E-Nose-Driven Advancements in Ammonia Gas Detection: A Comprehensive Review from Traditional to Cutting-Edge Systems in Indoor to Outdoor Agriculture
Abstract
1. Introduction
- Classifying the efficacy of various techniques for determining ammonia levels in agricultural settings, specifically analyzing indoor and outdoor methodologies and weighing the pros and cons of each.
- Investigating and comparing the performance of Electronic nose (E-nose) structures in agriculture, measuring the amount of ammonia in indoor and outdoor space and classifying advantages and disadvantages.
- Comprehensive review of indoor and outdoor ammonia detection methods in agricultural environments.
- Analysis of the advantages and disadvantages of each method for detecting low concentrations of agricultural ammonia volatilization (0.01–5 ppm).
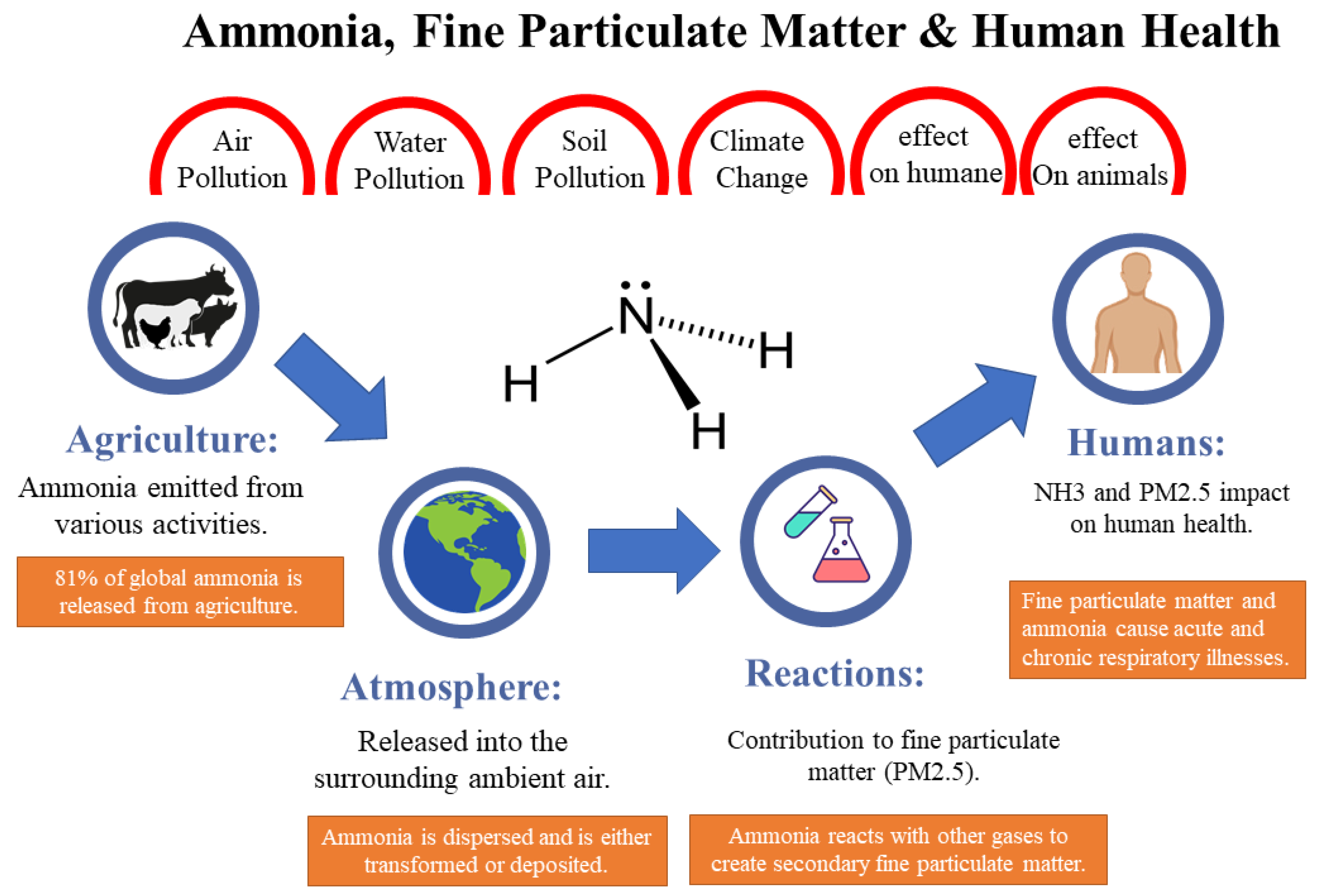
- Emphasis on the importance of monitoring and measuring sources of ammonia in agriculture to mitigate negative health and environmental impacts.
- Discussion of the need for real-time monitoring of ammonia volatilization in agriculture for environmental pollution control and loss prevention.
- Highlight the importance of selecting the right detection method to ensure the protection of human health and the environment from the harmful effects of ammonia.
- Highlight the potential for combining E-nose technology with recent algorithms like deep learning to improve the accuracy of detection.
- A valuable resource for understanding the methods and considerations involved in detecting and reducing ammonia volatilization in agriculture.
Data Collection
2. Methodology
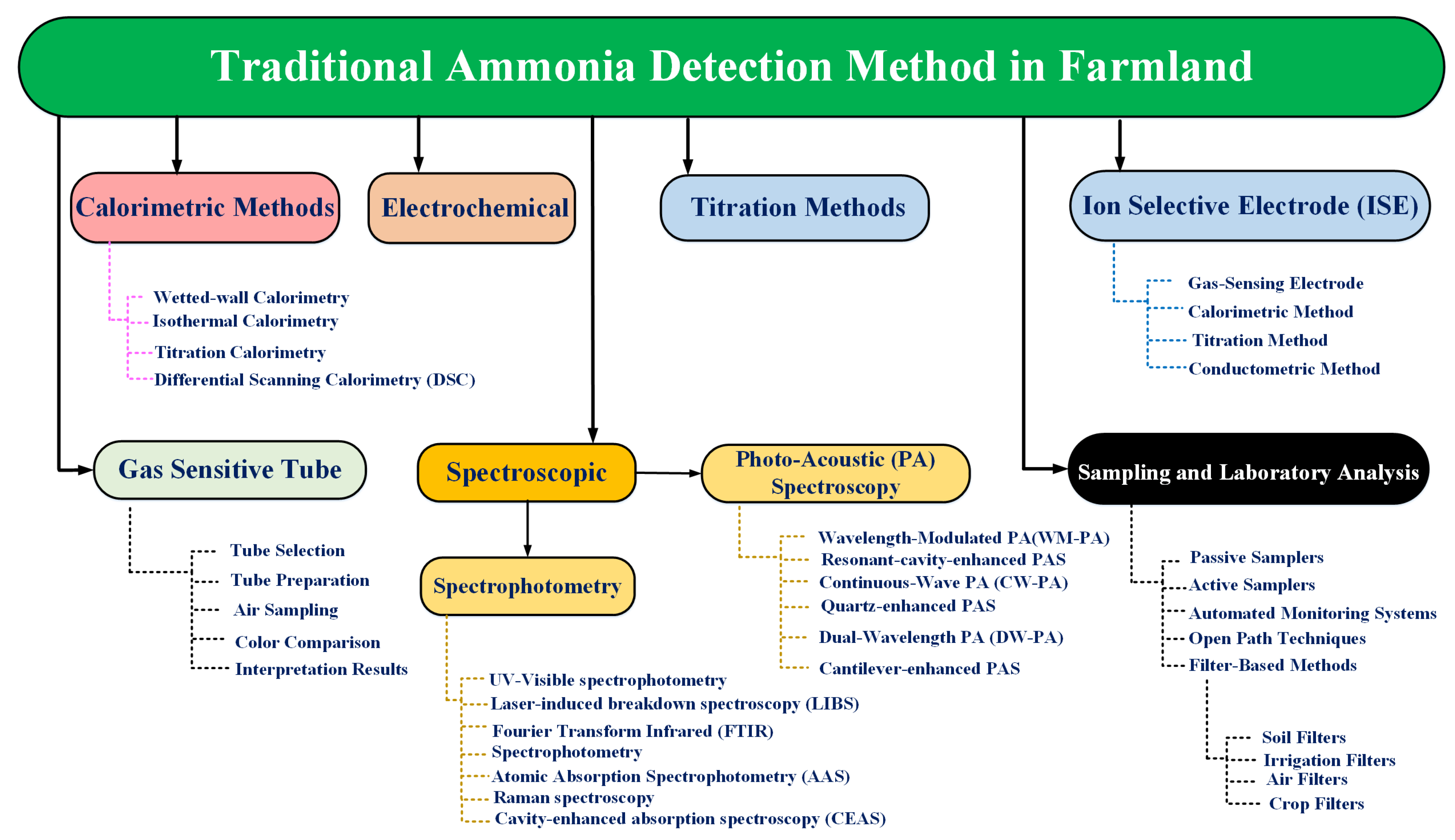
2.1. Traditional Ammonia Detection Method
2.1.1. Calorimetric Methods
- Wetted-wall calorimetry: In this method, a wetted wall is exposed to the air sample containing ammonia gas. The ammonia gas reacts with water on the wetted surface, resulting in heat release due to the exothermic nature of the reaction. The change in temperature of the wetted wall is then measured using a temperature sensor, and the amount of ammonia gas present in the air sample is determined based on the heat released [29].
- Isothermal Calorimetry: In this method, a sample of air containing ammonia gas is mixed with a known amount of an absorbent solution, such as sulfuric acid or hydrochloric acid, in a calorimeter. The absorption of ammonia gas by the solution is an exothermic reaction, resulting in a change in temperature that is proportional to the amount of ammonia gas present [30].
- Titration calorimetry: This method involves reacting an air sample containing ammonia gas with a titrant solution of known concentration in a calorimeter. The titration reaction between the ammonia gas and the titrant is exothermic, resulting in a change in temperature that is proportional to the amount of ammonia gas present [31].
- Differential scanning calorimetry (DSC): DSC is a technique used to measure the heat flow associated with chemical reactions. In the context of ammonia gas detection, a DSC instrument can be used to measure the heat flow when an air sample containing ammonia gas is exposed to a reference gas or a reference material. The heat flow is recorded as a function of temperature, and the concentration of ammonia gas in the air sample is determined based on the heat flow and the known properties of the reference gas or material [32].
2.1.2. Electrochemical
2.1.3. Titration Methods
2.1.4. Ion Selective Electrodes (ISE)
- Gas-Sensing Electrode: One traditional method for ammonia gas detection in farmland involves using a gas-sensing electrode based on ISE technology. The electrode is typically coated with a membrane that is selective to ammonia ions (NH), allowing only ammonia ions to pass through and interact with the electrode surface. As the ammonia gas from the farmland diffuses into the electrode, it dissolves in water to form ammonium ions, which then interact with the electrode and generate an electrical potential that is proportional to the ammonia concentration. This potential can be measured using a pH meter or a dedicated ion meter, and the concentration of ammonia in the farmland can be calculated based on the Nernst equation [38].
