Potentials of Biomass Waste Valorization: Case of South America
Abstract
1. Introduction
2. Mapping Available Waste Biomass in South America: Distribution, Source and Composition
Composition of Waste Biomass: Key Information towards Biorefinery Strategies
| Biomass Source | % Dry wt a | Reference | ||
|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | ||
| Willow sawdust | 42.0 | 30.0 | 26.0 | [25] |
| 42.5 | 26.1 | 23.0 | [26] | |
| 49.6 * | 20.0 * | 18.4 | [27] | |
| 29.7 * | 16.4 * | 24.1 | [27] | |
| Poplar wood chips | 43.5 * | 21.8 * | 26.2 | [28] |
| 43.7 * | 21.5 * | 23.9 | [29] | |
| 39.5 | 17.4 * | 26.2 | [30] | |
| Pine wood chips | 49.5 * | 24.1 * | 25.6 (AIL) | [31] |
| 42.5 * | 20.8 * | 27.9 | [29] | |
| 41.7 * | 22.8 * | 26.9 | [29] | |
| 45.0 * | 21.8 * | 28 | [29] | |
| 46.4 * | 20.6 * | 29.4 | [28] | |
| Eucalyptus wood chips | 22.3 (AIL) | [32] | ||
| 20.6 (AIL) | [33] | |||
| 4.8 (ASL) | ||||
| 48.1 * | 12.7 * | 29.6 | [29] | |
| Eucalyptus pruning residue | 46.1 | 26.0 | 25.1 | [34] |
| (AIL + ASL) | ||||
| Linden tree pruning residue | 42.0 | 21.4 | 27.8 | [34] |
| (AIL + ASL) | ||||
| Plane tree pruning residue | 34.0 | 24.2 | 38.8 | [34] |
| (AIL + ASL) | ||||
| Olive tree pruning residue | 25.0 | 15.8 | 16.6 (AIL) | [35] |
| 2.2 (ASL) | ||||
| 28.6 * | 13.6 * | 21.4 (AIL) | [36] | |
| 2.3 (ASL) | ||||
| Hazelnut tree pruning residue | 37.2 | 20.45 | 28.5 (AIL) | [37] |
| 2.5 (ASL) | ||||
| Brewer’s spent grain | 13.1–25.4 | 28.4–29.96 | 11.9–27.8 | [38] |
| 15.14 | 50.23 | 29.37 | [39] | |
| 14.47 | 4.38 | 29.57 | [40] | |
| Barley straw | 33.1 | 24.9 | 16.1 | [28] |
| 35.65 * | 16.86 * | 20.70 (AIL) | [41] | |
| 2.40 (ASL) | ||||
| Fallen leaves pellets # | 30.25 | 38.04 | 30.11 | [42] |
| % Dry wt a | |||||||
|---|---|---|---|---|---|---|---|
| Feedstock | Origin | Cellulose | Hemicellulose | Lignin b | Extractives c | Ashes | Reference |
| Sugarcane bagasse | Brazil | 42.2 | 27.6 | 21.6 | 5.6 | 2.8 | [43] |
| Argentina | 43.1 * | 27.1 * | 21.3 | 2.1 | 1.5 | [44] | |
| Colombia | 37.7 | 29.4 | 32.9 | - | - | [45] | |
| Colombia | 53.2 | 14.6 | 32.2 | - | 12.3 | [46] | |
| Panela cane | Colombia | 43.6 | 33.0 | 21.8 | - | - | [47] |
| Colombia | 36.1 | 24.2 | 33.3 | - | - | ||
| Corn | Perú | 40.9 | 38.9 | 16.5 | - | - | [48] |
| Brazil | 31.3 | 32.3 | 17.4 | - | 1.9 | [49] | |
| Soybean | Brazil | 35.0 * | 22.8 * | 7.6 | 6.8 | 1.1 | [50] |
| Cuba | 35.3 | 16.9 | 21.7 | 5.8 | 10.6 | [51] | |
| Wheat straw | Argentina | 48.8 * | 51.2 | - | - | 10.6 | [52] |
| - | 39.7 | 30.6 | 17.7 | - | 7.7 | [53] | |
| Rice hulls | Brazil | 36.2 * | 19.8 * | 23.9 | 2.32 | 12.5 | [50] |
| Argentina | 34.1 | 15.8 | 19.0 | 8.2 | 15.0 | [54] | |
| Tea | China | 17.5 | 16.4 | 19.5 | - | - | [55] |
| Grapevine | Argentina | 15.3 | 5.0 | 38.0 | - | 8.8 | [56] |
| Argentina | 16.0 | 5.8 | 30.8 | - | 10.2 | ||
| Olive | Argentina | 30.2 | 15.6 | 51.7 | -- | 7.2 | [57] |
| Banana | Brazil | 36.3 * | 9.2 * | 8.4 | 25.2 | 8.0 | [50] |
| Brazil | 26.8 * | 12.7 * | 10.7 | 22.9 | 8.0 | ||
| Ecuador | 38.0 | 8.7 | 8.9 | 24.1 | 17.6 | [58] | |
| Ecuador | 21.9 | 12.8 | 21.5 | 18.0 | 15.7 | ||
| Other fruits | Brazil | 8.7 * | 59.0 * | 17.3 | 9.5 | 0.7 | [50] |
| Brazil | 32.4 * | 18.0 * | 36.0 | 1.4 | 3.0 | ||
| Coffee | Brazil | 35.3 * | 27.2 * | 24.5 | 4.2 | 2.0 | [50] |
| Colombia | 35.4 | 18.2 | 23.2 | - | 1.4 | [59] | |
| Peanut | Argentina | 81.2 * | 18.8 | - | - | 1.47 | [52] |
| India | 35.7 | 18.7 | 30.2 | - | 4.7 | [60] | |
| Forest industry residues | Chile | 49.5 * | 24.1 * | 25.6 | 3.0 | 1.7 | [31] |
| Chile | 50.5 * | 21.9 * | 20.1 | 3.1 | 1.1 | ||
| Argentina | 43.2 | 24.7 | 27.7 | 4.7 | 0.3 | [61] | |
| Argentina | 40.6 | 20.2 | 29.2 | 2.2 | 0.5 | [62] | |
| Argentina | 41.8 | 12.1 | 31.3 | 7.9 | 0.7 | [63] | |
| Argentina | 34.1 | 15.2 | 33.2 | 14.6 | 0.5 | [64] | |
| Brazil | 38.8 * | 11.8 * | 33.0 | 8.1 | 0.1 | [50] | |
3. Enzymatic Saccharification towards Key Building Blocks for Waste Biomass Valorization
3.1. Saccharification through Commercial Enzymes: Applications in Biomass Waste Valorization in South America
| Feedstock | Country of Origin | Pretreatment | Commercial Enzyme | Reaction Conditions | Yield | Objective | Reference |
|---|---|---|---|---|---|---|---|
| Brewer spent grain (BSG) | Brazil | Alkaline | Cellic®CTec3 (Novozymes, Bagsværd, Denmark) | 50 °C, 200 rpm for 48 h in 0.1 M citrate buffer | >70% glucose | Pretreatment improvement | [40] |
| Alkaline–acid | Cellulase and β-glucosidase from Novozymes | 45 °C, 120 rpm, 72 h 8% (w/v) substrate with 2.2% (v/v) cellulase and 1% (v/v) β-glucosidase | 75 g L−1 glucose | Ethanol production | [68] | ||
| Acid–alkaline | Trichoderma reesei cellulase Celluclast 1.5 L (Novozymes) | 45 °C, 100 rpm, 96 h 8% (w/v) substrate. Enzyme/substrate ratio of 45 FPU g−1 | 57.8 g L−1 glucose | Lactic acid production | [69] | ||
