Growth, Nutrient Accumulation, and Drought Tolerance in Crop Plants with Silicon Application: A Review
Abstract
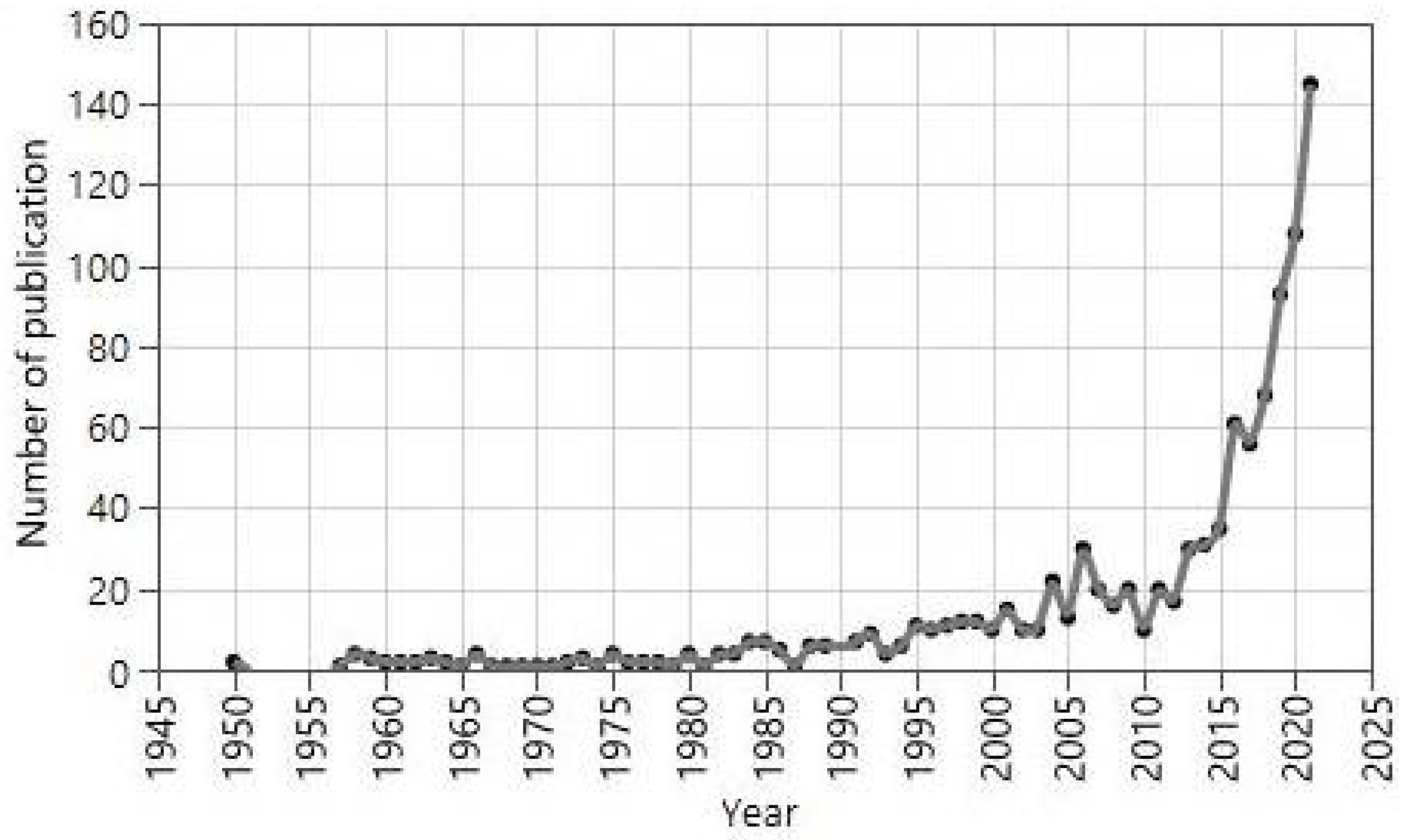
1. Introduction
2. Role of Si on Plant Growth and Yield
3. Role of Si on Nutrients Availability and Accumulation
3.1. Macronutrients
3.2. Micronutrients
3.3. Silicon
4. Silicon Application as Nano-Fertilizers and Nano-Pesticides
5. Effect of Si on Drought Tolerance in Plants
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Ma, J.F.; Takahashi, E. Soil, Fertilizer, and Plant Silicon Research in Japan; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A review of silicon in soils and plants and its role in us agriculture: History and future perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef]
- Sandhya, K.; Prakash, N.B.; Meunier, J.D.; Sandhya, K.; Prakash, N.B.; Meunier, J.D. Diatomaceous earth as source of silicon on the growth and yield of rice in contrasted soils of Southern India. J. Soil Sci. Plant Nutr. 2018, 18, 344–360. [Google Scholar] [CrossRef][Green Version]
- Artyszak, A. Effect of Silicon Fertilization on Crop Yield Quantity and Quality—A Literature Review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef]
- Janislampi, K.W. Effect of Silicon on Plant Growth and Droucht Stress Tolerance. All Graduate Theses and Dissertations, UtahState University, Logan, UT, USA, 2012. [Google Scholar] [CrossRef]
- Amin, M.; Ahmad, R.; Ali, A.; Hussain, I.; Mahmood, R.; Aslam, M.; Lee, D.J. Influence of Silicon Fertilization on Maize Performance Under Limited Water Supply. Silicon 2018, 10, 177–183. [Google Scholar] [CrossRef]
- Cuong, T.X.; Ullah, H.; Datta, A.; Hanh, T.C. Effects of Silicon-Based Fertilizer on Growth, Yield and Nutrient Uptake of Rice in Tropical Zone of Vietnam. Rice Sci. 2017, 24, 283–290. [Google Scholar] [CrossRef]
- Mali, M.; Aery, N.C. Effect of silicon on growth, biochemical constituents, and mineral nutrition of cowpea. Commun. Soil Sci. Plant Anal. 2009, 40, 1041–1052. [Google Scholar] [CrossRef]
- Mehrabanjoubani, P.; Abdolzadeh, A.; Sadeghipour, H.R.; Aghdasi, M. Impacts of silicon nutrition on growth and nutrient status of rice plants grown under varying zinc regimes. Theor. Exp. Plant Physiol. 2015, 27, 19–29. [Google Scholar] [CrossRef]
- Neu, S.; Schaller, J.; Dudel, E.G. Silicon availability modifies nutrient use efficiency and content, C:N:P stoichiometry, and productivity of winter wheat (Triticum aestivum L.). Sci. Rep. 2017, 7, 3–10. [Google Scholar] [CrossRef]
- Luo, W.; Ma, J.; Khan, M.A.; Liao, S.; Ruan, Z.; Liu, H.; Zhong, B.; Zhu, Y.; Duan, L.; Fu, L.; et al. Cadmium accumulation in rice and its bioavailability in paddy soil with application of silicon fertilizer under different water management regimes. Soil Use Manag. 2021, 37, 299–306. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, S.; Cai, K.; Huang, F.; Pan, B.; Wang, W. Cd accumulation, biomass and yield of rice are varied with silicon application at different growth phases under high concentration cadmium-contaminated soil. Chemosphere 2020, 242, 125128. