Green Energy Generated in Single-Chamber Microbial Fuel Cells Using Tomato Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of scMFCs
2.2. Collection and Preparation of Tomato Waste
2.3. Characterization of the scMFC
2.4. Isolation and Molecular Identification of Anode Microorganisms
2.4.1. Isolation of Bacteria
2.4.2. Yeast Isolation
2.4.3. Molecular Identification
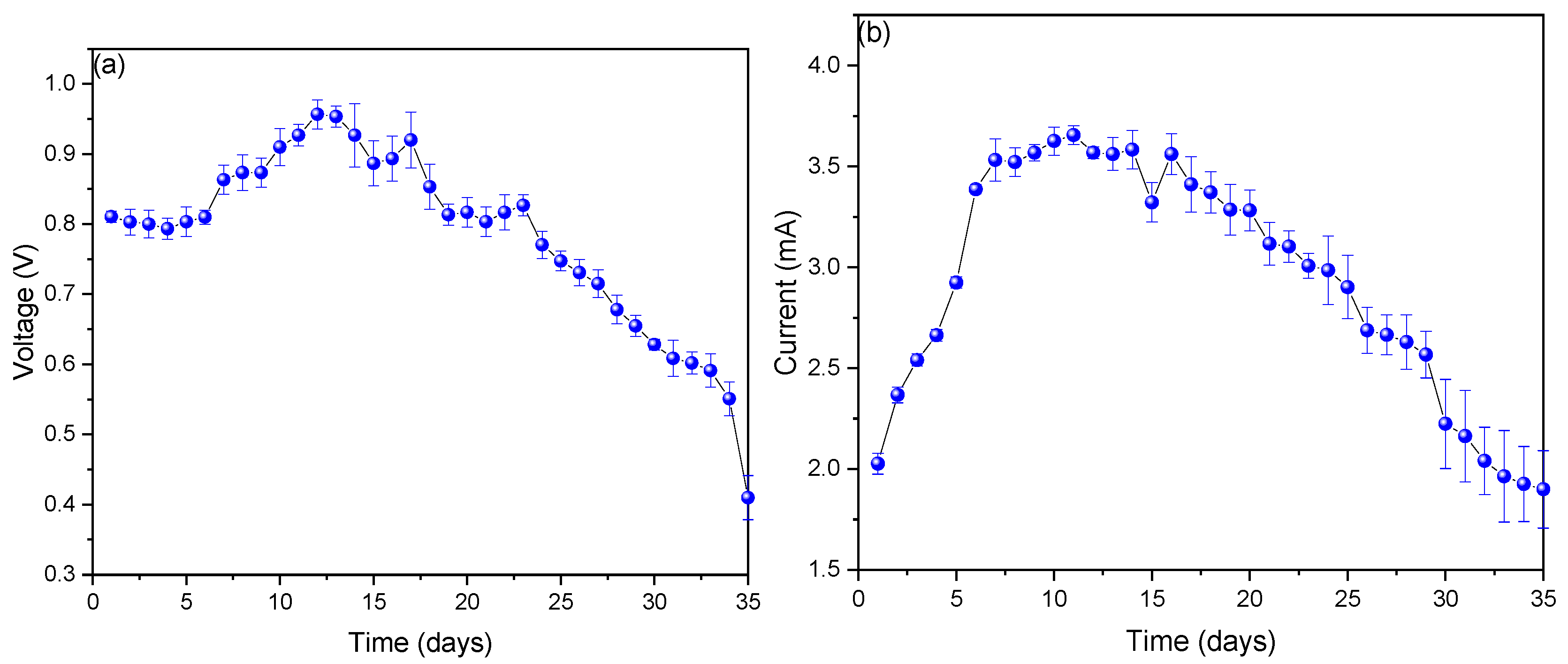
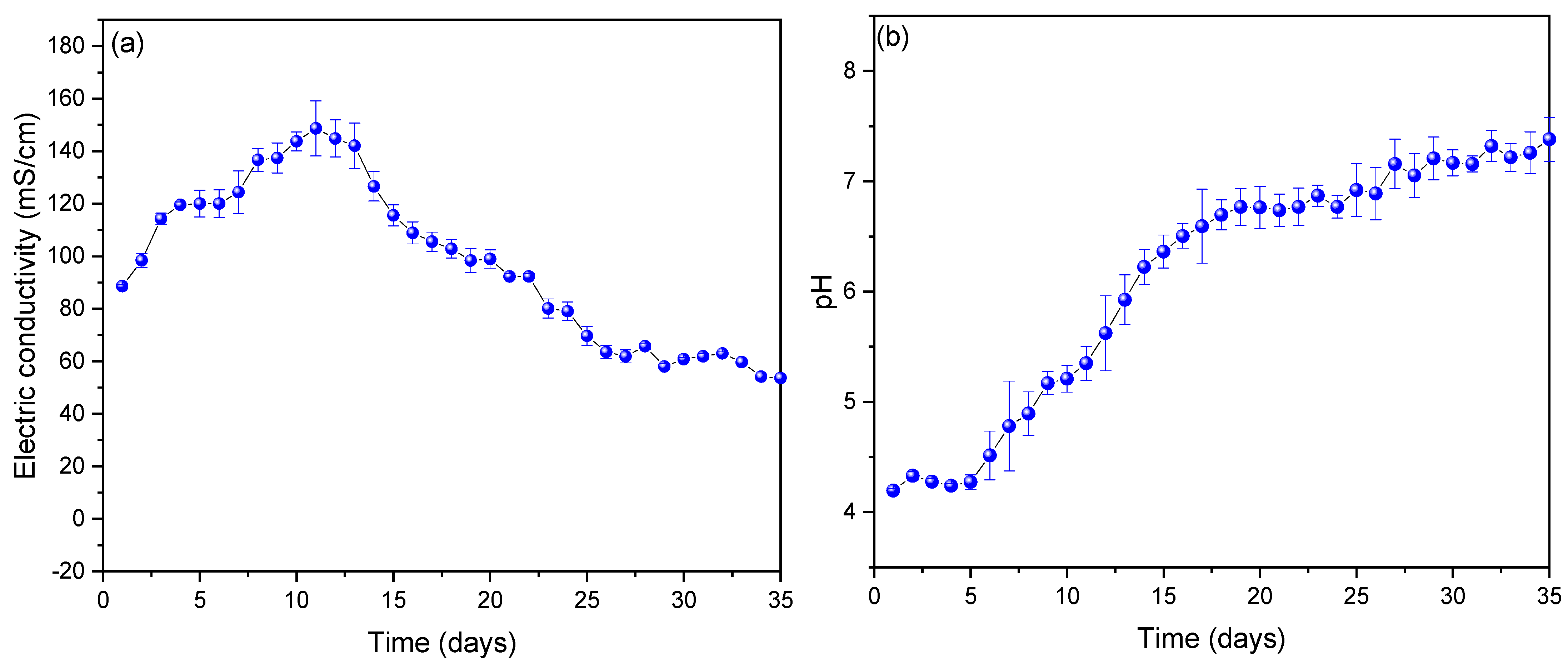
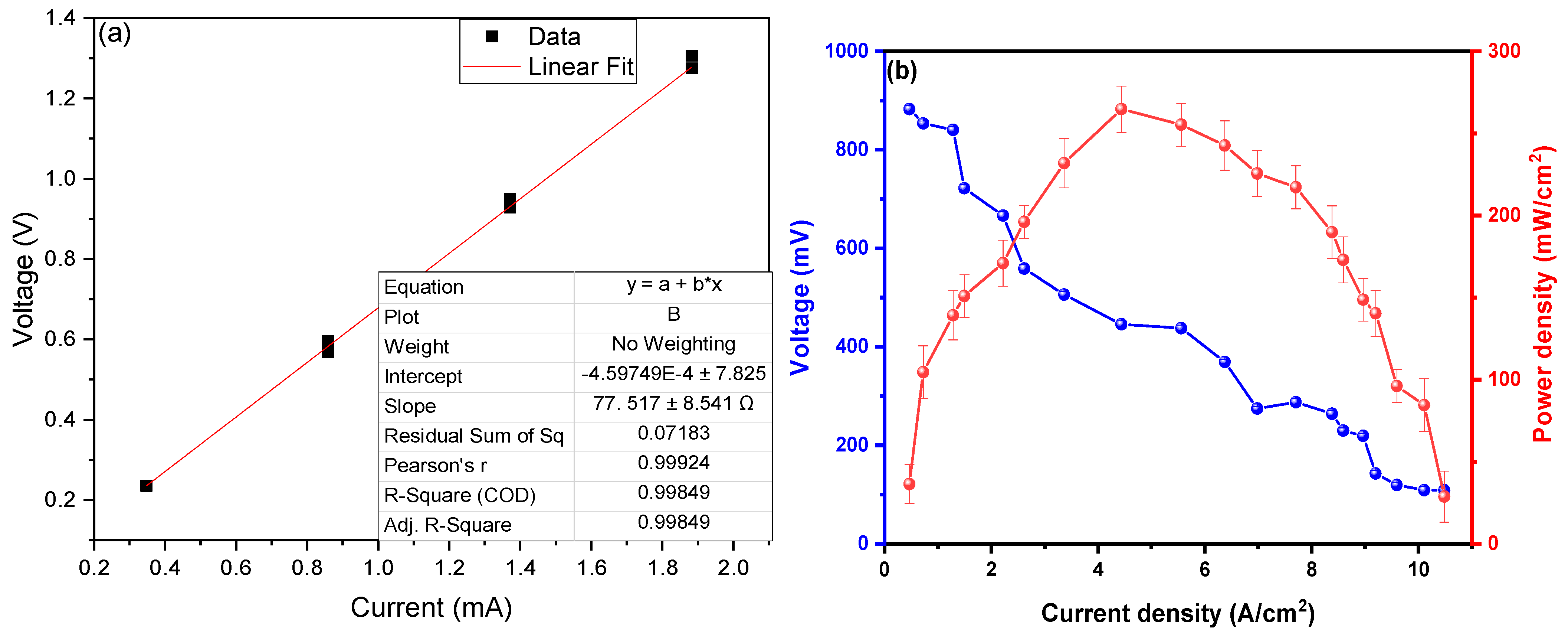
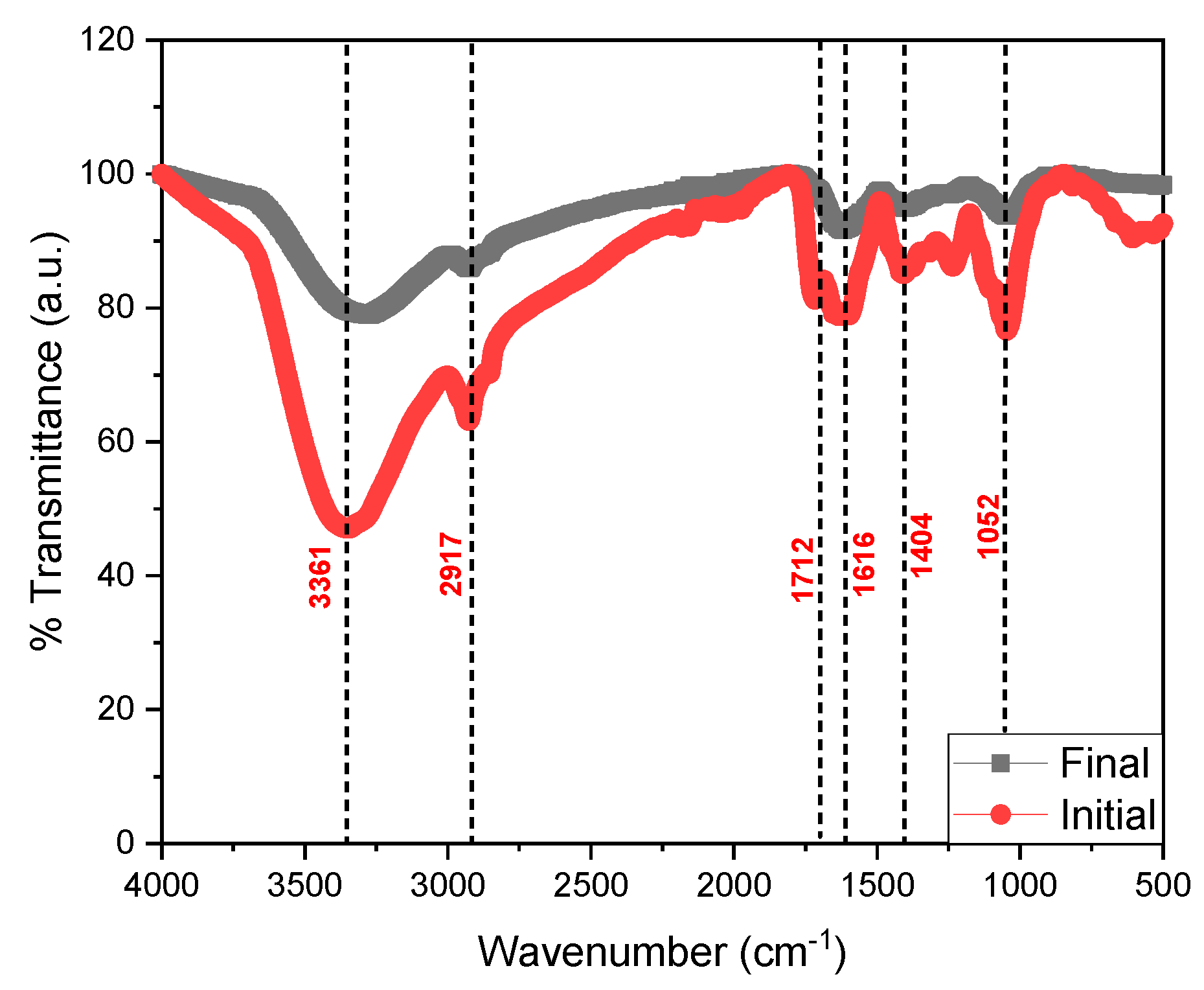
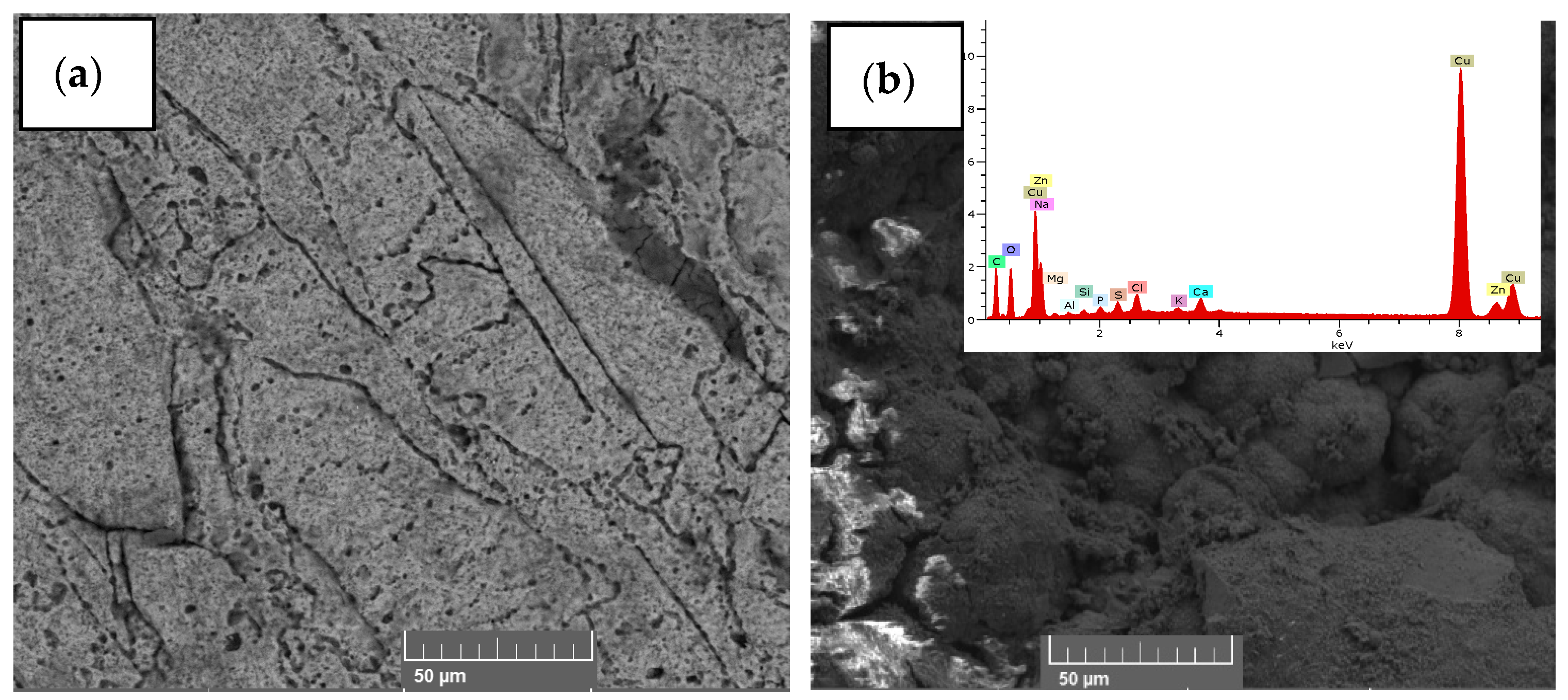
3. Results and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Opoku, R.; Obeng, G.Y.; Osei, L.K.; Kizito, J.P. Optimization of industrial energy consumption for sustainability using time-series regression and gradient descent algorithm based on historical electricity consumption data. Sustain. Anal. Model. 2022, 2, 100004. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Ma, G.; Chen, X.; Fan, J.; Yang, B. Prediction of electricity consumption during epidemic period based on improved particle swarm optimization algorithm. Energy Rep. 2022, 8, 437–446. [Google Scholar] [CrossRef]
- Ramos, D.; Faria, P.; Vale, Z.; Mourinho, J.; Correia, R. Industrial Facility Electricity Consumption Forecast Using Artificial Neural Networks and Incremental Learning. Energies 2020, 13, 4774. [Google Scholar] [CrossRef]
- vom Scheidt, F.; Medinová, H.; Ludwig, N.; Richter, B.; Staudt, P.; Weinhardt, C. Data analytics in the electricity sector–A quantitative and qualitative literature review. Energy AI 2020, 1, 100009. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Nazario-Naveda, R.; Benites, S.M.; Delfín-Narciso, D.; Rojas-Villacorta, W.; Romero, C.V. Bioelectricity through microbial fuel cells using avocado waste. Energy Rep. 2022, 8, 376–382. [Google Scholar] [CrossRef]
- Masebinu, R.; Kambule, N. Electricity consumption data of a middle-income household in Gauteng, South Africa: Pre and Post COVID-19 lockdown (2019–2021). Data Brief 2022, 43, 108341. [Google Scholar] [CrossRef]
