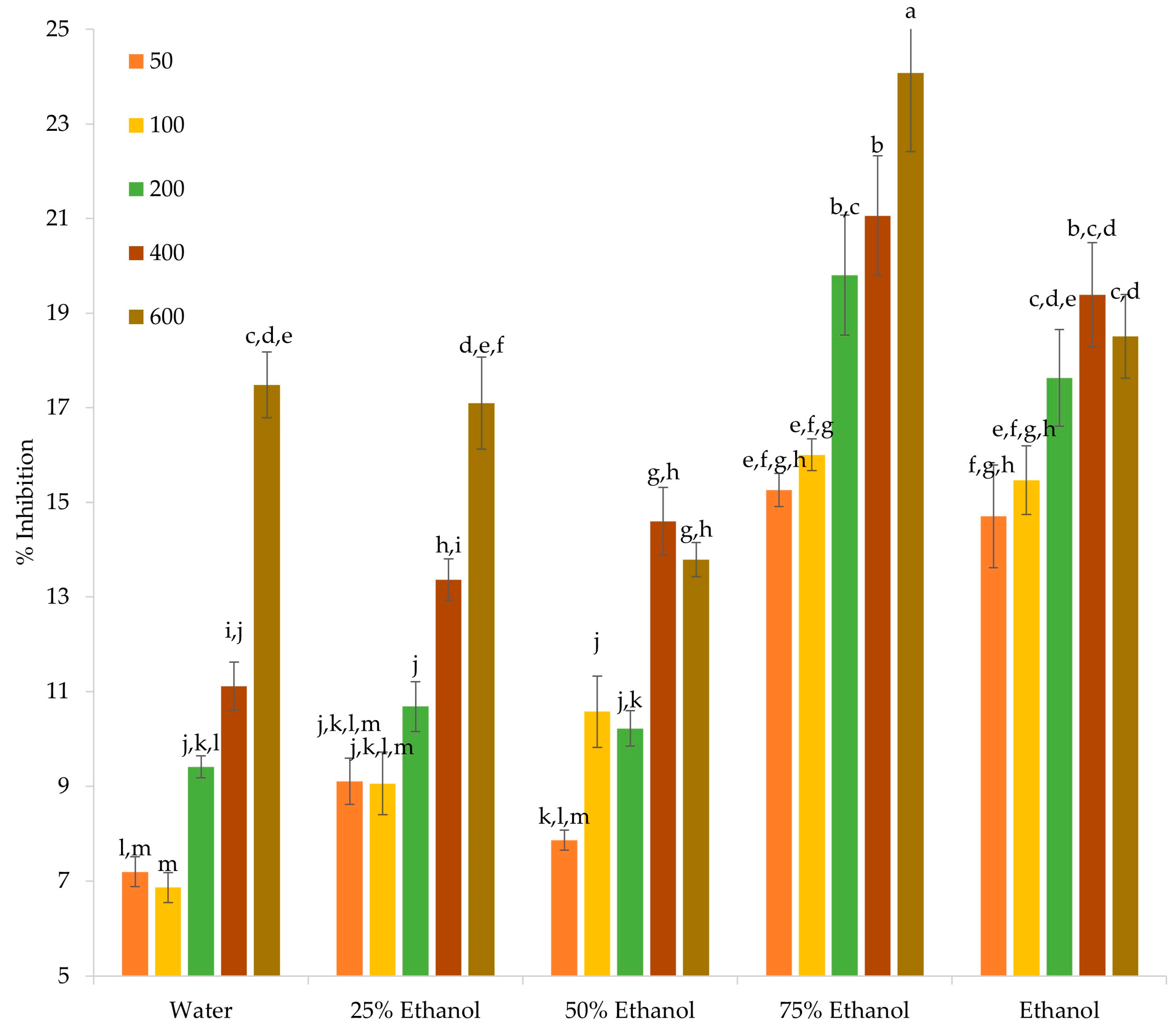
3.3. Evaluation of Anti-Inflammatory Properties
3.4. Evaluation of Antibacterial Properties
3.5. Evaluation of Anticancer Properties
3.5.1. Cell Growth Effect of OS Crude Extracts on U-87 MG and MCF-7 Cells
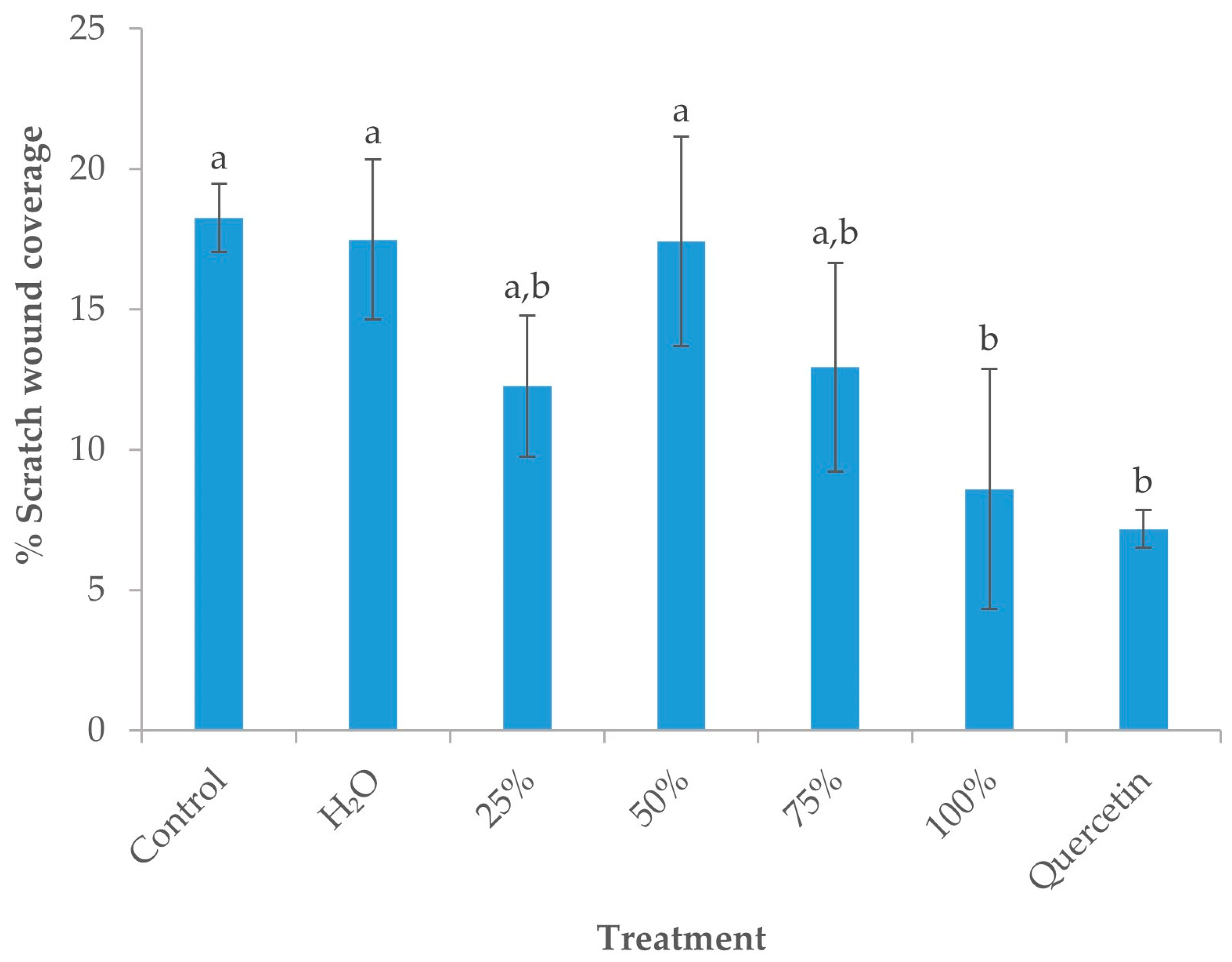
3.5.2. Effect of OS Crude Extracts on the Migratory Potential of MCF-7 Cells
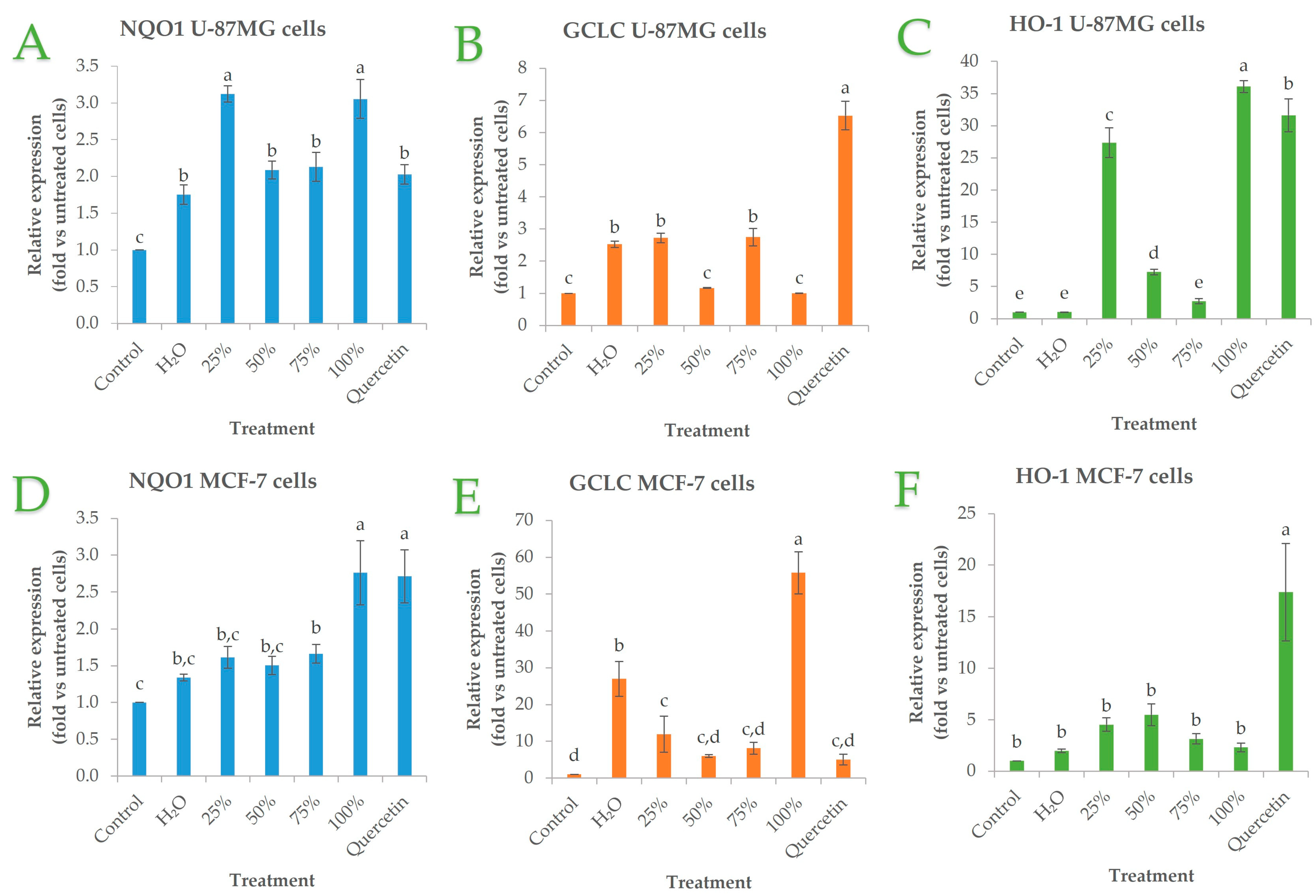
3.5.3. Effect of OS Crude Extracts on Antioxidant Gene Expression
3.5.4. Cytotoxic Activity of OS Crude Extracts and Cell Viability in MCF-7 Spheroids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osojnik Črnivec, I.G.; Skrt, M.; Šeremet, D.; Sterniša, M.; Farčnik, D.; Štrumbelj, E.; Poljanšek, A.; Cebin, N.; Pogačnik, L.; Smole Možina, S.; et al. Waste streams in onion production: Bioactive compounds, quercetin and use of antimicrobial and antioxidative properties. Waste Manag. 2021, 126, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.G.; Sukumaran, R.K. Enzyme Technologies for Bioconversion of Food Processing by-Products; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781439848876. [Google Scholar]
- Sagar, N.A.; Kumar, Y.; Singh, R.; Nickhil, C.; Kumar, D.; Sharma, P.; Om Pandey, H.; Bhoj, S.; Tarafdar, A. Onion waste based-biorefinery for sustainable generation of value-added products. Bioresour. Technol. 2022, 362, 127870. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.S.; Sridhar, A.; Vishali, S. Utilization of fruit and vegetable waste to produce value-added products: Conventional utilization and emerging opportunities-A review. Chemosphere 2022, 287, 132221. [Google Scholar] [CrossRef]
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and Bio-Based Food Packaging: A Review on Past and Current Design Innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef]
- Paesa, M.; Nogueira, D.P.; Velderrain-Rodríguez, G.; Esparza, I.; Jiménez-Moreno, N.; Mendoza, G.; Osada, J.; Martin-Belloso, O.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Valorization of Onion Waste by Obtaining Extracts Rich in Phenolic Compounds and Feasibility of Its Therapeutic Use on Colon Cancer. Antioxidants 2022, 11, 733. [Google Scholar] [CrossRef]