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chu, C.; Wei, H.; Zhang, L.; Ahmad, Z.; Wu, S.; Xie, B. Ameliorative effects of silicon fertilizer on soil bacterial community and pakchoi (Brassica chinensis L.) grown on soil contaminated with multiple heavy metals. Environ. Pollut. 2020, 267, 115411. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Santi, L.P.; Nurhaimi-Haris; Mulyanto, D. Effect of bio-silicon on drought tolerance in plants. In Proceedings of the Earth and Environmental Science, IOP Conference Series: Earth and Environmental Science, Jakarta, Indonesia, 18–20 October 2017; IOP Publishing: Bristol, UK, 2018; 183, p. 12014. [Google Scholar]
- Ahmad, M.; El-Saeid, M.H.; Akram, M.A.; Ahmad, H.R.; Haroon, H.; Hussain, A. Silicon fertilization—A tool to boost up drought tolerance in wheat (Triticum aestivum L.) crop for better yield. J. Plant Nutr. 2016, 39, 1283–1291. [Google Scholar] [CrossRef]
- Shakoor, S.A.; Bhat, M.A.; Mir, S.H. Phytoliths in Plants: A Review. J. Bot. Sci. 2014, 3, 10–24. [Google Scholar]
- Green, S.W.; Piperno, D.R. Phytolith Analysis: An Archaeological and Geological Perspective. Am. J. Archaeol. 1991, 95, 741. [Google Scholar] [CrossRef]
- Keeping, M.G. Uptake of Silicon by Sugarcane from Applied Sources May Not Reflect Plant-Available Soil Silicon and Total Silicon Content of Sources. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Khan, M.A.; Goyal, V.; Jain, N. Impact of Ortho Silicic Acid Formulation on Yield and Disease incidence of Potatoes. In Proceedings of the 7th International Conference on Silicon in Agriculture, Bengaluru, India, 24–28 October 2017; p. 137. [Google Scholar]
- Kaya, C.; Tuna, L.; Higgs, D. Effect of silicon on plant growth and mineral nutrition of maize grown under water-stress conditions. J. Plant Nutr. 2006, 29, 1469–1480. [Google Scholar] [CrossRef]
- Korndörfer, G.H.; Lepsch, I. Effect of silicon on plant growth and crop yield. Stud. Plant Sci. 2001, 8, 133–147. [Google Scholar] [CrossRef]
- Ashtiani, F.A.; Kadir, J.; Nasehi, A.; Rahaghi, S.R.H.; Sajili, H. Effect of silicon on rice blast disease. Pertanika J. Trop. Agric. Sci. 2012, 35, 1–12. [Google Scholar]
- Ning, D.; Song, A.; Fan, F.; Li, Z.; Liang, Y. Effects of slag-based silicon fertilizer on rice growth and brown-spot resistance. PLoS ONE 2014, 9, e102681. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.A.; Datnoff, L.E. Silicon and Plant Diseases; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783319229300. [Google Scholar]
- Ahammed, G.J.; Yang, Y. Mechanisms of silicon-induced fungal disease resistance in plants. Plant Physiol. Biochem. 2021, 165, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic Variation in the Silicon Composition of Plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, R.; Thakral, V.; Raturi, G.; Shinde, S.; Nikolić, M.; Tripathi, D.K.; Sonah, H.; Deshmukh, R. Significance of Silicon Uptake, Transport, and Deposition in plants. J. Exp. Bot. 2020, 71, 6703–6718. [Google Scholar] [CrossRef]