| Dilute acid and alkaline | Trichoderma reesei cellulase Celluclast 1.5 L (Novozymes) | 45 °C, 100 rpm for 96 h in sodium citrate buffer (pH 4.8) with 0.02% (w/v) sodium azide. Enzyme/substrate ratio of 45 FPU g−1 | 85.6% glucose | Pretreatment improvement | [70] | ||
| Acid–alkaline | Trichoderma reesei cellulase Celluclast 1.5 L (Novozymes) | 45 °C, 100 rpm, 96 h 8% (w/v) substrate in sodium citrate buffer (pH 4.8). Enzyme/substrate ratio of 45 FPU g−1 | 57.8 g/L glucose, 7.5 g/L cellobiose | Lactic acid production | [71] | ||
| Colombia | Acid | Trichoderma reesei cellulase Celluclast 1.5 L (Novozymes) | 45 °C, 100 rpm for 96 h in citrate buffer solution (pH 4.8) at a solid-to-liquid ratio of 1-to-8. Enzyme/substrate ratio of 45 FPU g−1 | 4.5% glucose | Xylitol, ethanol and polyhydroxybutyrate (PHB) production | [72] | |
| Olive tree pruning | Argentina | Alkaline | Cellulase from Trichoderma reesei ATCC 26921 (≥700 units g−1) (Sigma Aldrich, Søborg, Denmark) and hemicellulase from Aspergillus niger (0.3–3 units mg−1) (Sigma Aldrich, St. Louis, MO, USA). | 45 °C, 100 rpm for 24 h in 0.05 M sodium citrate buffer (pH 4.9). 4% (w/v) substrate concentration | 220 mg sugars g−1 dry biomass | Bioethanol production | [73] |
| Pine sawdust | Argentina | Alkaline–acid | Trichoderma reesei cellulases (51 FPU mL−1 of cellulose, Sigma Aldrich) | 50 °C, stirring for 72 h in acetate buffer 50 mM (pH 4.8). 2% total solids | 24.3% glucose | Study effect of pretreatment on substrate accessibility | [74] |
| Alkaline–acid | Celluclast 1.5 L (Sigma) | 50 °C, 150 rpm for 48 h in 0.05 M sodium acetate buffer (pH 4.8). Enzyme/substrate ratio of 20 U g−1 | 1.81 g L-1 glucose | Pretreatment improvement | [75] | ||
| Kraft–anthraquinone | Cellulase from Trichoderma reesei (Sigma Aldrich, Søborg, Denmark) | 50 °C, 130 rpm for 72 h in 0.05 M sodium citrate buffer (pH 4.8). Enzyme/substrate ratio of 20 FPU g−1 | EH% 100 | Pretreatment improvement | [76] | ||
| Soda–ethanol | Cellic®CTec2 (Novozymes) | 37°C, 130 rpm for 48 h in 0.05 M sodium citrate buffer (pH 5), 1% hydrolysable cellulose (dry matter). Enzyme/substrate ratio of 30 FPU g−1 | ≈100% EH; 11 g L−1 glucose | Bioethanol production | [77] | ||
| Pinus radiata wood chips | Chile | Acid–ethanol | Cellic®CTec3 (Novozymes) | 50 °C, 150 rpm for 72 h in 0.05 M citrate buffer (pH 4.8). Enzyme/substrate ratio of 0.044 g g−1 | 70 g L−1 glucose | Ethanol production | [78] |
| Pinus patula bark | Colombia | Alkaline | Celluclast 1.5 L and Viscozyme L | 60 °C, 100 rpm for 72 h in 0.1 M citrate buffer solution (pH 4.8). Enzyme/substrate ratio 25 FPU g−1 | 63 g L−1 hexose | Bioethanol and furfural production | [79] |
| Sugarcane bagasse (SB) | Brazil | Acid | Cellulase from Trichoderma reesei (I) and mix of cellulase and β-glucosidase (II)(Genecor and Novozymes) | 45 °C, 70 rpm for 24 h in 100 mM sodium citrate buffer (pH 4.8). Enzyme/substrate ratio of 30 FPU g−1. Tween 20/substrate ratio of 0.08 g g−1 | I: 47.7% glucose II: 48.1% glucose | Study cellulose digestibility by modifying variables | [80] |
| Acid–alkaline | Cellulase from Trichoderma reesei Multifect® (Genecor International Inc.) | 48 °C, 200 rpm for 24 h in 0.05 M citrate buffer (pH 5.0). Enzyme/substrate ratio of 25 FPU g−1 | 40.4 g L−1 glucose | Pretreatment improvement | [81] | ||
| Acid | Cellic®Ctec2 (Novozymes) | 50 °C, 200 rpm for 24 h in 0.1 M sodium citrate buffer (pH 5.0). Enzyme/substrate ratio of 30 FPU g−1 | Tops: 39.8 g L−1 | Ethanol production | [82] | ||
| Bagasse: 22.2 g L−1 | |||||||
| Straw: 31.0 g LL−1 | |||||||
| Steam explosion | Cellic®Ctec2 (Novozymes) | 50 °C, stirring for 96 h in 50 mM acetate buffer (pH 4.8). Enzyme/substrate ratio of 8.4 FPU g−1 | 60–70 g L−1 glucose | Cellulosic ethanol production | [83] | ||
| Hydrodynamic cavitation–alkaline pretreatment | Cellic C-Tec (Novozymes) | 48 h in 50 mM sodium citrate buffer (pH 4.8). Enzyme/substrate ratio of 20 FPU g−1 | 91% glucose | Pretreatment improvement | [84] | ||
| Acid | P4 from Trichoderma reesei (AB enzymes) | 40 °C, stirring, for 65 h in 0.05 M citrate buffer. Enzyme/substrate ratio 0.001 g L−1 | 29.11 mg mL−1 | Selection of cellulolytic enzyme | [85] | ||
| Acid–ultrasonic | Celluclast 1.5 L (I) and Cellic cTec2 (II) (Novozymes) | 50 °C, 300 rpm for 24 h in 0.2 M sodium acetate buffer (pH 4.8). Enzyme/substrate ratio of 20 FPU g−1 | I: RS % 189, TCY % 45 | Study effect of ultrasound treatment | [86] | ||
| II: RS % 192, TCY % 66 | |||||||
| Acid–SC-CO2 | Cellic cTec2 (Novozymes) | 50 °C, 300 rpm for 24 h in 0.2 M sodium acetate buffer (pH 4.8). Enzyme/substrate ratio of 10 FPU g−1 | RS % 132, TCY % 32 | Study effect of SC-CO2 treatment | [87] | ||
| Napiergrass | Uruguay | Acid–alkaline | Cellulase complex NS50013 and β-glucosidase NS50010 (Novozymes) | 50 °C, 100 rpm, for 130 h in pH 4.8 buffered solution. Enzyme/substrate ratio of 5 FPU g−1 cellulase and 10 CBU g−1 β-glucosidase. PEG 6000/substrate ratio of 0.05 g g−1 | 45% cellulose hydrolysis | Fuel bioethanol production | [88] |
| 27 g L−1 glucose | |||||||
