Metallurgical Wastes as Resources for Sustainability of the Steel Industry
Abstract
:1. Introduction
- waste prevention—by application of “clean technologies”;
- waste minimization—by implementing best practices in every waste generating activity;
- valorization—by reuse, material recycling and energy recovery;
- disposal—by incineration and landfill.
- enhancing recovery;
- reducing the hazardous nature of waste;
- reducing the final disposal of waste in such a way as to safeguard human health and the environment.
- securing the necessary waste disposal capacities by giving priority to waste disposal installations at area level;
- closing down waste disposal sites failing to meet EU requirements.
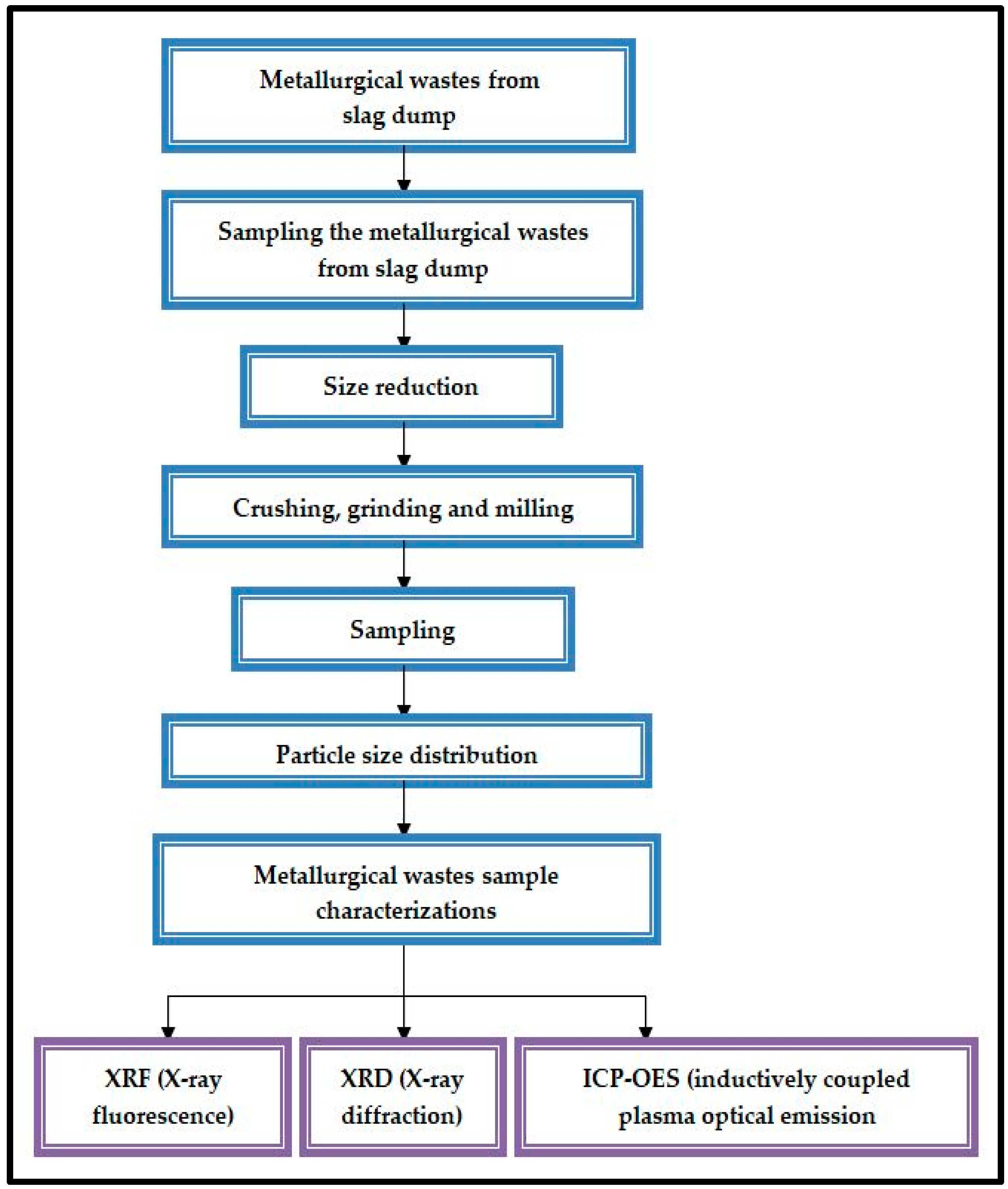
2. Materials and Methods
- X-ray diffraction (XRD) is one of the most powerful and modern techniques for qualitative and quantitative analysis of crystalline compounds. The technique provides information that cannot be obtained in any other way. The identification of the mineralogical phases found in metallurgical wastes is a helpful technique for finding their potential applications. Therefore, the analysis of these spectra is usually performed after chemical analysis, which orientates the search of the pattern peaks and leads to the identification of the sample structures. The minerals’ investigation by X-ray diffraction can detect traces of crystalline phases up to 1% by mass [24,25,28,29].
3. Results and Discussion
- the chemical composition of the metallurgical wastes varies from sample to sample;
- the predominant chemical compounds in the composition of the analyzed wastes are: SiO2, Fetotal, CaO and MgO;
- in their composition, no free CaO and no free MgO was identified;
- the waste samples have significant concentrations of total iron (Fetotal);
- the most important content of total iron (Fetotal) was found in sample 1 (24.2%).
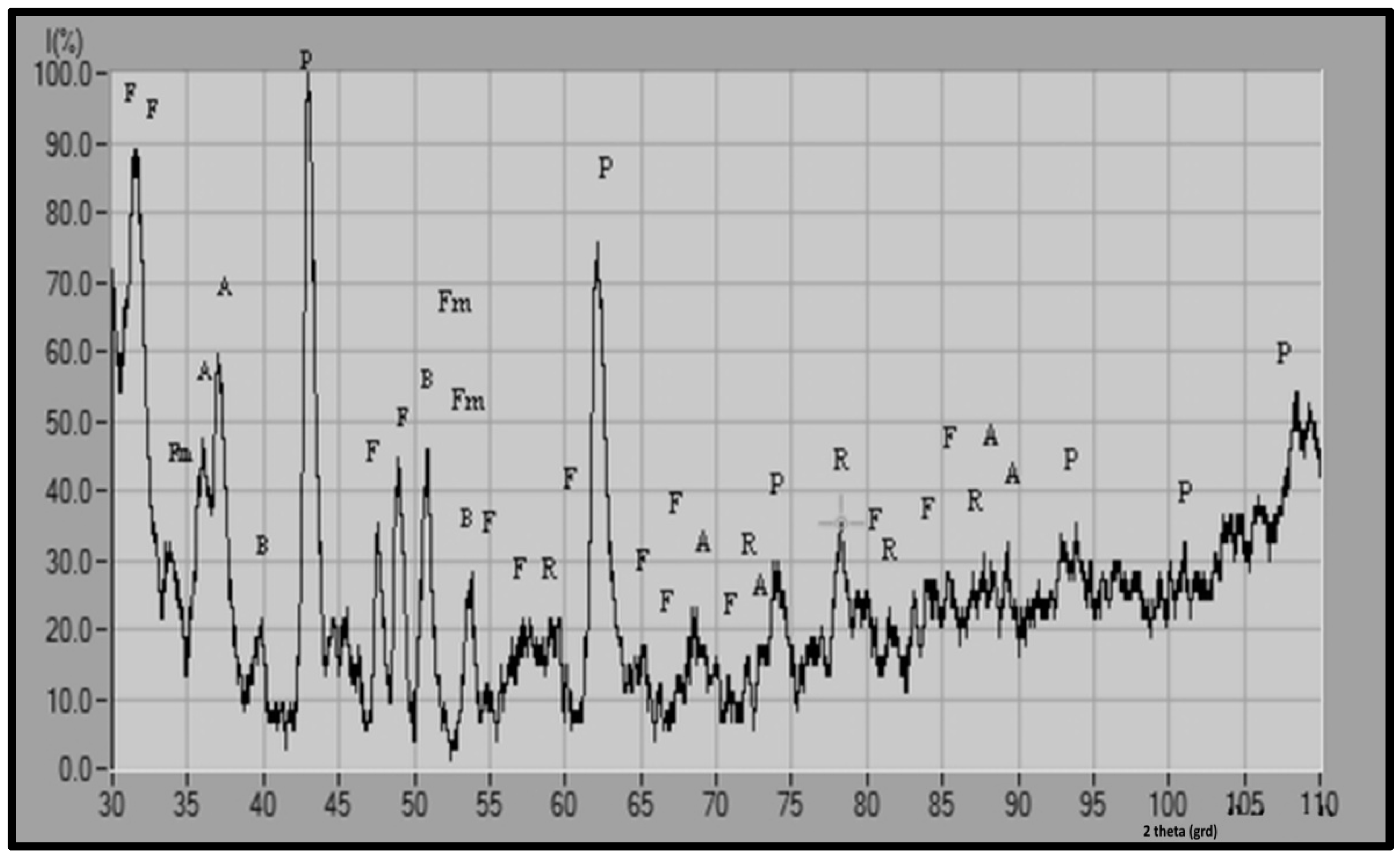
- the mineralogical composition of the metallurgical wastes from the slag dump is complex;
- the mineralogical composition of wastes varies from sample to sample;
- the major mineralogical phases identified in the metallurgical waste sample 1 are: Hedenbergite and Fe–Ringwoodite; the major mineralogical phases identified in the metallurgical waste sample 2 are: Periclase and Fayalite magnesian manganoan;