- Choi, I.S.; Cho, E.J.; Moon, J.H.; Bae, H.J. Onion skin waste as a valorization resource for the by-products quercetin and biosugar. Food Chem. 2015, 188, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Vojvodić Cebin, A.; Šeremet, D.; Mandura, A.; Martinić, A.; Komes, D. Onion Solid Waste as a Potential Source of Functional Food Ingredients. Eng. Power Bull. Croat. Acad. Eng. 2020, 15, 7–13. [Google Scholar]
- Bello, M.O.; Olabanji, I.O.; Abdul-Hammed, M.; Okunade, T.D. Characterization of domestic onion wastes and bulb (Allium cepa L.): Fatty acids and metal contents. Int. Food Res. J. 2013, 20, 2153–2158. [Google Scholar]
- Babbar, N.; Baldassarre, S.; Maesen, M.; Prandi, B.; Dejonghe, W.; Sforza, S.; Elst, K. Enzymatic production of pectic oligosaccharides from onion skins. Carbohydr. Polym. 2016, 146, 245–252. [Google Scholar] [CrossRef]
- Santiago, B.; Arias Calvo, A.; Gullón, B.; Feijoo, G.; Moreira, M.T.; González-García, S. Production of flavonol quercetin and fructooligosaccharides from onion (Allium cepa L.) waste: An environmental life cycle approach. Chem. Eng. J. 2020, 392, 123772. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Gonzalez-Aguilar, G.A. Quantification of flavonoids, total phenols and antioxidant properties of onion skin: A comparative study of fifteen Indian cultivars. J. Food Sci. Technol. 2020, 57, 2423–2432. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidative phenolic constituents of skins of onion varieties and their activities. J. Funct. Foods 2013, 5, 1191–1203. [Google Scholar] [CrossRef]
- Chadorshabi, S.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red onion skin active ingredients, extraction and biological properties for functional food applications. Food Chem. 2022, 386, 132737. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, S.; Rashidipour, M.; Khosravi, P.; Shahryarhesami, S.; Ashrafi, B.; Kaviani, M.; Sarabi, M.M. Biocompatibility, cytotoxicity, antimicrobial and epigenetic effects of novel chitosan-based quercetin nanohydrogel in human cancer cells. Int. J. Nanomed. 2020, 15, 5963–5975. [Google Scholar] [CrossRef]
- Mihaylova, D.; Lante, A. Water an Eco-Friendly Crossroad in Green Extraction: An Overview. Open Biotechnol. J. 2019, 13, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid. Based Complement. Altern. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef] [Green Version]