| King grass | Colombia | Alkaline | Acellerase 1500 (Genencor, New York, NY, USA) | 50 °C, 180 rpm for 24 h in 0.05 M citrate buffer (pH 4.8). Enzyme/substrate ratio of 30 FPU g−1 cellulase and 10 CBU g−1 β-glucosidase.PEG 6000/substrate ratio of 0.05 g g−1 | 78 g L−1 glucose | Fuel bioethanol production | [89] |
3.2. Native Fungal Enzymes Degrading Cellulosic Substrates and Their Potential Applications
4. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ubando, A.T.; Del Rosario, A.J.R.; Chen, W.-H.; Culaba, A.B. A state-of-the-art review of biowaste biorefinery. Environ. Pollut. 2011, 269, 116149. [Google Scholar] [CrossRef]
- Ohara, H. Biorefinery. Appl. Microbiol. Biotechnol. 2003, 62, 474–477. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. A comparative analysis of pretreatment strategies on the properties and hydrolysis of brewers’ spent grain. Bioresour. Technol. 2018, 248, 272–279. [Google Scholar] [CrossRef]
- Zhang, Z.; Harrison, M.D.; Rackemann, D.W.; Doherty, W.O.S.; O’Hara, I.M. Organozolv pretreatment of plant biomass for enhanced enzymatic saccharification. Green Chem. 2016, 18, 360–381. [Google Scholar] [CrossRef]
- Solier, Y.N.; Mocchiutti, P.; Cabrera, M.N.; Saparrat, C.M.N.; Zanuttini, M.A.; Inalbon, M.C. Alkali-peroxide treatment of sugar cane bagasse. Effect of chemical charges on the efficiency of xylan isolation and susceptibility of bagasse to saccharification. Biomass Conv. Bioref. 2022, 12, 567–576. [Google Scholar] [CrossRef]
- Madadi, M.; Tu, Y.; Abbas, A. Recent status on enzymatic saccharification on lignocellulosic biomass for bioethanol production. Electron. J. Biol. 2017, 13, 135–143. [Google Scholar]
- Alegre, M. FAO Update of Biomass Balance for Energy Purposes in Argentina (In Spanish). Technical Documents Collection N° 19, Buenos Aires, Argentina; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Rojas Ponce, Y.; Meza Robayo, J.A. Production and Consumption of Wood Fuels Spatial Analysis Using the Wisdom Method: Basis for a National Wood Energy Strategy; Instituto Forestal INFOR: Santiago, Chile, 2010; (In Spanish). [Google Scholar] [CrossRef]
- Pirelli, T.; Rossi, A. Sustainability of Forest Biomass for Energy and of Ethanol from Maize and Sugarcane in Paraguay. Results and Recommendations from the Implementation of the Global Bioenergy Partnership Indicators. Environmental and Natural Resources Management. Working Paper, No. 70, FAO. 2018. Available online: https://www.fao.org/publications (accessed on 10 April 2023). (In Spanish).
- Biometrans. Biomethane Production for Transport Fuel from Waste Biomass. Diagnosis of Biomass Resources available in Latin America. CYTED Ciencia y Tecnología para el Desarrollo. 2018. Available online: http://cyted.org/sites/default/files/d1._diagnostico_de_los_recursos_de_biomasa_disponibles.pdf (accessed on 10 April 2023). (In Spanish).
- Assureira Espinoza, E.G.; Assureira Espinoza, M.A. Energy Potential of Waste Biomass in Peru; Pontificia Universidad Católica del Perú: Lima, Perú, 2015. (In Spanish) [Google Scholar]
- Welfle, A. Balancing growing global bioenergy resource demands—Brazil’s biomass potential and the availability of resource for trade. Biomass Bioenergy 2017, 105, 83–95. [Google Scholar] [CrossRef]
- Forster-Carneiro, T.; Berni, M.D.; Dorileo, I.L.; Rostagno, M.A. Biorefinery study of availability of agriculture residues and wastes for integrated biorefineries in Brazil. Resour. Conserv. Recy. 2013, 77, 78–88. [Google Scholar] [CrossRef]
- Lorenzo, I. Circular Economy and Climate Change. Proyecto Biovalor, Ministerio de Industria, Energía y Minería, Uruguay. 2020. Available online: Biovalor.gov.uy (accessed on 10 April 2023). (In Spanish)
- Quantification of Waste Generated in Agroindustrial Sectors Uruguay 2016. Technical Data Sheets. Available online: https://biovalor.gub.uy/materiales/ (accessed on 10 April 2023). (In Spanish).
- Forestry Sector in Uruguay. Forestry Report. Uruguay XXI, Investment, Export and Country Brand Promotion Agency 2022. Available online: https://www.uruguayxxi.gub.uy/uploads/informacion/2ec25967b8d7bfd72de685fbe8d201e06b5507bd.pdf (accessed on 10 April 2023).
- del Pino, A.; Hernández, J.; Arrarte, G. Nutrient export with logs, and release from residues, after harvest of a Pinus taeda plantation in Uruguay. Open J. For. 2020, 10, 360–376. [Google Scholar] [CrossRef]
- Boragno, L.; Boscana, M. Forest Statistics 2022. Dirección General Forestal, Ministerio de Ganadería, Agricultura y Pesca, Uruguay. 2022. Available online: https://www.gub.uy/ministerio-ganaderia-agricultura-pesca/datos-yestadisticas/estadisticas/boletin-estadisticas-forestales-2022 (accessed on 10 April 2023). (In Spanish).
- Area, M.C.; Vallejos, M.E. Biorrefinería a Partir de Residuos Lignocelulósicos: Conversión de Residuos a Productos de alto Valor. Editorial Académica Española, España. 2012. Available online: https://www.researchgate.net/publication/262933028_Biorrefineria_a_partir_de_residuos_lignocelulosicos_Conversion_de_residuos_a_productos_de_alto_valor (accessed on 15 April 2023).