- Hadjout, D.; Torres, J.; Troncoso, A.; Sebaa, A.; Martínez-Álvarez, F. Electricity consumption forecasting based on ensemble deep learning with application to the Algerian market. Energy 2022, 243, 123060. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Yen, H.-W.; Nomanbhay, S.; Ho, Y.-C.; Show, P.L. Transformation of Biomass Waste into Sustainable Organic Fertilizers. Sustainability 2019, 11, 2266. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.; et al. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef]
- Ahuja, I.; Dauksas, E.; Remme, J.F.; Richardsen, R.; Løes, A.-K. Fish and fish waste-based fertilizers in organic farming–With status in Norway: A review. Waste Manag. 2020, 115, 95–112. [Google Scholar] [CrossRef]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.D.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste: A review of operational parameters and their impact on process performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef] [PubMed]
- Ronga, D.; Caradonia, F.; Parisi, M.; Bezzi, G.; Parisi, B.; Allesina, G.; Pedrazzi, S.; Francia, E. Using Digestate and Biochar as Fertilizers to Improve Processing Tomato Production Sustainability. Agronomy 2020, 10, 138. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone, E. Valorization of Tomato Waste as a Source of Carotenoids. Molecules 2021, 26, 5062. [Google Scholar] [CrossRef] [PubMed]
- Løvdal, T.; Van Droogenbroeck, B.; Eroglu, E.C.; Kaniszewski, S.; Agati, G.; Verheul, M.; Skipnes, D. Valorization of Tomato Surplus and Waste Fractions: A Case Study Using Norway, Belgium, Poland, and Turkey as Examples. Foods 2019, 8, 229. [Google Scholar] [CrossRef]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as Potential Source of Natural Additives for Meat Industry. A Review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef]
- Christwardana, M.; Hadiyanto, H.; Motto, S.A.; Sudarno, S.; Haryani, K. Performance evaluation of yeast-assisted microalgal microbial fuel cells on bioremediation of cafeteria wastewater for electricity generation and microalgae biomass production. Biomass Bioenergy 2020, 139, 105617. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Al-Zaqri, N.; Yaakop, A.S.; Umar, K. Potato waste as an effective source of electron generation and bioremediation of pollutant through benthic microbial fuel cell. Sustain. Energy Technol. Assess. 2022, 53, 102560. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rodríguez-Couto, S. Development and modification of materials to build cost-effective anodes for microbial fuel cells (MFCs): An overview. Biochem. Eng. J. 2020, 164, 107779. [Google Scholar] [CrossRef]