- the intermediate mineralogical phases identified in the metallurgical waste sample 1 are: Calcium iron oxide and Brownmillerite; the intermediate mineralogical phases identified in the metallurgical waste sample 2 are: Andradite and Brownmillerite;
- the minor mineralogical phases identified in the metallurgical waste sample 1 are: Magnetite, Magnesioferrite and Andradite; the minor mineralogical phases identified in the metallurgical waste sample 2 are: Fe–Ringwoodite and Fayalite manganoan;
- in both waste samples, the following mineralogical phases were identified: Fe–Ringwoodite, Andradite and Brownmillerite;
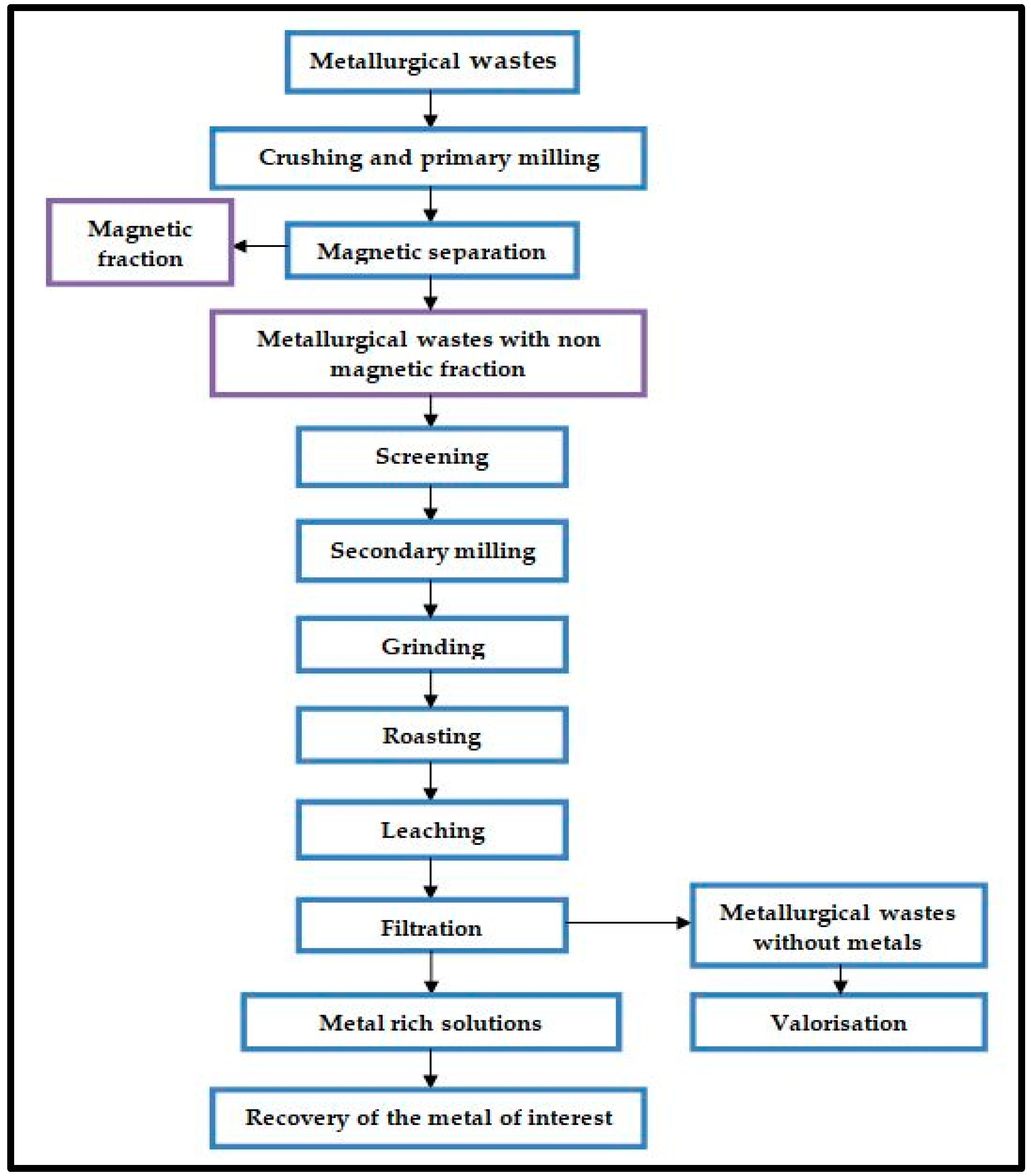
- they contain valuable components that can be reused in various processes;
- the existing ferrous components in the waste samples can be reused (after mechanical pre-processing and magnetic separation) as raw materials in the process from which they originate or other processes;
- from an economic point of view, the usage of mineralogical compounds from metallurgical wastes may reduce the cost of extracting and processing the natural resources;
- the identified mineralogical compounds have a great economic importance in terms of saving natural resources.
- Fayalite can be used as refractory sands, abrasives and mineral specimens;
- Magnetite can be used as: ore of iron; a heavy medium (magnetite is often mixed with a liquid for use as a heavy medium for specific gravity separations); an abrasive (synthetic emery is produced by mixing magnetite with aluminum oxides); a toner in electrophotography; a micronutrient in fertilizers; a pigment in paints; an aggregate in high-density concrete;
- Magnesioferrite can be used: in heterogeneous catalysis, adsorption, sensors, magnetic technologies and also for the adsorption of SiO2; for ferrite pigment production; it can be highly effective in cleaning water sources by degrading contaminants and removing other unwanted substances from the environment;
- Periclase is used as an additional material in the cement industry, at a site-batching plant, or by blending MgO clinker into the cement clinker before grinding them together. According to Walling [32], MgO-based cements provide a large-scale replacement for Portland cement in the production of steel-reinforced concretes for civil engineering applications.