- Studer, M.H.; DeMartini, J.D.; Davis, M.F.; Sykes, R.W.; Davison, B.; Keller, M.; Tuskan, G.A.; Wyman, C.E. Lignin content in natural Populus variants affects sugar release. Proc. Nat. Acad. Sci. USA 2011, 108, 6300–6305. [Google Scholar] [CrossRef]
- Rahikainen, J.; Mikander, S.; Marjamaa, K.; Tamminem, T.; Lappas, A.; Viikari, L.; Kruus, K. Inhibition of enzymatic hydrolysis by residual lignins from softwood-study of enzyme binding and inactivation on lignin-rich surface. Biotechnol. Bioeng. 2011, 108, 2823–2834. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Jung, S.; Ragauskas, A. Pseudo-lignin formation and its impact on enzymatic hydrolysis. Bioresour. Technol. 2012, 117, 7–12. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Alriols, M.G.; Labidi, J. Evaluation of different lignocellulosic raw materials as potential alternative feedstocks in biorefinery processes. Ind. Crops Prod. 2014, 53, 102–110. [Google Scholar] [CrossRef]
- Bak, J.S.; Ko, J.K.; Choi, I.G.; Park, Y.-C.; Seo, J.-H.; Kim, K.H. Fungal pretreatment of lignocellulose by Phanerochaete chrysosporium to produce ethanol from rice straw. Biotechnol. Bioeng. 2009, 104, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Lempiäinen, H.; Lappalainen, K.; Haverinen, J.; Tuuttila, T.; Hu, T.; Jaakkola, M.; Lassi, U. The effect of mechanocatalytic pretreatment on the structure and depolymerization of willow. Catalysts 2020, 10, 255. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Szczukowski, S.; Tworkowski, J.; Wróblewska, H.; Krzyżaniak, M. Short rotation willow coppice biomass as an industrial and energy feedstock. Ind. Crops Prod. 2011, 33, 217–223. [Google Scholar] [CrossRef]
- Han, S.-H.; Cho, D.H.; Kim, Y.H.; Shin, S.-J. Biobutanol production from 2-year-old willow biomass by acid hydrolysis and acetone–butanol–ethanol fermentation. Energy 2013, 61, 13–17. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; Oliva, J.M.; Ballesteros, M. Realistic approach for full-scale bioethanol production from lignocellulose: A review. J. Sci. Ind. Res. 2008, 67, 874–884. [Google Scholar]
- Álvarez, C.; Reyes-Sosa, F.M.; Díez, B. Enzymatic hydrolysis of biomass from wood. Microb. Biotechnol. 2016, 9, 149–156. [Google Scholar] [CrossRef]
- Negro, M.J.; Manzanares, P.; Ballesteros, I.; Oliva, J.M.; Cabañas, A.; Ballesteros, M. Hydrothermal pretreatment conditions to enhance ethanol production from poplar biomass. Appl. Biochem. Biotechnol. 2003, 105, 87–100. [Google Scholar] [CrossRef]
- Muñoz, C.; Mendonça, R.; Baeza, J.; Berlin, A.; Saddler, J.; Freer, J. Bioethanol production from bio-organosolv pulps of Pinus radiata and Acacia dealbata. J. Chem. Technol. Biotechnol. 2007, 82, 767–774. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Rencoret, J.; Cadena, E.M.; Rico, A.; Barth, D.; del Río, J.C.; Martínez, A.T. Demonstration of laccase-based removal of lignin from wood and non-wood plant feedstocks. Bioresour. Technol. 2012, 119, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sampedro, R.; Eugenio, M.E.; García, J.C.; López, F.; Villar, J.C.; Díaz, M.J. Steam explosion and enzymatic pre-treatments as an approach to improve the enzymatic hydrolysis of Eucalyptus globulus. Biomass Bioenergy 2012, 42, 97–106. [Google Scholar] [CrossRef]
- Gallina, G.; Cabeza, A.; Grénman, H.; Biasi, P.; García-Serna, J.; Salmi, T. Hemicellulose extraction by hot pressurized water pretreatment at 160 °C for 10 different woods: Yield and molecular weight. J. Supercrit. Fluids 2018, 133, 716–725. [Google Scholar] [CrossRef]
- Cara, C.; Ruiz, E.; Ballesteros, M.; Manzanares, P.; Negro, M.J.; Castro, E. Production of fuel ethanol from steam-explosion pretreated olive tree pruning. Fuel 2008, 87, 692–700. [Google Scholar] [CrossRef]
- Mateo, S.; Roberto, I.C.; Sánchez, S.; Moya, A.J. Detoxification of hemicellulosic hydrolyzate from olive tree pruning residue. Ind. Crops Prod. 2013, 49, 196–203. [Google Scholar] [CrossRef]
- Sabanci, K.; Buyukkileci, A.O. Comparison of liquid hot water, very dilute acid and alkali treatments for enhancing enzymatic digestibility of hazelnut tree pruning residues. Bioresour. Technol. 2018, 261, 158–165. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S.; Sarma, S.J.; Brar, S.K.; Misra, K.; Oberoi, H.S. Waste biomass: A prospective renewable resource for development of bio-based economy/processes. In Biotransformation of Waste Biomass into High Value Biochemical; Springer: New York, NY, USA, 2014; pp. 3–28. [Google Scholar] [CrossRef]
- Borel, L.D.M.S.; Lira, T.S.; Ribeiro, J.A.; Ataíde, C.H.; Barrozo, M.A.S. Pyrolysis of brewer’s spent grain: Kinetic study and products identification. Ind. Crops Prod. 2018, 121, 388–395. [Google Scholar] [CrossRef]
- Lobo Gomes, C.; Gonçalves, E.; Galeano Suarez, C.A.; de Souza Rodrigues, D.; Cavalcanti Montano, I. Effect of reaction time and sodium hydroxide concentration on delignification and enzymatic hydrolysis of brewer’s spent grain from two Brazilian brewers. Cell Chem. Technol. 2021, 55, 101–112. [Google Scholar] [CrossRef]