- Prathiba, S.; Kumar, P.S.; Vo, D.-V.N. Recent advancements in microbial fuel cells: A review on its electron transfer mechanisms, microbial community, types of substrates and design for bio-electrochemical treatment. Chemosphere 2022, 286, 131856. [Google Scholar] [CrossRef]
- Gul, H.; Raza, W.; Lee, J.; Azam, M.; Ashraf, M.; Kim, K.-H. Progress in microbial fuel cell technology for wastewater treatment and energy harvesting. Chemosphere 2021, 281, 130828. [Google Scholar] [CrossRef] [PubMed]
- Asefi, B.; Li, S.-L.; Moreno, H.A.; Sanchez-Torres, V.; Hu, A.; Li, J.; Yu, C.-P. Characterization of electricity production and microbial community of food waste-fed microbial fuel cells. Process. Saf. Environ. Prot. 2019, 125, 83–91. [Google Scholar] [CrossRef]
- Mohamed, S.N.; Hiraman, P.A.; Muthukumar, K.; Jayabalan, T. Bioelectricity production from kitchen wastewater using microbial fuel cell with photosynthetic algal cathode. Bioresour. Technol. 2020, 295, 122226. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Guerrero–Barajas, C.; Ibrahim, M.N.M.; Umar, K.; Yaakop, A.S. Local fruit wastes driven benthic microbial fuel cell: A sustainable approach to toxic metal removal and bioelectricity generation. Environ. Sci. Pollut. Res. 2022, 29, 32913–32928. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Mohanakrishna, G.; Kumar, A.; Lai, C.; Lee, J.-K.; Kalia, V.C. Exploitation of Citrus Peel Extract as a Feedstock for Power Generation in Microbial Fuel Cell (MFC). Indian J. Microbiol. 2019, 59, 476–481. [Google Scholar] [CrossRef]
- Segundo, R.-F.; De La Cruz-Noriega, M.; Nazario-Naveda, R.; Benites, S.M.; Delfín-Narciso, D.; Angelats-Silva, L.; Díaz, F. Golden Berry Waste for Electricity Generation. Fermentation 2022, 8, 256. [Google Scholar] [CrossRef]
- Zafar, H.; Peleato, N.; Roberts, D. A comparison of reactor configuration using a fruit waste fed two-stage anaerobic up-flow leachate reactor microbial fuel cell and a single-stage microbial fuel cell. Bioresour. Technol. 2023, 374, 128778. [Google Scholar] [CrossRef]
