Ambient and Heat-Cured Geopolymer Composites: Mix Design Optimization and Life Cycle Assessment
Abstract
:1. Introduction
2. Experimental Program
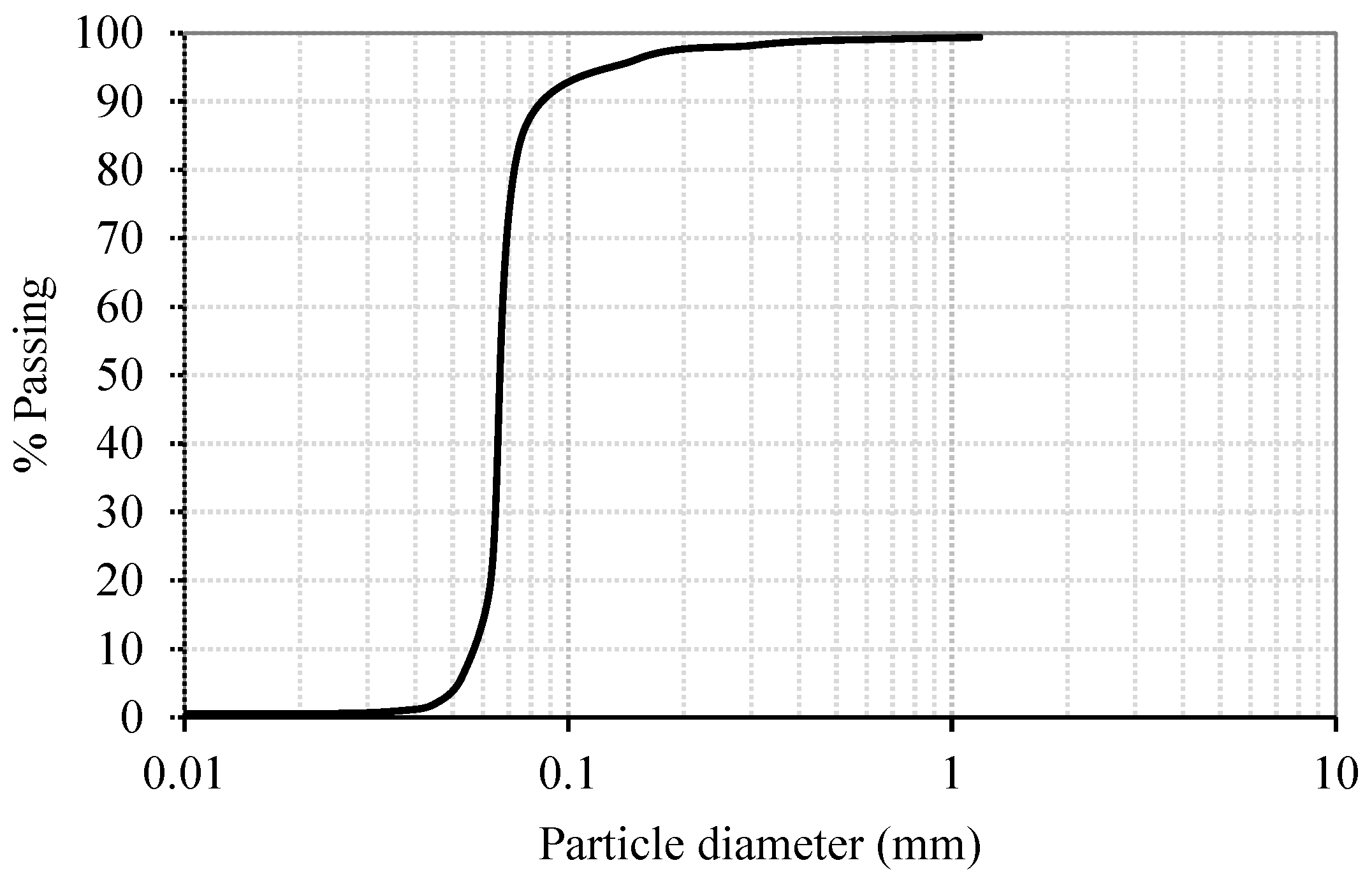
2.1. Materials
2.2. Mix Proportions, Mortar Casting, and Specimen Preparations
2.3. Test Procedures
2.3.1. Compressive Strength
2.3.2. Flow Table Test
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Life Cycle Assessment (LCA)