- Periclase (MgO) is one of the raw materials used for making Portland cement. It has been found that adding MgO powder to concrete will influence mechanical properties, but will have very little effect on thermal properties. Long-term studies have demonstrated that because the hydration process is irreversible and Mg(OH)2 is stable, the mechanical behavior of MgO concrete is stable [42,46,47,48].
- -
- lower added value applications, are basically direct applications that utilize the physical aspects of the metallurgical wastes, such as construction aggregates;
- -
- higher added value applications, utilize the chemical and mineralogical compositions of the metallurgical wastes and require further processing procedures, such as: crushing or grinding, screening and magnetic separation; higher added value recycling applications of metallurgical wastes are as a raw material for steel industry, ceramic building materials, Portland cement, etc.
- -
- “Dissemination of results of the European projects dealing with reuse and recycling of by-products (REUSteel)”; focused on the reuse and recycling of by-products in the steel sector;
- -
- “Recycling of residues from metallurgical industry with the arc furnace technology (Recarc)”; focused on the recycling of residues from the metallurgical industry;
- -
- “Slag NO Waste: Innovative system for 100% recycling of white slag and for ZERO WASTE electric steel production (SNOW-LIFE)”; focused on demonstrating the potential of SNOW technology to act as a cost-effective waste reduction and reuse solution for white slag, from EU steel plants.
4. Recovery Potential of Metals from Slag Dump
5. Critical Metals Connected with Market Demand
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| No. | Symbol | 2θ (Degree) | Interplanar Distance d | Interplanar Distance from the Reference Chart dref | Chart No | Mineralogical Name and Chemical Formula | Miller Indexes n k l |
| 1 | R | 30.92 | 2.89 | 2.89 | 83–2074 | Fe–Ringwoodite Fe2(SiO4) | 2 2 0 |
| 2 | C | 34.25 | 2.63 | 2.63 | 31–0274 | Calcium Iron Oxide CaFe3O5 | 0 0 3 |
| 3 | C | 35.08 | 2.49 | 2.49 | 31–0274 | Calcium Iron Oxide CaFe3O5 | 3 2 0 |
| 4 | R | 36.25 | 2.46 | 2.46 | 83–2074 | Fe–Ringwoodite Fe2(SiO4) | 3 1 1 |
| 5 | B | 36.93 | 2.43 | 2.43 | 74–1346 | Brownmillerite FeAlO3(CaO)2 | 2 1 1 |
| 6 | H | 37.99 | 2.36 | 2.36 | 87–1705 | Hedenbergite CaFe(Si2O6) | 1 3 1 |
| 7 | C | 42.80 | 2.11 | 2.11 | 31–0274 | Calcium Iron Oxide CaFe3O5 | 3 2 2 |
| 8 | B | 50.50 | 1.79 | 1.79 | 74–1346 | Brownmillerite FeAlO3(CaO)2 | 0 6 2 |
| 9 | B | 50.66 | 1.79 | 1.79 | 74–1346 | Brownmillerite FeAlO3(CaO)2 | 2 3 2 |
| 10 | B | 53.99 | 1.69 | 1.69 | 74–1346 | Brownmillerite FeAlO3(CaO)2 | 1 0 3 |
| 11 | B | 56.32 | 1.63 | 1.63 | 74–1346 | Brownmillerite FeAlO3(CaO)2 | 0 7 2 |
| 12 | H | 58.48 | 1.57 | 1.57 | 87–1705 | Hedenbergite CaFe(Si2O6) | 5 3 0 |
| 13 | A | 61.68 | 1.50 | 1.50 | 10–0288 | Andradite (Calcium Iron Silicate) Ca3Fe2 + 3(SiO4)3 | 8 0 0 |
| 14 | M | 62.41 | 1.48 | 1.48 | 88–1941 | Magnesioferrite MgFe2O4 | 4 4 0 |
| 15 | Ma | 67.21 | 1.39 | 1.39 | 73–2273 | Magnetite Mg.04Fe2.96O4 | 4 4 2 |
| 16 | H | 67.62 | 1.38 | 1.38 | 87–1705 | Hedenbergite CaFe(Si2O6) | 1 5 2 |
| 17 | Ma | 71.28 | 1.32 | 1.32 | 73–2273 | Magnetite Mg.04Fe2.96O4 | 6 2 0 |
| 18 | H | 72.29 | 1.30 | 1.30 | 87–1705 | Hedenbergite CaFe(Si2O6) | −7 1 2 |
| 19 | M | 73.90 | 1.28 | 1.28 | 88–1941 | Magnesioferrite MgFe2O4 | 5 3 3 |
| 20 | Ma | 74.46 | 1.27 | 1.27 | 73–2273 | Magnetite Mg.04Fe2.96O4 | 5 3 3 |
| 21 | R | 77.59 | 1.23 | 1.23 | 83–2074 | Fe–Ringwoodite Fe2(SiO4) | 6 2 2 |
| 22 | H | 80.21 | 1.19 | 1.19 | 87–1705 | Hedenbergite CaFe(Si2O6) | 1 7 1 |
| 23 | H | 80.60 | 1.19 | 1.19 | 87–1705 | Hedenbergite CaFe(Si2O6) | 5 5 1 |
| 24 | Ma | 83.28 | 1.16 | 1.16 | 73–2273 | Magnetite Mg.04Fe2.96O4 | 7 1 1 |
| 25 | R | 84.95 | 1.14 | 1.14 | 83–2074 | Fe–Ringwoodite Fe2(SiO4) | 7 1 1 |
| 26 | H | 88.91 | 1.10 | 1.10 | 87–1705 | Hedenbergite CaFe(Si2O6) | 5 1 3 |
| 27 | M | 89.15 | 1.09 | 1.09 | 88–1941 | Magnesioferrite MgFe2O4 | 7 3 1 |
| 28 | A | 99.75 | 1.00 | 1.00 | 10–0288 | Andradite (Calcium Iron Silicate) Ca3Fe2 + 3(SiO4)3 | 12 0 0 |
| 29 | A | 101.43 | 0.99 | 0.99 | 10–0288 | Andradite (Calcium Iron Silicate) Ca3Fe2 + 3(SiO4)3 | 12 2 0 |
Appendix B
| No. | Symbol | 2θ (Degree) | Interplanar Distance d | Interplanar Distance from the Reference Chart dref | Chart no | Mineralogical Name and Chemical Formula | Miller Indexes n k l |
| 1 | F | 31.51 | 2.83 | 2.83 | 88–1998 | Fayalite Magnesian manganoan Mg.145Fe1.742Mn.113SiO4 | 1 3 0 |
| 2 | F | 31.63 | 2.82 | 2.82 | 88–1997 | Fayalite magnesian manganoan Mg.347Fe1.548Mn.105SiO4 | 1.3 0 |
| 3 | Fm | 33.5 | 2.66 | 2.66 | 12–0220 | Fayalite manganoan (Fe.Mn)2SiO4 | 2 4 0 |
| 4 | A | 33.71 | 2.66 | 2.66 | 03–1136 | Andradite (Calcium Iron Silicate) Ca3Fe2 + 3(SiO4)3 | 4 2 0 |
| 5 | A | 36.97 | 2.43 | 2.43 | 03–1136 | Andradite (Calcium Iron Silicate) Ca3Fe2 + 3(SiO4)3 | 4 2 2 |
| 6 | B | 39.88 | 2.2 | 2.2 | 11–0124 | Brownmillerite (Calcium Aluminium Iron Oxide) Ca4Al2Fe2 + 3O10 | 2 3 1 |
| 7 | P | 42.94 | 2.10 | 2.10 | 43–1022 | Periclase MgO | 2 0 0 |