- Han, M.; Kang, K.E.; Kim, Y.; Choi, G.-W. High efficiency bioethanol production from barley straw using a continuous pretreatment reactor. Process Biochem. 2013, 48, 488–495. [Google Scholar] [CrossRef]
- González, W.A.; López, D.; Pérez, J.F. Biofuel quality analysis of fallen leaf Pellets: Effect of moisture and glycerol contents as binders. Renew. Energy 2020, 147, 1139–1150. [Google Scholar] [CrossRef]
- de Moraes Rocha, G.J.; Marcos Nascimento, V.; Gonçalves, A.R.; Fernandes Nunes Silva, V.; Martín, C. Influence of mixed sugarcane bagasse samples evaluated by elemental and physical–chemical composition. Ind. Crops Prod. 2015, 64, 52–58. [Google Scholar] [CrossRef]
- Area, M.; Felissia, F.; Vallejos, M. Ethanol-water fractionation of sugar cane bagasse catalyzed with acids. Cell Chem. Technol. 2009, 43, 271–279. [Google Scholar]
- Larrahondo, J.E. Calidad de la Caña de Azucar. In El Cultivo de la Caña en la Zona Azucarera de Colombia; Cenicaña: Cali, Colombia, 1995; pp. 337–354. [Google Scholar]
- Marrugo, G.; Valdés, C.F.; Chejne, F. Characterization of Colombian agroindustrial biomass residues as energy resources. Energy Fuels 2016, 30, 8386–8398. [Google Scholar] [CrossRef]
- Sagastume Gutiérrez, A.; Eras, J.J.C.; Hens, L.; Vandecasteele, C. The energy potential of agriculture, agroindustrial, livestock, and slaughterhouse biomass wastes through direct combustion and anaerobic digestion. The case of Colombia. J. Clean. Prod. 2020, 269, 122317. [Google Scholar] [CrossRef]
- Echeverría, C.; Bazán, G.; Sánchez-Gonzalez, J.; Lescano, L.; Pagador, S.; Linares, G. Pre-treatment by acidification and freezing on corncob polymers and its enzymatic hydrolysis. Asian J. Sci. Res. 2018, 11, 222–231. [Google Scholar] [CrossRef]
- Correia Vieira, R.; Corrêa Antunes, D.P.; Rodrigues dos Santos-Rocha, M.S.; Lopes Barbosa, K.; Cabral dos Santos Silva, M.; Gomes, M.A.; Garcia Almeida, R.M.R. Enzymatic hydrolisis optimization from corn wastes by experimental design. Int. J. Eng. Sci. 2017, 6, 9–15. [Google Scholar]
- Rambo, M.K.D.; Schmidt, F.L.; Ferreira, M.M.C. Analysis of the lignocellulosic components of biomass residues for biorefinery opportunities. Talanta 2015, 144, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, E.; Muñoz, M.J.; Martín, R.; Caro, I.; Curbelo, C.; Díaz, A.B. Comparison of industrially viable pretreatments to enhance soybean straw biodegradability. Bioresour. Technol. 2015, 194, 1–6. [Google Scholar] [CrossRef]
- Fermanelli, C.S.; Córdoba, A.; Pierella, L.B.; Saux, C. Pyrolysis and copyrolysis of three lignocellulosic biomass residues from the agro-food industry: A comparative study. Waste Manag. 2020, 102, 362–370. [Google Scholar] [CrossRef]
- Espinosa, E.; Sánchez, R.; Otero, R.; Domínguez-Robles, J.; Rodríguez, A. A comparative study of the suitability of different cereal straws for lignocellulose nanofibers isolation. Int. J. Biol. Macromol. 2017, 103, 990–999. [Google Scholar] [CrossRef]
- Dagnino, E.P.; Chamorro, R.E.; Romano, S.D.; Felissia, F.E.; Area, M.C. Optimization of rice hulls acid pretreatment and its characterization as a potential substrate for bioethanol production. Ind. Crops Prod. 2013, 42, 363–368. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, Z.; Lin, X.; Ren, Z.; Li, B.; Zhang, Y. Preparation and characterization of microcrystalline cellulose (MCC) from tea waste. Carbohydr. Polym. 2018, 184, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.; Mazza, G.; Fernández, A.; Saffe, A.; Echegaray, M. Prediction of the lignocellulosic winery wastes behavior during gasification process in fluidized bed: Experimental and theoretical study. J. Environ. Chem. Eng. 2018, 6, 5570–5579. [Google Scholar] [CrossRef]
- Giménez, M.; Rodríguez, M.; Montoro, L.; Sardella, F.; Rodríguez-Gutierrez, G.; Monetta, P.; Deiana, C. Two phase olive mill waste valorization. Hydrochar production and phenols extraction by hydrothermal carbonization. Biomass Bioenergy 2020, 143, 105875. [Google Scholar] [CrossRef]
- Guerrero, A.B.; Aguado, P.L.; Sánchez, J.; Curt, M.D. GIS Based assessment of banana residual biomass potential for ethanol production and power generation: A case ctudy. Waste Biomass Valor. 2015, 7, 405–415. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt Boix, A. Isolation and characterization of microcrystalline cellulose and cellulose nanocrystals from coffee husk and comparative study with rice husk. Carbohydr. Polym. 2018, 191, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Raju, G.U.; Kumarappa, S.; Gaitonde, V.N. Mechanical and physical characterization of agricultural waste reinforced polymer composites. J. Mater. Environ. Sci. 2012, 3, 907–916. [Google Scholar]