Life Cycle Inventory (LCI)
3. Results and Discussion
3.1. Compressive Strength
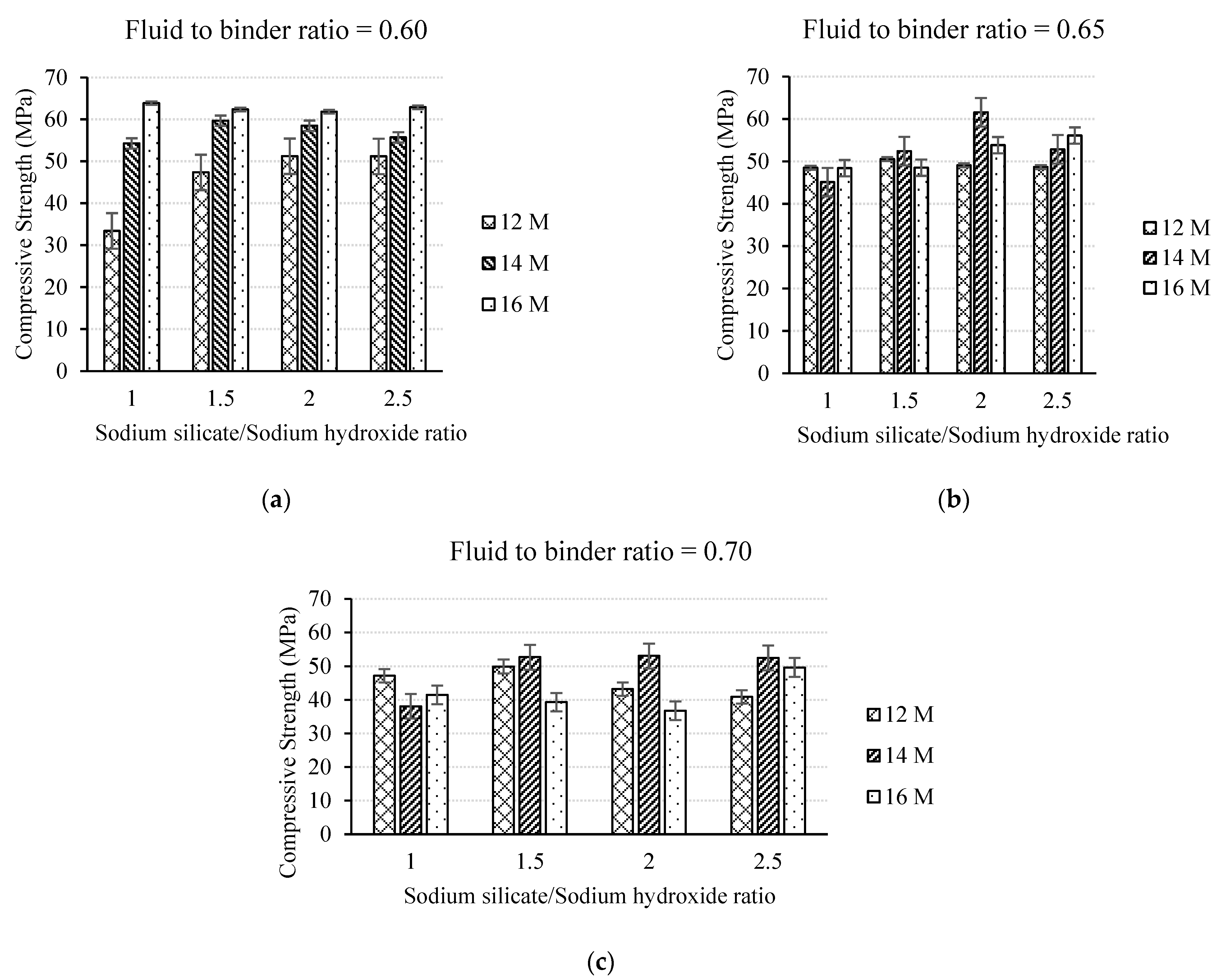
3.1.1. Effect of the Fluid to Binder Ratio and Activator Solution Characteristics
3.1.2. Effect of Curing Condition
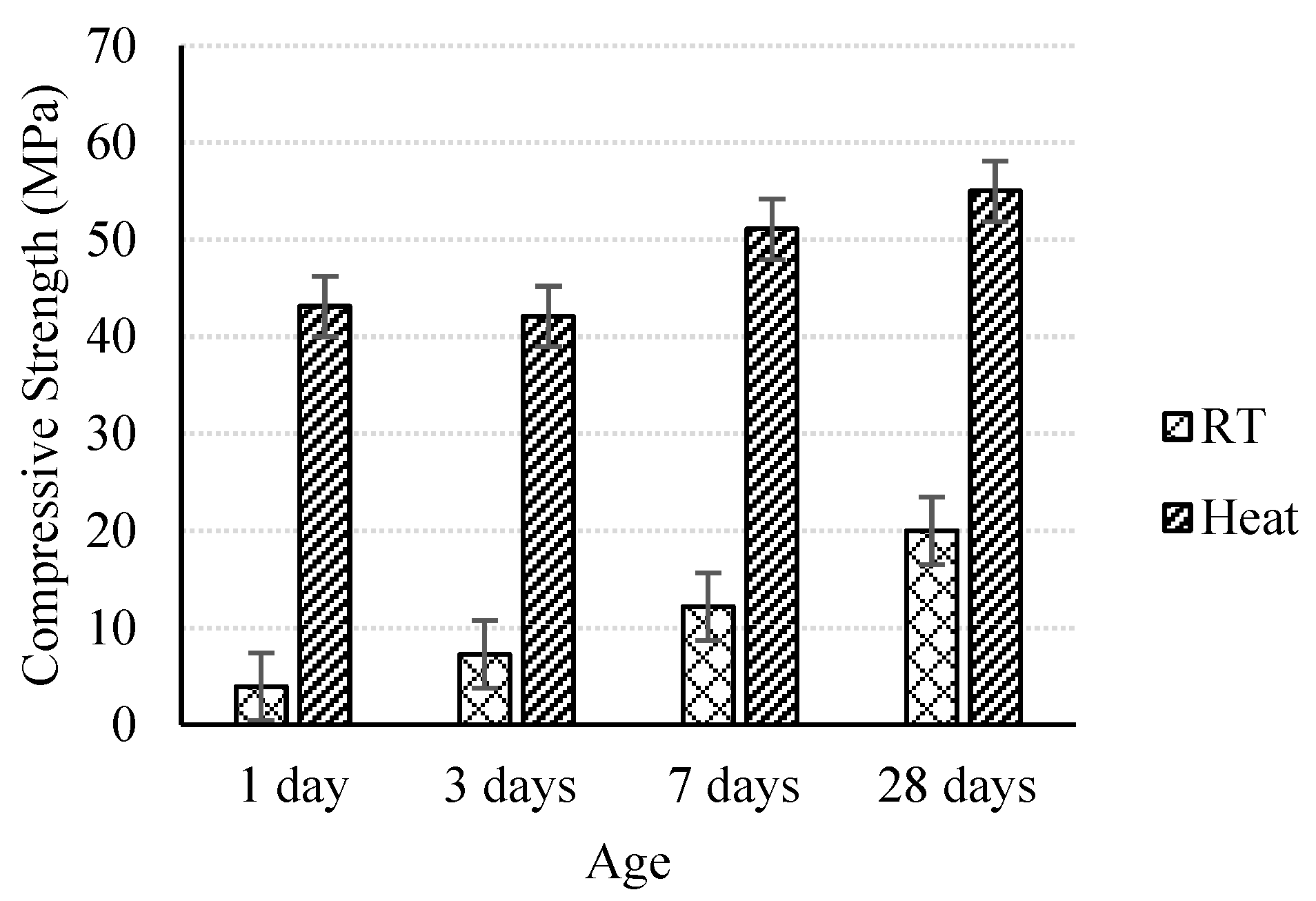
3.1.3. Effect of Age on Compressive Strength Development