- Makabe, S.; Kakuda, K.; Sasaki, Y.; Ando, T.; Fujii, H.; Ando, H. Relationship between mineral composition or soil texture and available silicon in alluvial paddy soils on the Shounai Plain, Japan. Soil Sci. Plant Nutr. 2009, 55, 300–308. [Google Scholar] [CrossRef]
- Agostinho, F.; Tubana, B.; Martins, M.; Datnoff, L. Effect of Different Silicon Sources on Yield and Silicon Uptake of Rice Grown under Varying Phosphorus Rates. Plants 2017, 6, 35. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Liu, X.; Yin, L.; Deng, X.; Gong, D.; Du, S.; Wang, S.; Zhang, Z. Combined application of silicon and nitric oxide jointly alleviated cadmium accumulation and toxicity in maize. J. Hazard. Mater. 2020, 395, 122679. [Google Scholar] [CrossRef]
- Zajaczkowska, A.; Korzeniowska, J.; Sienkiewicz-Cholewa, U. Effect of soil and foliar silicon application on the reduction of zinc toxicity in wheat. Agriculture 2020, 10, 522. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Bala, M.; Pareek, A.; Singla-Pareek, S.L.; KumarSingh, A. Silicon-mediated abiotic and biotic stress mitigation in plants: Underlying mechanisms and potential for stress resilient agriculture. Plant Physiol. Biochem. 2021, 163, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s Role in Abiotic and Biotic Plant Stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Chen, K. The regulatory role of silicon on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions. Acta Physiol. Plant. 2012, 34, 1589–1594. [Google Scholar] [CrossRef]
- Pilon, C.; Soratto, R.P.; Moreno, L.A. Effects of soil and foliar application of soluble silicon on mineral nutrition, gas exchange, and growth of potato plants. Crop Sci. 2013, 53, 1605–1614. [Google Scholar] [CrossRef]
- Zaheer, M.M.; Yasin, N.A.; Ahmad, S.R.; Khan, W.U.; Ahmad, A.; Ali, A.; Rehman, S.U. Amelioration of cadmium stress in gladiolus (Gladiolus grandiflora L.) by application of potassium and silicon. J. Plant Nutr. 2018, 41, 461–476. [Google Scholar] [CrossRef]
- Bocharnikova, E.A.; Khomiakov, D.M.; Zhang, P.; Matichenkov, V.V.; Liu, Y.; Pakhnenko, E.P. Effect of Amorphous Silicon Dioxide on Cadmium Behavior in the Soil–Rice Plant System. Moscow Univ. Soil Sci. Bull. 2018, 73, 34–38. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon Influences Soil Availability and Accumulation of Mineral Nutrients in Various Plant Species. Plants 2018, 7, 41. [Google Scholar] [CrossRef]
- Savant, N.K.; Datnoff, L.E.; Snyder, G.H. Depletion of plant-available silicon in soils: A possible cause of declining rice yields. Commun. Soil Sci. Plant Anal. 1997, 28, 1245–1252. [Google Scholar] [CrossRef]
- Taha, R.S.; Seleiman, M.F.; Shami, A.; Alhammad, B.A.; Mahdi, A.H.A. Integrated Application of Selenium and Silicon Enhances Growth and Anatomical Structure, Antioxidant Defense System and Yield of Wheat Grown in Salt-Stressed Soil. Plants 2021, 10, 1040. [Google Scholar] [CrossRef]