- Kaltsa, O.; Grigorakis, S.; Lakka, A.; Bozinou, E.; Lalas, S.; Makris, D.P. Green Valorization of Olive Leaves to Produce Polyphenol-Enriched Extracts Using an Environmentally Benign Deep Eutectic Solvent. Agriengineering 2020, 2, 226–239. [Google Scholar] [CrossRef] [Green Version]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimized Isolation Procedure for the Extraction of Bioactive Compounds from Spent Coffee Grounds. Appl. Sci. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Kasouni, A.I.; Chatzimitakos, T.G.; Troganis, A.N.; Stalikas, C.D. Citric acid-based carbon dots: From revealing new insights into their biological properties to demonstrating their enhanced wound healing potential by in vitro and in vivo experiments. Mater. Today Commun. 2021, 26, 102019. [Google Scholar] [CrossRef]
- Bozinou, E.; Lakka, A.; Poulianiti, K.; Lalas, S.; Makris, D.P. Cyclodextrins as high-performance green co-solvents in the aqueous extraction of polyphenols and anthocyanin pigments from solid onion waste. Eur. Food Res. Technol. 2021, 247, 2831–2845. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Development of a low-temperature and high-performance green extraction process for the recovery of polyphenolic phytochemicals fromwaste potato peels using hydroxypropyl β-cyclodextrin. Appl. Sci. 2020, 10, 3611. [Google Scholar] [CrossRef]
- Kasouni, A.I.; Chatzimitakos, T.G.; Stalikas, C.D.; Trangas, T.; Papoudou-Bai, A.; Troganis, A.N. The Unexplored Wound Healing Activity of Urtica dioica L. Extract: An In Vitro and In Vivo Study. Molecules 2021, 26, 6248. [Google Scholar] [CrossRef]
- Guleria, I.; Kumari, A.; Lacaille-Dubois, M.A.; Saini, A.K.; Kumar, V.; Saini, R.V.; Lal, U.R.; Gaur, N.A.; Kumari, S.; Seth, A.; et al. In-vitro antimicrobial, antioxidant, anti-inflammatory, and cytotoxic activities of Populus ciliata bark and leaves: A comparative study. S. Afr. J. Bot. 2022, 148, 238–250. [Google Scholar] [CrossRef]
- Maritan, S.M.; Lian, E.Y.; Mulligan, L.M. An Efficient and Flexible Cell Aggregation Method for 3D Spheroid Production. J. Vis. Exp. 2017, 2017, e55544. [Google Scholar] [CrossRef]
- Lagies, S.; Schlimpert, M.; Neumann, S.; Wäldin, A.; Kammerer, B.; Borner, C.; Peintner, L. Cells grown in three-dimensional spheroids mirror in vivo metabolic response of epithelial cells. Commun. Biol. 2020, 3, 246. [Google Scholar] [CrossRef] [PubMed]