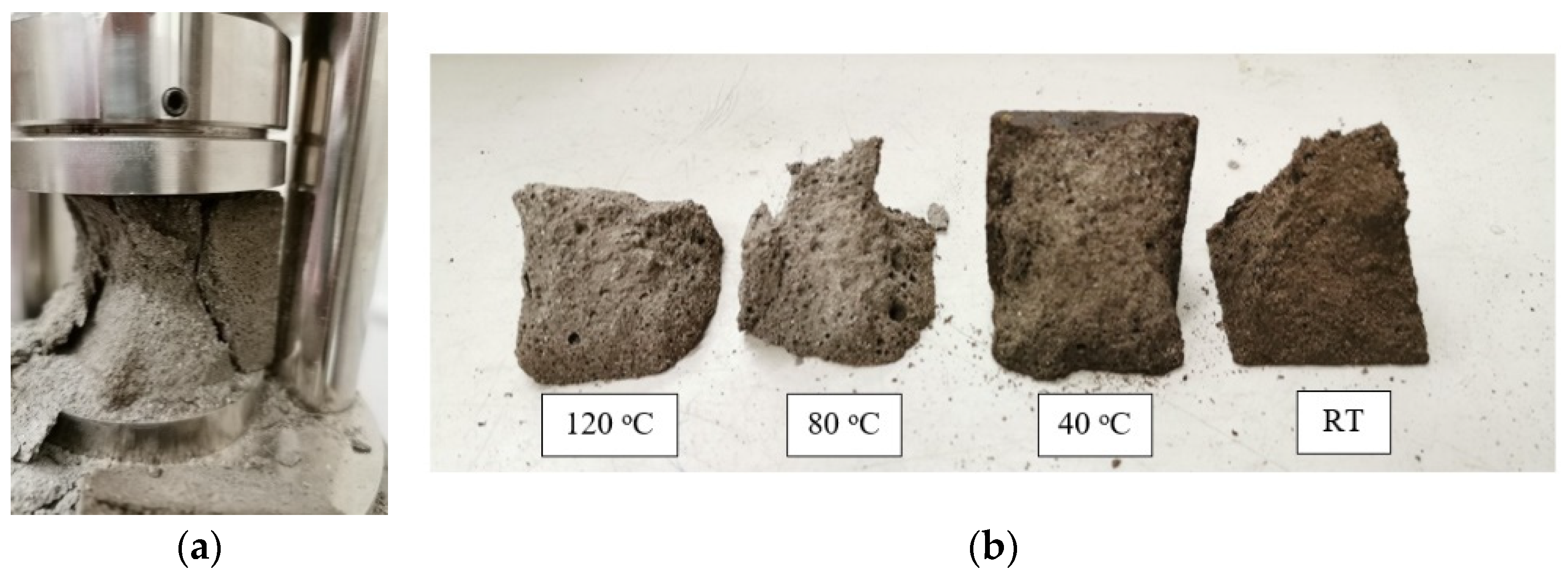
3.2. Failure Modes
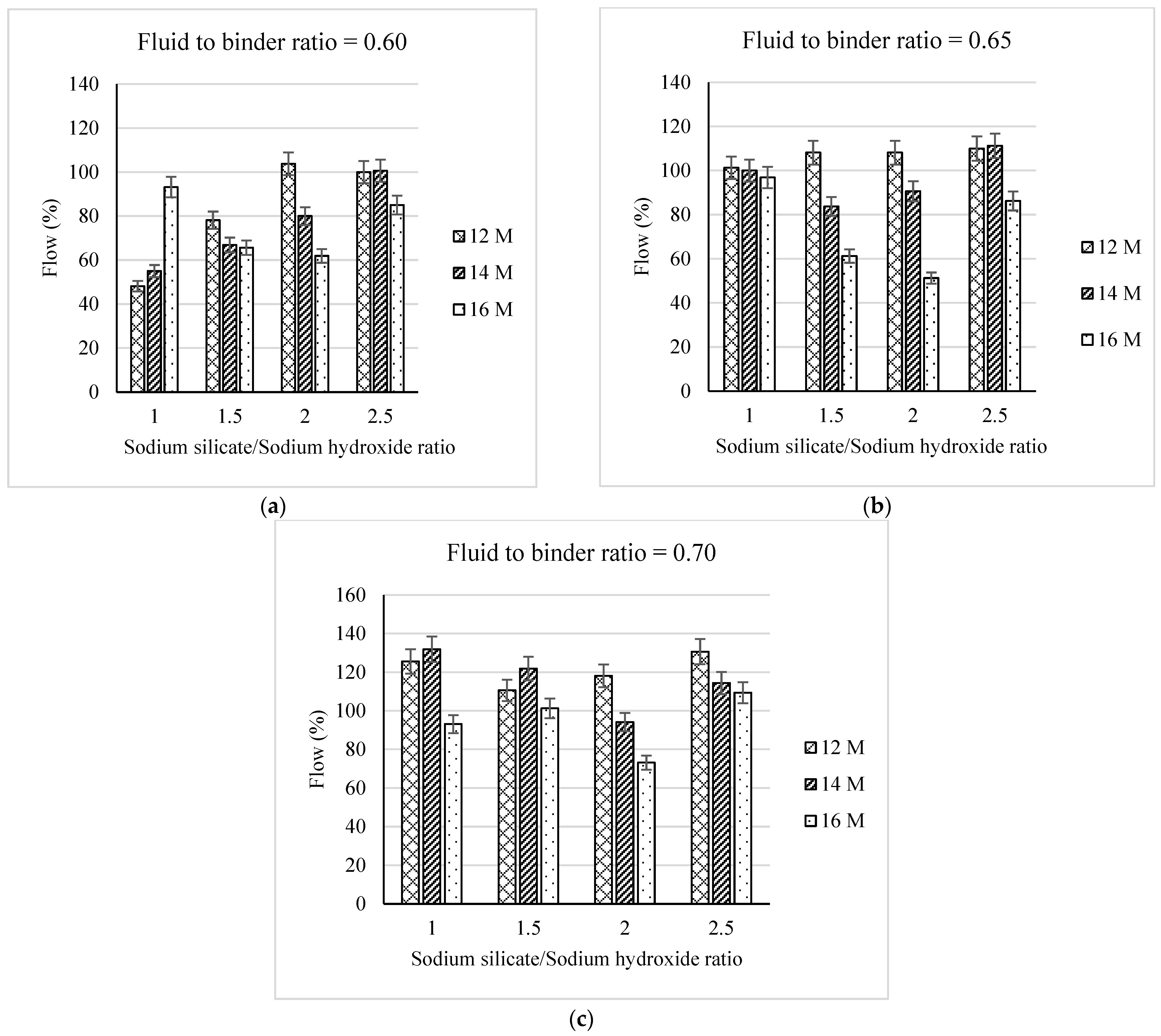
3.3. Flowability of the Geopolymer Mortar
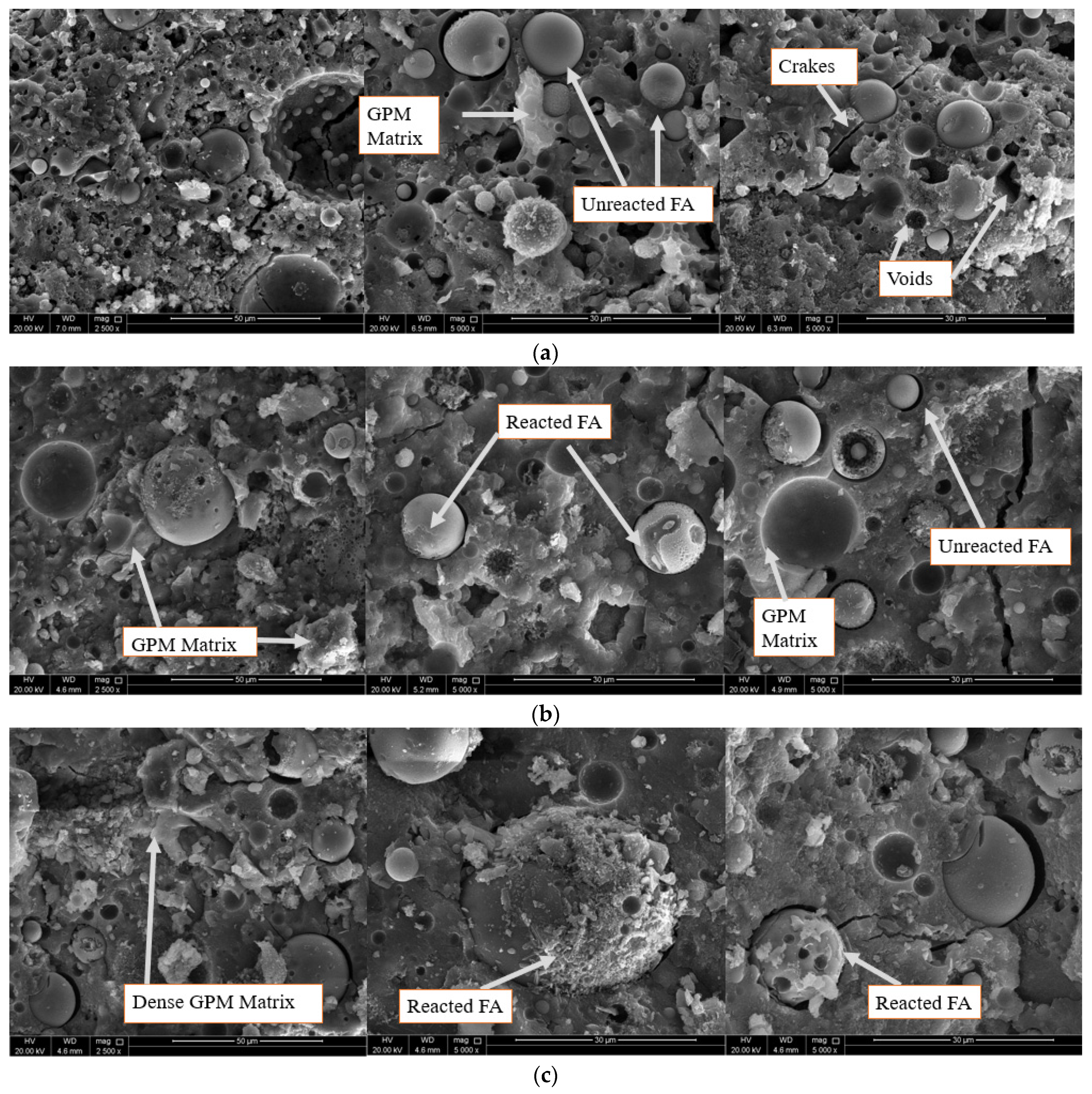
3.4. Microstructure Investigation Using SEM Imaging
3.5. Life Cycle Assessment Results
4. Conclusions
- The strength and flowability of heat-treated geopolymer mortar are highly dependent on alkaline activator solution characteristics such as the NaOH solution molarity, the Na2SiO3/NaOH ratio, and the fluid to binder ratio. Among the studied specimens, the optimum mix design of the fly ash-based geopolymer mortar in terms of compressive strength was achieved for specimens prepared with a NaOH molarity of 16 M, a Na2SiO3/NaOH ratio of one, and a fluid to binder ratio of 0.6.