- Colorimetric Method: Another traditional method for ammonia gas detection in farmland involves using a colorimetric method with an ISE. In this method, the gas-sensing electrode is coated with a membrane that contains a pH indicator dye, which changes color in the presence of ammonia ions. As the ammonia gas diffuses into the electrode and forms ammonium ions, the pH of the membrane changes, causing the color of the dye to change. The intensity of the color change is proportional to the concentration of ammonia in the farmland, and it can be visually or spectrophotometrically measured to determine the ammonia concentration [39].
- Titration Method: The titration method is another traditional method that uses ISE for ammonia gas detection in farmland. In this method, a gas-sensing electrode is immersed in a solution containing the ammonia gas, and a titrant solution of known concentration is added dropwise to the solution. The titrant solution contains a counter ion that reacts with the ammonia ions to form a precipitate or a complex, causing a change in the electrode potential. The titration is continued until the endpoint is reached, which is determined by a sudden change in the electrode potential. The amount of titrant solution required to reach the endpoint is proportional to the concentration of ammonia in the farmland and can be used to calculate the ammonia concentration [40].
- Conductometric Method: The conductometric method, a conventional technique for ammonia gas detection in farmland, harnesses the capabilities of Ion Selective Electrodes (ISE) to facilitate accurate measurements. In this method, a gas-sensing electrode is used as one of the two electrodes in a conductometric cell, and the other electrode is a reference electrode. When ammonia gas diffuses into the cell, it dissolves in water to form ammonium ions, which increase the electrical conductivity of the solution. The change in conductivity is proportional to the concentration of ammonia in the farmland and can be measured using a conductivity meter. The conductometric method provides a simple and rapid measurement of ammonia concentration without the need for additional reagents or titration [41].
2.1.5. Gas-Sensitive Tubes
- Tube selection: There are different types of gas-sensitive tubes available, each designed to measure a specific concentration range of ammonia gas. The appropriate tube is selected based on the expected concentration of ammonia in the air.
- Tube preparation: The gas-sensitive tube is attached to a hand-operated pump, which is used to draw a known volume of air through the tube. The tube is usually calibrated to a specific flow rate, and the pump is operated accordingly [44].
- Air sampling: The pump is used to draw a known volume of air from the farmland area being tested. The air is passed through the gas-sensitive tube, and any ammonia gas present in the air reacts with the reagent in the tube, causing a color change [45].
- Color comparison: After the air has been sampled, the gas-sensitive tube is removed from the pump, and the color of the reagent is compared to the scale printed on the tube. The intensity of the color change is used to estimate the concentration of ammonia gas in the air.
- Interpretation of results: The concentration of ammonia gas is estimated based on the scale provided on the gas-sensitive tube. The results are typically reported as a range or an approximate value, depending on the accuracy of the tube and the specific application.
2.1.6. Spectroscopic Methods
- Photo-acoustic (PA) spectroscopy: This method combines the principles of photonics and acoustics to produce a measurable acoustic signal proportional to the absorption of light by a gas. This method known as a well-established technique for ammonia detection which can be considered an indoor method for measuring ammonia concentrations as a type of direct-reading instrument [46,47]. A variety of PA spectroscopy sensors are employed for ammonia detection, encompassing the following types:
- -
- Wavelength-Modulated PA (WM-PA) Sensors: These sensors use a modulated laser source to excite the sample and a detector to measure the PA signal. This type of PA sensor is commonly used for ammonia detection due to its high sensitivity and stability [48].
- -
- Continuous-Wave PA (CW-PA) Sensors: These sensors employing a continuous wave laser source and a detector to measure the PA signal. This type of PA sensor is typically used for the detection of low concentrations of ammonia in gas mixtures [49].
- -
- Dual-Wavelength PA (DW-PA) Sensors: These sensors utilize two laser sources at different wavelengths to excite the sample and a detector to measure the PA signal. This type of PA sensor is used for the detection of ammonia in the presence of interfering species [50].
- -
- Resonant-cavity-enhanced PAS: In this method, the sample gas is contained within a resonant cavity that is designed to enhance the acoustic signal generated by the absorbed light [51].
- -
- Quartz-enhanced PAS: In this approach, a quartz tuning fork is utilized both for the generation and detection of the photoacoustic signal [52].
- -
- Cantilever-enhanced PAS: This method uses a cantilever to enhance the acoustic signal generated by the absorbed light [53].
In the case of ammonia detection, a laser source is used to provide a short pulse of light that is absorbed by the ammonia molecules. This absorption generates a thermal expansion that results in a pressure wave, which is detected by a microphone or a piezoelectric transducer. The resulting acoustic signal is proportional to the concentration of ammonia in the sample. PA spectroscopy has been used for the detection of ammonia in various applications, including environmental monitoring, industrial process control, and breath analysis for medical diagnostics. The technique offers several advantages over traditional gas sensing methods, including high sensitivity, fast response time, and the ability to detect trace amounts of gas [54]. The use of photoacoustic methods to detect ammonia concentration has been extensively explored by researchers.In 2003, ref. [55] described the continuous detection of trace levels of ammonia in the air, with the ability to detect concentrations as low as sub-ppb. The experiment was conducted in a 40% carbon dioxide and 40% relative humidity environment. In 2004, the use of photoacoustic spectroscopy for ammonia detection with a detection limit of less than 0.1 ppb was reported [56]. In 2005, ref. [57] demonstrated the use of photoacoustic spectroscopy for detecting ammonia gas in agricultural environments. The results showed good agreement with ion chromatography and were promising for real-time monitoring. A study by [58] reported that the photoacoustic method can detect ammonia concentrations in the range of 0.1 to 20 ppm in laboratory conditions, with high sensitivity and accuracy. Furthermore, a study by [59] found that the photo-acoustic method could detect ammonia in less than 5 s with a detection limit of 2 ppm. These findings suggest that photoacoustic spectroscopy can be an effective and efficient technique for the detection of ammonia in various environments. - Spectrophotometry: Knwon as a common technique that uses light to measure the concentration of a substance. This method can be used to detect ammonia by measuring the absorption of light at specific wavelengths. Spectrophotometry can be considered an indoor method for measuring ammonia concentration in agriculture. Spectrophotometry is a laboratory-based analytical technique that measures the absorption of light by a sample at a specific wavelength [60,61]. A study conducted by [21] found that the range for detecting ammonia using spectrophotometry is typically between 0.1 and 2 ppm, but with proper sample preparation and instrument calibration, it can be extended up to 5 ppm. The diverse range of spectrophotometry sensors are utilized for the detection of ammonia as shown in Figure 5, including:
- -
- Laser-induced breakdown spectroscopy (LIBS) This method involves the use of a high-powered laser to create a plasma on the surface of a sample [62].
- -
- Raman spectroscopy: This method involves the use of a laser to excite molecules in the sample, which then emit light at a different wavelength [63].
- -
- Cavity-enhanced absorption spectroscopy (CEAS): This method involves the use of a high-finesse optical cavity to increase the path length of light passing through the sample [64].
- -
- UV-Visible spectrophotometry: This type of spectrophotometry uses ultraviolet (UV) or visible light to measure the absorbance of light by a sample. Ammonia can be detected in solution using this method by measuring the absorbance of light at specific wavelengths [65].
- -
- Fourier Transform Infrared (FTIR) spectrophotometry: FTIR spectrophotometry is a type of infrared spectrophotometry that uses interferometry to measure the infrared absorption of a sample. Ammonia can be detected in gas form using this method by measuring the absorbance of light at specific infrared wavelengths [66].
- -
- Atomic Absorption Spectrophotometry (AAS): AAS is a type of spectrophotometry that uses the absorption of light by free atoms to determine the concentration of a sample. Ammonia can be detected in solution using this method by measuring the absorbance of light at specific wavelengths [67].
2.1.7. Sampling and Laboratory Analysis
- Passive samplers: Passive samplers are a simple yet efficient tool that relies on the principle of diffusion to collect ammonia gas. They consist of a sorbent material, such as silica gel, that effectively absorbs the ammonia gas from the surrounding air. Passive samplers are a preferred option for long-term monitoring due to their easy usage and durability in the field. These small devices are widely used to measure the levels and presence of contaminants in the environment, including pesticides, herbicides, and other chemicals commonly used in agriculture. The mechanism of passive samplers is based on the process of adsorption and absorption of contaminants from the environment. These devices do not require any external power or pumps to operate, making them convenient to use and maintain. They can be deployed for extended periods, ranging from several days to several months, to collect samples of contaminants in the air, water, or soil [69].In farmland, passive samplers play a crucial role in monitoring the levels of pesticides in the air or water that can pose a potential threat to human health and the environment. The information collected by these samplers can be used by farmers and agricultural workers to adjust their practices and reduce the use of harmful chemicals, leading to a safer and healthier farming environment. Passive samplers are also useful in understanding the movement of contaminants within the environment. By deploying samplers at various locations within farmland, it is possible to identify areas that are more vulnerable to contamination and develop strategies to prevent or reduce the spread of pollutants [70]. This model is known as a valuable tool for monitoring and assessing the health of farmland. By providing accurate and reliable data on the levels of contaminants in the environment, they can help farmers and agricultural workers make informed decisions about their practices and protect the health of the land and the people who work on it [71].
- Active samplers: Active samplers are devices that utilize a pump to draw air through a sorbent material actively. Although active samplers are more efficient at gathering ammonia gas than passive samplers, they necessitate a power source and can be more expensive. These devices play a critical role in monitoring soil quality on farmland [72]. They collect soil samples from various depths and locations in a field and analyze them for multiple parameters such as pH levels, organic matter content, and nutrient composition. In recent years, the use of active samplers in farmland has gained importance as farmers and agricultural researchers seek to optimize crop yields and minimize environmental impact. By gaining insights into the soil composition, farmers can make well-informed decisions about management practices like fertilization, irrigation, and more. The active samplers used on farmland come in different types, such as the cone penetrometer and soil corer, which are deployed depending on the specific needs of the farmer or researcher. After collecting the soil samples, they are typically sent to a laboratory for further analysis [73]. The analysis provides critical information on nutrient levels and the presence of any potential contaminants, which can guide farmers to make appropriate decisions on maximizing crop yields while minimizing the environmental impact. In summary, active samplers are essential tools for anyone involved in agriculture, from small-scale farmers to large agricultural corporations. By employing these devices to monitor the quality of soil in their fields, farmers can optimize their yields while taking necessary steps to safeguard the environment.