- Rahman, W.; Yusup, S.; Mohammad, S.A. Screening of fruit waste as substrate for microbial fuel cell (MFC). In AIP Conference Proceedings; AIP Publishing LLC: Arau, Malaysia, 2021; Volume 2332, p. 020003. [Google Scholar]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Cabanillas-Chirinos, L.; Nazario-Naveda, R.; Gallozzo-Cardenas, M.; Diaz, F.; Murga-Torres, E. Potential Use of Coriander Waste as Fuel for the Generation of Electric Power. Sustainability 2023, 15, 896. [Google Scholar] [CrossRef]
- Prasidha, W. Electricity Production from Food Waste Leachate (Fruit and Vegetable Waste) using Double Chamber Microbial Fuel Cell: Comparison between Non-aerated and Aerated Configuration. ROTASI 2020, 22, 162–168. [Google Scholar]
- Naveenkumar, M.; Senthilkumar, K. Microbial fuel cell for harvesting bio-energy from tannery effluent using metal mixed biochar electrodes. Biomass Bioenergy 2021, 149, 106082. [Google Scholar] [CrossRef]
- Aiyer, K.S. How does electron transfer occur in microbial fuel cells? World J. Microbiol. Biotechnol. 2020, 36, 19. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.; Lee, S.-M.; Oh, S.-E.; Kim, E.J.; Hwang, Y.; Seo, D.; Kim, J.Y.; Kahng, Y.H.; Lee, Y.W.; Chung, S.-Y.; et al. Addition of reduced graphene oxide to an activated-carbon cathode increases electrical power generation of a microbial fuel cell by enhancing cathodic performance. Electrochim. Acta 2019, 297, 613–622. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Serrà, A.; Ibrahim, M.N.M.; Yaakop, A.S. Self-assembled oil palm biomass-derived modified graphene oxide anode: An efficient medium for energy transportation and bioremediating Cd (II) via microbial fuel cells. Arab. J. Chem. 2021, 14, 103121. [Google Scholar] [CrossRef]
- Hung, Y.-H.; Liu, T.-Y.; Chen, H.-Y. Renewable Coffee Waste-Derived Porous Carbons as Anode Materials for High-Performance Sustainable Microbial Fuel Cells. ACS Sustain. Chem. Eng. 2019, 7, 16991–16999. [Google Scholar] [CrossRef]
- Guan, C.-Y.; Tseng, Y.-H.; Tsang, D.C.; Hu, A.; Yu, C.-P. Wetland plant microbial fuel cells for remediation of hexavalent chromium contaminated soils and electricity production. J. Hazard. Mater. 2019, 365, 137–145. [Google Scholar] [CrossRef]
- Yaqoob, A.Y.; Hussian, N.A.; Alhejuje, M.M. Application of Heavy metals pollution index on two types of constructed wetland. Marsh Bull. 2022, 17, 22–29. [Google Scholar]