- Savant, N.K.; Snyder, G.H.; Datnoff, L.E. Silicon Management and Sustainable Rice Production. Adv. Agron. 1997, 58, 151–199. [Google Scholar]
- Maghsoudi, K.; Emam, Y.; Ashraf, M. Foliar Application of Silicon at Different Growth Stages Alters Growth and Yield of Selected Wheat. J. Plant Nutr. 2016, 39, 1194–1203. [Google Scholar] [CrossRef]
- Sirisuntornlak, N.; Ullah, H.; Sonjaroon, W.; Anusontpornperm, S.; Arirob, W.; Datta, A. Interactive Effects of Silicon and Soil pH on Growth, Yield and Nutrient Uptake of Maize. Silicon 2021, 13, 289–299. [Google Scholar] [CrossRef]
- Walsh, O.S.; Shafian, S.; Mcclintick-chess, J.R.; Belmont, K.M.; Blanscet, S.M. Potential of Silicon Amendment for Improved Wheat Production. Plants 2018, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Owino-Gerroh, C.; Gascho, G.J. Effect of Silicon on Low pH Soil Phosphorus Sorption and on Uptake and Growth of Maize. Commun. Soil Sci. Plant Anal. 2005, 35, 2369–2378. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Pulz, A.L.; Lemos, L.B.; Soratto, R.P.; Lima, G.P.P. Eff ects of Silicon and Drought Stress on Tuber Yield and Leaf Biochemical Characteristics in Potato. Crop Sci. 2009, 49, 949–954. [Google Scholar] [CrossRef]
- Montesano, F.F.; D’Imperio, M.; Parente, A.; Cardinali, A.; Renna, M.; Serio, F. Green bean biofortification for Si through soilless cultivation: Plant response and Si bioaccessibility in pods. Sci. Rep. 2016, 6, 31662. [Google Scholar] [CrossRef]
- Barros, T.C.; De Mello Prado, R.; Garcia Roque, C.; Ribeiro Barzotto, G.; Roberto Wassolowski, C. Silicon and salicylic acid promote different responses in legume plants. J. Plant Nutr. 2018, 41, 2116–2125. [Google Scholar] [CrossRef]
- Olle, M. the Effect of Silicon on the Organically Grown Iceberg Lettuce Growth and Quality. Agraarteadus 2017, 28, 82–86. [Google Scholar] [CrossRef]
- Ashok, L.B.; Swamy, G.S.K.; Prakash, N.B. Diatomaceous earth as source of silicon in tomato crop. In Proceedings of the 7th International Conference on Silicon in Agriculture, Bengaluru, India, 24–28 October 2017; p. 128. [Google Scholar]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon with Essential and Beneficial Elements in Plants. Front. Plant Sci. 2021, 12, 697592. [Google Scholar] [CrossRef]
- Nazirkar, R.B.; Narale, B.; Durgude, A.G. Effect of sources and levels of silicon on soil properties, uptake, yield and quality of Kharif Onion. In Proceedings of the 7th International Conference on Silicon in Agriculture, Bengaluru, India, 24–28 October 2017; p. 141. [Google Scholar]
- Gou, T.; Yang, L.; Hu, W.; Chen, X.; Zhu, Y.; Guo, J.; Gong, H. Silicon improves the growth of cucumber under excess nitrate stress by enhancing nitrogen assimilation and chlorophyll synthesis. Plant Physiol. Biochem. 2020, 152, 5361. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M.; Oku, H.; Nahar, K.; Hawrylak-Nowak, B. Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; ISBN 9789811090431. [Google Scholar]