- Heat curing is necessary for geopolymer mortar to achieve high early compressive strength. The optimum curing conditions among the studied specimens are heat treatment at 80 °C and for 24 h.
- The chemical composition of the fly ash used in the production of the geopolymer mortar (e.g., fly ash) is an important factor that affects the mechanical behavior of the geopolymer mortar. The variations in the chemical composition of fly ash leads to unreliable results in the mechanical behavior; thus, this needs to be carefully investigated.
- Strength development with age is more obvious for ambient-cured specimens than heat-cured specimens. However, the heat-treated geopolymer mortar can gain, in one day, more than twice the compressive strength of the ambient-cured specimens at 28 days.
- The microstructure of the fly ash-based geopolymer mortar is affected by the molarity of the NaOH solution. The higher the molarity and as the amount of reacted FA particles increased, the better the microstructure became and the fewer pores in the matrix were found.
- The production of geopolymer mortar is more environmentally friendly than the production of cement mortar. The production of 1.0 m3 of geopolymer mortar generates, for GWP, AP, EP and ADP fossil, emissions 72%, 65%, 41%, and 29% less than the production of 1.0 m3 of cement mortar.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer, C. The greening of the concrete industry. Cem. Concr. Compos. 2009, 31, 601–605. [Google Scholar] [CrossRef]
- Kajaste, R.; Hurme, M. Cement industry greenhouse gas emissions–management options and abatement cost. J. Clean. Prod. 2016, 112, 4041–4052. [Google Scholar] [CrossRef]
- Aïtcin, P.-C. Cements of yesterday and today: Concrete of tomorrow. Cem. Concr. Res. 2000, 30, 1349–1359. [Google Scholar] [CrossRef]
- Rattanasak, U.; Chindaprasirt, P. Influence of NaOH solution on the synthesis of fly ash geopolymer. Miner. Eng. 2009, 22, 1073–1078. [Google Scholar] [CrossRef]
- Davidovits, J. High-Alkali Cements for 21st Century Concretes. Spec. Publ. 1994, 144, 383–398. [Google Scholar] [CrossRef]
- Atis, C.; Görür, E.; Karahan, O.; Bilim, C.; Ilkentapar, S.; Luga, E. Very high strength (120 MPa) class F fly ash geopolymer mortar activated at different NaOH amount, heat curing temperature and heat curing duration. Constr. Build. Mater. 2015, 96, 673–678. [Google Scholar] [CrossRef]
- Irshidat, M.R.; Abdel-Jawad, Y.A.; Al-Sughayer, R. Feasibility of producing sustainable geopolymer composites made of locally available natural pozzolan. J. Mater. Cycles Waste Manag. 2018, 20, 1751–1760. [Google Scholar] [CrossRef]
- Pereira, D.S.D.T.; da Silva, F.J.; Porto, A.B.R.; Candido, V.S.; da Silva, A.C.R.; Filho, F.D.C.G.; Monteiro, S.N. Comparative analysis between properties and microstructures of geopolymeric concrete and portland concrete. J. Mater. Res. Technol. 2018, 7, 606–611. [Google Scholar] [CrossRef]
- Hooton, R.D. Current developments and future needs in standards for cementitious materials. Cem. Concr. Res. 2015, 78, 165–177. [Google Scholar] [CrossRef]
- Mermerdaş, K.; Algın, Z.; Ekmen, Ş. Experimental assessment and optimization of mix parameters of fly ash-based lightweight geopolymer mortar with respect to shrinkage and strength. J. Build. Eng. 2020, 31, 101351. [Google Scholar] [CrossRef]
- Hadi, M.; Zhang, H.; Parkinson, S. Optimum mix design of geopolymer pastes and concretes cured in ambient condition based on compressive strength, setting time and workability. J. Build. Eng. 2019, 23, 301–313. [Google Scholar] [CrossRef]
- Tenepalli, J.S.; Neeraja, D. Properties of class F fly ash based geopolymer mortar produced with alkaline water. J. Build. Eng. 2018, 19, 42–48. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Phoo-Ngernkham, T.; Chindaprasirt, P.; Sata, V.; Pangdaeng, S.; Sinsiri, T. Properties of high calcium fly ash geopolymer pastes with Portland cement as an additive. Int. J. Miner. Met. Mater. 2013, 20, 214–220. [Google Scholar] [CrossRef]
- Kubba, Z.; Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Asaad, M.A.; Ismail, M.; Tahir, M.M.; Mirza, J. Impact of curing temperatures and alkaline activators on compressive strength and porosity of ternary blended geopolymer mortars. Case Stud. Constr. Mater. 2018, 9, 00205. [Google Scholar] [CrossRef]