- Automated monitoring systems: These monitoring systems utilize sophisticated sensors to monitor the real-time levels of ammonia gas continously and precisely. These intricately designed systems are interconnected, forming a comprehensive network that enables remote access to the collected data, allowing for in-depth analysis. The use of automated monitoring systems in farmland has gained popularity in recent years, as farmers seek to improve their efficiency and reduce costs. These systems collect data on soil moisture, temperature, humidity, and other environmental factors, as well as data on plant growth and health, using a range of sensors and monitoring tools [74]. The primary advantage of using automated monitoring systems in farmland is that they allow farmers to make data-driven decisions about irrigation, fertilization, and pest control. The system can trigger irrigation to ensure that plants receive the required amount of water to grow, and pesticides can be applied to control the infestation if pests are detected. Furthermore, the use of automated monitoring systems can help farmers to minimize waste and improve sustainability.By optimizing irrigation and fertilization, farmers can decrease the quantity of water and chemicals used, resulting in a positive environmental impact. By monitoring plant growth and health, farmers can identify areas that require additional attention, such as areas where plants are not growing as well as they should. Additionally, automated monitoring systems can save farmers time and labor [75]. Instead of manually monitoring every field, farmers can rely on the data collected by the system to make informed decisions about crop management. This is particularly useful for large farms, where manual monitoring may not be practical.
- Filter-based methods: Filter-based methods are a fundamental tool in farmland management, aiming to improve crop yields and minimize the risks of plant diseases by eliminating pollutants and impurities from the soil, water, and air. This approach involves using physical and chemical processes to remove contaminants, including gases such as ammonia, from the environment. One common example of a filter-based method is passing air through a filter to capture particulate matter and gases, followed by laboratory analysis to determine the concentration of the target gas [76].
- Open-path techniques: Open-path techniques are a set of remote sensing methods that employ lasers to gauge the concentration of ammonia gas in the air, enabling real-time measurement of ammonia gas concentrations over a vast area. The technique is non-invasive and can measure various environmental parameters such as temperature, humidity, carbon dioxide, and methane concentrations, making it particularly useful in farmland. This technique operates by directing a laser beam across the farmland to gauge the absorption of light by atmospheric gases. One of the key benefits of open-path techniques is their ability to provide real-time measurements of atmospheric conditions over a large area. By continuously monitoring these conditions, farmers can better comprehend the impact of climate change, pests, and diseases on their crops and take appropriate actions to mitigate their effects.Moreover, open-path techniques are useful in detecting leaks from agricultural facilities such as animal feedlots or silage storage areas, thus reducing the risk of environmental contamination and greenhouse gas emissions [77]. With open-path techniques, farmers can continuously monitor atmospheric conditions over a large area, which is especially useful in farmland where environmental conditions can fluctuate significantly over short distances [78].Several filter-based techniques are employed in farmland management, including soil, irrigation, air, and crop filters.
- -
- Soil filters: use filter media such as activated carbon or sand to remove impurities from the soil, rendering it suitable for farming.
- -
- Irrigation filters: remove sediment and debris from water, improving the efficiency of the water usage and preventing the clogging of irrigation systems.
- -
- Air filters: remove airborne particles from the atmosphere, particularly in areas with high levels of air pollution.
- -
- Crop filters: utilize specific crops to filter out harmful substances from the soil, such as heavy metals, which can be eliminated from the farm system by harvesting and disposing of the crops.
Passive samplers are inexpensive and easy to use in comparison to sampling and laboratory analysis procedures, however they have limited detection capability. Active samplers can take bigger air samples and deliver faster findings, but they must be maintained and trained. Automated monitoring systems provide constant monitoring but are expensive. Filter-based approaches are accurate, but they are costly and time-consuming. Soil, irrigation, air, and crop filters all give information about specific habitats, but they have limits. Open-path approaches provide real-time monitoring but need specialised equipment that can be costly. One common point among all these methods is that all of these approaches seek to measure and monitor pollution levels and offer information on the effects of pollution on various habitats. The difference among them is the cost, ease of use, detection abilities, sampling volume, speed of results, maintenance requirements, and the specific environments they target. Table 1 shows the comparison study of different methods used by the researchers for the detection of ammonia in farmland.
2.2. Electronic Nose (E-Nose) Ammonia Detection Method
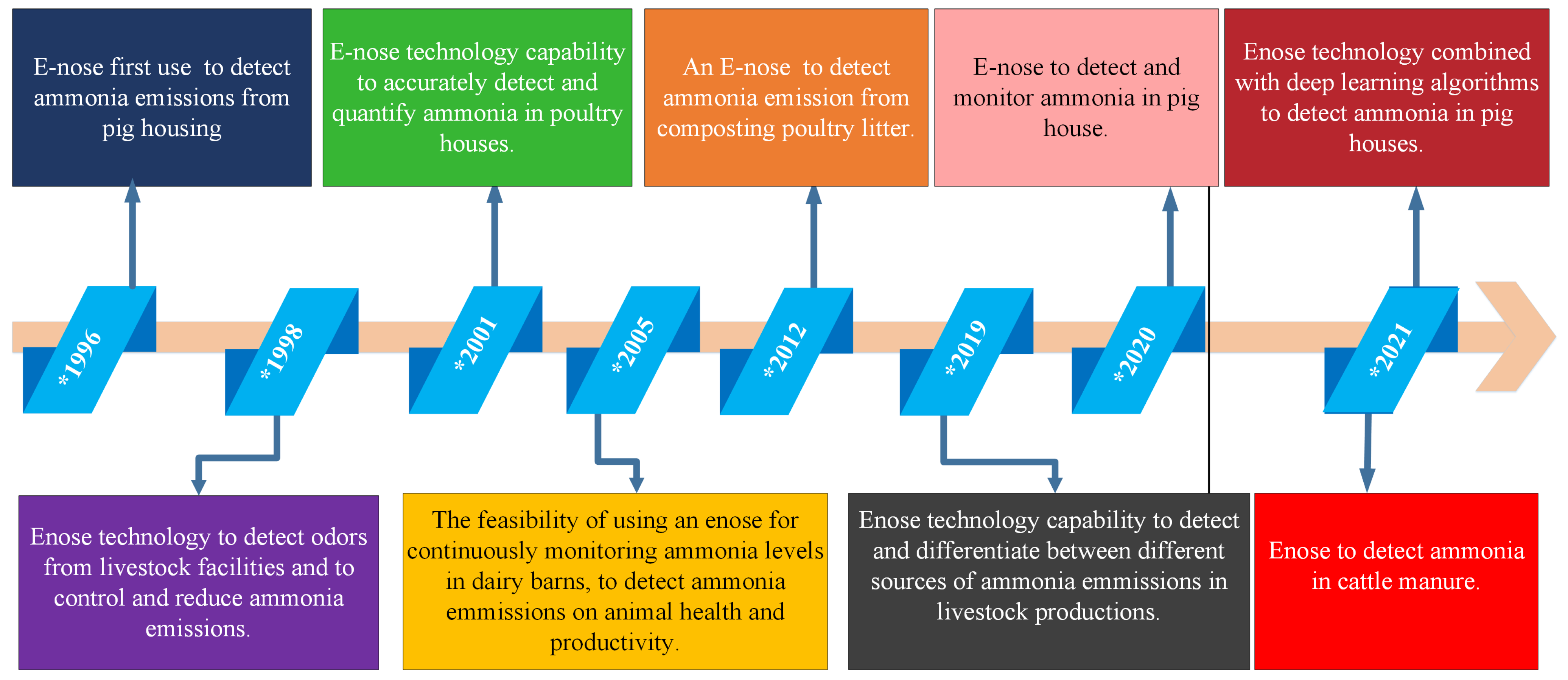
2.2.1. E-Nose History Application in Agriculture
2.2.2. E-Nose Structure
- Sensor: The E-nose can be equipped with multiple sensors, allowing for the simultaneous detection of multiple VOCs, including ammonia, providing a more comprehensive analysis of the air quality [93]. Some of the most commonly used sensor types for this purpose are listed below:
- -
- Metal-oxide Semiconductor (MOS) Gas Sensor: MOS sensors operate by measuring the change in electrical resistance when exposed to gases such as ammonia. They are cost-effective and can be used for continuous monitoring of ammonia levels in farmland. Some commonly known models of these sensor types include the Figaro TGS2600, SPEC Sensors MQ-137, and Winsen MQ135 [94].
- -
- Electrochemical Gas Sensor: Electrochemical sensors are commonly used for gas detection, including ammonia gas. They operate by measuring the electrical current produced when ammonia gas reacts with an electrode, providing real-time data on ammonia concentration in the surrounding air. The most commonly used models for these sensors include the Alphasense NH-B1, City Technology 4NH-100 C, and Membrapor EC-NH [95].
- -
- Photoionization Detector (PID): PID sensors use ultraviolet light to ionize gas molecules, including ammonia, and measure the resulting electrical current. They are highly sensitive and can provide instant readings of ammonia concentration in the air, making them suitable for farmland applications. The following models, such as the Honeywell MiniRAE 3000, Ion Science TigerLT, and RKI Instruments Eagle 2 PID, are widely recognized and commonly used for this sensor group [96].
- -
- Non-Dispersive Infrared (NDIR) Sensors: Infrared sensors work by measuring the absorption of infrared light by gases, including ammonia. They are highly selective and can provide accurate readings of ammonia concentration in the air, making them ideal for farmland applications. Some notable models for this sensor type include the SGX Sensortech AMMONIA-1C-N, and Winsen ME2-NH. These models have gained significant recognition in the industry [42].
- -
- Wireless Sensor Networks (WSN): WSNs consist of multiple sensor nodes deployed in a farmland area, interconnected wirelessly to collect and transmit data, including ammonia gas concentrations, in real-time. They can provide comprehensive coverage of farmland areas and enable remote monitoring of ammonia levels. The gas sensing boards commonly employed for these types of applications include the Libelium Waspmote Gas Sensor Board, which integrates a range of sensors capable of detecting gases such as ammonia [97].
2.2.3. E-Nose Signal Processing Method
- Principal Component Analysis (PCA): PCA is a widely used technique for feature extraction and dimensionality reduction. It analyzes the correlation between sensor responses and identifies the principal components that capture the most significant variations in the data. By applying PCA to the sensor data, the E-nose system can reduce the dimensionality of the input and improve classification accuracy. This method offers several advantages, including the reduction of data dimensional, capturing significant variations, facilitating data visualization, and enabling feature extraction. However, it is important to note that this method assumes linearity in the data and may not effectively handle nonlinear relationships [98].
- Artificial Neural Networks (ANN): ANN algorithms are frequently employed in E-nose systems for pattern recognition and classification. These algorithms consist of interconnected nodes or “neurons” that learn from the sensor data and make predictions based on the acquired knowledge. ANN can be trained to recognize specific patterns associated with ammonia gas, enabling accurate detection and classification. When considering the advantages of this method, it is reported that it excels in handling complex and nonlinear relationships, demonstrating robustness in pattern recognition and classification tasks. Additionally, it possesses the ability to learn from data and generalize its findings. However, it should be noted that this method does have certain disadvantages, including the requirement for substantial computational resources and training time. Furthermore, it is sensitive to factors such as network architecture and hyperparameters, which need to be carefully considered during implementation [99].