- Pati, S.; Pal, B.; Badole, S.; Hazra, G.C. Effect of Silicon Fertilization on Growth, Yield, and Nutrient Uptake of Rice. Commun. Soil Sci. Plant Anal. 2016, 3624, 1532–2416. [Google Scholar] [CrossRef]
- Eneji, A.E.; Inanaga, S.; Muranaka, S.; Li, J.; Hattori, T.; An, P.; Tsuji, W.; Eneji, A.E.; Inanaga, S.; Muranaka, S.; et al. Growth and Nutrient Use in Four Grasses Under Drought Stress as Mediated by Silicon Fertilizers. J. Plant Nutr. 2008, 31, 355–365. [Google Scholar] [CrossRef]
- Pulz, A.L.; Crusciol, C.A.C.; Lemos, L.B.; Soratto, R.P. Silicate and Limestone Effects on Potato Nutrition, Yield and Quality under Drought Stress. Rev. Bras. Ciência do Solo 2008, 32, 1651–1659. [Google Scholar] [CrossRef]
- Reboredo, F.; Lidon, F.C.; Pessoa, F.; Duarte, M.P.; Silva, M.J. The uptake of macronutrients by an active silicon accumulator plant growing in two different substrata. Emirates J. Food Agric. 2013, 25, 986–993. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Gouharrizi, Z.Z.; Khorassani, R.; Halajnia, A. The effect of silicon on phosphorus uptake and wheat growth under drought stress in a calcareous soil. Environ. Stresses Crop Sci. 2021, 14(3), 665–673. [Google Scholar]
- Ma, J.F.; Takahashi, E. Function of silicon in plant growth. In Soil, Fertilizer and Plant Silicon Research in Japan, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 151–154. [Google Scholar]
- Hawk, C.; Hyland, J.; Rupert, R.; Colonvega, M.; Hall, S. Assessment of balance and risk for falls in a sample of community-dwelling adults aged 65 and older. Chiropr. Osteopat. 2006, 14, 3. [Google Scholar] [CrossRef][Green Version]
- Vieira da Cunha, K.P.; Williams Araújo do Nascimento, C.; José da Silva, A. Silicon alleviates the toxicity of cadmium and zinc for maize (Zea mays L.) grown on a contaminated soil. J. Plant Nutr. Soil Sci. 2008, 171, 849–853. [Google Scholar] [CrossRef]
- Fan, X.; Wen, X.; Huang, F.; Cai, Y.; Cai, K. Effects of silicon on morphology, ultrastructure and exudates of rice root under heavy metal stress. Acta Physiol. Plant. 2016, 38, 197. [Google Scholar] [CrossRef]
- Li, J.; Leisner, M.; Frantz, J. Alleviation of copper toxicity in Arabidopsis thaliana by silicon addition to hydroponic solutions. J. Am. Soc. Hortic. Sci. 2008, 133, 70–77. [Google Scholar] [CrossRef]
- Flora, C.; Khandekar, S.; Boldt, J.; Leisner, S. Silicon alleviates long-term copper toxicity and influences gene expression in Nicotiana tabacum. J. Plant Nutr. 2019, 42, 864–878. [Google Scholar] [CrossRef]
- Kim, Y.H.; Khan, A.L.; Kim, D.H.; Lee, S.Y.; Kim, K.M.; Waqas, M.; Jung, H.Y.; Shin, J.H.; Kim, J.G.; Lee, I.J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant. Boil. 2014, 14, 13. [Google Scholar] [CrossRef]
- Savić, J.; Marjanović-Jeromela, A. Effect of silicon on sunflower growth and nutrient accumulation under low boron supply. Helia 2013, 36, 61–68. [Google Scholar] [CrossRef][Green Version]