- Pappas, I.S.; Siomou, S.; Bozinou, E.; Lalas, S.I. Moringa oleifera leaves crude aqueous extract down-regulates of BRCA1, mta-1 and oncogenes c-myc and p53 in AsPC-1, MCF-7 and HTC-116 cells. Food Biosci. 2021, 43, 101221. [Google Scholar] [CrossRef]
- Georgiou-Siafis, S.K.; Tsiftsoglou, A.S. Activation of KEAP1/NRF2 stress signaling involved in the molecular basis of hemin-induced cytotoxicity in human pro-erythroid K562 cells. Biochem. Pharmacol. 2020, 175, 113900. [Google Scholar] [CrossRef]
- Yeligar, S.M.; Machida, K.; Kalra, V.K. Ethanol-induced HO-1 and NQO1 are differentially regulated by HIF-1α and Nrf2 to attenuate inflammatory cytokine expression. J. Biol. Chem. 2010, 285, 35359–35373. [Google Scholar] [CrossRef] [Green Version]
- Sakasai-Sakai, A.; Takeuchi, M.; Takata, T. Intracellular toxic advanced glycation end-products promote the production of reactive oxygen species in HEPG2 cells. Int. J. Mol. Sci. 2020, 21, 4861. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Ebrahimi, F.; Agar, O.T.; Dunshea, F.R.; Barrow, C.J.; Suleria, H.A.R. Comparative Study on the Effect of Phenolics and Their Antioxidant Potential of Freeze-Dried Australian Beach-Cast Seaweed Species upon Different Extraction Methodologies. Pharmaceuticals 2023, 16, 773. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef] [PubMed]
- Hikmawanti, N.P.E.; Fatmawati, S.; Asri, A.W. The effect of ethanol concentrations as the extraction solvent on antioxidant activity of Katuk (Sauropus androgynus (L.) Merr.) leaves extracts. IOP Conf. Ser. Earth Environ. Sci. 2021, 755, 012060. [Google Scholar] [CrossRef]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Phenolic Compound Family Extracted from Raspberries (Rubus idaeus): A General Review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef]
- Bakrim, S.; El Omari, N.; El Hachlafi, N.; Bakri, Y.; Lee, L.H.; Bouyahya, A. Dietary Phenolic Compounds as Anticancer Natural Drugs: Recent Update on Molecular Mechanisms and Clinical Trials. Foods 2022, 11, 3323. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Singh, D.P.; Sarma, B.K.; Upadhyay, G.; Singh, H.B. Polyphenolics from various extracts/fractions of red onion (Allium cepa) peel with potent antioxidant and antimutagenic activities. Food Chem. Toxicol. 