- Support Vector Machines (SVM): SVM is a supervised machine learning algorithm used for classification tasks. It separates data into different classes by creating an optimal hyperplane in a high-dimensional feature space. SVM can be trained on labeled sensor data to distinguish between ammonia and non-ammonia samples, making it suitable for ammonia gas detection in E-nose systems. The Support Vector Machines (SVM) offers several advantages in data analysis and classification, including effectiveness for binary classification tasks, good performance in high dimensional feature spaces, robustness against over fitting, and capability of handling nonlinear relationships through kernel functions. However, it is important to consider the following disadvantages when using SVM: it is computationally intensive, especially for large datasets, may not scale well with extensive amounts of data, and requires careful selection of the appropriate kernel and tuning of hyper parameters. Despite these challenges, SVM remains a powerful tool in machine learning and data analysis, particularly for binary classification problems in high-dimensional spaces [100].
- Artificial Olfactory System (AOS): AOS is an approach inspired by the olfactory system of living organisms. It involves modeling the sensor responses based on biological principles and implementing algorithms that mimic the odor perception and recognition process. AOS methods can be applied to E-nose systems for ammonia detection, enabling more accurate and reliable results. Advantages of an Artificial Olfactory System (AOS) include its inspiration from biological olfactory systems, enabling the capture of complex odor perception and recognition, thereby potentially enhancing accuracy in odor analysis. However, AOS implementation and modeling can be complex, requiring extensive training and optimization. Additionally, the availability of standardized AOS methods is limited [97].
- Fuzzy Logic (FL): Fuzzy logic is a mathematical framework that deals with uncertainty and imprecision. It is often utilized in E-nose systems to handle the inherently fuzzy nature of sensor data and decision-making processes. By incorporating fuzzy logic techniques, the E-nose can effectively handle variations in sensor responses and provide more robust ammonia detection results. The advantages of fuzzy logic can be summarized as follows: it effectively deals with uncertainty and imprecision in data, provides a suitable framework for handling fuzzy relationships, and allows for the incorporation of expert knowledge into the system. However, it is important to note that fuzzy logic also has its limitations, such as the subjective design of fuzzy rule sets and the need for tuning membership functions and fuzzy operators to achieve optimal performance [93].
2.2.4. Pattern Recognition (PR)
- Long Short-Term Memory (LSTM): LSTM is a recurrent neural network (RNN) architecture that has been successfully applied in various fields, including ammonia detection in E-nose systems. LSTM networks are designed to capture long-term dependencies and temporal patterns in sequential data, making them well-suited for time-series analysis. The method offers several advantages, including its ability to capture temporal dependencies in sequential data, effectiveness in time-series analysis, and the capability to learn long-term dependencies. However, it is worth noting that this method has a few disadvantages, such as the requirement of a large amount of training data, the complexity of its architecture, and its computational intensity [103].
- Partial Least Squares (PLS): PLS is a statistical method commonly used in E-nose systems for data analysis and modeling. PLS is a valuable tool in E-nose detection, allowing for effective modeling of sensor data, feature selection, and prediction of target substance concentrations. It helps to overcome multicollinearity and provides insights into the underlying relationships between sensor responses and the target substance, contributing to accurate and reliable detection in E-nose systems. PLS offers several advantages in E-nose detection. It excels in handling multicollinearity in data, making it suitable for modeling complex relationships. It can also enable feature selection and analysis of variable importance, allowing for a more focused and efficient analysis of the sensor data. However, it is important to consider the limitations of PLS. One drawback is its sensitivity to outliers in the data, which can impact the accuracy of the model. Additionally, when dealing with small datasets, PLS has the potential to overfit the model, resulting in overly complex and less generalizable predictions. In whole, PLS provides valuable benefits in E-nose detection, including its ability to handle multicollinearity, model complex relationships, and conduct feature selection. Nonetheless, its sensitivity to outliers and the risk of overfitting with small datasets should be taken into account during its implementation and interpretation [104].
- Linear Discriminant Analysis (LDA): LDA is a commonly used method in E-nose systems for ammonia gas detection and konwn as a supervised classification algorithm that aims to find a linear combination of features that maximizes the separation between different classes of data. The combination of E-nose and LDA can provide accurate and reliable detection of ammonia gas, contributing to applications in environmental monitoring, industrial safety, and agriculture. The LDA offers benefits such as dimensionality reduction, multiclass classification capability, and statistical inference. Its assumptions of linearity and limited ability to model nonlinear decision boundaries should be considered when applying it in the context of ammonia gas detection using E-nose systems [105].
2.2.5. E-Nose Sensor Type vs. Signal Processing Method
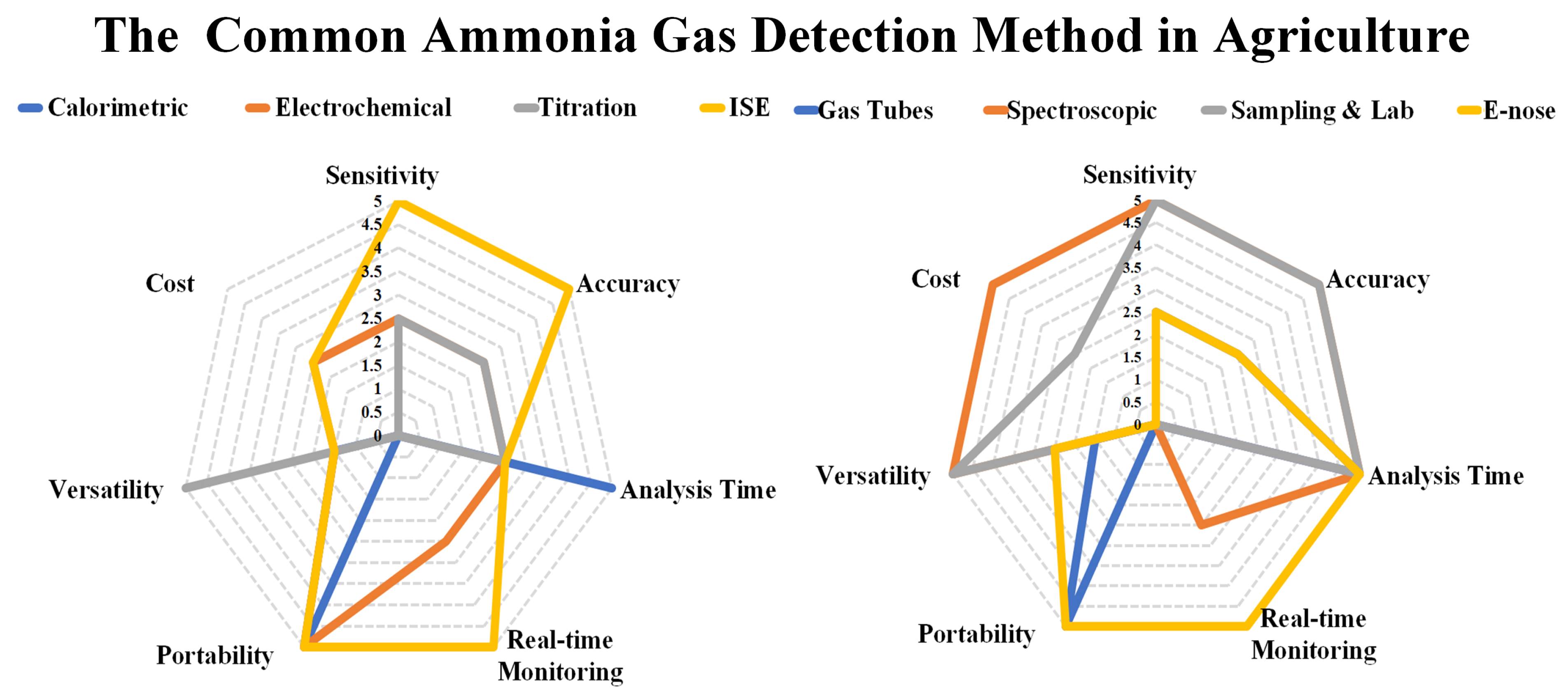
3. Performance Evaluation of Traditional Methods vs. E-Nose
4. Conclusions
- Ammonia detection is crucial for successful crop cultivation to predict soil fertility issues, as excess ammonia harms plants, animals, and the environment due to increased acidity levels.
- Traditional methods for ammonia detection are simple, affordable, but have limitations such as manual sampling, measurement errors, and delays in obtaining results.
- E-nose technologies offer real-time, non-invasive, and automated monitoring advantages, but may require calibration, data analysis, and higher costs.
- E-nose technology shows promise in agriculture for ammonia detection, improving air quality, reducing emissions, and enhancing animal health.
- The cost of E-nose ammonia detection varies based on sensor type and system complexity, with simpler systems being less expensive.
- E-nose ammonia detection methods vary in speed, with some providing results in seconds, while others take minutes.
- Accuracy of E-nose ammonia detection methods varies based on the sensor type, with some sensors known for high accuracy.