- Elsokkary, I.H. Silicon as a Beneficial Element and as an Essential Plant Nutrient: An Outlook (Review). Alexandria Sci. Exch. J. 2018, 39, 534–550. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech. 2019, 9, 90. [Google Scholar] [CrossRef]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef]
- O’Farrell, N.; Houlton, A.; Horrocks, B.R. Silicon nanoparticles: Applications in cell biology and medicine. Int. J. Nanomed. 2006, 1, 451–472. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol. Biochem. 2017, 110, 70–81. [Google Scholar] [CrossRef]
- Cui, J.; Liu, T.; Li, F.; Yi, J.; Liu, C.; Yu, H. Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environ. Pollut. 2017, 228, 363–369. [Google Scholar] [CrossRef]
- Abdel-Haliem, M.E.F.; Hegazy, H.S.; Hassan, N.S.; Naguib, D.M. Effect of silica ions and nano silica on rice plants under salinity stress. Ecol. Eng. 2017, 99, 282–289. [Google Scholar] [CrossRef]
- Wanyika, H.; Gatebe, E.; Kioni, P.; Tang, Z.; Gao, Y. Mesoporous silica nanoparticles carrier for urea: Potential applications in agrochemical delivery systems. J. Nanosci. Nanotechnol. 2012, 12, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Lodriche, S.S.; Soltani, S.; Mirzazadeh, R. Silicon Nanocarrier for Delivery of Drug, Pesticides and Herbicides, and for Waste Water Treatment. U.S. Patent Application No. 13/406, 28 February 2012. [Google Scholar]
- Janmohammadi, M.; Amanzadeh, T.; Sabaghnia, N.; Ion, V. Effect of nano-silicon foliar application on safflower growth under organic and inorganic fertilizer regimes. Bot. Lithuan. 2016, 22, 53–64. [Google Scholar] [CrossRef]
- Torney, F.; Trewyn, B.G.; Lin, V.S.Y.; Wang, K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotechnol. 2007, 2, 295–330. [Google Scholar] [CrossRef] [PubMed]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef] [PubMed]
- Ulrichs, C.; Mewis, I.; Goswami, A. Crop Diversification Aiming Nutritional Security in West Bengal: Biotechnology of Stinging Capsules in Nature’s Water-Blooms. Nanotechnol. Sci. Appl. 2014, 31–53. [Google Scholar]
- Rouhani, M.; Samih, M.A.; Kalamtari, S. Insecticidal effect of silica and silver nanoparticles on the cowpea seed beetle, Callosobruchus maculatus F. (Col.: Bruchidae). J. Entomol. Res. 2012, 4, 297. [Google Scholar]
- El-Bendary, H.M.; El-Helaly, A.A. First record nanotechnology in agricultural: Silica nano-particles a potential new insecticide for pest control. App. Sci. Rep. 2013, 4, 241–246. [Google Scholar]
- Magda, S.; Hussein, M.M. Determinations of the effect of using silica gel and nano-silica gel against Tutaabsoluta (Lepidoptera: Gelechiidae) in tomato fields. J. Chem. Pharm. Res. 2016, 8, 506–512. [Google Scholar]
- Ziaee, M.; Ganji, Z. Insecticidal efficacy of silica nanoparticles against Rhyzopertha dominica F. and Tribolium confusum Jacquelin du Val. J. Plant Protect. Res. 2016, 56, 250–256. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293. [Google Scholar] [CrossRef]