2009, 47, 1161–1167. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Radha; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; et al. Onion (Allium cepa L.) peels: A review on bioactive compounds and biomedical activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes 2014, 7, 560. [Google Scholar] [CrossRef] [Green Version]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Kim, W.J.; Lee, K.A.; Kim, K.T.; Chung, M.S.; Cho, S.W.; Paik, H.D. Antimicrobial effects of onion (Allium cepa L.) peel extracts produced via subcritical water extraction against Bacillus cereus strains as compared with ethanolic and hot water extraction. Food Sci. Biotechnol. 2011, 20, 1101–1106. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S. Antimicrobial assessment of polyphenolic extracts from onion (Allium cepa L.) skin of fifteen cultivars by sonication-assisted extraction method. Heliyon 2020, 6, e05478. [Google Scholar] [CrossRef]
- Lee, K.A.; Kim, K.T.; Nah, S.Y.; Chung, M.S.; Cho, S.W.; Paik, H.D. Antimicrobial and antioxidative effects of onion peel extracted by the subcritical water. Food Sci. Biotechnol. 2011, 20, 543–548. [Google Scholar] [CrossRef]
- Santhosh, A.; Theertha, V.; Prakash, P.; Smitha Chandran, S. From waste to a value added product: Green synthesis of silver nanoparticles from onion peels together with its diverse applications. Proc. Mater. Today Proc. 2019, 46, 4460–4463. [Google Scholar] [CrossRef]
- Oyawoye, O.M.; Olotu, T.M.; Nzekwe, S.C.; Idowu, J.A.; Abdullahi, T.A.; Babatunde, S.O.; Ridwan, I.A.; Batiha, G.E.; Idowu, N.; Alorabi, M.; et al. Antioxidant potential and antibacterial activities of Allium cepa (onion) and Allium sativum (garlic) against the multidrug resistance bacteria. Bull. Natl. Res. Cent. 2022, 46, 214. [Google Scholar] [CrossRef]
- Yüksel, T.N.; Bozgeyik, E.; Yayla, M. The Effect of Quercetin and Quercetin-3-d-xyloside on Breast Cancer Proliferation and Migration. J. Basic Clin. Health Sci. 2022, 6, 569–578. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential mechanisms of quercetin in cancer prevention: Focus on cellular and molecular targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.-C.; Jiang, Q.; Yu, Y.; Mei, J.-P.; Cui, Y.-K.; Zhao, W.-J. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochem. Int. 2015, 80, 60–71. [Google Scholar] [CrossRef]