- Kantarcı, F.; Türkmen, I.; Ekinci, E. Optimization of production parameters of geopolymer mortar and concrete: A comprehensive experimental study. Constr. Build. Mater. 2019, 228, 116770. [Google Scholar] [CrossRef]
- Arnoult, M.; Perronnet, M.; Autef, A.; Rossignol, S. How to control the geopolymer setting time with the alkaline silicate solution. J. Non-Cryst. Solids 2018, 495, 59–66. [Google Scholar] [CrossRef]
- Saxena, S.K.; Kumar, M.; Singh, N.B. Influence of alkali solutions on properties of pond fly ash-based geopolymer mortar cured under different conditions. Adv. Cem. Res. 2018, 30, 1–7. [Google Scholar] [CrossRef]
- Görhan, G.; Kürklü, G. The influence of the NaOH solution on the properties of the fly ash-based geopolymer mortar cured at different temperatures. Compos. Part B Eng. 2014, 58, 371–377. [Google Scholar] [CrossRef]
- Budh, C.D.; Warhade, N.R. Effect of molarity on compressive strength of geopolymer mortar. Int. J. Civil Eng. Res. 2014, 5, 83–86. [Google Scholar]
- Madheswaran, C.K.; Gnanasundar, G.; Gopalakrishnan, N. Effect of molarity in geopolymer concrete. Int. J. Civil Struct. Eng. 2013, 4, 106–115. [Google Scholar]
- Narayanan, A.; Shanmugasundaram, P. An Experimental Investigation on Flyash-based Geopolymer Mortar under different curing regime for Thermal Analysis. Energy Build. 2017, 138, 539–545. [Google Scholar] [CrossRef]
- Mo, B.-H.; Zhu, H.; Cui, X.-M.; He, Y.; Gong, S.-Y. Effect of curing temperature on geopolymerization of metakaolin-based geopolymers. Appl. Clay Sci. 2014, 99, 144–148. [Google Scholar] [CrossRef]
- De Rossi, A.; Simão, L.; Ribeiro, M.J.; Hotza, D.; Moreira, R.D.F.P.M. Study of cure conditions effect on the properties of wood biomass fly ash geopolymers. J. Mater. Res. Technol. 2020, 9, 7518–7528. [Google Scholar] [CrossRef]
- Choi, Y.C.; Park, B. Effects of high-temperature exposure on fractal dimension of fly-ash-based geopolymer composites. J. Mater. Res. Technol. 2020, 9, 7655–7668. [Google Scholar] [CrossRef]
- Irshidat, M.R.; Al-Nuaimi, N.; Rabie, M. Sustainable utilization of waste carbon black in alkali-activated mortar production. Case Stud. Constr. Mater. 2021, 15, e00743. [Google Scholar] [CrossRef]
- ISO. 14040; Environmental Management–Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- Passuello, A.; Rodríguez, E.D.; Hirt, E.; Longhi, M.; Bernal, S.A.; Provis, J.; Kirchheim, A.P. Evaluation of the potential improvement in the environmental footprint of geopolymers using waste-derived activators. J. Clean. Prod. 2017, 166, 680–689. [Google Scholar] [CrossRef]
- Abdulkareem, M.; Havukainen, J.; Horttanainen, M. How environmentally sustainable are fibre reinforced alkali-activated concretes? J. Clean. Prod. 2019, 236, 117601. [Google Scholar] [CrossRef]
- ThinkStep. GaBi Solutions. GaBi 2019. Available online: https://gabi.sphera.com/international/index/ (accessed on 2 March 2022).
- Knoeri, C.; Sanyé-Mengual, E.; Althaus, H.-J. Comparative LCA of recycled and conventional concrete for structural applications. Int. J. Life Cycle Assess. 2013, 18, 909–918. [Google Scholar] [CrossRef] [Green Version]
- Teh, S.H.; Wiedmann, T.; Moore, S. Mixed-unit hybrid life cycle assessment applied to the recycling of construction materials. J. Econ. Struct. 2018, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- van den Heede, P.; De Belie, N. Environmental impact and life cycle assessment (LCA) of traditional and ‘green’ concretes: Literature review and theoretical calculations. Cem. Concr. Compos. 2012, 34, 431–442. [Google Scholar] [CrossRef]
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A. Environmental impact of cement production: Detail of the different processes and cement plant variability evaluation. J. Clean. Prod. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Chithambaram, S.J.; Kumar, S.; Prasad, M.M.; Adak, D. Effect of parameters on the compressive strength of fly ash based geopolymer concrete. Struct. Concr. 2018, 19, 1202–1209. [Google Scholar] [CrossRef]