- Sreelekshmy, B.R.; Basheer, R.; Sivaraman, S.; Vasudevan, V.; Elias, L.; Shibli, S.M.A. Sustainable electric power generation from live anaerobic digestion of sugar industry effluents using microbial fuel cells. J. Mater. Chem. A 2020, 8, 6041–6056. [Google Scholar] [CrossRef]
- Iigatani, R.; Ito, T.; Watanabe, F.; Nagamine, M.; Suzuki, Y.; Inoue, K. Electricity generation from sweet potato-shochu waste using microbial fuel cells. J. Biosci. Bioeng. 2019, 128, 56–63. [Google Scholar] [CrossRef]
- Priya, A.D.; Setty, Y.P. Cashew apple juice as substrate for microbial fuel cell. Fuel 2019, 246, 75–78. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Ntaikou, I.; Pastore, C.; di Bitonto, L.; Bebelis, S.; Lyberatos, G. An overall perspective for the energetic valorization of household food waste using microbial fuel cell technology of its extract, coupled with anaerobic digestion of the solid residue. Appl. Energy 2019, 242, 1064–1073. [Google Scholar] [CrossRef]
- Daud, N.N.M.; Ahmad, A.; Yaqoob, A.A.; Ibrahim, M.N.M. Application of rotten rice as a substrate for bacterial species to generate energy and the removal of toxic metals from wastewater through microbial fuel cells. Environ. Sci. Pollut. Res. 2021, 28, 62816–62827. [Google Scholar] [CrossRef] [PubMed]
- Simeon, M.I.; Asoiro, F.U.; Aliyu, M.; Raji, O.A.; Freitag, R. Polarization and power density trends of a soil-based microbial fuel cell treated with human urine. Int. J. Energy Res. 2020, 44, 5968–5976. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; Nazario-Naveda, R.; Benites, S.M.; Gallozzo-Cardenas, M.; Delfín-Narciso, D.; Díaz, F. Use of Pineapple Waste as Fuel in Microbial Fuel Cell for the Generation of Bioelectricity. Molecules 2022, 27, 7389. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Bin Abu Bakar, M.A.; Kim, H.-C.; Ahmad, A.; Alshammari, M.B.; Yaakop, A.S. Oxidation of food waste as an organic substrate in a single chamber microbial fuel cell to remove the pollutant with energy generation. Sustain. Energy Technol. Assess. 2022, 52, 102282. [Google Scholar] [CrossRef]
- Du, H.; Shao, Z. Synergistic effects between solid potato waste and waste activated sludge for waste-to-power conversion in microbial fuel cells. Appl. Energy 2022, 314, 118994. [Google Scholar] [CrossRef]