| 8 | F | 45.56 | 1.98 | 1.98 | 88–1998 | Fayalite magnesian manganoan Mg.145Fe1.742Mn.113SiO4 | 0 4 2 |
| 9 | F | 45.90 | 1.98 | 1.98 | 88–1997 | Fayalite magnesian manganoan Mg.347Fe1.548Mn.105SiO4 | 2 3 0 |
| 10 | B | 47.61 | 1.92 | 1.92 | 11–0124 | Brownmillerite (Calcium Aluminium Iron Oxide) Ca4Al2Fe2 + 3O10 | 2 1 2 |
| 11 | B | 44.06 | 1.86 | 1.86 | 11–0124 | Brownmillerite (Calcium Aluminium Iron Oxide) Ca4Al2Fe2 + 3O10 | 2 2 2 |
| 12 | Fm | 50.83 | 1.79 | 1.79 | 12–0220 | Fayalite manganoan (Fe.Mn)2SiO4 | 2 4 0 |
| 13 | Fm | 53.58 | 1.71 | 1.71 | 12–0220 | Fayalite manganoan (Fe.Mn)2SiO4 | 2 4 1 |
| 14 | F | 53.79 | 1.70 | 1.70 | 88–1997 | Fayalite magnesian manganoan Mg.347Fe1.548Mn.105SiO4 | 2 4 1 |
| 15 | F | 56.50 | 1.62 | 1.62 | 88–1998 | Fayalite magnesian manganoan Mg.145Fe1.742Mn.113SiO4 | 1 5 2 |
| 16 | R | 57.64 | 1.59 | 1.59 | 74–1002 | Fe–Ringwoodite Fe2(SiO4) | 5 1 1 |
| 17 | F | 60.15 | 1.53 | 1.53 | 88–1998 | Fayalite magnesian manganoan Mg.145Fe1.742Mn.113SiO4 | 2 5 1 |
| 18 | P | 62.14 | 1.49 | 1.49 | 43–1022 | Periclase MgO | 2 2 0 |
| 19 | F | 66.26 | 1.41 | 1.41 | 88–1998 | Fayalite magnesian manganoan Mg.145Fe1.742Mn.113SiO4 | 2 6 0 |
| 20 | F | 68.10 | 1.37 | 1.37 | 88–1998 | Fayalite magnesian manganoanMg.145Fe1.742Mn.113SiO4 | 2 6 1 |
| 21 | F | 68.46 | 1.37 | 1.37 | 88–1998 | Fayalite magnesian manganoan Mg.145Fe1.742Mn.113SiO4 | 3 2 2 |
| 22 | A | 69.98 | 1.34 | 1.34 | 03–1136 | Andradite(Calcium Iron Silicate)Ca3Fe2 + 3(SiO4)3 | 8 4 0 |
| 23 | F | 70.81 | 1.33 | 1.33 | 88–1998 | Fayalite magnesian manganoan Mg.145Fe1.742Mn.113SiO4 | 3 4 1 |
| 24 | R | 72.03 | 1.31 | 1.31 | 74–1002 | Fe–Ringwoodite Fe2(SiO4) | 6 2 0 |
| 25 | A | 73.83 | 1.28 | 1.28 | 03–1136 | Andradite (Calcium Iron Silicate) Ca3Fe2 + 3(SiO4)3 | 6 6 4 |
| 26 | P | 74.08 | 1.27 | 1.27 | 43–1022 | Periclase MgO | 3 1 1 |
| 27 | R | 75.62 | 1.25 | 1.25 | 74–1002 | Fe–Ringwoodite Fe2(SiO4) | 6 2 2 |
| 28 | F | 81.44 | 1.18 | 1.18 | 88–1998 | Fayalite magnesian manganoan Mg.145Fe1.742Mn.113SiO4 | 3 3 3 |
| 29 | R | 83.10 | 1.16 | 1.16 | 74–1002 | Fe–Ringwoodite Fe2(SiO4) | 7 1 1 |
| 30 | F | 84.44 | 1.14 | 1.14 | 88–1998 | Fayalite magnesian manganoan Mg.145Fe1.742Mn.113SiO4 | 0 6 4 |
| 31 | F | 83.56 | 1.13 | 1.13 | 88–1998 | Fayalite magnesian manganoan Mg.145Fe1.742Mn.113SiO4 | 1 9 0 |
| 32 | R | 87.02 | 1.11 | 1.11 | 74–1002 | Fe–Ringwoodite Fe2(SiO4) | 6 4 2 |
| 33 | A | 87.78 | 1.11 | 1.11 | 03–1136 | Andradite (Calcium Iron Silicate) Ca3Fe2 + 3(SiO4)3 | 9 6 1 |
| 34 | A | 89.32 | 1.09 | 1.09 | 03–1136 | Andradite (Calcium Iron Silicate) Ca3Fe2 + 3(SiO4)3 | 8 7 3 |
| 35 | P | 93.90 | 1.05 | 1.05 | 43–1022 | Periclase MgO | 4 0 0 |
| 36 | P | 105.90 | 0.96 | 0.96 | 43–1022 | Periclase MgO | 3 3 1 |
| 37 | P | 108.50 | 0.97 | 0.94 | 43–1022 | Periclase MgO | 4 2 0 |
References
- Branca, T.A.; Colla, V.; Algermissen, D.; Granbom, H.; Martini, U.; Morillon, A.; Pietruck, R.; Rosendahl, S. Reuse and Recycling of By-Products in the Steel Sector: Recent Achievements Paving the Way to Circular Economy and Industrial Symbiosis in Europe. Metals 2020, 10, 345. [Google Scholar] [CrossRef] [Green Version]
- Birat, J.-P. Society, Materials, and the Environment: The Case of Steel. Metals 2020, 10, 331. [Google Scholar] [CrossRef] [Green Version]
- Terrones-Saeta, J.M.; Suárez-Macías, J.; Iglesias-Godino, F.J.; Corpas-Iglesias, F.A. Evaluation of the Use of Electric Arc Furnace Slag and Ladle Furnace Slag in Stone Mastic Asphalt Mixes with Discarded Cellulose Fibers from the Papermaking Industry. Metals 2020, 10, 1548. [Google Scholar] [CrossRef]
- Lis, T.; Nowacki, K.; Żelichowska, M.; Kania, H. Innovation in metallurgical waste management. Metabk 2015, 54, 283–285. [Google Scholar]
- Iluţiu-Varvara, D.A. Researching the hazardous potential of metallurgical solid wastes. Pol. J. Environ. Stud. 2016, 25, 147–152. [Google Scholar] [CrossRef]
- Sankaran, K.J.; Suman, S.; Sahaw, A.; Balaji, U.; Sakthivel, R. Improved LPG sensing properties of nickel doped cobalt ferrites derived from metallurgical wastes. J. Magn. Magn. Mater. 2021, 537, 168231. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Worldsteel Association: Steel Industry by-Products. Available online: https://www.worldsteel.org/en/dam/jcr:1b916a6d-06fd-4e84-b35d-c1d911d18df4/Fact_By-products_2018.pdf (accessed on 29 December 2021).
- National Waste Management Strategy–Ministry of Environment and Water Management, Romania. 2014. Available online: http://mmediu.ro/new/wp-content/uploads/2014/01/NationalWasteStrategy.pdf (accessed on 26 December 2021).
- Iluţiu-Varvara, D.A. Study concerning the analysis of the solid waste generation dynamics specific to the steelmaking in Romania. Metal. Int. 2012, 17, 70–75. [Google Scholar]
- Environmental Report for Romania’s Energy Strategy 2020–2030, with a View to 2050, Romania. 2020. Available online: http://www.mmediu.ro/app/webroot/uploads/files/Raport%20de%20mediu_aug%202020.pdf (accessed on 27 December 2021).
- National Plan of the Waste Management, Romania. 2018. Available online: http://mmediu.ro/app/webroot/uploads/files/2018-01-10_MO_11_bis.pdf (accessed on 26 December 2021).