- Tang, J.; Oroudjev, E.; Wilson, L.; Ayoub, G. Delphinidin and cyanidin exhibit antiproliferative and apoptotic effects in MCF7 human breast cancer cells. Integr. Cancer Sci. Ther. 2015, 2, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-W.; Hou, W.-C.; Shen, S.-C.; Juan, S.-H.; Ko, C.-H.; Wang, L.-M.; Chen, Y.-C. Quercetin inhibition of tumor invasion via suppressing PKC /ERK/AP-1-dependent matrix metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis 2008, 29, 1807–1815. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Li, X.; Wu, L.; Zhou, D.; Song, Y.; Zhang, L.; Wu, Q.; He, Q.; Wang, G.; Liu, X.; et al. Quercetin Suppresses Human Glioblastoma Migration and Invasion via GSK3β/β-catenin/ZEB1 Signaling Pathway. Front. Pharmacol. 2022, 13, 963614. [Google Scholar] [CrossRef] [PubMed]
- Valerio, L.G.; Kepa, J.K.; Pickwell, G.V.; Quattrochi, L.C. Induction of human NAD(P)H:quinone oxidoreductase (NQO1) gene expression by the flavonol quercetin. Toxicol. Lett. 2001, 119, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Chang, S.-Y.; Jang, H.-J.; Cho, J.M.; Kim, D.-B.; Lee, S.S.; Ko, S.H.; Park, Y.-M.; Needs, P.W.; Jo, Y.-H.; et al. Quercetin-induced upregulation of human GCLC gene is mediated by cis -regulatory element for early growth response protein-1 (EGR1) in INS-1 beta-cells. J. Cell. Biochem. 2009, 108, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Song, J.-H.; Oh, M.-H.; Lee, Y.-J.; Kim, Y.-B.; Im, J.-H.; Lee, S.-H. ERK1/2 activation in quercetin-treated BEAS-2B cell plays a role in Nrf2-driven HO-1 expression. Mol. Cell. Toxicol. 2011, 7, 347–355. [Google Scholar] [CrossRef]
- Shahi Thakuri, P.; Gupta, M.; Singh, S.; Joshi, R.; Glasgow, E.; Lekan, A.; Agarwal, S.; Luker, G.D.; Tavana, H. Phytochemicals inhibit migration of triple negative breast cancer cells by targeting kinase signaling. BMC Cancer 2020, 20, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Solvents | TPC (mg GAE/g dw) | TFC (mg RtE/g dw) | DPPH (μmol DPPH/g dw) | FRAP (μmol AAE/g dw) | AAHP (μmol AAE/g dw) |
|---|---|---|---|---|---|
| Water | 348.71 ± 7.85 d | 11.16 ± 0.51 d | 177.15 ± 2.8 d | 127.77 ± 5.86 e | 25.66 ± 0.11 c,d |
| 25% Ethanol | 377.37 ± 10.98 c | 20.73 ± 0.04 c | 299.84 ± 0.81 c | 230.95 ± 3.57 d | 27.09 ± 0.8 c |