- Stoffel, R.B.; Felissia, F.E.; Curvelo, A.A.S.; Gassa, L.M.; Area, M.C. Desresinación Alcalina y Tratamiento Ácido de Aserrín de Pino Destinado a Biorrefinería. In Proceedings of the 45° Congresso Internacional de Celulose e Papel da ABTCP/VII Congresso Ibero-Americano de Pesquisa de Celulose e Papel, Sao Paulo, Brasil, 9–11 October 2012. Available online: https://www.celso-foelkel.com.br/artigos/outros/2012_Desresinacao_serragem_pinus.pdf (accessed on 15 April 2023).
- Stoffel, R.B.; Felissia, F.E.; Gassa, L.; Area, M.C. Biorrefinería a Partir de Residuos de la Industrialización Primaria de Pino: Caracterización de Materias Primas. In Proceedings of the VIII Jornadas Científico-Tecnológicas, FCEQYN, UNaM, Posadas, Argentina, 2–4 November 2011. [Google Scholar]
- Hornus, M. Optimización de la Extracción de Hemicelulosas de Aserrín de Eucalyptus Mediante Tratamiento Hidrotérmico; Informe Beca de Estímulo a las Vocaciones Científicas: Posadas, Argentina, 2012. [Google Scholar]
- Dagnino, E.P.; Chamorro, E.R.D.; Romano, S.; Felissia, F.E.; Area, M.C. Optimización de prehidrólisis de aserrín de Prosopis nigra para la producción de azúcares fermentables. In Proceedings of the 45° Congresso Internacional de Celulose e Papel da ABTCP/VII Congresso Ibero-Americano de Pesquisa de Celulose e Papel, Sao Paulo, Brasil, 9–11 October 2012. [Google Scholar]
- Magalhães, A.I.; de Carvalho, J.C.; de Melo Pereira, G.V.; Karp, S.G.; Camara, M.C.; Coral Medina, J.D.; Soccol, C.R. Lignocellulosic biomass from agro-industrial residues in South America: Current developments and perspectives. Biofuels Bioprod. Bioref. 2019, 13, 1505–1519. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Valencia, E.Y.; Chambergo, F.S. Mini-review: Brazilian fungi diversity for biomass degradation. Fungal Genet. Biol. 2013, 60, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Liguori, R.; Soccol, C.R.; de Souza Vandenberghe, L.; Woiciechowski, A.L.; Faraco, V. Second generation ethanol production from brewer’s spent grain. Energies 2015, 8, 2575–2586. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Fernandes, M.; Dragone, G.; Mancilha, I.M.; Roberto, I.C. Brewer´s spent grain as raw material for lactic acid production by Lactobacillus delbrueckii. Biotechnol. Lett. 2007, 29, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Fernandes, M.; Milagres, A.M.F.; Roberto, I.C. Effect of hemicellulose and lignin on enzymatic hydrolysis of cellulose from brewer´s spent grain. Enz. Microb. Technol. 2008, 43, 124–129. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Fernandes, M.; Mancilha, I.M.; Roberto, I.C. Effect of medium supplementation and pH control on lactic acid production from brewer´s spent grain. Biochem. Eng. J. 2008, 40, 437–444. [Google Scholar] [CrossRef]
- Dávila, J.A.; Rosenberg, M.; Cardona, C.A. A biorefinery approach for the production of xylitol, ethanol and polyhydroxybutyrate from brewer’s spent grain. AIMS Agric. Food 2016, 1, 52–66. [Google Scholar] [CrossRef]
- Mamaní, A.M.; Maturano, Y.; Herrero, L.; Montoro, L.; Sardella, F. Increase in fermentable sugars of olive tree pruning biomass for bioethanol production: Application of an experimental design for optimization of alkaline pretreatment. Period Polytech. Chem. Eng. 2022, 66, 269–278. [Google Scholar] [CrossRef]
- Stoffel, R.B.; Vinholi Neves, P.; Felissia, F.E.; Pereira Ramos, L.; Gassa, L.M.; Area, M.C. Hemicellulose extraction from slash pine sawdust by steam explosion with sulfuric acid. Biomass Bioenergy 2017, 107, 93–101. [Google Scholar] [CrossRef]
- Rodriguez, M.D.; Castrillo, M.L.; Velázquez, J.E.; Kramer, G.R.; Sedler, C.I.; Zapata, P.D.; Villalba, L. Obtención de azúcares fermentables a partir de aserrín de pino pretratado secuencialmente con ácido-base. Rev. Int. Contam. Ambie 2017, 33, 317–324. [Google Scholar] [CrossRef]
- Kruyeniski, J.; Ferreira, P.J.T.; Videira Sousa Carvalho, M.G.; Vallejos, M.E.; Felissia, F.E.; Area, M.C. Physical and chemical characteristics of pretreated slash pine sawdust influence its enzymatic hydrolysis. Ind. Crops Prod. 2019, 130, 528–536. [Google Scholar] [CrossRef]
- Mendieta, C.M.; Felissia, F.E.; Arismendy, A.M.; Kruyeniski, J.; Area, M.C. Enzymatic hydrolysis and fermentation strategies for biorefining of pine sawdust. Bioresources 2021, 16, 7474–7491. [Google Scholar] [CrossRef]
- Valenzuela, R.; Priebe, X.; Troncoso, E.; Ortega, I.; Parra, C.; Freer, J. Fiber modifications by organosolv catalyzed with H2SO4 improves the SSF of Pinus radiata. Ind. Crops Prod. 2016, 86, 79–86. [Google Scholar] [CrossRef]
- Moncada, J.; Cardona, C.A.; Higuita, J.C.; Vélez, J.J.; López-Suarez, F.E. Wood residue (Pinus patula bark) as an alternative feedstock for producing ethanol and furfural in Colombia: Experimental, technoeconomic and environmental assessments. Chem. Eng. Sci. 2016, 140, 309–318. [Google Scholar] [CrossRef]