- World Commission on Environment and Development (WCED). Our Common Future; Oxford University Press: Oxford, UK; New York, NY, USA, 1987. [Google Scholar]
- Rosen, M.A.; Kishawy, H.A. Sustainable Manufacturing and Design: Concepts, Practices and Needs. Sustainability 2012, 4, 154–174. [Google Scholar] [CrossRef] [Green Version]
- Iluţiu-Varvara, D.A.; Tintelecan, M.; Aciu, C.; Sas-Boca, I.M. Reuse of the Steel Mill Scale for Sustainable Industrial Applications. Proceedings 2020, 63, 14–17. [Google Scholar]
- Kucuker, M.A. Biomining Concept for Recovery of Rare Earth Elements (REEs) from Secondary Sources. In Hamburger Berichte; Verlag Abfall aktuell der Ingenieurgruppe RUK GmbH: Stuttgart, Germany, 2018; ISBN 9783981757286. [Google Scholar]
- Szyczewski, P.; Siepak, J.; NiedzielskI, P.; Sobczyński, T. Research on heavy metals in Poland. Pol. J. Environ. Stud. 2009, 18, 755–768. [Google Scholar]
- Tumuklu, A.; Yalcin, M.G.; Sonmez, M. Detection of heavy metal concentrations in soil caused by Nigde City Garbage Dump. Pol. J. Environ. Stud. 2007, 16, 651–658. [Google Scholar]
- Djokic, J.; Minic, D.; Kamberovic, Z. Reuse of metallurgical slag from the silicothermic magnesium production and secondary lead metallurgy. Metal. Int. 2012, 3, 46–52. [Google Scholar]
- Pasetto, M.; Baldo, N. Recycling of steel slags in road foundations. Environ. Eng. Manag. J. 2010, 9, 773–777. [Google Scholar] [CrossRef]
- Sofilić, T.; Mladenovič, A.; Sofilić, U. Defining of EAF steel slag application possibilities in asphalt mixture production. J. Environ. Eng. Landsc. 2011, 19, 148–157. [Google Scholar]
- Jonczy, I. Diversification of phase composition of metallurgical wastes after the production of cast iron and cast steel. Arch. Metall. Mater. 2014, 59, 481–485. [Google Scholar] [CrossRef]
- Jonczy, I.; Stanek, J. Phase composition of metallurgical slag studied by Mössbauer spectroscopy. Nukleonika 2013, 58, 127–131. [Google Scholar]
- Arghir, G. Crystallographic Characterization of Metals and Alloys by X-ray Diffraction; Technical University Publishing: Cluj-Napoca, Romania, 1993. [Google Scholar]
- Querait, I.; Margui, E.; Grieken, R.V. Sample Preparation for X-Ray Fluorescence Analysis. In Enciclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar]
- EN 15309:2007; Characterization of Waste and Soil—Determination of Elemental Composition by X-ray Fluorescence. Available online: https://standards.iteh.ai/catalog/standards/cen/9e1fdc1f-ee02-4ff2-9cfb-e337d95e97d7/en-15309-2007 (accessed on 8 August 2021).
- Lavina, B.; Dera, P.; Downs, R.T. Modern X-ray diffraction methods in mineralogy and geosciences. Rev. Mineral. Geochem 2014, 78, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Franus, W. Characterization of X-type zeolite prepared from coal fly ash. Pol. J. Environ. Stud. 2012, 21, 337–343. [Google Scholar]
- Sahan, M.; Kucuker, M.A.; Demirel, B.; Kuchta, K.; Hursthouse, A. Determination of metal content of waste mobile phones and estimation of their recovery potential in Turkey. Int. J. Environ. Res. Public Health 2019, 16, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- London Metal Exchange. 2021. Available online: https://www.lme.com/ (accessed on 8 August 2021).
- Romanian Commodities Exchange. 2021. Available online: https://www.brm.ro/ (accessed on 8 August 2021).
- Available online: https://en.wikipedia.org/wiki/Prices_of_chemical_elements (accessed on 16 October 2021).
- Shanghai Metals Market. 2020. Available online: https://www.metal.com/ (accessed on 27 December 2021).
- Federal Institute for Geosciences and Natural Resources (Bundesanstalt für Geowissenschaften und Rohstoffe or BGR). 2021. Available online: https://www.bgr.bund.de/DE/Themen/Min_rohstoffe/Produkte/Preisliste/pm_19_12.pdf (accessed on 27 December 2021).
- Wang, G. Determination of the expansion force of coarse steel slag aggregate. Constr. Build. Mater. 2010, 24, 1961–1966. [Google Scholar] [CrossRef]
- Iluţiu-Varvara, D.A.; Mârza, C.M.; Brânduşan, L.; Aciu, C.; Balog, A.; Cobȋrzan, N. Assessment of the metallic iron content from steelmaking slags in order to conserve natural resources. Proc. Technol. 2014, 12, 615–620. [Google Scholar] [CrossRef] [Green Version]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 2001. [Google Scholar]
- Paceagiu, J.; Rădulescu, E.; Dragomir, A.M.; Hotnog, R. Implications of the use of steel slag to clinker manufacture: Laboratory test results. Rom. J. Mater. 2010, 40, 306–314. [Google Scholar]
- Iluţiu-Varvara, D.A.; Brânduşan, L.; Arghir, G.; Pică, E.M. Researches about the characterization of metallurgical slags for landfilled wastes minimization. Environ. Eng. Manag. J. 2015, 14, 2115–2126. [Google Scholar] [CrossRef]