- Kamperidis, T.; Pandis, P.K.; Argirusis, C.; Lyberatos, G.; Tremouli, A. Effect of Food Waste Condensate Concentration on the Performance of Microbial Fuel Cells with Different Cathode Assemblies. Sustainability 2022, 14, 2625. [Google Scholar] [CrossRef]
- Segundo, R.-F.; Magaly, D.L.C.-N.; Benites, S.M.; Daniel, D.-N.; Angelats-Silva, L.; Díaz, F.; Luis, C.-C.; Fernanda, S.-P. Increase in Electrical Parameters Using Sucrose in Tomato Waste. Fermentation 2022, 8, 335. [Google Scholar] [CrossRef]
- Rehal, J.K.; Aggarwal, P.; Dhaliwal, I.; Sharma, M.; Kaushik, P. A Tomato Pomace Enriched Gluten-Free Ready-to-Cook Snack’s Nutritional Profile, Quality, and Shelf Life Evaluation. Horticulturae 2022, 8, 403. [Google Scholar] [CrossRef]
- Ramya, S.; Chandran, M.; King, I.J.; Jayakumararaj, R.; Loganathan, T.; Pandiarajan, G.; Kaliraj, P.; Pushpalatha, G.G.L.; Abraham, G.; Vijaya, V.; et al. Phytochemical Screening, GCMS and FTIR Profile of Bioactive Compounds in Solanum lycopersicum Wild Fruits collected from Palani Hill Ranges of the Western Ghats. J. Drug Deliv. Ther. 2022, 12, 56–64. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Xi, H.; Yu, Y.; Song, Y.; Wu, C.; Zhou, Y. Evaluation methods of inhibition to microorganisms in biotreatment processes: A review. Water Cycle 2023, 4, 70–78. [Google Scholar] [CrossRef]
- Su, F.; Wang, F.; Zhang, C.; Lu, T.; Zhang, S.; Zhang, R.; Qi, X.; Liu, P. Ameliorating substance accessibility for microorganisms to amplify toluene degradation and power generation of microbial fuel cell by using activated carbon anode. J. Clean. Prod. 2022, 377, 134481. [Google Scholar] [CrossRef]
- Nawaz, A.; Haq, I.U.; Qaisar, K.; Gunes, B.; Raja, S.I.; Mohyuddin, K.; Amin, H. Microbial fuel cells: Insight into simultaneous wastewater treatment and bioelectricity generation. Process. Saf. Environ. Prot. 2022, 161, 357–373. [Google Scholar] [CrossRef]
- Hou, B.; Liu, X.; Zhang, R.; Li, Y.; Liu, P.; Lu, J. Investigation and evaluation of membrane fouling in a microbial fuel cell-membrane bioreactor systems (MFC-MBR). Sci. Total Environ. 2022, 814, 152569. [Google Scholar] [CrossRef]
- Hirose, S.; Inukai, K.; Nguyen, D.T.; Taguchi, K. Use of loofah electrodes coated with rice husk smoked charcoal and Japanese ink in a microbial fuel cell for muddy water treatment. Energy Rep. 2023, 9, 160–167. [Google Scholar] [CrossRef]