- Li, Z.Z.; Chen, J.F.; Liu, F.; Liu, A.Q.; Wang, Q.; Sun, H.Y.; Wen, L.X. Study of UV-shielding properties of novel porous hollow silica nanoparticle carriers for avermectin. Pest Manag. Sci. 2007, 63, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, W.; Xu, Y.; Zhang, X. Slow-release formulation of a new biological pesticide, pyoluteorin, with mesoporous silica. J. Agric. Food Chem. 2011, 59, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhang, L.; Fu, G.; Yang, Y.; Zhu, C.; Tao, L. Drought-induced proline accumulation is uninvolved with increased nitric oxide, which alleviates drought stress by decreasing transpiration in rice. J. Plant Res. 2012, 125, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2006. [Google Scholar]
- Perez, C.E.A.; Rodrigues, F.Á.; Moreira, W.R.; Damatta, F.M. Leaf Gas Exchange and Chlorophyll a Fluorescence in Wheat Plants Supplied with Silicon and Infected with Pyricularia oryzae. Biochem. Cell Biol. 2014, 104, 143–149. [Google Scholar] [CrossRef]
- Hattori, T.; Inanaga, S.; Araki, H.; An, P.; Morita, S.; Luxová, M.; Lux, A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant. 2005, 123, 459–466. [Google Scholar] [CrossRef]
- Chen, W.; Yao, X.; Cai, K.; Chen, J. Silicon Alleviates Drought Stress of Rice Plants by Improving Plant Water Status, Photosynthesis and Mineral Nutrient Absorption. Biol. Trace Res. 2011, 142, 67–76. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Merwad, A.M.; Elnaka, E.A. Rice (Oryza Sativa L.) Tolerance to Drought Can Be Improved by Silicon Application. Commun. Soil Sci. Plant Anal. 2018, 49, 945–957. [Google Scholar] [CrossRef]
- Nolla, A.; Faria, R.J.; Korndorfer, G.H.; Silva, T.R.B. Effect of Silicon on Drought Tolerance of Upland Rice. J. Food Agric. Environ. 2012, 10, 269–272. [Google Scholar]
- Gong, H.J.; Chen, K.M.; Zhao, Z.G.; Chen, G.C.; Zhou, W.J. Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages. Biol. Plant. 2008, 52, 592–596. [Google Scholar] [CrossRef]
- Ullah, U.; Ashraf, M.; Shahzad, S.M.; Siddiqui, A.R.; Piracha, M.A.; Suleman, M. Growth behavior of tomato (Solanum lycopersicum L.) under drought stress in the presence of silicon and plant growth promoting rhizobacteria Characterization bacterial strain. Soil Environ. 2016, 35, 65–75. [Google Scholar]
- Kaushik, P.; Saini, D.K. Silicon as a Vegetable Crops Modulator—A Review. Plants 2019, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Sonobe, K.; Inanaga, S.; An, P.; Morita, S. Effects of silicon on photosynthesis of young cucumber seedlings under osmotic stress. J. Plant Nutr. 2008, 31, 1046–1058. [Google Scholar] [CrossRef]
- Gunes, A.; Pilbeam, D.J.; Inal, A.; Coban, S. Influence of silicon on sunflower cultivars under drought stress, I: Growth, antioxidant mechanisms, and lipid peroxidation. Commun. Soil Sci. Plant Anal. 2008, 39, 1885–1903. [Google Scholar] [CrossRef]
- Dupree, P.; Babu, T.; White, B.; Tubana, B.; Datnoff, L. Survey of the plant-available silicon status of agricultural soils in Louisiana. J. Plant Nutr. 2017, 41, 273–287. [Google Scholar] [CrossRef]