- Kong, Z.Y.; Wong, X.N.; Lum, S.W.; Tan, S.Y.; Khan, M.R.; Cheng, C.K. The application of magnesium ferrite photocatalyst for photo treatment of methylene blue. J. Eng. Sci. Technol. 2015, 10, 1–10. [Google Scholar]
- Du, C. A review of magnesium oxide in concrete. Concr. Int. 2005, 27, 45–50. [Google Scholar]
- Schwarz, M.; Veverka, M.; Michalková, E.; Lalík, V.; Veverková, D. Utilisation of industrial waste for ferrite pigments production. Chem. Zvesti 2012, 66, 248–258. [Google Scholar] [CrossRef]
- Rao, S.R. Resource Recovery and Recycling from Metallurgical Wastes; Elsevier: London, UK, 2011. [Google Scholar]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]
- Li, C.M. Mechanical properties and durability of MgO concrete. Adv. Sci. Technol. Water Resour. 2000, 20, 30. [Google Scholar]
- Li, C.M. Effect of temperature on autogenous volume expansion of MgO concrete. Des. Hydropower Stn. 1999, 15, 96. [Google Scholar]
- Serjun, V.Z.; Mirtic, B.; Mladenovic, A. Evaluation of ladle slag as a potential material for building and civil engineering. Mater. Technol. 2013, 47, 543–550. [Google Scholar]
- Friedrich, B. Sustainable Utilization of Metals-Processing, Recovery and Recycling. Metals 2019, 9, 769. [Google Scholar] [CrossRef] [Green Version]
- Aciu, C.; Manea, D.L.; Puia, C.; Cadar, O. Mortars for the enhancement of the indoor environmental quality. Studia UBB Chemia 2015, 60, 45–54. [Google Scholar]
- Vavilapalli, D.S.; Banik, S.; Peri, R.G.; Muthuraaman, B.; Miryala, M.; Murakami, M.; Klimkowicz, A.; Asokan, K.; Ramachandra Rao, M.S.; Singh, S. Nitrogen Incorporated photoactive brownmillerite Ca2Fe2O5 for energy and environmental applications. Nature 2020, 10, 2713. [Google Scholar] [CrossRef] [Green Version]
- Willey, R.J.; Noirclerc, P.; Busca, G. Preparation and characterization of magnesium chromite and magnesium ferrite aerogels. Chem. Eng. Commun. 1993, 123, 1. [Google Scholar] [CrossRef]
- Swapan, K.P.; Sumanta, S.; Hema, D. Microstructure characterization of nanocrystalline magnesium ferrite annealed at elevated temperatures by Rietveld Method. ISRN Ceramics 2011, 2011, 194575. [Google Scholar]
- Katz, E. Synthesis, Properties and applications of magnetic nanoparticles and nanowires-a brief introduction. Magnetochemistry 2019, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- Wroblewski, C.; Volford, T.; Martos, B.; Samoluk, J.; Martos, P. High Yield Synthesis and Application of Magnetite Nanoparticles (Fe3O4). Magnetochemistry 2020, 6, 22. [Google Scholar] [CrossRef]
- Gao, D.; Wang, F.-P.; Wang, Y.-T.; Zeng, Y.-N. Sustainable Utilization of Steel Slag from Traditional Industry and Agriculture to Catalysis. Sustainability 2020, 12, 9295. [Google Scholar] [CrossRef]
- Ciocan, A.; Potecașu, F.; Radu, T. Correlation between the properties of old slags and the recycling solutions. Ann. Dunarea De Jos Univ. Galati. Metall. Mater. Sci. 2014, 3, 65. [Google Scholar]
- Brand, A.S.; Fanijo, E.O. A review of the influence of steel furnace slag type on the properties of cementitious composites. Appl. Sci. 2020, 10, 8210. [Google Scholar] [CrossRef]
- Shakhpazov, E.H.; Svyazhin, A.G. Slag recycling in ferrous metallurgy. In Proceedings of the EOSC’97: 2nd European Oxygen Steelmaking Congress, Toronto, Italy, 13–15 October 1997; p. 499. [Google Scholar]
- Reuter, M.; Xiao, Y.; Boin, U. Recycling and environmental issues of metallurgical slags and salt fluxes. In VII International Conference on Molten Slags Fluxes and Salts; The South African Institute of Mining and Metallurgy: Johannesburg, South Africa, 2004. [Google Scholar]
- Horckmans, L.; Möckel, R.; Nielsen, P.; Kukurugya, F.; Vanhoof, C.; Morillon, A.; Algermissen, D. Multi-Analytical Characterization of Slags to Determine the Chromium Concentration for a Possible Re-Extraction. Minerals 2019, 9, 646. [Google Scholar] [CrossRef] [Green Version]
- Chamling, P.K.; Haldar, S.; Patra, S. Physico-Chemical and Mechanical Characterization of Steel Slag as Railway Ballast. Indian Geotech. J. 2020, 50, 267. [Google Scholar] [CrossRef]
- Brand, A.S.; Roesler, J.R. Concrete with Steel Furnace Slag and Fractionated Reclaimed Asphalt Pavement; Report No.ICT-14-015; Illinois Center for Transportation: Urbana, IL, USA, 2014. [Google Scholar]
- Wang, X.; Geysen, D.; Van Gerven, T.; Jones, P.T.; Blanpain, B.; Guo, M. Characterization of landfilled stainless steel slags in view of metal recovery. Front. Chem. Sci. Eng. 2017, 11, 353. [Google Scholar] [CrossRef]
- Herbelin, M.; Bascou, J.; Lavastre, V.; Guillaume, D.; Benbakkar, M.; Peuble, S.; Baron, J.-P. Steel Slag Characterisation—Benefit of Coupling Chemical, Mineralogical and Magnetic Techniques. Minerals 2020, 10, 705. [Google Scholar] [CrossRef]