- Mbugua, J.K.; Mbui, D.N.; Mwaniki, J.M.; Mwaura, F.B. Electricity Generation by Clostridiumspp and Proteus Vulgaris from Rotten Tomatoes in a Double Chamber Microbial Fuel Cell. Int. J. Res. Stud. Microbiol. Biotechnol. 2018, 4, 32–33. [Google Scholar]
- Heard, G.M.; Fleet, G.H. CANDIDA|Yarrowia (Candida) Lipolytica. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 360–365. ISBN 9780122270703. [Google Scholar]
- Logan, B.E.; Regan, J.M. Electricity-Producing Bacterial Communities in Microbial Fuel Cells. Trends Microbiol. 2006, 14, 512–518. [Google Scholar] [CrossRef]
- Gatti, M.N.; Milocco, R.H. A Biofilm Model of Microbial Fuel Cells for Engineering Applications. Int. J. Energy Environ. Eng. 2017, 8, 303–315. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; Cabanillas-Chirinos, L.; Nazario-Naveda, R.; Gallozzo-Cardenas, M.; Diaz, F.; Delfin-Narciso, D.; Rojas-Villacorta, W. Use of Tangerine Waste as Fuel for the Generation of Electric Current. Sustainability 2023, 15, 3559. [Google Scholar] [CrossRef]
- Greenman, J.; Gajda, I.; You, J.; Mendis, B.A.; Obata, O.; Pasternak, G.; Ieropoulos, I. Microbial Fuel Cells and Their Electrified Biofilms. Biofilm 2021, 3, 100057. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electricigens in the Anode of Microbial Fuel Cells: Pure Cultures versus Mixed Communities. Microb. Cell Fact. 2019, 18, 39. [Google Scholar] [CrossRef]
- Park, D.H.; Zeikus, J.G. Electricity Generation in Microbial Fuel Cells Using Neutral Red as an Electronophore. Appl. Environ. Microbiol. 2000, 66, 1292–1297. [Google Scholar] [CrossRef]
- Kim, N.J.; Choe, Y.J.; Jeong, S.H.; Kim, S.H. Development of Microbial Fuel Cells Using Proteus Vulgaris. Bull. Korean Chem. Soc. 2000, 21, 44–48. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, V. Recent Trends in Upgrading the Performance of Yeast as Electrode Biocatalyst in Microbial Fuel Cells. Chemosphere 2021, 284, 131383. [Google Scholar] [CrossRef] [PubMed]
- Rojas Flores, S.; Pérez-Delgado, O.; Naveda-Renny, N.; Benites, S.M.; De La Cruz-Noriega, M.; Delfin Narciso, D.A. Generation of Bioelectricity Using Molasses as Fuel in Microbial Fuel Cells. Environ. Res. Eng. Manag. 2022, 78, 19–27. [Google Scholar] [CrossRef]
- Gunawardena, A.; Fernando, S.; To, F. Performance of a Yeast-Mediated Biological Fuel Cell. Int. J. Mol. Sci. 2008, 9, 1893–1907. [Google Scholar] [CrossRef] [PubMed]