- The utilization of E-nose technology in agriculture enhances monitoring efficiency, enables real-time monitoring, and facilitates non-destructive sampling.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michaels, R.A. Emergency planning and the acute toxic potency of inhaled ammonia. Environ. Health Perspect. 1999, 107, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Xia, D. Ammonia control represents the key for PM2.5 elimination: Insights for global air pollution control interconnected from PM2.5 events in China. Clean Technol. Environ. Policy 2021, 23, 829–841. [Google Scholar] [CrossRef]
- Simion, C.E.; Florea, O.G.; Mercioniu, I.; Dinu, I.V.; Stanoiu, A. Gas sensing mechanism involved in NH3 detection with NiO material. In Proceedings of the 2022 International Semiconductor Conference (CAS), Brasov, Romania, 12–14 October 2022; pp. 109–112. [Google Scholar]
- Pigni, A.; Tugnolo, A.; Beghi, R.; Cocetta, G.; Finzi, A. Rapid and continuous monitoring of air ammonia concentration in dairy milking parlors. In Proceedings of the 2021 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Bolzano, Italy, 3–5 November 2021; pp. 167–171. [Google Scholar]
- Bulbul, A.; Kim, H. ppb level gas quantification by bubble chromatography. In Proceedings of the 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Kaohsiung, Taiwan, 18–22 June 2017; pp. 660–663. [Google Scholar]
- Pauluhn, J. Acute inhalation toxicity of ammonia: Revisiting the importance of RD50 and LCT01/50 relationships for setting emergency response guideline values. Regul. Toxicol. Pharmacol. 2013, 66, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Andre, R.S.; Facure, M.H.; Mercante, L.A.; Correa, D.S. Electronic nose based on hybrid free-standing nanofibrous mats for meat spoilage monitoring. Sens. Actuators B Chem. 2022, 353, 131114. [Google Scholar] [CrossRef]
- Guido, V.; Finzi, A.; Piazzi, P.; Ferrari, O.; Ricco, C.R.; Riva, E.; Provolo, G. Effect of mitigation techniques on ammonia emissions and nutrients recovery: The role of fertigation with digestate. In Proceedings of the 2020 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Trento, Italy, 4–6 November 2020; pp. 39–43. [Google Scholar]
- Liu, T.; Chen, Z.; Fu, Q.; Shi, B.; Yang, L. Acute toxicity test of landfill leachates using protozoan communities. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar]
- Zhang, B.; Wu, B.; Liu, J. PM2.5 pollution-related health effects and willingness to pay for improved air quality: Evidence from China’s prefecture-level cities. J. Clean. Prod. 2020, 273, 122876. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Y.; Yuan, L.; Chen, N.; Kong, S. Ambient observations indicating an increasing effectiveness of ammonia control in wintertime PM2.5 reduction in Central China. Sci. Total Environ. 2022, 824, 153708. [Google Scholar] [CrossRef]
- Yuanxi, W.; Zhang, W.; Jun, W. Research on Preparation and Performance of a New Solid Electrolyte Based Nitrogen Oxides Sensor. IEEE Sens. J. 2022, 22, 13908–13914. [Google Scholar] [CrossRef]
- Singh, A.K.; Pandey, A.; Chakrabarti, P. Poly [2,5-bis (3-tetradecylthiophen-2-yl) thieno [3,2-b] thiophene] organic polymer based-interdigitated channel enabled thin film transistor for detection of selective low ppm Ammonia sensing at 25 C. IEEE Sens. J. 2019, 20, 4047–4055. [Google Scholar] [CrossRef]
- Insausti, M.; Timmis, R.; Kinnersley, R.; Rufino, M.C. Advances in sensing ammonia from agricultural sources. Sci. Total Environ. 2020, 706, 135124. [Google Scholar] [CrossRef]
- Swotinsky, R.B.; Chase, K.H. Health effects of exposure to ammonia: Scant information. Am. J. Ind. Med. 1990, 17, 515–521. [Google Scholar] [CrossRef]
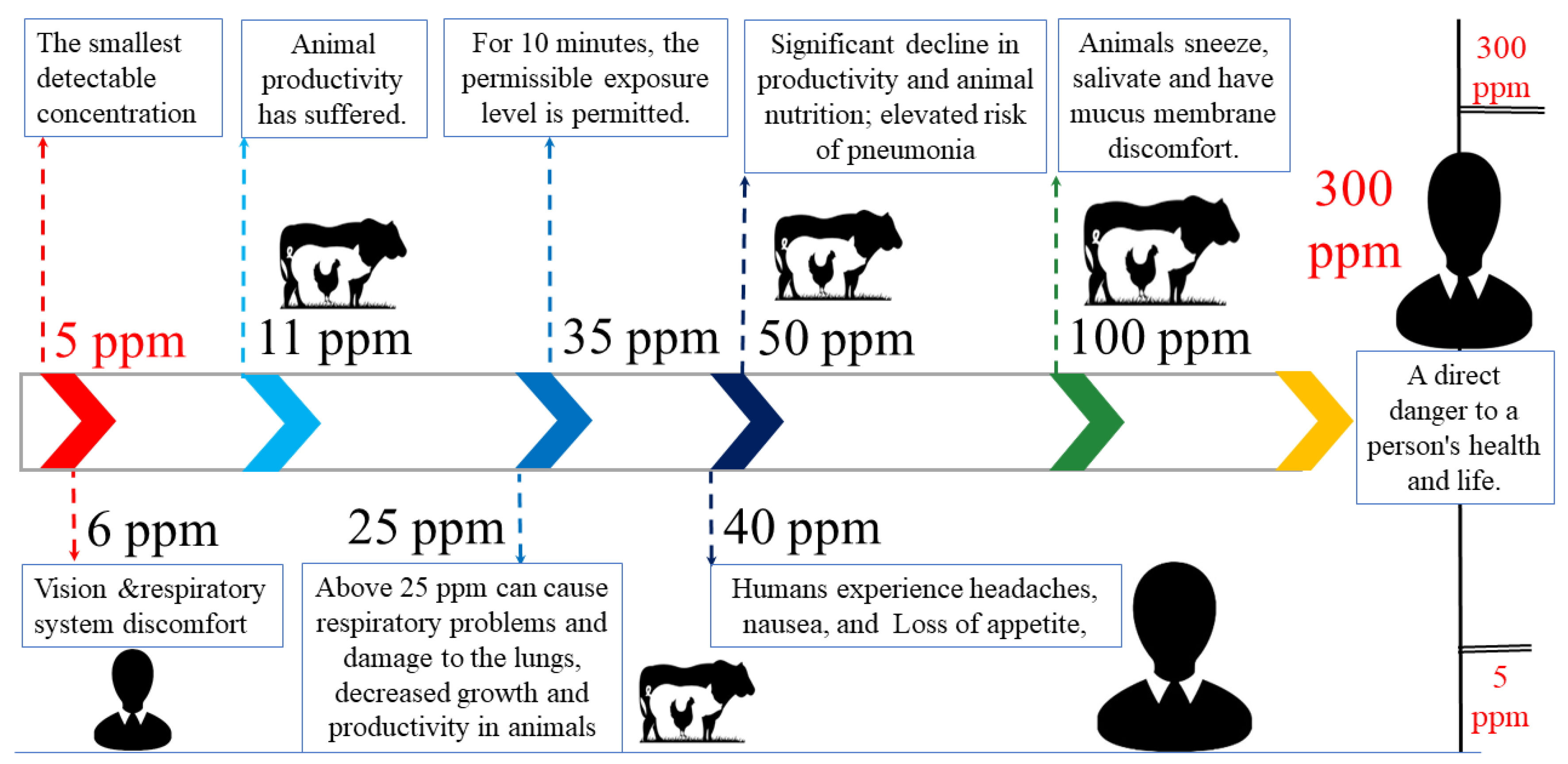
- Conti, C.; Borgonovo, F.; Guarino, M. Ammonia concentration and recommended threshold values in pig farming: A review. In Proceedings of the 2021 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Bolzano, Italy, 3–5 November 2021; pp. 162–166. [Google Scholar]
- Liu, Q.X.; Zhou, Y.; Li, X.M.; Ma, D.D.; Xing, S.; Feng, J.H.; Zhang, M.H. Ammonia induce lung tissue injury in broilers by activating NLRP3 inflammasome via Escherichia/Shigella. Poult. Sci. 2020, 99, 3402–3410. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, A.F.; Lee, D.S.; Asman, W.A.; Dentener, F.J.; Van Der Hoek, K.W.; Olivier, J.G.J. A global high-resolution emission inventory for ammonia. Glob. Biogeochem. Cycles 1997, 11, 561–587. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Q.X.; Li, X.M.; Ma, D.D.; Xing, S.; Feng, J.H.; Zhang, M.H. Effects of ammonia exposure on growth performance and cytokines in the serum, trachea, and ileum of broilers. Poult. Sci. 2020, 99, 2485–2493. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, H.H.; Wathes, C.M. Ammonia and poultry welfare: A review. World’s Poult. Sci. J. 2000, 56, 235–245. [Google Scholar] [CrossRef]
- Lin, T.H.; Li, Y.T.; Hao, H.C.; Fang, I.C.; Yang, C.M.; Yao, D.J. Surface acoustic wave gas sensor for monitoring low concentration ammonia. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 1140–1143. [Google Scholar]
- Ansari, M.; Moravvej-Farshi, M.K. Ammonia Vapor Sensor based on Tapered Multimode Fiber Coated with Silica-Gel. In Proceedings of the 2020 28th Iranian Conference on Electrical Engineering (ICEE), Tabriz, Iran, 4–6 August 2020; pp. 1–4. [Google Scholar]
- Moshayedi, A.J.; Gharpure, D.C. Evaluation of bio inspired Mokhtar: Odor localization system. In Proceedings of the 2017 18th International Carpathian Control Conference (ICCC), Sinaia, Romania, 28–31 May 2017; pp. 527–532. [Google Scholar]
- Geng, K.; Ata, J.M.; Chen, J.; Hu, J.; Zhang, H. ENOSE Performance in Transient Time and Steady State Area of Gas Sensor Response for Ammonia Gas: Comparison and Study. In Proceedings of the 2023 2nd Asia Conference on Algorithms, Computing and Machine Learning, Shanghai, China, 17–19 March 2023; pp. 247–252. [Google Scholar]
- Chen, D.; Miao, Z.; Peng, M.; Xing, H.; Zhang, H.; Teng, X. The co-expression of circRNA and mRNA in the thymuses of chickens exposed to ammonia. Ecotoxicol. Environ. Saf. 2019, 176, 146–152. [Google Scholar] [CrossRef]
- Moshayedi, A.J.; Khan, A.S.; Yang, S.; Geng, K.; Hu, J. ENose design and structures from statistical analysis to application in robotic: A compressive review. EAI Endorsed Trans. AI Robot. 2023, 1, e13. [Google Scholar]
- Wang, X.; Yang, J.; Salla, M.; Xi, S.; Yang, Y.; Li, M.; Zhang, F.; Zhu, M.K.; Huang, S.; Huang, S.; et al. Redox-Mediated Ambient Electrolytic Nitrogen Reduction for Hydrazine and Ammonia Generation. Angew. Chem. Int. Ed. 2021, 60, 18721–18727. [Google Scholar] [CrossRef]
- Ahn, C.K.; Han, K.; Lee, M.S.; Kim, J.Y.; Chun, H.D.; Kim, Y.; Park, J.M. Experimental studies of additives for suppression of ammonia vaporization in the ammonia based CO2 capture process. Energy Procedia 2013, 37, 7108–7116. [Google Scholar] [CrossRef]
- Jaikang, P.; Paengnakorn, P.; Grudpan, K. Simple colorimetric ammonium assay employing well microplate with gas pervaporation and diffusion for natural indicator immobilized paper sensor via smartphone detection. Microchem. J. 2020, 152, 104283. [Google Scholar] [CrossRef]
- Dewantari, A.A.; Yongwattana, N.; Payongsri, P.; Seemakhan, S.; Borwornpinyo, S.; Ojida, A.; Wongkongkatep, J. Fluorescence detection of deoxyadenosine in Cordyceps spp. by indicator displacement assay. Molecules 2020, 25, 2045. [Google Scholar] [CrossRef]
- Drout, R.J.; Kato, S.; Chen, H.; Son, F.A.; Otake, K.I.; Islamoglu, T.; Snurr, R.Q.; Farha, O.K. Isothermal titration calorimetry to explore the parameter space of organophosphorus agrochemical adsorption in MOFs. J. Am. Chem. Soc. 2020, 142, 12357–12366. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Vedachalam, S.; Sathyaraj, G.; Garai, S.; Arthanareeswaran, G.; Sankaranarayanan, K. Fast sensing ammonia at room temperature with proline ionic liquid incorporated cellulose acetate membranes. J. Mol. Liq. 2020, 305, 112820. [Google Scholar] [CrossRef]
- Fang, C.S.; Oh, K.H.; Park, J.K.; Yang, H. Rapid and sensitive electrochemical detection of carbaryl based on enzyme inhibition and thiocholine oxidation mediated by a ruthenium (III) complex. Electroanalysis 2017, 29, 339–344. [Google Scholar] [CrossRef]
- Li, D.; Xu, X.; Li, Z.; Wang, T.; Wang, C. Detection methods of ammonia nitrogen in water: A review. TrAC Trends Anal. Chem. 2020, 127, 115890. [Google Scholar] [CrossRef]
- Ali, M.A.; Dong, L.; Dhau, J.; Khosla, A.; Kaushik, A. Perspective—electrochemical sensors for soil quality assessment. J. Electrochem. Soc. 2020, 167, 037550. [Google Scholar] [CrossRef]
- Sanger, J.B.; Sitanayah, L.; Ahmad, I. A Sensor-based Garbage Gas Detection System. In Proceedings of the 2021 IEEE 11th Annual Computing and Communication Workshop and Conference (CCWC), Las Vegas, NV, USA, 27–30 January 2021; pp. 1347–1353. [Google Scholar]