- Das, B.; Prakash, S.; Reddy, P.S.R.; Misra, V.N. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recycl. 2007, 50, 40–57. [Google Scholar] [CrossRef]
- European Commission. Dissemination of Results of the European Projects Dealing with Reuse and Recycling of By-Products (REUSteel). Available online: https://www.reusteel.eu/details.html (accessed on 22 February 2022).
- European Commission. Recycling of Residues from Metallurgical Industry with the Arc Furnace Technology (Recarc). Available online: https://webgate.ec.europa.eu/life/publicWebsite/project/details/2089 (accessed on 22 February 2022).
- European Commission. Slag NO Waste: Innovative System for 100% Recycling of White Slag and for ZERO WASTE Electric Steel Production (SNOW-LIFE). Available online: https://webgate.ec.europa.eu/life/publicWebsite/index.cfm?fuseaction=search.dspPage&n_proj_id=5107 (accessed on 22 February 2022).
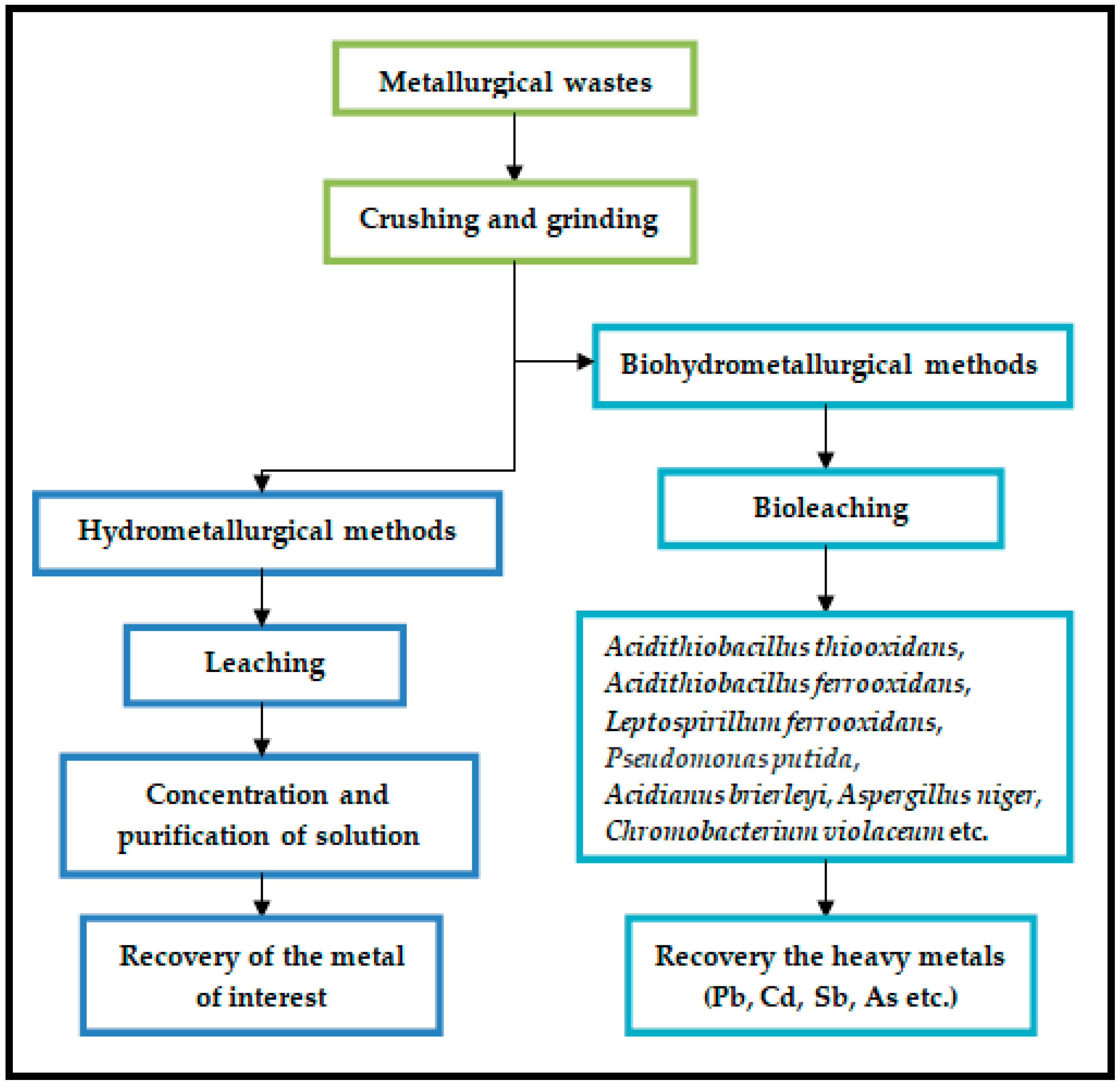
- Habib, A.; Bhatti, H.N.; Iqbal, M. Metallurgical processing strategies for metals recovery from industrial slags. Zeitschrift für Physikalische Chemie 2020, 234, 201–231. [Google Scholar] [CrossRef]
- Shiel, A.E.; Weis, D.; Orians, K.J. Evaluation of zinc, cadmium and lead isotope fractionation during smelting and refining. Sci. Total Environ. 2010, 408, 2357–2368. [Google Scholar] [CrossRef]
- Jadhav, H.; Hocheng, J. A review of recovery of metals from industrial waste. Achiev. Mater. Manuf. Eng. 2012, 54, 159–167. [Google Scholar]
- Ruiz, O.; Clemente, C.; Alonso, M.; Alguacil, F.J. Recycling of an electric arc furnace flue dust to obtain high grade ZnO. J. Hazard. Mater. 2007, 141, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.; Stevens, G. Innovations in separations technology for the recycling and re-use of liquid waste streams. Chem. Eng. J. 2001, 84, 149–159. [Google Scholar] [CrossRef]
- Silva, J.E.; Paiva, A.; Soares, D.; Labrincha, A.; Castro, F. Solvent extraction applied to the recovery of heavy metals from galvanic sludge. J. Hazard. Mater. 2005, 120, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, G. Biohydrometallurgy; McGraw-Hill: Himburg, Germany, 1990; Volume 1. [Google Scholar]
- Lee, J.C.; Pandey, B.D. Bio-processing of solid wastes and secondary resources for metal extraction—A review. Waste Manag. 2012, 32, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Properties and Utilization of Steel Slag in Engineering Applications. Ph.D. Thesis, University of Wollongong, New South Wales, Australia, 1992. [Google Scholar]
- Primavera, A.; Pontoni, L.; Mombelli, D.; Barella, S.; Mapelli, C. EAF slag treatment for inert materials’ production. J. Sustain. Metall. 2016, 2, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.bushveldminerals.com/about-vanadium/ (accessed on 21 February 2022).
- Roskill 2021: Vanadium. Outlook to 2030, 19th Edition. Available online: https://roskill.com/market-report/vanadium/ (accessed on 21 February 2022).
- European Commission. Study on the EU’s list of Critical Raw Materials (2020)–Critical Raw Materials Factsheets (Final). 2020. Available online: https://ec.europa.eu/docsroom/documents/42883/attachments/2/translations/en/renditions/native (accessed on 21 February 2022).
- Roskill 2021: Titanium Metal Outlook to 2031. Available online: https://www.woodmac.com/reports/metals-roskill-titanium-metal-outlook-to-2031-528375 (accessed on 21 February 2022).
- Watari, T.X.; Nansai, K.; Nakajima, K. Review of critical metal dynamics to 2050 for 48 elements. Resour. Conserv. Recycl. 2020, 155, 104669. [Google Scholar] [CrossRef]
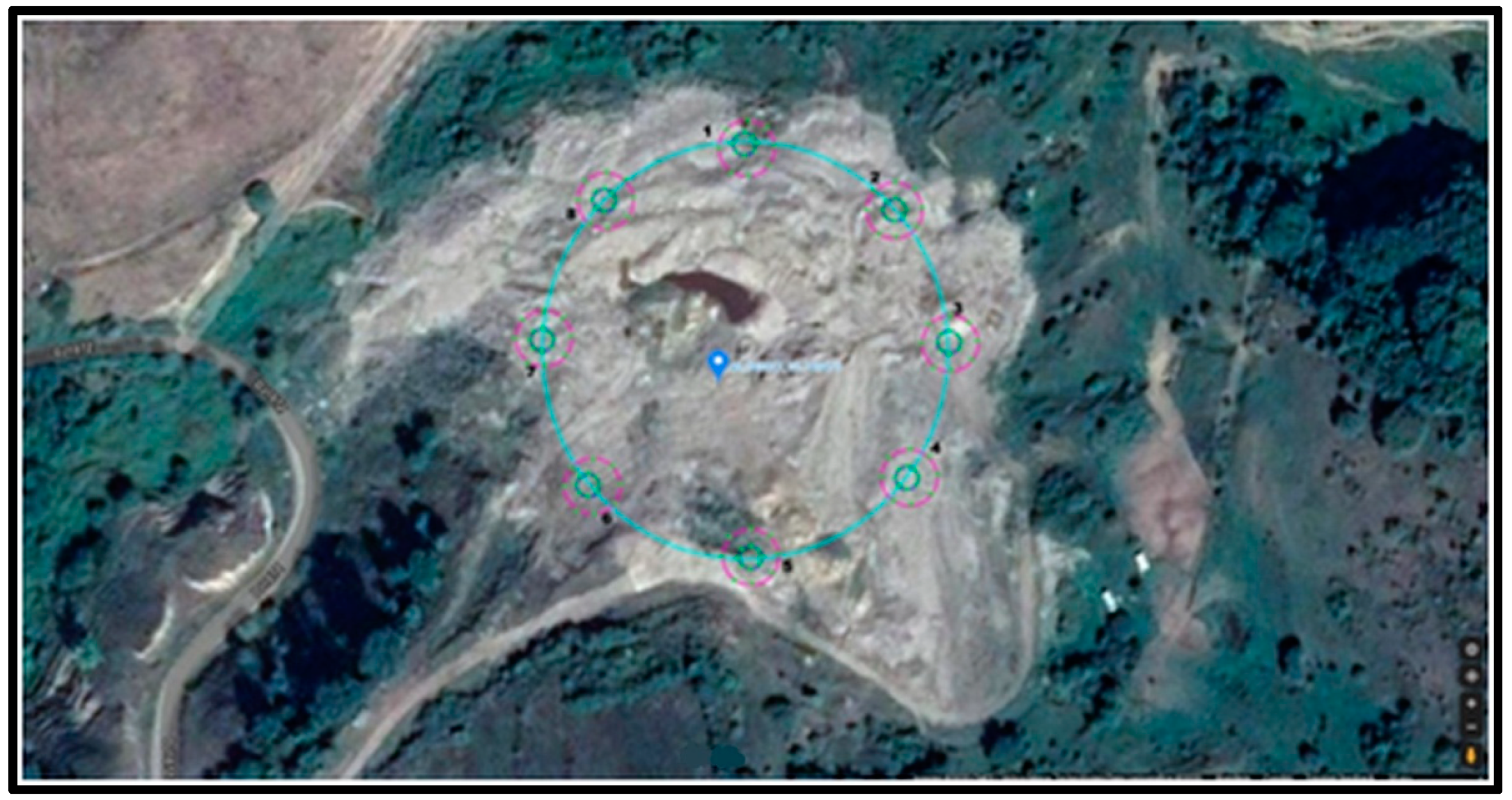

| No. | GPS Values |
|---|---|
| 1 | 46°34′06.12″ N, 23°74′95.83″ E |
| 2 | 46°34′05.21″ N, 23°75′01.30″ E |
| 3 | 46°34′01.70″ N, 23°75′03.79″ E |
| 4 | 46°33′98.33″ N, 23°75′00.67″ E |
| 5 | 46°33′95.89″ N, 23°74′96.17″ E |
| 6 | 46°33′97.48″ N, 23°74′89.95″ E |
| 7 | 46°34′02.48″ N, 23°74′87.32″ E |
| 8 | 46°34′05.37″ N, 23°74′89.73″ E |
| Distribution before Grinding (%) Sample 1 | Distribution after Grinding (%) Sample 1 | Distribution before Grinding (%) Sample 2 | Distribution after Grinding (%) Sample 2 | Grain Size (mm) |
|---|---|---|---|---|
| 3 | 7 | 6 | 9 | d < 0.005 |
| 5 | 11 | 9 | 14 | 0.005 < d < 0.05 |
| 6 | 15 | 15 | 29 | 0.05 < d < 2 |
| 34 | 38 | 44 | 28 | 2 < d < 5 |
| 31 | 10 | 18 | 12 | 5 < d < 10 |
| 21 | 19 | 8 | 8 | d > 10 |
| No. | MgO (%) | CaO (%) | Al2O3 (%) | Fetotal (%) | SiO2 (%) | MnO (%) | P2O5 (%) | V2O5 (%) | TiO2 (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12.9 ± 1.8 | 20.1 ± 1.9 | 6.9 ± 0.6 | 24.2 ± 1.8 | 25.8 ± 2.9 | 7.5 ± 0.5 | 0.36 ± 0.5 | 0.37 ± 0.6 | - |