- Santos, V.T.O.; Esteves, P.J.; Milagres, A.M.F.; Carvalho, W. Characterization of commercial cellulases and their usein the saccharification of a sugarcane bagasse sample pretreatedwith dilute sulfuric acid. J. Ind. Microbiol. Biotechnol. 2011, 38, 1089–1098. [Google Scholar] [CrossRef]
- Araújo Barcelos, C.; Nobuyuki Maeda, R.; Vargas Betancur, G.J.; Pereira Jr, N. The essentialness of delignification on enzymatic hydrolysis of sugar cane bagasse cellulignin for second generation ethanol production. Waste Biomass Valor. 2013, 4, 341–346. [Google Scholar] [CrossRef]
- Cerqueira Pereira, S.; Maehara, L.; Monteiro Machado, C.M.; Sanchez Farinas, C. 2G ethanol from the whole sugarcane lignocellulosic biomass. Biotechnol. Biofuels 2015, 8, 44. [Google Scholar] [CrossRef]
- Neves, P.V.; Pitarelo, A.P.; Ramos, L.P. Production of cellulosic ethanol from sugarcane bagasse by steam explosion: Effect of extractives content, acid catalysis and different fermentation technologies. Bioresour. Technol. 2016, 208, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Terán Hilares, R.; dos Santos, J.C.; Ahmed, M.A.; Jeon, S.H.; da Silva, S.S.; Han, J.I. Hydrodynamic cavitation-assisted alkaline pretreatment as a new approach for sugarcane bagasse biorefineries. Bioresour. Technol. 2016, 214, 609–614. [Google Scholar] [CrossRef]
- Aguiar, M.M.; Pietroboni, V.C.; Moura de Salles, M.; Pupo, M.; Hortense Torres, N.; Américo, J.H.P.; Richard Salazar-Banda, G.; Silva, D.P.; Rosim Monteiro, D.T.; Romanholo Ferreira, L.F. Evaluation of comercial cellulolytic enzymes for sugarcane bagasse hydrolysis. Cellul. Chem. Technol. 2018, 52, 695–699. [Google Scholar]
- de Carvalho Silvello, M.A.; Martínez, J.; Goldbeck, R. Increase of reducing sugars release by enzymatic hydrolysis of sugarcane bagasse intensified by ultrasonic treatment. Biomass Bioenergy 2019, 122, 481–489. [Google Scholar] [CrossRef]
- de Carvalho Silvello, M.A.; Martínez, J.; Goldbeck, R. Alternative technology for intensification of fermentable sugars released from enzymatic hydrolysis of sugarcane bagasse. Biomass Conv. Bioref. 2022, 12, 2399–2405. [Google Scholar] [CrossRef]
- Camesasca, L.; Ramírez, M.B.; Guigou, M.; Ferrari, M.D.; Lareo, C. Evaluation of dilute acid and alkaline pretreatments, enzymatic hydrolysis and fermentation of napiergrass for fuel ethanol production. Biomass Bioenergy 2015, 74, 193–201. [Google Scholar] [CrossRef]
- Gallego, L.J.; Escobar, A.; Peñuela, M.; Peña, J.D.; Rios, L.A. King Grass: A promising material for the production of second-generation butanol. Fuel 2015, 143, 399–403. [Google Scholar] [CrossRef]
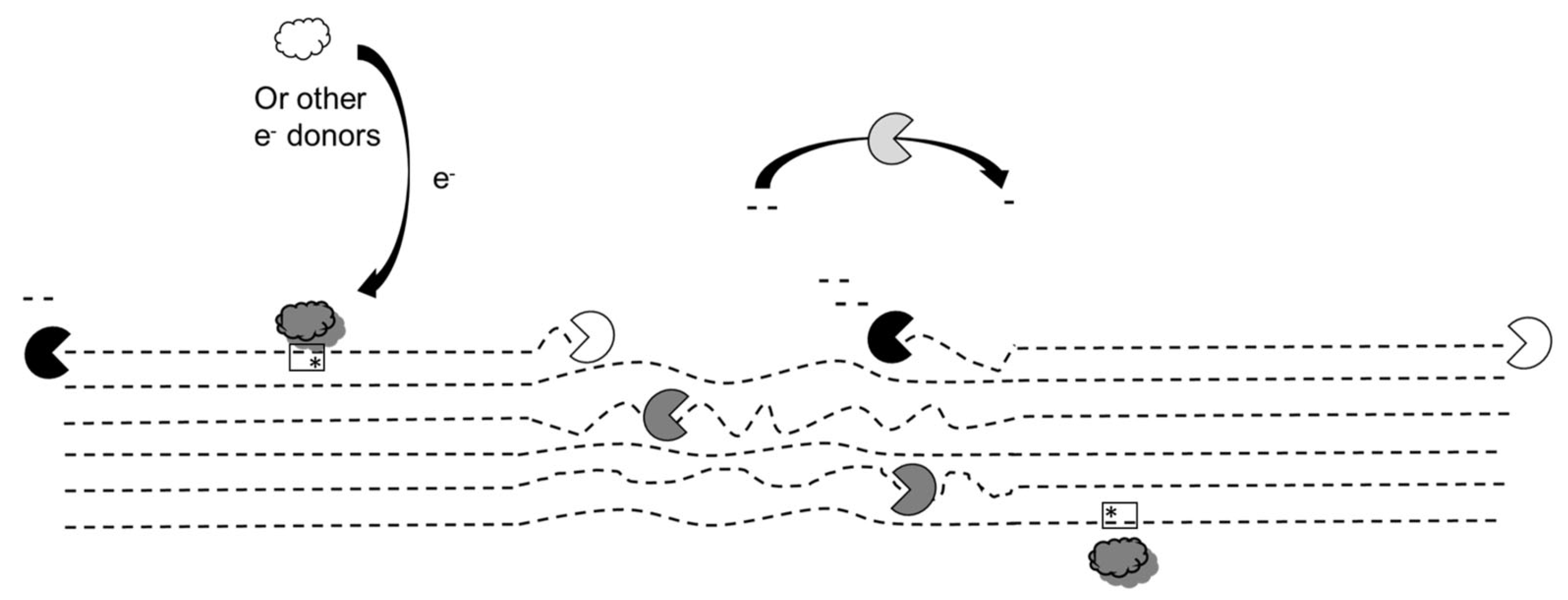
- Dimarogona, M.; Topakas, E.; Christakopoulos, P. Recalcitrant polysaccharide degradation by novel oxidative biocatalysts. Appl. Microbiol. Biotechnol. 2013, 97, 8455–8465. [Google Scholar] [CrossRef] [PubMed]
- Gourlay, K.; Arantes, V.; Saddler, J.N. Use of substructure-specific carbohydrate binding modules to track changes in cellulose accessibility and surface morphology during the amorphogenesis step of enzymatic hydrolysis. Biotechnol. Biofuels 2012, 5, 51. [Google Scholar] [CrossRef]
- Várnai, A.; Mäkelä, M.R.; Djajadi, D.T.; Rahikainen, J.; Hatakka, A.; Viikari, L. Carbohydrate-binding modules of fungal cellulases: Occurrence in nature, function, and relevance in industrial biomass conversion. Adv. Appl. Microbiol. 2014, 88, 103–165. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Liu, X.; Hakulinen, N.; Taherzadeh, M.J.; Wang, Y.; Wang, Y.; Qin, X.; Wang, X.; Yao, B.; Luo, H.; et al. Boosting enzymatic degradation of cellulose using a fungal expansin: Structural insight into the pretreatment mechanism. Bioresour. Technol. 2022, 358, 127434. [Google Scholar] [CrossRef]
- Saparrat, M.C.N.; Arambarri, A.M.; Balatti, P.A. Growth response and extracellular enzyme activity of Ulocladium botrytis LPSC 813 cultured on carboxy-methylcellulose under a pH range. Biol. Fertil. Soil 2007, 44, 383–386. [Google Scholar] [CrossRef]
- Coniglio, R.O.; Fonseca, M.I.; Villalba, L.L.; Zapata, P.D. Screening of new secretory cellulases from different supernatants of white rot fungi from Misiones, Argentina. Mycology 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Teixeira, W.F.A.; Batista, R.D.; do Amaral Santos, C.C.A.; Chagas Freitas Júnior, A.; Franchini Terrasan, C.R.; Pinheiro, R.; de Santana, M.W.; Gonçalves de Siqueira, F.; Coutinho de Paula-Elias, F.; de Almeida, A.F. Minimal enzymes cocktail development by filamentous fungi consortia in solid-state cultivation and valorization of pineapple crown waste by enzymatic saccharification. Waste Biomass Valor. 2021, 12, 2521–2539. [Google Scholar] [CrossRef]
- Garrido, M.M.; Landoni, M.; Sabbadin, F.; Valacco, M.P.; Couto, A.; Bruce, N.C.; Wirth, S.A.; Campos, E. PsAA9A, a C1-specific AA9 lytic polysaccharide monooxygenase from the white-rot basidiomycete Pycnoporus sanguineus. Appl. Microbiol. Biotechnol. 2022, 104, 9631–9643. [Google Scholar] [CrossRef] [PubMed]