| 50% Ethanol | 505.57 ± 28.63 b | 32.14 ± 0.22 b | 378.45 ± 0.76 b | 356.87 ± 5.06 a | 32.71 ± 0.8 b |
| 75% Ethanol | 522.1 ± 25.58 b | 36.2 ± 0.26 a | 396.01 ± 7.78 a | 334.91 ± 1.23 b | 55.04 ± 2.07 a |
| Ethanol | 795.11 ± 19.01 a | 31.11 ± 1.3 b | 299.19 ± 2.49 c | 304.21 ± 2.31 c | 23.09 ± 1 d |
| Compounds | Water | 25% Ethanol | 50% Ethanol | 75% Ethanol | Ethanol |
|---|---|---|---|---|---|
| Protocatechuic acid | 42.04 ± 1.72 a | 24.53 ± 0.93 b | 25.28 ± 0.91 b | 27.27 ± 0.95 b | 25.7 ± 0.98 b |
| Spiraeoside | 141.12 ± 5.64 c | 137.27 ± 5.22 c | 267.94 ± 11.25 b | 356.46 ± 11.41 a | 348.2 ± 12.54 a |
| Quercetin | 29.63 ± 1.24 c | 143.08 ± 5.87 b | 259.27 ± 9.59 a | 241.1 ± 7.47 a | 257.76 ± 10.83 a |
| Cyanidin 3-O-glucoside | 11.35 ± 0.37 d | 13.53 ± 0.55 c | 22.27 ± 0.82 a | 23.51 ± 0.92 a | 16.81 ± 0.71 b |
| Delphinidin 3,5-di-O-galactoside | 7.27 ± 0.24 c | 9.83 ± 0.3 b | 12.31 ± 0.52 a | 11.97 ± 0.47 a | 9.19 ± 0.31 b |
| Delphinidin 3,5-di-O-glucoside | 76.08 ± 2.81 d | 114.54 ± 4.12 c | 138.98 ± 5.28 b | 158 ± 6 a | 111.52 ± 3.46 c |
| Cyanidin 3-O-(6″-malonylglucoside) | 26.35 ± 0.95 e | 35.66 ± 1.32 c | 41.98 ± 1.55 b | 49.21 ± 1.77 a | 30.5 ± 1.13 d |
| Bacterial Species | Solvents | 25 mg/L | 50 mg/L | 100 mg/L |
|---|---|---|---|---|
| L. monocytogenes | Water | 10 ± 0.1 b | 20 ± 0.3 a,b | 32.5 ± 12.6 a |
| 25% Ethanol | 20 ± 14.1 a | 25 ± 10 a | 30 ± 14.1 a | |
| 50% Ethanol | – | 10 ± 0.1 a,b | 15 ± 10 a | |
| 75% Ethanol | 27.5 ± 15 a | 30 ± 0.3 a | 40 ± 0.4 a | |
| Ethanol | – | – | – | |
| E. coli O157:H7 | Water | 8 ± 1.4 c | 23 ± 2.4 b | 45 ± 3.6 a |
| 25% Ethanol | 32.5 ± 5 a | 35 ± 17.3 a | 50 ± 0.8 a | |
| 50% Ethanol | 10 ± 0.3 b | 17.5 ± 5 a,b | 22.5 ± 5 a | |
| 75% Ethanol | 25 ± 5.8 b | 37.5 ± 12.6 a,b | 42.5 ± 5 a | |
| Ethanol | – | – | – | |
| B. cereus | Water | – | – | 17.5 ± 5 |
| 25% Ethanol | – | – | – | |
| 50% Ethanol | – | – | – | |
| 75% Ethanol | – | – | – | |
| Ethanol | – | – | – | |
| E. faecalis | Water | 12.5 ± 5 b | 30 ± 14.1 a,b | 32.5 ± 5 a |
| 25% Ethanol | 30 ± 14.1 a | 37.5 ± 15 a | 45 ± 10 a | |
| 50% Ethanol | 32.5 ± 5 a | 35 ± 23.8 a | 40 ± 0.9 a | |
| 75% Ethanol | 40 ± 18.3 a | 42.5 ± 18.9 a | 52.5 ± 18.9 a | |
| Ethanol | – | – | 10 ± 0 | |
| P. aeruginosa | Water | – | 25 ± 10 a | 30 ± 14.1 a |