- Choosang, J.; Numnuam, A.; Thavarungkul, P.; Kanatharana, P.; Radu, T.; Ullah, S.; Radu, A. Simultaneous detection of ammonium and nitrate in environmental samples using on ion-selective electrode and comparison with portable colorimetric assays. Sensors 2018, 18, 3555. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Huang, L.; Cai, G.; Zeng, R.; Yu, Z.; Tang, D. Contactless Photoelectrochemical Biosensor Based on the Ultraviolet–Assisted Gas Sensing Interface of Three-Dimensional SnS2 Nanosheets: From Mechanism Reveal to Practical Application. Anal. Chem. 2022, 94, 9487–9495. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Chuang, M.Y.; Zan, H.W.; Meng, H.F.; Lu, C.J.; Yeh, P.H.; Chen, J.N. One-minute fish freshness evaluation by testing the volatile amine gas with an ultrasensitive porous-electrode-capped organic gas sensor system. ACS Sens. 2017, 2, 531–539. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Wang, Y.; Zhang, R.; Li, J.; Li, X.; Zang, Z. Conductometric room temperature ammonia sensors based on titanium dioxide nanoparticles decorated thin black phosphorus nanosheets. Sens. Actuators B Chem. 2021, 349, 130770. [Google Scholar] [CrossRef]
- Kashour, H.; Soubh, L. Comparative between Ammonia Ion selective electrode and dye binding method to study effect of processing methods on protein content of plain Yogurt. Res. J. Pharm. Technol. 2021, 14, 6257–6261. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; She, C.; Cheng, J.; Li, H.; Liu, S.; Jing, C.; Cheng, Y.; Chu, J. High performance tube sensor based on PANI/Eu3+ nanofiber for low-volume NH3 detection. Anal. Chim. Acta 2020, 1093, 115–122. [Google Scholar] [CrossRef]
- Lewicki, R.; Kosterev, A.A.; Thomazy, D.M.; Risby, T.H.; Solga, S.; Schwartz, T.B.; Tittel, F.K. Real time ammonia detection in exhaled human breath using a distributed feedback quantum cascade laser based sensor. In Proceedings of the Quantum Sensing and Nanophotonic Devices VIII, San Francisco, CA, USA, 23–27 January 2011; Volume 7945, pp. 141–147. [Google Scholar]
- Erisman, J.W.; Otjes, R.; Hensen, A.; Jongejan, P.; van den Bulk, P.; Khlystov, A.; Möls, H.; Slanina, S. Instrument development and application in studies and monitoring of ambient ammonia. Atmos. Environ. 2001, 35, 1913–1922. [Google Scholar] [CrossRef]
- Ilke, M.; Bauer, R.; Lengden, M. A calibration-free methodology for resonantly enhanced photoacoustic spectroscopy using quantum cascade lasers. IEEE Sens. J. 2020, 20, 10530–10538. [Google Scholar] [CrossRef]
- Yang, T.; Liu, R.; Yao, Y.; Luo, W.; Zhou, K.; Jin, L. Photoacoustic Spectroscopy for Detection of Trace CH4 using Optimized Photoacoustic Cell. In Proceedings of the 2022 IEEE International Conference on High Voltage Engineering and Applications (ICHVE), Berlin, Germany, 25–29 September 2022; pp. 1–4. [Google Scholar]
- Chen, K.; Zhang, B.; Guo, M.; Deng, H.; Yang, B.; Gong, Z.; Peng, W.; Yu, Q. All-optical photoacoustic multigas analyzer using digital fiber-optic acoustic detector. IEEE Trans. Instrum. Meas. 2020, 69, 8486–8493. [Google Scholar] [CrossRef]
- Wang, G.; Lahib, A.; Duncianu, M.; Gou, Q.; Stevens, P.S.; Dusanter, S.; Tomas, A.; Sigrist, M.W.; Chen, W. Monitoring of peroxy radicals by chemical amplification enhanced photoacoustic spectroscopy. In Proceedings of the European Conference on Lasers and Electro-Optics, Munich, Germany, 21–25 June 2021. [Google Scholar]
- McKechnie, A.E. Regulation of body temperature: Patterns and processes. In Sturkie’s Avian Physiology; Academic Press: New York, NY, USA, 2022; pp. 1211–1244. [Google Scholar]
- Henderson, B.; Khodabakhsh, A.; Metsälä, M.; Ventrillard, I.; Schmidt, F.M.; Romanini, D.; Ritchie, G.A.; te Lintel Hekkert, S.; Briot, R.; Risby, T.; et al. Laser spectroscopy for breath analysis: Towards clinical implementation. Appl. Phys. B 2018, 124, 161. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tong, Y.; He, Y.; Jin, X.; Tittel, F.K. Compact and sensitive mid-infrared all-fiber quartz-enhanced photoacoustic spectroscopy sensor for carbon monoxide detection. Opt. Express 2019, 27, 9302–9312. [Google Scholar] [CrossRef]
- Ma, F.; Liao, Z.; Zhao, Y.; Qiu, Z.; Wan, L.; Li, K.; Zhang, G. Detection of trace C2H2 in N2 buffer gas with cantilever-enhanced photoacoustic spectrometer. Optik 2021, 232, 166525. [Google Scholar] [CrossRef]
- Durmuş, H.O.; Birlikseven, C.; Karaböce, B.; Seyitsoy, M. Investigation of Photoacoustic Effect on Different Materials. In Proceedings of the 2022 Medical Technologies Congress (TIPTEKNO), Antalya, Turkey, 31 October–2 November 2022; pp. 1–4. [Google Scholar]
- Pushkarsky, M.B.; Webber, M.E.; Patel, C.K.N. Ultra-sensitive ambient ammonia detection using CO2-laser-based photoacoustic spectroscopy. Appl. Phys. B 2003, 77, 381–385. [Google Scholar] [CrossRef]
- Schilt, S.; Thévenaz, L.; Niklès, M.; Emmenegger, L.; Hüglin, C. Ammonia monitoring at trace level using photoacoustic spectroscopy in industrial and environmental applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 3259–3268. [Google Scholar] [CrossRef]
- Webber, M.E.; MacDonald, T.; Pushkarsky, M.B.; Patel, C.K.N.; Zhao, Y.; Marcillac, N.; Mitloehner, F.M. Agricultural ammonia sensor using diode lasers and photoacoustic spectroscopy. Meas. Sci. Technol. 2005, 16, 1547. [Google Scholar] [CrossRef]
- Petrus, M.; Popa, C.; Bratu, A.M. Ammonia concentration in ambient air in a Peri-urban area using a laser photoacoustic spectroscopy detector. Materials 2022, 15, 3182. [Google Scholar] [CrossRef] [PubMed]
- Essing, S.; Trautmann, M.; Tumpold, D.; Schrag, G. Humidity Sensing for free—Advanced thermoacoustic signal models in miniaturized photoacoustic gas sensors. In Proceedings of the 2023 24th International Conference on Thermal, Mechanical and Multi-Physics Simulation and Experiments in Microelectronics and Microsystems (EuroSimE), Graz, Austria, 16–19 April 2023; pp. 1–4. [Google Scholar]
- Li-qiong, C.; Yong, H. Influencing factors on determination of ammonia nitrogen in water by nessler’s reagent spectrophotometry. In Proceedings of the 2011 International Symposium on Water Resource and Environmental Protection, Xi’an, China, 20–22 May 2011; Volume 2, pp. 1173–1176. [Google Scholar]
- Hasan, A.A. The behaviour of aluminium ion in treatment of dairy wastewater. J. Eng. Sustain. Dev. 2022, 26, 92–101. [Google Scholar] [CrossRef]
- Fu, X.; Zhao, C.; Ma, S.; Tian, H.; Dong, D.; Li, G. Determining available potassium in soil by laser-induced breakdown spectroscopy combined with cation exchange membrane adsorption. J. Anal. At. Spectrom. 2020, 35, 2697–2703. [Google Scholar] [CrossRef]
- Rafferty, C.; Johnson, K.; O’Mahony, J.; Burgoyne, B.; Rea, R.; Balss, K.M. Analysis of chemometric models applied to Raman spectroscopy for monitoring key metabolites of cell culture. Biotechnol. Prog. 2020, 36, e2977. [Google Scholar] [CrossRef]
- Banik, G.D.; Mizaikoff, B. Exhaled breath analysis using cavity-enhanced optical techniques: A review. J. Breath Res. 2020, 14, 043001. [Google Scholar] [CrossRef]
- Amiri, V.; Roshan, H.; Mirzaei, A.; Neri, G.; Ayesh, A.I. Nanostructured metal oxide-based acetone gas sensors: A review. Sensors 2020, 20, 3096. [Google Scholar] [CrossRef]
- Gupta, P.; Maurya, S.; Pandey, N.K.; Verma, V. Metal-Oxide Based Ammonia Gas Sensors: A Review. Nanosci. Nanotechnol.-Asia 2021, 11, 270–289. [Google Scholar] [CrossRef]
- Bratovcic, A. Recent developments on metal oxide-based gas sensors for environmental pollution control. In New Technologies, Development and Application IV; Springer: Cham, Switzerland, 2021; pp. 952–963. [Google Scholar]
- Xu, X.; Bai, Z.; Wang, T. Portable device for on-site detection of ammonia nitrogen. Inf. Process. Agric. 2022, 9, 475–484. [Google Scholar] [CrossRef]
- Pan, Y.; Gu, M.; Song, L.; Tian, S.; Wu, D.; Walters, W.W.; Yu, X.; Lü, X.; Ni, X.; Wang, Y.; et al. Systematic low bias of passive samplers in characterizing nitrogen isotopic composition of atmospheric ammonia. Atmos. Res. 2020, 243, 105018. [Google Scholar] [CrossRef]
- Kawashima, H.; Ogata, R.; Gunji, T. Laboratory-based validation of a passive sampler for determination of the nitrogen stable isotope ratio of ammonia gas. Atmos. Environ. 2021, 245, 118009. [Google Scholar] [CrossRef]
- Šraj, L.O.C.; Almeida, M.I.G.; Sharp, S.M.; McKelvie, I.D.; Morrison, R.; Kolev, S.D. Monitoring of ammonia in marine waters using a passive sampler with biofouling resistance and neural network-based calibration. Environ. Pollut. 2020, 267, 115457. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Yoshida, O.; Joy, K.S.; Raju, R.A.; Islam, K.N.; Jeba, F.; Salam, A. Sources identification of ammonium in PM2.5 Monsoon Seas. Dhaka, Bangladesh. Sci. Total Environ. 2022, 838, 156433. [Google Scholar] [CrossRef]
- Gu, M.; Pan, Y.; Sun, Q.; Walters, W.W.; Song, L.; Fang, Y. Is fertilization the dominant source of ammonia in the urban atmosphere? Sci. Total Environ. 2022, 838, 155890. [Google Scholar] [CrossRef]
- Paul, K.; Chatterjee, S.S.; Pai, P.; Varshney, A.; Juikar, S.; Prasad, V.; Bhadra, B.; Dasgupta, S. Viable smart sensors and their application in data driven agriculture. Comput. Electron. Agric. 2022, 198, 107096. [Google Scholar] [CrossRef]