- Picart, P.; Diaz, P.; Pastor, F.I. Cellulases from two Penicillium sp. strains isolated from subtropical forest soil: Production and characterization. Lett. Appl. Microbiol. 2007, 45, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, M.; Villarreal, P.; Barahona, S.; Alcaíno, J.; Cifuentes, V.; Baeza, M. Screening and characterization of amylase and cellulase activities in psychrotolerant yeasts. BMC Microbiol. 2016, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Vega, K.; Villena, G.K.; Sarmiento, V.H.; Ludueña, Y.; Vera, N.; Gutiérrez-Correa, M. Production of alkaline cellulase by fungi isolated from an undisturbed rain forest of Peru. Biotechnol. Res. Int. 2012, 2012, 934325. [Google Scholar] [CrossRef] [PubMed]
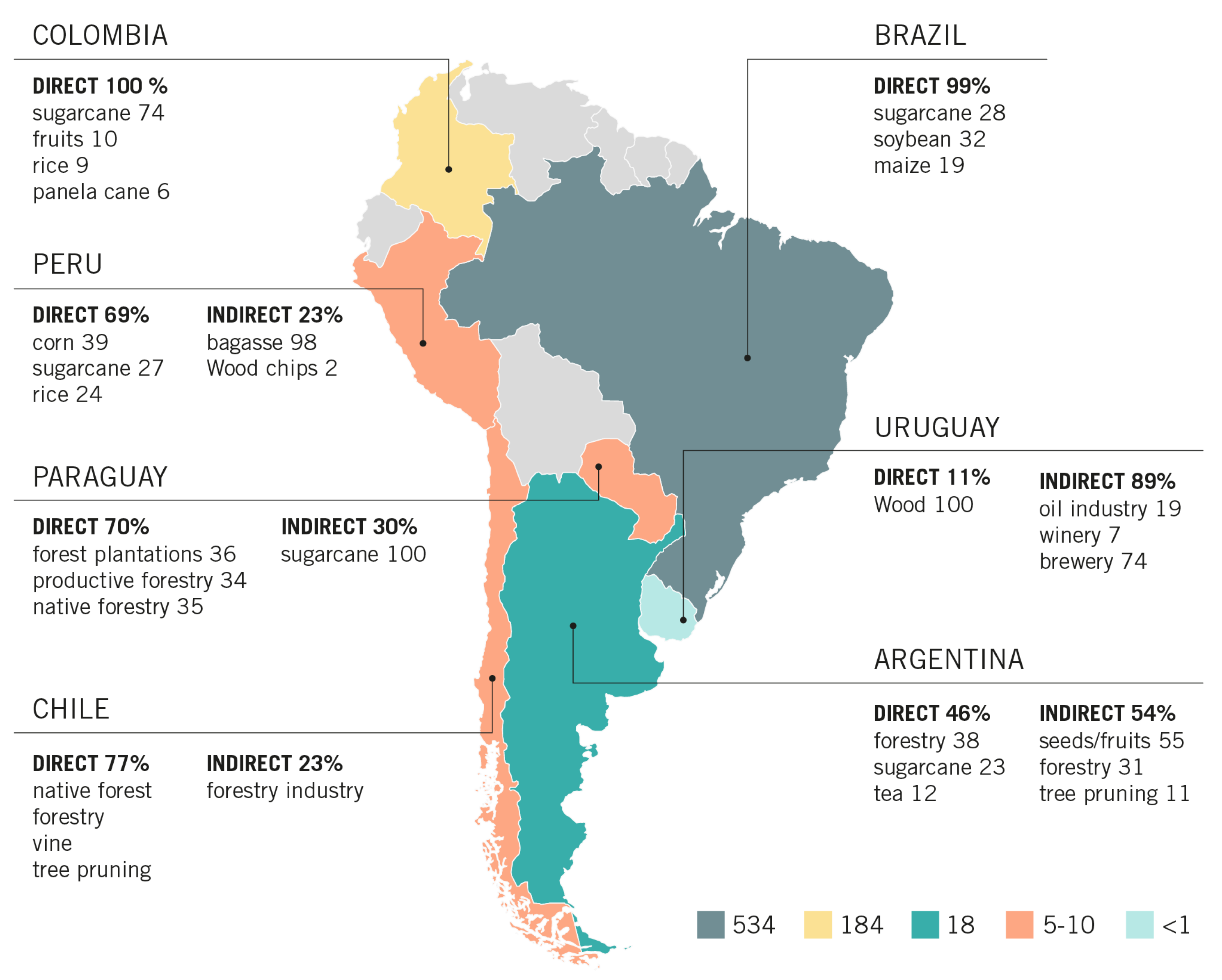
| Country | Amount (t/Year) and Source | Nature and Source (%) | Reference | ||
|---|---|---|---|---|---|
| Direct | Indirect | Direct | Indirect | ||
| Argentina | 8,475,731 | 10,131,736 | forestry (38%), sugarcane bagasse (23%), tea (12%), grapevine (7%), banana (6%), rice (5%), others | mills (55%), forestry industries (31%), peanut processing (3%), others such as tree pruning residue (11%) | [7] |
| Chile | 4,999,477 | 1,531,710 | native forest, forestry, vine and various pruning residues | forestry industries | [8] |
| Paraguay | 2,568,562–3,186,132 | 1,369,990 | forest plantations (36%), productive forestry (34%), native forestry (35%) | sugarcane bagasse of bioethanol production | [9] |
| Colombia | 182,643,563 | 254,255 | sugarcane bagasse (74%), rice (8.7%), fruits (9.9%), panela cane (5.6%) | tree pruning residue (52.7%) | [10] |
| Perú | 7,083,496 | 3,164,174 | corn (39%), sugarcane (27%), rice (24%) | bagasse (98%), wood chips (2%) | [11] |
| Brazil | 518,390,000 (agriculture residues—crops) 9,420,000 (forestry residues) | 5,810,000 | sugarcane (28%), soybean (32%), maize (19%) | industrial residues (47.7%) | [12,13] |
| Uruguay | 2222 | 17,967 | Wood chips and wood waste | oil industry (19%), wineries (6.5%), breweries (74%) | [14,15,16,17,18] |
| Cellulolytic Enzyme Components and Other Ones Associated with Fungal Degradation of Plant Cell Wall | Fungal Sources | Production Systems | Reference |
|---|---|---|---|
| β-1,4 Endoglucanase, E.C. 3.2.1.4; cello-biohydrolase; E.C. 3.2.1.91; β-glucosidase, E.C. 3.2.1.21 | Ulocladium botrytis LPSC 813 (Pleosporaceae) | Solid-state fermentation on Scutia buxifolia litter | [94] |
| Extracellular proteins showing cellobiohydrolase, β-glucosidase and endoglucanase activity | Fourteen white rot fungi isolated from the Misiones rainforest (Argentina) belonging to the genera Pycnoporus and Trametes | Agar and liquid cultures using specific inducers | [95] |
| β-1,4-endoglucanase, E.C. 3.2.1.4; β-glucosidase, EC 3.2.1.21; endo-1,4-β-xylanase, E.C. 3.2.1.8; pectin esterase, E.C. 3.1.1.11 | Six compatible consortia of Trichoderma strains with Aspergillus niger or Pleurotus ostreatus | Solid-state fermentation on pineapple crown waste | [96] |
| C1-specific AA9 lytic polysaccharide monooxygenase | Recombinant protein from Pycnoporus sanguineus expressed in Pichia pastoris | Liquid cultures induced with methanol | [97] |
| Hydrolytic activity on different polysaccharides such as carboxy-methyl cellulose (CMC), Avicel, acid swollen cellulose, bacterial microcrystalline cellulose, laminarin, lichenan, starch, birchwood xylan and oat spelt xylan | Penicillium sp. CR-316 and Penicillium sp. CR-313 isolated from the subtropical soil of Puerto Iguazu’ (Argentina) | Shaken liquid cultures on potato dextrose broth and mineral medium supplemented with CMC, Avicel or rice straw at 1% | [98] |
| CMCase | Yeasts isolated from the Antarctic region | Shaken liquid cultures and semi-solid ones supplemented with CMC | [99] |
| Alkaline cellulases | Fungi isolated from an undisturbed rainforest in Peru | Agar and liquid cultures using specific inducers | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampaolesi, S.; Briand, L.E.; Saparrat, M.C.N.; Toledo, M.V. Potentials of Biomass Waste Valorization: Case of South America. Sustainability 2023, 15, 8343. https://doi.org/10.3390/su15108343
Sampaolesi S, Briand LE, Saparrat MCN, Toledo MV. Potentials of Biomass Waste Valorization: Case of South America. Sustainability. 2023; 15(10):8343. https://doi.org/10.3390/su15108343
Chicago/Turabian StyleSampaolesi, Sofía, Laura Estefanía Briand, Mario Carlos Nazareno Saparrat, and María Victoria Toledo. 2023. "Potentials of Biomass Waste Valorization: Case of South America" Sustainability 15, no. 10: 8343. https://doi.org/10.3390/su15108343
APA StyleSampaolesi, S., Briand, L. E., Saparrat, M. C. N., & Toledo, M. V. (2023). Potentials of Biomass Waste Valorization: Case of South America. Sustainability, 15(10), 8343. https://doi.org/10.3390/su15108343