| ID Sample | BLAST Characterization | Length of Consensus Sequence (nt) | % Identity | Accession Number | Phylogeny |
|---|---|---|---|---|---|
| Bacteria | |||||
| MT-B01 | Proteus vulgaris | 1467 | 100.00 | CP023965.1 | Cellular organisms; Bacteria; Proteobacteria; Gammaproteobacteria; Enterobacterales; Morganellaceae; Proteus |
| MT-B02 | Proteus vulgaris | 1466 | 100.00 | CP023965.1 | Cellular organisms; Bacteria; Proteobacteria; Gammaproteobacteria; Enterobacterales; Morganellaceae; Proteus |
| Yeast | |||||
| MT-L01 | Yarrowia lipolytica | 368 | 100.00 | MN124085.1 | Cellular organisms; Eukaryota; Opisthokonta; Fungi; Dikarya; Ascomycota; saccharomyceta; Saccharomycotina; Saccharomycetes; Saccharomycetales; Dipodascaceae; Yarrowia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Flores, S.; De La Cruz-Noriega, M.; Cabanillas-Chirinos, L.; Benites, S.M.; Nazario-Naveda, R.; Delfín-Narciso, D.; Gallozzo-Cardenas, M.; Diaz, F.; Murga-Torres, E.; Rojas-Villacorta, W. Green Energy Generated in Single-Chamber Microbial Fuel Cells Using Tomato Waste. Sustainability 2023, 15, 10461. https://doi.org/10.3390/su151310461
Rojas-Flores S, De La Cruz-Noriega M, Cabanillas-Chirinos L, Benites SM, Nazario-Naveda R, Delfín-Narciso D, Gallozzo-Cardenas M, Diaz F, Murga-Torres E, Rojas-Villacorta W. Green Energy Generated in Single-Chamber Microbial Fuel Cells Using Tomato Waste. Sustainability. 2023; 15(13):10461. https://doi.org/10.3390/su151310461
Chicago/Turabian StyleRojas-Flores, Segundo, Magaly De La Cruz-Noriega, Luis Cabanillas-Chirinos, Santiago M. Benites, Renny Nazario-Naveda, Daniel Delfín-Narciso, Moisés Gallozzo-Cardenas, Félix Diaz, Emzon Murga-Torres, and Walter Rojas-Villacorta. 2023. "Green Energy Generated in Single-Chamber Microbial Fuel Cells Using Tomato Waste" Sustainability 15, no. 13: 10461. https://doi.org/10.3390/su151310461
APA StyleRojas-Flores, S., De La Cruz-Noriega, M., Cabanillas-Chirinos, L., Benites, S. M., Nazario-Naveda, R., Delfín-Narciso, D., Gallozzo-Cardenas, M., Diaz, F., Murga-Torres, E., & Rojas-Villacorta, W. (2023). Green Energy Generated in Single-Chamber Microbial Fuel Cells Using Tomato Waste. Sustainability, 15(13), 10461. https://doi.org/10.3390/su151310461