| 25% Ethanol | 20 ± 0.7 a | 22.5 ± 5 a | 25 ± 3.6 a | |
| 50% Ethanol | 10 ± 0.6 b | 15 ± 2.2 b | 25 ± 5.8 a | |
| 75% Ethanol | – | 20 ± 0.4 b | 30 ± 0.9 a | |
| Ethanol | 30 ± 7.1 a | 35 ± 17.3 a | 37.5 ± 15 a | |
| S. aureus | Water | – | – | – |
| 25% Ethanol | – | – | 30 ± 0.3 | |
| 50% Ethanol | – | 20 ± 0.6 a | 20 ± 0.7 a | |
| 75% Ethanol | 25 ± 5.8 a | 30 ± 4.1 a | 35 ± 10 a | |
| Ethanol | – | – | – | |
| S. typhimurium | Water | – | – | 12.5 ± 5 |
| 25% Ethanol | – | – | 15 ± 5.8 | |
| 50% Ethanol | 20 ± 0.8 a | 25 ± 5.8 a | 30 ± 7.1 a | |
| 75% Ethanol | – | 20 ± 0.2 a | 20 ± 0.1 a | |
| Ethanol | 20 ± 0.6 a | 20 ± 0.5 a | 20 ± 0.7 a | |
| Y. enterocolitica | Water | 12.5 ± 5 a | 17.5 ± 5 a | 25 ± 10 a |
| 25% Ethanol | 15 ± 10 a | 27.5 ± 15 a | 32.5 ± 6.5 a | |
| 50% Ethanol | 20 ± 4.1 a | 25 ± 5.8 a | 30 ± 20 a | |
| 75% Ethanol | 20 ± 3.4 a | 22 ± 4 a | 25 ± 10 a | |
| Ethanol | 12.5 ± 5 b | 20 ± 0.3 a,b | 27.5 ± 9.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozinou, E.; Pappas, I.S.; Patergiannakis, I.-S.; Chatzimitakos, T.; Palaiogiannis, D.; Athanasiadis, V.; Lalas, S.I.; Chatzilazarou, A.; Makris, D.P. Evaluation of Antioxidant, Antimicrobial, and Anticancer Properties of Onion Skin Extracts. Sustainability 2023, 15, 11599. https://doi.org/10.3390/su151511599
Bozinou E, Pappas IS, Patergiannakis I-S, Chatzimitakos T, Palaiogiannis D, Athanasiadis V, Lalas SI, Chatzilazarou A, Makris DP. Evaluation of Antioxidant, Antimicrobial, and Anticancer Properties of Onion Skin Extracts. Sustainability. 2023; 15(15):11599. https://doi.org/10.3390/su151511599
Chicago/Turabian StyleBozinou, Eleni, Ioannis S. Pappas, Iason-Spyridon Patergiannakis, Theodoros Chatzimitakos, Dimitrios Palaiogiannis, Vassilis Athanasiadis, Stavros I. Lalas, Arhontoula Chatzilazarou, and Dimitris P. Makris. 2023. "Evaluation of Antioxidant, Antimicrobial, and Anticancer Properties of Onion Skin Extracts" Sustainability 15, no. 15: 11599. https://doi.org/10.3390/su151511599
APA StyleBozinou, E., Pappas, I. S., Patergiannakis, I.-S., Chatzimitakos, T., Palaiogiannis, D., Athanasiadis, V., Lalas, S. I., Chatzilazarou, A., & Makris, D. P. (2023). Evaluation of Antioxidant, Antimicrobial, and Anticancer Properties of Onion Skin Extracts. Sustainability, 15(15), 11599. https://doi.org/10.3390/su151511599