- Mahfuz, S.; Mun, H.S.; Dilawar, M.A.; Yang, C.J. Applications of smart technology as a sustainable strategy in modern swine farming. Sustainability 2022, 14, 2607. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.; Hua, Z.; Li, Z.; He, X.; Yan, R.; Li, Y.; Zhi, Z.; Tian, C. A low cost and high performance NH3 detection system for a harsh agricultural environment. Sens. Actuators B Chem. 2022, 361, 131675. [Google Scholar] [CrossRef]
- Bai, M.; Loh, Z.; Griffith, D.W.; Turner, D.; Eckard, R.; Edis, R.; Denmead, O.T.; Bryant, G.W.; Paton-Walsh, C.; Tonini, M.; et al. Performance of open-path lasers and Fourier transform infrared spectroscopic systems in agriculture emissions research. Atmos. Meas. Tech. 2022, 15, 3593–3610. [Google Scholar] [CrossRef]
- Molleman, B.; Alessi, E.; Krol, D.; Morton, P.A.; Daly, K. Application of metal oxide semiconductor for detection of ammonia emissions from agricultural sources. Sens. Bio-Sens. Res. 2022, 38, 100541. [Google Scholar] [CrossRef]
- Franco, F.F.; Manjakkal, L.; Shakthivel, D.; Dahiya, R. ZnO based screen printed aqueous ammonia sensor for water quality monitoring. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar]
- Khairudin, N.; Najmi, M.H.M.; Zain, A.M.; Omar, N.; Manut, A.; Zolkapli, M.; Burham, N.; Rani, R.A.; Zoolfakar, A.S. Enhancing humidity sensing performance: The effect of Nitrogen doped on Electrochemical Reduced Graphene Oxide (ERGO). In Proceedings of the 2020 IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 28–29 July 2020; pp. 112–115. [Google Scholar]
- Moufid, M.; Tiebe, C.; Bari, N.E.; Bartholmai, M.; Bouchikhi, B. Advance in Electronic nose technology developed for the detection and discrimination of ethanol, ammonia, and hydrogen sulfide gases. In Proceedings of the 2022 IEEE International Symposium on Olfaction and Electronic nose, Aveiro, Portugal, 29 May–1 June 2022; pp. 1–4. [Google Scholar]
- Borowik, P.; Adamowicz, L.; Tarakowski, R.; Siwek, K.; Grzywacz, T. Odor detection using an e-nose with a reduced sensor array. Sensors 2020, 20, 3542. [Google Scholar] [CrossRef]
- Misselbrook, T.H.; Hobbs, P.J.; Persaud, K.C. Use of an Electronic nose to measure odour concentration following application of cattle slurry to grassland. J. Agric. Eng. Res. 1997, 66, 213–220. [Google Scholar] [CrossRef]
- Stuetz, R.M.; Fenner, R.A.; Engin, G. Assessment of odours from sewage treatment works by an Electronic nose, H2S analysis and olfactometry. Water Res. 1999, 33, 453–461. [Google Scholar] [CrossRef]
- Nimmermark, S. Use of Electronic noses for detection of odour from animal production facilities: A review. Water Sci. Technol. 2001, 44, 33–41. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Brugger, M.; Manuzon, R.; Arnold, G.; Imerman, E. Study of air quality spatial and temporal distributions on large dairy farms in Ohio. In Proceedings of the Livestock Environment VII, Beijing, China, 18–20 May 2005; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2005; p. 188. [Google Scholar]
- Delgado-Rodríguez, M.; Ruiz-Montoya, M.; Giraldez, I.; López, R.; Madejón, E.; Díaz, M.J. Use of Electronic nose and GC-MS in detection and monitoring some VOC. Atmos. Environ. 2012, 51, 278–285. [Google Scholar] [CrossRef]
- Swe, M.M.; Eamsa-Ard, T.; Srikhirin, T.; Kerdcharoen, T. Monitoring the freshness level of beef using nanocomposite gas sensors in Electronic nose. In Proceedings of the 2019 IEEE International Conference on Consumer Electronics-Asia (ICCE-Asia), Bangkok, Thailand, 12–14 June 2019; pp. 100–103. [Google Scholar]
- Aouadi, B.; Zaukuu, J.L.Z.; Vitális, F.; Bodor, Z.; Fehér, O.; Gillay, Z.; Bazar, G.; Kovacs, Z. Historical evolution and food control achievements of near infrared spectroscopy, Electronic nose, and electronic tongue—Critical overview. Sensors 2020, 20, 5479. [Google Scholar] [CrossRef] [PubMed]
- Aunsa-Ard, W.; Pobkrut, T.; Kerdcharoen, T.; Siyang, S.; Prombaingoen, N. Development of intelligent Electronic nose for livestock industries. In Proceedings of the 2021 7th International Conference on Engineering, Applied Sciences and Technology (ICEAST), Pattaya, Thailand, 1–3 April 2021; pp. 221–225. [Google Scholar]
- Peng, S.; Zhu, J.; Liu, Z.; Hu, B.; Wang, M.; Pu, S. Prediction of Ammonia Concentration in a Pig House Based on Machine Learning Models and Environmental Parameters. Animals 2022, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Ariyakul, Y. Odor Source Localization Using Optimization Algorithm for Sensor Network with Movable Nodes. In Proceedings of the 2022 9th International Conference on Information Technology, Computer, and Electrical Engineering (ICITACEE), Semarang, Indonesia, 25–26 August 2022; pp. 159–163. [Google Scholar]
- Zhang, H.; Tai, H. Sensor Array Design for Toxic Gas Detection in Electronic nose System. In Proceedings of the 2021 IEEE 4th Advanced Information Management, Communicates, Electronic and Automation Control Conference (IMCEC), Chongqing, China, 18–20 June 2021; Volume 4, pp. 2017–2020. [Google Scholar]
- Ricci, P.P.; Gregory, O.J. Sensors for the detection of ammonia as a potential biomarker for health screening. Sci. Rep. 2021, 11, 7185. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Park, J.K.; Yun, G.H.; Choi, H.H.; Lee, H.J.; Yook, J.G. Radio-frequency/microwave gas sensors using conducting polymer. Materials 2020, 13, 2859. [Google Scholar] [CrossRef]
- Misbah, M.; Rivai, M.; Kurniawan, F. Quartz crystal microbalance based Electronic nose system implemented on Field Programmable Gate Array. TELKOMNIKA (Telecommun. Comput. Electron. Control) 2019, 17, 370–376. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Doan, T.L.L.; Prabhakaran, S.; Tran, D.T.; Kim, D.H.; Lee, J.H.; Kim, N.H. Hierarchical Co and Nb dual-doped MoS2 nanosheets shelled micro-TiO2 hollow spheres as effective multifunctional electrocatalysts for HER, OER, and ORR. Nano Energy 2021, 82, 105750. [Google Scholar] [CrossRef]
- Deshpandey, N.; Shaligram, A.D.; Botre, B.A.; Bindal, S.; Sadistap, S.S. Embedded E-nose application to sense the food grain storage condition. In Proceedings of the 2010 International Conference on Computational Intelligence and Communication Networks, Bhopal, India, 26–28 November 2010; pp. 608–611. [Google Scholar]
- Jia, W.; Liang, G.; Tian, H.; Sun, J.; Wan, C. Electronic nose-based technique for rapid detection and recognition of moldy apples. Sensors 2019, 19, 1526. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Xu, J. Applications of Electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Kumbhar, A.; Gharpure, D.C.; Botre, B.A.; Sadistap, S.S. Embedded e-nose for food inspection. In Proceedings of the 2012 1st International Symposium on Physics and Technology of Sensors (ISPTS-1), Pune, India, 8–10 March 2012; pp. 311–314. [Google Scholar]
- Jia, W.; Liang, G.; Jiang, Z.; Wang, J. Advances in Electronic nose development for application to agricultural products. Food Anal. Methods 2019, 12, 2226–2240. [Google Scholar] [CrossRef]
- Bakiler, H.; Güney, S. Estimation of concentration values of different gases based on long short-term memory by using Electronic nose. Biomed. Signal Process. Control 2021, 69, 102908. [Google Scholar] [CrossRef]
- Wongrat, E.; Nuengnit, T.; Panyathip, R.; Chanlek, N.; Hongsith, N.; Choopun, S. Highly selective room temperature ammonia sensors based on ZnO nanostructures decorated with graphene quantum dots (GQDs). Sens. Actuators B Chem. 2021, 326, 128983. [Google Scholar] [CrossRef]
- Supchocksoonthorn, P.; Thongsai, N.; Moonmuang, H.; Kladsomboon, S.; Jaiyong, P.; Paoprasert, P. Label-free carbon dots from black sesame seeds for real-time detection of ammonia vapor via optical Electronic nose and density functional theory calculation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 118–128. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.; Aheto, J.H.; Osae, R. Application of Electronic nose as a non-invasive technique for odor fingerprinting and detection of bacterial foodborne pathogens: A review. J. Food Sci. Technol. 2020, 57, 1977–1990. [Google Scholar] [CrossRef]
- Edita, R.; Darius, G.; Vinauskienė, R.; Eisinaitė, V.; Balčiūnas, G.; Dobilienė, J.; Tamkutė, L. Rapid evaluation of fresh chicken meat quality by Electronic nose. Czech J. Food Sci. 2018, 36, 420–426. [Google Scholar]
- Madhaiyan, G.; Sun, A.T.; Zan, H.W.; Meng, H.F.; Horng, S.F.; Chen, L.Y.; Hung, H.W. Solution-processed chloroaluminum phthalocyanine (ClAlPc) ammonia gas sensor with vertical organic porous diodes. Sensors 2021, 21, 5783. [Google Scholar] [CrossRef]
- Illahi, A.A.C.; Dadios, E.P.; Bandala, A.A.; Vicerra, R.R.P.; Sybingco, E. Automatic Harmful Gas Detection Using Electronic nose Technology. In Proceedings of the 2021 IEEE 13th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management (HNICEM), Manila, Philippines, 28–30 November 2021; pp. 1–4. [Google Scholar]
- Yang, Y.; Qian, M.C.; Deng, Y.; Yuan, H.; Jiang, Y. Insight into aroma dynamic changes during the whole manufacturing process of chestnut-like aroma green tea by combining GC-E-Nose, GC-IMS, and GC× GC-TOFMS. Food Chem. 2022, 387, 132813. [Google Scholar] [CrossRef]
- Weng, X.; Kong, C.; Jin, H.; Chen, D.; Li, C.; Li, Y.; Ren, L.; Xiao, Y.; Chang, Z. Detection of Volatile Organic Compounds (VOCs) in livestock houses based on Electronic nose. Appl. Sci. 2021, 11, 2337. [Google Scholar] [CrossRef]