- Pedreira, N.G.; de Souza Junior, J.P.; dos Santos, A.C.A.; Flores, R.A.; Aragão, A.S.; de Mello Prado, R.; Arruda, E.M.; da Costa, C.F. Nutrition and production of Helianthus annuus in a function of application of leaf silicon. J. Plant Nutr. 2018, 42, 137–144. [Google Scholar] [CrossRef]



| Application Rate (kg/ha) | Number of Panicles (×104/ha) | Number of Spikelets/Panicle | Yield (kg/ha) |
|---|---|---|---|
| 0 | 4.84 | 74.7 | 7010 |
| 75 | 4.94 | 73.9 | 7870 |
| 105 | 5.03 | 74.8 | 8160 |
| 135 | 5.03 | 76.8 | 8230 |
| Plant Species | Si% in Plant Biomass |
|---|---|
| Rice (O. sativa) | 4.17 |
| Wheat (Triticum aestivum) | 2.45 |
| Barley (Hordeum vulgare) | 1.82 |
| Tomato (Lycopersicon esculentum) | 1.54 |
| Sugarcane (Saccharum officianum) | 1.51 |
| Soybean (Glycine max) | 1.39 |
| Lettuce (Lactuca serriola) | 0.97 |
| Corn (Zea mays) | 0.82 |
| Potato (Solanum tuberosum) | 0.4 |
| Crop Species | Place & Time | Materials Used | Dosage | Application Method | Findings | References |
|---|---|---|---|---|---|---|
| Rice (O. sativa) |
|
|
|
|
| |
| Wheat (Triticum aestivum) |
| K2SiO3 |
| SA |
| |
| Potato (Solanum tuberosum) |
|
|
|
|
| |
| Lettuce (Lactuca sativa), Pea (Pisum sativum), Carrot (Daucus carota) | Sweden (2018) | K2SiO3 | 80 and 1000 kg Si/ha Soil 100, 500, 1000 and 5000 µM Si in nutrient medium | SA | Increases mineral nutrient (Ca, P, S, Mn, Zn, Cu) accumulation | Bocharnikova et al. [41], Greger et al. [42] |
| Chard (Beta vulgaris), Kale (B. oleracea var. sabellica) | Brazil (2019) | NaKSiO3, K2SiO3 | 0.00; 0.84; 1.68 and 2.52 g/L | FA | Increased accumulated Si in both sources and vegetables Increased fresh matter content | Pedreira et al. [107] |
| Corn (Zea mays) | Turkey (2017) | Exogenous Si | 0, 300, 750 kg Si/ha | SA (broadcasting) | Formation of less soluble zinc silicates in the cytoplasm | Keeping et al. [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rea, R.S.; Islam, M.R.; Rahman, M.M.; Nath, B.; Mix, K. Growth, Nutrient Accumulation, and Drought Tolerance in Crop Plants with Silicon Application: A Review. Sustainability 2022, 14, 4525. https://doi.org/10.3390/su14084525
Rea RS, Islam MR, Rahman MM, Nath B, Mix K. Growth, Nutrient Accumulation, and Drought Tolerance in Crop Plants with Silicon Application: A Review. Sustainability. 2022; 14(8):4525. https://doi.org/10.3390/su14084525
Chicago/Turabian StyleRea, Rafea Sultana, Mohammad Rafiqul Islam, Mohammad Mahmudur Rahman, Bibhash Nath, and Ken Mix. 2022. "Growth, Nutrient Accumulation, and Drought Tolerance in Crop Plants with Silicon Application: A Review" Sustainability 14, no. 8: 4525. https://doi.org/10.3390/su14084525
APA StyleRea, R. S., Islam, M. R., Rahman, M. M., Nath, B., & Mix, K. (2022). Growth, Nutrient Accumulation, and Drought Tolerance in Crop Plants with Silicon Application: A Review. Sustainability, 14(8), 4525. https://doi.org/10.3390/su14084525