| 2 | 18.3 ± 1.8 | 26.2 ± 1.9 | 3.9 ± 0.5 | 19.6 ± 1.6 | 22.3 ± 1.5 | 5.7 ± 0.4 | 0.45 ± 0.5 | 0.72 ± 0.9 | 0.39 ± 0.4 |
| No. | Elements | Sample 1 (ppm) | Sample 2 (ppm) |
|---|---|---|---|
| 1 | V | 2983 | 3214 |
| 2 | Cr | 152 | 89 |
| 3 | Ni | 795 | 898 |
| 4 | Cu | 2841 | 3567 |
| 5 | Zn | 148 | 49 |
| 6 | As | 113 | 64 |
| 7 | Mo | 1398 | 1198 |
| 8 | Sn | 329 | 39 |
| 9 | Sb | 199 | 63 |
| 10 | Pb | 59 | 25 |
| 11 | Cd | 783 | 894 |
| Total | 9800 |
| No. | Mineralogical Phase | Symbol | Sample 1 (%) | Sample 2 (%) |
|---|---|---|---|---|
| 1 | Fe–Ringwoodite | R | 22 ± 0.6 | 5 ± 0.6 |
| 2 | Calcium iron oxide | C | 18 ± 0.5 | - |
| 3 | Brownmillerite | B | 16 ± 0.7 | 14 ± 1.0 |
| 4 | Hedenbergite | H | 26 ± 2.3 | - |
| 5 | Andradite (calcium iron silicate) | A | 3 ± 0.4 | 17 ± 0.5 |
| 6 | Magnesioferrite | M | 6 ± 0.5 | - |
| 7 | Magnetite | Ma | 9 ± 0.6 | - |
| 8 | Fayalite magnesian manganoan | F | - | 22 ± 0.8 |
| 9 | Fayalite manganoan | Fm | - | 3 ± 0.4 |
| 10 | Periclase | P | - | 39 ± 2.3 |
| Mineralogical Phase | Applications | References |
|---|---|---|
| Brownmillerite | Material for energy and environmental applications (fuel cells, supercapacitors, batteries). | Vavilapalli et al. [51] |
| Magnesioferrite | Semiconductor material; Heterogeneous catalysis; Adsorption; Sensors; Magnetic Technologies | Willey et al. [52]; Swapan et al. [53] |
| Magnetite | Medicine; Technology; Bioremediation; Analytical analysis. | Katz, E. [54]; Wroblewski et al. [55] |
| Periclase | Concrete; Construction of dams; Agricultural fertilizers. | Du, C. [42] Gao et al. [56] |
| Types of Metallurgical Wastes | Mineralogical Compounds Identified | References |
|---|---|---|
| Metallurgical wastes from slag dump (slag from EAF is prevalent) | Fe–Ringwoodite; Calcium iron oxide; Brownmillerite; Hedenbergite; Andradite (calcium iron silicate); Magnesioferrite; Magnetite; Fayalite magnesian manganoan; Periclase. | This study |
| EAF slag from carbon steel | Spinels; Quartz; Calcite; Wustite; Hematite; Larnite; Gehlenite; Brownmillerite. | Horckmans et al. [61] |
| Stainless steel slag | Spinels; Quartz; Calcite; Periclase; Dicalcium silicate; Cuspidine; Larnite; Wollastonite; Akermanite; Merwinite; Bredigite. | Horckmans et al. [61] |
| Steel slag | Larnite; Wuestite; Mayenite; Srebrodolskite; Portlandite. | Chamling et al. [62] |
| EAF slag | Dicalcium silicate, Merwinite, Gehlenite, Wüstite, Hematite and Magnetite, Mayenite, Brownmillerite; Periclase. | Brand et al. [63] |
| Landfilled stainless steel slag from EAF | Dicalcium silicate; Magnesiochromite; Quartz; Gehlenite; Bredigite; Magnesite; Merwinite; Calcite; Cuspidine; Akermanite; Iron carbide; Magnetite; Calcium chromate; Wollastonite. | Wang et al. [64] |
| EAF slag | Wustite; Spinel; Chromite; Brownmillerite; Calcium chromite; Larnite; Calcite; Quartz. | Herbelin et al. [65] |
| Elements | Average Concentrations | Average Concentrations | Recovery Potential | Price of Metals | Economic Value |
|---|---|---|---|---|---|
| (mg/kg) | (Metal Ton/Waste Ton) | (Metals Tons from Slag Dump) | (USD/ton) [29,30,31,32,33] | (USD Millions) | |
| V | 3098.5 | 0.0030985 | 2602.74 | 385,000.00 | 1002.0549 |
| Cr | 120.5 | 0.0001205 | 101.22 | 9400.00 | 0.9514 |
| Ni | 846.5 | 0.0008465 | 711.06 | 19,675.00 | 13.9901 |
| Cu | 3204 | 0.003204 | 2691.36 | 9503.00 | 25.5759 |
| Zn | 98.5 | 0.0000985 | 82.74 | 3006.00 | 0.24871 |
| As | 88.5 | 0.0000885 | 74.34 | 1310.00 | 0.0973 |
| Mo | 1298 | 0.001298 | 1090.32 | 42,450.00 | 46.2840 |
| Sn | 184 | 0.000184 | 154.56 | 36,475.00 | 5.6375 |
| Sb | 131 | 0.000131 | 110.04 | 8132.00 | 0.8948 |
| Pb | 42 | 0.000042 | 35.28 | 2469.00 | 0.0871 |
| Cd | 838.5 | 0.0008385 | 704.34 | 2730.00 | 1.9228 |
| Fe | 219,000 | 0.219 | 183,960 | 424.00 | 77.9990 |
| Total | 1175.7440 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iluţiu-Varvara, D.-A.; Aciu, C. Metallurgical Wastes as Resources for Sustainability of the Steel Industry. Sustainability 2022, 14, 5488. https://doi.org/10.3390/su14095488
Iluţiu-Varvara D-A, Aciu C. Metallurgical Wastes as Resources for Sustainability of the Steel Industry. Sustainability. 2022; 14(9):5488. https://doi.org/10.3390/su14095488
Chicago/Turabian StyleIluţiu-Varvara, Dana-Adriana, and Claudiu Aciu. 2022. "Metallurgical Wastes as Resources for Sustainability of the Steel Industry" Sustainability 14, no. 9: 5488. https://doi.org/10.3390/su14095488
APA StyleIluţiu-Varvara, D.-A., & Aciu, C. (2022). Metallurgical Wastes as Resources for Sustainability of the Steel Industry. Sustainability, 14(9), 5488. https://doi.org/10.3390/su14095488