- Ma, P.; Hu, N.; Ruan, J.; Song, H.; Chen, X. In-situ measurement of ammonium in wastewater using a tilted fiber grating sensor. J. Light. Technol. 2021, 39, 4055–4061. [Google Scholar] [CrossRef]
- Hobbs, P.J.; Misselbrook, T.H.; Pain, B.F. Assessment of odours from livestock wastes by a photoionization detector, an Electronic nose, olfactometry and gas chromatography-mass spectrometry. J. Agric. Eng. Res. 1995, 60, 137–144. [Google Scholar] [CrossRef]

| Method Name | Description | Reference |
|---|---|---|
| Calorimetric Methods | Setup: Chemical reaction between ammonia and a reagent, produces a measurable change in heat. | [28] |
| Range: 1–100 ppm or even lower | ||
| Sensor: Rely on the calorimeter or other heat-sensing device to measure the heat change. | ||
| Electrochemical | Setup: Different types of electrodes. | [35] |
| Range: 1 to 100 ppm | ||
| Sensor: Glassy carbon, gold, and platinum electrodes | ||
| Electrochemical | Setup: multi-gas detection system using different sensor arrays. | [36] |
| Range: 0.0904 ppm | ||
| Sensor: MQ-136, MQ-137, TGS-2611 | ||
| Electrochemical | Setup: screen-printed electrode (SPE). | [33] |
| Range: 50 ppm | ||
| Titration Methods | Setup: Titration methods for ammonia detection typically involve the use of a reagent that reacts with ammonia to produce a visible change in color or pH. | [27] |
| Range: 0.1 ppm to 100 ppm | ||
| Sensor: Rely on the reagent and titration equipment to measure the change in color or pH resulting from the chemical reaction with ammonia | ||
| Ion Selective Electodes (ISE) | Setup: Involve the use of an ammonia-specific membrane. The membrane generates an electrical potential which can be measured using an electrode. | [37] |
| Range: 1–100 ppm or 10–500 ppm | ||
| Sensor: Ammonia-specific membrane and an electrode to detect and measure the concentration of ammonia ions in a solution. | ||
| Gas Sensitive Tubes | Setup: Typically use a tube filled with a reactive substance that changes color or produces a visible reaction in the presence of ammonia. The tube is placed in the path of a gas stream and the concentration of ammonia can be estimated by measuring the length of the tube that has changed color or undergone a reaction. | [38] |
| Range: 1–100 ppm or 10–500 ppm | ||
| Sensor: Rely on a reactive substance contained within the tube to produce a visible reaction in the presence of ammonia. | ||
| Photo-acoustic | Setup: The continuous detection of trace ammonia in the air and the sub-ppb level are realized. | [55] |
| Range: 32 parts per trillion (ppt) | ||
| Sensor: Photoacoustic spectroscopy, TGA300, TGA310, TGA320 | ||
| Photo-acoustic | Setup: Depending on laser PAS, a portable ammonium analyzer has been designed and tested.A single-mode CO2 laser was employed, and for the auditory sensing, a resonant configuration’s high sensitivity was completely used. | [56] |
| Range: 0.1ppb to 3 ppm | ||
| Sensor: Laser Photoacoustic Spectroscopy | ||
| Photo-acoustic | Setup: Detection of ammonia gas in the agricultural environment. | [57] |
| Range: up to 8 ppm | ||
| Sensor: Laser Photoacoustic Spectroscopy, Diode Laser | ||
| Photo-acoustic | Setup: Laboratory Conditions | [58] |
| Range: 0.1 to 20 ppm | ||
| Sensor: Microphone | ||
| Photo-acoustic | Setup: Light Source, Sample holder, Acoustic detector, Signal amplifier and processing | [59] |
| Range: 2 ppm | ||
| Sensor: Interferometer | ||
| Spectrophotometry | Setup: Silicone Force Sensor (SXTSC1) | [68] |
| Range: 0.1 to 2 ppm and extended upto 5 ppm | ||
| Sensor: UV-VIS spectrophotometry | ||
| Sampling and Laboratory analysis | Setup: For ammonia detection in air or water, samples are collected and analyzed in a lab using methods like titration or ion selective electrodes. | [69] |
| Range Ammonia detection ranges vary (0.1–100 ppm) due to different methods and sensors. | ||
| Sensor: pH sensors, Ion selective electrodes (ISEs), Mass spectrometry detectors, Colorimetric sensors, Fluorescence sensors. |
| Sensor Type | Principle | Advantages | Disadvantages |
|---|---|---|---|
| MOS | Change in electrical resistance when exposed to gases absorption of infrared light by gases, including ammonia | Cost-effective; Compact size; Relatively fast response time; Suitable for continuous monitoring of ammonia levels in farmland | Limited sensitivity; susceptible to environmental factors and interfering gases; requires periodic recalibration |
| Electrochemical | Electrochemical reaction of ammonia gas with an electrode | High sensitivity; Good selectivity; Wide measurement range; Relatively low power consumption; Real-time data on ammonia concentration in the surrounding air; commonly used for gas detection | Limited lifespan; sensitivity to temperature and humidity variations; Requires periodic calibration and maintenance |
| PID | Ionization of gas molecules by ultraviolet light | High sensitivity; fast response time; capable of detecting low concentrations; can detect a wide range of volatile organic compounds (VOCs) | Higher cost compared to other sensors; potential interference from other gases; requires periodic calibration. |
| NDIR | Absorption of infrared light by gases, including ammonia | High accuracy; Good selectivity; Long lifespan; Stable and reliable performance | Higher cost compared to some other sensors; Slower response time; Larger physical size |
| WSN | Multiple sensor nodes interconnected wirelessly | Remote monitoring capability; Real-time data collection; scalability; Flexibility in sensor placement; Potential for comprehensive coverage | Costly infrastructure setup; potential for signal interference; complex data management and analysis; requires power and communication resources. |
| A: Sensor: | ||||
|---|---|---|---|---|
| Cost | Speed | Accuracy | Reference | |
| MOS | Low-cost | Fast | Depend | [109] |
| ES | Vary in cost | Fast | Accurate | [108] |
| PID | More expensive | Fast | Accurate | [96] |
| NDIR | More expensive | Slow | High Accuracy | [42] |
| WSN | Higher upfront costs | Fast | High accuracy | [97] |
| B: Method: | ||||
| Cost | Speed | Accuracy | Reference | |
| PCA | NO additional costs | Fast | Accurate | [98] |
| ANN | Vary in cost | Depend | High Accuracy | [99] |
| SVM | Cost-effective | Fast | High Accuracy | [100] |
| AOS | Vary in cost | Depend | High Accuracy | [97] |
| FL | NO additional costs | Fast | Accurate | [93] |
| PR | NO specific costs | Fast | Depend | [101,102] |
| LSTM | Vary in cost | Slower | Depend | [103] |
| PLS | NO additional costs | Fast | Accurate | [104] |
| LDA | NO additional costs | Fast | Accurate | [105] |
| Advantages | E-Nose | Traditional Methods |
|---|---|---|
| Sensitivity | High detection limits in the low ppm or even ppb range | Highly selective for ammonia |
| Precision and Accuracy | Precise and accurate measurements | Familiarity and wide adoption |
| Real-time Monitoring and Rapid Results | Provides real time monitoring and quick results | Easier to implement and validate |
| Portability and Ease of Use | Very portable and easy to use | High accuracy and precision in ammonia measurement |
| Potential Selectivity towards Specific Analytes | Including ammonia | Potentially reducing interference from other compounds in the sample |
| Disadvantages | E-Nose | Traditional Methods |
| Sensor Limitations | It has sensor limitations | Require complex sample preparation steps |
| Cost | E-nose devices can have varying costs depending on the complexity of the sensor array | Longer analysis times compared to E-nose |
| Standardization | Lack standardized methods or regulatory guidelines for ammonia measurement | Require specialized equipment, reagents, and skilled personnel for operation and maintenance |
| Application Range | Limited | Less portable compared to E-nose devices |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moshayedi, A.J.; Sohail Khan, A.; Hu, J.; Nawaz, A.; Zhu, J. E-Nose-Driven Advancements in Ammonia Gas Detection: A Comprehensive Review from Traditional to Cutting-Edge Systems in Indoor to Outdoor Agriculture. Sustainability 2023, 15, 11601. https://doi.org/10.3390/su151511601
Moshayedi AJ, Sohail Khan A, Hu J, Nawaz A, Zhu J. E-Nose-Driven Advancements in Ammonia Gas Detection: A Comprehensive Review from Traditional to Cutting-Edge Systems in Indoor to Outdoor Agriculture. Sustainability. 2023; 15(15):11601. https://doi.org/10.3390/su151511601
Chicago/Turabian StyleMoshayedi, Ata Jahangir, Amir Sohail Khan, Jiandong Hu, Abdullah Nawaz, and Jianxiong Zhu. 2023. "E-Nose-Driven Advancements in Ammonia Gas Detection: A Comprehensive Review from Traditional to Cutting-Edge Systems in Indoor to Outdoor Agriculture" Sustainability 15, no. 15: 11601. https://doi.org/10.3390/su151511601
APA StyleMoshayedi, A. J., Sohail Khan, A., Hu, J., Nawaz, A., & Zhu, J. (2023). E-Nose-Driven Advancements in Ammonia Gas Detection: A Comprehensive Review from Traditional to Cutting-Edge Systems in Indoor to Outdoor Agriculture. Sustainability, 15(15), 11601. https://doi.org/10.3390/su151511601