- Patankar, S.V.; Jamkar, S.S.; Ghugal, Y.M. Effect of water-to-geopolymer binder ratio on the production of fly ash based geopolymer concrete effect of water-to-geopolymer binder ratio on the production of fly ash based geopolymer concrete. Int. J. Adv. Technol. Civil Eng. 2013, 2, 79–83. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J. Effect of different superplasticizers and activator combinations on workability and strength of fly ash based geopolymer. Mater. Des. 2014, 57, 667–672. [Google Scholar] [CrossRef]
- Ilkentapar, S.; Atis, C.; Karahan, O.; Avşaroğlu, E.G. Influence of duration of heat curing and extra rest period after heat curing on the strength and transport characteristic of alkali activated class F fly ash geopolymer mortar. Constr. Build. Mater. 2017, 151, 363–369. [Google Scholar] [CrossRef]
- Abdullah, A.; Hussin, K.; Abdullah, M.; Yahya, Z.; Sochacki, W.; Razak, R.; Błoch, K.; Fansuri, H. The Effects of Various Concentrations of NaOH on the Inter-Particle Gelation of a Fly Ash Geopolymer Aggregate. Materials 2021, 14, 1111. [Google Scholar] [CrossRef]
- Zejak, R.; Nikolić, I.; Blecić, D.; Radmilović, V.; Radmilović, V.R. Mechanical and microstructural properties of the fly-ash-based geopolymer paste and mortar. Mater. Tehnol. 2013, 47, 535–540. [Google Scholar]
- Ng, C.; Alengaram, U.J.; Wong, L.S.; Mo, K.H.; Jumaat, M.Z.; Ramesh, S. A review on microstructural study and compressive strength of geopolymer mortar, paste and concrete. Constr. Build. Mater. 2018, 186, 550–576. [Google Scholar] [CrossRef]
- Fawer, M.; Concannon, M.; Rieber, W. Life cycle inventories for the production of sodium silicates. Int. J. Life Cycle Assess. 1999, 4, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Huntzinger, D.N.; Eatmon, T. A life-cycle assessment of Portland cement manufacturing: Comparing the traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Habert, G.; d’Espinose de Lacaillerie, J.B.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Bajpai, R.; Choudhary, K.; Srivastava, A.; Sangwan, K.S.; Singh, M. Environmental impact assessment of fly ash and silica fume based geopolymer concrete. J. Clean. Prod. 2020, 254, 120147. [Google Scholar] [CrossRef]
- Al Bakri, M.M.; Mohammed, H.; Kamarudin, H.; Niza, I.K.; Zarina, Y. Review on fly ash-based geopolymer concrete without Portland Cement. J. Eng. Technol. Res. 2011, 3, 1–4. [Google Scholar]
- McLellan, B.C.; Williams, R.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Zerfu, K.; Ekaputri, J.J. Review on Alkali-Activated Fly Ash Based Geopolymer Concrete. Mater. Sci. Forum 2016, 841, 162–169. [Google Scholar] [CrossRef]



| Oxide | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | SO3 | K2O | Na2O | Cl− | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| % | 49.9 | 17.1 | 11.8 | 7.83 | 4.9 | 0.42 | 0.2772 | 0.1428 | 0.011 | 3.5 |
| Phases | Molarity | Fluid/Binder Ratio | Na2SiO3/NaOH | Curing Temperature (°C) | Curing Period (h) | Age (Days) |
|---|---|---|---|---|---|---|
| Phase One | 12 | 0.60 | 1 | 80 | 24 | 1 |
| 14 | 0.65 | 1.5 | ||||
| 16 | 0.70 | 2 | ||||
| 2.5 | ||||||
| Phase Two | 16 | 0.60 | 1 | 25 (ambient) | 24 | 1 |
| 40 | 48 | |||||
| 80 | 72 | |||||
| 120 | ||||||
| Phase Three | 16 | 0.60 | 1 | 80 | 24 | 1 |
| 3 | ||||||
| 7 | ||||||
| 28 |
| Mix Design ID | NaOH Concentration (M) | F/B | Na2SiO3 /NaOH | Fly Ash (kg/m3) | Sand (kg/m3) | NaOH Solution (kg/m3) | Na2SiO3 Solution (kg/m3) |
|---|---|---|---|---|---|---|---|
| M1 | 12 | 0.6 | 1 | 711 | 1956 | 213 | 213 |
| M2 | 1.5 | 711 | 1956 | 171 | 256 | ||
| M3 | 2 | 711 | 1956 | 142 | 284 | ||
| M4 | 2.5 | 711 | 1956 | 122 | 305 | ||
| M5 | 0.65 | 1 | 711 | 1956 | 231 | 231 | |
| M6 | 1.5 | 711 | 1956 | 185 | 277 | ||
| M7 | 2 | 711 | 1956 | 154 | 308 | ||
| M8 | 2.5 | 711 | 1956 | 132 | 330 | ||
| M9 | 0.7 | 1 | 711 | 1956 | 249 | 249 | |
| M10 | 1.5 | 711 | 1956 | 199 | 299 | ||
| M11 | 2 | 711 | 1956 | 166 | 332 | ||
| M12 | 2.5 | 711 | 1956 | 142 | 356 | ||
| M13 | 14 | 0.6 | 1 | 711 | 1956 | 213 | 213 |
| M14 | 1.5 | 711 | 1956 | 171 | 256 | ||
| M15 | 2 | 711 | 1956 | 142 | 284 | ||
| M16 | 2.5 | 711 | 1956 | 122 | 305 | ||
| M17 | 0.65 | 1 | 711 | 1956 | 231 | 231 | |
| M18 | 1.5 | 711 | 1956 | 185 | 277 | ||
| M19 | 2 | 711 | 1956 | 154 | 308 | ||
| M20 | 2.5 | 711 | 1956 | 132 | 330 | ||
| M21 | 0.7 | 1 | 711 | 1956 | 249 | 249 | |
| M22 | 1.5 | 711 | 1956 | 199 | 299 | ||
| M23 | 2 | 711 | 1956 | 166 | 332 | ||
| M24 | 2.5 | 711 | 1956 | 142 | 356 | ||
| M25 | 16 | 0.6 | 1 | 711 | 1956 | 213 | 213 |
| M26 | 1.5 | 711 | 1956 | 171 | 256 | ||
| M27 | 2 | 711 | 1956 | 142 | 284 | ||
| M28 | 2.5 | 711 | 1956 | 122 | 305 | ||
| M29 | 0.65 | 1 | 711 | 1956 | 231 | 231 | |
| M30 | 1.5 | 711 | 1956 | 185 | 277 | ||
| M31 | 2 | 711 | 1956 | 154 | 308 | ||
| M32 | 2.5 | 711 | 1956 | 132 | 330 | ||
| M33 | 0.7 | 1 | 711 | 1956 | 249 | 249 | |
| M34 | 1.5 | 711 | 1956 | 199 | 299 | ||
| M35 | 2 | 711 | 1956 | 166 | 332 | ||
| M36 | 2.5 | 711 | 1956 | 142 | 356 |
| Type of Data | Source |
|---|---|
| Fly Ash | GaBi database 2020, Fly Ash |
| Cement | GaBi database 2020 RER: Portland cement (CEM I ELCD/CEMBUREAU) |
| Sand | GaBi database 2020 (Sand 0/2 ts) |
| Sodium hydroxide | GaBi database 2020, (EU-28, sodium hydroxide, 100% caustic soda) |
| Sodium silicate | GaBi database 2020, (sodium silicate, waterglass) |
| Water | GaBi database 2020, Water (desalinated; deionized) |
| Electricity | GaBi database 2020, EU-28: Electricity grid mix ts |
| Transportation | GaBi database 2020 Truck, Euro 3, up to 7.5 t gross weight/2.7 t payload capacity |
| Diesel | GaBi database 2020, EU-28: Diesel mix at refinery ts |
| Mix Design | Compressive Strength (MPa) | Flow Davg. (cm) | Flow (%) |
|---|---|---|---|
| M1 | 33.42 | 14.81 | 48.13 |
| M2 | 47.37 | 17.81 | 78.13 |
| M3 | 51.23 | 20.38 | 103.75 |
| M4 | 51.19 | 20.00 | 100.00 |
| M5 | 48.53 | 20.13 | 101.25 |
| M6 | 50.53 | 20.81 | 108.13 |
| M7 | 49.09 | 20.81 | 108.13 |
| M8 | 48.66 | 21.00 | 110.00 |
| M9 | 47.15 | 22.56 | 125.63 |
| M10 | 49.87 | 21.06 | 110.63 |
| M11 | 43.17 | 21.81 | 118.13 |
| M12 | 40.85 | 23.06 | 130.63 |
| M13 | 54.28 | 15.50 | 55.00 |
| M14 | 59.70 | 16.69 | 66.88 |
| M15 | 58.49 | 18.00 | 80.00 |
| M16 | 55.74 | 20.06 | 100.63 |
| M17 | 45.13 | 20.00 | 100.00 |
| M18 | 52.43 | 18.38 | 83.75 |
| M19 | 61.58 | 19.06 | 90.63 |
| M20 | 52.87 | 21.13 | 111.25 |
| M21 | 38.06 | 23.19 | 131.88 |
| M22 | 52.64 | 22.19 | 121.88 |
| M23 | 52.98 | 19.42 | 94.17 |
| M24 | 52.40 | 21.44 | 114.38 |
| M25 | 63.86 | 19.31 | 93.13 |
| M26 | 62.38 | 16.56 | 65.63 |
| M27 | 61.83 | 16.19 | 61.88 |
| M28 | 62.89 | 18.50 | 85.00 |
| M29 | 48.42 | 19.69 | 96.88 |
| M30 | 48.53 | 16.13 | 61.25 |
| M31 | 53.85 | 15.13 | 51.25 |
| M32 | 56.12 | 18.63 | 86.25 |
| M33 | 41.46 | 19.31 | 93.13 |
| M34 | 39.30 | 20.13 | 101.25 |
| M35 | 36.77 | 17.31 | 73.13 |
| M36 | 49.59 | 20.94 | 109.38 |
| M25(D1T1) | 19.83 | 19.31 | 93.13 |
| M25(D1T2) | 43.13 | 19.31 | 93.13 |
| M25(D1T3) | 37.21 | 19.31 | 93.13 |
| M25(D2T1) | 18.17 | 19.31 | 93.13 |
| M25(D2T2) | 39.80 | 19.31 | 93.13 |
| M25(D2T3) | 36.10 | 19.31 | 93.13 |
| M25(D3T1) | 24.37 | 19.31 | 93.13 |
| M25(D3T2) | 29.98 | 19.31 | 93.13 |
| M25(D3T3) | 29.24 | 19.31 | 93.13 |
| M25(RT-1 day) | 3.93 | 19.31 | 93.13 |
| M25(RT-3 days) | 7.26 | 19.31 | 93.13 |
| M25(RT-7 days) | 12.20 | 19.31 | 93.13 |
| M25(RT-28 days) | 20.00 | 19.31 | 93.13 |
| M25 (Heat-1 day) | 43.13 | 19.31 | 93.13 |
| M25 (Heat-3 days) | 42.11 | 19.31 | 93.13 |
| M25 (Heat-7 days) | 51.08 | 19.31 | 93.13 |
| M25 (Heat-28 days) | 55.00 | 19.31 | 93.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabie, M.; Irshidat, M.R.; Al-Nuaimi, N. Ambient and Heat-Cured Geopolymer Composites: Mix Design Optimization and Life Cycle Assessment. Sustainability 2022, 14, 4942. https://doi.org/10.3390/su14094942
Rabie M, Irshidat MR, Al-Nuaimi N. Ambient and Heat-Cured Geopolymer Composites: Mix Design Optimization and Life Cycle Assessment. Sustainability. 2022; 14(9):4942. https://doi.org/10.3390/su14094942
Chicago/Turabian StyleRabie, Mohamed, Mohammad R. Irshidat, and Nasser Al-Nuaimi. 2022. "Ambient and Heat-Cured Geopolymer Composites: Mix Design Optimization and Life Cycle Assessment" Sustainability 14, no. 9: 4942. https://doi.org/10.3390/su14094942
APA StyleRabie, M., Irshidat, M. R., & Al-Nuaimi, N. (2022). Ambient and Heat-Cured Geopolymer Composites: Mix Design Optimization and Life Cycle Assessment. Sustainability, 14(9), 4942. https://doi.org/10.3390/su14094942








